Table of Contents
| Preamble | 4 |
| Key Revisions | 5 |
| Chapter 1. Summary of the Guidelines | 7 |
| Chapter 2. Clinical Diagnosis of Atherosclerosis | 12 |
| Chapter 3. Comprehensive Risk Assessment | 14 |
| 3-1. Risk Factor Assessment | 14 |
| 3-2. Disease Concept and Diagnostic Criteria for Metabolic Syndrome | 27 |
| Chapter 4. Comprehensive Risk Management | 29 |
| 4-1. Absolute Risk of Atherosclerotic Cardiovascular Diseases (ASCVD) and Lipid-Management Targets | 29 |
| 4-2. Lifestyle Modification | 34 |
| 4-3. Drug Therapy | 47 |
| 4-4. Managing Major High-Risk Conditions | 59 |
| 4-5. Implementation of Comprehensive Risk Assessment and Management | 66 |
| Chapter 5. Familial Hypercholesterolemia | 75 |
| Chapter 6. Other Types of Primary Dyslipidemias | 80 |
| Chapter 7. Elderly | 83 |
| Chapter 8. Women | 86 |
| Chapter 9. Children | 90 |
| References | 92 |
| Appendix 1: Physical Activity Guidelines for Health Promotion 2013 | 137 |
| Appendix 2: Exercise Guidelines for Health Promotion 2006 | 138 |
| Appendix 3: Method for Achilles Tendon Radiography | 139 |
Preamble
Every 5 years, the JAS publishes guidelines for the treatment of dyslipidemia and atherosclerosis. To date, this society has released four such guidelines. Since 2007, the JAS has included objectives that consider all the risk factors for atherosclerotic cardiovascular diseases (ASCVD) and has accordingly been publishing manuals, such as the “Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases.” Because guidelines should be based on evidence of diagnoses and treatments that have already been validated, regular revisions are necessary to administer medical care of high quality.
Apart from age, gender, and family history, for which clinical intervention is not possible, the major risk factors for ASCVD include diabetes, hypertension, smoking, and dyslipidemia. Dyslipidemia is a huge risk factor for coronary artery disease (CAD), a form of ASCVD, and in the current set of guidelines, we will be dealing with CAD as the main disease of interest. Nevertheless, other risk factors also ought to be thoroughly managed as part of the efforts for preventing ASCVD.
In 1987, a consensus conference on hyperlipidemia was held at the JAS, and reference values for diagnosing hyperlipidemia were proposed. Although the reference values were established when there was a lack of evidence in Japan, it was an unprecedented proposal that provided the clinical limits of lipids for the prevention of ASCVD. In 1988, the evidence-based National Cholesterol Program (NCEP) was announced in the United States, and since then, the reference values have undergone several revisions. At that time, there was a strong momentum for the creation of a set of Japan-specific guidelines; therefore, the “Guidelines for Diagnosis and Treatment of Hyperlipidemia” were established in 1997. Following the publication of epidemiological studies, such as the Hisayama study, and observational studies, such as J-LIT, we gradually gathered sufficient evidence in our country. Subsequently, the “Guidelines for Diagnosis and Treatment of Atherosclerotic Cardiovascular Diseases”, which took risk factors into consideration, were established in 2002.
The “Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2007” were later published in 2007. A change of terms from “hyperlipidemia” to “dyslipidemia” and replacement of total cholesterol with LDL cholesterol (LDL-C) as the major risk factor were features of this guideline. Establishing LDL-C as the major risk factor has led to an even more direct approach toward managing the risk factors for ASCVD. Furthermore, raising the topic on the importance of measures against metabolic syndrome and smoking, which have gained considerable attention, serves as a reminder to pay attention also to measures for the prevention of ASCVD in our daily lives. For healthy individuals, the risk of ASCVD has traditionally been assessed through the relative risk; however, this was replaced by the use of absolute risk in the guidelines released in 2012. This change resulted in a higher awareness of the importance of combined risk factors. Considering the need to comprehensively manage the wide range of atherosclerotic risks, we have compiled information on the “Comprehensive Management of Atherosclerotic Cardiovascular Diseases” and have created charts, which incorporate the guidelines of various scientific societies.
We have striven to include more updated information in the current revision; hence, you will find several newly introduced Clinical Questions (CQs), which are the mainstream of recent guidelines.
Shizuya Yamashita
President, the Japan Atherosclerosis Society
Key Revisions
1. CQs and Systematic Review (SR)
In the subsections on dyslipidemia in the assessment of risk factors, absolute risk of ASCVD, lipid management targets as well as drug therapy and diet therapy in improving lifestyle habits, we created CQs and performed an SR based on the MINDS method. For our SR, we essentially chose the literature published before the end of 2015.
2. Calculation of Absolute Risk
Following the 2012 version, the assessment of risk has been performed using the absolute risk calculation described in this set of guidelines. The NIPPON DATA80, which was used to calculate the absolute risk in the 2012 version, was the result of baseline surveys conducted when statins were not available. It is suited to the observation of the natural course of disease, and the data are highly useful; however, using death instead of disease onset as the outcome and the absence of information on LDL-C and HDL-C are major issues, in addition to some others. SR indicated that the Suita study, which used CAD as its outcome, is most suitable for risk calculation in this set of guidelines. We believe that the determination of the incidence rate of CAD instead of the overall risk assessment has enabled a clearer demonstration of the importance of each risk.
3. Addition of High-Risk Conditions
In view of the plan to compile an extensive list of risks for atherosclerosis, we included hyperuricemia and sleep apnea syndrome (SAS) as conditions to be considered. Although these conditions may contribute to atherosclerotic lesions to different extents, it is necessary to consider them from the perspective of comprehensive management.
4. Stricter LDL-C Control in High-Risk Conditions for Secondary Prevention
For the secondary prevention of high-risk conditions, such as familial hypercholesterolemia (FH) and acute coronary syndrome (ACS), we proposed an even stricter LDL-C control level than the current LDL-C control level of < 100 mg/dL.
5. Elaborating on FH
In conjunction with the launch of new drugs, the addition of pediatric FH as an indication for statins, and so on, we have provided a detailed description of the diagnosis and treatment of FH. To facilitate easy comprehension, we have used a flow chart for describing the treatment methods.
6. Evidence Levels and Recommendation Levels
Similar to the previous guidelines, we have included statements at the beginning of each section. The evidence levels and recommendation levels have also been provided (Tables below).
The evidence levels are separately presented as evidence from therapeutic interventions and that from epidemiological studies. The evidence in Japan was regarded as the core of the evidence levels; however, please note that important data from various other countries may have been used wherever the data from Japan was insufficient.
Classification of Evidence Levels in Relation to Treatment and Diagnosis
| 1+ | High-quality RCT* and their MA/SR |
| 1 | Other RCT* and their MA/SR |
| 2 | Prospective cohort studies, their MA/SR, and (pre-determined) RCT sub-analysis |
| 3 | Non-randomized comparative studies, before–after comparative studies, and retrospective cohort studies, case–control studies, their MA/SR, and RCT post hoc sub-analysis |
| 4 | Cross-sectional studies and case series |
RCT: Randomized controlled trial, MA: Meta-analysis, SR: Systematic review
A high-quality RCT is defined as a study that (1) involves a large number of subjects (high statistical power), (2) is double-blinded and independently assessed, (3) has a high follow-up rate (low drop-out rate) and few of protocol deviations, (4) includes a clear method for random allocation, etc.
Classification of Evidence Levels of Epidemiological Studies
| E-Ⅰa: | Meta-analysis of cohort studies |
| E-Ⅰb: | Cohort studies |
| E-Ⅱ: | Case-control studies and cross-sectional studies |
| E-Ⅲ: | Descriptive studies (case series) |
Recommendation Levels
| A | Strong recommendation |
| B | Weak recommendation |
Recommendations made according to consensus are indicated by the word “consensus.”
Conflict of Interest
In accordance with the “COI Management Guidelines for Clinical Research” established by the Japan Association of Medical Sciences' COI committee, a conflict of interest (COI) statement has been obtained from each member of the committee involved in drafting the Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. The names of the enterprises disclosed in the COI statement are provided below. The applicable period is January 01, 2013, to December 31, 2015.
Amgen Astellas BioPharma K.K., Astellas Pharma Inc., AstraZeneca plc, Abbott Japan Co., Ltd., Izumisano city, Eisai Co., Ltd., Aegerion Pharmaceuticals, Inc., MSD K.K., Otsuka Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Omron Healthcare Co., Ltd., Curves Japan Co., Ltd., Kaizuka city, Kaneka Medix Corp., Kissei Pharmaceutical Co., Ltd. Kyowa Hakko Kirin Co., Ltd., Kyowa Medex Co., Ltd., Kirin Company, Limited, Quintiles Transnational Japan, GlaxoSmithKline K.K., Kowa Company, Ltd., Kowa Pharmaceutical Co., Ltd., Signpost Corporation, Sanofi K.K., Sanwa Chemistry Co., Ltd, Sanwa Kagaku Kenkyusho Co., Ltd., JCR Pharmaceuicals Co., Ltd., Shionogi & Co., Ltd. Skylight Biotech, Inc. Zuyou Co., Ltd., Daiichi Sankyo Company, Limited., Taisho Pharmaceutical Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Company Limited., Mitsubishi Tanabe Pharma Corporation, Chugai Pharmaceutical Co., Ltd., Teijin Home Healthcare Limited., Teijin Pharma Limited., Terumo Corporation, Tomiyama Scientific Industry Co., Ltd., Eli Lilly Japan K.K., Japan Blood Product Organization, Nippon Boehringer Ingelheim Co., Ltd., Medtronic Japan Co., Ltd., Novartis Pharma K.K., Novo Nordisk Pharma Ltd., Bayer Yakuhin, Ltd., Biogen Idec, Inc., PPD Japan, K.K., Lake Biwa Consortium of Health and Welfare, Pfizer Japan Inc., Philips Respironics GK, Fukuda Denshi Co., Ltd. Bristol-Myers Squibb Company, Boston Scientific Corporation Japan, Maruha Nichiro Corporation, Meiji Seika Pharma Co., Ltd., Medical Review Co., Ltd., Merck & Co., Mochida Pharmaceutical Co., Ltd., Rinku General Medical Center, ResMed Inc.
Chapter 1. Summary of the Guidelines
1. Clinical Diagnosis of Atherosclerosis
To prevent ASCVD, it is essential to identify the presence of atherosclerotic lesions and understand their severity before the clinical symptoms appear. The risk factors need to be managed and treated, with consideration for the prevention of progression and regression of such lesions. Therefore, it is necessary to diagnose the extent to which atherosclerosis has progressed.
Angiography, intravascular ultrasound, and invasive diagnostic methods involving a vascular endoscope are occasionally used for diagnosis in patients who present symptoms and for the secondary prevention of ASCVD. Conversely, non-invasive methods are mainly used for assessing atherosclerosis for the primary prevention of ASCVD in patients who do not present with any symptoms. These non-invasive methods of assessment are generally classified into either morphological examinations [ultrasound, multi-detector computed tomography (MDCT), and magnetic resonance imaging/magnetic resonance angiography (MRI/MRA)] or vascular function tests [ankle–brachial index (ABI), brachial–ankle pulse wave velocity (baPWV), cardio–ankle vascular index (CAVI), and flow-mediated vasodilation (FMD)]. We anticipate that carotid intima–media thickness (IMT)/plaque, ABI, baPWV, CAVI, and FMD will be useful means for predicting the future risks of ASCVD. In addition, MDCT is a simple and highly specific examination method, which can easily detect coronary lesions. Each patient should be assessed using one or a combination of methods as applicable to his or her condition.
2. Comprehensive Risk Assessment and Management
1). Risk Assessment and Management
Assessing each risk factor for atherosclerosis and managing the factors that can be resolved through interventions are important for preventing ASCVD. Evidence from numerous epidemiological studies has revealed many risk factors for atherosclerosis. They include dyslipidemia, smoking, hypertension, diabetes, chronic kidney disease (CKD), aging, male sex, family history of CAD, history of CAD, non-cardiogenic cerebral infarction, peripheral artery disease (PAD), abdominal aortic aneurysm (AAA), hyperuricemia, and SAS as well as metabolic syndromes that are caused by the accumulation of visceral fats and insulin resistance. Led by The Japanese Society of Internal Medicine, 11 scientific societies and The Japanese Association of Medical Science/Japan Medical Association published the “Comprehensive Risk Management Chart for the Prevention of Cerebro- and Cardiovascular Diseases” in 2015 as a comprehensive set of guidelines for the prevention of ASCVD. The methods of assessment and management were thoroughly explained in six steps in the guidelines, which covered topics from screening (based on the rationale behind the chart) to drug therapy.
2). Diagnostic Criteria and Management Standards for Dyslipidemia
The incidence of CAD is high when the LDL-C or triglyceride (TG) levels are high or when the HDL-C level is low. The diagnostic criteria for dyslipidemia are established in this set of guidelines (Table 1) with regard to preventive screening for ASCVD.
Table 1. Diagnostic Criteria for Dyslipidemia (Blood Collected from Patients in Fasting State)*.
| LDL-C | ≥ 140 mg/dL | Hyper-LDL cholesterolemia |
| 120–139 mg/d | Borderline hyper-LDL cholesterolemia** | |
| HDL-C | < 40 mg/dL | Hypo-HDL cholesterolemia |
| TG | ≥ 150 mg/dL | Hypertriglyceridemia |
| Non-HDL cholesterol | ≥ 170 mg/dL | Hyper-non-HDL cholesterolemia |
| 150–169 mg/dL | Borderline hyper-non-HDL cholesterolemia** |
“Fasting state” is defined as fasting for at least 10 h. However, consumption of liquids with no calories, such as water and tea without milk or sugar, is allowed.
If screening shows borderline hyper-LDL cholesterolemia and borderline hyper-non-HDL cholesterolemia, investigate whether a high-risk condition is present and consider the need for treatment.
- LDL-C is derived using the Friedewald formula (TC.HDL-C.TG/5) or through a direct method.
- Non-HDL-C (TC.HDL-C) or a direct method is used if the TG level is 400 mg/dL and greater or if postprandial blood is collected. However, if hypertriglyceridemia is absent during screening, the risk shall be assessed with the consideration that the difference from LDL-C can possibly turn out to be smaller than + 30 mg/dL.
When employing this set of diagnostic criteria, the basis is to measure the total cholesterol (TC), TG, and HDL-C levels using blood collected in a fasting state and subsequently calculating the LDL-C using the Friedewald formula (LDL-C=TC–HDL-C–TG/5). However, this formula cannot be applied if the TG level is 400 mg/dL and greater, or when using postprandial blood. In such cases, non-HDL-C ( = TC– HDL-C) shall be used. The accuracy of the LDL-C direct measurement method has improved over time, and it can therefore be used in place of the Friedewald formula.
In the 2012 version of the guidelines, for primary prevention, patients are stratified based on the absolute risk, and we had established management standards for dyslipidemia, which catered to the stratification. We followed the same plan while formulating the current guidelines and searched for methods of assessment according to absolute risk. Specifically, we formulated the CQ, “Does an assessment method that predicts the incidence of or death due to ASCVD in the Japanese exist?”, and performed an SR. Although nine studies were selected by SR, we eventually chose the Suita study because (1) LDL-C and HDL-C were both included as predictive indicators, (2) the LDL-C levels were categorized in detail, (3) stroke was not included as an endpoint, and (4) the outcome was set as event onset instead of death. We performed the stratification based on the Suita score (Fig. 1). As shown in Fig. 2, due to the complexity of calculating the Suita score, we created an application for categorization so that this method can be easily applied in our daily medical practice (http://www.j-athero.org/publications/gl2017_app.html). In addition, we created a chart describing the stratification by gender, age, and number of risk factors (Fig. 3). Categorization based on Fig. 2 and that based on Fig. 1 have been verified through simulation and found to be almost consistent.
Fig. 1.
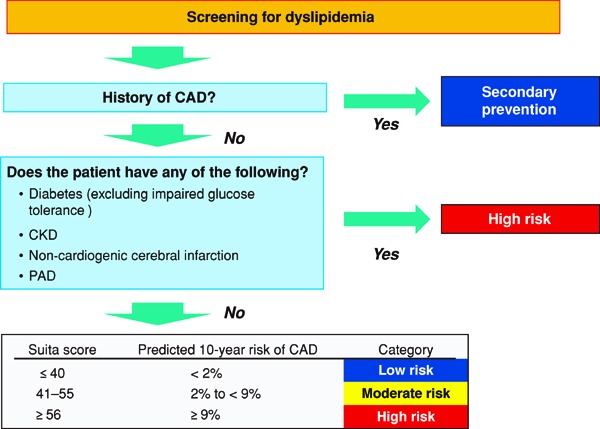
Flowchart Using the Suita Score to Establish LDL-C Management Targets, from the Perspective of CAD Prevention
• The Suita score is calculated based on Fig. 2.
• Note: For patients diagnosed with FH and those diagnosed with familial type Ⅲ hyperlipidemia, do not use this chart and refer to Chapter 5 (Familial Hypercholesterolemia) and Chapter 6 (Other Types of Primary Dyslipidemias), respectively.
Fig. 2.
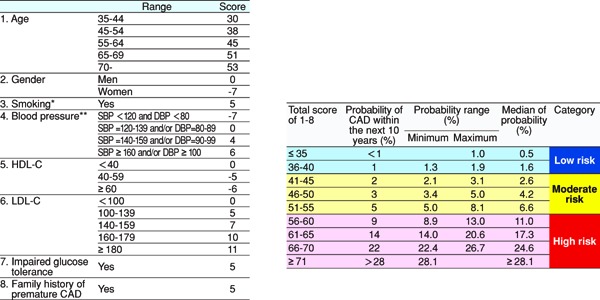
Model for Predicting CAD Onset Using the Suita Score
* Ex-smokers should be regarded as nonsmokers. Note that the risk of CAD decreases by almost half 1 year after smoking cessation and drops to the same level as in nonsmokers after 15 years of smoking cessation.
** The current values are used even if the patient is currently undergoing treatment or not. However, counsel the patient while keeping in mind that patients undergoing treatment for hypertension have a higher risk of CAD than those who have the same blood pressure value without undergoing treatment.
Fig. 3.
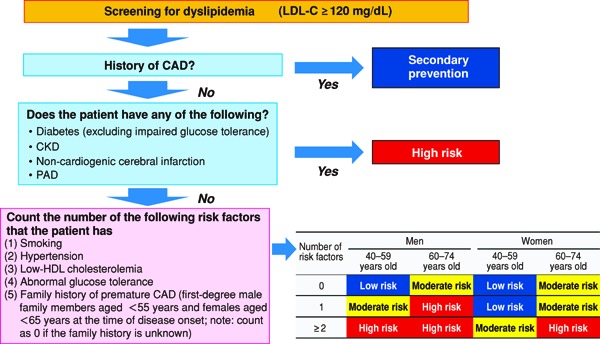
Flowchart for Establishing LDL-C Management Targets from the Perspective of CAD Prevention (Simplified Version Using Risk Factors)
The lipid management targets that catered to this categorization are shown in Table 2. For primary prevention, the administration of drug therapy should generally be considered after lifestyle modifications have been made for a certain period and when the effects have been ascertained. The management targets are challenging goals by utmost effort for patients with low or moderate risk. A 20–30% decrease in LDL-C also indicates a reduction in the risk of CAD by 30%; hence, we decided that a reduction of 20–30% in LDL-C levels can be considered another target for such patients. For secondary prevention, it is advisable to initiate drug therapy, with the management targets shown in Table 2 as the goal, along with lifestyle modifications.
Table 2. Lipid Management Targets for Patients with Different Risk Category.
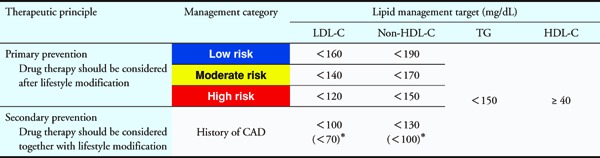 |
For patients who are also suffering from high-risk conditions, such as FH, ACS, and diabetes complicated by other high-risk conditions shown in Table 3b, stricter LDL-C control should be considered, with a level of < 70 mg/dL as the target.
- Although non-drug therapy is used as a standard means for achieving the management target in primary prevention, drug therapy should be considered for patients with low risk if the LDL-C level is ≥ 180 mg/dL. The possibility of FH should also be considered (refer to Chapter 5).
- Achieving the LDL-C management target should be the first goal, and reaching the non-HDL-C management target should be the next goal after the first goal has been achieved. Managing the TG and HDL-C levels is important during this process.
- These values are challenging goals by utmost effort; a 20%–30% reduction in LDL-C levels for primary prevention (low or moderate risk) and a decrease of ≥ 50% for secondary prevention are also possible targets.
- For elderly patients (aged ≥ 75 years), refer to Chapter 7.
3). Lifestyle Modification
As previously mentioned, lifestyle modification is required for both primary prevention and secondary prevention patients. The cessation of smoking is important for preventing atherosclerosis, and controlling the energy intake to reduce obesity would lead to improvements not just in obesity but also in other risk factors. Although a limited number of studies have followed the onset of ASCVD as an endpoint, diet therapy based mainly on the Japanese dietary pattern contributes to decrease risk factors, including improvement of lipid metabolism. In terms of exercise therapy, epidemiological studies have shown that the amount of exercise and level of physical fitness are negatively correlated with CVD, indicating the importance of appropriate exercise.
4). Drug Therapy
Given that statin therapy has been shown to be beneficial for prevention of ASCVD not just overseas but also in Japan, we believe that it is appropriate to consider statins as the first medication of choice for controlling LDL-C levels. In high-risk primary prevention patients, the management target for LDL-C should be < 120 mg/dL. On the other hand, for secondary prevention, aggressive treatment with the aim of lowering LDL-C level at least < 100 mg/dL should be initiated immediately after the disease onset; furthermore, an even lower target value should be considered when patients are complicated with other high risk clinical conditions. The application, effectiveness, and safety of oral hypolipidemic agents other than statins have already been validated; thus, before prescribing them, clinicians should always pay attention to the indications as well as clinical status of contraindication and careful administration. Ezetimibe, PCSK9 inhibitors, and EPA are the medications that have been proven to be effective for the prevention of ASCVD when used in combination with statins. When prescribing hypolipidemic agents, efforts should be made to improve adherence for medication because good adherence has proven to lead to better efficacy for prevention of ASCVD.
The effects on plasma lipids' levels of each agent are listed in Table 4. Taking into account of the importance for prevention of ASCVD as well as the attainability of the target level for each lipid through drug therapy, we set the order of intervention starting with LDL-C, followed by non HDL-C and TG. In the main chapter of “Drug Therapy”, we also referred to the significance or meaning of management for hypertriglyceridemia.
Table 4. Classification of Medications Used to Treat Dyslipidemia According to Their Efficacy.
| Category | LDL-C | NonHDL-C | TG | HDL-C | Major drug name |
|---|---|---|---|---|---|
| Statin | ↓↓∼ ↓↓↓ |
↓↓∼ ↓↓↓ |
↓ | -∼↑ | Pravastatin, Simvastatin, Fluvastatin, Atorvastatin, Pitavastatin, Rosuvastatin |
| Intestinal cholesterol transporter inhibitor (Cholesterol absorption inhibitor) | ↓↓ | ↓↓ | ↓ | ↑ | Ezetimibe |
| Anion exchange resin | ↓↓ | ↓↓ | ↑ | ↑ | Colestimide, Cholestyramine |
| Probucol | ↓ | ↓ | – | ↓↓ | Probucol |
| PCSK9 inhibitor | ↓↓↓↓ | ↓↓↓↓ | ↓∼↓↓ | –∼↑ | Evolocumab, Alirocumab |
| MTP inhibitor* | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓ | Lomitapide |
| Fibrate | ↑∼↓ | ↓ | ↓↓↓ | ↑↑ | Bezafibrate, Fenofibrate, Clinofibrate, Clofibrate |
| Selective peroxisome proliferator-activated receptor α modulator (SPPARMα) | ↑∼↓ | ↓ | ↓↓↓ | ↑↑ | Pemafibrate |
| Nicotinic acid derivative | ↓ | ↓ | ↓↓ | ↑ | Niceritrol, Nicomol, Tocopheryl Nicotinate |
| N-3 polyunsaturated fatty acid | – | – | ↓ | – | Ethyl icosapentate, Omega-3-acid ethyl ester |
Applicable only to patients with homozygous FH
↓↓↓↓: ≤ – 50% ↓↓↓: −50∼30% ↓↓: −20∼30% ↓: −10∼ −20%
↑: 10∼20% ↑↑: 20∼30% –: −10∼10%
5). Major High-Risk Conditions
A history of CAD, diabetes, and cerebrovascular diseases are observed in patients requiring more active treatment due to unsatisfactory disease management, the presence of complications, and overlapping risk factors. Therefore, we created a special subsection in this set of guidelines to provide detailed explanations on cases requiring enhanced management.
3. Primary Dyslipidemias
FH is a hereditary disease, and its heterozygote exists in 1 of 200–500 individuals. FH poses a high risk of CAD and is frequently encountered by general practitioners. Because the rate of diagnosis in Japan is still low, there is a need to accurately diagnose and treat this condition by assessing the family history, tendon xanthoma, and LDL-C levels, which are part of the diagnostic criteria for FH. In this set of guidelines, we will discuss the guiding principles for treating FH during pregnancy and childbirth and in children. In addition to the treatment methods involving LDL apheresis, we have included explanations on the types of primary dyslipidemia other than FH. Patients who are suspected to be suffering from any of these diseases should be referred to specialists for further assessment.
4. The Elderly, Women, and Children
In the elderly persons 65–74 years of age, a high LDL-C level is an important risk factor for CAD, and the effect of statin therapy on CAD prevention and primary prevention of non-cardiogenic cerebral infarction is promising. Meanwhile, because the effect of statin therapy on primary prevention in the elderly persons ≥ 75 years of age is not evident, the objective is to manage cases individually, according to the decisions of the attending physician. The treatment for women is basically lifestyle modification, but for secondary prevention patients and patients with FH or at a high risk of CAD, drug therapy shall be considered. It is also important for children to maintain the intake of proper meals and continue with exercise therapy. Similar to adults, the management of secondary dyslipidemia in children should focus on treating the primary disease. It should be kept in mind that the diagnostic criteria for pediatric cases of dyslipidemia, including FH, are different from those for adult cases.
Chapter 2. Clinical Diagnosis of ASCVD
From the perspective of preventing ASCVD, it is important to identify the presence of arteriosclerosis and atherosclerotic lesions and understand its severity before clinical symptoms appear. The management and treatment of risk factors with the prevention of progression or even regression taken into consideration is essential. Invasive diagnostic methods, such as angiography, are necessary in the secondary prevention of ASCVD. However, noninvasive methods are mainly used in primary prevention to assess arteriosclerosis and atherosclerosis. In this section, we will be discussing the methods for assessing arteriosclerosis and atherosclerosis.
1. Morphological Examinations
1). Ultrasonography
Ultrasonography is a widely used noninvasive imaging method. Lesions in the peripheral arteries, such as the carotid arteries, and arteries in the lower extremities, can be assessed using an ultrasound machine that comes with a high-frequency linear probe of ≥ 7 MHz.
The Japan Society of Ultrasonics in Medicine and The Japan Academy of Neurosonology recommend using ultrasonography as a standard method to measure the intima–media thickness (IMT), plaques (localized protruding lesions of ≥ 1.1 mm), stenosis and such for assessing the degree of arteriosclerosis, particularly in the carotid arteries1–3). IMT is assessed as the thickness adjusted by age4). It is also used to reflect the degree of systemic arteriosclerosis or as an alternative assessment factor for predicting the risk of co-existence or onset of ASCVD (e.g., CAD, PAD, cerebrovascular diseases, etc.)5–8). Although the presence of plaque has a stronger significance in disease prediction compared to the IMT, a high IMT value in cases where plaques are not detected is the underlying pathogenetic mechanism for the development of plaques. With reference to the Mannheim consensus, it is also necessary to assess the properties of plaques that have a maximum IMT of > 1.5 mm3, 9), and assessment is especially important for some plaques that can possibly lead to cerebral embolism (e.g., echolucent plaques, ulcers, mobile lesions, and lipid-rich plaques). If short-axis scanning shows a buildup of plaque in ≥ 50% of the vascular lumen, then the degree of stenosis needs to be assessed. When the stenosis is significant (i.e., ≥ 70%), in addition to active medical treatment, carotid endarterectomy or carotid artery stenting should also be considered.
Performing the assessment to determine the properties of the plaques and the percentage of stenosis in the same way as it is done in the carotid arteries is likewise important for arteries in the lower extremities10). Furthermore, confirming the presence of collateral circulation, the patterns of blood flow waveforms and the below-knee transit time of vessel flow (TVF) would make it possible to estimate where the stenotic portions are11).
Ultrasonography is also a useful method for diagnosing atherosclerotic renal artery stenosis in the renal arteries12, 13).
2). Computed Tomography (CT)
CT is a method of examination that can diagnose arteriosclerosis in a short amount of time. It can also determine whether aneurysm is present by measuring the size of the artery. Furthermore, as the degree of calcification, fats, and fiber content can be estimated to some extent based on the CT number, it is an excellent means for confirming the presence of calcified lesions in the aorta and peripheral arteries. Multidetector CT (MDCT) offers superior imaging speed and spatial resolution, and by injecting a contrast medium through the peripheral veins, it enables the visualization of diseases in all the arteries and coronary arteries. It is commonly used to screen for CAD. It has excellent specificity14–17), and organic coronary stenosis can almost be ruled out if no abnormalities are detected using this technique.
3). Magnetic Resonance Imaging/Angiography (MRI/MRA)
MRI is especially useful for detecting lesions in the brain, which include ischemic changes and cerebral infarction. MRA provides an excellent means for visualizing stenosis and obstructive lesions in the intracranial arteries, carotid arteries, aorta, renal arteries and other blood vessels. Nowadays, non-contrast enhanced MRA is sometimes used in place of angiography. The properties of the plaques can also be assessed using MRI plaque imaging.
4). Catheterization
To date, angiography using a catheter remains as one of the main methods for diagnosing arterial stenosis despite being an invasive examination. Stenotic portions are assessed on the basis of the percentage of stenosis, which is calculated using the luminal diameter of normal-appearing portions and stenotic portions. However, limitations such as eccentric plaques and compensatory remodeling hinder the accurate determination of plaque volume. Meanwhile, intravascular ultrasound (IVUS), optical coherence tomography (OCT) and the vascular endoscope are some other methods that provide an excellent assessment of the plaque volume as well as the properties of the plaques.
2. Vascular Function Tests18)
1). Ankle–Brachial Index (ABI) and Toe–Brachial Index (TBI)
ABI is the ratio of the blood pressure (BP) in the brachial artery to the BP at the ankle joint. This ratio is an indicator of narrowing of the central major arteries from the ankle joint or the presence of obstructive lesions and the degree of compensation by collateral circulation. Methods of measurement include the Doppler technique and the oscilloscope. The Korotkoff sounds should be verified when measuring the BP in the extremities using a sphygmomanometer and a stethoscope. On the other hand, the oscillometric method is employed when performing automated measurement using an automated sphygmomanometer or specialized equipment. Although the correlation between both methods is generally good, the precision of the oscillometric method is low when used in cases of critical limb ischemia. If the ABI is ≤ 0.9, then the presence of obstructive lesions in the lower extremities should be suspected19, 20). TBI is the ratio of the BP in the brachial artery to the blood pressure at the toes. Measuring both the ABI and TBI allows us to gauge the peripheral artery obstructive lesions distal than ankle joints. The reference TBI value is ≥ 0.7, and the presence of obstructive lesions in the arteries of the lower extremities should be suspected if the resulting value is ≤ 0.6. It is necessary to note that diabetes patients and patients undergoing dialysis are prone to calcification in the walls of arteries below the knee, and hence, there are cases in which the ABI cannot be accurately measured.
2). Brachial-Ankle Pulse-Wave Velocity (baPWV)
The pulse-wave velocity (PWV) produced by cardiac output reflects the stiffness of arteries21). It can be easily determined by measuring the pulse waves in the extremities using a specialized device. However, it is necessary to note that the PWV is an indicator of arterial stiffness and does not necessarily reflect atherosclerosis. The PWV is the speed at which the aortic vibration (i.e., pulse wave) generated by the beating of the heart is transmitted to the peripheral artery. It is proportionate to the stiffness and thickness of the arterial walls. The carotid-femoral PWV (cfPWV) and baPWV are the two forms of PWV measurement. baPWV is currently being used in clinical practice in Japan. For baPWV, influences during BP measurement must be taken note of.
Aging22), hypertension23), diabetes24), and pulse rate22) are CVD risk factors that have been reported to cause increased baPWV, and they show a good correlation with the Framingham Risk Score. A baPWV of 1,400 cm/sec corresponds to the moderate risk level in the Framingham Risk Score.
3). Stiffness Parameter β and Cardio-ankle Vascular Index (CAVI)
Stiffness parameter β is an index that represents the localized intrinsic stiffness of the arterial walls. Arterial elasticity is hardly affected by BP, and stiffness parameter β has been designed as an index of arterial elasticity that is corrected using BP during measurement. It is calculated using the formula ln (Ps/Pd)/[(Ds-Dd)/Dd], with BP and changes in carotid artery caliber as the variables25). Additionally, it has been reported that stiffness parameter β is correlated with carotid atherosclerosis26, 27).
The CAVI is an index that represents the elasticity of the entire artery, from its aortic root to the ankle, and it is derived by applying the concept of the stiffness parameter β to the long arteries. A feature of CAVI is its nondependence on BP during measurement28). The CAVI increases with age28), and patients with cerebral infarction, CVD29), chronic kidney disease (CKD), and vasculitis have high CAVI values. It is also increased in patients who suffer from hypertension, diabetes, metabolic syndromes, sleep apnea syndrome (SAS), smoking, stress caused by disasters, etc., but at the same time, it has been reported that the CAVI improves with treatment of these contributing factors25). Prospective surveys on cardiovascular events have revealed that a high CAVI value is associated with a high frequency of cardiovascular events30).
In addition to these, the central BP is also an index that reflects vascular function.
4). Vascular Endothelial Function
Reactive hyperemia following a 5-minute avascularization in the forearm and certain drugs such as acetylcholine cause a vascular endothelium-dependent increase in blood flow, and vascular endothelial function is assessed by measuring the resulting increase in arterial diameter and blood flow. The first method of assessment is strain-gauge plethysmography, which measures the changes in circumference of the extremities as the arterial pulse is produced. The second method is flow-mediated dilatation (FMD), in which the changes in diameter of arteries in the upper arm are measured by ultrasonography. FMD is a test that assesses the extent of brachial artery dilation caused by reactive hyperemia after 5 minutes of forearm ischemia. The formula used for calculation is FMD (%) = (diameter of the most dilated blood vessel - resting blood vessel diameter)/resting blood vessel diameter × 100. The normal FMD value is ≥ 6–7%, and a malfunction in the endothelial cells results in poor production of nitric oxide (NO), which in turn lowers the amount of FMD. FMD starts decreasing from early stages of arteriosclerosis31, 32), and it is therefore useful for the initial assessment of ASCVD.
3. Predicting the Risk for ASCVD by Assessing Arterial Walls
It has been reported that IMT/plaque of carotid artery, ABI, baPWV, CAVI, FMD, and such are independent predictive factors of future risk for ASCVD. However, reports of other countries have revealed that adding the result of IMT measurement does not increase the ability of risk prediction by the Framingham Risk Score33). Although there are reports on the significance of noninvasive arterial wall assessment in Japan34), there is still a lack of sufficient evidence. Recent reports by Japan have shown that35) when data from a meta-analysis that integrated personal-level data of 14,673 Japanese was used, the addition of baPWV to the classic risk factors increased the ability to predict the risk for CVD. These reports have particularly demonstrated the usefulness of baPWV in risk prediction for low-risk groups. In addition, it is suggested that a baPWV > 1,800 cm/s is a risk that is equivalent to the high-risk conditions18, 21). However, baPWV that reflects a high risk may possibly be different in the target group, and further accumulation of data would be advantageous. To indicate the abnormal findings of these indices except ABI in the risk categories in this set of guidelines, which will lead to stricter management, more evidence needs to be built.
Chapter 3. Comprehensive Risk Assessment
1. Risk Factor Assessment
1). Dyslipidemia
CQ1. Is LDL-C a predictor of the ASCVD incidence and mortality in the Japanese people?
Elevated LDL-C predicts the CAD incidence and mortality in the future. Among the types of stroke, LDL-C has been shown to be positively related to cerebral infarction and negatively related to hemorrhagic stroke, but in regard to the Japanese, the evidence cannot be considered adequate. (Evidence level: E-1b)
Many epidemiological studies have been conducted in Europe and America, including the Framingham Study in the US. Similar to the results of these studies, the increase in hazard ratio for the CAD incidence and mortality following an elevation in LDL-C has been validated in cohort studies involving the Japanese36–40). It was shown in the Circulatory Risk in Communities Study (CIRCS) that in comparison with the LDL-C < 80 mg/dL group, the risk was increased by 1.4 times in the 80–99 mg/dL group, 1.7 times in the 100–119 mg/dL group, 2.2 times in the 120–139 mg/dL group, and 2.8 times in the ≥ 140 mg/dL group37). The results have therefore made it clear that the risk of CAD incidence also increases by two times or more in the Japanese if the LDL-C level is ≥ 140 mg/dL. As for deaths from CAD, gender differences were observed in regard to risk, and a significant increase was seen only in men38).
An elevated LDL-C level also increased the risk for cerebral thrombosis (except lacunar infarction) among the subtypes of ischemic stroke36). However, on the contrary, it has been reported that there was a negative relationship between hemorrhagic stroke (primarily intracerebral hemorrhage) and a decrease in hazard ratio in the groups with high LDL-C levels40).
Interventions for hypercholesterolemia, including lifestyle modifications, have been clearly shown to significantly suppress CAD events according to the Western studies. Large-scale clinical studies conducted in Japan have also reported similar results41–44), in which it was evident that the occurrence of CAD in the Japanese subjects decreased as a result of treating the hyper-LDL cholesterolemia. Furthermore, it has not been observed in these studies that decreasing the LDL-C level adds to the risk for intracerebral hemorrhage.
Overlapping risk factors also increase the incidence and mortality rates of CAD in the Japanese45, 46). It has been shown that even with the same degree of hypertension, the addition of hyper-LDL cholesterolemia contributes to an increased risk for cardiovascular diseases47).
Considering all the points mentioned above, in this set of guidelines, we have set LDL-C ≥ 140 mg/dL as the screening reference value for the Japanese. Furthermore, we have set a range of 120–139 mg/dL as the borderline level for which the effects of overlapping risk factors should be carefully assessed.
CQ2. Is total cholesterol (TC) a predictor of the ASCVD incidence and mortality in the Japanese people?
Elevated TC predicts the CAD incidence and mortality in the future. Regarding stroke, it has been shown that TC is positively related to cerebral infarction and negatively related to hemorrhagic stroke, a result that many studies have in common. TC therefore predicts the occurrence of and death from stroke. (Evidence level: E-1a)
Similar to the above mention about LDL-C, a number of cohort studies conducted in Japan have reported an increase in the incidence and mortality rates of CAD following an elevation in TC level48–54). A 24-year follow-up in NIPPON DATA80 showed that the hazard ratio for CAD death was 1.55 times higher in the TC ≥ 220 mg/dL group than in the TC < 220 mg/dL group, and the population attributable fraction (PAF) was 18.2%54). When the TC level was ≥ 240 mg/dL, even though the hazard ratio further increased to 1.79 times, the PAF dropped to 11.9%. The association between TC and the incidence/mortality rate of CAD is almost linear, but a statistically significant increase in risk with TC levels around 220 mg/dL has been observed in many studies. Although a relationship between TC and risk of CAD death was seen in both the men and women, some reports have stated that this relation is attenuated in elderly individuals who are ≥ 65 years old55).
Regarding stroke, the association between TC and hazard ratio differed because of cerebral infarction and hemorrhagic stroke (primarily intracerebral hemorrhage). For hemorrhagic strokes, in contrast with the increase in risk of occurrence at low TC levels55–57), an increased risk because of high TC levels was observed in ischemic stroke, as with in CAD58, 59).
The synergy between BP and TC in regard to CAD death was demonstrated in the EPOCH-JAPAN study60). When a systolic BP of ≥ 160 mmHg overlaps with TC levels of ≥ 220 mg/dL, the adjusted hazard ratio for CAD death increased to 4.4 times that of the group with BPs < 120 mmHg and TC levels < 180 mg/dL. On the other hand, there was a smaller number of deaths caused by intracerebral hemorrhage in the group with TC levels of ≥ 220 mg/dL even when the BP readings were within the normal range.
CQ3. Is non-HDL-C a predictor of the ASCVD incidence and mortality in the Japanese people?
Elevation of non-HDL-C predicts the CAD incidence and mortality in the future. But at the same time, there are reports stating that an association does not exist in regard to stroke. (Evidence level: E-1b)
As non-HDL-C includes all arteriosclerosis-causing lipoproteins, such as remnant lipoproteins, there are views that claim its relatively superior ability in predicting the CAD incidence in comparison with LDL-C61, 62). The association between non-HDL-C and CAD has been reported in the results of many epidemiological surveys conducted in Japan51, 63–70). As with LDL-C, non-HDL-C was similarly associated with the occurrence of myocardial infarction (MI), and they both had the same ability to predict MI64). On the other hand, non-HDL-C surpasses TC in the ability to predict the occurrence of MI51). On the basis of analyses performed using data obtained from men, or both men and women, there have been reports stating that the risk of and death from CAD and MI increase when the non-HDL-C levels are approximately ≥ 140 mg/dL67, 71, 72). In all of these studies, the risk of onset and risk of death were evidently increased when non-HDL-C levels were ≥ 170–180 mg/dL or more, but there was no fixed trend observed in the women51, 64, 71, 73).
Meanwhile, studies concerning the association between non-HDL-C and stroke have reported varying results. There are reports saying that the association is unclear51, 67), and there are also those that revealed an increase in risk when non-HDL-C levels were low70). In reports of studies that reviewed the effect of hypertriglyceridemia on the ability of non-HDL-C to predict MI65), an obvious increase in risk for MI was seen in the presence of hypertriglyceridemia (TG ≥ 150 mg/dL) and non-HDL-C levels of ≥ 190 mg/dL. Similar to the US, the non-HDL-C levels of Japanese individuals with dyslipidemia was shown to be +30 mg/dL that of LDL-C74, 75).
On the basis of the results above, we concluded that non-HDL-C is possibly a useful indicator for predicting the risk of CAD incidence and mortality. Therefore, in this set of guidelines, the screening reference value for non-HDL-C has been set at ≥ 170 mg/dL. Furthermore, we have set a range of 150–169 mg/dL as the borderline level for which the effects of overlapping risk factors should be carefully assessed.
CQ4. Is HDL-C a predictor of the ASCVD incidence and mortality in the Japanese people?
A decrease in HDL-C level predicts the CAD and ischemic stroke incidence and mortality in the future. (Evidence level: E-1b)
A low HDL-C level is a risk for developing CAD and ischemic stroke. On the contrary, the risk decreases when HDL-C levels are high39, 53, 76–79). A 9.6-year observational period in NIPPON DATA90 showed that HDL-C was significantly inversely correlated with all deaths as well as stroke-related deaths80). It has been revealed in regional and occupational cohort studies that the risk for CAD increases when HDL-C levels are < 40 mg/dL46, 53, 78, 79). Likewise, in the J-LIT study that involved a cohort of simvastatin users, the relative risk was 1.3 times higher in the < 40 mg/dL group than in the 40–49 mg/dL group in primary prevention81). In addition, the relative risk was higher at 1.6 times in secondary prevention82). Studies conducted in 23 countries in Asia and Oceania, including Japan, have shown that a decreased HDL-C level alone also becomes a risk factor for CAD when both the LDL-C and TG levels are within the normal range, and this is especially so in Asia83). However, there have also been reports from large-scale cohort studies involving only the Japanese that low levels of HDL-C by itself does not constitute a risk for CAD84).
Considering the above findings, we have defined a HDL-C level of < 40 mg/dL in these guidelines as the screening reference value for hypo-HDL cholesterolemia. Women generally have a higher HDL-C level than men46, 80, 85). However, there is still insufficient evidence on the association between the discrepancy in HDL-C levels because of gender differences and CAD in each gender78). Therefore, in these guidelines, we have set the reference value for women to be the same as men.
CQ5. Is triglyceride (TG) a predictor of the ASCVD incidence and mortality in the Japanese people?
An increase in TG level, whether fasting or nonfasting, predicts the CAD and ischemic stroke incidence and mortality in the future. (Evidence level: E-1b)
There have been numerous reports on the association between a high TG level and the risk for CAD not just in Europe and the US86), but also in Asia, Oceania87) and even in Japan46, 78, 88–91). Despite a normal range of HDL-C, the association between TG and CAD was still observed in several of these studies86–89). Subsequent to the Framingham Study, hypertriglyceridemia is currently defined as a TG level of ≥ 150 mg/dL (fasting) in the US92). Although TG is traditionally tested with blood collected in a fasting state, some reports have stated that its ability to predict the risk for cardiovascular events is higher using blood collected in a nonfasting state91). It has been shown in epidemiological surveys in Japan that the occurrence of CAD increases when fasting TG levels of are ≥ 150 mg/dL46, 90). These surveys have also shown that a nonfasting TG level of ≥ 165 mg/dL adds to the risk for MI, exercise-induced angina, sudden death88) or ischemic CVD91). Furthermore, there have been many reports on hypertriglyceridemia being a risk factor for ischemic stroke, though the association is weaker than that with CAD46, 65, 87, 93–95).
Considering the above findings, hypertriglyceridemia has been defined as a TG level of ≥ 150 mg/dL in these guidelines. However, hypertriglyceridemia often comes with other important implications, such as an increase in the amount of remnant lipoproteins or small dense LDL and the co-existence of hypo-HDL cholesterolemia. It could also be a finding of metabolic syndrome, and hence, other factors accompanying an elevated TG level should also be thoroughly considered.
Diagnostic Criteria for Dyslipidemia
As mentioned in CQ1 to CQ5, the positive correlation between a higher incidence rate of CAD and high levels of LDL-C, TC, non-HDL-C and TG, or low levels of HDL-C, has been shown not just in epidemiological surveys conducted in Europe and the US but also in Japan. Among the different types of stroke, the association with cerebral infarction has been found to be almost similar to that with CAD. However, for hemorrhagic stroke (primarily intracerebral hemorrhage), the incidence and mortality rates are instead higher at low LDL-C and TC levels. At present, the absolute risk of CAD (incidence and mortality rate) in Japan is very much lower than that in the Western countries96). However, there have been reports and such revealing that the LDL-C and TC levels of the Japanese have been on the rise because of recent westernization of lifestyle habits. As a result of this change, the TC levels of the Japanese have already reached the levels in the US, or even higher97). These reports have also pointed out the increasing incidence rate of CAD in some regions in Japan98, 99), and this is why the management of dyslipidemia is of importance. In these guidelines, we have therefore established the diagnostic criteria for dyslipidemia with a focus on preventing the development of CAD. The diagnostic criteria are shown in Table 1.
The sequence of diagnosis is to first measure the TC, TG and HDL-C levels in a fasting state in the morning and subsequently calculating the LDL-C level using the Friedewald formula (LDL-C = TC–HDL-C–TG/5). However, measurement using the direct method is also allowed. Even though problems with the accuracy of the direct method have been pointed out100), there have recently been the discontinuation of sales and production, improvement and revision of standard pricing of reagents that were found to be mediocre over time. As a result of the improved performance of reagents, the validity of LDL-C measurement within daily clinical practice has been substantiated101). However, most of the studies providing evidence relating to the treatment of hyper-LDL cholesterolemia have used the Friedewald formula to determine LDL-C levels. It should be noted that the diagnostic criteria, treatment targets and such are based on the Friedewald formula. Non-HDL-C or the direct method of LDL-C measurement is used when the patient is in a nonfasting state or when the TG level is ≥ 400 mg/dL. However, the accuracy of the direct method and the non-HDL-C calculation cannot be guaranteed when the TG level is ≥ 1000 mg/dL101) and ≥ 600 mg/dL, respectively. Other methods of assessment should be considered in such cases.
2). Smoking
[Statement]
Smoking is a risk factor for CAD, stroke, abdominal aortic aneurysm and peripheral artery disease (PAD). (Evidence level: E-1a)
Passive smoking is a risk factor for CAD and stroke. (Evidence level: E-1a)
Smoking has been reported to be a risk factor for CAD and stroke in a number of sources, such as cohort studies conducted overseas and in Japan, as well as their meta-analyses. Compared to nonsmokers, smokers face a higher risk for CAD and stroke, and there is a dose-response relationship in this association102). In addition, there has been no data showing that low-tar, low-nicotine tobacco decreases the risk for these diseases. The risk increases even when the number of cigarettes smoked per day is less than five102). Reviews in Japan alone have shown that smoking has been consistently reported in many cohort studies103–111) to be a risk factor. Compared to lifetime nonsmokers, the relative risk for developing CAD and death from it was 2.15 times higher for those who smoke ≤ 20 cigarettes a day and 3.28 times for those who smoke > 20 sticks in a meta-analysis. Furthermore, the relative risk of getting stroke and stroke-related death was 1.41 times higher for those who smoke ≤ 20 cigarettes a day and 1.56 times for those who exceed 20 sticks112). Other than that, a metaanalysis of cohort studies in Japan have shown that for abdominal aortic aneurysm(AAA), the relative risk is 3.89 times higher for men and 4.30 times for women113). The dose–response relationship has also been made apparent in the analysis. The association with PAD has also been shown in cohort studies, including the Framingham Study. Even in cross-sectional studies conducted in Japan, the proportion of PAD, as determined using the ABI, was shown to be 3.7 times higher in current smokers (4.2 times for those with ≥ 45 pack-years) and 3.7 times in ex-smokers. The dose–response relationship has similarly been demonstrated in these studies114).
On the other hand, for passive smokers, it has been revealed in a meta-analysis that the relative risks for CAD and stroke are 1.31115) and 1.25116), respectively.
Smoking increases the risk for developing type 2 diabetes to 1.4 times117), and the risk for metabolic syndrome increases in accordance with the number of cigarettes smoked118). A meta-analysis has shown that smokers have lower HDL-C levels and higher LDL-C and TG levels, and the dose-response relationship has again been observed119). Smoking by itself is not just a risk factor for ASCVD. It also increases the risk for diabetes, dyslipidemia and metabolic syndrome, in turn contributing to an added risk for ASCVD.
Recently, new forms of tobacco (heat-not-burn tobacco products and electronic cigarettes, etc) that differ from the conventional combustion cigarette have been in circulation. As these new forms of tobacco have only been in circulation for a short period of time, their effects on health, such as the risk for ASCVD and related death, cannot be determined at this point of time. Nonetheless, although heat-not-burn tobacco products do not contain substances that are produced by combustion, users still inhale and exhale the aerosol generated by heating the tobacco leaves and additives, including nicotine120). Moreover, from the fact that various carcinogens have been reported to be found in the aerosol, regardless of whether electronic cigarettes contain nicotine121), the use of any of them has a possibility of adversely affecting health.
3). Hypertension
[Statement]
Blood pressures that exceed the optimal reading (i.e., systolic BP (SBP) < 120 mmHg and diastolic BP (DBP) < 80 mmHg) increase the risk for developing CVD, stroke, MI, CKD, etc. (Evidence level: E-1a)
Hypertension is an important risk factor for cerebro- and CVD, such as cerebrovascular disease and CAD, as well as heart failure, CKD and many more. Hypertension at middle age also increases the risk for dementia when one reaches old age122). In the results of EPOCH-JAPAN, a meta-analysis of 10 domestic cohort studies (70,000 men and women in total), the hazard ratio for cerebro- and cardiovascular disease-related death increases progressively with an elevation of BP levels that exceed the optimal level (< 120/80 mmHg). This association was stronger in the middle-aged adults than in the elderly123).
The estimation by EPOCH-JAPAN showed a death rate of 50% from all forms of cerebro- and CVD, 52% from stroke and 59% from CAD. They were all assessed to be caused by high BPs that exceed the optimum level. Among all deaths from these causes, subjects with grade-I hypertension made up the highest proportion123). A comparison between subjects with hypertension and those without hypertension in the lipid intervention study J-LIT showed that the relative risk for CAD in primary prevention subjects was 2.05 times higher in women and 2.15 times in men124).
The basis for diagnosing hypertension is usually the BP reading measured at the outpatient clinic. However, it has been reported that BP measurements at home and 24-hour ambulatory BP monitoring (ABPM) can predict the incidence of cardiovascular events better than in-clinic BP measurements. In The Japanese Society of Hypertension's Guidelines for the Management of Hypertension 2014 (JSH 2014), it has been explicitly stated that if the diagnosis made with in-clinic BP readings differs from that with home BP readings, BP-lowering effects should be determined and diagnosis should preferably be made on the basis of the BP measured at home122). The reference value for hypertension differs for in-clinic BP, 24-hour ABP and home BP. In-clinic BP readings of ≥ 140/90 mmHg, home BP readings of ≥ 135/85 mmHg and ABP readings of ≥ 130/80 mmHg shall be managed as hypertension122).
4). Diabetes Mellitus (DM)
[Statement]
DM is a strong risk factor for ASCVD. (Evidence level: E-1a)
DM is an important risk factor for ASCVD125, 126). In NIPPON-DATA80, diabetic patients showed significantly higher risk of 2.8 times for death from CAD than non-diabetic subjects127). The Hisayama study reported that the incidence rate of CAD and cerebral infarction in diabetic patients were both high after adjusting multiple factors, such as gender and age. In this study, the incidence rate of CAD was 5.0/1,000 person-years for diabetic patients and 1.6/1,000 person-years for subjects with normal glucose tolerance (NGT). Similarly, the incidence rate of cerebral infarction was 6.5/1,000 person-years in diabetic subjects, that significantly higher than the 1.9/1,000 personyears in subjects with NGT128). The CIRCS study also showed that the incidence rate of cerebral infarction for diabetic patients was higher at 1.9 times in men and 2.2 times in women as compared to non-diabetics129). Although the absolute risk of CAD among Japanese diabetic patients is thought to be 30–70% compared to that of diabetic Europeans and Americans130, 131), the difference has been closing in the near future.
Silent myocardial ischemia often coexists in diabetic patients, and this may result in delayed diagnosis132). Features of coronary lesions in diabetic patients include (1) multiple-vessel disease, (2) highly complicated and diffuse133, 134) and (3) multiple calcified lesions135).
As for cerebral infarction, the JPHC study has shown that lacunar infarction, atherothrombotic infarction and thromboembolic infarction occur more often in diabetic patients than non-diabetic subjects136).
Furthermore, prognosis of CAD in diabetic patients is worse than in non-diabetic subjects137–139), and also have a higher risk for recurrence of cerebral infarction140, 141). The risk for PAD is as much as three to four times higher in diabetic patients142), and this risk increases by 26% with every 1% increase in the HbA1c level143).
The risk of CVD begins to increase since impaired glucose tolerance (IGT) state144). In the Hisayama study, the incidence rate of CAD in patients with IGT is 1.9 times higher than that of subjects with NGT, even though it was lower than the level of 2.6 times in diabetics128). The incidence rate of cerebral infarction increased significantly following the HbA1c level was ≥ 5.5–6.4%145). The JPHC study showed that the risk for CAD was 1.65 times higher in the borderline glucose tolerance group and 3.05 times higher in the diabetic group as compared to subjects with NGT, indicating the risk increases before the incidence of diabetes146). The two hours glucose level post glucose load was more strongly associated with the risk for CAD than the fasting level in subjects with IGT147, 148). These suggest that the significance of postprandial glucose level as a risk factor of atherosclerosis.
Women generally have a lower risk for CAD than men. But in diabetic patients, it has been reported that the increase in relative risk of CAD was higher in women than men, resulting in reduced gender differences149, 150). It has also reported that compared to male subjects with NGT male diabetic patients increased the risk of CAD by 17 times higher at ages 31–40 years, and by 2 to 3 times higher at ages 41 to 61 years, which suggests that the impact of diabetes on CAD was markedly greater in younger men compared with men in middle age151).
Thus, prevention of ASCVD in addition to microvascular disease is quite important issue even in Japanese diabetic subjects152).
5). Chronic Kidney Disease (CKD)
[Statement]
CKD is a high-risk condition for ASCVD. (Evidence level: E-1a)
In the Evidence-based Clinical Practice Guideline for CKD 2013 published by the Japanese Society of Nephrology153), CKD is defined as continuously having for ≥ 3 months, (1) an obvious renal damage based on abnormalities in urine, diagnostic images, blood, and pathology; the presence of proteinuria of ≥ 0.15 g/gCr (albuminuria of ≥ 30 mg/gCr) is especially important, and/or (2) a glomerular filtration rate (GFR) of < 60 mL/min/1.73 m2. An estimated GFR (eGFR) is used for GFR. CKD is not merely a highrisk condition for end-stage kidney disease but also for ASCVD. As the risk of these composite outcomes differs greatly depending on the cause, GFR and amount of proteinuria (albuminuria), the severity of CKD is classified (CGA classification)153, 154) using these three primary factors. It has been estimated that 13% adults in Japan have CKD155), and screening for CKD is therefore an important element in the comprehensive risk management for ASCVD.
In the screening for CKD156), urinary protein and urinary occult blood are assessed by a qualitative urine test, and eGFR is determined by measuring serum creatinine levels. Whenever necessary, the degree of proteinuria is further determined quantitatively using the casual spot urinary protein-to-creatinine ratio. If a patient is identified with CKD, even when he/she is in the group for primary prevention of ASCVD, stricter control of risk factors, including dyslipidemia, is recommended as a high-risk patient.
CKD may include conditions for which diagnosis by kidney biopsy is preferable so as to treat for remission, conditions requiring urgent treatment (immune suppression therapy with corticosteroids, immune suppressants, molecular-targeted agents, or in combination), and also conditions that may need specialized medical care with renal replacement therapy such as renal transplantation and dialysis. The Japanese Society of Nephrology recommends the following criteria for referral to nephrologists: (1) advanced proteinuria (urinary protein-to-creatinine ratio of ≥ 0.50 g/gCr or ≥ 2+ by dipstick test), (2) both urinary protein and urinary blood are positive (≥ 1 + by dipstick test) and (3) eGFR < 45 mL/min/1.73 m2 154).
Traditional risk factors, such as BP, lipids and carbohydrate metabolism, are exacerbated in CKD. On top of that, non-traditional risk factors, such as abnormal phosphate-calcium metabolism play roles in advanced stages of CKD, and the relative contributions of certain risk factors for ASCVD are altered. According to a large-scale cohort study in Canada157), the association between LDL-C and ASCVD is lower when eGFR is lower, and the association was no longer significant when eGFR was < 15 mL/min/1.73 m2. This finding is consistent with the results of randomized controlled trials revealing that the use of statin did not decrease the risk for ASCVD significantly in dialysis patients158, 159), suggesting the importance of implementing effective measures on patients with earlier stages of CKD.
6). Aging and Gender Differences
[Statement]
Aging is the strongest risk factor for ASCVD, such as CAD and cerebrovascular disease. (Evidence level: E-1b)
Women have a lower risk for MI and MI-related death than men, but the gender difference decreases with aging. (Evidence level: E-2)
The risk for ASCVD, such as MI, and death from it increases as one moves up to the next age category. In terms of absolute risk, aging enhances the risk for ASCVD and death from it much more than any other risk factors160–162).
Women have a lower risk for MI and MI-related death than men. In a survey conducted in Takashima town, Shiga Prefecture from 1999 to 2001, the age-adjusted incidence rate of acute MI (100,000 person-years) was 35.7 for women, and this was one-third the figure of 100.7 for men163). Furthermore, according to the vital statistics of 2014, the (approximate) rate of mortality due to ischemic heart disease in a target population of 100,000 is 49.8 for men and 36.6 for women. The mortality rates (target population of 100,000) according to age are as follows: 2.6 for men and 0.6 for women in the 30s, 11.0 for men and 2.3 for women in the 40s, 30.2 for men and 6.6 for women in the 50s, 66.5 for men and 17.3 for women in the 60s, 131.9 for men and 60.3 for women in the 70s, 334.9 for men and 209.5 for women in the 80s, 675.6 for men and 460.2 for women in the 90s, and last but not least, 787.5 for men and 549.0 for women in the ≥ 100s. The mortality rate of ischemic heart disease was lower in the women than men in all age groups. However, the mortality rate of ischemic heart disease for women starts increasing when they reach the 60s. Women in the 70s have a mortality rate that is almost the same as men in the 60s, which goes to show that the risk for ASCVD is not low for elderly women164). There was almost no difference between the mortality rates of cerebral infarction for men and women (target population of 100,000), which were 25.4 and 28.8, respectively. The mortality rates (target population of 100,000) according to age are: 0.2 for men and 0.1 for women in the 30s, 0.9 for men and 0.4 for women in the 40s, 3.6 for men and 1.1 for women in the 50s, 15.9 for men and 4.7 for women in the 60s, 61.9 for men and 24.9 for women in the 70s, 242.3 for men and 156.2 for women in the 80s, 662.7 for men and 605.3 for women in the 90s, and lastly, 1125.0 for men and 1182.4 for women in the ≥ 100s. Up to the 90s age group, the mortality rate of cerebral infarction was lower in women than men.
7). Family History of CAD
[Statement]
A family history of CAD is a risk factor for developing CAD. (Evidence level: E-1b)
It has been reported in the Western countries since 1970s that a family history of CAD is a risk factor for the disease itself165–172). A family history of CAD, especially in first-degree close relatives (parents, children, brothers and sisters), and a family history of premature CAD (age of incidence: < 55 years for men and < 65 years for women) are strong risk factors for CAD.
It was reported in the Framingham Study that if at least one parent has CAD, the age-adjusted odds ratio for the risk of developing CAD is 2.6 for men and 2.3 for women. After adjusting all the variables in the multivariate analysis, the ratios were 2.0 and 1.7 for men and women, respectively168). The J-LIT study in Japan has shown that a family history of CAD increases the relative risk of developing CAD by three times173). The recent CREDO-Kyoto study has also reported that a family history of CAD contributes to the occurrence of major cardiovascular events at a young age174).
Traditional risk factors (high LDL-C, low HDL-C, hypertension, diabetes, and smoking) are associated with genetic predisposition and are influenced by habits within the family. In other words, a family history of CAD is considered to also include genetic and environmental risk factors, a fact that is already known. There has been attention on other risk factors that should be considered, such as Lp (a), small dense LDL and homocysteine, which are all genetically regulated. However, it is assumed that unknown genetic factors play a role170) as family history remains a strong risk factor even after adjusting all the traditional risk factors in multivariate analyses80, 166–168, 175, 176).
Therefore, most studies relating to family history have concluded that a family history of CAD is an independent risk factor for CAD. Individuals with a family history of premature CAD (age of incidence: < 55 years for men and < 65 years for women) should particularly be considered to be at high risk for CAD.
8). History of CAD
[Statement]
A history of CAD poses a higher risk compared to primary prevention. (Evidence level: E-1b)
It is evident in epidemiological studies and intervention trials conducted in the Western countries that the incidence rate of cardiovascular events is higher in patients with CAD than in primary prevention patients177–179). Similar results have also been reported in studies in Japan. The MEGA study, a primary prevention trial involving the use of statin41), showed that the incidence rate of cardiovascular events for the group that underwent diet therapy was 2.1/1,000 person-years. In the J-LIT study, the incidence rate in primary prevention patients was 0.9/1,000 person-years 180) and 4.5/1,000 person-years in CAD patients173). In JELIS, primary prevention patients had an incidence rate of 1.6/1,000 person-years while the rate was 6.8/1,000 person-years in patients with CAD181). In addition, the incidence rate of cardiovascular events was ≥ 15/1,000 person-years in the JCAD182) and CREDO-Kyoto studies183), which are registry studies involving patients with CAD.
9). Noncardiogenic Cerebral Infarction
[Statement]
A history of noncardiogenic cerebral infarction is a high-risk condition for cerebrovascular disease and CAD. (Evidence level: E-1b)
Patients with a history of cerebrovascular disease are known to be a high-risk group for CAD. In Japan, there has been reports revealing that the 1-year incidence rate for patients with a history of stroke is 0.40–0.45% (4.0–4.5 people/1,000 person-years)184, 185). Patients in Japan who have a history of stroke are considered to be at a high risk for developing CAD, especially those with noncardiogenic cerebral infarction originating from arteriosclerotic lesions.
In addition, findings of arteriosclerosis in the carotid arteries are an independent risk factor for CVD6, 8). It has been reported that an increase in the intima-media thickness (IMT) of common carotid arteries is particularly a significant predictive factor in cerebral infarction and CAD186–188). However, it was reported in the results of a meta-analysis that both CVD and stroke were not seen to be significantly correlated with the progress of IMT189).
On the other hand, it has been reported that the incidence rate of cerebrovascular disease is higher than the recurrence rate of CAD in patients with a history of CAD. Moreover, the risk of developing cerebrovascular disease is also higher than the risk of CAD in those with a history of PAD184). Therefore, a history of cerebrovascular disease is something that should be taken note of.
10). Peripheral Artery Disease (PAD)
i). Lower extremity PAD
[Statement]
PAD causes CAD and cerebrovascular disease to occur easily at high incidence rates. (Evidence level: E-1b)
Although “ASO” has traditionally been used in Japan to refer to PAD, the term used in these guidelines is “PAD”190). It mainly refers to diseases based on stenosis and obstructive lesions in the arteries of the lower extremities due to atherosclerosis. Coldness in the lower extremities, intermittent claudication, ulcers, and necrosis are some of the symptoms observed in PAD. In Europe and America, it has been clearly demonstrated in epidemiological studies that PAD patients are more prone to developing other forms of ASCVD, such as CAD and cerebrovascular disease. Similar reports have also been surfacing in Japan recently.
In the Hisayama study, in which ordinary citizens were involved, 2,954 subjects who were ≥ 40 years old and did not suffer from CVD were followed up for an average of 7.1 years. The results showed that the risk for developing CAD was 4.13 times higher in subjects who had an ABI of ≤ 0.9 than those who had a normal ABI191). The CIRCS study was another study that involved ordinary citizens, in which 939 subjects who were 60–74 years old and did not suffer from CVD were followed up for an average of 9.3 years. The results have likewise showed that subjects with an ABI of ≤ 0.9 had a higher risk of 2.04 times for developing CAD than those with an ABI of ≥ 1.1, and the risk for developing cerebrovascular disease was also higher at 3.39 times192). The REACH Registry, a prospective cohort study, had 5,193 Japanese entered by 2004. Among them, the incidence of CVD within 1 year was studied in 603 individuals who were suffering from coexisting PAD. The resulting incidence rates were generally high - 1.25% for all deaths, 0.55% for cardiovascular death, 0.77% for nonfatal MI, and 1.56% for nonfatal stroke184). A prospective observational study was conducted on 557 patients with PAD by Shigematsu et al. It was observed in the study that the three-year incidence rate was 6.3% for cardiovascular death, 11.3% for heart disease, 7.0% for cerebrovascular disease and 16.9% for events in the lower extremities193).
As mentioned above, other forms of ASCVD, such as CAD and cerebrovascular disease, easily occur in PAD patients at high incidence rates, and this has also been clearly shown in Japan. Therefore, when encountering a PAD patient, a careful full body examination is necessary to check for the presence of ASCVD.
ii). Abdominal Aortic Aneurysm (AAA)
[Statement]
Atherosclerotic disease often coexists in patients with AAA. (Evidence level: E-2)
High LDL-C194), high TC195), hypertension196), and smoking are the factors that contribute to the development of AAA, and this is a commonality with ASCVD. A survey conducted by Akai et al. on 374 Japanese patients with AAA showed that hypertension contributed to the increase in diameter of the aneurysms. However, the association of its increased size with a history of ASCVD or TC was ruled out197). Hollier et al. studied the long-term prognosis of 1,087 patients who had undergone open surgical repair for AAA. Among the causes of death, they reported that cardiovascular-related causes made up 37% and MI made up 22%198). However, they were unable to obtain any results showing that AAA is a risk factor of ASCVD. There are currently no papers relating to studies on the long-term prognosis of patients with AAA in Japan. Nonetheless, in a study where preoperative coronary angiography was performed on 94 patients with AAA who were to undergo elective surgery for, 45.7% of them were found to having coexisting CAD199). In a separate study, ATP-loading myocardial single-photon emission computed tomography (SPECT) was performed on 788 Japanese patients, including 500 patients with AAA who had no history of CAD, 183 patients with PAD and 105 patients with both AAA and PAD. As a result, myocardial ischemia was seen in 37% the patients with AAA, 55% patients with PAD and 77% patients with both200). From these results, even though there are no longitudinal studies demonstrating AAA as a risk factor for ASCVD, there are some cross-sectional studies showing the association between AAA and ASCVD. Therefore, it is recommended to screen for ASCVD in patients with AAA.
iii). Atherosclerotic Renal Artery Stenosis
[Statement]
Atherosclerotic Renal Artery Stenosis Is a High-risk Condition for ASCVD. (Evidence Level: E-2)
Ninety five percent of renal artery stenosis (RAS) is caused by arteriosclerosis and it coexists with other forms of ASCVD at a high rate. RAS is a progressive condition, and individuals with RAS have a tendency for worsened renal function. However, there is also an added risk for cardiovascular complications at the same time201, 202). There has been a report stating that upon examining the renal arteries during cardiac catheterization as a screening test, 30% patients who went through the procedure were found to be having RAS203), and that the survival rate decreases with higher degrees of RAS204). However, there are currently no relevant detailed reports or reports from cross-sectional studies. Even though coronary artery lesions are found at high rates when RAS is present and RAS is an important high-risk condition in CVD, there is insufficient evidence showing that it is a direct primary risk factor for ASCVD.
11). Other Diseases to Be Considered
i). Hyperuricemia
[Statement]
Hyperuricemia can be regarded as a risk factor for ASCVD. (Evidence level: E2)
The Guideline for the Management of Hyperuricemia and Gout (Second Edition) released by the Japanese Society of Gout and Nucleic Acid Metabolism was revised in 2010. In the revised guideline, it was mentioned that serum uric acid levels (1) can be regarded as an independent predictive factor for the development of hypertension, and (2) it can possibly be a predictive factor for the risk of incipient stroke and recurrence, as well as for the re-hospitalization and prognosis caused by heart failure. At the same time, it was mentioned in the guideline that conflicting reports on whether serum uric acid level is an independent risk factor for CVD have been produced205).
According to subsequent reports, including a meta-analysis, it has been revealed that uric acid level is an independent risk factor for the development of hypertension, stroke, and coronary events206–208). Uric acid levels was also reported as an independent risk factor for cerebro- and cardiovascular death in EPOCH-JAPAN, in which 13 cohort studies in Japan were summarized209).
On the other hand, there is still a lack of evidence from interventional studies on whether uric acid-lowering treatments contribute to the suppression of ASCVD and the improvement of prognosis, and this remains a question to be answered. Nonetheless, a meta-analysis of interventional studies has shown that uric acid-lowering treatment using allopurinol lowers BP210). In a study involving elderly patients with hypertension, the group that received allopurinol was compared to the propensity score-matched control group. The results showed that the suppressive effect on the incidence of stroke and coronary events (acute MI and ACS) was especially seen when high doses of allopurinol were administered211).
ii). Sleep Apnea Syndrome (SAS)
[Statement]
Obstructive sleep apnea is an independent risk factor for ASCVD. (Evidence level: E-1b)
Abnormal breathing pattern (occurrence of apnea and hypopnea) and ventilatory failure during sleep are two characteristics of SAS, a representative condition of sleep-disordered breathing (SDB) which presents various symptoms. SAS is divided into obstructive sleep apnea (OSA), which is based on obstruction of the respiratory tract, and central sleep apnea (CSA), which is caused by an absence of respiratory drive from the respiratory center. Among the two types of SAS, CSA is relatively less common. Although it is regarded in the field of circulatory system to be a condition that brings about heart failure, OSA is on the other hand, seen as a common disease that is closely related to lifestyle habits and is strongly associated with ASCVD. It is possible that OSA causes arteriosclerosis to progress through various mechanisms.
A meta-regression analysis has clearly shown that patients with OSA have lower endothelial function than those without OSA, in addition to increased stiffness of the blood vessels and high-sensitivity C-reactive protein level212). It has also been evident in a meta-analysis that OSA is an independent risk factor for intima–media thickening213). Many cohort studies and prospective studies involving large numbers of patients have confirmed that OSA poses a risk for developing type 2 diabetes214). It has also been substantiated in these studies that OSA increases the rate of new future occurrences of hypertension215). We can hence see from these results that OSA directly or indirectly contributes to the incidence and progression of ASCVD.
A comparison between subjects with severe OSA and the control group in prospective observational studies has revealed a significant increase in the occurrence of both fatal and nonfatal cardiovascular events in the group with severe OSA216, 217). Therefore, it is advisable to treat OSA as one of the risk factors for ASCVD and carry out screening accordingly. Regardless of whether an underlying heart disease is present, active screening is especially recommended for patients who are noticed to be experiencing two or more of the following: excessive daytime sleepiness or a choking sensation during sleep, asthma, repeatedly waking up from sleep, feels unrefreshed when waking up, feels tired during the day and lacks concentration218). Continuous positive airway pressure (CPAP) is an effective therapy for patients who are diagnosed with severe OSA. However, the effect of CPAP on primary or secondary prevention of CVD has not proven yet in recent RCTs219, 220).
12). Other Risk Factors and Markers to Be Considered
[Statement]
Hyper-Lp(a)-lipoproteinemia is a risk factor for ASCVD. (Evidence level: E-1a)
Measuring the level of malondialdehyde-modified LDL (MDA-LDL) is useful for prognostic prediction of the incidence of CAD in diabetic patients who have a history of CAD. (Evidence level: E-1b)
Hyper-remnant-lipoproteinemia is a risk factor for ASCVD. (Evidence level: E-1b)
Postprandial hyperlipidemia is a risk factor for CAD. (Evidence level: E-1b)
A high small dense LDL level is a risk factor for ASCVD. (Evidence level: E-1a)
A high apolipoprotein B (apo B) level is a risk factor for ASCVD. (Evidence level: E-1a)
The TC/HDL-C ratio, non-HDL-C/HDL-C ratio, LDL-C/HDL-C ratio, and apo B/AI ratio are markers for ASCVD. (Evidence level: E-1a)
High levels of fibrinogen and plasminogen activator inhibitor-1 (PAI-1) are markers for ASCVD. (Evidence level: E-1a)
It is necessary to take note that these factors include genuine risk factors that contribute to the progression of ASCVD, but at the same time, there may also be factors within that are very likely to be markers for ASCVD.
Risk factors or markers for ASCVD that should be considered are proposed separately from the risk factors established in the preceding sections.
i). Lp(a)
Lp(a) is an independent risk factor for CAD and stroke. When the size of apolipoprotein (a) [apo(a)] is small, the concentration of Lp(a) is high, and there is a high risk of CVD. Single-nucleotide polymorphisms have been revealed in genes that reflect this correlation221–228). There have been some hypotheses regarding the mechanism through which Lp(a) causes ASCVD. One hypothesis is that apo(a) protein is highly homologous to plasminogen222, 227), and it interferes with the actions of plasminogen, thereby promoting thrombus formation228, 229). It has also been suggested that apo(a) protein preferentially binds to oxidized phospholipids, which influence the risk for CAD230, 231); furthermore, it has been suggested that apo(a) is easily anchored to the arterial walls232, 233). High Lp(a) concentrations are found in patients with FH, and it is presumed that hyper-Lp (a)-lipoproteinemia further increases the risk for CVD in patients with FH222).
ii). MDA-LDL
MDA-LDL is a form of oxidized LDLs, which are lipoproteins produced through the oxidative modification of lipids, such as phospholipids, and apolipoproteins when LDL is subjected to oxidative stress234, 235). MDA-LDL is involved in a wide range of processes of atherosclerosis, such as the damaging of endothelial cells, acceleration of monocyte penetration into the vascular walls, and formation of foam cells235). MDALDL is useful for prognostic prediction of the incidence of CAD and the recurrence of stenosis after percutaneous coronary intervention (PCI) in diabetic patients with a history of CAD236). In patients with stable angina who were receiving lipid-lowering treatment, the risk for cardiovascular events after treatment using a drug-eluting stent has been shown to increase by 1.14 times with every 10 U/L elevation in MDALDL levels237).
iii). Remnant Lipoprotein
Remnant lipoprotein is an intermediate lipoprotein that is produced during the process in which chylomicrons and very low-density lipoproteins (VLDL) are metabolized. Remnant lipoproteins deposit themselves in the vascular intima, causing the progression of atherosclerosis238). Patients with MI have a high risk for cardiovascular events when the remnant lipoprotein level is high. In addition, hyper-remnant-lipoproteinemia is an independent risk factor even when LDL-C is maintained at < 100 mg/dL239, 240). Studies have shown that the measurement of remnant lipoprotein levels is useful for risk assessment in secondary prevention for patients with ACS who have undergone coronary interventions and are taking statins. Its usefulness has also been demonstrated in the assessment of risk for ASCVD in primary and secondary prevention for patients with coexisting type 2 diabetes and CKD241, 242). In addition, hyper-remnant-lipoproteinemia can explain part of the residual risk of allcause mortality in patients with CAD243). Some of the conditions in which the amount of remnant lipoprotein increases include familial combined hyperlipidemia, familial type Ⅲ hyperlipidemia, DM, and metabolic syndrome.
iv). Postprandial Hyperlipidemia
Zilversmit et al. proposed that the increase in the postprandial levels of remnant lipoprotein after meals can cause atherosclerosis. Considerable evidence has been accumulated thereafter, and this hypothesis has therefore been established as the disease concept238, 243–245). Epidemiological studies in Japan have also demonstrated that a high nonfasting TG level adds to the risk of CAD. Overall, every 1 mmol/L increase in TG levels increases the relative risk of CAD by 1.34 times (men, 1.29 times; women, 1.42 times)88). The risk starts increasing when the nonfasting TG level reaches 115 mg/dL, and at ≥ 167 mg/dL, the risk increases by ≥ 3 times. The result remains the same even with correction using HDL-C88). In the sub-analysis of MRFIT, the nonfasting TG level was as useful as, or even more useful than, the fasting TG level for the risk for CAD; a nonfasting TG level of ≥ 200 mg/dL indicates a high risk246). The diagnostic criteria for hypertriglyceridemia in a nonfasting state have recently been gaining attention, especially in Europe and America247, 248). In addition, measurement of the amount of apolipoprotein B-48 in a fasting state is expected to be useful as a screening marker for postprandial hyperlipidemia249).
v). Small Dense LDL
Among all the LDL particles, small dense LDL particles250, 251) have a high density. The association between the levels of small dense LDL and CAD has been reported in numerous sources251–256), and its relationship with PAD and aneurysms has also been demonstrated257, 258). In addition, small dense LDL is more strongly associated with the risk for CAD259), the severity of coronary atherosclerosis256, 260), and the incidence of ASCVD in secondary prevention261) than is LDL-C among Japanese populations. There have been several hypotheses regarding the mechanism of small dense LDL as a strong atherogenic factor. One hypothesis is that small dense LDL is easily oxidized262) and processed in pathways other than that of the LDL receptor263). Small dense LDL is also easily incorporated into the arterial wall264), where it binds readily to the matrix265), and this is another proposed mechanism. Small dense LDL is closely associated with hypertriglyceridemia and hypo-HDL cholesterolemia259, 266). In addition, its levels are increased in conditions such as type 2 DM, metabolic syndrome, and insulin resistance259, 267).
vi). Apo B
Apo B (apo B-100) is an apolipoprotein found in atherosclerosis-causing lipoprotein particles, such as LDL and remnant lipoprotein. As each lipoprotein particle contains one apo B molecule, the apo B value is proportionate to the number of these lipoprotein particles. According to a meta-analysis of epidemiological studies, apo B is a stronger risk factor for cardiovascular events than are LDL-C and HDL-C268, 269). The Framingham Study has revealed that apo B improves the risk assessment for CAD more than do LDL-C and non-HDL-C270). A meta-analysis of studies using statins has clearly shown that compared to a reduction in LDL-C and non-HDL-C levels, a reduction in the amount of apo B has a stronger association with the decrease in the risk of CAD271). Another meta-analysis has demonstrated that measurement of the amount of apo B, in addition to LDL-C and non-HDL-C, improves risk prediction272).
vii). Ratios of Lipids and Apolipoproteins
The levels of lipids, such as LDL-C and HDL-C, are commonly considered as risk factors. However, rather than the levels of these lipids and apolipoproteins, the ratio of different cholesterols and the ratio of different apolipoproteins contained in each lipoprotein are risk factors for ASCVD. These ratios are namely the TC/HDL-C ratio, non-HDL-C/HDL-C ratio, LDL-C/HDL-C ratio, and apo B/AI ratio268, 273–275). Nonetheless, these data are mostly the results of studies in various Western countries; therefore, the current management targets to be achieved in Japan should be the absolute values of each lipid. In Chinese diabetic patients, the TC/HDL-C, non-HDL-C/HDL-C, LDL-C/HDL-C, and apo B/AI ratios were involved in the spread of coronary lesions. The apo B/AI ratio, in particular, was strongly involved in the coronary atherosclerosis; however, the significance disappeared after adjustment for confounding factors276). Studies conducted in Japan have reported that the TC/HDL-C ratio is significantly associated with coronary artery calcification, even after correction for the confounding factors277).
viii). Inflammatory Markers (C-reactive Protein and Pentraxin-3)
C-reactive protein (CRP) is an acute-phase protein that is used as an inflammatory marker. It has been already revealed that chronic inflammation of the blood vessels is an important factor for the progression of atherosclerosis. However, in recent years, high-sensitive CRP (hs-CRP) has been reported as a possible risk factor for ASCVD278, 279). There are reports in Japan stating that a significant association was seen between hs-CRP and stroke (especially cerebral infarction and lacunar infarction)280). hs-CRP has also been associated with the onset risk of MI and stroke, of which the association is particularly strong in MI281). Conversely, in a study on the genotype related to CRP concentration in the blood and the frequency of CAD, there was no association between the concentration of CRP and the frequency of CAD282). This result suggests that the concentration of CRP is probably not a cause of ASCVD but instead reflects the extent to which atherosclerosis has progressed. Pentraxin (PTX)-3 belongs to the same pentraxin family of proteins as CRP. Unlike CRP, which is found in the liver, PTX-3 is specifically expressed in the endothelial cells, smooth muscle cells, and WBCs. Recent reports have suggested that the association of CRP and PTX-3 in coronary artery plaques in pathological autopsies shows that both CRP and PTX-3 reflect unstable plaques. However, the distributions of CRP and PTX-3 in the plaques are not the same, and there is a possibility that they play different roles. There have also been reports on the PTX-3-lowering effect of statins. PTX-3 is expected to serve as a specific marker reflecting CVD in the future283–286).
ix). Homocysteine
The elevation of homocysteine concentration in the blood has been reported to be a risk factor not just for CAD but also for stroke and PAD287–289). In a study involving elderly individuals who were aged ≥ 85 years and had no history of cardiovascular disease, a high homocysteine concentration in the blood increased the relative risk for MI290). Furthermore, analyses in the recent years have suggested that homocysteine is a stronger marker than is CRP291). However, therapies for lowering the concentration of homocysteine through vitamin supplementation did not result in the suppression of events292, 293). Furthermore, it has also been reported that there is no association between ASCVD and hereditary genetic mutations that cause an increase in homocysteine levels294). In a meta-analysis related to the risk for CAD at a young age, the effect of homocysteine was not seen in all subjects. However, in the group with homocysteine levels of ≥ 15 µmol/L or in Asians with a 677C→T mutation in the methylenetetrahydrofolate reductase gene, a significant increase in risk was observed295). The background for this finding has been suggested to be the relatively lower intake of folic acid by Asians than that by other races, and this implies a need to study hyperhomocysteinemia as an independent risk factor in Japan. The various genetic polymorphisms affecting homocysteine concentration have been evaluated in studies that involved individuals of European descent, using genome-wide association study. The results revealed that the concentration of homocysteine did not contribute to the risk for CAD in the Caucasians296).
x). Blood Coagulation and Fibrinolytic Factors
Fibrinogen is an independent risk factor for cardiovascular disease297–299). The conclusion of an integrated analysis of 52 prospective studies was that both CRP and fibrinogen are risk factors for primary cardiovascular disease278). By contrast, some recent reports on the association of CRP and fibrinogen with mean IMT and coronary artery calcium score in the Japanese, Japanese–American, and Caucasian populations have stated that a significant association was not seen in any of the races after adjustment for multiple variables (age, SBP, LDL-C, HDL-C, fasting glucose level, smoking, and alcohol consumption) or for age and BMI300, 301). PAI-1 is a fibrinolytic factor secreted by the endothelial cells, and its activity increases in the acute phase of acute MI. Sakamoto et al. have shown that although its activity was decreased at the time of discharge at approximately 1 month later, the levels were still higher in patients with acute MI than in the control group302). Reports have demonstrated that PAI-1 is associated with ASCVD and metabolic syndrome, which involves an accumulation of visceral fats and insulin resistance303). The risk may possibly increase further depending on the genotypes that exist for PAI-1304, 305).
2. Disease Concept and Diagnostic Criteria for Metabolic Syndrome
[Statement]
Metabolic syndrome is a condition that poses a high risk for the development of cardiovascular disease (Evidence level: E-1b).
Japanese eating habits and dietary components have clearly changed in recent years306). Lifestyles that include overnutrition and physical inactivity are threatening to increase the incidence of CAD and stroke. Among the conditions underlying the development of ASCVD, particular importance is ascribed to a cluster of multiple risk factors, including hyperglycemia, dyslipidemia, and elevated blood pressure, which are closely related to lifestyle. This pathologic condition used to be called “Syndrome X,”307), “the deadly quartet”308), “visceral fat syndrome”309), or “insulin resistance syndrome”310) but those terms were unified as “metabolic syndrome”311) in 1999. Metabolic syndrome is recognized as a condition in which the risk factors of atherosclerosis cluster on the basis of obesity, particularly visceral fat accumulation, due to overnutrition and physical inactivity312, 313). The dysregulated secretion of adipose tissue-derived bioactive molecules (adipocytokines), which accompanies the accumulation of visceral fat tissue, is important for the development of metabolic syndrome and related cardiovascular diseases.
1). Importance of Risk Factor Accumulation
The Group of ‘The Research for the Association between Host Origin and Atherosclerotic Diseases under the Preventive Measure for Work-related Diseases of the Japanese Ministry of Labour’ performed a case-control study in approximately 120,000 office workers314, 315). The records of annual medical health checkups performed 10 years prior to the onset of CAD were reviewed. The surveys revealed that BMI, BP, fasting blood glucose, and serum lipids levels were significantly higher in cases compared with those in controls for the evaluated 10-year period, even though these abnormalities were mild or moderate. The NIPPON DATA80 epidemiological study also showed that the relative risk of death due to CAD and stroke increased with the number of combined risk factors314–317) [Fig. 4: Relationship between the number of concurrent risk factors and death due to CAD and stroke (NIPPON DATA80: 1980–1994)]. These results clearly indicate the importance of a cluster of multiple risk factors in the development of CAD in Japan, even if the severity of each risk factor is mild. According to a survey of middle-aged and elderly Japanese, the frequency of having multiple risk factors were high in subjects with visceral fat accumulation318). The Japan Society for the Study of Obesity proposed a definition of “obesity disease” based on susceptibility to the clustering of obesity-associated risk factors319, 320). The fat distribution in CAD patients revealed that approximately half of the patients had excess visceral fat accumulation321). A 10-year follow-up study of middleaged and elderly Japanese-American men revealed that approximately 30% of subjects developed CAD and that the accumulation of visceral fat, hypertension, and hyperglycemia were important risk factors322).
Fig. 4.
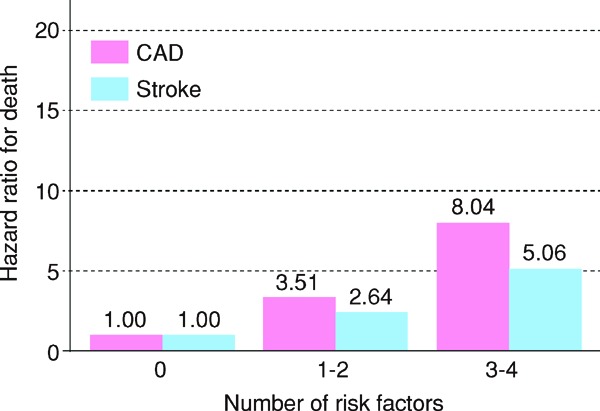
Relationship between the number of concurrent risk factors and death due to CAD and stroke (NIPPON DATA80: 1980–1994)316
Risk factors: Obesity, hypertension, hyperglycemia, hypercholesterolemia
Conditions, such as metabolic syndrome, that involve the clustering of multiple risk factors have been shown to increase the risk of cardiovascular diseases in epidemiological studies323–326) and meta-analyses327), as well as in CIRCS study78), the Tanno and Sobetsu study328) and the Hisayama study317). (Fig. 5: Relationship between the number of concurrent risk factors and incidences of CAD and cerebral infarction317)). Concerning the secondary prevention of CAD, it was also reported that the presence of metabolic syndrome increased the incidence of subsequent cardiac events. Accordingly, metabolic syndrome is positioned as an important high risk pathologic condition for CAD312).
Fig. 5.
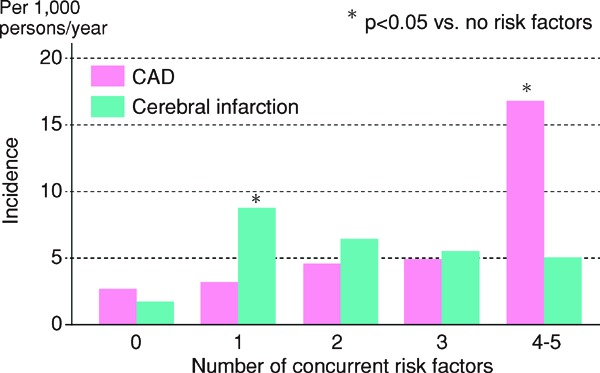
Rerationship between the number of concurrent risk factors and incidences of CAD and cerebral infarction317
Components of metabolic syndrome: Obesity, impaired glucose tolerance, lipidosis, hyperttention, hyperinsulinemia
After adjustment for age, 5-year (1988–1993) follow-up of 1097 men and women aged ≥ 60 years in Hisayama-cho
2). Diagnostic Criteria for Metabolic Syndrome
The definition of metabolic syndrome in Japan, which is characterized by the accumulation of visceral fat accompanied by the concurrence of multiple risk factors including elevated blood pressure, dyslipidemia, and hyperglycemia, was established in 2005 (Table 5). Diagnostic Criteria for Metabolic Syndrome in Japan312)). In the diagnostic criteria, waist circumference is used as an index of visceral fat accumulation for practical convenience, and individuals with metabolic syndrome are defined as those having visceral fat accumulation demonstrated by increased waist circumference and 2 or more risk factors312). The International Diabetes Federation also published similar diagnostic criteria313). However, European and American scientific societies issued a joint declaration later proposing that an individual with three out of the five risk factors - visceral obesity, hypertriglyceridemia, hypo-HDL cholesterolemia, high BP reading, and high glucose level - can be diagnosed as metabolic syndrome329). Visceral fat accumulation was not considered a necessary condition in this criteria.
Table 5. Japanese diagnostic criteria for metabolic syndrome.
| Visceral fat accumulation | |
| Waist circumference | Men ≥ 85 cm |
| Women ≥ 90 cm | |
| (The values for both men and women correspond to visceral fat ≥. 100 cm2). | |
| Two or more of the items mentioned below in addition to the above | |
| Hypertriglyceridemia | ≥ 150 mg/dL |
| and/or | |
| Hypo-HDL cholesterolemia | < 40 mg/dL |
| for both men and women | |
| Systolic blood pressure | ≥ 130 mmHg |
| and/or | |
| Diastolic blood pressure | ≥ 85 nnHg |
| Fasting hyperglycemia | ≥ 110 mg/dL |
Measurement of visceral fat by methods such as CT scanning is recommended.
The waist circumference is measured at the umbilical level in the standing position during light breathing. If the umbilicus is displaced due to marked fat accumulation, measurement is performed at the level of the midpoint between the lower costal margin and iliac crest.
If a diagnosis of metabolic syndrome has been made, a glucose tolerance test is recommended, but it is not essential for the diagnosis.
If the examinee is undergoing drug treatments for hypertriglyceridemia, hypo-HDL-cholesterolemia, hypertension, and/or diabetes mellitus, each item is considered to be positive.
(Evaluation Committee on Diagnostic Criteria for Meabolic Syndrome: Internal Medicine, 2005; 94: 794–809, in Japanese)
The criteria for waist circumference in Japan were determined by the absolute visceral fat area (VFA) of 100 cm2. According to a recent large-scale cross-sectional study conducted in Japan on visceral fat area and accumulated risk factors, the average number of obesity-related cardiovascular risk factors (dyslipidemia, high blood pressure, and high blood glucose) was more than 1.0 at 100 cm2 for visceral fat area in both men and women330). It is obvious that the Japanese criteria of waist circumference is based on scientific evidence. On the other hand, Western criteria of waist circumferences are merely based on the value corresponding to the obesity criteria in each country.
Visceral fat accumulation is required for the diagnostic criteria in Japan, and the aim is to solve the integration of risk factors through the interventions to reduce visceral fat. Since 2008, the measurement of waist circumference has been made compulsory in specific medical examinations and occupational health examinations, with the intent to prevent diabetes and atherosclerotic cardiovascular disease based on the concept of metabolic syndrome.
Chapter 4. Comprehensive Risk Management
1. Absolute Risk of Atherosclerotic Cardiovascular Disease (ASCVD) and Lipid-Management Targets
CQ 6. Are there any assessment tool for predicting the ASCVD incidence and mortality in the Japanese people?
There are several assessment tool that predict the absolute risk of ASCVD in the Japanese population. Hypertension, diabetes, and smoking are regarded as predictive factor for ASCVD. In addition, total cholesterol (TC), LDL cholesterol (LDL-C) and HDL cholesterol (HDL-C) are predictive indicators in the assessment tool that predicts the coronary artery disease (CAD) incidence and/or mortality. (Evidence level: E-1b)
Relative risk is usually used to indicate the risk of future ASCVD incidence and/or mortality. Recently, methods of assessing the actual incidence rate in individuals, otherwise referred to as absolute risk, have been developed and are being used in clinical guidelines in various countries. These include the Framingham Score331) in the USA and the SCORE Risk Assessment Charts in Europe332). The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines include a new pooled cohort ASCVD risk equation to assess absolute risk333). Not like the Framingham Score which predicts only CAD incidence, this model predicts cerebro-cardiovascular disease incidence including stroke and CAD within 10 years.
The NIPPON DATA80 Risk Chart161) was used in the 2012 Japanese Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases to estimate absolute risk. The management target is set after categorizing the target individuals (patients) according to 10-year CAD mortality. An understanding of absolute risk is important for managing ASCVD risk factors, implementing effective preventive strategies, and establishing the priority of future treatments. We performed a systematic review to answer CQ1: “Are there any assessment tool that predict the ASCVD incidence and mortality in the Japanese?” The Japanese population in this review included adults with no history of ASCVD. It did not include individuals treated for secondary prevention or studies only consisted of patients with dyslipidemia. Nine articles published between January 01, 1990 and September 30, 2016161, 162, 334–340) were selected for this review and are summarized in Table 6.
Table 6. Tools for Predicting the Absolute Risk of ASCVD On the Basis of Cohort Studies Conducted in Japan.
| Name of cohort | Method of risk assessment | Period of risk assessment | Risk factors used for assessment | Outcome (event to be predicted) |
|---|---|---|---|---|
| (Communiy-based cohort) | ||||
| NIPPON DATA80 (CAD)161) | Risk assessment chart | 10 years | (Gender-specific tables), age, TC, smoking, SBP and random blood glucose | CAD mortality |
| NIPPON DATA80 (all forms of CVD)161) | Risk assessment chart | 10 years | (Gender-specific tables), age, TC, smoking, SBP and random blood glucose | all forms of CVD mortality (including stroke risk assessment chart) |
| Hisayama Study334) | Scoring table | 10 years | Gender, age, LDL-C, HDL-C, diabetes, SBP and smoking | MI incidence, sudden cardiac death, coronary revascularization and stroke incidence |
| JMS cohort (MI)335) | Risk assessment chart | 10 years | (Gender-specific tables), age, TC, SBP, smoking (for men only) and diabetes (for women only) | MI incidence |
| JMS cohort (stroke)336) | Risk assessment chart | 10 years | (Gender-specific tables), age, SBP, smoking and diabetes | Stroke incidence |
| JALS-ECC337) | Scoring table | 5 years | Gender, age, TC (or non-HDL-C), HDL-C, hypertension (grades 1 and 2), smoking and diabetes | MI incidence |
| Ibaraki cohort338) | Website | 5–15 years | Gender, age, body weight, SBP, HDL-C, TG, AST, blood glucose level (including status of treatment), blood withdrawal conditions, smoking and alcohol consumption | Death due to each cause (stroke, cancer, ischemic heart disease, all forms of CVD and total death) |
| JPHC339) | Scoring table | 10 years | Age, gender, smoking (gender-specific scoring), BMI (25–30 and ≥ 30), BP (scoring by whether the patient is taking antihypertensive drug; The Japanese Society of Hypertensionfs criteria are adopted in the classification) and diabetes | Stroke incidence |
| Suita study162) | Scoring table | 10 years | Age, gender, smoking, diabetes, BP classification (The Japanese Society of Hypertensionfs standards are adopted in the classification; however, grade II hypertension and above are one category), TC or LDL-C, HDL-C and CKD | CAD incidence (MI, sudden cardiac death, coronary revascularization) |
| JPHC (cohort II)340) | Scoring table | 10 years | Age (logarithmic value), gender, smoking, whether the patient is taking any antihypertensive drug, presence of diabetes, SBP (logarithmic value), HDL-C (logarithmic value), non-HDL-C (logarithmic value, only for CAD) | Incidence of CAD (MI + sudden cardiac death) or cerebral infarction |
1). Establishing Absolute Risk
In this guideline revision, we reviewed the continued use of the NIPPON DATA80 risk chart for death due to CAD that had been adopted in the 2012 guidelines. The NIPPON DATA80 had various advantages, including the fact that groups were selected from all over Japan by random sampling, the chart was based on a baseline survey conducted before statins were available and its high suitability for the observation of the natural course of disease, making it very useful for the assessment of absolute risk. However, some aspects of NIPPON DATA80 are problematic such as (1) the fact that its predicted outcome is death and not CAD incidence, (2) the fact that it does not include information on LDL-C or HDL-C and (3) the fact that the baseline year is 1980, and when this baseline hazard is applied to more recent cohort populations, the estimated mortality of the high-risk group is higher than the actual measured mortality of recent populations341).
Like the NIPPON DATA80 survey, the Ibaraki Prefectural Health Study used death as the outcome. The other seven articles selected for review used disease incidence as the outcome. Stroke was the outcome in two articles; MI in two; and CAD, combined CAD and stroke, and CAD or cerebral infarction in one article each. Hardly any cohort studies conducted in Japan have demonstrated that dyslipidemia was a risk factor for stroke342), and hypercholesterolemia (e.g., TC or non-HDL-C) was not included as a risk factor in any of the assessment tool in which the outcome was stroke or cerebral infarction. In addition, absolute risk was assessed over 5 years in two of the reviewed articles and 10 years in the other seven. Furthermore, only two articles included both MI and coronary intervention as the outcomes, which also included both LDL-C and HDL-C as predictive indicators, i.e., important risk factors for establishing the management targets for dyslipidemia. Accordingly, we concluded that the Hisayama score334) and the Suita score162) include the most suitable criteria for assessment of absolute risk relevant to the primary prevention of ASCVD in Japanese adults by managing dyslipidemia. The biggest difference between these risk assessment tools is the fact that the Suita score considers only CAD (MI, PCI, coronary artery bypass, and sudden cardiac death), whereas the Hisayama score considers a combined outcome of CAD, with diagnostic criteria nearly the same as the Suita score, and stroke. In both scores, the fasting LDL-C value is calculated using the Friedewald formula. Stroke comprises a large proportion of the cases of cerebro- and cardiovascular disease in Japan. The ratio of stroke to CAD in the Hisayama study was approximately 2: 1; however, among stroke cases, the percentage of atherothrombotic cerebrovascular disease in which LDL-C was a risk factor is approximately 20%36). Therefore, it is difficult to determine detailed risk by LDL-C level with the Hisayama score, as it uses a combined outcome. The Suita score permits detailed risk assessment by specific dyslipidemia values because it includes five LDL-C level criteria in contrast to the Hisayama score, which includes two levels.
2). Management of Dyslipidemia Using Absolute Risk
The 2013 ACC/AHA guidelines343) recommended avoiding setting an LDL-C control target and using statins to treat four groups of patients for whom they are considered beneficial. The JAS position on those guidelines, published as a press release in February 2014, is that having a management target is advisable in clinical practice in Japan from the viewpoint of patient adherence. Many practicing clinicians use management targets as treatment references, and our view is that their use should be continued. The 2016 European Guidelines for the Management of Dyslipidemias published in August 2016 continue to require the use of a management target344). We have also included management targets in the management of ASCVD with absolute risk in this revision of the guidelines.
As the threshold of each risk grade cannot be determined statistically by using the absolute risk, the criteria were based on clinical consensus and social conventions. In the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) Ⅲ guidelines of the USA, a ≥ 20% 10-year risk of incidence of fatal CAD and nonfatal MI based on the Framingham Score was considered high risk. However, this cannot be used in Japan because ASCVD types and incidence rates of ASCVD differ from those in the USA. The aforementioned 2013 ACC/AHA Guidelines, based on novel pooled risk calculations, recommend statins for anyone with a predicted 10-year incidence of cerebro- and cardiovascular disease (CAD and stroke) of ≥ 7.5%333) which, however, is a rough indication to select the patients to for statin therapy. We have not accumulated enough evidence from clinical studies in Japan to establish a similar reference for selecting patients for statin therapy. Our society's guidelines must maintain continuity and consistency with the risk categorization used in our guidelines before. Management targets for dyslipidemia are also provided in the 2015 Comprehensive Risk Management Chart for the Prevention of Cerebrovascular and Cardiovascular Diseases jointly published by 13 academic societies, including JAS and the Japanese Society of Internal Medicine156). We considered these recent changes in existing recommendations when creating this set of guidelines and established risk categories and lipid-management targets.
3). CAD Risk Categories
In the 2012 version of our guidelines, the NIPPON DATA80 risk chart was the basis for categorizing absolute risk. While the chart detail allows to determine detailed individual risk, it is complex in appearance. The guidelines published in 2007, were simple and convenient to use, but risk categorization depended on a number of risk factors other than LDL-C. Application of the 2007 guidelines results in lack of association with absolute risk because of the huge influence of age and sex. The effect of age is especially substantial. This means that the estimated absolute risk is low in young people even with multiple risk factors and high in older individuals even with few risk factors. Therefore, reference material 2 of the 2012 guidelines (http://www.j-athero.org/publications/gl2012_114.pdf) notes that the number of risk factors can be used in estimates of absolute risk if sex and age taken into account. Accordingly, we presented two types of flowchart for determining the lipid-management target; one uses an absolute risk calculation by the Suita score and the other uses the number of risk factors, consistent with the Comprehensive Risk Management Chart for the Prevention of Cerebrovascular and Cardiovascular Diseases 2015.
Fig. 1 shows the flowchart using the absolute risk derived from the Suita score. When screening for dyslipidemia, whether patients are candidates for secondary prevention is first confirmed. Next, whether these patients have a high-risk condition such as diabetes (not including impaired glucose tolerance), CKD, non-cardiogenic cerebral infarction, or PAD is evaluated. The Suita score calculation is performed in patients with none of these conditions. Diabetes and CKD are included in the original Suita score; but they are not used in calculating the score here as they would already have been treated as high-risk conditions. If the patient has a family history of premature CAD, i.e., first-degree relatives with incidence before 55 years of age for men or 65 years of age for women, or impaired glucose tolerance, it is assumed that they have a risk equivalent to smoking or low HDL-C; five points is added to the Suita score. The predictive model for CAD incidence based on the Suita score is shown in Fig. 2.
In terms of the probability of CAD incidence, apart from the lowest risk category (≤ 35), the minimum value of score range is shown as an integer in each risk category in the original article of the Suita score. In this guideline, ranges (smallest score-highest score) and the median of risk scores are shown in each category. For consistency with the absolute risk assessment by NIPPON DATA80 used in the 2012 guidelines, the absolute risk of CAD is classified by scores ≤ 40 as low (a 10-year probability of CAD incidence of < 2%), 41–55 as moderate (2–8%), or ≥ 56 as high (≥ 9%). Committee members have confirmed that the distribution of low risk, moderate risk, and high risk individuals in two different populations is almost identical to the distribution of categories Ⅰ, Ⅱ, and Ⅲ calculated by the NIPPON DATA80 as in the 2012 guidelines.
On the other hand, determination of the risk for each sex and age group (i.e., age 40–59 and 60–74 years) is easily achieved with the Comprehensive Risk Management Chart for the Prevention of Cerebrovascular and Cardiovascular Diseases 2015. Using that categorization, we estimated the actual absolute risk of CAD using the Suita score (i.e., the LDL model). We made a few changes to make the evaluation easier. We adopted the Suita score age groups of 45–54 years in place of 40–59 years and 65–69 years in place of 60–74 years. Smoking, hypertension, and low HDL-C were added as risk factors. We used the median Suita hypertension scores of grade 1 and ≥ grade 2, and we increased the number of points awarded to the low HDL-C category (< 40 mg/dL) compared with the 40–59 mg/dL category. All were awarded five points. An LDL-C level of ≥ 180 mg/dL has always indicated a high risk for CAD, and may also suggest the presence of familial hypercholesterolemia (FH). Therefore, these guidelines clearly recommend drug therapy for patients with an LDL-C level ≥ 180 mg/dL regardless of the number of other risk factors. A flowchart of the recommended LDL-C management target based on the number of risk factors is shown in Fig. 3. The flowchart conforms to the low-risk, moderate-risk, and high-risk criteria of the simplified Comprehensive Risk Management Chart for the Prevention of Cerebrovascular and Cardiovascular Diseases 2015. However, if the estimation is performed on the basis of the Suita score, the distribution is 39–41 points for the low-risk, 42–56 points for the moderate-risk, and 49–71 points for the high-risk groups. Overlapping of groups is unavoidable, but considered acceptable in this simplified method. In addition, a family history of premature CAD, which is absent in the Suita score, and impaired glucose tolerance are considered to carry the same level of risk as the other factors included in the assessment method and are therefore also counted as risk factors. A management target that corresponds to the respective risk level, from low to high, is identified and compared with the actual lipid level. If the target has not been achieved, the aim of lowering is set.
As the risk of CAD changes with age and risk factor status, the management category should be reassessed at least once per year according to the most recent test findings. In addition, the risk scores have been basically created assuming that they apply to patients with risk factors, including dyslipidemia, that are not being treated. Two scores from the Japan Health Center Study took into account patients with or without antihypertensive drugs for blood pressure control339, 340). However, no risk scores that consider whether the patient is taking any medicines for control of dyslipidemia or diabetes are available in Japan, the USA, or Europe. The currently available risk scoring tools can be used even if the patient is on medication, but the actual risks of cerebro- and cardiovascular diseases differ in patients with or without antihypertensive drug treatment. Usually patients whose blood pressure cannot be controlled by lifestyle modification are medicated and their risk of cerebro-and cardiovascular diseases are lowered by medication. However, as their blood pressure are assumed to remained high for long time before medicated, it is difficult to lower their risk to the same level of those who are not medicated. If they both have the same blood pressure, then the patient on medication is at a higher risk345). It should be kept in mind that the actual risk of disease in patients on medication may be higher than the risk indicated by the prediction score.
The incidence of CAD differs in the various regions and populations in Japan, and is higher in cities than in rural areas98), and employees of large companies have an extremely low incidence rate of MI compared to the general population346). The NIPPON DATA80 outcome was the number of deaths in populations that were selected by stratified random sampling from all regions of Japan and should be representative of the general population. There are doubts as to whether the absolute risk assessed using the Suita score is also representative of the general population of Japan. The incidence of CAD is likely to be higher in the Suita study than in other regional cohort studies and it differs from many other regional cohort studies in the use of coronary intervention as one of outcomes. Consequently, the estimated absolute risk for CAD may be much higher than in other regional cohort studies. In Japan, the Hisayama score is the only other method in which coronary intervention is also assessed as an outcome, and coronary intervention is not used for an outcome in the scoring system of the 2013 ACC/AHA Guidelines in the USA333).
4). Management Targets for Dyslipidemia from the Perspective of Preventing CAD
The category-specific dyslipidemia management targets are shown in Table 2. For primary prevention, drug therapy should be considered after lifestyle modifications have been applied for 3–6 months and when the effects have been ascertained. However, for patients in any of the risk categories, including the low-risk category, if the LDL-C level remains at ≥ 180 mg/dL, then the addition of drug therapy and lifestyle modifications can both be considered. The validity of an LDL-C management target has been partially confirmed by the systematic review data (see Chapter 4, CQ17). The management targets for high-risk category is < 120 mg/dL, and for the the low-risk and moderate-risk groups are the same as in the previous guidelines, i.e., < 160 mg/dL and < 140 mg/dL, respectively. Achieving these management targets is challenging. The results of a meta-analysis of randomized controlled trials involving the use of statins revealed that a 20–30% decrease in LDL-C level lowers the risk for CAD by approximately 30%347, 348). Considering long-term effects and safety, a reduction rate of 20–30% in the LDL-C level can also be used as a target for the low-risk and moderate-risk groups. However, this management target cannot be applied to patients with FH or familial type Ⅲ hyperlipidemia. As patients with FH are difficult to treat, and their risk of CAD in the future is extremely high, it is recommended that these patients be referred to a specialist (see Chapter 5, Familial Hypercholesterolemia and Capter6, Other Types of Primary Dyslipidemias).
This set of guidelines was developed for use in adults aged < 75 years of age. The advantages and disadvantages of lipid management in patients aged < 40 years of age are at the discretion of the attending physician; however, if lipid management is to be carried out, then the Suita score absolute risk for 35–44 years of age group is used.
As patients with a history of CAD most likely require more aggressive treatment for secondary prevention, they are managed differently than patients in need of primary prevention. The LDL-C management target for secondary prevention should be set at a lower value than for primary prevention. Large clinical studies conducted in Western countries have shown that lowering the mean pretreatment LDL-C level decreases the risk of recurrence and total mortality of CAD and it is also effective in decreasing stroke incidence. Observational studies and clinical trials conducted in Japan found that the decrease in frequency of recurrence was proportional to the decrease in LDL-C level until it reached a level of 100 mg/dL. Consequently, by means of drug therapy together with lifestyle modification, the LDL-C management target for secondary prevention shall be at < 100 mg/dL; however, if a level of < 100 mg/dL is difficult to achieve, the target shall be a 50% reduction in the LDL-C level.
As in the previous guidelines, during secondary prevention, if non-cardiogenic cerebral infarction, PAD, CKD, or metabolic syndrome are also present or if the patient has multiple risk factors or is still smoking, it is advisable to make it mandatory to achieve an LDL-C level of < 100 mg/dL considering the higher risk. Some reports in Japan have also indicated the usefulness of controlling the LDL-C level to < 70 mg/dL for a regressive effect on coronary plaques in patients complicated with ACS or diabetes mellitus. In view of the extremely high risk of CAD with FH, if the patient's condition is complicated with FH or ACS, then it would be appropriate to consider stricter lipid management with a target LDL-C level of < 70 mg/dL and a non-HDL-C level of < 100 mg/dL during secondary prevention. In addition, because diabetic patients who are complicated with conditions shown in Table 3b had a high risk for recurrence of CAD, their management target should be set as that of ACS or FH (see Chapter 4.4-1 ‘History of CAD’ for details).
Table 3. Patient Conditions that Require Stricter Management in Secondary Prevention.
| a | Familial hypercholesterolemia (FH) |
| Acute coronary syndrome (ACS) | |
| Diabetes mellitus (DM) | |
| b | Noncardiogenic cerebral infarction |
| Peripheral artery disease (PAD) | |
| Chronic kidney disease (CKD) | |
| Metabolic syndrome | |
| Overlap of major risk factors | |
| Smoking | |
Although management targets for each lipid of concern in dyslipidemia have been established, the primary objective should be the achievement of the LDL-C management target. The non-HDL-C targets can be reviewed when the LDL-C target has been achieved. NCEP-ATP III guidelines recommend a non-HDL-C target 30 mg/dL higher than that of LDL-C, similar to the guidelines in Japan74, 75). We thus include a non-HDL-C management target that is 30 mg/dL higher than the LDL-C target in this set of guidelines. If the TG level is ≥ 400 mg/dL, or if blood is collected after a meal, then the non-HDL-C target shall be used for the initial management of dyslipidemia instead of the LDL-C target. If non-HDL-C levels are used for screening in the general population, then it should also be noted that the difference between the non-HDL-C and LDL-C levels would be < 30 mg/dL, at approximately 20 mg/dL349, 350). As in the previous guidelines, a TG level of < 150 mg/dL and an HDL-C level of ≥ 40 mg/dL are the recommended targets for both primary and secondary prevention. Even if the LDL-C management target has been achieved, a high non-HDL-C level may be accompanied by hypertriglyceridemia, and management of this condition is important. Few available medications are effective for increasing low HDL-C, and some reports have found that CAD risk is low if a low HDL level is not accompanied by other lipid abnormalities351). On the basis of this evidence, the approach for managing HDL-C, should basically involve lifestyle modification after management of LDL-C, non-HDL-C and TG levels.
2. Lifestyle Modification
1). Overview of Lifestyle Modification
ASCVD is developed by various environmental factors such as excessive total energy intake and insufficient physical activity in addition to genetic predisposition. Many epidemiological studies in Japan and other countries have revealed that excessive intake of cholesterol and animal fats (saturated fatty acids) results in increased serum LDL-C levels. Excessive total energy intake and insufficient physical activity are the primary causes of metabolic syndrome, inducing the accumulation of visceral fat, abnormal glucose metabolism, elevated blood pressure and increased triglyceride levels, and decreased HDL-C levels. These lifestyle abnormalities result in ASCVD such as myocardial infarction. Therefore, the basic management to prevent ASCVD is the adoption of lifestyle changes such as smoking cessation and avoiding passive smoking, achieving and maintaining an appropriate body weight by re-evaluating total energy intake and physical activity, adhering to traditional Japanese dietary pattern with a low salt content, refraining from excessive alcohol intake, and performing aerobic exercise for at least 30 minutes per day. It is recommended that medical practitioners take improvements in patient lifestyle and ASCVD risk factors into account.
Table 7. Lifestyle Modifications for ASCVD prevention.
| • Stop smoking and avoid passive smoking |
| • Pay attention to excessive total energy intake and insufficient physical exercise, and maintain an appropriate body weight |
| • Refrain from the consumption of large amounts of fatty meat, animal fat, eggs, and processed foods with fructose |
| • Increase the intake of fish, green and yellow vegetables, seaweed, soy products, and unrefined grains |
| • Moderately consume fruits with low carbohydrate content |
| • Avoid excessive alcohol consumption |
| • Perform moderate- or high-intensity aerobic exercise for a target of at least 30 minutes a day |
Table 8. Dietary Advice for Prevention of ASCVD.
| • Total energy intake (kcal/day) is generally calculated as body weight [kg, (height in meter)2 × 22] × physical activity (25–30 for light, 30–35 for normal, and ≥ 35 for strenuous activity) |
| • Limit fat intake to 20–25%, saturated fatty acids intake between 4.5% ≤ and < 7% of total energy intake, and cholesterol intake to < 200 mg/day |
| • Increase the intake of n-3 polyunsaturated fatty acids |
| • Refrain from consuming industrial trans fatty acids |
| • Limit carbohydrate intake to 50–60% of total energy intake and increase dietary fiber intake |
| • Keep salt intake to < 6 g/day |
| • Limit alcohol consumption to ≤ 25 g/day |
2). Smoking Cessation
[Statement]
For primary and secondary prevention of ASCVD, one should stop smoking and avoid passive smoking. (Evidence level: 2, recommendation level: A)
Smoking is an independent risk factor for ASCVD, and regardless a history of ASCVD, smoking cessation decreases the development and subsequent progression of ASCVD, and the risk of death due to ASCVD. The effects of smoking are not dependent on age or gender102). In ASCVD patients, the benefits of smoking cessation are apparent soon after quitting, and the longer the duration of abstinence from smoking, the greater is the decrease of risk107). As decreasing the number of cigarettes smoked or switching to low-nicotine or low-tar tobacco does not lead to a reduction in risk, cessation is a must for primary and secondary prevention of ASCVD. All smokers should be encouraged to quit regardless of age. Passive smoking also increases CAD and stroke risk115, 116), and it is also important to advise patients to avoiding secondhand smoke exposure. A meta-analysis found that in other countries, the implementation of smokefree legislation decreased the number of cases of ACS and stroke352).
For all patients, the first step is to determine whether they have a history of smoking or passive smoking156). The rate of successful smoking cessation increases by 1.7 times if physicians advise their patients to quit smoking, compared with not doing so353). The “5A approach,” i.e., Ask, Advise, Assess, Assist, and Arrange can be effective in such cases in a short time354). As with other drug dependencies, symptoms of nicotine withdrawal appear soon after quitting, and that makes it difficult for many smokers to quit. In Japan, when both medical facilities and patients satisfy certain requirements, health insurance coverage is applied for smoking cessation treatment for 12 weeks355). The 2016 medical fee revision expanded coverage to qualifying minors. The use of effective drugs to treat nicotine-dependence (e.g., nicotine patches, nicotine gum, and varenicline)356, 357) combined with counseling for psychological dependence increase the success rate of smoking cessation.
While the long-term benefits of smoking cessation are evident, a recent meta-analysis reported a 4–5 kg increase in body weight 1 year after quitting smoking, with the increase quite pronounced within the first 3 months after cessation358). Short-term worsening of blood glucose and lipid levels may be observed during that interval, but improvement in insulin resistance117), increase in HDL-C359) and reduction in oxidized LDL360) have been reported regardless of weight gain. Although the expectation of weight gain hinders the start of smoking cessation and causes resumption of smoking, it is suggested that discontinuing smoking surpasses the disadvantages caused by weight gain and reduce the risk of CVD361). Patients must be made aware of the benefits of quitting smoking and given support to discontinue smoking during the process.
3). Management of Obesity and Metabolic Syndrome
[Statement]
For the management of obesity and metabolic syndrome, lifestyle modification is essential for the reduction of body weight and visceral fat. (Evidence level: 2, recommendation level: A)
To achieve and maintain an ideal body weight and optimal waist circumference are important targets for lifestyle modification. Obesity, especially excess visceral fat accumulation, is considered to be an independent risk factor for CVD and promotes atherosclerosis directly or indirectly via dyslipidemia, impaired glucose tolerance, hypertension and the dysregulated production of adipocytokine362–365). Therefore, it is important to achieve lifestyle modification through dietary management and exercise.
i). Obesity Disease
Obesity disease is an obesity with complications that require medical treatment of weight loss for the improvement. The status of body weight is evaluated based on the body mass index, BMI (body weight (kg)/[height(m)]2). In Japan, the ideal body weight is defined to be a BMI of 22, which has the lowest morbidity, and a BMI ≥ 25 is diagnosed as obesity366). However, the obesity is not immediately categorized as a disease. If obesity is complicated by or associated with health problems that require weight loss for the improvement, or associated with visceral fat accumulation likely accompanied by health problems, it is diagnosed as the obesity disease367). For screening of obesity disease in daily clinical practice, a waist circumference at the umbilical level of ≥ 85 cm for men and ≥ 90 cm for women is used as the criteria of visceral fat accumulation368). Patients with suspected visceral fat accumulation should be evaluated by abdominal CT, and those with a visceral fat area of ≥ 100 cm2 at umbilical level are diagnosed as visceral obesity, which is common to metabolic syndrome. The reduction of visceral fat is expected to normalize the multiple abnormalities in plasma lipids, glucose and blood pressure caused by obesity312).
ii). Metabolic Syndrome
Metabolic syndrome is a condition with excessive accumulation of visceral fat complicated by anomalies of hyperglycemia, dyslipidemia and elevated blood pressure, even though the criterion for obesity (BMI ≥ 25) is not met. Metabolic syndrome is significant because it is a high risk of ASCVD even if the levels of hyperglycemia, dyslipidemia and elevated blood pressure are relatively mild323–327). The difference between the diagnostic criteria in Japan and the joint statement by Western academic societies329) is whether they are based on visceral fat accumulation or not. It is generally accepted that ASCVD risk is high in conditions with coincident hyperglycemia, hypertension and dyslipidemia without visceral fat accumulation, but multiple treatments are required in such patients, and weight loss is not likely to be effective for the improvement of multiple risk factors. For the management of metabolic syndrome with excess visceral fat accumulation, however, the benefits of lifestyle modification and behavioral therapy are evident.
iii). Treatments for Obesity and Metabolic Syndrome
The target body weight in the treatment of obese patients should not immediately be set as a BMI of < 25. Rapid weight loss resulting from extensive calorie restriction may lead to rapid rebound weight gain. Weight reduction by diet and exercise therapy is expected to provide relatively rapid improvement in moderate abnormalities of plasma lipids, glucose and blood pressure caused by obesity, even if the BMI is within the range of obesity369). Even if medications are needed to treat coexisting diabetes, dyslipidemia, and hypertension, it is necessary that both medical staffs and patients realize the risk reduction through measuring body weight and assessing waist circumference.
Accordingly, it is important to achieve ≥ 3% reduction body weight or waist circumference over 3–6 months and to review the patient's accomplishments over time320, 323, 370).
iv). Relationship of Hyper-LDL Cholesterolemia with Metabolic Syndrome
Hyper-LDL cholesterolemia is a major risk factor for ASCVD, and its management protocol has been established. Metabolic syndrome has been proposed as a high-risk condition for ASCVD independent of hyper-LDL cholesterolemia. Therefore, the diagnostic criteria of metabolic syndrome include no criterion concerning the LDL-C level. However, subjects with metabolic syndrome sometimes have elevated plasma LDL-C levels. The combination of metabolic syndrome and hyper-LDL-cholesterolemia will further increase the risk of ASCVD, a comprehensive approach for both risk factor control are necessary in such cases.
4). Diet Therapy
CQ7: Is limiting total energy intake and maintaining an appropriate body weight effective in preventing ASCVD?
Limiting total energy intake and maintaining an appropriate body weight is effective in improving serum lipid levels. (Evidence level: 1, recommendation level: A)
Although there is no direct evidence showing that limiting total energy intake and maintaining an appropriate body weight suppresses ASCVD, improvement in serum lipid levels by lifestyle modification including body weight reduction in response to diet therapy may prevent ASCVD. (Recommendation level: A)
There is no direct evidence that diet alone, by decreasing the total energy intake, suppresses ASCVD. However, lifestyle modification including body weight reduction has positive effects on ASCVD risk factors including serum lipid levels, which may in turn suppress ASCVD.
There is no clear evidence of setting total energy intake to improve serum lipid levels. Rather, the total energy intake should be optimized on the basis of an ideal body weight and the amount of daily activity. The total energy intake (kcal/day) can be calculated as ideal body weight (kg) x physical activity (25–30 min for light activity, 30–35 for normal activity, and ≥ 35 for strenuous activity)371). In Japan, active support of lifestyle modification resulted in a weight loss of ≥ 3% in obese individuals within 1 year, could reduce LDL-C, TG, uric acid, and blood pressure levels, improve blood glucose control, and increase HDL-C levels significantly320, 372).
An RCT comparing an energy-restrictive diet with an energy-restrictive diet+exercise in overweight postmenopausal women found decreases in body weight and amount of body fat; decreased TG, TC, LDL-C, and the LDL-C/HDL-C ratio; and a decrease in SBP in both groups373).
Another RCT compared the effects of lifestyle modification, including total energy intake of 1,300 kcal/day (lowered fat intake to 25% of total energy intake and saturated fat intake to 7% of total energy intake, and lowered to 100 mg/day cholesterol) and 1,000–1,500 kcal/week of physical activity for an average of 54 months in premenopausal women. Although 35% of the women had become postmenopausal during the intervention period, the increases of LDL-C, TG, and blood glucose levels in the intervention group were significantly less than in the assessment-only group. Body weight and waist circumferences in the intervention group also decreased significantly374). A meta-analysis of RCTs revealed that lifestyle and weight-loss intervention including diet in overweight and obese adults with type 2 diabetes resulted in a reduction of TC, LDL-C, and TG as well as an increase in HDL-C level in the subjects that achieved a weight loss of > 5% over 1 year375).
Therefore, maintaining an appropriate body weight is effective in improving serum lipid levels, and it is possible to prevent ASCVD by improving serum lipid levels. However, in such a case as sarcopenia and malnutrition in the elderly, caution should be exercised when decreasing the total energy intake, and the appropriate ratio and intake of nutrients should be considered.
CQ8: Is limiting the percentage of energy derived from fat in the appropriate total energy intake effective in preventing ASCVD?
Limiting the percentage of energy derived from fat in the total appropriate energy intake is effective in improving serum lipid levels. (Evidence level: 1, recommendation level: B)
Although there is no direct evidence showing that limiting the percentage of energy derived from fat in the total appropriate energy intake suppresses ASCVD, lifestyle modifications including weight loss and modified and/or decreased fat intake may prevent ASCVD. (Recommendation level: A)
There is no direct evidence showing that reduction of the percentage of energy derived from fat decreases ASCVD. However, many trials comparing low-fat* and low-carbohydrate meals in the appropriate total energy intake have been performed to reveal these effects on weight loss as the primary outcome and on ASCVD risk factors as the second outcomes in obese subjects with BMI more than 25 kg/m2.
A meta-analysis of RCTs comparing ≥ 1 year outcomes of high-fat (> 30% of total energy intake) and low-fat diets in obese subjects with BMI > 25 kg/m2, found significant reductions of TC and LDL-C levels following a low-fat diets compared with high-fat diets. The TG levels were lower and the HDL-C levels were higher following a high-fat diet. The differences in TC and LDL-C levels observed in the two groups were abolished when total energy intake was restricted. A high carbohydrate intake was associated with an increase in the TG levels376). Another meta-analysis of RCTs compared the effects of balanced diets (fat to 25–35% of total energy intake, and carbohydrate to 45–65% of total energy intake) with low-carbohydrate (< 45% of total energy intake) diets under restricting total energy intake in obese subjects with BMI ≥ 26 kg/m2. This analysis found no differences in body weight or ASCVD risk factors (serum lipids, blood pressure, and fasting blood glucose) at 3–6 months and 1–2 years377). Another meta-analysis of RCTs that compared low-fat and low-carbohydrate diets found that the 6-month weight loss was greater in participants with an extremely low-carbohydrate diet containing ≤ 20 g or < 30 g/day of carbohydrates, compared with a low-fat diet with meals containing fat to ≤ 25–30% of total energy intake. However, the difference in body weight was no longer significant at 1 year. TG decreased and HDL-C increased in the low-carbohydrate diet group; TC and LDL-C were lower in the low-fat diet than in the low-carbohydrate diet group378). In another RCT, obese participants with BMI of 25 to 40 kg/m2 were divided into four energy-restricted diet groups that had different percentages of energy of protein in total energy intake (15–25%), fat (20–40%), and carbohydrate (35–65%) for two years. There were no significant differences in the body weights and waist circumferences among four groups after 2 years, and the body weights remained decreased in subjects who were able to continue the diet therapy for 2 years in all groups. In this study, the largest decrease in LDL-C levels was observed in the low-fat diet, TG also decreased regardless of the diet, and the largest increase in HDL-C occurred in those with the low-carbohydrate diet379). A meta-analysis of RCTs comparing high-protein and low-protein diets for ≥ 12 months found improved fasting insulin levels in the high-protein diet compared with the low-protein diet, but there were no differences in body weight, serum lipid levels or blood glucose levels380).
Thus, limiting the percentage of energy derived from fat in total energy intake in a diet with appropriate total energy intake improves blood lipid levels, which in turn may help prevent ASCVD. A metaanalysis of RCTs that evaluated diet therapy in which the fat content was modified and/or decreased did not find significant effects on total mortality or mortality due to ASCVD. However, modified and/or decreased fat intake decreased CVD events by 14% in men with high or moderate risk of CVD who continued the therapy for at least 2 years381).
No findings contradict the setting of the percentage of energy derived from fat of total energy intake at 20–25% and the percentage of energy derived from carbohydrate at 50–60%, which have been the recommendations for some time based on the appropriate total energy intake according to the current situation in Japan and various conditions. Most importantly, limiting the percentage of energy derived from fat is effective in lowering blood LDL-C levels. For managing hypertriglyceridemia and low HDL-C, it is recommended to intake a somewhat low percentage of energy derived from carbohydrate in consideration of complications such as obesity, diabetes, and hypertension.
CQ9: Is reduction of the intake of saturated fatty acids (SFA) or substituting with monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) effective in preventing ASCVD?
In an appropriate total energy intake, reduction of the amount of SFA or substituting with PUFA is effective in improving serum lipid levels, and in preventing CAD. (Evidence level: 1+, recommendation level: A)
Serum lipid levels are expected to improve by substituting SFA with MUFA. (Evidence level: 1, recommendation level: A)
Considerable reduction in SFA intake may be associated with the incidence of cerebral hemorrhage. (Evidence level: 2)
A meta-analysis of RCTs did not find significant decreases in overall mortality or CVD mortality associated with a reduction of SFA intake for ≥ 2 years, but did find a 17% decrease in CVD events. Substitution of SFA with PUFA resulted in a 27% reduction, but the effect of substitution of SFA with MUFA was unclear382). Greater reduction in SFA intake reduced CVD events, and increases in PUFA and MUFA intake were protective of CVD events. Other meta-analysis of RCTs also found that consuming PUFA in place of SFA reduced CAD events383). Some meta-analyses of cohort studies have reported an association of SFA intake and the incidence of CVD events, but others have denied its involvement384–390). The effects of substituting SFA with carbohydrate or protein is unclear382).
Cohort studies in American women and Japanese population have shown that cerebral hemorrhage increased when the intake of animal fat and protein was low391, 392). The Japan Collaborative Cohort (JACC) Study found an inverse association between SFA intake and mortality from total stroke, cerebral hemorrhage, and ischemic stroke393). The Japan Public Health Center (JPHC) study found inverse associations between SFA intake and cerebral hemorrhage and ischemic stroke, and positive association between SFA intake and myocardial infarction394).
The effect of reduction in SFA intake on serum lipid levels has been investigated in RCTs, and a metaanalysis revealed an association with decreases in TC and LDL-C with no significant effects on HDL-C and TG levels382). Other meta-analysis have also found decreases in TC and LDL-C levels, but the effect of decreased SFA intake on HDL-C is less clear, and many reports have not found significant changes in TG levels395–401). NIPPON DATA90 also found that SFA intake is positively associated with TC and LDL-C levels402), and in the INTERLIPID Study, a dietary increase in the PUFA/SFA ratio was associated with decreases of the TC and LDL-C but not the TG and HDL-C levels403). A meta-analysis including the above-mentioned RCTs reported that the reduction in CVD events was clearer in subgroup with greater reduction in saturated fats, and that degree of reduction in CVD events was strongly related to degree of reduction of serum TC level, and there was a modest suggestion of greater increase in PUFA and MUFA382). Substitution of 9.5–9.6% of total energy intake of dietary SFA with either MUFA or n-6 PUFA significantly decreased both TC and LDL-C levels in RCT among subjects with moderate risk of CVD397).
The available evidence shows that reduction of SFA in an appropriate total energy intake or substitution of SFA with PUFA improves serum lipid levels and prevents CAD. Substitution of SFA with MUFA also helps to improve serum lipid levels. Considerable reduction in SFA intake may also be associated with increased risk of cerebral hemorrhage. The evidence is not strong enough to establish a recommended SFA intake. However, taking account of the intake at which the incidence of cerebral hemorrhage increases and the current average intake in Japan, no findings contradict the setting of SFA intake between 4.5% ≤ and < 7% of total energy intake for the patients with dyslipidemia.
CQ10: Is increasing the intake of n-3 PUFA effective in preventing ASCVD?
Increasing the intake of n-3 PUFA is effective in decreasing the TG level. (Evidence level: 1, recommendation level: A)
Increasing the intake of n-3 PUFA may lead to suppression of CAD. (Evidence level: 2, recommendation level: A)
A meta-analysis of RCTs found that increased intake of n-3 PUFA (fish oil and α-linolenic acid) resulted in a significant reduction of vascular death (i.e., death due to MI, stroke, and sudden death), but there was no overall effect on composite CVD events or on total mortality404). Participants in the JPHC cohort study with a higher fish consumption had a lower incidence of nonfatal CAD405), and CVD mortality was low in JACC Study and NIPPON DATA80 participants with high fish consumption406, 407). However, the effect of fish consumption on CVD was not clear in cohort studies conducted in Western countries408–414). A meta-analysis of cohort studies did not find a decrease in combined CVD events, but did report a reduction in overall mortality, CVD death, fatal MI and sudden death with increasing the intake of n-3 PUFA415). Another meta-analysis of cohort studies reported a decrease in sudden cardiac death and a trend to a decrease of fatal CAD in subjects who consumed ≥ 250 mg/day n-3 PUFA, compared with those who consumed < 250 mg/day416). A similar analysis reported a significant reduction of CVD events in subjects with a high intake of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and EPA+DHA +docosapentaenoic acid (DPA)415, 417). Thus, it is expected that CAD would be decreased in populations with a diet high in fish, which contain EPA and DHA.
Meta-analyses of RCTs including both healthy participants and those with dyslipidemia have shown that increased intake of fish oil resulted in decreased TG levels415, 418–420). It has also been shown to decrease postprandial elevation of TG levels421). Adding fish meals to energy-restricted diets for obese patients with hypertension, improved blood glucose, fasting insulin level, and oral glucose tolerance test results in addition to decreasing TG level422). Increasing n-3 PUFA intake is thus effective in decreasing TG levels. Although meta-analysis of cohort studies have shown that there was an inverse association (not significant) between intake of α-linolenic acid and CAD in men423) and non-significant association of α-linolenic acid with low incidence of CVD424), the association of α-linolenic acid with ASCVD has not been confirmed by well-controlled RCTs425).
CQ11: Is increasing the intake of n-6 PUFA effective in preventing ASCVD?
In an appropriate total energy intake, increasing the intake of n-6 PUFA may improve serum lipid levels. (Evidence level: 1, recommendation level: A)
It is not clear whether increasing the intake of n-6 PUFA prevents ASCVD. (Evidence level: 1)
Many meta-analysis of RCTs to analyze the effects of increasing the intake of n-6 PUFA has been reported. A significant 27% reduction of CVD events was observed in a meta-analysis of RCTs that evaluated the effects of substituting SFA with PUFA382), and in another analysis, substitution of dietary SFA of 5% of total energy intake with n-6 PUFA resulted in a 19% reduction in CAD events383). However, other meta-analysis of RCTs and cohort studies did not find that increasing the intake of n-6 PUFA was associated with a reduction of CAD426). Other cohort study of US also found no association with CVD for n-6 PUFA417). After publication of these reports, a negative correlation of the concentration of linoleic acid in the blood and both overall and CAD mortality was reported in the cohort study of the elderly427), and an analysis of cohort studies found that CAD events were 15% lower in patients with a high compared with low linoleic acid intake, and CAD mortality was 21% lower428). The preventive effect of an increasing the intake of n-6 PUFA on ASCVD incidence is thus unclear.
Regarding the effect on serum lipid levels, substitution of dietary SFA of 9.5–9.6% of total energy intake with n-6 PUFA significantly decreased both TC and LDL-C levels in RCT among subjects with moderate risk of CVD397), and a diet with n-6 PUFA as 19% of the total energy intake in the form of corn oil decreased TC, LDL-C, and TG levels in patients with dyslipidemia400). Thus, increasing the intake of n-6 PUFA may improve serum lipid levels.
CQ12: Is increasing MUFA intake effective in preventing ASCVD?
In an appropriate total energy intake, increasing the intake of MUFA may improve serum lipid levels. (Evidence level: 1, recommendation level: A)
It is not clear whether increasing the intake of MUFA prevents ASCVD. (Evidence level: 1)
Evidence from RCTs indicates that substituting SFA with MUFA had no effect on overall mortality, CVD events, or the incidence of myocardial infarction, stoke, and coronary death382). The PREDIMED trial found that a Mediterranean diet supplemented with olive oil or nuts, decreased the incidence of CVD events compared with a controlled diet429). However, a meta-analysis of cohort studies reported that the association of dietary MUFA with CVD events and CVD death was inconsistent386). Another meta-analysis of cohort studies reported that increasing the intake of olive oil among MUFA seems to be negatively correlated with overall and CVD mortality, CVD events, and stroke incidence430).
In patients with dyslipidemia, a diet high in MUFA was shown to decrease TC, LDL-C, and HDL-C levels compared with a diet high in SFA431), and the substitution of SFA with MUFA has been shown to decrease TC, LDL-C, and HDL-C432) or TC and LDL-C levels397). However, the addition of MUFA to the American Heart Association (AHA) STEP 1 Diet, increasing from 30.1% to 37.8% of total energy intake, resulted in no significant improvement of serum lipid levels433). A comparison of high-SFA (stearic acid), high-MUFA (oleic acid) and high-PUFA (linoleic acid) diets of approximately 38% of total energy intake in healthy individuals found no significant differences in serum lipid levels434). A metaanalysis of RCTs reported that substitution of carbohydrate with MUFA decreased TC and LDL-C levels non-significantly, and decreased TG level significantly and increased HDL-C level significantly. However, the lowering effect on LDL-C level was less pronounced than after substitution of carbohydrate with the same amount of PUFA435). Other RCT has reported improvements in body weight, body fat mass, and systolic and diastolic blood pressure with diets in which the MUFA > 12% of total energy intake, compared with ≤ 12%, but significant effects on the serum lipid levels have not been confirmed436). Meta-analysis of RCT to analyze the effect of substitution of SFA with MUFA also did not have significant effect on serum lipid levels382). The current evidence thus shows that increasing MUFA intake may improve serum lipid levels, but that this effect may disappear with excessive intake.
CQ13. Is reducing the intake of trans fatty acids effective in preventing ASCVD?
Reducing the intake of trans fatty acids is effective in preventing ASCVD. (Evidence level: 2, recommendation level: B)
Trans fatty acids are constituents of naturally occurring foods such as beef, mutton, milk, and dairy products, and may also be industrially produced (hydrogenation) or refinement (deodorization or high-heat processing) of fat. Trans fatty acids produced by hydrogenation are contained in hard margarine, fatty spreads, and vegetable shortening, deep-fried food and confectionery made using these products. Trans fatty acids are also contained in refined vegetable oils such as salad oil.
Industrially produced trans fatty acids increase LDL-C437–441) and lipoprotein (a) [Lp(a)] which is a lipoprotein that promotes atherosclerosis437, 442, 443) and decrease HDL-C439, 442, 444), compared with other fatty acids, whereas the effect on TG is inconsistent440–442). Cohort studies and their meta-analyses provide concordant evidence that the intake of trans fatty acids is associated with CAD384, 438, 445–448). No significant relationship with ischemic stroke has been observed447). A cross-sectional study reported high blood concentration of elaidic acid, an industrial trans fatty acid, in Japanese patients with metabolic syndrome or young Japanese patients with CAD449). Currently, there is no consensus on whether naturally occurring trans fatty acids should be regarded in the same way as industrially produced ones439, 444, 445, 450, 451).
A meta-analysis of RCTs comparing the effects of vegetable oils containing industrially produced trans fatty acids with oils substituted with other fatty acids reported that TC, LDL-C, and TG levels were significantly decreased, and the HDL-C level significantly increased, when trans fatty acids were substituted with MUFA or PUFA448). A meta-analysis of cohort studies in the same article found that substitution of trans fatty acids with SFA, MUFA or PUFA has decreased CAD risk448).
The average daily trans fatty acid intake of the Japanese is 0.92–0.96 g, or 0.44–0.47% of the total energy intake452), which is lower than the < 1% target recommended by the World Health Organization453, 454). Caution is required as intake may exceed the average value if one consumes an imbalanced diet with an excessive amount of confectionery and high-fat foods. Trans fatty acids should be cut back on to prevent CAD.
CQ14: Is restricting cholesterol intake effective in preventing ASCVD?
Decreasing cholesterol intake to < 200 mg/day can result in a lower LDL-C level for patients with hyper-LDL cholesterolemia. (Evidence level: 1, recommendation level: A)
Restricting the intake of cholesterol may prevent ASCVD in patients with hyper-LDL cholesterolemia. (Recommendation level: A)
Among cohort studies, the Seven Countries Study and the Honolulu Heart Program reported that cholesterol intake was associated with CAD mortality in men384, 455). Whereas, the Framingham Study did not find any significant relationships between cholesterol intake and the prevalence of CAD or CADrelated death in men456). Cholesterol intake was not also significantly associated with death due to CAD in both men and women in the Strong Heart Study457). In the Framingham Study, TC and LDL-C levels were significantly and positively associated with SFA but not cholesterol intake458). However, an RCT found that a high-cholesterol diet (600 mg/day) did significantly increase LDL-C levels more than a low-cholesterol diet (200 mg/day)459). Cholesterol in the same dietary concentrations produced a larger increase in LDL-C levels when combined with SFAs than with PUFAs460). A meta-analysis of RCTs and cohort studies reported that increasing the cholesterol intake increased TC, LDL-C and HDL-C levels, and increases of LDL-C were no longer statistically significant when intervention doses exceeded 900 mg/day461).
Restriction of energy of fat to 30% of total energy intake, energy of SFA to 10% of total energy intake, and cholesterol intake to < 250 mg/day in the AHA Step 1 diet has been shown to significantly decrease TC levels433). Similar RCTs reported significant reductions in TC, LDL-C, and HDL-C levels with the AHA Step 1 diet with cholesterol to < 300 mg/day462, 463) or the Step 2 diet, with fat to 30% of total energy intake, SFA to 7%, and cholesterol to < 75 mg/1,000 kcal/day plus lifestyle modification464). A diet intervention containing SFA to 8% of total energy intake and cholesterol to < 200 mg/day also decreased LDL-C levels465). Many RCTs studying the effects of varying types and amounts of fatty acids on serum lipid levels do take the amount of cholesterol into account by restricting cholesterol intake to 200–300 mg/day.
Cholesterol-containing meals also contain SFA, the cholesterol absorption differs greatly from person to person, and serum cholesterol levels are regulated by the synthesis in the liver. Consequently, the effects of cholesterol intake on serum lipid levels vary among individuals466, 467), and have also been demonstrated in intervention studies459, 468–472). For example, although hens' eggs are high in cholesterol content, the relationship between egg consumption and serum lipid levels is variable in both healthy individuals and in patients with dyslipidemia469–471, 473–482). Meta-analysis of clinical studies demonstrated that TC, LDL-C, and HDL-C levels have been shown to increase with the dietary consumption of egg yolks483). The average daily intake of cholesterol in the Japanese ≥ 20 years of age was estimated as 340 mg in men and 290 mg in women from the 2015 National Health and Nutrition Survey. In diabetic subjects, some cohort studies and a meta-analysis of these have found that CVD, especially the incidence of or death due to CAD, was increased with a high intake of hens' eggs484–488).
Although it is difficult to establish a limit of cholesterol intake for patients with hyper-LDL cholesterolemia, an intake of cholesterol to < 200 mg/day and of SFA to < 7% of total energy intake may decrease the LDL-C level, and the improvement of serum lipid levels may prevent ASCVD. Individuals who do not have serum high-LDL cholesterol levels should also refrain from excessive intake of cholesterol, because excessive intake may elevate their LDL-C levels in future.
CQ15: Can increasing the intake of vegetables be recommended for ASCVD prevention?
Consumption of vegetables may decrease the risk for CAD and stroke. (Evidence level: 2, recommendation level: A)
Consumption of green and yellow vegetables may decrease stroke risk. (Evidence level: 2, recommendation level: A)
Meta-analyses of cohort studies have shown that overall mortality, death due to CVD489), and risks of CVD490) and CAD491, 492) decrease when vegetable intake is high. Before 2004, an association between stroke and the vegetable consumption had not been demonstrated493, 494), but a recent study reported that stroke risk was 14% lower in a group with the highest vegetable intake than in a group with the lowest intake. The association was strongest for the intake of leafy vegetables495). Cruciferous, green and yellow vegetables may have preventive effects on stroke496).
Cohort studies in Japan reported the reduced mortality risk due to CVD in men and women who consumed vegetables in large amounts497) and in women who consumed more vegetables dishes498). In one study, stroke mortality rate has been shown to be low in with frequent consumption of green and yellow vegetables499), however this was not shown in another study500).
Very few RCTs have evaluated the effects of increased vegetable intake. Adding 350 g of Okinawan vegetables, such as bitter gourd, green papaya, and gynura bicolor, for 2 weeks had no effect on the serum lipid levels in healthy young women501, 502).
The consumption of vegetables including green and yellow vegetables may be useful for preventing ASCVD incidence. However, as pickled vegetables habitually consumed in Japan have a significant influence on the salt intake503, 504), the intake of a vegetable dish containing reduced-salt content should be recommended.
CQ16: Can increasing the intake of seaweed be recommended for ASCVD prevention?
Consumption of seaweed may be useful for preventing ASCVD. (Evidence level: 2, recommendation level: B)
Japan is one of the few countries in the world in which seaweed is regularly consumed. The consumption of seaweed has been routinely recommended in energy-restricted diets in Japan as it provides little energy505), and is rich in viscous fibers and minerals such as potassium, calcium, magnesium, iron, zinc, and iodine.
In recent years, much attention has been focused on the health effects of the sulfated polysaccharides and carotenoids contained in seaweed.
Although, very few studies have focused on the benefits of seaweed consumption, a cohort study in Japan reported that a high frequency of seaweed consumption was associated with decreased all-cause mortality in both men and women, as well as decreased risk of death due to cerebrovascular disease in women, but no association with CAD was found506). No studies have investigated the association between the dietary consumption of seaweed and cardiovascular risk factors. At present, information on the effectiveness of seaweed consumption to prevent ASCVD incidence is insufficient.
Incidentally, as seaweed contains a high concentration of iodine, and some kinds of seaweed contain a high amounts of arsenic, it is necessary to avoid excessive consumption of seaweed507).
CQ17: Can increasing the intake of fruit be recommended for ASCVD prevention?
Consumption of fruit may decrease the risk for CAD and stroke; moderate consumption of fruit with low sugar content is recommended. (Evidence level: 2, recommendation level: A)
Meta-analyses of cohort studies have shown that the regular intake of fruit decreases overall and CVD mortality489) and CAD492, 508), stroke493), and type 2 diabetes risk509), and the association is especially strong with the regular intake of citrus fruits495, 496), apples, and pears495).
In Japan, cohort studies have revealed that as the amount or frequency of fruit consumption increases, the risk of mortality due to CVD497) and stroke497, 499, 500) lowers. A high frequency of citrus fruit consumption is related to a reduction in overall mortality500), CVD mortality500), and CVD incidence510), and an increased amount of citrus fruit consumption is associated with a lower risk of CVD511). Furthermore, increased fruit intake was reported to decrease the prevalence of hypertension512). Some RCTs have reported the effects of consuming additional fruit with low sugar content on serum lipid parameters. The addition of grapefruit or oroblanco to the diet resulted in small decreases in the level of LDL-C and TGs513, 514), however, the addition of kiwifruit and berries increased the levels of HDL-C515, 516).
The meta-analysis of RCTs showed that the consumption of fruit juice could decrease diastolic blood pressure, but did not influence blood cholesterol level517). However, the sweetie juice supplemented diet decreased levels of LDL-C and TGs518) and daily consumption of a high amount (750 mL) of orange juice increased TG levels by 30% and HDL-C levels by 21%519), in an RCT study on patients with high TC levels. It should be noted that the effects of fruit consumption on ASCVD risk factors differ according to the variety and quantity of fruit.
The evidence shows that increasing the intake of citrus fruits or fruits with low sugar content reduces stroke and CAD risk, but excessive fruit consumption may lead to increased TG concentration.
CQ18: Can decreasing the intake of processed foods containing fructose be recommended for ASCVD prevention?
Consuming large amounts of processed foods containing fructose may increase ASCVD risk; reducing the intake of such foods is recommended. (Evidence level: 3, recommendation level: B)
Decreasing the intake of fructose in processed food may decrease the TG concentration. (Evidence level: 3, recommendation level: B)
Fruit and honey contain natural fructose. Its sweetness is stronger than that of sugar and increases when chilled. Because of its potent sweetness, fructose, sucrose which is a disaccharide containing glucose and fructose, and isomerized sugars (high-fructose corn syrup), are used as sweeteners in soft drinks, milk beverages, and ice sweets such as sherbet and gelato. Consuming large quantities of fructose in processed foods has been considered to increase CAD risk through excess energy intake, obesity, increased TG level, worsened insulin resistance, and the incidence of type 2 diabetes.
The results of cohort studies that investigated the association of sugar-sweetened beverage consumption with CVD and stroke risk are inconsistent520, 521). In the JPHC cohort study in Japan, soft drink was positively associated with the risk of ischemic stroke in women, but the risk tended to be lower in men522).
A meta-analysis of RCTs that studied the effects of sugars containing fructose in healthy and obese participants and type 2 diabetes patients reported significant increases in TG levels when 18–35% energy derived from fructose was added to the control diet. No difference was found between the control and fructose diets in isocaloric trials523). Another metaanalysis of RCTs on healthy and obese subjects found an association of fructose with elevated TG levels and blood pressure as well as decreased HDL-C levels, but the significance disappeared when studies that lead to the highest effect on the heterogeneity test were excluded524). The effects of fructose on the risk factors for ASCVD were not observed in other RCTs525, 526).
As demonstrated above, evidence on the direct effects of a high intake of processed foods containing fructose on ASCVD is lacking, however, it is necessary to note that this would be the cause of excessive energy intake.
CQ19: Can the consumption of soy and soy products be recommended for ASCVD prevention?
Consuming soy and soy products is recommended because they may decrease CAD and stroke risk. (Evidence level: 2, recommendation level: A)
Investigations of the association of ASCVD with soy consumption are limited to cohort studies in Japan, where soy and soy products are habitually consumed. The JPHC study reported that the risk of cerebral infarction and MI in women who consumed soy ≥ 5 times/week was lower by 36% and 45%, respectively, compared to the corresponding risk in women who consumed soy 0–2 times/week527). A study in a cohort with a very high prevalence of hypertension did not find the same association528).
The association of soy products that are consumed as food, such as soy milk, soybeans, kinako (toasted soybean flour), tofu, fermented soybeans and cookies with ASCVD risk factors, has been evaluated in RCTs. Some reported a decrease in TC or LDL-C levels529–541), and others have not reported such a decrease542–553). Differences in the subjects, type and quantity of food products, and the intervention period make it difficult to compare studies. In a Japanese RCT, decrease in TC levels was observed in premenopausal women with a diet supplemented with soy milk554). Some studies have reported decrease in blood pressure associated with soy consumption555, 556), whereas others have not reported such a decrease540, 544–546, 557). A meta-analysis of studies evaluating consumption of isolated soy protein reported a reduction of LDL-C levels in individuals with high TC levels558). The consumption of soy or soy products may play a part in decreasing CAD and stroke risk.
CQ20: Can meals following the Japanese dietary pattern (The Japan Diet) be recommended for ASCVD prevention?
A low-salt Japanese dietary pattern with reduced fat on meat and animal fats such as beef tallow, lard, and butter and the consumption of a combination with soy, fish, vegetables, seaweed, mushrooms, fruits, and unpolished grains are recommended for ASCVD prevention. (Recommendation level: A)
Daily meal preparation involves combining a variety of food items. It is therefore useful to evaluate the effects of meals on the incidence of diseases and their risk factors, taking into account the combination of food items consumed (dietary pattern) and not just the individual nutrients559).
Epidemiological findings of the Seven Countries Study obtained in the 1960s and 1970s revealed that the CAD mortality rate in Japan was extremely low compared with that in Northern Europe and the USA. The notable diet in Japan was very small amounts of meat, fats and oils, and dairy products with large amounts of rice, soy, and fish560). Other than polished rice, barley and slightly polished rice were consumed in Japan up to the 1960s561). Domestic cohort studies with baseline data up to the 1990s showed a low CVD mortality rate patients with dietary pattern containing high percentages of soy, fish, vegetables, seaweed, mushrooms and fruits562, 563). The mortality rate due to overall and CVD were approximately 20% lower with a Japanese-style dietary pattern with salt reduction564). However, the CVD mortality rate was higher if the dietary pattern contained of high percentages of meat, butter, and high-fat dairy products562–564).
As described above, the consumption of food items that constitute the Japanese dietary pattern has been reported to decrease ASCVD risk. Furthermore, a meta-analysis of cohort studies conducted in Northern Europe, the USA, and China found that the consumption of unrefined grains resulted in a lower risk of CAD565).
Therefore, the traditional Japanese dietary pattern in which fat on meat and animal fats such as beef tallow, lard, and butter, are avoided, and barley, millet and unpolished grains are consumed in combination with soy, fish, vegetables, seaweed, mushrooms, and fruits, is believed to contribute to preventing ASCVD566).
The high salt content of a Japanese diet, with an average salt intake of 10.0 g/day567) is of concern. As excessive salt intake leads to increased blood pressure and promotes atherosclerosis, a target intake of < 6 g/day is thus recommended122). In addition, although moderate alcohol intake may have preventive effects in terms of CAD risk, it should be noted that excessive alcohol intake causes high blood pressure and hypertriglyceridemia568).
Diet to Improve Risk Factors
Diet modification is essential for the treatment of dyslipidemia and ASCVD. Based on “the Japan diet” with a lower salt content, it is important to instruct the patients' diet in consideration of eating behavior, condition, and lifestyle. Timely evaluations of the effects are needed to modify their diet appropriately.
• Hyper-LDL Cholesterolemia and Diet
Intake of SFAs, cholesterol and trans fatty acids, which increase the LDL-C level, should be decreased with the appropriate management of total energy intake. Replace SFAs with MUFAs or PUFAs, reduce intake of SFA to < 7% of energy, and cholesterol to < 200 mg a day. Specifically, reduce intake of high fatty meat and/or animal fats (beef tallow, lard, butter), milk, offals, and eggs. The consumption of vegetables, including green and yellow vegetables, and soy or soy products is recommended.
• Hypertriglyceridemia and Diet
Patients should achieve and maintain a desirable body weight. Lower intake of carbohydrates, and avoide excessive consumption of alcohol. Excessive consumption of fruits and fructose-containing processed foods should be avoided as they may contribute to an increase in TG level. The intake of n-3 PUFA should also be increased. In patients with hyperchylomicronemia, the total amount of dietary fats should be redused (≤ 15% of energy); the use of medium-chain fatty acids569, 570).
• Low HDL-C and Diet
Patients should maintain an ideal body weight or consider a total energy intake to help achieve it. The percentage of energy derived from carbohydrates should be slightly decreased, and the amount of trans fatty acids should be decreased.
• Metabolic Syndrome and Diet
The total energy intake should be optimized on the basis of the ideal body weight and the amount of daily activity. Patients should aim to decrease their body weight or waist circumference by ≥ 3% within 3–6 months320), rapid weight loss should be avoided. The percentage of energy derived from carbohydrates should be slightly decreased while consuming enough protein to help prevent a reduction of muscle mass320). Preparing meals with foods with a low glycemic index and a low glycemic load lower the risk ASCVD incidence571–574). Concomitant exercise therapy is effective for improving obesity, serum lipid levels, and blood pressure373, 575).
• Hypertension and Diet
Salt intake should be restricted, with positive consumption of vegetables and fruits. It should work toward sodium restriction and potassium sufficiency, which promote the urinary excretion of sodium. It is recommended to treat obesity, maintain an ideal body weight, refrain from the consumption of cholesterol and SFAs, and positively consume fish (fish oil). The excessive consumption of alcohol should be avoided as it increases blood pressure.
• Diabetes Mellitus and Diet
Improving obesity is the most important lifestyle component for controlling type 2 diabetes. Total energy intake should be adequate for individual physical activity (energy consumption), and whenever possible, it should be divided equally into three daily meals i.e., breakfast, lunch, and dinner. Food should be chewed well and consumed without rushing. Keep in mind that sugars should not be overly consumed, and should comprise < 60% of the energy intake. There should not be an excess or insufficient intake of any nutrients. Patients should increase dietary fiber. Eating the vegetables first during meals suppresses the elevation of postprandial blood glucose, which can help in decreasing body weight576). The dietary percentages of energy from SFAs and PUFAs should be ≤ 7% and ≤ 10%, respectively.
5). Exercise Therapy
[Statement]
Habitual physical activity and aerobic exercise are effective for ASCVD prevention. (Evidence level: 1, recommendation level: A)
Lack of physical activity is associated with increased body fat (obesity), dyslipidemia, metabolic syndrome, hypertension, diabetes/impaired glucose tolerance, vascular endothelial dysfunction, and decreased physical fitness. It is also a risk factor for ASCVD such as CAD and cerebrovascular disease577–587). Increased sedentary behavior, defined as “any waking behavior with an energy expenditure of 1.5 metabolic equivalents (METs) or less in a sitting or reclining posture” may be a risk factor for various health outcomes independent of physical activity588–590).
Increasing physical activity maintains or adds to physical fitness, improves serum lipid levels, decreases blood pressure, increases insulin sensitivity and glucose tolerance, improves vascular endothelial function, and decreases thrombosis risk591–593). It also decreases mental stress and slows the decline in cognitive function594, 595). The amount of daily physical activity, leisure time physical activity and physical fitness level have been found to be negatively correlated with morality due to cerebrovascular disease and cancer, as well as all-cause mortality in studies conducted worldwide596–606) and cohort studies in Japan577).
Exercise therapy includes both aerobic and resistance (muscle) exercise. Aerobic exercise is effective in improving lipid metabolism607–615). The effects of aerobic exercise (walking, brisk walking, supervised or nonsupervised training) compared with nonexercising controls on LDL-C, TG, and HDL-C levels have been extensively studied. However, study results are difficult to compare because of differences in subjects, exercise intensity and duration, and pre-intervention lipid levels. Among 8 reports of systematic review and metaanalysis of RCTs with comparison of exercise and non-exercise control including Japanese participants and were published after 2,000, significant decreases in LDL-C and TG levels were reported in two articles, respectively. Six reported a significant increase in HDL-C levels, which was the most frequently observed effect of exercise on serum lipids577, 607–613). A meta-analysis of 25 RCTs that compared aerobic exercise for training periods of at least 15 minutes for ≥ 8 weeks with nonexercise control reported that exercise therapy significantly increased the HDL-C levels612). The increase in HDL-C was positively correlation with the duration of exercise, and exercising for ≥ 121 minutes/week significantly increased the levels. A meta-analysis of four RCTs conducted in Japan comparing the effects of low to moderate-intensity aerobic exercise for 10 weeks to 24 months with nonexercise therapy found that exercise significantly increased the HDL-C levels577).
Table 9 shows simplified guidelines for exercise therapy. Efforts should be made to increase daily physical activity and to incorporate exercises suited to individual lifestyles. Increasing aerobic exercise is the primarily goal, and brisk walking or slow jogging are recommended. Moderate-intensity exercise (e.g., walking at normal speed) is effective and safe and can be performed for extended periods without strain. The increase in blood pressure during moderate exercise is modest, and blood lactate does not accumulate. The aim is to exercise for at least 30 minutes a day at least three times a week, daily if possible. For the elderly with a muscle wasting, aerobic exercise combined with mild resistance training is useful. Bench-stepping exercises, which can be performed indoors, are recommended577). In 2013, the Ministry of Health, Labor and Welfare of Japan published “Japanese official physical activity guidelines for health promotion (Active Guide)”616) (see Appendices 1 and 2). To prevent lifestyle-related diseases, starting with “Plus 10,” be active for an additional 10 min every day” helps to achieve the ultimate aim of 60 minutes of activity per day for adults and 40 minutes per day for the elderly.
Table 9. Guidelines for Exercise Therapy.
| Type | Implement with an emphasis on aerobic exercises such as walking, brisk walking, swimming, aerobic dance, slow jogging, cycling, and bench-stepping |
| Intensity | Aim for a moderate intensity* or above |
| Frequency and duration | Aim to exercise for at least 30 min per day at least 3 days a week |
| Others | Walk or perform other, similar activities frequently and at times other than during exercise therapy and avoid a sedentary lifestyle as much as possible |
- An exercise intensity equivalent to walking at normal speed (= walking)
- In terms of METs (a unit that expresses the intensity of exercise as the equivalent number of times the resting metabolism), it is typically 3 METs (walking) but it differs according to individual physical fitness
- The perceived exertion during exercise corresponds to 11–13 on the Borg scale, i.e., fairly light to somewhat hard)
The Borg Scale.
| Scale | Perceived |
|---|---|
| 20 | |
| 19 | Very, very hard |
| 18 | |
| 17 | Very hard |
| 16 | |
| 15 | Hard |
| 14 | |
| 13 | Somewhat hard |
| 12 | |
| 11 | Fairly light |
| 10 | |
| 9 | Very light |
| 8 | |
| 7 | Very, very light |
| 6 |
(Borg GA: Med Sci Sports Exerc. 1973; 5: 90–93)
Note that unaccustomed exercises carry a risk of musculoskeletal disorders, and in addition to bone and joint injury, strenuous exercise may add to the risk of sudden death and MI in patients with CVD617, 618). This should be taken into careful consideration, and when exercise therapy is performed, underlying ASCVD and bone and joint diseases should be taken into account. It is necessary to choose exercises and physical activities that are adapted to individual physical fitness, exercise history, and the current status of physical activity.
3. Drug Therapy
1). Drug Therapy
CQ21. Can the evidence on ASCVD prevention in Western countries be applied in Japan?
It has been proven that the effects of LDL-C-lowering therapy using statins to decrease the relative risk of ASCVD incidence are observed regardless of race and absolute risk. (Evidence level 1+, recommendation level A)
Although the absolute risk of CAD is lower in the Japanese population than in Western countries, the relative risk of LDL-C and CAD is similar. The MEGA study41), a large-scale clinical trial conducted in Japan, confirmed the effectiveness of LDL-C-lowering therapy using statins to prevent cardiovascular events in Japanese patients with hypercholesterolemia. Epidemiology studies, however, have not found a significant correlation of serum cholesterol level and the incidence of noncardiogenic cerebral infarction38, 57, 64, 337). LDL-C is also a risk factor for atherothrombotic cerebral infarction36), and the effectiveness of statins to decrease the incidence of atherothrombotic cerebral infarction was confirmed in a Japanese population by the J-STARS study619). The effectiveness of statins for preventing the first occurrence and recurrence of stroke in Japanese populations was confirmed in a sub-analysis of the MEGA study620, 621) and in the JELIS study622).
Meta-analyses conducted by the Cholesterol Treatment Trialists' Collaboration on large-scale clinical trials using statins confirmed that a decrease in the incidence of cardiovascular events was proportionate to the decrease in LDL-C level, regardless of pretreatment LDL-C level and individual absolute risk177–179, 348). The HOPE-3 study was a large international study with 21 participating countries, including some in the Asian region, and it evaluated primary prevention in populations with a risk that was equivalent to high risk in Japan. It has been proven by the study that LDL-C-lowering therapy using statins exhibits suppressive effects on cardiovascular events regardless of race623).
2). Indications for Drug Therapy
CQ22. Is an LDL-C management target of < 120 mg/dL appropriate for high-risk category of primary prevention?
The LDL-C level for high-risk patients in primary prevention should be controlled at a target of < 120 mg/dL. (Evidence level 2, recommendation level B)
The APPROACH-J study was a prospective Japanese study that investigated the 2-year incidence rate of cardiovascular events for high-risk patients in primary prevention who had recently started statin therapy. The risk of CAD incidence plateaued at LDL-C levels ≤ 119 mg/dL, which demonstrates the importance of maintaining an LDL-C level of < 120 mg/dL624). The importance of the comprehensive management of overlapping complicating risk factors in addition to LDL-C, such as smoking, hypertension, and diabetes, was affirmed in this study. Furthermore, for diabetes patients, who are a high-risk group in primary prevention, a sub-analysis of the J-LIT study results found that keeping the LDL-C level < 120 mg/dL was useful for preventing CAD incidence625). The HOPE-3 study was a randomized comparative intervention trial of statins for primary prevention in populations with characteristics corresponding to high risk in Japan. Significant reduction of cardiovascular events was seen in the statin-treated group that maintained LDL-C levels of < 120 mg/dL. The average pretreatment LDL-C level of both study groups was approximately 128 mg/dL623). Thus, controlling the LDL-C level at a target of < 120 mg/dL for high-risk populations in primary prevention appears to be appropriate. However, despite recent efforts to increase the awareness of and familiarity with guidelines, it has been reported that the achievement of the LDL-C management target by high-risk patients with type 2 diabetes, noncardiogenic cerebral infarction, or PAD is still not adequate in Japanese clinical practice626, 627).
CQ23. When should drug therapy be initiated for hyper-LDL cholesterolemia?
For high-risk patients in primary prevention, if lifestyle modification is not likely to be effective, the combined use of drug therapy should be considered as soon as possible. (Evidence level 3, recommendation level A)
It has been proven that initiating aggressive LDL-C-lowering therapy at an early stage after disease incidence is effective for secondary prevention. (Evidence level 1+, recommendation level A)
In addition to plasma LDL-C level, physicians prescribing drug therapy in primary prevention should also be aware of other patient characteristics, including smoking habits, the presence of hypertension, diabetes, CKD, a family history of premature CAD or other ASCVD, such as noncardiogenic cerebral infarction, PAD or carotid atherosclerosis. An assessment of individual risk should include this information when drug therapy is considered for patients with high absolute risk. The long-term follow-up of large clinical trials of statins that were conducted in the 1990s, such as the West of Scotland Coronary Prevention Study (WOSCOPS) and the Heart Protection Study (HPS)628, 629) confirmed that early initiation of LDLC-lowering therapy regardless of the plasma LDL-C level was useful for the long-term prevention of cardiovascular events and all-cause mortality in high-risk patients. No increases in new-incidence cancer or non-CVD deaths were observed, which reconfirms the safety of long-term LDL-C-lowering therapy using statins.
Lifetime cumulative LDL-C and the threshold for CAD incidence are recent concepts. Aggressive LDL-C-lowering therapy is recommended as early as possible for patients with familial hypercholesterolemia who present with hyper-LDL cholesterolemia at a young age followed by developing the premature CAD. Cardiovascular events may occur sooner than expected in patients with usual hyper-LDL cholesterolemia because of the accumulation of LDL-C that is promoted by complicating atherosclerosis risk factors. A Mendelian randomization analysis found that the risk of cardiovascular events was extremely low in patients with hypo-LDL cholesterolemia that was caused by gene mutations630). There is a need to consider LDL-C-lowering therapy as soon as possible for hyper-LDL cholesterolemic patients with high absolute risk. Long-term observational included in large clinical trials found that the early initiation of LDLC-lowering therapy decreased lifetime cumulative LDL-C, which may have made it possible to decrease the occurrence of cardiovascular events.
Low-risk patients without additional risk factors, young individuals, and premenopausal women are at low absolute risk. Even if the LDL-C management target is not achieved, it is possible that the progress may be observed with lifestyle modification only. Drug therapy should be considered on the basis of the importance of the existing risks only if strict lifestyle guidance does not improve lifestyle habits and does not achieve the management target.
CQ24. Can an LDL-C management target be set for preventing occurrence of ASCVD for secondary prevention in patients with a history of CAD?
In secondary prevention, the LDL-C control target should be at a level of < 100 mg/dL. However, if it is difficult to achieve an LDL-C level < 100 mg/dL, the alternative target would be to decrease the level by ≥ 50%. (Evidence level 3, recommendation level A)
Clinical trials have confirmed that the effects of statins on the reduction of cardiovascular events can improved by administering statins with more potent LDL-C-lowering effects177, 631). However, there are no lipid intervention studies that have set an LDL-C management target. Therefore, when LDL-C-lowering therapy is given to prevent cardiovascular events, the question of whether to “treat to target” or to use a potent statin without setting a target value i.e., “fire and forget” is under debate. Currently, it is necessary to consider the results of epidemiological studies and cohort studies to choose the LDL-C management target. In a long-term Japanese observational study of secondary prevention, the incidence of cardiovascular events was significantly decreased in patients whose LDL-C levels were controlled at < 100 mg/dL632). In the CREDO-Kyoto Registry Cohort-2, the 3-year incidence of cardiovascular events in patients treated with potent and standard statins were compared. Patients underwent analysis stratified by LDL-C management level633). The incidence of cardiovascular events was significantly lower with the potent statin than with the standard statin. In addition, in patients with LDL-C levels ≥ 120 mg/dL, the risk of the incidence of cardiovascular events was 1.74 times greater than in patients with LDL-C levels of 80–99 mg/dL, which was a significant difference. A significant difference in the risk of cardiovascular events was not seen when the LDL-C level was < 80 mg/dL. The mean LDL-C levels that were analyzed by the strength of statin were 92 mg/dL in patients treated with potent statins and 101 mg/dL in patients with standard statins, respectively. The study outcome supports the benefits of controlling the LDL-C level at < 100 mg/dL rather than basing the treatment on the strength of statin. It has been reported that the risk of cardiovascular events increased in patients with the escape phenomenon (i.e., re-elevation in LDL-C level of ≥ 10% after a reduction) following the start of statin treatment634). However, patients with the escape phenomenon had an LDL-C level of 106 mg/dL; whereas the comparison group had an LDL-C level of 90 mg/dL. In addition to the choosing the strength of the statin, regular blood testing to monitor the LDL-C level is recommended. A recent survey in Japan, found that 65% patients with ACS, and 55% for other secondary prevention patients, achieved an LDL-C management target of < 100 mg/dL. Thus, LDL-C management with lipid-lowering therapy for secondary prevention in Japan is still not satisfactory626). A meta-analysis of international trials reported that the risk for ASCVD was significantly decreased in patients with an LDL-C reduction of ≥ 50%, regardless of the LDL-C level achieved635). To prevent ASCVD in cases where it is difficult to control the LDL-C at < 100 mg/dL, lipid management with the aim of lowering pretreatment LDL-C by 50% or more is recommended177, 631, 633, 635).
CQ25. Is risk stratification possible in secondary prevention? In addition, is it possible to set an LDL-C management target?
Even in secondary prevention, the risk for cardiovascular events is high if FH, ACS, DM, noncardiogenic cerebral infarction, PAD, CKD, and metabolic syndrome are present, or if there is an overlap in the major risk factors and the presence of smoking. Stricter LDL-C management is therefore necessary. (Evidence level 3, recommendation level B)
Even in secondary prevention, the incidence of cardiovascular events is clearly high if FH, ACS, DM, noncardiogenic cerebral infarction, PAD, CKD, and metabolic syndrome are also present, or if there is an overlap of the major risk factors or continued smoking (Table 3) (refer to Chapter 3, 1–8: History of CAD). After the results of IMPROVE-IT636) and clinical trials using PCSK9 inhibitors637–639) conducted in recent years, the clinical significance of maintaining the LDL-C level at < 70 mg/dL is garnering attention in secondary prevention complicated with high-risk conditions640).
In Japan, only a few interventional studies have been conducted using cardiovascular events as the endpoint for secondary prevention. Therefore, results from interventional studies involving patients with ACS who underwent IVUS as a surrogate endpoint have instead been reported. The results of one metaanalysis revealed that the rate of change in plaque (atheroma) volume [percent atheroma volume (PAV)] correlates significantly with cardiovascular event risk641). The results of ESTABLISH642), JAPAN-ACS643), the ZEUS study644), and PRECISE-IVUS645) have shown that in cases stratified into a high-risk category even in secondary prevention, such as ACS or ACS complicated by DM, controlling the LDL-C at a target level of < 70 mg/dL is useful for plaque regression. A meta-analysis of clinical trials that combined primary and secondary prevention has revealed a linear relationship between the LDL-C level following statin treatment and the suppressive effects of statins on cardiovascular events. A review performed outside of Japan reported that larger suppressive effects on cardiovascular events can be achieved by maintaining the LDL-C level at < 50 mg/dL646). We consider these findings to be supportive of the hypothesis of “lower is better.” As FH puts the patient at extremely high risk for CAD, it is likely that the risk in primary prevention is equivalent to that in secondary prevention. Therefore, in secondary prevention complicated with FH or ACS, stricter LDL-C management to achieve a level < 70 mg/dL can be considered appropriate. The secondary prevention for DM patients complicated with conditions described in Table 3b, should also be treated based on the target level of FH or ACS, as they are considered to be at high risk of recurrence of CAD. In cases where the target cannot be achieved with monotherapy using statins, the combined use of medications such as ezetimibe should be considered.
CQ26. If the LDL-C management target has been achieved, should lipid management be carried out using the non-HDL-C level as an indicator?
If the TG level is high even when the LDL-C management target has been achieved, lipid management should be carried out using the non-HDL-C level as the target. (Evidence level 3, recommendation level B)
Undoubtedly, the LDL-C level is the most important target in lipid management. However, in managing dyslipidemia expressed by hypertriglyceridemia, the non-HDL-C level is more useful than the LDL-C level. This has been reported in a number of studies177–179, 623, 624, 626, 627, 647). The standard non-HDL-C level is defined as a value 30 mg/dL higher than LDL-C in NCEP-ATP Ⅲ. Studies from Japan have shown similar findings74, 75). Even when the LDL-C management target has been achieved, if hypertriglyceridemia is present, the non-HDL-C level should be the secondary target for lipid management aimed at preventing ASCVD. However, if the patient's TG level is ≥ 400 mg/dL or if blood sample is postprandial period, then the non-HDL-C level should be used initially instead of the LDL-C level. We hope that additional evidence will be gathered from interventional studies using the non-HDL-C level as a marker.
Q1. Is therapeutic intervention for hypertriglyceridemia effective in suppressing the incidence of ASCVD?
There is insufficient evidence regarding the preventive effect of drug therapy for hypertriglyceridemia upon the incidence of ASCVD. However, keeping in mind that patients with a marked increase in fasting triglyceride levels face the risk of acute pancreatitis, it is important that we consider the concurrent administration of fibrates or other drugs with dietary advice, such as limiting the amount of fat intake and abstinence from alcohol. (Recommendation level: B)
As yet, no RCT has reported an effect on the incidence of ASCVD after conducting drug therapy targeted only at patients with hypertriglyceridemia. However, there are reports of RCTs using TG-lowering nicotinic acid derivatives and fibrates on patients with dyslipidemia, and some have shown a preventive effect on the incidence of ASCVD. The first of such RCTs is the Helsinki Heart Study, a large clinical study involving patients with hypertriglyceridemia648). In this study, gemfibrozil significantly suppressed the incidence of CAD by 34% in middle-aged men in primary prevention; these men had non-HDL-C levels of 200 mg/dL or higher. Subsequent to the Helsinki Heart Study, there were the FIELD649) and ACCORD650) studies, both conducted with fenofibrate. With respect to primary endpoints of these studies, although the effectiveness of fenofibrate was not demonstrated, a meta-analysis in primary prevention using fibrates revealed that deaths from cerebrovascular and cardiovascular diseases, non-fatal myocardial infarction, and non-fatal stroke significantly decreased by 16%651). These deaths were also reported to significantly decrease by 12% in secondary prevention652). Meanwhile, the co-administration of a nicotinic acid derivative (niacin) and statin to increase HDL-C levels did not have a suppressive effect on cardiovascular events in the AIM-HIGH653) and HPS2-THRIVE654) studies.
Therefore, we cannot confirm that drugs for hypertriglyceridemia, such as fibrates and nicotinic acid derivatives, have a preventive effect on ASCVD. Nonetheless, TGs have been demonstrated to be correlated with acute pancreatitis655). In addition, we should consider the risk of acute pancreatitis in patients with markedly increased fasting TG levels (500 mg/dL or higher) and consider the concurrent administration of fibrates or other drugs with dietary advice.
3). Characteristics and Criteria for Selecting the Various Drugs
Q2. Have the indications, efficacy, and safety been established for drugs used to treat dyslipidemia?
The indications, efficacy, and safety of statins, ezetimibe, the anion-exchange resins, probucol, fibrates, n3-PUFAs, and nicotinic acid derivatives for treating dyslipidemia have been established. Although the indications and efficacy of PCSK9 inhibitors have been established, the safety of long-term administration has not been validated.
Table 4 shows the classification of drugs used to treat dyslipidemia according to efficacy. These are drugs for which the effects have been ascertained through double-blind studies completed in Japan. There is a need to select safe and effective medications based on understanding of the characteristics of various drugs, while taking into account any complicating diseases, drug interactions, etc. The characteristics of various drugs used to treat dyslipidemia are described below.
i). HMG-CoA Reductase Inhibitors (Statins): Pravastatin, Simvastatin, Fluvastatin, Atorvastatin, Pitavastatin, and Rosuvastatin
Statins are indicated for cases of dyslipidemia with a high LDL-C level. They are currently one of the most effective medications for lowering the LDL-C level and have been so because their effects on FH were shown656). Statins competitively inhibit HMG-CoA reductase, which is a rate-limiting enzyme of cholesterol synthesis, in turn suppressing cholesterol biosynthesis657). The subsequent activation of SREBP2 promotes LDL receptor synthesis and results in a reduction in the blood LDL-C level658). The range of the LDL-C-lowering effect is 20%–50%. Although a reduction in cholesterol biosynthesis in the liver also results in a decrease in the TG level through simultaneous suppression of very low-density lipoprotein (VLDL) synthesis and secretion659), the decrease ranges from approximately 10%–20%. Myopathy-like symptoms, such as hepatic disorders, an increased creatine kinase (CK) level, and muscular weakness, have been reported as adverse reactions to statins. Rhabdomyolysis, characterized by an increased myoglobin level in the blood and urine, has also been reported, though this complication is very rare. Rhabdomyolysis risk increases with the combined use of statins with fibrates, nicotinic acid derivatives, cyclosporine, or erythromycin, etc. Immune-mediated necrotizing myopathy, characterized by muscle fiber necrosis without infiltration of inflammatory cells on histological examination and the positivity for anti-HMG-CoA reductase antibodies on serological examination, has also been reported even in Japan660, 661). The symptoms which are characterized by muscle weakness predominantly in proximal muscle and marked muscle pain with an increased CK level, persist even after stopping administration of oral statins, and there have been cases in which symptoms progress rapidly. Administration of oral statins should therefore be stopped immediately if myopathy-like symptoms appear and the patient's condition should be monitored closely.
Moreover, there have been reports on suspected teratogenicity in pregnant patients who incidentally took statins during early pregnancy662); therefore, at present, it appears that statins must not be used in women who are pregnant, possibly pregnant, trying to conceive, or lactating. Pitavastatin (1–2 mg) has been approved for pediatric FH in patients aged ≥ 10 years.
ii). Intestinal Cholesterol Transporter Inhibitor (Cholesterol Absorption Inhibitor): Ezetimibe
Ezetimibe inhibits a small intestine cholesterol transporter (NPC1L1) that exists in the small intestinal mucosa and controls the absorption of cholesterol derived from diet and bile in the small intestine, thereby exerting a blood cholesterol-lowering effect663). Unlike resins, this drug is absorbed into the body. After passing through the intestinal circulatory system, approximately 78% of it is excreted in the feces. Because this drug selectively inhibits cholesterol absorption, it does not affect the absorption of fat-soluble vitamins, such as vitamins A and D. The usual oral dose (10 mg/day) decreases the LDL-C level by approximately 18%. Similar to resins, it enhances cholesterol synthesis in the liver. Therefore, the combined use of ezetimibe with statins is ideal and provides a synergistic effect: combination therapy with 10 mg of ezetimibe and a statin at the usual dose decreases the LDL-C level by approximately 35–50%664–666), which is equivalent to the effects achieved using a maximum statin dose alone. A meta-analysis of large-scale clinical trials involving combination therapy with ezetimibe and statins in patients with high-risk conditions such as FH, ACS, and PAD, confirmed the safety of combination therapy and cardiovascular event suppression resulting from its LDL-C-lowering effects667). It also increased the HDL-C level by 8–9% while decreasing the TG level by 20–30%. Although gastrointestinal symptoms were found to be common adverse reactions, there were no significant differences compared to a placebo. Similar to statins, myopathylike symptoms such as an increased CK level and muscle weakness, have been reported. These effects are rare, and there are no reports indicating that the combined use with statins intensifies adverse reactions. It has recently been reported that vitamin K absorption in the intestines is mediated by NPC1L1; therefore, it is necessary to note that the action of ezetimibe combination therapy in patients who take oral warfarin may be intensified668).
iii). Anion-Exchange Resins (Resins): Colestimide and Cholestyramine
Resins are indicated for dyslipidemia with a high LDL-C level (type Ⅱa). Although the first-line drugs of choice for hyper-LDL cholesterolemia are statins, resins may be the first-line drugs for patients who cannot tolerate statins because of adverse reactions, and for women who require drug therapy while pregnant or possibly pregnant. The greatest merit of resin therapy lies in combination therapy with statins.
Cholestyramine is the first medication that was proven through large-scale clinical trials to have suppressive effects on CAD incidence669, 670). Resin adsorbs bile acids in the intestines and inhibits intestinal circulation of bile acid via reabsorption, thereby promoting catabolism from cholesterol to bile acids. It is believed that this catabolism leads to decreased sterol pools in the body and an enhanced synthesis of LDL receptors in the liver, resulting in a decreased LDL-C level in the blood671). However, HMG-CoA reductase activity in the liver may simultaneously increase, enhancing cholesterol biosynthesis. Combining these drugs with statins, which are HMG-CoA reductase inhibitors, is therefore extremely logical. In contrast, bile acids act as ligands for the nuclear receptor FXR and are involved in regulating TG metabolism by controlling SREBP1c expression and enhancing lipoprotein lipase (LPL) activity. Resin administration results in a decreased LDL-C level, as well as VLDL synthesis and an increased blood TG level because of bile acid absorption. Adverse drug reactions primarily include gastrointestinal symptoms, such as constipation and abdominal bloating; however, no serious adverse reactions have been observed so far because these drugs are nonabsorbable. It has been pointed out that resins adsorb concomitant drugs, such as statins, digitalis, warfarin, thiazide diuretics, and thyroid gland preparations. Therefore, when these medications are concomitantly used, instructions to take them at intervals must be provided. Furthermore, as they may also inhibit the absorption of fat-soluble vitamins (A, D, E, and K) and folic acid, vitamin supplementation should be considered during long-term resin administration.
iv). Probucol
Probucol is indicated for dyslipidemia with a high LDL-C level (type Ⅱa). This drug is characterized by its ability to cause xanthoma regression. In addition to decreasing the LDL-C level, this drug also decreases the HDL-C level.
LDL-C level reduction resulting from probucol is 15–25%. The mechanism underlying this decrease is thought to involve enhanced LDL catabolism, particularly the promotion of cholesterol excretion into bile. On the other hand, the mechanism underlying the decrease in the HDL-C level is thought to involve the suppression of ABCA1, a membrane protein essential for HDL production. Other possible mechanisms include enhanced activity of cholesterol ester transfer proteins (CETPs) and of SR-BI, an HDL receptor. From the viewpoint of cell biology672, 673), immunohistology674, 675) and other factors, it has become clear that LDL oxidation is an important aspect of the pathogenic mechanism of atherosclerosis. As probucol is made up of two butylated hydroxytoluene (BHT) antioxidants and is fat-soluble, it is taken up by lipoproteins and exerts potent antioxidant effects. Clinically, the suppressive effects on post-percutaneous transluminal coronary angioplasty (PTCA) restenosis676, 677), carotid artery IMT progression, and cardiovascular events678) have been reported from RCTs, albeit small-scales ones. Moreover, a cohort study has also revealed secondary-prevention effects of probucol in patients with heterozygous FH679). In PQRST, however, suppressive effects on atherosclerosis progression in the femoral arteries could not be achieved with probucol administration in addition to diet therapy and cholestyramine treatment680). In any case, because no large-scale clinical studies have been conducted, probucol use is limited to certain situations such as combination therapy with statins or in monotherapy in patients who cannot tolerate statins. Other than gastrointestinal symptoms, hepatic disorders, and rashes, adverse drug reactions include QT prolongation and torsades de pointes on electrocardiograms.
v). PCSK9 Inhibitors (Human Anti-PCSK9 Monoclonal Antibodies): Evolocumab and Alirocumab
PCSK9 inhibitors are currently indicated for FH or patients with hypercholesterolemia who have a high risk for cardiovascular events and do not benefit sufficiently from even at the maximum tolerated dose statins.
These drugs bind specifically to and inhibit the actions of the PCSK9 (proprotein convertase subtilisin/kexin type 9) protein, which is involved in the degradation of LDL receptors in the liver. In doing so, it increases the recycling of LDL receptors, thereby showing a blood LDL-C-lowering effect681). Although increasing the synthesis of LDL receptors, statins, which inhibit cholesterol synthesis in the liver and activate SREBP2, simultaneously increase PCSK9 synthesis. Therefore, it is reasonable to use statins in combination with PCSK9 inhibitors. Their LDL-C-lowering effect is the strongest among all the existing medications. In a phase-3 study involving subjects with a high risk for cardiovascular events including those with heterozygous FH, administrating PCSK9 inhibitor once every two weeks decreased LDL-C levels by 70–75% with combination oral statins682, 683). These inhibitors also decrease the Lp(a) level, on which statins do not show a lowering effect. PCSK9 inhibitors decrease TG levels by 20–25% and increases HDL-C levels by 10–15%.
As these medications are injected subcutaneously, the main adverse drug reaction reported is a reaction at the injection site, whereas others include nasopharyngitis and gastroenteritis. No reports have stated that the combined use with statins intensifies the adverse reactions in individuals with hepatic or musculoskeletal disorders. Although adverse events caused by low LDL-C levels have not been reported, the long-term efficacy and safety of PCSK9 inhibitors should be monitored carefully.
vi). MTP Inhibitor: Lomitapide
Lomitapide is currently the only microsomal triglyceride transfer protein (MTP) inhibitor approved in Europe, America, and Japan. The inhibition of MTP decreases VLDL production, which in turn causes decreased LDL-C and TG levels. Even in patients with homozygous FH (HoHF) for whom other drug therapies show no effect, lomitapide decreases the LDL-C level by approximately 50%. However, major adverse drug reactions include accumulation of fat in the liver, abdominal pain, and diarrhea. As a result of these major adverse drug reactions, its long-term safety needs to be studied further in the future. The indication for lomitapide in Japan is limited to patients with hoFH.
Four weeks following commencement of a low-fat diet and administration of lomitapide in patients with hoFH, the blood LDL-C level and apo-B level decreased by 50.9% and 55.6%, respectively (average pretreatment LDL-C level: 615 mg/dL)684). During lomitapide treatment, the AST and ALT levels were found to be significantly elevated, as was the liver fat content. However, these levels returned to normal in all patients 14 weeks after treatment cessation. Furthermore, a dose-dependent decrease in the LDL-C and apo-B levels was observed following lomitapide monotherapy or combination therapy with ezetimibe, in conjunction with a low-fat diet in 85 patients with high TC (average pretreatment LDL-C level: 170 mg/dL)685). The maximum lomitapide dose alone decreases the LDL-C level by 30% and the apo-B level by 24% from pretreatment levels. In contrast, the combination with ezetimibe showed decreases of 46% and 37%, respectively.
vii). Fibrates: Bezafibrate, Fenofibrate, Clinofibrate and Clofibrate
Fibrates are the most effective medications for hypertriglyceridemia. Fibrates are particularly effective for type Ⅲ hyperlipidemia because they enhance the catabolism of remnant lipoproteins. They are also highly effective in increasing the HDL-C level.
Their primary mechanism of action is the activation of PPARα, a nuclear receptor, through binding of fibrates as ligands686, 687). This results in (1) enhanced β-oxidation of fatty acids and decreased TG production in the liver; (2) increased LPL production; (3) decreased Apo C-Ⅲ production and enhanced LPL activity, which lead to the promotion of TG degradation and catabolism from VLDL to LDL; and (4) increased production of Apo A-Ⅰ and A-Ⅱ. As a result, the TG level decreases and the HDL-C level increases. Bezafibrate has a TG-lowering effect of 30–40%, a TC-lowering effect of approximately 10%, and an HDL-C-increasing effect of 35–45%. Fenofibrate is characterized by its long half-life, and exerts a uric acid-lowering effect in addition to its effect on lipids. The main adverse drug reaction is rhabdomyolysis, which is likely to occur in patients with renal dysfunction, so caution should be taken.
viii). SPPARMα: Pemafibrate
Selective peroxisome proliferator-activated receptor α modulators (SPPARMα) (pemafibrate already approved in Japan) are considered to be safer than conventional fibrates in combination with statins because they have few drug interactions with the statins currently used in Japan and are not renally excreted.
ix). Nicotinic Acid Derivatives: Niceritrol, Nicomol, and Tocopheryl Nicotinate
Nicotinic acid derivatives are indicated for hyper-LDL cholesterolemia, hypertriglyceridemia, and dyslipidemia accompanied by increased remnant lipoproteins.
The mechanism of action of these drugs involves the suppression of hormone-sensitive lipase activation, in turn inhibiting lipolysis in peripheral fat tissue and decreasing the influx of free fatty acids into the liver. This results in lipoprotein synthesis suppression in the liver. Furthermore, nicotinic acid derivatives exert HDL-C-increasing effects by suppressing apolipoprotein A-1 catabolism. Nicotinic acid monotherapy (3.0 g/day) causes a decrease in the TG level by 26%688). Nicotinic acid derivatives also have Lp(a)-lowering effects689–691). The main adverse drug reactions include itching and facial flushing because of peripheral vasodilation. Moreover, as nicotinic acid derivative use may exacerbate insulin resistance, these drugs must be administered carefully in patients with DM.
x). N-3 Polyunsaturated Fatty Acids (PUFAs): Ethyl Icosapentate and Omega-3-Acid-Ethyl Ester
PUFAs are indicated for dyslipidemia accompanied by an increased TG level, particularly for type Ⅱb and type Ⅳ hyperlipidemia. EPA and DHA suppress VLDL synthesis in the liver, thereby decreasing the TG level and exerting a slight HDL-C-increasing effect. The results of epidemiological studies and secondary-prevention studies have previously demonstrated that the consumption of fish oil and n-3 PUFA helps prevent cardiovascular events. The JELIS study692) conducted in Japan found that the addition of EPA to statins showed a significant preventive effect on major cardiovascular events compared to statin monotherapy. This indicates the efficacy of EPA in preventing cardiovascular events. However, recent large-scale clinical studies involving high-risk patients with both myocardial infarction and type 2 diabetes were unable to demonstrate that n-3 PUFA administration had suppressive effect on cardiovascular events693, 694). In addition to their effects on lipids, EPA and DHA are expected to have antiplatelet and anti-inflammatory effects in ASCVD prevention. Other than gastrointestinal symptoms, such as diarrhea, the main adverse drug reaction is bleeding diathesis.
4). Combination Therapy
CQ27. Does combination therapy using statin and other cholesterol-lowering drugs (ezetimibe, anion-exchange resin, probucol, and PCSK9 inhibitor) prevent ASCVD incidence?
Combination therapy using statin and ezetimibe prevents ASCVD incidence in patients with ACS. (Evidence level 1+, recommendation level: B).
Combination therapy using statin and PCSK9 inhibitor prevents ASCVD incidence in patients with a history of myocardial infarction, nonhemorrhagic stroke, or PAD. (Evidence level 1+, recommendation level: B).
The IMPROVE-IT study636) which enrolled ACS subjects (secondary prevention) with controlled LDL-C levels (50–100 mg/dL) under simvastatin treatment, revealed a significantly lower ASCVD incidence in the group for which ezetimibe was added on to simvastatin treatment. This study also revealed that the relationship between the reduced LDL-C value and the event inhibition rate attained with the addition of ezetimibe was similar to that observed in previous statin studies. The FOURIER study639) was a study which enrolled high-risk subjects (patients with a history of myocardial infarction, nonhemorrhagic stroke, or PAD) with an LDL-C level of ≥ 70 mg/dL or a non-HDL-C level of ≥ 100 mg/dL under statin treatment, and examined add-on effect of PCSK9 inhibitor to the statin; this study showed that the combined use of PCSK9 inhibitors with statins suppressed ASCVD incidence. There are no large-scale RCTs which examined the effect of the combined use of statin with anion-exchange resin or probucol in preventing ASCVD.
The SHARP study695), although the design of which does not fit into this “CQ22”, showed that combination therapy using statin and ezetimibe in patients with CKD (including those for both primary prevention and secondary prevention) suppressed ASCVD incidence compared to placebo.
CQ28. For patients with dyslipidemia complicated by hypertriglyceridemia or low HDL-C, would fibrate, nicotinic acid derivative or n-3 PUFA possess beneficial effect in suppressing ASCVD incidence when used in combination with statin?
Combination therapy using ethyl icosapentate (EPA) preparation or fibrate with statin is effective in suppressing ASCVD incidence. (Evidence level 2, recommendation level B).
Although no clinical studies have investigated the efficacy of combination therapy as a primary endpoint in subjects with the dyslipidemia profile assumed in this CQ, some clinical studies have investigated it in a sub-analysis.
In a sub-analysis of JELIS study696) which enrolled subjects with hypercholesterolemia, it was shown that EPA administration added-on to statins prevented coronary event incidence for patients in primary prevention whose TG and HDL-C levels were ≥ 151 mg/dL and < 40 mg/dL, respectively. The ACCORD-LIPID study, which was conducted in North America, involved type 2 diabetes subjects (primary prevention and secondary prevention). Its subanalysis suggested that fibrate administration in addition to statins could prevent ASCVD incidence in groups with a TG level of ≥ 204 mg/dL and an HDL-C level of < 34 mg/dL697).
The AIM-HIGH study698), which examined the effect of nicotinic acid derivative (NA) added-on to statin in the subjects with low HDL-C and hypertriglyceridemia together with ASCVD history, did not show suppressive effects of NA on ASCVD incidence. The sub-analysis of the HPS2-THRIVE study699), which included subjects with HDL-C levels < 34.8 mg/dL or TG levels ≥ 151 mg/dL and a ASCVD history, also could not show beneficial effect of NA added to statin. However, there would be some pitfalls for these two studies. Statin dose as well as the prescription rate of ezetimibe for the control group in the AIM-HIGH study was higher than the NA group. Regarding HPS2-THRIVE, the subjects were those who had already attained an average LDL-C level of 63 mg/dL, and the sample size for the sub-analysis was small (19.1% with an HDL-C level < 38.4 mg/dL and 25.6% with a TG level ≥ 151 mg/dL).
5). Follow-Up of Drug Therapy
Q3. Do we need to perform blood test regularly after initiating drug therapy?
It is advisable to perform regular blood testing after initiating drug therapy. Test items should be chosen upon consideration of the medications administered and the patient's background. (Recommendation level B).
Following drug therapy initiation, symptoms associated with adverse drug reactions should be monitored, and it is also advisable to perform regular blood testing two to three times within the first 6 months and once every 3–6 months thereafter. The blood test results will allow for the review of drug effects, dose adjustment, confirmation of adverse reactions and lifestyle guidance. The tests to be performed in addition to lipid tests should be selected taking the patient's background and the medications used into consideration; these tests include liver function tests (AST, ALT, and γGT), muscle enzyme tests (CK), renal function tests (BUN and Cre) and blood glucose-related tests (HbA1c and blood glucose level). Because it is usually very rare to detect serious complications in a timely manner from regular tests, there have been reports insisting that testings before drug administration and upon the appearance of symptoms are sufficient. However, regular blood testing is likely to help build a good patient-physician relationship and inhibit cardiovascular events because of better drug adherence.
Adverse reactions caused by statins (rhabdomyolysis, liver failure, etc.) are extremely rare if statins are not used in combination with fibrates and other drugs which affect statin metabolism. In a meta-analysis of 21 RCTs700) and in another meta-analysis of 30 RCTs701), the incidence rate of muscles-related adverse reactions with statin use was not found to be significantly different from that with a placebo. Furthermore, although the doses are much different from those in Japan, it has been suggested that a high statin dose, old age, small physical build, and female gender are risk factors for adverse reactions in Western countries702–705). It is also shown that adverse reaction develops often within 6 months after starting the medication706, 707). Post-marketing surveys in Japan have shown the same trend. We also have to keep in mind that if the liver enzyme and muscle enzyme levels are found to be elevated or abnormal, causes other than statins (such as an increase in the liver enzymes and muscle enzymes because of fatty liver and exercise, respectively) must be excluded704). Moreover, although it has been suggested that the risk for diabetes increases with statin use by 9–13%, meta-analyses of large-scale RCTs suggest that incidence frequency is low (approximately one to two cases per 1,000 patients per year)708–710). Furthermore, it has been suggested that diabetes incidence increases in individuals already at a high risk for diabetes (e.g., the elderly, those with metabolic syndrome and impaired glucose tolerance)708, 711). There have been three confirmed cases of diabetic ketoacidosis or hyperosmolar hyperglycemic nonketotic syndrome in Japan for all statins; however, two of the cases were already under diabetes treatment, and the remaining case would have been slowly progressive type 1 diabetes.
Elevation in creatinine levels resulting from fibrates administration is usually mild and reversible; however, in some cases, creatinine levels may become abnormally high, thus we always need pay attention. When administering nicotinic acid derivatives (NA), an increase in the blood glucose level and the development of diabetes from metabolic syndrome should be taken into account; however, these conditions can be treated and avoided with appropriate therapy and attention. Caution should be taken when using fibrates and NA in combination with statins as this can easily bring about liver and muscle adverse effects712).
6). Combination Therapy with Other Agents to Prevent Atherosclerosis
Q4. Does statin use in combination with drugs metabolized by CYP increase the incidence rate of adverse reactions?
Because many cases of rhabdomyolysis caused by the combined use of hydrophobic statins and CYP-metabolized drugs have been reported, it is advisable to pay thorough attention to the adverse reactions that appear when using these drugs concomitantly. (Evidence level 4, recommendation level A).
Xenobiotic substances, such as statins, are metabolized in the liver by the cytochrome (CYP) P450 protein. Among the statins, many hydrophobic statins are CYP substrates, such as CYP3A4 and CYP2C9. Such statins are metabolized by CYP and subsequently excreted. Pravastatin is a hydrophilic statin that is barely metabolized by CYP, whereas rosuvastatin is slightly metabolized by CYP2C9.
Drugs such as antifungal agents (fluconazole, itraconazole, etc.), macrolide antibiotics (erythromycin, clarithromycin, etc.) and protease inhibitors used to treat HIV are known to be CYP substrates. Drugs used in the fields of cardiovascular medicine and metabolism, namely calcium antagonists, warfarin, nateglinide, and glimepiride, are also CYP substrates. The use of these drugs in combination with statins may cause adverse events because of the intensified effects and increased drug levels in the blood. Some macrolide antibiotics, antifungal agents, protease inhibitors, and bergamottin contained in grapefruit juice, exert an inhibitory effect on CYP, and using them in combination with statins may increase statin levels in the blood. It has also been recently shown that the combined use of statins with CYP-inducing drugs, such as rifampicin and barbiturates, may decrease the effects of statins. Statins and the main metabolic and cardiovascular drugs metabolized by CYP are shown in Table 10.
Table 10. Statins and Cardiovascular and Metabolic Drugs Metabolized by CYP.
| CYP | Statin(s) metabolized | Cardiovascular and metabolic drug(s) metabolized |
|---|---|---|
| CYP3A4 | Atorvastatin Simvastatin |
Calcium antagonists (diltiazem, verapamil, nifedipine, amlodipine, cilnidipine, azelnidipine, and benidipine), warfarin, and repaglinide* |
| CYP2C9 | Fluvastatin Rosuvastatin |
Angiotensin receptor blockers (ARB) (losartan, valsartan, candesartan, Irbesartan, and azilsartan), warfarin, glinides (nateglinide and mitiglinide), and glimepiride |
Repaglinide is mainly metabolized by CYP2C8, but CYP3A4 is also sometimes involved.
It has been reported that the AUC of the blood statin levels increases when they are used in combination with drugs that are CYP substrates713). However, a search for reports published between 1990 and the present did not produce any that have investigated whether the adverse reactions intensify with this drug combination. Nonetheless, many cases of rhabdomyolysis resulting from statin use in combination with CYP substrates have been reported714). Although it involved only a small number of patients, one report from overseas revealed that the combined use of atorvastatin and ezetimibe in patients who were undergoing anticoagulation therapy for atrial fibrillation had their doses stabilized in approximately 3 months and did not experience an increase in the incidence of complications such as hemorrhage715) These findings were demonstrated despite the need to slightly decrease anticoagulant dose in the treatment group.
In addition to CYP, hypolipidemic agents are also influenced by transporters such as the breast cancer resistance proteins, organic anion transporter protein B1 (OATP1B1), organic anion-transporting poly-peptide-C (OATP-C), and P-glycoprotein. Even for rosuvastatin, a water-soluble statin, coadministration with cyclosporine is contraindicated as concomitant use increases rosuvastatin levels in the blood. This is because transporters of hepatocytes, such as the breast cancer resistance proteins and OATP1B1, are thought to be inhibited by cyclosporine, thereby causing a decrease in drug uptake by the hepatocytes.
Q5. Is the use of compound drugs in hyperlipidemia therapy effective in improving serum lipid levels and preventing the incidence of atherosclerotic cerebrovascular and cardiovascular diseases?
Although the use of compound drugs has been reported to increase the adherence to medication compared to prescribing each drug separately, there are no reports of compound drugs being more effective in preventing ASCVD incidence or at changing the serum lipid levels. (Recommendation level: B).
Many compound drugs are being used in the metabolic and cardiovascular drug categories to decrease the burden of taking medicines for the elderly and to improve adherence. In addition to those made up of multiple hypertensive agents or hypolipidemic agents, compound drugs containing hypolipidemic agents and other drugs are also available. For example, a compound drug made up of atorvastatin and amlodipine, a calcium antagonist, is being used in Japan. In other countries, compound drugs consisting of statins and DPP4 inhibitors are also being used. The use of compound drugs not only enhances patient QOL but also adherence to medication, which may lead to improved serum lipid levels and decrease CVD incidence. A report overseas found that a calcium antagonist–statin compound drug significantly improved serum lipid levels and better controlled blood pressure compared to placebo716). However, there are no reports of comparisons between the administration of a compound drug and the administration of the various drugs separately. In regard to CVD incidence, there are similarly no reports in which the effects of prescribing a compound drug were compared to those of drugs prescribed separately.
It has been reported that compound drugs improve patient adherence to medication compared to prescribing the drugs separately717). The maintenance of adherence is greatest when a single medication is added for patients who take only one drug orally718). Although the preventive effects on CVD have not been shown, the use of compound drugs is likely beneficial considering Japan's currently aging society, improvement of adherence, patient convenience, and healthcare economics.
7). Adherence
Q6. What are the factors that affect adherence?
Adherence to hypolipidemic agents is low in individuals aged < 50 years, ≥ 70 years, women, and low-income earners. Adherence is high in patients who have a CVD history.
It has been suggested that frequent lipid testing, low copayment, and the use of generic drugs are associated with an increase in adherence.
As with other therapeutic agents, adherence decreases as the number of doses per day increases. (Evidence level: 2)
Meta-analyses conducted overseas have demonstrated that adherence to oral statins differs according to factors, including age, gender, income, and whether other treatments are being implemented for comorbid disease. Women and low-income earners have been shown to have low adherence rates. In individuals aged < 50 years or ≥ 70 years, the adherence was low, with a “U-shaped” distribution. Secondary-prevention patients who have a CAD history showed high adherence, whereas primary-prevention patients showed low adherence. There have been many reports indicating that adherence is also high in patients receiving treatment for hypertension or diabetes. The frequency of lipid testing and low cost to the patient has also been shown to be associated with good adherence719). In addition, there are differences in adherence between drugs. It is known that the adherence to anionexchange resins is particularly low, whereas the adherence to fibrates, omega-3-acid-ethyl esters, and nicotinic acid derivatives is lower than that to statins720).
Lipid level improvement and CVD prevention are not likely to occur if the patient does not actually take the drugs prescribed. The adherence to medication is high in clinical studies and trials because of strict dosing management by study coordinators, but adherence is lower in actual clinical practice (Table 11), and high discontinuation rates have been reported overseas.
Table 11. Adherence to Statins Reported Outside of Japan719).
| Name of Country | Sample Size | Adherence Rate | Reference |
|---|---|---|---|
| United States | 19,422 | 1 year: 30% 2 years: 20% 3 years: 25% |
728) |
| United Kingdom | 6,262 | 1 year: 66% 5 years: 75% 10 years: 68% |
729) |
| United States | 4,776 | 6 months: 80% 1 year: 74% 2 years: 65% 3 years: 61% |
730) |
| United States | 34,501 | 3 months: 79% 6 month: 56% 1 years: 50% 10 years: 42% |
731) |
Adherence Rate: PDC [No. of prescription days/observation period × 100 (%)]
Adherence is associated with the preventive effects of CVD. In a retrospective cohort study on statins conducted overseas, the 4–5-year mortality rate in the group that had a high ≥ 90%) adherence rate was 45% lower than the group with a low (< 10%) adherence rate721). Likewise, in Japan's JELIS study, the group of secondary prevention patients who achieved an adherence rate of 80% had significantly fewer primary endpoints consisting of sudden cardiac death and fatal/nonfatal myocardial infarction compared to the group that did not722).
To improve drug adherence, guidance on lifestyle modification, such as diet and exercise therapy, should be provided to individuals who have low adherence, namely women, young individuals, the elderly, and primary prevention patients. Moreover, medical professionals must make an effort thoroughly explain the association between dyslipidemia and CVD incidence to patients, as well as aid patient understanding of the purpose of their treatment. It is advisable for other members of the medical team, such as nurses and pharmacists, to also provide regular explanations to patients regarding the importance of taking their medicine. In a recent comparative study, the study group that met with a pharmacist regularly had a lower discontinuation rate than the group that did not723). Discontinuation often occurs 1–2 years after treatment initiation; however, the discontinuation rate is known to decrease after that724). For this reason, to prevent discontinuation, it is particularly important to repeatedly explain the need for treatment to patients in the 1–2 years after treatment initiation. Regular lipid testing has also been reported to help increase adherence719).
It is known that the adherence to medication decreases when the number of daily doses increases725, 726). Therefore, when writing prescriptions, physicians should try to keep the number of doses as low as possible. In addition, the timing of medication doses (e.g. before or after meals) should not be confusing for the patient. The use of compound drugs has been known to increase adherence compared to prescribing drugs separately717); hence, the use of compound drugs should be considered for patients who require multiple drugs. A low copayment for the drugs is also associated with adherence. A cohort study conducted in the United States compared patients who were prescribed name-brand statins to those prescribed generic statins. It was found that patients who were prescribed generic statins not only had better adherence rates than those who were prescribed name-brand statins but also fewer composite endpoints such as hospitalization because of ACS and stroke, and all-cause mortality727).
4. Managing Major High-Risk Conditions
1). History of CAD
[Statement]
Even in secondary prevention, the risk for cardiovascular events is high if FH, ACS, DM, noncardiogenic cerebral infarction, PAD, CKD, and metabolic syndrome are present, or if there is an overlap in the major risk factors and the presence of smoking. (Evidence level: Ep-Ib)
In secondary prevention in patients with a history of CAD, the LDL-C control target should be at a level of < 100 mg/dL. As the risk of ASCVD incidence is high in patients with FH or ACS, stricter LDL-C management to achieve a level < 70 mg/dL should be considered. The secondary prevention for DM patients complicated with conditions described in Table 3b, should also be treated based on the target level of FH or ACS, as they are considered to be at high risk of recurrence of CAD.
i). Familial Hypercholesterolemia (FH)
It has been reported that the cumulative LDL-C (total LDL-C from the time of birth) plays a role in cardiovascular incidence732). In addition, observational studies have reported that LDL-C-lowering therapy using statins decreases cardiovascular event risk733), and delays the age of incidence of these events734). From an ethical viewpoint, it is difficult to conduct randomized comparative studies involving secondary prevention patients with FH. However, based on the findings that patients with early-incidence CAD have are more likely to have FH735, 736), and that FH patients have a higher risk of CAD recurrence than non-FH patients737), it is recommended that the LDL-C level be controlled at a stricter level (as much as possible to LDL-C < 70 mg/dL) in FH patients738).
ii). Acute Coronary Syndrome (ACS)
Patients who have experienced ACS have a higher risk of recurrence for cardiovascular events than patients with stable CAD. The OACIS-LIPID study in Japan investigated the effect of early administration of statins on suppressing cardiovascular events in patients with acute myocardial infarction739). The study showed an incidence rate of 40/1,000 person-years for all-cause mortality and nonfatal myocardial infarction in the group that underwent lipid-lowering therapy using drugs other than statins. However, at 30/1,000 person-years, the incidence rate of cardiovascular events was also remarkably high in the group that received statins. In the PACIFIC study740), which was a multicenter observational registry study on ACS, the incidence rate of fatal and nonfatal myocardial infarction was high, at 35/1,000 person-years, despite approximatively 80% subjects being administered statins.
Meanwhile, it has been reported that providing LDL-C-lowering therapy at an early stage following ACS incidence is effective in suppressing cardiovascular events741), and that stricter LDL-C-lowering therapy suppresses cardiovascular events significantly more than conventional LDL-C-lowering therapy742). Regarding the LDL-C management target, the IMPROVE-IT636) study revealed that lowering the LDL-C level to 53.6 mg/dL using statin–ezetimibe combination therapy suppressed cardiovascular events by 6.4% more than the group that had their LDL-C level controlled at 69.5 mg/dL with statins only. In meta-analyses of RCTs in which statins were administered within 14 days after ACS, cardiovascular events shown to be significantly suppressed after ≥ 2 years743). However, these preventive effects were not able to be proven in a shorter observational period of 4 months744).
The effectiveness of early LDL-C-lowering therapy for patients with ACS has been studied in Japan by observing the coronary plaques using intravascular ultrasound (IVUS). In the ESTABLISH study642), strict LDL-C-lowering therapy was administered early after ACS. The therapy decreased the average LDL-C level to 70 mg/dL after 6 months, which resulted in a 13.1% decrease in plaque volume. Moreover, it was reported that the change in plaque volume showed a significant positive correlation with the post-treatment LDL-C level and the rate of decrease in the LDL-C level. Additional cases were added and follow-up surveys (completed an average of 4.2 years later) were conducted in the same study. The results of the follow-up surveys revealed that starting a strict LDL-C-lowering therapy at an early stage significantly suppressed cardiovascular events745). In JAPAN-ACS643), it was similarly demonstrated that the early initiation of a strict LDL-C-lowering therapy using statins for patients with ACS suppresses plaque progression. However, no significant relationship was demonstrated between the rate of decrease in the LDL-C levels, or in the post-treatment LDL-C level and the rate of plaque regression. It was reported in the recent PRECISE-IVUS study that decreasing the LDL-C level to < 70 mg/dL using a combination of statin and ezetimibe resulted in a marked plaque volume regression in patients with ACS, compared to those who were treated with statins only645). In recent years, the development of vascular imaging techniques other than IVUS has made it possible to assess the characteristics of plaques, and the association of these characteristics with clinical events is also being studied746–748).
iii). Diabetes Mellitus (DM)
For patients with a myocardial infarction history, it has been reported that the risk of recurrence of cardiovascular events increases with the presence of diabetes137, 749–751). Epidemiological studies in Japan involving subjects with CAD have also revealed that diabetic patients have a high risk for all-cause mortality and cardiovascular events138, 182, 752). Likewise, in the analysis of the J-LIT study on patients with CAD, it was shown that the presence of diabetes causes the relative risk for cardiovascular events to increase by approximately 2.5-fold173).
According to a meta-analysis by the Cholesterol Treatment Trialists' (CTT) Collaboration, the effects of LDL-C-lowering therapy using statins on cardiovascular event are equal regardless of whether the patient had diabetes751). In the sub-analysis of the TNT study, which involved subjects with CAD complicated with diabetes, treatment using a high statin dose significantly suppressed cardiovascular and cerebrovascular events by 25% and 31%, respectively, compared to treatment using the usual statin dose753).
A meta-analysis of clinical studies using IVUS, which has been implemented overseas, reported that diabetes was an independent risk factor in cases where the coronary plaques were found to be progressing, regardless of whether their LDL-C levels had been decreased to ≥ 70 mg/dL with treatment754). It has been demonstrated that coronary plaque volume progression, the incidence rate of cardiovascular events, and the post-treatment LDL-C level show a significant positive correlation with one another. This suggests the importance of a stricter LDL-C-lowering therapy if CAD is complicated with diabetes. The sub-analysis of JAPAN-ACS755), a study conducted in Japan involving patients with ACS, showed that the presence of diabetes posed a strong negative risk on plaque regression. Even if the LDL-C level is controlled to the same extent as that of nondiabetic patients, the rate of plaque regression in diabetic patients is markedly low. However, this study also reported that significant plaque-regressing effects can be achieved if the LDL-C level is controlled at < 75 mg/dL756). Furthermore, the ZEUS study, despite being small-scale, reported that strict LDL-C-lowering therapy using statins and ezetimibe in combination is useful for regressing plaques in CAD complicated with diabetes644).
iv). Noncardiogenic Cerebral Infarction
ASCVD that occurs with atherosclerosis as an underlying basis, such as CAD, cerebrovascular diseases, and PAD, are mutually high-risk conditions for vascular complications. It was demonstrated in the REACH registry, a registry study on patients with ASCVD or overlapping risks factors for ASCVD, that approximately 16% registered cases are complicated with two or more ASCVD757). A comparison between the database of the Texas Heart Association in the United States and CREDO-Kyoto study, a registry study that involved subjects who had undergone coronary revascularization in Japan, showed a significantly higher rate of complication with cerebrovascular diseases in Japan (16.4% vs. 5.0%). However, it was confirmed that the complication of cerebrovascular disease is a high-risk condition for cardiovascular event in both Japan and the United States752).
Results of secondary prevention studies on CAD conducted in western countries, such as 4S, LIPID, and CARE, have revealed a high risk for the recurrence of cerebrovascular and cardiovascular events in patients with CAD who have a cerebrovascular disease history. However, it has been reported that LDL-C-lowering therapy using statins suppresses the risk for recurrence of both cerebrovascular and cardiovascular events758–760).
v). Peripheral Arterial Disease (PAD)
In the comparison between the aforementioned database of the Texas Heart Association in the US and CREDO-Kyoto study in Japan, it was confirmed that PAD is a high-risk condition for cardiovascular event in both Japan and the US752), though the complication rate of PAD was significantly higher in the US.
Patients with PAD who have a CAD history are at extremely high risk for total mortality and fatal cardiovascular events761–767). There are no reports of lipid intervention studies involving subjects with PAD complicated by CAD. However, in the sub-analysis of IDEAL, a study that involved subjects with myocardial infarction complicated with PAD, it was reported that an LDL-C-lowering therapy using a high statin dose significantly suppressed cardiovascular events compared to a moderate statin dose767). It was also confirmed that the therapy prevented the new incidence of PAD by 30% in cases that were not already complicated with PAD.
A meta-analysis of clinical studies in which coronary plaque progression was analyzed by IVUS showed that the inhibition of the progression and plaque regression is observed when the LDL-C level is controlled at < 70 mg/dL, regardless of the presence or absence of PAD. Thus, strict LDL-C lowering therapy in patients with PAD expect to suppress CAD events because plaque regression has been shown to be associated with fewer cardiovascular events768).
vi). Chronic Kidney Disease (CKD)
Stratified analyses of the eGFR have been performed in long-term observational studies involving subjects with ACS and on post-PCI patients. The results of these studies have found that, compared to patients with normal renal function, the risk for cardiovascular events, including cerebrovascular disease, cardiac death, and total mortality increases by 2–3-fold in those with CKD even when the CKD is mild. It has been reported that the risk increases in association with the severity of the disease740, 769, 770). In addition, according to the observational CREDO-Kyoto study, the risk for cardiovascular death is 2.9-fold higher for post-PCI patients with CKD, and the risk for total mortality increases by 2.1-fold183). In particular, patients aged ≥ 55 years have a 3.7-fold increased risk for cardiovascular events, including cerebrovascular diseases174). Furthermore, patients with CKD and a serum creatinine level ≥ 2.0 mg/dL were found to have a 7.0-fold increased risk for total mortality. This suggests that the risk for cardiovascular events increases markedly with the presence of CKD in patients who have undergone PCI752).
For patients with CKD complicated with CAD, the preventive effects of lipid-lowering therapy on cardiovascular events have been investigated in post hoc analyses of early-stage secondary prevention studies using statins. These analyses have reported that statin use suppresses cardiovascular events in patients with mild CKD and an eGFR < 75 mL/min/1.73 m2 771, 772). However, suppressive effects on cerebrovascular diseases were not demonstrated. Furthermore, post hoc analyses of secondary prevention studies have shown that high-dose statin treatment decreases cardiovascular event significantly, by approximately 30%, in patients with moderate CKD compared to the usual dose773, 774). In CREDO-Kyoto PCI/CABG Registry Cohort-2, a secondary-prevention cohort study on CAD in Japan, patients with mild CKD and an eGFR of 30–60 mL/min/1.73 m2 who took oral statins had a significantly lower incidence rate of major cardiovascular events. The incidence rate of cerebrovascular disease was also significantly lower in patients who were taking oral statins775). However, similar effects were not seen in patients with severe CKD whose eGFRs were < 30 mL/min/1.73 m2 or in patients undergoing hemodialysis.
vii). Metabolic Syndrome and an Overlap of Major Risk Factors
The results of a meta-analysis of 87 clinical studies have shown that the presence of metabolic syndrome results in an added risk for total mortality, coronary events, and cerebrovascular events for patients with CAD327).
A sub-analysis was performed with just those complicated by metabolic syndrome among all the subjects with CAD in the TNT study, and it was shown that an overlap of the major risk factors poses a risk for cardiovascular events. The incidence rate of cardiovascular events is particularly high in cases where three or more major risk factors are present. However, it has been shown that cardiovascular event is significantly suppressed by 29% with high-dose statin therapy compared to a usual-dose statin therapy776).
JCAD observational study in Japan also revealed that if three or more major risk factors are present, the risk for cardiovascular events is 1.3-fold higher compared to having two or fewer risk factors182). In a study where patients who underwent PCI were followed up with for a long period, the relative risk for cardiovascular events in those with metabolic syndrome was 2.1-fold higher777). However, statin administration resulted in a significant decrease in total mortality by 44% and in coronary death by 47%778).
viii). Smoking
In patients with CAD who continue to smoke, the risk of recurrence of cardiovascular events is higher than that observed in nonsmokers. The total mortality, cardiac death, and sudden cardiac death risks are also significantly higher779–783). In the REACH Registry, a registry study on patients with ASCVD, CAD, cerebrovascular diseases, and PAD or overlapping risks factors for ASCVD, the incidence rate of cardiovascular events in patients who continued to smoke was approximately 1.3-fold higher than that observed in lifelong nonsmokers784). In the OACIS study, even after adjusting for age, gender, DM, hypertension, dyslipidemia, and therapeutic agents, the risk for total mortality in post-myocardial infarction patients who continued smoking was 2.3-fold higher than in lifelong nonsmokers. In contrast, the total mortality risk in patients who quitted smoking after myocardial infarction incidence decreased to the same level as the nonsmokers. Compared to those who continued smoking, the total mortality risk of patients who quitted smoking decreased significantly by 61%785). Many epidemiological studies have reported that regardless of age and gender, the risk of recurrence of cardiovascular events decreases by approximately half after the first year of smoking cessation and continue to decrease thereafter. Such studies have also reported that the risk further decreases to almost the same level as that of the lifelong nonsmokers approximately 10 years after smoking cessation779–786).
On the basis of a composite analysis of secondary prevention studies, TNT and IDEA787), and the post hoc analysis of the GREACE study788), even with a strict LDL-C-lowering therapy using statins, the risk for cardiovascular events is higher in patients who continue smoking than in lifelong nonsmokers or the ex-smokers. Therefore, providing guidance on smoking cessation to patients who continue to smoke is extremely important.
2). Diabetes Mellitus (DM)
[Statement]
Not only hyperglycemia but also dyslipidemia and hypertension should be comprehensively controlled soon after the onset of diabetes. (Evidence level: 1, recommendation level: A)
More strict control of LDL-C should be considered among diabetic subjects with FH, noncardiogenic cerebral infarction, PAD, microvascular complications (retinopathy and nephropathy), metabolic syndrome, continuation of poor glycemic control, an overlap of major risk factors of ASCVD, or smoking. (Consensus, recommendation level: A)
i). Prevention and Treatment of ASCVD in Patients with DM
a). Risk Factors for ASCVD
It has been reported that LDL-C, HDL-C, and HbA1c levels, as well as and systolic blood pressure are risk factors for CAD, whereas hypertension, male gender, and atrial fibrillation are risk factors for cerebral infarction789). However, a study conducted in Japan (JDCS) has suggested that LDL-C and TG levels are risk factors for CAD, and that systolic blood pressure is a risk factor for stroke790). Other Japanese studies have reported that smoking, male gender, and high Lp(a)791) are risk factors for CAD. It is important to manage these risk factors comprehensively in patients with DM to prevent ASCVD. Lifestyle intervention is especially important in diabetic subjects with metabolic syndrome. The presence of microvascular complications such as diabetic nephropathy792, 793) or retinopathy794) is a predictive factor for CAD.
b). Blood Glucose
Meta-analyses have clearly shown that tight blood glucose control leads to the suppression of ASCVD incidence795, 796). However, a longer duration of 8–15 years after strict glycemic control is necessary for the suppression of ASCVD risk797, 798). There is little evidence showing that tight blood glucose control with specific drug can suppress the occurrence of ASCVD events. However, recent investigations reported that the suppression of composite cardiovascular events and cardiovascular death by SGT2 inhibitors799), and that the suppressive effects of glucagonlike peptide-1 (GLP-1) receptor agonists on ASCVD incidence800, 801). On the other hand, strict blood glucose control increases the risk for hypoglycemia, and hypoglycemia has been reported to be related to cardiovascular death in the Japanese802). Strict blood glucose control must be carefully carried out.
According to the J-EDIT study, a Japanese study that involved elderly subjects, stroke risk was 2.63-fold higher in the group with HbA1c levels ≥ 8.5% than in the group with HbA1c levels of 7.0–8.4%. On the other hand, subjects with HbA1c levels < 7.0% also had a 2.35-fold higher risk. Thus, elderly patients with DM require both hyperglycemia prevention while avoiding excess strict glucose control803).
c). Lipids
Patients with DM are often complicated with hyper-LDL cholesterolemia, hypertriglyceridemia, and low HDL-C levels. In a meta-analysis including CARDS804), LDL-C-lowering therapy using statins greatly decreases CAD-related mortality, CVD, and cerebral infarction in diabetic patients. These effects were similar to those in nondiabetic subjects751).
In contrast, it was reported in the FIELD study that fibrates decrease CAD events in primary prevention805). In the ACCORD study involving high-risk subjects with DM, the sub-analysis suggested that the risk for cardiovascular events might be significantly suppressed by adding fibrate in patients who have hypertriglyceridemia and low HDL-C after statin administration806). The additional administration of EPA was also reported to significantly decrease coronary events in Japanese patients who were taking statins for treating hypercholesterolemia complicated with impaired glucose metabolism807). Furthermore, CVD risk decreases significantly because of a decrease of LDL-C level from 94 mg/dL to 54 mg/dL when statins and ezetimibe are used for treatment in patients with ACS. The effects were evident especially in patients with DM808).
d). Blood Pressure
Elevated blood pressure leads to an increased risk of ASCVD in DM subjects809). In meta-analyses, blood pressure-lowering therapy was found to decrease risk of cerebrovascular diseases810). However, risk reduction of CAD has only been observed in subjects with hypertension before treatment initiation811), which indicates that the effects are limited for CAD prevention. Angiotensin-converting enzyme inhibitors812), angiotensin Ⅱ receptor blockers813), and calcium channel antagonists814) have been reported to be effective for preventing cardiovascular events in patients with DM.
e). Comprehensive Risk Management
Early comprehensive management of risk factors, such as hyperglycemia, hypertension, dyslipidemia, smoking habit, and visceral fat obesity, is important in prevention of ASCVD among diabetic subjects815, 816). Lifestyle modification such as diet therapy817), increasing physical activity818), and smoking cessation819) are important, but there is no evidence that lifestyle modifications alone decrease the risk of ASCVD in patients with DM820, 821). Several studies have suggested that a comprehensive and intensive therapy for the risk factors, combined with lifestyle modification and drug therapy, has a protective effect on ASCVD. These findings have also been reported in Japan822, 823).
The ADA has recommended that aspirin use for the primary ASCVD prevention should be considered824) for high-risk men and women aged ≥ 50 years. However, the JPAD studies conducted in Japan did not show any protective effects of aspirin on cardiovascular events in patients with DM825, 826).
ii). LDL-C control for ASCVD prevention
a). Lipid Management for Primary Prevention
Recently published US guidelines did not refer definitive achievement goals of plasma lipids, but recommended statin administration without monitoring of lipid levels during the course of therapy827). However, in the ESC/EAS guidelines, LDL-C level < 100 mg/dL is recommended as the primary target for patients with type 2 DM828). In Japan, the use of JAS guidelines for control of plasma lipids in diabetic subjects has been recommended829).
There are only a few studies involving Japanese patients with DM; however, a TG level < 150 mg/dL and a HDL-C level ≥ 40 mg/dL are recommended as targets regardless of the presence or absence of DM. The target for the primary prevention of CAD is < 120 mg/dL for LDL-C and < 150 mg/dL for non-HDL-C. Some clinical conditions in patients with DM have been shown to have CAD risk. These conditions include the following: (1) FH; (2) noncardiogenic cerebral infarction830), (3) PAD830); (4) microvascular complications (retinopathy, nephropathy, etc.)792, 794, 831–833); (5) persistently poor glycemic control143, 795, 834); (6) metabolic syndrome-related complications (visceral obesity)835); (7) overlap of major risk factors836, 837); and (8) smoking836).
Table 12. Diabetic Patients at Higher Risk for Developing CAD.
| Familial hypercholesterolemia (FH) |
| Noncardiogenic cerebral infarction |
| Peripheral arterial disease (PAD) |
| Microvascular complications (retinopathy, nephropathy, etc.) |
| Persistent poor glycemic control |
| Metabolic syndrome |
| Overlap of major risk factors |
| Smoking |
Although all primary prevention patients with DM should predict LDL-C level < 120 mg/dL, patients who have any of the above listed characteristics should implement stricter management (i.e., compulsory achievement of the target value). Furthermore, diabetic patients with FH or more than one of above eight characteristics are at extremely high ASCVD risk, the targets of secondary prevention should be considered.
In the CTT study, it was reported that decreasing LDL-C level by 38.7 mg/dL using statins, cerebrovascular disease risk in DM patients is decreased by 21%. Thus, it is likely that control of LDL-C is effective for the prevention of cerebrovascular disease same as CAD prevention838).
b). Lipid Management for Secondary Prevention
Regardless of the presence or absence of DM, LDL-C level < 100 mg/dL and non-HDL-C level < 130 mg/dL are recommended. Secondary-prevention of CAD in diabetic patients shows a high rate of recurrence137–139, 839), and it is difficult to achieve plaque regression in patients with DM even with the recommended control of LDL-C-lowering therapy840). In addition, DM has been reported to be the most important risk factor for CAD recurrence in the Japanese841). Thus, it is reasonable to set an LDL-C level of < 100mg/dL as the compulsory target in secondary prevention for patients with DM842). As mentioned in CQ20, among secondary prevention cases with diabetes complicated with risk factors such as noncardiogenic cerebral infarction, PAD, CKD, metabolic syndrome, overlap of major risk factors or smoking habit have a more higher risk of recurrence. Thus, it is reasonable to control of LDL-C as the same target level of FH or ACS, by achieving LDL-C level of < 70 mg/dL754, 756).
3). Cerebrovascular Disease
[Statement]
Statin therapy may prevent the development of cerebral infarction. (Evidence level: 2, recommendation level: A)
i). Frequency
Cerebrovascular disease is classified into three types (cerebral hemorrhage, cerebral infarction, and subarachnoid hemorrhage). According to the 2015 Stroke Data Bank, the cerebrovascular disease incidence in Japan is reported to be approximately 18.5% for cerebral hemorrhage, approximately 5.6% for subarachnoid hemorrhage, and 75.9% for cerebral infarction842). Compared to the cerebrovascular disease incidence in western countries, the cerebral hemorrhage incidence in Japan is higher whereas the cerebral infarction incidence is relatively low843).
Cerebral infarction is further classified into three clinical types (lacunar infarction, atherothrombotic cerebral infarction, and cardiogenic cerebral embolism). In the Hisayama study, lacunar infarction accounted for approximately 50% cases of cerebral infarction, whereas the incidence rates of atherothrombotic cerebral infarction and cardiogenic cerebral embolism were just under 30% and just over 20%, respectively844). The J-MUSIC study published in 2,000 saw a change in the incidence rates, with a 38.8% rate for lacunar infarction, a 33.3% rate for atherothrombotic cerebral infarction, and a 21.8% rate for cardiogenic cerebral embolism845). The numbers were also different in the 2015 Stroke Data Bank, with an incidence rate of 31.2% for lacunar infarction, 33.2% for atherothrombotic cerebral infarction, and 27.7% for cardiogenic cerebral embolism842). Cerebral infarction incidence in the western Caucasian population has been reported to be approximately 30% for both lacunar infarction and atherothrombotic cerebral infarction, and approximately 40% for cardiogenic cerebral embolism846). It has been reported that in Japan, the number of cases of lacunar infarction is decreasing whereas the number of cases of cardiogenic cerebral embolism is increasing.
ii). Risk Factors for the Development of Cerebrovascular Disease
The results of NIPPON DATA80 indicate that the factors affecting mortality rate of cerebrovascular disease in the Japanese are age, systolic blood pressure, smoking, and hyperglycemia. Lipid levels, such as the TC level, are not recognized as risk factors50). Similarly, the consolidated results of 61 observational studies conducted in western countries (approximately 0.9 million subjects) found no relationship between the TC level and the mortality rate of cerebrovascular disease847). The results of a meta-analysis of 18 cohort studies in Japan and China showed that blood pressure is the most important risk factor for cerebrovascular disease, and that the TC level has much less involvement than blood pressure848).
In terms of the individual cerebrovascular diseases, studies have clearly revealed that hypertension is a risk factor for cerebral hemorrhage, and that hypertension, smoking, alcohol, and the presence of cerebral aneurysm are the major risk factors for subarachnoid hemorrhage. With respect to cerebral infarction, the major risk factors for cardiogenic cerebral embolism are hypertension and cardiac thrombi849, 850).
A study of the risk factors for noncardiogenic cerebral infarction only revealed that in the 2015 Stroke Data Bank, the dyslipidemia incidence in patients with noncardiogenic cerebral infarction is 40–50%, a number that has been increasing in recent years842). However, the results of epidemiological studies conducted in Japan have indicated that there is no significant relationship between the serum cholesterol levels (TC, LDL-C, and non-HDL-C) and the incidence rate of noncardiogenic cerebral infarction36, 38, 40, 57, 64, 78, 337). In western countries, epidemiological studies such as MRFIT have reported that an increased TC level is associated with an increased risk for cerebral infarction851–853). Furthermore, the consolidated results of nine cohort studies indicated that cerebral infarction incidence is significantly decreased by 15% in patients with a decrease of 1 mmol/L (38.6 mg/dL) in the LDL-C level854). Similarly reported in the result from a meta-analysis of 21 large clinical trials by CTT Collaboration, stroke incidence is decreased by 15% and cerebral infarction incidence is decreased by 20% in patients with a decrease of 1 mmol/L (38.6 mg/dL) in the LDL-C level177). On the contrary, some reports have stated that the TC level is either not a risk factor for cerebral infarction or has very little involvement855, 856).
The results of the Hisayama study, which investigated cerebral infarction risk by type, demonstrated that the LDL-C level is a risk factor for atherothrombotic cerebral infarction. However, the LDL-C level is not related to the incidence of other types of cerebral infarction36). Furthermore, the Hisayama study reported that blood pressure has a strong effect on lacunar infarction, atherothrombotic cerebral infarction, and, in women, cardiogenic cerebral embolism857). Reports published in various other countries have also indicated that the major risk factor for cerebral infarction, including cardiogenic cerebral embolism, is hypertension855). Thus, cholesterol level is recognized to be a risk factor only for atherothrombotic cerebral infarction, whereas hypertension is considered to be the major risk factor for all types of cerebral infarction, including atherothrombotic cerebral infarction849).
Many reports, including those from Japan, have stated that hypocholesterolemia is a risk factor for cerebral hemorrhage40, 858). According to a meta-analysis of cohort studies, a decrease of 1 mmol/L (38.6 mg/dL) in the cholesterol level increases the cerebral hemorrhage incidence by 19%854). In Japan, an LDL-C level of 80 mg/dL or less has also been reported to increase the cerebral hemorrhage incidence40). However, as described later, the results of a meta-analysis of prevention studies on CAD did not indicate that cholesterol-lowering therapy leads to an increased cerebral hemorrhage incidence348).
There have been many reports, including those from Japan, stating that the lower the HDL-C level, the higher the incidence rate of cerebral infarction46, 859–861).
Many reports have also indicated that there is no clear relationship between the TG level and cerebrovascular disease856, 862, 863). However, the results of a meta-analysis of epidemiological surveys conducted in the Asia-Pacific region, in which the fasting TG level was divided into four groups, reported that the group of patients with the highest TG levels had a 50% higher risk of ischemic stroke compared to the group with the lowest TG levels87). Furthermore, the results of cohort studies involving approximately 14,000 subjects reported that ischemic stroke incidence increases in both men and women when postprandial hypertriglyceridemia is present864). These results indicate that a 1-mmol/L (88.5 mg/dL) increase in the postprandial TG level increases ischemic stroke risk by 15%.
iii). Lipid-Lowering Therapy and Cerebrovascular Disease
Although it has been established that statins are important in preventing stroke incidence, there are still relatively few studies that have investigated their use for stroke recurrence prevention. Stroke is investigated as a secondary endpoint in most studies. The results of a meta-analysis of prevention studies conducted in the western counties showed that cholesterol-lowering therapy using statins significantly decreased cerebral infarction incidence by 19%. On the other hand, cerebral hemorrhage incidence did not change significantly348). It remains unclear as to why the statin administration decreases cerebrovascular disease in observational studies given the fact that the cholesterol level is not recognized as a risk factor for cerebrovascular disease.
There are two studies in which the recurrence of stroke was the primary endpoint: the SPARCL865) study and the J-STARS619) study. In SPARCL, high doses of statins were administered to subjects who had no CAD with a stroke or transient ischemic attack (TIA) history within 6 months following the incidence. The recurrence rate of stroke in these subjects was compared with that of subjects who received a placebo, and the results revealed that apart from a significant decrease in the recurrence of stroke (−16%, p = 0.03), the incidence rate of CAD was also significantly lower (− 35%, p = 0.003). A post hoc analysis found that cerebral infarction incidence was significantly decreased (hazard ratio 0.78), but that of cerebral hemorrhage was significantly increased (hazard ratio 1.66). However, it must be noted that statin dose in this study was much higher than the upper limit approved in Japan. The J-STARS study involved Japanese subjects with ischemic stroke, excluding those with cardiogenic cerebral embolism. The results of a comparison between the pravastatin group and the non-pravastatin group revealed that the former had a significantly decreased incidence rate of atherothrombotic cerebral infarction (hazard ratio 0.33). Meanwhile, the incidence rate of intracranial hemorrhage was the same as the non-statin group (hazard ratio 1.00)619). In terms of secondary prevention of noncardiogenic cerebral infarction, these results suggest that statin therapy has suppressive effects on atherothrombotic cerebral infarction incidence. Moreover, in regard to the results of SPARCL, which are contrary to those of a meta-analysis348) in which increased incidence of cerebral hemorrhage because of statin administration was not observed, it has been shown that hemorrhage incidence does not increase in the Japanese. However, regarding cerebral hemorrhage risk following cholesterol-lowering therapy, further preventive studies need to be conducted.
Among the other studies conducted in Japan, the MEGA study, which involved subjects with no history of CAD or stroke, showed that statin administration tends to decrease stroke incidence, with hazard ratios of 0.66 (men) and 0.63 (women)620). In particular, ischemic stroke incidence in men and stroke in women who were ≥ 55 years were significantly decreased620, 621). The results of a sub-analysis of JELIS showed that treatment with statins and EPA in patients with a stroke history significantly suppressed the recurrence of stroke by approximately 20% compared with statin monotherapy622).
There are also other reports suggesting that the outcome of cerebral infarction that occurs during the course of treatment with statins is satisfactory866), and that cerebral infarction risk increases as a result of discontinuing statin treatment867).
iv). Measures to Prevent Cerebrovascular Disease
It is vital to control blood pressure because the greatest risk for cerebrovascular disease is hypertension. Atrial fibrillation is a major risk factor for cardiogenic cerebral embolism, and cerebral aneurysm is a significant risk factor for subarachnoid hemorrhage. Therefore, it is necessary to appropriately manage these risk factors. The relevant guidelines should be referenced when managing these risk factors868).
In western countries, based on the results of a meta-analysis, lipid-lowering therapy is recommended for the prevention of noncardiogenic cerebral infarction847, 869). In Japan, because the proportion of atherothrombotic cerebral infarction has been increasing, and that the MEGA study showed that statins are effective in preventing cerebral infarction, appropriate lipid management should be carried out in addition to adequate antihypertensive therapy to prevent cerebral infarction. For the prevention of noncardiogenic cerebral infarction with underlying atherosclerosis, it is advisable to implement a set of management criteria based on the criteria for the ischemic heart disease prevention.
5. Implementation of Comprehensive Risk Assessment and Management
[Statement]
For the prevention of cerebrovascular and cardiovascular diseases, comprehensive management of the major risk factors, such as dyslipidemia, smoking, hypertension, and DM, should be carried out as early as possible. (Recommendation level: A)
Lifestyle modification, including diet therapy, exercise therapy, and smoking cessation, forms the basis of cerebrovascular and cardiovascular disease prevention. It is important to continue to provide guidance on lifestyle modification even after initiating drug therapy. (Recommendation level: A)
Cerebrovascular and cardiovascular diseases require multiple types of risk assessment and management870). In view of this, 11 academic societies led by The Japanese Society of Internal Medicine, together with the Japan Medical Association and The Japanese Society of Medical Sciences, jointly published an integrated management guideline titled “Comprehensive Risk Management Chart for the Prevention of Cerebrovascular and Cardiovascular Diseases” in April 2015 (hereafter abbreviated as “comprehensive chart”)156). As shown in Fig. 6, its basic concept was to decrease the risk factors (obesity, hypertension, hyperglycemia, serum lipid abnormalities, renal dysfunction, etc.) by comprehensively managing the patient's lifestyle habits. However, in cases where various risk factors overlap, other than comprehensive management including drug intervention, the comprehensive chart also emphasized that specialized drug therapy for treating the underlying causes is essential if the disease is hereditary or secondary.
Fig. 6.
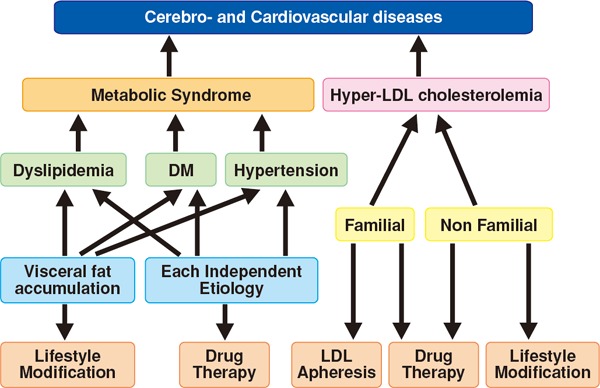
Comprehensive Risks and Risk Factors for Lifestyle Habits
Joint Committee for Comprehensive Risk Management Chart for the Prevention of Cerebro- and Cardiovascular Diseases, The Journal of The Japanese Society of Internal Medicine 2015, Vol. 104, No. 4, 824–860
In this section, we will describe the comprehensive risk assessment and their management in six steps, based on the concept of the comprehensive chart (Fig. 7). The main target population is primarily first-visit patients assessed as “requiring further investigation” because of the presence of risk factors for ASCVD. However, for patients who have an ASCVD history, such as CAD, and for those already receiving treatment or are being followed up for dyslipidemia, DM, or hypertension, reassessment of the risk factors and determination of the effectiveness of their case management should be carried out periodically.
Fig. 7.
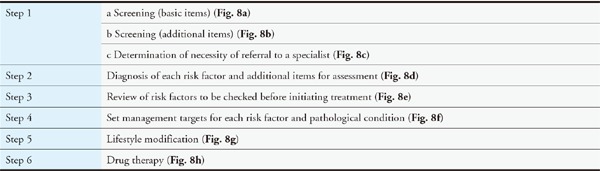
Comprehensive risk assessment and their management in six steps
[Screening]
Step 1: Screening for Cerebro- and Cardiovascular Disease Risk Assessment
Exhaustive screening of the major risk factors is important for the comprehensive risk management of cerebro- and cardiovascular diseases. In addition to blood biochemistry tests, careful medical history-taking and examination should be carried out.
The screening consists of Step 1a (basic items), Step 1b (additional items), and Step 1c, (criteria for determining if the patient should be referred to a specialist).
If possible, it is advisable to collect fasting blood for laboratory testing in Step 1a. Blood for Step 1b should generally be collected in a fasting state.
Step 1 consists of the basic items for screening and the additional items presented in Steps 1a and 1b, as well as the criteria for determining the necessity of patient referral to a specialist in Step 1c.
i). Step 1a
Step 1a consists of the basic screening items. The items for the medical history-taking, physical findings, and tests necessary when assessing each patient's risk for ASCVD are shown in Fig. 8a. In addition to subjective symptoms, complications, medical history, lifestyle habits (smoking, passive smoking, and alcohol consumption), exercise habits, and sleep habits, which are standard items specific to a physical examination, it is recommended to check the patient's home blood pressure and family history during the medical history-taking. Age, gender, height, body weight, and BMI (kg/m2) are the recommended items to include in the physical findings, and the recommended examinations are in-clinic blood pressure, pulse rate (regular or irregular), and chest auscultation. It is preferable to collect fasting blood for the blood tests as much as possible. Other than TC, HDL-C, non-HDL-C (TC − HDL-C), eGFR (serum creatinine), ALT, γ-GT, HbA1c, and blood glucose, it is recommended to perform urinalysis (qualitative) and electrocardiography. If atrial fibrillation is detected, the patient should be referred to a specialist depending on the degree of abnormality.
Fig. 8a.
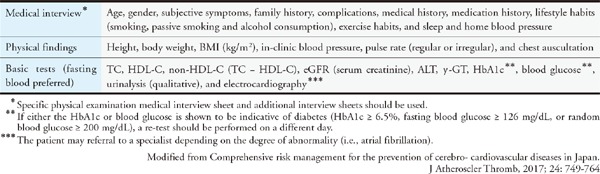
Step 1a Screening (Basic Items)
ii). Step 1b
Step 1b consists of the additional items for screening, which are to be carried out concurrently with 1a or if abnormalities are observed in 1a (Fig. 8b). For the physical findings, measurement of the abdominal circumference (waist circumference), standing blood pressure (after 1–3 minutes of standing), ankle–brachial index (ABI), arterial palpation in the extremities, and auscultation of vascular murmurs in the neck and abdomen should be performed. In principle, the blood count, blood glucose, TG, and LDL-C measurements should be tested using fasting blood, and in addition to a chest X-ray and uric acid test, the urinary protein/creatinine ratio should be measured if abnormalities are found in the urinalysis (qualitative). Whenever necessary, the plasma aldosterone concentration/renin activity ratio should also be measured.
Fig. 8b.
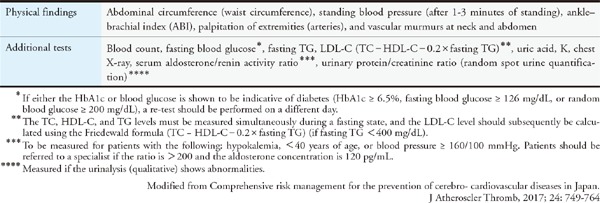
Step 1b Screening (Additional Items)
iii). Step 1c
Step 1c lists the circumstances in which referral to a specialist is likely to be necessary based on the outcome of the above-mentioned screening (Fig. 8c).
Fig. 8c.
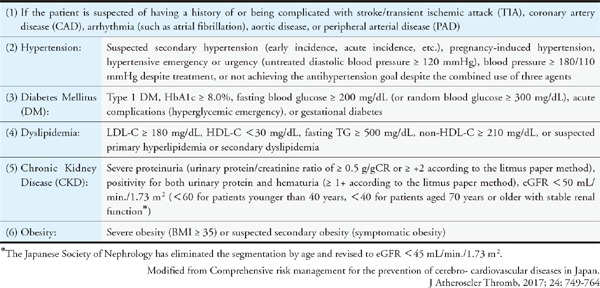
Step 1c Determination of Necessity for Referral to a Specialist
 If the patient is suspected of having a history of or being complicated with stroke/ TIA, CAD, arrhythmia such as atrial fibrillation, aortic disease, or PAD
If the patient is suspected of having a history of or being complicated with stroke/ TIA, CAD, arrhythmia such as atrial fibrillation, aortic disease, or PAD For patients with hypertension, if secondary hypertension (early incidence, acute incidence, etc.), pregnancy-induced hypertension, hypertensive emergency or urgency (untreated diastolic blood pressure ≥ 120 mmHg) is suspected, or if refractory hypertension (blood pressure ≥ 180/110 mmHg despite undergoing treatment, or not achieving the antihypertension goal with the combined use of three agents) is present
For patients with hypertension, if secondary hypertension (early incidence, acute incidence, etc.), pregnancy-induced hypertension, hypertensive emergency or urgency (untreated diastolic blood pressure ≥ 120 mmHg) is suspected, or if refractory hypertension (blood pressure ≥ 180/110 mmHg despite undergoing treatment, or not achieving the antihypertension goal with the combined use of three agents) is present For patients with DM, if type 1 DM, HbA1c ≥ 8.0%, fasting blood glucose ≥ 200 mg/dL (or random blood glucose ≥ 300 mg/dL), acute complications (hyperglycemic emergency), or gestational diabetes is present
For patients with DM, if type 1 DM, HbA1c ≥ 8.0%, fasting blood glucose ≥ 200 mg/dL (or random blood glucose ≥ 300 mg/dL), acute complications (hyperglycemic emergency), or gestational diabetes is present For patients with dyslipidemia, if LDL-C ≥ 180 mg/dL, HDL-C < 30 mg/dL, TG ≥ 500 mg/dL, non-HDL-C ≥ 210 mg/dL, or if primary or secondary dyslipidemia is suspected (Table 13)
For patients with dyslipidemia, if LDL-C ≥ 180 mg/dL, HDL-C < 30 mg/dL, TG ≥ 500 mg/dL, non-HDL-C ≥ 210 mg/dL, or if primary or secondary dyslipidemia is suspected (Table 13) For patients with CKD, if advanced proteinuria (urinary protein/creatinine ratio ≥ 0.50 g/gCr or ≥ 2+ by the litmus paper method), positivity for both urinary protein and urinary blood (≥ 1 + by the litmus paper method), or eGFR < 50 mL/min/1.73 m2 (< 60 for those aged < 40 years and < 40 for those aged ≥ 70 years with stable renal function) is observed. (The Japanese Society of Nephrology has eliminated the segmentation by age and revised to eGFR < 45 mL/min./1.73 m2 in 2017154).)
For patients with CKD, if advanced proteinuria (urinary protein/creatinine ratio ≥ 0.50 g/gCr or ≥ 2+ by the litmus paper method), positivity for both urinary protein and urinary blood (≥ 1 + by the litmus paper method), or eGFR < 50 mL/min/1.73 m2 (< 60 for those aged < 40 years and < 40 for those aged ≥ 70 years with stable renal function) is observed. (The Japanese Society of Nephrology has eliminated the segmentation by age and revised to eGFR < 45 mL/min./1.73 m2 in 2017154).) For patients with obesity, if severe obesity (BMI ≥ 35) or if secondary obesity (symptomatic obesity) is suspected
For patients with obesity, if severe obesity (BMI ≥ 35) or if secondary obesity (symptomatic obesity) is suspected
Table 13. Major Secondary Dyslipidemias.
| • Hypothyroidism |
| • Nephrotic syndrome |
| • Renal failure/uremia |
| • Primary biliary cirrhosis |
| • Obstructive jaundice |
| • Diabetes mellitus |
| • Cushing's syndrome |
| • Obesity |
| • Alcohol consumption |
| • Autoimmune diseases [systemic lupus erythematosus (SLE), etc.] |
| • Drug-induced dyslipidemia (diuretics, β-blockers, steroids, estrogen, retinoic acid, cyclosporin, etc.) |
| • Pregnancy |
Step 2:
Step 2 consists of the diagnosis of each risk factor as well as additional assessments. It should be carried out using the five items in Table 4–15 as guidelines. Moreover, for any of the conditions, carotid ultrasonography, echocardiography, coronary computed tomography (CT), chest, and abdominal CT, magnetic resonance imaging (MRI), magnetic resonance (MR) angiography, brachial–ankle pulse wave velocity (baPVW), or cardiac-ankle vascular index (CAVI) should be performed as necessary.
Table 15. Pediatric FH diagnostic criteria.
| • Hyper-LDL cholesterolemia: LDL-C level of ≥ 140 mg/dL when untreated (If total cholesterol level is ≥ 220 mg/dL, measure the LDL-C level) |
| • Family history of FH or premature CAD (blood relative closer than the two parents) |
Excluding secondary hyperlipidemia, if two items are satisfied, FH is diagnosed.
During the growth phase, there are fluctuations in LDL-C; therefore, careful observation is required.
In pediatric cases, there are few clinical symptoms such as xanthomatosis; therefore, it is important to investigate the family history for FH. Use the family survey results of those beyond the parents as a reference if necessary.
Early CAD is defined as CAD with an onset at < 55 years of age for males and < 65 years of age in females, respectively
If xanthoma is present, LDL-C is suspected to be extremely high (homozygote).
For patients with hypertension (in-clinic blood pressure ≥ 140/90 mmHg or home blood pressure ≥ 135/85 mmHg), 24-hour blood pressure should be measured as necessary (differentiation of nocturnal hypertension and workplace hypertension).
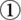 If DM is suspected and cannot be ruled out (HbA1c 5.6–6.4%, fasting blood glucose 100–125 mg/dL or random blood glucose 140–199 mg/dL, or a strong family history of DM or obesity), a 75-g OGTT should be performed (except when symptoms of DM are clearly present).
If DM is suspected and cannot be ruled out (HbA1c 5.6–6.4%, fasting blood glucose 100–125 mg/dL or random blood glucose 140–199 mg/dL, or a strong family history of DM or obesity), a 75-g OGTT should be performed (except when symptoms of DM are clearly present). If the patient is clearly diagnosed with DM, i.e., if both the HbA1c and blood glucose level are indicative of diabetes in the same blood test, the blood glucose level is indicative of diabetes and classic symptoms (thirst, polydipsia, polyuria, and weight loss) are present, diabetic retinopathy is present, or a test done on a different day was able to reconfirm findings indicative of diabetes (the blood glucose level must at least be indicative of diabetes in either the first test or re-test871)), funduscopy should be performed and the urine albumin/creatinine ratio (random spot urine quantification) should be assessed.
If the patient is clearly diagnosed with DM, i.e., if both the HbA1c and blood glucose level are indicative of diabetes in the same blood test, the blood glucose level is indicative of diabetes and classic symptoms (thirst, polydipsia, polyuria, and weight loss) are present, diabetic retinopathy is present, or a test done on a different day was able to reconfirm findings indicative of diabetes (the blood glucose level must at least be indicative of diabetes in either the first test or re-test871)), funduscopy should be performed and the urine albumin/creatinine ratio (random spot urine quantification) should be assessed.
For patients with dyslipidemia (LDL-C ≥ 140 mg/dL, HDL-C < 40 mg/dL, fasting TG ≥ 150 mg/dL, or non-HDL-C ≥ 170 mg/dL872)), in addition to checking for the presence of arcus corneae, Achilles tendon thickening, cutaneous/tendon xanthomas and eruptive xanthomas, lipoprotein agarose gel electrophoresis or polyacrylamide gel electrophoresis should be performed to measure the apoproteins (AⅠ, AⅡ, B, CⅡ, CⅢ, E), small dense LDL particles, Lp (a), remnant lipoprotein cholesterol particles, lipoprotein lipases, hepatic lipases, and lecithin–cholesterol acyltransferases (LCAT).
CKD is diagnosed if the patient's eGFR persistently remains at < 60 mL/min/1.73 m2 or proteinuria is present for ≥ 3 months153).
The diagnosis of metabolic syndrome should be based on the diagnostic criteria by the eight academic societies for internal medicine.
Fig. 8d.
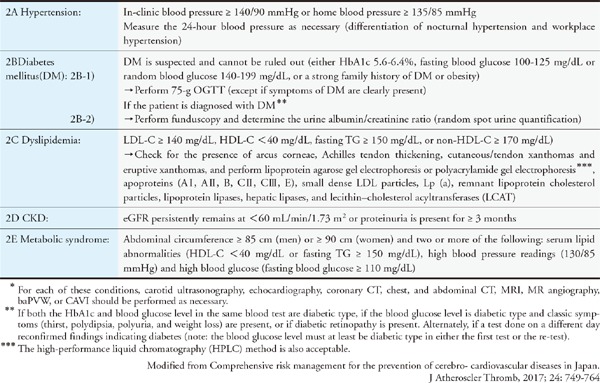
Step 2 Diagnosis of Each Risk Factor and Additional Items for Assessment*
Step 3:
Step 3 lists the risk factors that should be specifically taken note of for the prevention of cerebrovascular and cardiovascular diseases and before initiating treatment. These risk factors include: (1) smoking; (2) hypertension; (3) DM, including impaired glucose intolerance; (4) dyslipidemia; (5) CKD; (6) obesity, particularly visceral fat obesity; (7) aging and gender, i.e., men or postmenopausal women; and (8) family history, i.e., history or complication of cerebrovascular or cardiovascular diseases and lifestyle diseases (hypertension, DM, or dyslipidemia) in biological grandparents, biological parents, or blood siblings (especially cases of early incidence. It should always be kept in mind that strict management is necessary in cases where multiple risk factors are present (Fig. 8e).
Step 4:
• The risk factors for dyslipidemia are stratified as described in Chapter 4-1.
1) Patients with a history of CAD are considered for secondary prevention
2) Among primary prevention patients, those with any of the following are considered at high risk: (1) diabetes mellitus (excluding impaired glucose tolerance); (2) chronic kidney disease (CKD); (3) noncardiogenic cerebral infarction; or (4) peripheral arterial disease.
3) For primary prevention patients, if additional risks are absent, they should be stratified into low-, moderate- and high-risk based on the absolute risk derived from the Suita score or the total number of additional risk factors other than LDL-C, including: (1) smoking; (2) hypertension; (3) low-HDL-C; (4) impaired glucose tolerance; or (5) family history of early onset CAD (first-degree relatives aged < 55 years (men) or < 65 years (women) at the time of onset).
4) In primary prevention, the patient is considered to be at high risk if the LDL-C level is ≥ 180 mg/dL, regardless of the number of additional risk factors present.
• The management targets for hypertension are < 140/90 mmHg for patients aged < 75 years (home blood pressure < 135/85 mmHg), < 150/90 mmHg for those aged ≥ 75 years (home blood pressure < 145/85 mmHg), < 140/90 mmHg if drug tolerance is noted (home blood pressure < 135/85 mmHg), and < 130/80 mmHg for those complicated with diabetes or proteinuria-positive CKD (home blood pressure < 125/75 mmHg).
• An HbA1c reading < 6.0% is recommended if the aim of diabetes management is normalization of the blood glucose level. The recommended reading for preventing complications is HbA1c < 7.0%. If it is difficult to intensify the treatment, the recommended HbA1c reading is < 8.0%.
• For obesity management, the aim is to reduce body weight by 3–5%.
• The basis of metabolic syndrome is the excessive accumulation of visceral fat. Multiple risk factors of ASCVD are present in this condition. The correction of obesity, especially visceral fat, should be considered to be a management target. Likewise, for other diseases such as hyperuricemia, keep in mind the prevention of ASCVD by deciding on the appropriate therapy and management targets that are suited to each patient.
Fig. 8e.

Step 3 Risk Factors to be Reviewed before Initiating Treatment
Fig. 8f shows the management targets suitable for each risk factor and pathological condition. As shown in the table, the management targets for hypertension and DM are in accordance the guidelines of The Japanese Society of Hypertension122) and The Japan Diabetes Society871), respectively. However, particularly for the elderly, the management targets should be established with the circumstances of individuals taken into consideration, such as the activities of daily living (ADL), cognitive function, and QOL.
Fig. 8f.

Step 4 Setting Management Targets Suited to the Risk Factors for Each Pathological Condition*
As mentioned in Chapter 3-1, regarding the management of dyslipidemia for the young elderly aged ≥ 65 years but aged < 75 years, the risk for each patient is stratified into secondary prevention, high-risk, moderate risk, or low risk162). For all risk categories, the management target is ≥ 40 mg/dL for HDL-C and < 150 mg/dL for TG. With respect to LDL-C and non-HDL-C, the LDL-C management target for the low-risk category is < 160 mg/dL (non-HDL-C < 190 mg/dL). The LDL-C management target is < 140 mg/dL (non-HDL-C < 170 mg/dL) for the moderate-risk category and < 120 mg/dL (non-HDL-C < 150 mg/dL) for the high-risk category. A patient is at high risk if he/she has a history of or is complicated with DM, CKD, cerebral infarction, or PAD, regardless of age and gender. Similarly, a patient who has an LDL-C level ≥ 180 mg/dL is also considered to be at high risk regardless of the number of additional risk factors present. This section can be applied to the risk stratification for patients with secondary hyperlipidemia. Refer to “Chapter 7. The Elderly” for old old patients (≥ 75 years).
As mentioned in Chapter 3-2, the management targets for hypertension are < 140/90 mmHg (home blood pressure < 135/85 mmHg) for patients aged < 75 years, < 150/90 mmHg for those aged ≥ 75 years (home blood pressure < 145/85 mmHg), < 140/90 mmHg if drug tolerance is noted (home blood pressure of < 135/85 mmHg), and < 130/80 mmHg for those complicated with DM or proteinuria-positive CKD (home blood pressure < 125/75 mmHg).
For DM, an HbA1c reading < 6.0% is the recommended management target when the aim of diabetes management is to normalize the blood glucose level. For preventing complications, the recommended goal is HbA1c < 7.0%, and if it is difficult to intensify the treatment, the recommended goal is HbA1c < 8.0%. In lipid management, patients with DM are stratified into the high-risk category, and the management targets are LDL-C < 120 mg/dL and non-HDL-C < 150 mg/dL. However, for patients predicted to be at a particularly high risk for CAD, which include those who: (1) are complicated with FH; (2) are complicated with noncardiogenic cerebral infarction; (3) are complicated with PAD; (4) are complicated with microvascular complications (retinopathy, nephropathy, etc.); (5) have persistent poor glycemic control; (6) are complicated with metabolic syndrome (visceral fat obesity); (7) have multiple overlapping major risk factors, or (8) smoking, stricter management with compulsory achievement of the management targets is necessary. Overlapping conditions are considered to be particularly high-risk, and the same management targets as those for secondary prevention should therefore also be considered.
The aim of obesity management should be an improvement of hypertension, DM, and dyslipidemia by a 3–5% reduction in body weight369, 873). Visceral fat accumulation is considered an independent risk factor for CVD. It has been demonstrated in epidemiological studies in western countries as well as in Japan that the presence of multiple risk factors, such as metabolic syndrome, increases the risk for ASCVD (refer to Chapter 3-2, “Metabolic Syndrome”). Besides the management of each risk factor, emphasis should be placed on the reduction of visceral fat–the basis of metabolic syndrome–or in other words, the correction of obesity.
The serum uric acid level is a predictive factor for the future incidence of hypertension and is related to CKD incidence and progression874). An elevated serum uric acid level is believed to be associated with an increase in metabolic syndrome incidence. Therefore, therapeutic intervention should be considered for patients with a history of hypertension, DM, or CAD if the serum uric acid level is ≥ 8.0 mg/dL, even if they are asymptomatic with no gout attacks or gout stones. However, lifestyle modification should still be the basis for therapy875).
Step 5: Lifestyle Modification
Lifestyle modification is the core of ASCVD prevention, and physicians must refrain from initiating drug therapy without further consideration of lifestyle. Nondrug therapies should be continued during the course of treatment with drugs. In other words, guidance on lifestyle modification should not be neglected. The main improvements are shown in Fig. 8g.
Fig. 8g.

Step 5 Lifestyle modifications
With reference to and as mentioned in Chapter 3–4, among all the causes of ASCVD, smoking cessation is the easiest intervention. Regardless of gender, smoking cessation should be recommended for patients of all age groups to prevent ASCVD (Standard Procedure for Smoking Cessation Therapy Version 6, 2014 by The Japanese Circulation Society, The Japan Lung Cancer Society, Japanese Cancer Society, and The Japanese Respiratory Society). The increased risk of CAD in nonsmokers because of passive smoking is also a serious issue. Smoking cessation therapy is covered by health insurance if certain conditions are met876).
For obese patients (BMI ≥ 25), especially those with an accumulation of visceral fat resulting from metabolic syndrome, a weight loss ≥ 3% in 3–6 months should be the goal (refer to “4. Step 4” of this section). The optimization of energy and nutritional intake, and the correction of improper dietary habits and behaviors form the basis of treatment for patients with risk factors such as dyslipidemia, hypertension, DM, or obesity. High-fat meats, animal fats, and excessive alcohol consumption should be avoided while adopting a salt-reduced Japanese dietary pattern combined with an intake of fish, soy, vegetables, seaweed, mushrooms, fruits, and unrefined grains.
Exercise has been shown to result in improvements in dyslipidemia (increase in HDL-C level, etc.), decrease blood pressure, improve insulin resistance, and decrease blood glucose levels. Patients should aim to perform aerobic exercise of moderate intensity for ≥ 30 minutes a day, three times or more per week (daily if possible). Metabolic Equivalent of Task (MET) is a unit that expresses the intensity of exercise in terms of the equivalent number of times the metabolism is at rest, and moderate intensity is defined as an intensity of ≥ 3 METs. Normal walking is equivalent to 3 METs, while brisk walking is 4 METs and jogging is 7 METs; however, these values differ according to each individual's physical fitness. Patients who do not regularly exercise should be instructed to start with light and short exercises. However, patients complicated with hypertension are subjected to exercise therapy only if they have a moderate or lower blood pressure level (< 180/110 mmHg) and no CVD. Exercise therapy needs to be prohibited or restricted for diabetic patients suffering from DM with extremely poor metabolic control (fasting blood glucose ≥ 250 mg/dL or positivity for urine ketone bodies of moderate severity or higher), new occurrence of fundal hemorrhage because of proliferative retinopathy, CAD, or renal failure. If the patient is at high risk, a medical checkup should be performed beforehand and the opinion of a specialist should be sought on whether exercise therapy is possible or if there is a need for exercise restriction.
Step 6: Drug Therapy
Drug therapy (Fig. 8h) should be carefully initiated or continued depending on the individual risk and condition while continuously implementing lifestyle modification. Meanwhile, rigid drug therapy is necessary for high-risk cases. For the details on drug therapy for hypertension and DM, physicians should follow the guidelines for each disease122, 871).
Special attention should be paid to adverse drug reactions if the patient is ≥ 75 years old or has renal dysfunction877).
Fig. 8h.

Step 6 Drug Therapy*
Chapter 5. Familial Hypercholesterolemia
[Statement]
Familial hypercholesterolemia (FH) is a frequent autosomal hereditary disease associated with a high risk of CAD. Its early diagnosis and intensive treatment are recommended. (Evidence level: 3, Recommendation level: A)
For the treatment of heterozygous FH, strict lipid management, primarily with statin therapy, is recommended. (Evidence level: 3, Recommendation level: A)
For the treatment of homozygotes FH and drug therapy-resistant severe heterozygous FH, strict LDL cholesterol control by LDL apheresis is recommended. (Evidence level: 3, Recommendation level: A)
1. Pathophysiology and Clinical Features of FH
FH is an autosomal dominant disease with 3 major features: (1) hyper-LDL-cholesterolemia, (2) premature CAD and (3) tendon and skin xanthomas. FH is dominantly inherited except for autosomal recessive hypercholesterolemia (ARH), a very rare form. FH patients have hyper-LDL-cholesterolemia from their birth, resulting in having a considerably high risk of CAD. Untreated heterozygous FH (HeFH) men 30 to 50 years of age and women 50 to 70 years of age are likely to develop CAD such as myocardial infarction and angina pectoris878). Early diagnosis and intensive treatment as well as family screening (cascade screening) will contribute to the prevention of premature death in FH patients. HeFH patients in Japan are observed in one in 200–500 of the general population, similar to those in other countries, suggesting that there are over 300,000 patients. Accordingly, FH is the most frequently encountered genetic disease in daily practice. Its diagnosis and treatment in childhood are important when progression of atherosclerosis begins.
2. Causative Genes of FH
Genetic analysis is not necessarily required for a diagnosis of FH. However, in addition to hyper-LDL-cholesterolemia, the presence of mutation in the LDL receptor or other genes involved in LDL receptor pathway gives a definitive diagnosis of FH. FH is caused by pathogenic mutations in genes of LDL receptor, apolipoprotein B-100 (Apo B-100) and PCSK9 which play an important role in LDL receptor pathway. In 60–80% of clinically diagnosed HeFH have a mutation in these causative genes. Homozygous FH (HoFH) are defined as having 2 pathogenic mutations in 2 alleles of the causative genes. ARH is an extremely rare disease, caused by mutations in LDLRAP1 which protein is involved in LDL receptor uptake.
3. Diagnosis of FH
1). Diagnostic Criteria
Diagnostic criteria are shown in Table 14. When diagnosing FH, it is necessary to pay great attention to family history in the patient interview. This is especially noted for young patients because they have a less chance to have Achilles tendon thickening. When serious illness develops concomitantly, including acute myocardial infarction, there may be a temporary drop in LDL-C. Therefore, palpation should be performed to examine the Achilles tendon and survey of the family history must be conducted for all the patients with acute myocardial infarction.
Table 14. Diagnostic criteria for heterozygous FH in adults (15 years of age or older).
| • Hyper-LDL-cholesterolemia (an untreated LDL-C level ≥ 180 mg/dL) |
| • Tendon xanthomas (thickening of tendons on dorsal side of the hands, elbows, knees or Achilles tendon hypertrophy) or xanthoma tuberosum |
| • Family history of FH or premature CAD (within the patient's second-degree relatives) |
The diagnosis should be made after excluding secondary dyslipidemia.
If a patient meets two or more of the above-mentioned criteria, the condition should be diagnosed as FH. In case of suspected heterozygous FH, making a diagnosis using genetic testing is desirable.
Xanthelasma is not included in xanthoma tuberosum.
Achilles tendon hypertrophy is diagnosed if the Achilles tendon thickness is ≥ 9 mm on X-ray imaging. (See Appendix)
An LDL-C level of ≥ 250 mg/dL strongly suggests FH.
If a patient is already receiving drug therapy, the lipid level that led to treatment should be used as the reference for diagnosis.
Premature CAD is defined as the occurrence of CAD in men < 55 years of age or women < 65 years of age, respectively.
If FH is diagnosed, it is preferable to also examine the patient's family members.
These diagnostic criteria also apply to HoFH.
HoFH can be diagnosed based on clinical features: serum total cholesterol of 600 mg/dL or more, cutaneous xanthomas and ASCVD from childhood and parents' family history of heFH. Cutaneous and tendon xanthomas frequently occur in parts subjected to mechanical stimulation, such as the finger joints, elbow joints, knee joints and so on. If it is difficult to distinguish between HoFH and severe HeFH, genetic analysis is useful.
Diagnostic criteria for pediatric FH are shown in Table 15. As there are few physical symptoms such as xanthomas in pediatric HeFH, diagnosis has to be made on the basis of LDL-C and family history.
2). X-ray Examination of Achilles Tendon
Achilles tendon thickening is diagnosed when the greatest dimension is ≥ 9 mm. While it is also possible to use ultrasonography for evaluation, criteria have still to be standardized. (Refer to Achilles tendon radiography procedure in appendix).
3). Differential Diagnosis
Diseases that must be distinguished from FH include conditions that cause secondary dyslipidemia (e.g. diabetes mellitus, hypothyroidism, nephrotic syndrome, cholestatic liver disease, drug-induced diseases (due to steroids) and a similar disease, familial combined hyperlipidemia (FCHL). FCHL can be distinguished from FH by the absence of tendon xanthomas, the presence of small dense LDL, the presence of other types of dyslipidemia (type IIa, type IIb, type IV) in patient's family and elevation of LDL-C to a lesser extent than for FH patients.
4. Treatment of Heterozygous FH
1). Management Target Levels
Because FH is a disease associated with a very high risk of CAD, FH should be considered to correspond to secondary prevention, and it is desirable to set a management target for the LDL-C level at < 100 mg/dL. However, in many cases, it is difficult to achieve the target level in clinical practice. Therefore, it is also acceptable to aim for < 50% of the pretreatment level if the management target for LDL-C is not achieved.
In HeFH patients for secondary prevention, the LDL-C management target level is set at < 70 mg/dL because they can be considered to be at even higher risk.
However, there is no clear evidence for the validity of these numerical targets because clinical studies on FH without lipid-lowering therapy are ethically not permissible. The achievement of the management target does not always assure the absence of future cardiovascular events. In the treatment of FH, risk assessment cannot be applied using the risk charts provided in these guidelines.
2). Lifestyle Modification
Lifestyle modification should also be performed in FH patients as described in Chapter 4-2 Lifestyle Modification. However, due to the high risk of CAD, screening for CAD before administering exercise therapy is essential. CAD should be evaluated using patient interviews to determine the presence or absence of effort angina, and exercise electrocardiography and echocardiography should be performed. If the existence of CAD is suspected, administering treatment for CAD before initiating exercise therapy is thus preferred. Smoking cessation and obesity management are also important.
3). Drug Therapy
In many HeFH patients, adequate lipid management is not achieved through lifestyle habit interventions alone, so drug therapy is usually combined with them. Statins are the first-line drugs for FH treatment. A retrospective analysis of 329 HeFH patients conducted in Japan revealed that statin use delayed the incidence of CAD734). When sufficient efficacy is not obtained with the initial statin dose, increase the dosage to the maximum tolerated dose and co-administer with ezetimibe. If efficacy is still not adequate, PCSK9 inhibitors, resins and probucol are used (Fig. 9). When the attending physician determines that the risk is particularly high, such as in secondary prevention patients and those with underlying diabetes, LDL-C should be lowered as early as possible.
Fig. 9.
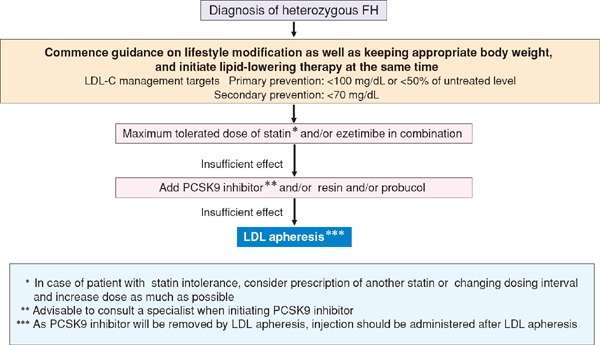
Treatment flow chart for adult (15 years or over) heterozygous FH
A retrospective study suggested that probucol delayed recurrence of CAD in HeFH. In addition, it has been reported that the addition of the PCSK9 inhibitor evolocumab (Rutherford-Ⅱ study879)) or alirocumab (Odyssey FHI and FHII studies880)) in HeFH patients already being treated with statin (and ezetimibe) the further lowering of LDL-C (approx 60%) and Lp(a) was achieved relatively safely. However, it remains unclear whether such combination therapies are more effective in suppressing cardiovascular events in FH patients as compared to statin alone.
4). Application of LDL Apheresis Therapy
In HeFH patients, LDL apheresis therapy should be considered if the total cholesterol (TC) level does not decrease to 250 mg/dL or below following lifestyle habit improvement and intensive drug therapy in the presence of CAD, LDL apheresis is indicated, and it is desirable to consult a specialist.
5. Treatment of Homozygous FH
1). Target Levels for Management
In HoFH, it is essential to start intensive therapy including lowering LDL-C as early as possible. LDL-C target levels for management in HoFH are < 100 mg/dL in primary prevention patients and < 70 mg/dL in secondary prevention patients but in many cases they are difficult to achieve.
2). Lifestyle Modification
Similar to that recommended for patients with HeFH, lifestyle modification, including diet therapy, exercise therapy, smoking cessation and obesity management, provides the basis for treatment in patients with HoFH, although intensive LDL-C-lowering treatment is necessary at an earlier age because patients with HoFH have an extremely high risk for the development and progression of CAD. It is necessary to assess CAD, valvular disease (aortic valve stenosis and supravalvular aortic stenosis in particular) and aortic aneurysm, and make a careful judgment based on the findings before giving any guidance on exercise therapy because progression of atherosclerosis is remarkable in HoFH patients.
3). Drug Therapy
In HoFH, in order to prevent the incidence and progression of CAD, intensive lipid-lowering therapy should be initiated as early as possible at an early age (Fig. 10). The major mechanisms of action of statins, bile acid adsorbing resins and PCSK9 inhibitors are to enhance expression (activation) of LDL receptors. For the defective type, in which only a small amount of LDL receptor activity remains, slight efficacy is observed but in the negative type in which LDL receptor activity is completely absent, no LDL-C lowering effect is observed881, 882). A retrospective study found that the administration of statin and other drugs was effective in reducing mortality rates in HoFH883). It has been reported that MTP inhibitors, which were developed for HoFH patients, lowered LDL-C by approximately 50%693, 884). However, as the frequencies of the adverse events of fatty liver and diarrhea are high, it is essential to control the fat and alcohol intake strictly. Probucol reportedly exerts a certain LDL-C lowering effect on HoFH and may cause the regression or disappearance of xanthoma in the skin or Achilles tendon885). Nevertheless, for LDL-C control, LDL apheresis therapy once every 1–2 weeks is still required in many cases. When patients are resistant to all of the above treatments or show intolerance, liver transplantation may be considered.
Fig. 10.
Treatment flow chart for adult (15 years or over) homozygous FH
4). LDL Apheresis in HoFH
In patients with HoFH, it is difficult to decrease the LDL-C level sufficiently using existing drug therapies, and many patients require continued LDL apheresis with extracorporeal circulation starting in childhood. Considering the inhibition of the progression of CAD, the earlier LDL apheresis is initiated, the better; however, it is difficult to perform LDL apheresis until the affected child can be kept in bed during apheresis. Realistically, the timing of treatment initiation is 4 to 6 years of age, when children can lie on bed and extracorporeal circulation can be performed; however, it is recommended that the treatment be initiated as early as possible.
5). Pregnancy and Delivery of Patients with HoFH
It is important to permit patients with HoFH to become pregnant as planned. Before pregnancy, screening for atherosclerosis should be performed using carotid ultrasonography, echocardiography and exercise tolerance tests to assess the status of atherosclerosis. By three months before the planned pregnancy, treatment with lipid-lowering drugs other than bile acid-binding resins should be discontinued. Because the cardiovascular system is greatly stressed during late pregnancy, particularly at delivery, performing LDL apheresis during pregnancy is desirable. LDL apheresis can also be safely administered during pregnancy.
6). HoFH Designated as an Intractable Disease
HoFH has been designated as an intractable disease in the Specified Disease Treatment Research Program since 2009. The criteria for designation are as follows: patients with HoFH definitively diagnosed using a genetic analysis of genes involved in the LDL metabolic pathway or measurement of the LDL receptor activity are definitively designated, and patients with remarkable hypercholesterolemia and those with cutaneous xanthoma starting in childhood who are refractory to drug treatment should be designated.
6. Treatment of Pediatric HeFH
When HeFH is diagnosed, patients should be provided with guidance regarding lifestyle modification such as diet and exercise as quickly as possible in order to reduce the risk for atherosclerosis by means of LDL-C lowering. Fig. 11 shows a treatment flow chart for pediatric HeFH886). For both boy and girl patients from the age of ≥ 10 years, pharmacotherapy needs to be considered if the LDL-C level is persistently above 180 mg/dL. Statins are the first line drugs starting from the minimal dose. In Japan, pitavastatin has been indicated for pediatric FH patients of ≥ 10 years since June 2015. The target LDL-C level should ideally be < 140 mg/dL. When there is a family history of premature CAD or diabetes is also present, ensure that the LDL-C level is kept below 140 mg/dL. While it will be difficult to achieve targets in serious cases, efforts should be made to achieve a level as close to the target as possible through combination drug therapy. Guidance on lifestyle modification including diet should be continued even after commencing drug therapy.
Fig. 11.
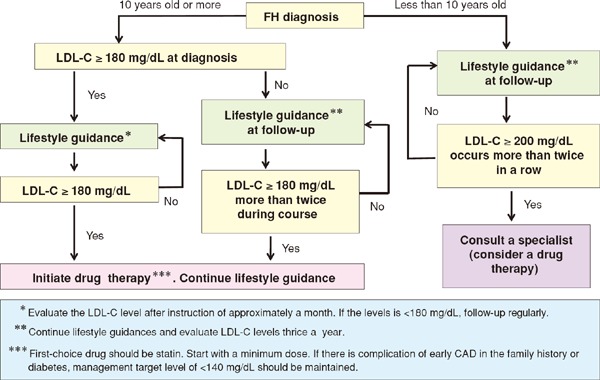
Algorithm for treatment of pediatric FH heterozygote
Chapter 6. Other Types of Primary Dyslipidemias
1. Primary Hyperlipidemias other than Familial Hypercholesterolemia
Besides FH, there are various types of primary hyperlipidemias which are caused by a mutation of single-gene and highly heritable. Classification of these diseases have been proposed based on their pathogenesis and/or and genetic abnormalities (Table 16). Familial lipoprotein lipase (LPL) deficiency manifests as severe hyperchylomicronemia and may present with eruptive cutaneous xanthomas or acute pancreatitis, although it does not necessarily accompany ASCVD. On the other hand, familial type Ⅲ hyperlipoproteinemia and FCHL are frequently associated with CVD; therefore, early diagnosis and initiation of treatment is important. It is recommended to refer the patients with these disorders to specialists. The clinically important diseases are described in depth below.
Table 16. Categories of primary dyslipidemias.
| Primary hyperlipidemia | Primary hyperchylomicronemia | Familial lipoprotein lipase deficiency GPIHBP1 deficiency LMF1 deficiency Apoprotein A-V deficiency Apoprotein C-Ⅱ deficiency Primary type V hyperlipidemia Others |
| Primary hypercholesterolemia | Familial hypercholesterolemia [LDL receptor deficiency or abnormality, PCSK9 abnormality, familial apoB100 abnormality, LDLRAP1 abnormality (autosomal recessive hypercholesterolemia), and others] Homozygous FH Heterozygous FH Polygenic hypercholesterolemia Familial combined hyperlipidemia |
|
| Familial type Ⅲ hyperlipidemia | Apoprotein E abnormality Apoprotein E deficiency |
|
| Primary hypertriglyceridemia | Familial type Ⅲ hyperlipidemia | |
| Primary high HDL cholesterolemia | CETP deficiency HL deficiency Others |
|
| Primary hypolipidemia | Abetalipoproteinemia (MTP abnormality) | |
| Familial low betalipoproteinemia (ApoB or PCSK9 abnormality) | ||
| Familial low HDL lipoproteinemia | Tangier disease Familial LCAT deficiency and fish eye disease Apoprotein A-Ⅰ deficiency Apoprotein A-Ⅰ abnormality Others |
|
| Other dyslipidemias | Sitosterolemia and cerebrotendinous xanthomatosis | |
2. FCHL
1). Causes
FCHL was initially proposed as hyperlipidemia with mixed phenotypes associated with high prevalence of myocardial infarction887). The patients with FCHL typically manifest type Ⅱb (or combined) hyperlipidemia reflecting the variously combined effects of genetic and acquired factors (e.g., lifestyle habits). The phenotype may change to type Ⅱa or Ⅳ hyperlipidemia in response to changes in diet or age. The first degree relatives of affected patients are commonly associated with type Ⅱa, Ⅱb or Ⅳ hyperlipidemia. FCHL, which was previously believed to be an autosomal dominant monogenic disorder, is now considered to be an oligogenic disease complicated with other multiple environmental factors888). Genetic analyses have linked various genes to FCHL: LPL, USF-1, apoproteins B, C-Ⅱ, A-Ⅰ/C-Ⅲ/A-Ⅳ gene cluster, LDLR, and PCSK9. Besides the genetic factors, environmental factors such as over-nutrition, obesity, and lack of physical activity are believed to contribute to the manifestation of FCHL. Moreover, the prevalence of this disease is as high as 1% of the general population, and was reported to be 0.4% of the general population of children in Japan889).
2). Clinical Symptoms
In patients with FCHL, the increase in the serum LDL-C levels is relatively mild compared with that observed in patients with FH. In contrast to FH, Achilles tendon thickening is not observed in patients with FCHL. The incidence of CAD in patients with FCHL is high, although not as high as that in patients with FH890, 891). In Japanese patients with FCHL, myocardial infarctions are commonly observed in men ≥ 35 years of age and women ≥ 55 years of age. FCHL was reported to be found in 32% of patients ≤ 65 years of age with myocardial infarctions in Japan892).
3). Laboratory Findings and Diagnosis
In patients with FCHL, the severity of hypercholesterolemia and hypertriglyceridemia is mild to moderate. FCHL is associated with an increase in the amount of apolipoprotein B and shift of the size of LDL to small (appearance of small dense LDL). The diagnosis should be made according to the diagnostic criteria of the Research Committee for Primary Hyperlipidemia, Research on Measures against Intractable Diseases established by the Japanese Ministry of Health, Labour and Welfare (Table 17). FCHL is diagnosed if apolipoprotein B100/LDL-C ratio is > 1.0 or presence of small dense LDL is confirmed using lipoprotein polyacrylamide gel disc electrophoresis (PAG). A survey of familial history is optional for this diagnosis.
Table 17. Diagnostic criteria of FCHL.
Criteria
|
|
Diagnosis The diagnosis is confirmed if all of the above criteria ((1) to (4)) are met. However, in daily practice, a diagnosis may simply be made if criteria (1) to (3) are met. |
(Cited from the 2000 report of the Research Committee for Primary Hyperlipidemia, Research on Measures Against Intractable Diseases established by the Japanese Ministry of Health, Labour and Welfare)
4). Treatment
Patients with FCHL should be treated according to a treatment guideline for FH. Lifestyle modification and obesity management achieved via dietary and exercise therapy are most important. Patients with FCHL respond well to dietary therapy, and the effects of drugs are greater than those observed in patients with FH. With respect to drug therapy, statins, fibrates and nicotinic acid derivatives are effective. The presence or absence of ASCVD, such as CAD, is a prognostic factor.
3. Familial Type Ⅲ Hyperlipidemia
1). Causes
Familial type Ⅲ hyperlipidemia, a hereditary type of hyperlipidemia also called broad β disease, is characterized by increased plasma levels of remnant lipoproteins, such as intermediate-density lipoprotein (IDL), chylomicron remnants and β-VLDL893, 894). Three isoforms are present for apoE, which is necessary for the hepatic uptake of remnant lipoproteins: wild-type E3 and isoforms E2 and E4. Familial type Ⅲ hyperlipidemia is caused by genetic abnormalities in apoE. Patients with familial type Ⅲ hyperlipidemia typically have APOE2/E2 genotype. Other mutations of APOE gene, such as APOE1, abnormal APOE3 and APOE deficiency are also known as the predisposing factor. In Western countries, the incidence of familial type Ⅲ hyperlipidemia is estimated to be approximately 0.4% and 0.2% for adult men and women, respectively895). The incidence of E2/E2 is estimated to be approximately 0.2% in Japan; however, only a small portion of the patients with E2/E2 develop familial type Ⅲ hyperlipidemia, with an incidence of 0.01–0.02% in a general population.
Abnormalities in apoE impairs the hepatic uptake of chylomicron remnants and IDL, leading to the accumulation of these lipoproteins in the blood. However, in many cases, remarkable hyperlipidemia does not develop despite the presence of apo E2/E2, other environmental conditions such as DM, obesity or hypothyroidism are necessary to develop hyperlipidemia.
2). Clinical Symptoms
Patients with familial type Ⅲ hyperlipidemia are characterized by the high incidence of typical xanthoma such as xanthoma striatum palmare and/or xanthoma tuberosum, and premature ASCVD [e.g., CAD, carotid atherosclerosis, renal arteriosclerosis or PAD] and may develop renovascular hypertension or intermittent claudication due to PAD. In Western countries, the risk of CAD is increased by five- to eight-fold896). The incidence of complications of CAD is also high in Japan897).
3). Laboratory Findings and Diagnosis
Both the serum TC and TG levels are raised in this patient population. However, the ranges of these parameters vary from slightly increased in patients with normal TC or TG levels to up to 500 mg/dL or 2,000 mg/dL, respectively. The diagnosis is made based on the diagnostic criteria of the Specific Disease Primary Hyperlipidemia Research Group of the Ministry of Health and Welfare (Table 18). This disease can be screened by a positive broad β pattern on lipoprotein polyacrylamide gel disc electrophoresis in patients with increases in both TC and TG and apoE/TC ratios of > 0.05. Other indicators, such as apoE/B > 0.20 and apoE/CⅢ > 1.0898), TC/apoB > 6.2 and TG/apoB < 10899), RLP-C/TG > 0.1895), non-HDL-C/apoB > 3900), and apoB48/TG > 0.11901), have also been proposed. LDL-C levels are low on lipoprotein analysis using ultracentrifugation or HPLC. Ultracentrifugation can be used to confirm a significant increase of cholesterol in IDL fraction (1.006 < d < 1.019) and high cholesterol/TG ratio (≥ 0.42) in the VLDL fractions (d < 1.006). Finally, isoelectric focusing, Western Blotting, or genetic analysis can be used to demonstrate an apoE isoform abnormality.
Table 18. Diagnostic criteria of familial type Ⅲ hyperlipidemia.
Major criteria
|
Minor criteria
|
Diagnosis
|
(Cited from the 1987 report of the Research Committee for Primary Hyperlipidemia, Research on Measures Against Intractable Diseases established by the Japanese Ministry of Health, Labour and Welfare)
4). Treatment
Dietary fat restriction is essential. Patients with familial type Ⅲ hyperlipidemia respond relatively well to lifestyle modification including dietary and exercise therapy; thus, early diagnosis and treatment are extremely important. Treatment of associating comorbidities, such as DM, obesity or hypothyroidism is also effective for treating dyslipidemia. With respect to drug therapy, fibrates are the first-line drugs; however, nicotinic acid derivatives and statins are also effective. The prognosis is favorable as long as diagnosis is appropriately made and treatment is initiated early. Periodic examinations is essential to prevent the development of CAD, carotid atherosclerosis and PAD. Consultations with specialists are also recommended.
4. Other primary dyslipidemias
Primary hyperchylomicronemia (e.g., familial LPL deficiency and apoC-Ⅱ deficiency) exhibits severe hypertriglyceridemia due to marked hyperchylomicronemia. In a typical case, primary hyperchylomicronemia manifests as type I hyperlipidemia; however, some patients may also exhibit type V hyperlipidemia. Primary hyperchylomicronemia often causes acute pancreatitis; therefore, a strict fat restriction is required (≤ 15–20 g/day). In hypertriglyceridemia accompanying ApoA-V gene abnormalities, it is necessary to prevent ASCVD. The following rare primary lipoprotein disorders have been listed as intractable diseases in 2015: sitosterolemia that are frequently associated with early-onset CAD, cerebrotendinous xanthomatosis, and Tangier disease were recently listed as Detailed diagnostic and treatment guidelines can be found at the Japan Intractable Diseases Information Center website under “Metabolic diseases” (www.nanbyou.or.jp/entry/504#04). PROLIPID (PROspective registry study of primary hyperLIPIDemia) study, which aims to investigate the prognostic outcome of three diseases of primary hyperlipidemia (Homozygous and heterozygous FH, hyperchylomicronemia and familial type Ⅲ hyperlipidemia), is currently underway since 2015.
Chapter 7. Elderly
[Statement]
As is the case for nonelderly adults, hyper-LDL cholesterolemia is an important risk factor of CAD among the elderly persons 65–74 years of age. (Evidence level: E-1a)
Statin therapy for hyper-LDL cholesterolemia in the elderly persons may be effective for the secondary prevention of CAD as is the case for nonelderly adults. (Evidence level: 1+, recommendation level: A)
Statin therapy for hyper-LDL cholesterolemia in the elderly persons 65–74 years of age may be effective for the primary prevention of CAD and non-cardiogenic cerebral infarction. (Evidence level: 1+, recommendation level: A)
The effects of lipid lowering therapy for hyper-LDL cholesterolemia in the primary prevention of CAD are not clear in the elderly patients ≥ 75 years of age, and should be handled for individual patients at the discretion of the attending physician. (Evidence level: 1)
1. Relationship between Dyslipidemia and ASCVD in the Elderly
As aging is a significant risk factor of ASCVD, the risk for ASCVD is higher in elderly people than in nonelderly ones. The prognosis after the incidence of cerebrovascular disease and CAD is also poor, which increases the risk for disability. Therefore, primary and secondary preventions are extremely important. However, because aging is a stronger risk factor than other risk factors associated with ASCVD, the weighting of other risk factors such as dyslipidemia become relatively less. Nevertheless, these risk factors should also be managed accordingly.
Epidemiological studies in Western countries have revealed that hyper-LDL cholesterolemia is also a risk factor for CAD among the elderly (primarily in the young elderly: 65–74 years of age) as well as in nonelderly adults902–907). Although, many of the studies in the elderly aged ≥ 75 years reported that there was no relationship between the LDL-C level and the risk of CAD908–910), a meta-analysis of 10 cohort studies in Japan (65,594 subjects, aged 70–89 years, EPOCH-JAPAN) reported that in men, total cholesterol ≥ 240mg/dL correlated with significantly increased death from CAD. No significant relationship was found in women. There was no significant relationship for stroke mortality in either sex55). Furthermore, according to the NIPPON DATA 90 cohort data relating to the relationship between non-HDL-C and ASCVD, a significant relationship was found between non-HDL-C and CAD in elderly subjects aged ≥ 65 years, but no significant relationship was found between non-HDL-C and cerebral infarction or stroke911). A meta-analysis of 61 prospective studies including approximately 900 thousand adult men and women in Europe and North America, confirmed the deaths of 55,000 subjects from cardiovascular events during the reported observation periods, such as CAD and cerebrovascular disease. Of these, a significant correlation was found between total serum cholesterol at the beginning of the observation period and death from CAD in the elderly (aged 70–89 years), although a relationship with stroke was not identified912). In a further meta-analysis of 29 cohort studies in the Asia-Pacific region, a significant positive correlation was found between total cholesterol and death from CAD in not only individuals < 60 years but also in the 60–74 year group and ≥ 75 year group; however, no relationship was identified between stroke and lipid levels913). As such, some independent cohort studies did not find a positive correlation between serum lipid levels and ASCVD in the elderly aged ≥ 65 years, but meta-analyses have reported positive correlations between CAD and total cholesterol, non-HDL-C and LDL-C. However, even meta-analyses have revealed no correlation between stroke and these lipid levels.
In the Japan Cholesterol and Diabetes Mellitus Study, an observational study including elderly Japanese people with diabetes, a significant relationship was found between CAD and HDL-C as well as the LDL-C/HDL-C ratio, and between cerebrovascular disease and HDL-C in the elderly aged ≥ 75 years; however, no relationship was found between LDL-C and non-HDL-C and either of these diseases914).
There is almost no clinical evidence related to the elderly aged ≥ 85 years, a cohort which is expected to become larger in the future. This is because such subjects are unlikely to participate in longitudinal studies. However, the Cardiovascular Health Study has shown that in the elderly aged ≥ 85 years, LDL-C was not a risk factor for cardiovascular events915). It appears that aging itself has a greater impact on the prognosis of this elderly age group.
2. The Efficacy of LDL-C Lowering Therapy for Preventing ASCVD in the Elderly
1). Preventive Effects of Statins on ASCVD
PROSPER, a large clinical study involving only elderly subjects, revealed that statin therapy was able to prevent CAD916). In this study, 3.2 years of statin administration on patients aged 70–82 years, including secondary prevention patients, lowered the primary endpoint (CAD death + nonfatal myocardial infarction + fatal and nonfatal stroke) by 15%, clearly demonstrating that intervention with statins could also be applied to the elderly. The reduction of CAD risk was more pronounced in men than in women as well as in secondary prevention patients than primary prevention, although no significant difference was observed in the interaction analysis.
Several meta-analyses by Cholesterol Treatment Trialists have been published, and showed the following: in the > 65 years group, the relative risk of statin therapy on major coronary artery events was 0.81 (95% confidence interval [CI]: 0.76–0.88] (2005)917), the relative risk on a major vascular event of statin and high-dose statin groups in subjects aged 66–75 years was 0.78 (95% CI: 0.74–0.83) relative to the control group or a low-dose statin group, and 0.84 (95% CI: 0.73–0.97) in the group > 75 years (2010). Both meta-analyses demonstrated that statin therapy showed increased effects than the control group, and that high-dose statins had greater effects than a low-dose statin group in preventing an event918).
A meta-analysis of 51,351 elderly subjects aged ≥ 60 years, including primary and secondary prevention patients, who received intervention with statins showed that statin therapy decreased total mortality by 15% (95% CI: 7–22%), death from CAD by 23% (95% CI: 15–29%), fatal and nonfatal myocardial infarction by 26% (95% CI: 22–30%), and fatal and nonfatal stroke by 24% (95% CI: 10–35%) compared to the placebo. However, the relative risk of cancer onset with statin therapy was 1.06 (95% CI: 0.95–1.18) relative to the placebo, and the difference was not statistically significant. There was no significant difference between statins and the placebo in terms of adverse events, such as a ≥ 3-fold increase of AST and ALT, ≥ 10-fold increase of CK or termination of the trial. However, muscle pain and gastrointestinal symptoms occurred significantly more often in the statin group. Moreover, new-onset diabetes was significantly higher in the statin group among those ≥ 65 years919). A meta-analysis of CAD mortality risk in a secondary prevention intervention trial, including elderly subjects aged ≥ 65 years, showed that the effects of secondary prevention on the elderly are far greater than that would be predicted from the outcomes in younger individuals920). As such, the secondary prevention effects of statin in the elderly with CAD history are evident and are recommended. The recently published J-STARS is a research study investigating the efficacy of 10 mg pravastatin on patients with ischemic stroke aged 45–80 years. Although no significant difference was found in terms of the primary endpoint, statins decreased the incidence of atherothrombotic cerebral infarction by 67%921). Considering the mean patient age of 66 years in this study, approximately half of the subjects were presumed to be elderly, and considering that the effects of statin therapy were observed regardless of age group, these results suggest that statins are capable of preventing the recurrence of atherothrombotic cerebral infarction in the elderly.
Meanwhile, the MEGA Study on primary prevention in the elderly showed that the concurrent risk of CAD and cerebral infarction decreased significantly in individuals aged ≥ 65 years [hazard ratio: 0.60 (0.39–0.93)], which was related to the efficacy of statin treatment in the elderly with high LDL-C922). Another meta-analysis, involving only primary prevention studies, also indicated that statin administration lowered the risk of all-cause mortality, major cardiovascular events and major cerebrovascular events923). Another meta-analysis of primary prevention including 24,674 elderly patients (mean age: 73 years, 43% women) confirmed that statins decreased myocardial infarction and stroke by approximately 40% and 25%, respectively924). The efficacy of statin treatment has thus been established, at least for primary prevention in younger elderly patients. Consequently, pharmaceutical therapy can be recommended on the basis of the risk factors present. However, there is still a lack of strong evidence for primary prevention in the elderly aged ≥ 75 years. As there is no method of risk assessment for elderly patients aged ≥ 75 years, as indicated in Chapter 2, treatment should be given, or dismissed, at the discretion of the attending physician. In addition, as geriatric syndrome complications, such as frailty and sarcopenia, are common in elderly aged ≥ 75 years, it is important to confirm whether patients in this particular group have adequate protein intake when dietary counseling is provided.
2). The Effects of Statins in Dementia Prevention
A selection of observational and interventional studies has attempted to investigate the effects of statin treatment on dementia prevention. However, many of these reports relate to subanalysis and secondary endpoints, and studies that set cognitive function as the primary endpoint involved sample sizes that were too small. An observational study suggested that statin treatment improves cognitive function925), many research results do not support these effects926, 927). A meta-analysis of interventional trials of four statins on patients with Alzheimer's disease patients in a recent Cochrane review, however, failed to demonstrate that statins could improve cognitive function928). Therefore, we can conclude that the effects of statins in the prevention and treatment of Alzheimer's disease are not positive. Although there is no research data relating to the assessment of statin effects on vascular dementia, we do know that stroke increases dementia risk by approximately 2-fold and that statins decrease the incidence of stroke; consequently, the preventive effects of statins on vascular dementia incidence can be expected. However, there are some case reports in which statins decreased cognitive function, and must, therefore, be approached with caution.
3). Frailty, a New Risk Factor that should be Considered in the Elderly
In the elderly, frailty is another factor which can require long-term care, much like dementia. In fact, there have been recent studies reporting that the incidence of cardiovascular events increases in elderly patients with frailty, or those who show vulnerability to acute stressors, a condition that appears with age. For example, White et al. conducted a prospective follow-up study dividing non-ST-elevating ACS patients aged ≥ 65 years into three groups: frail, pre-frail, and nonfrail groups using Fried criteria and found that the frail group had significantly higher primary endpoints of cardiovascular, myocardial infarction and stroke mortality than the nonfrail group (HR: 1.76; 95% CI: 1.36–2.28), and that all-cause mortality also increased significantly (HR: 1.98; 95% CI: 1.47–2.68)929). In another study, Sergi et al. divided 1,567 nonfrail elderly patients aged 65–96 years into three groups: a group meeting 1 Fried criterion, a group meeting 2 Fried criteria and a third group that did not meet any of the criteria; these groups were followed-up for 4.4 years in relation to CAD, heart failure, stroke, PAD and cardiovascular death as the primary endpoints. Even after adjustment for the confounding factors, event incidence occurred significantly more frequently in the groups meeting 1 or 2 Fried criteria; in other words, those in the pre-frail groups, compared to the robust group930). This suggested that frailty, characterized by low grip strength and slower walking speed, may represent a new risk factor for cardiovascular events in the elderly, and that there may be a need to make more comprehensive assessments that include geriatric syndromes such as frailty.
Sarcopenia is a pathological condition related to frailty. Sarcopenia is characterized by decreased muscle mass associated with age, which decreases muscular strength and walking speed, and has been suggested to not only cause falls and fractures but also exert impact on cardiovascular events931). Because statins cause muscle disorder as a side effect, it is concerned that long-term continuous use may be related to sarcopenia incidence. For example, Scott et al. showed that the use of statins in the community-dwelling elderly was linked to decreased leg muscle strength932); however, a subsequent meta-analysis by Krishnan et al. concluded that the earlier study by Scott et al. was lacking in convincing evidence933). Lynch et al. further reported that the use of statins in 3,422 elderly patients in rehabilitation (mean age = 81.4 years) was associated with an improvement in the activities of daily living934). As such, at present, there is simply not enough evidence to support the fact that statin treatment increases the risk of sarcopenia.
4). The Advantages and Disadvantages of Statin Treatment during End-of-Life Care
Sometimes doctors cannot decide whether or not a patient's medication should be continued or terminated, even during end-of-life care. Most clinical research tests the effects of pharmaceutical drugs by initiating them; however, Kutner et al. randomly assigned 381 patients estimated to have ≤ 1 year of life expectancy into two groups: one group for which treatment would be terminated and a second group that would continue taking the prescribed statin. These authors set death as the primary endpoint, and also studied quality of life (QOL) and financial effects. Results showed that there was no increase in deaths or cardiovascular events when statin treatment was terminated, and instead showed that QOL improved and that patients were able to save on their medical costs935). At the end of life, it is sometimes difficult to continue taking medication due to decreased appetite or dysphagia; hence, it is logical that being able to decrease oral medication in a safe manner will lead to an improvement in patient QOL.
Because statins in primary prevention are thought to take 3–4 years to have an effect upon the suppression of vascular events, the use of statins should only be considered in cases with at least ≥ 3 years of life expectancy.
3. Care of the Elderly
For preventing ASCVD in the elderly and maintaining QOL, it is important to manage dyslipidemia, particularly hyper-LDL cholesterolemia, which is an important risk factor. At the same time, it is important to consider the increase in secondary dyslipidemia, which occurs with complications such as hypothyroidism. There are many aspects to carefully consider in providing treatment to the elderly, such as the comorbidities other than ASCVD that may impact upon vital prognosis, latent organ disorder, atypical symptoms, decreased organ spare ability, decreased drug metabolism ability, malnutrition, frailty, polypharmacy, and due to variability in physical functions, careful considerations should be paid to treatment.
The basic treatment for dyslipidemia is dietary and exercise therapy for the elderly. Drug therapy should not be given at ease without implementing nonpharmaceutical therapies first. However, because adherence to strict diet therapies can worsen the nutritional state of the elderly, particularly in the latterstage elderly population, and because it is difficult for many to take on the same-level of physical activity as nonelderly adults for exercise therapy, it is best to make an intervention which is adapted to each individual's capacity. Pharmaceutical therapy should be prescribed with extreme care with the understanding that elderly patients are more prone to side effects.
Chapter 8. Women
[Statement]
It is important to manage risk factors, such as hypertension, diabetes, and smoking regardless of menopause status. In particular, diabetes and smoking are related to an increased the coronary artery disease (CAD) risk in women than in men. (Evidence level: E-1a)
The intensity of treatment for hypertension and diabetes should be personalized for each patient; however, smoking cessation is important for women across all age groups. (Evidence level: 2, recommendation level: A)
In premenopausal women, lifestyle modification is the most important aspect of dyslipidemia treatment. (Evidence level: 2, recommendation level: A)
Even before menopause, pharmacotherapy should be considered for high-risk women, such as those with familial hypercholesterolemia or CAD, and primary prevention subjects with high CAD risk. (Evidence level: 3, recommendation level: A)
Lifestyle modification is the fundamental aspect of treatment for dyslipidemia in postmenopausal women; however, pharmacotherapy should be considered in patients with high CAD risk. (Evidence level: 2, recommendation level: A)
1. Current Status of ASCVD in Japanese Women
According to the gender-specific causes of death in the 2014 Population Census of Japan, mortality rates for cardiovascular disease, including heart failure, and cerebrovascular disease in women is 26.8%, which is higher than the rate of 22.3% in men and the rate of 24.4% for malignant neoplasms936). However, myocardial infarction incidence is lower in women than in men937, 938) The epidemiological survey conducted in Japan between 1990 and the beginning of 2000 revealed that the age-adjusted incidence (100,000 persons/year) of myocardial infarction in women is 20–50% of that in men98, 163, 939, 940). Although the incidence rate of myocardial infarction increases after menopause, the rate is still lower than that in men163). According to the 2013 demographic statistics, mortality rate (per 100,000 persons) due to myocardial infarction in women is lower than that in men in the corresponding age groups: approximately 20% of the men's rate in the 50s, 20–30% in the 60s, and 37–48% even in their 70s936). In contrast, it has been reported that the mortality rate after a coronary event is higher in women than in men among both Western941–944) and Japanese subjects945, 946). Aging among Japanese women is progressing and is accompanied by an increased morbidity and mortality due to myocardial infarction163, 936). Thus, it is important to take future measures for prevention and care even in Japanese women.
Among the Japanese, the age-adjusted incidence of cerebral infarction is higher than that of myocardial infarction. However, the incidence in women is approximately 50–70% of that in men98, 939, 940, 947–949). The incidence rate of cerebral infarction in women increases with age, and it reaches 60–90% of that of the men for women aged ≥ 75 years. Compared to myocardial infarction incidence, the gender difference in the rate of cerebral infarction is small163, 948, 949). Mortality rate due to cerebral infarction in women in 2013 (per 100,000 persons) is also lower than that in men936). On the other hand, cross-sectional study of acute cerebral infarction reported that the duration of hospitalization is longer for women than for men and that patient status at the onset and after discharge is poorer for women than for men950).
Among the Japanese, cerebral infarction incidence is higher than that of myocardial infarction, and the gender differences in cerebral infarction incidence are smaller than that of myocardial infarction. Furthermore, proportion of older women continues to increase in Japan. Thus, the prevention and management of cerebral infarction among women is an important issue for the future.
2. Relationship between Risk Factors for Atherosclerosis and ASCVD in Women
1). Serum Lipids
Age-related changes in serum lipid levels are significantly different between men and women. Total cholesterol (TC) and low-density-lipoprotein cholesterol (LDL-C) levels are higher in men than in women until the fourth decade of their lives; however, because of menopause, the levels become higher in women than in men after the age ≥ 50 years85). High-densitylipoprotein cholesterol (HDL-C) levels are higher in women than in men among all age groups. Triglyceride (TG) levels are also higher in men than in women, but this increases with age in women, and the difference between genders diminishes after age 50 years85). It is likely that the changes in serum lipid levels, especially changes in LDL-C after menopause, may influence the ASCVD risk in women.
JALS-ECC337), a longitudinal epidemiological study reported that CAD risk was significantly higher in the high-TC group than in the low-TC group after adjustment for multiple confounding factors in women. CIRCS also showed that myocardial infarction risk after adjustment of multiple factors increase 1.42 times with each increase LDL-C by 30 mg/dl37).
EPOCH-JAPAN studied the relationship between TC levels and CAD mortality risk, and found that the risk was significantly higher in the high-TC group than in the low-TC group for women aged between 40 and 69 years951). The NIPPON DATA 80 also showed a significantly higher cardiovascular mortality risk among women with hypercholesterolemia group54). However, in the Ibaraki Prefectural Health Study, no significant correlation was found between LDL-C and CAD mortality38). Thus, cholesterol level is a significant risk factor for the CAD incidence among Japanese women, and also might be a risk factor related to CAD mortality.
The JPHC Study59) and EPOCH-JAPAN951) examined the relationship between TC and cerebral infarction risk, and concluded that no significant correlation existed among women.
Iso et al. reported that elevated TG level was also a significant risk factor for the incidence of myocardial infarction and ischemic cardiovascular diseases in women88, 93). In addition, JALS-ECC337) and CIRCS63) reported that non-HDL-C is a significant risk factor for CAD incidence but not CAD mortality66).
Thus, abnormalities in TC, LDL-C, TG, and non-HDL-C levels might be an important risk factors of CAD for Japanese women.
2). Smoking
The JPHC Study Cohort1786) and Suita Study108) found that the incidence rate of myocardial infarction was three to eight times higher among smokers than among nonsmokers even among women. In addition, CAD-related mortality risk was significantly higher in women smokers952, 953). Meta-analyses involving studies conducted in Japan have reported that the influence of smoking on CAD risk in women is greater than that in men954). JACSS, a multicenter collaborative research of acute coronary syndrome (ACS) in Japan, found that women who smoke had extremely higher ACS risk than men with smoking, with an odds ratio of 8.2 in women compared to that of 4.0 in men955).
Smoking is also a significant risk factor for cerebral infarction in women108). Moreover, passive smoking increases cerebral hemorrhage risk but not cerebral infarction risk among Japanese women956).
Thus, smoking should be considered as an important risk factor for CAD and cerebral infarction among Japanese women.
3). Hypertension
Epidemiological studies in Japan did not demonstrate that hypertension was a significant CAD risk factor in women957, 958), although there was a trend for increase the CAD risk958). However, it has been reported that hypertension was a significant risk factor for cerebral infarction incidence among women857, 957, 958). NIPPON DATA 80, which studied the relationship between hypertension and cardiovascular mortality risk, reported that the association between the two was significantly stronger for in younger women aged 30–59 years than in women aged ≥ 60 years959).
Thus, hypertension is an important risk factor for cerebral infarction among women, and it is necessary to treat hypertension from a younger age.
4). Diabetes
The JPHC136, 146, 960), Hisayama961), and Suita studies962) reported that CAD and cerebral infarction risk as well as the related mortality risk were significantly higher among patients with diabetes than among those without. The NIPPON DATA 80 showed that CAD mortality risk was high among older women whose random blood glucose ≥ 200 mg/dL161). JACCS reported that the odds ratio for myocardial infarction incidence for female diabetics was 6.12 compared to 2.90 for male diabetics, indicating a significant increased myocardial infarction risk among women954). In the meta-analyses, including the research in Japan, it was reported that CAD risk and all-stroke risk were 44%149) and 27%150) higher, respectively for diabetic women than for diabetic men.
3. Primary and Secondary ASCVD Prevention in Women
The fundamental strategy for ASCVD prevention is lifestyle modification. The Nurses' Health Study (NHS) in US revealed that the increasing of favorable lifestyle factors (e.g., smoking cessation, increase in physical activity, maintenance of ideal body weight, restriction of alcohol consumption, and healthy eating habits) were associated with lower risk of CAD963) and sudden cardiac deaths964). In addition, a collaborative analysis of NHS and Health Professionals Follow-up Study reported that the relative cerebral infarction risk among women who had all five factors described above was extremely low at 0.19, compared to women without any of favorable lifestyle factors965). Moreover, NHS, which included young women aged 27–44 years, showed that CAD incidence was decreased by 98% who had six factors (the above described five factors plus restricted TV-watching time) compared with women without any of these factors966). Thus, maintaining a healthy lifestyle from a younger age is quite important for preventing ASCVD in women.
Although effect of smoking on CAD risk is greater for women than for men953), this effect is diminished after smoking cessation108). Because smoking has a negative effect on pregnancy967) and smoking cessation decreases ASCVD risk regardless of age107), it is extremely important for women to stop smoking from a younger age.
Few large scale trials have investigated the primary prevention of CAD using statins in women. In the MEGA Study conducted in Japan, 68% subjects were postmenopausal women aged ≤ 70 years. In this study, statins did not decrease the risk of CAD and cerebral infarction significantly in women41). However, in the sub-analysis among women, risk of CAD combined with cerebral infarction was significantly decreased among the women aged ≥ 55 years620). In JUPITER, statins were administered to 3,426 female subjects, and the risks of unstable angina and reperfusion therapy were significantly decreased compared to the placebo group. However, significant risk reduction of myocardial infarction and cerebrovascular events was not observed in women with statin therapy968). In addition, the risk for the primary endpoints that include these events was significantly lower among the women aged ≥ 65 years but not in women aged < 65 years620). CTT reported that the significant risk reduction of CVD for 0.72 with each decrease in LDL-C by 38.7 mg/dL for men without history of vascular diseases. On the other hand, risk reduction was 0.85 for women, and this was not statistically significant179).
Because effect of statins on the primary prevention of ASCVD is not evident in women, the primary treatment strategy for women is lifestyle modification. However, drug therapy should be considered for women with FH and CAD as well as for primary prevention subjects who are at high CAD risk. There is little evidence that dyslipidemia increased CAD risk in premenopausal women. Thus, lifestyle modification is fundamental for treating dyslipidemia in premenopausal women after ruling out dyslipidemia due to secondary causes. A consensus has not been reached about the teratogenic risks on a fetus by statins during pregnancy662, 969, 970), and there is insufficient knowledge on the secretion of statins into breast milk. Thus, statin administration during pregnancy or lactation must be prohibited.
In secondary prevention, a meta-analysis of 11 studies, including 4S and CARE, reported that statins significantly decreased cardiovascular event risk to 0.82 in men and 0.81 in women971). The results of a CTT also showed a significant risk reduction of 0.84 for CVD with each decrease in LDL-C by 38.7 mg/dL in women with a history of vascular diseases, similar to that in men179). J-STARS investigated the effect of statins on the prevention of recurrent stroke in patients with cerebral infarction history. During 5-year study revealed that atherothrombotic cerebral infarction risk decreased significantly to 67%; however, risk reduction in women was not significant compared to that in men619).
Thus, it is necessary to treat women for the secondary prevention of CAD; however, the effect of statins on preventing the recurrence of cerebral infarction is not evident in women.
Although strict glycemic control contributes to the CAD prevention in diabetics972), it requires a longer duration to reveal a protective effect973, 974). In addition, its effect is small compared to the risk reduction seen for microvascular complications834). Because of increase in risk of hypoglycemia, strict glycemic control should be carefully considered by patients' conditions972, 975). The effect of diabetes on ASCVD incidence is greater for women than for men149, 150). Thus, it is important to initiate early comprehensive management, including risk factors other than hyperglycemia for ASCVD prevention.
Hypertension is an important risk factor for cerebral infarction in women and has been suggested to be a CAD risk factor. The number of hypertensive subjects increases with age both men and women, and the number of female patients surpasses that of males from age 60 years976). After menopause, LDL-C increases in women950) and ASCVD risk increases. Thus, hypertension management becomes important in postmenopausal women. Meta-analysis reported that there was no clear difference in the risk reduction of CVD by hypertension treatment between men and women977).
Intervention trial of hypertension among premenopausal women has not performed yet. Thus, lifestyle modification is quite important among premenopausal women after ruling out hypertension due to secondary causes. For the control of pregnancy-related and postmenopausal hypertension, the guidelines of the Japanese Society of Hypertension should be applied122).
Hormone-replacement therapy (HRT) is effective treating postmenopausal disorders and osteoporosis prevention. Multiple clinical trials on the relationship between HRT and CVD risk have been reported. In HERS, women who had CAD were given HRT, did not reveal any risk reduction of CAD or cerebrovascular disease978, 979). In a study including 16,608 postmenopausal women, Women's Health Initiative (WHI) examined the effect of HRT (conjugated estrogen + medroxyprogesterone acetate) on middle-aged and older US women who suffered from disorders that disturbed their quality of life. The results of this study showed a significant increase in cerebral infarction risk (1.44)980) and CAD (1.24) among women with HRT than among control women981). In this study, only women with conjugated estrogen replacement therapy showed a significant increase in cerebral infarction risk of 1.55982). Although, the increase in the risk of HRT-related CAD and cerebrovascular diseases are associated with age; there was no increased risk for either diseases among women aged < 60 years and the risk for CAD was relatively low982, 983).
The Japan Society of Obstetrics and Gynecology and Japan Society for Menopause and Women's Health developed a 2012 version of HRT guidelines984). The guidelines prohibit the use of HRT for patients who have a history of myocardial infarction, other CAD, and stroke. They also recommend the careful consideration of HRT when patients are obese, aged ≥ 60 years, have been postmenopausal for ≥ 10 years or have a history of coronary spasms and microvascular angina, severe hypertriglyceridemia, or uncontrollable diabetes or hypertension. To date, several studies have reported the negative effect of HRT on CVD risk; however, favorable effects of estrogen on lipid metabolism and vascular function have been established, and transdermal estrogen has been reported to significantly decrease myocardial infarction risk985). Thus, the benefits and safety of different types and dosages of estrogen and progestin not used in WHI, and the route of administration cannot be overlooked and further research is needed986).
Currently, CAD incidence among women is considerably lower in Japan than in Western countries938). A decrease in cerebrovascular disease incidence has also been observed because hypertension control938). On the other hand, new concerns related ASCVD risk such as westernized eating habits and decreasing of physical activity become apparent. Moreover, in a 2014 survey, 10–15% women in their 20s and 30s reported smoking987). Because women live longer than men, it is particularly important to introduce healthy lifestyle and control of risk factors of ASCVD from a younger age in woman.
Chapter 9. Children
[Statement]
Early detection and correct diagnosis of dyslipidemia are important. (Consensus, recommendation level: A)
In cases of familial hypercholesterolemia, attempt to identify new patients (children) in the family (cascade screening). (Consensus, recommendation level: A)
Provide patients with familial hypercholesterolemia with guidance on lifestyle habits, including diet, while considering the indication of drug therapy. (Consensus, recommendation level: A)
Nonpharmacological therapy is the main treatment for primary dyslipidemia other than familial hypercholesterolemia. (Consensus, recommendation level: A)
In cases of secondary dyslipidemia, adequately treat the primary disease. (Consensus, recommendation level: A)
It is recommended that children establish appropriate dietary and exercise habits and maintain proper body weight. (Consensus, recommendation level: A)
1. Early Detection of Dyslipidemia
Children do not have many opportunities to have their blood tested. If there is an opportunity to collect and test their blood, TC and TG should be tested at least once. Any abnormalities should be investigated in detail. For blood samples collected in a fasting state, LDL-C is calculated based on TC, TG, and HDL-C. For blood samples collected in a nonfasting state, LDL-C measured using the direct method and non-HDL-C should be used as a reference.
2. Criteria for Dyslipidemia in Children
Diagnostic criteria for dyslipidemia in children (i.e., elementary and junior high school students) are presented in Table 19. These values are derived from a report by Okada et al.988) based on a nationwide survey conducted in the 1990s. TC, LDL-C, and TG are based on the 95th percentile values, and HDL-C is based on the 5th percentile value. Although some age-related differences are seen, the entire group of children is represented by a single value. Recent studies have also reported that lipid levels do not substantially differ from the previously determined levels989). Regarding the reference value for non-HDL-C, the 95th percentile value is reported to be approximately 140 mg/dL in junior high school boys, whereas it is approximately 150 mg/dL in elementary school boys, as well as elementary and junior high school girls989).
Table 19. Diagnostic Criteria for Dyslipidemia in children (elementary and junior high school students) (fasting blood samples).
| Total cholesterol (TC) | ≥ 220 mg/dL |
| LDL cholesterol (LDL-C) | ≥ 140 mg/dL |
| Triglycerides (TG) | ≥ 140 mg/dL |
| HDL cholesterol (HDL-C) | < 40 mg/dL |
TC, LDL-C, and TG are derived from the 95th percentile values and HDL-C is derived from the 5th percentile value988).
Postprandial TG levels in children were studied by Kobayashi et al.990) during screening for lifestylerelated diseases and were found to increase within 1 h after eating and remained nearly constant until 3 h later, after which they decreased. The 95th percentile value for postprandial TG is approximately 200 mg/dL.
3. Primary Dyslipidemia
Primary hypercholesterolemia and primary hyperchylomicronemia are primary concerns for children. Type Ⅲ hyperlipidemia is considered to be rare in children.
1). Primary Hypercholesterolemia (Hyper-LDL-Cholesterolemia)
FH requires appropriate medical attention beginning from childhood. This is because LDL-C values become extremely high because of abnormalities in gene encoding for the LDL receptor system. Genetic testing results suggest that the incidence of FH is higher than that previously described. When a child is suspected of having FH, interviews to obtain a detailed family history and blood tests of family members (i.e., lipid tests) should be conducted to identify any newly affected children and adults in the family (e.g., cascade screening). It is recommended that proactive treatment be initiated in childhood because CAD often occur at a young age. When LDL-C is ≥ 180 mg/dL even with continuing guidance regarding lifestyle habits, it is recommended to consider drug therapy from approximately 10 years of age (See Chapter 5 “Familial Hypercholesterolemia”).
For FCHL, there is no evidence indicating the necessity of proactive drug therapy during childhood.
2). Primary Hypertriglyceridemia
It is important to detect primary hyperchylomicronemia, which can cause pancreatitis, particularly LPL deficiency, in children. Many reports have documented LPL gene mutations. Other possible causes include a deficiency of apolipoprotein C-II, which activates LPL, and mutations in apolipoprotein A-V genes.
Homozygotes have very severe hypertriglyceridemia (≥ 1,000 mg/dL). Diet therapy (fat intake restriction and the use of medium-chain fatty-acid milk for infants) is the primary form of treatment.
4. Secondary Dyslipidemia
Various causes are involved in the development of secondary dyslipidemia. Among these, obesity is frequently involved. In addition, thyroid hormones should always be tested when the cause is unclear because some patients with hypothyroidism (e.g., Hashimoto's thyroiditis) have severe hyper-LDL-cholesterolemia. Attention should also be paid to druginduced dyslipidemia.
Obese patients require treatment to reduce the degree of obesity. Hyper-LDL-cholesterolemia accompanying diabetes is also particularly problematic in children. Diabetes itself is a major risk factor for arteriosclerosis, and blood glucose control alone seems to be insufficient for preventing ASCVD (see Chapter 4 “Comprehensive Risk Management, 4-2) Diabetes”). The International Society for Pediatric and Adolescent Diabetes guidelines991) state lipid management targets of < 100 mg/dL for LDL-C, > 35 mg/dL for HDL-C, and < 150 mg/dL for TG. When LDL-C is above the target level, blood glucose control is intensified and diet and exercise therapies for hyperlipidemia are added. For hypo-HDL-cholesterolemia and hypertriglyceridemia, the basic approach is to improve lifestyle habits991). In Japan, the general consensus is that LDL-C should be maintained at least within the normal range (< 140 mg/dL); however, no explicit criteria are currently available for drug therapy for children992). Therefore, it is important to take care of children's diets and continue with strict blood glucose control.
5. Maintaining Proper Body Weight with Appropriate Diet and Exercise Habits
Pathological blood vessel changes associated with arteriosclerosis have been reported to gradually occur in childhood993, 994). It is important to prevent such changes from occurring and progressing as much as possible. To achieve this, it is important to develop correct lifestyle habits (e.g., diet) and maintain a proper body weight from childhood. Even in children, obesity causes problems such as abnormal blood test values (e.g., adipocytokine secretion) and blood pressure995, 996). Thus, obesity in children is also considered to promote ASCVD similar to that in adults, and attention paid to obesity during childhood helps reduce the risk of future lifestyle-related diseases.
The 2015 Dietary Reference Intakes for Japanese997) lists the desirable daily energy intake according to age and body size. Regarding nutritional balance, the target ratios of energy intake from fat and carbohydrates are 20–30% and 50–65%, respectively, which are same for all age groups (from 1 year to ≥ 70 years). Fat intake should be moderate because in recent years, due to the westernization of dietary habits, fat intake has increased. Therefore, it is recommended that individuals consume a well-balanced amount of fish, soybeans (products), vegetables, fruits, and seaweed, by utilizing Japanese food patterns as a main method, with no preference to a particular type of food. Care should also be taken to avoid excess salt intake.
Because BMI percentile (or standard deviation) method is inappropriate to evaluate overweight and obesity in children, because of the large variability in body height, it is better to assess the percentage of overweight (POW) based on comparison with standard body weight998). The POW is calculated as [(measured body weight–standard body weight)/standard body weight] × 100 (%). In general, elementary and junior high school students are classified as obese if the calculated POW is + 20% or higher (≥ 120% of the standard body weight)998). Like in obese adults, LDL-C and TG tend to be high in obese children, whereas HDL-C tends to be low. Even if the calculated POW is not indicative of severe obesity (≥ 50%), the child is considered to be “obesity disease”, a target of treatment for reducing the severity of obesity, when he or she exhibits any obesity-related complications996).
In obesity, the energy intake is increased and exceeds the necessary level; it should be reversed to normal levels. Obese children should increase their vegetable intake and avoid certain drinks and seasonings. The degree of obesity improves more easily during childhood because body height continues to increase. Moreover, an exercise habit should be established at the same time as dietary restrictions. In particular, adequate guidance should be given to children who are obese or not accustomed to exercise. In cases of severe obesity, energy intake may also need to be restricted.
Smoking is also an independent major risk factor for ASCVD, and smoking cessation is known to reduce the risk of developing such diseases. Because passive smoking has also been reported to increase the risk for CAD and diabetes, attention should be paid to smoking not only by the patient themselves but also by other family members.
Footnotes
Many studies performed in the Western countries consider a diet containing fat to ≤ 25% or to ≤ 30% of total energy intake as being a low-fat diet. Hence, the low-fat diet mentioned here refers to a dietary pattern in which the percentage of energy of fat is 30% or less of the total energy intake. It is necessary to take note that this is different from diets containing fat to 20–25% of total energy intake that are recommended in Japan and the fatrestricted diets for hyperchylomicronemia.
Footnotes
This is an English version of the Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017 published in Japanese in June 30, 2017.
Appendix 1. Physical Activity Guidelines for Health Promotion 2013
[Chapter 4 2-5. Exercise Therapy]
| Status on blood glucose/pressure/lipids | Physical activity (activity of daily living/exercise) | Exercise | Physical fitness (physical endurance) | |||
|---|---|---|---|---|---|---|
| Health checkup results are within the reference range | ≥ 65 years | Physical activity of any intensity for 40 min/day (10 METs·hour/week) | Increase even if slightly more than the current activity level (e.g., additional 10 min of walking) | - | Try to establish exercise habits (≥ 30 min for ≥ 2 days/week) | - |
| 18–64 years | Physical activity of ≥ 3 METs intensity3) for 60 min/day (23 METs·hour/week) | Exercise of ≥ 3 METs intensity for 60 min/ week (4 METs·hour/week) | Can continue exercise of intensity specified for sex/age range for approximately 3 min | |||
| < 18 years | - | - | - | |||
| Any of the blood glucose/pressure/lipids levels are at the recommended health guidance levels | Help the subject to assess his or her own physical condition before and during exercise; then, provide proactive exercise instructions as a part of health guidance if the subject is not visiting a medical institution and is confirmed to not have any risk according to “Screening sheet for physical activity risks.” | |||||
| Those with multiple risks or requiring immediate medical consultation | Patients with lifestyle-related diseases should consult their primary physicians before commencing active exercise because safety considerations are particularly important. | |||||
Adapted/modified from: Ministry of Health, Labour and Welfare “Japanese official physical activity guidelines for health promotion 2013 (outline)”
[Commentary]
“Japanese official physical activity guidelines for health promotion 2013” is the successor of “Exercise Guidelines for Health Promotion 2006,” revised to serve as a tool to help achieve the goal of Health Japan 21 (the second term).
“Physical activity” is defined as “all bodily movement that accompanies energy expenditure above resting energy expenditure” including both “activities of daily living” and “exercise.”
Guidelines on “physical activity” now include the all-generation direction of “ + 10 (add 10-min of activity to the current life)” in addition to age-specific criteria.
For exercise, performing “exercise” for ≥ 30 min at least 2 days/week in addition to “activities of daily living” was also shown to be desirable for individuals other than those in the 18–64 years age range.
In the “Japanese official physical activity guidelines for health promotion 2013,” messages are sent to those who are likely to be at risk in the future and those who are currently at risk.
Appendix 2. Exercise Guidelines for Health Promotion 2006
[Chapter 4 2–5. Exercise Therapy]
Adapted/modified from: Ministry of Health, Labour and Welfare “Exercise guidelines for health promotion 2006”
Appendix 3. Method for Achilles Tendon Radiography
[Chapter 5: Diagnosis of familial hypercholesterolemia]
Imaging position
In the sitting and lateral positions, attach the lower thigh and lateral malleolus side of the ankle to the receiver surface so that the lower thigh and the sole form a 90° angle. Use radiographic aids whenever possible to improve reproducibility and accuracy of the position because extension, flexion, medial rotation, and external rotation of the ankle affect the measurements of Achilles tendon hypertrophy.
Imaging conditions
When using a digital system, use 50 kV and 5.0 mAs (e.g., 100 mA × 0.05 s, 50 mA × 0.1 s). Increase or decrease the mAs value as needed.
Imaging distance (distance from the focus of the X-ray tube to the X-ray receiver surface)
Use a distance of 120 cm to eliminate the impact of the magnification ratio on X-ray images as much as possible. If possible, take images with a lead scale or the like (known size and radio-opaque) placed at the same height as that of the Achilles tendon to correct the magnification ratio.
X-ray central line
The position of incidence is the posterior margin of the medial malleolus of the tibia perpendicular to the receiver surface.
Image processing conditions
Conditions for which it is possible to clearly detect the Achilles tendon, adipose tissue, and skin are recommended when a digital X-ray imaging system is used for evaluation.
Achilles tendon hypertrophy measurement
When measuring on an image reference terminal, measure the most thickened part of the Achilles tendon using a measurement tool. When measuring on a film, measure the thickening on an actual-size output using a caliper or a ruler. The development of measurement software programs for improved measurement accuracy is required.
References
- 1). The joint committee of “The Japan Academy of Neurosonology” and “The Japan Society of Embolus Detection and Treatment” on guideline for nuerosonology. Carotid ultrasound examination. Neurosonology, 2006; 19: 49-67 (in Japanese) [Google Scholar]
- 2). Subcommittee for preparing guidelines for ultrasound diagnosis of carotid artery. Standard method for ultrasound evaluation of carotid artery lesions. Jpn J Med Ultrasonics, 2009; 36: 501-518 (in Japanese) [Google Scholar]
- 3). Subcommittee for preparing guidelines for ultrasound diagnosis of carotid artery. Standard method for ultrasound evaluation of carotid artery lesions 2017. Jpn J Med Ultrasonics, 2018. https://www.jsum.or.jp/committee/diagnostic/pdf/jsum0515_guideline.pdf (in Japanese) [Google Scholar]
- 4). Homma S, Hirose N, Ishida H, Ishii T, Araki G. Carotid plaque and intima-media thickness assessed by b-mode ultrasonography in subjects ranging from young adults to centenarians. Stroke, 2001; 32: 830-835 [DOI] [PubMed] [Google Scholar]
- 5). Nezu T, Hosomi N, Aoki S, Matsumoto M. Carotid intima-media thickness for atherosclerosis. J Atheroscler Thromb, 2016; 23: 18-31 [DOI] [PubMed] [Google Scholar]
- 6). O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular health study collaborative research group. N Engl J Med, 1999; 340: 14-22 [DOI] [PubMed] [Google Scholar]
- 7). del Sol AI, Moons KG, Hollander M, Hofman A, Koudstaal PJ, Grobbee DE, Breteler MM, Witteman JC, Bots ML. Is carotid intima-media thickness useful in cardiovascular disease risk assessment? The Rotterdam Study. Stroke, 2001; 32: 1532-1538 [DOI] [PubMed] [Google Scholar]
- 8). Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and metaanalysis. Circulation, 2007; 115: 459-467 [DOI] [PubMed] [Google Scholar]
- 9). Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS. Mannheim carotid intimamedia thickness and plaque consensus. (2004–2006–2011)An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis, 2012; 34: 290-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Ultrasonic diagnostic criteria committee of aortic and peripheral arterial lesions. Standard method for ultrasound evaluation of aortic and peripheral arterial lesions. Jpn J Med Ultrasonics, 2014; 41: 405-414 (in Japanese) [DOI] [PubMed] [Google Scholar]
- 11). Mizuta R, Kubota Y, Takeshita S, Akutsu H, Nakamoto F, Tanaka N, Sano M, Okajima T, Tsutsumi Y. Transit time of vessel flow in below-knee is useful for screening of vessel in below-knee lesions. Japanese journal of medical ultrasound technology, 2009; 34: 543-547 (in Japanese) [Google Scholar]
- 12). Japanese Society of Nephrology. Evidence-based clinical practice guideline for CKD 2009. (in Japanese) [Google Scholar]
- 13). Subcommittee for preparing guidelines on ultrasound diagnosis of renal arteries. Guidelines on methods of standard assessment of ultradound diagnosis of renal arterial lesions. Jpn J Med Ultrasonics, 2015; 42: 185-200 (in Japanese) [Google Scholar]
- 14). Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, Decramer I, Van Hoe LR, Wijns W, Hunink MG. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: Meta-analysis. Radiology, 2007; 244: 419-428 [DOI] [PubMed] [Google Scholar]
- 15). Schroeder S, Achenbach S, Bengel F, Burgstahler C, Cademartiri F, de Feyter P, George R, Kaufmann P, Kopp AF, Knuuti J, Ropers D, Schuijf J, Tops LF, Bax JJ, Working Group Nuclear Cardiology and Cardiac CT; European Society of Cardiology; European Council of Nuclear Cardiology Cardiac computed tomography: Indications, applications, limitations, and training requirements: Report of a writing group deployed by the working group nuclear cardiology and cardiac CT of the European society of cardiology and the European council of nuclear cardiology. Eur Heart J, 2008; 29: 531-556 [DOI] [PubMed] [Google Scholar]
- 16). Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter accuracy (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial. J Am Coll Cardiol, 2008; 52: 1724-1732 [DOI] [PubMed] [Google Scholar]
- 17). Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med, 2008; 359: 2324-2336 [DOI] [PubMed] [Google Scholar]
- 18). The Japanese Circulation Society. Guidelines for noninvasive vascular function test (JCS 2013). 2013. http://www.j-circ.or.jp/guideline/pdf/JCS2013_yamashina_h.pdf (in Japanese)
- 19). Carter SA. Indirect systolic pressures and pulse waves in arterial occlusive diseases of the lower extremities. Circulation, 1968; 37: 624-637 [DOI] [PubMed] [Google Scholar]
- 20). Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Br J Surg, 1969; 56: 676-679 [DOI] [PubMed] [Google Scholar]
- 21). Tomiyama H, Matsumoto C, Shiina K, Yamashina A. Brachial-ankle pwv: Current status and future directions as a useful marker in the management of cardiovascular disease and/or cardiovascular risk factors. J Atheroscler Thromb, 2016; 23: 128-146 [DOI] [PubMed] [Google Scholar]
- 22). Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement--a survey of 12517 subjects. Atherosclerosis, 2003; 166: 303-309 [DOI] [PubMed] [Google Scholar]
- 23). Yamashina A, Tomiyama H, Arai T, Koji Y, Yambe M, Motobe H, Glunizia Z, Yamamoto Y, Hori S. Nomogram of the relation of brachial-ankle pulse wave velocity with blood pressure. Hypertens Res, 2003; 26: 801-806 [DOI] [PubMed] [Google Scholar]
- 24). Ohnishi H, Saitoh S, Takagi S, Ohata J, Isobe T, Kikuchi Y, Takeuchi H, Shimamoto K. Pulse wave velocity as an indicator of atherosclerosis in impaired fasting glucose: The Tanno and Sobetsu study. Diabetes Care, 2003; 26: 437-440 [DOI] [PubMed] [Google Scholar]
- 25). Saiki A, Sato Y, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N, Kawana H, Nagumo A, Nagayama D, Ohira M, Endo K, Tatsuno I. The role of a novel arterial stiffness parameter, cardio-ankle vascular index (CAVI), as a surrogate marker for cardiovascular diseases. J Atheroscler Thromb, 2016; 23: 155-168 [DOI] [PubMed] [Google Scholar]
- 26). Ogawa T, Shimada M, Ishida H, Matsuda N, Fujiu A, Ando Y, Nitta K. Relation of stiffness parameter beta to carotid arteriosclerosis and silent cerebral infarction in patients on chronic hemodialysis. Int Urol Nephrol, 2009; 41: 739-745 [DOI] [PubMed] [Google Scholar]
- 27). Wada T, Kodaira K, Fujishiro K, Maie K, Tsukiyama E, Fukumoto T, Uchida T, Yamazaki S. Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. J Arterioscler Thromb, 1994; 14: 479-482 [DOI] [PubMed] [Google Scholar]
- 28). Shirai K, Hiruta N, Song M, Kurosu T, Suzuki J, Tomaru T, Miyashita Y, Saiki A, Takahashi M, Suzuki K, Takata M. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: Theory, evidence and perspectives. J Atheroscler Thromb, 2011; 18: 924-938 [DOI] [PubMed] [Google Scholar]
- 29). Takenaka T, Hoshi H, Kato N, Kobayashi K, Takane H, Shoda J, Suzuki H. Cardio-ankle vascular index to screen cardiovascular diseases in patients with end-stage renal diseases. J Atheroscler Thromb, 2008; 15: 339-344 [DOI] [PubMed] [Google Scholar]
- 30). Sato Y, Nagayama D, Saiki A, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N, Kawana H, Nagumo A, Ohira M, Endo K, Kurosu T, Tomaru T, Shirai K, Tatsuno I. Cardio-ankle vascular index is independently associated with future cardiovascular events in outpatients with metabolic disorders. J Atheroscler Thromb, 2016; 23: 596-605 [DOI] [PubMed] [Google Scholar]
- 31). Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R, Force International Brachial Artery Reactivity Task Force Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the international brachial artery reactivity task force. J Am Coll Cardiol, 2002; 39: 257-265 [DOI] [PubMed] [Google Scholar]
- 32). Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol, 1994; 24: 1468-1474 [DOI] [PubMed] [Google Scholar]
- 33). Den Ruijter HM, Peters SA, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, Engstrom G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Koffijberg H, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O'Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Witteman JC, Moons KG, Bots ML. Common carotid intima-media thickness measurements in cardiovascular risk prediction: A metaanalysis. JAMA, 2012; 308: 796-803 [DOI] [PubMed] [Google Scholar]
- 34). Kadota A, Miura K, Okamura T, Fujiyoshi A, Ohkubo T, Kadowaki T, Takashima N, Hisamatsu T, Nakamura Y, Kasagi F, Maegawa H, Kashiwagi A, Ueshima H, SESSA Research Group, NIPPON DATA80/90 Research Group Carotid intima-media thickness and plaque in apparently healthy Japanese individuals with an estimated 10-year absolute risk of CAD death according to the Japan Atherosclerosis Society (JAS) guidelines 2012: The Shiga Epidemiological Study of Subclinical Atherosclerosis (SESSA). J Atheroscler Thromb, 2013; 20: 755-766 [DOI] [PubMed] [Google Scholar]
- 35). Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, Inoguchi T, Maeda Y, Kohara K, Tabara Y, Nakamura M, Ohkubo T, Watada H, Munakata M, Ohishi M, Ito N, Nakamura M, Shoji T, Vlachopoulos C, Yamashina A, Collaborative Group for J-BAVEL (Japan Brachial-Ankle Pulse Wave Velocity Individual Participant Data Meta-Analysis of Prospective Studies) Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: An individual participant data meta-analysis. Hypertension, 2017; 69: 1045-1052 [DOI] [PubMed] [Google Scholar]
- 36). Imamura T, Doi Y, Arima H, Yonemoto K, Hata J, Kubo M, Tanizaki Y, Ibayashi S, Iida M, Kiyohara Y. LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population: The Hisayama study. Stroke, 2009; 40: 382-388 [DOI] [PubMed] [Google Scholar]
- 37). Imano H, Noda H, Kitamura A, Sato S, Kiyama M, Sankai T, Ohira T, Nakamura M, Yamagishi K, Ikeda A, Shimamoto T, Iso H. Low-density lipoprotein cholesterol and risk of coronary heart disease among Japanese men and women: The Circulatory Risk in Communities Study (CIRCS). Prev Med, 2011; 52: 381-386 [DOI] [PubMed] [Google Scholar]
- 38). Noda H, Iso H, Irie F, Sairenchi T, Ohtaka E, Ohta H. Gender difference of association between LDL cholesterol concentrations and mortality from coronary heart disease amongst Japanese: The Ibaraki Prefectural Health Study. J Intern Med, 2010; 267: 576-587 [DOI] [PubMed] [Google Scholar]
- 39). Yokokawa H, Yasumura S, Tanno K, Ohsawa M, Onoda T, Itai K, Sakata K, Kawamura K, Tanaka F, Yoshida Y, Nakamura M, Terayama Y, Ogawa A, Okayama A. Serum low-density lipoprotein to high-density lipoprotein ratio as a predictor of future acute myocardial infarction among men in a 2.7-year cohort study of a Japanese northern rural population. J Atheroscler Thromb, 2011; 18: 89-98 [DOI] [PubMed] [Google Scholar]
- 40). Noda H, Iso H, Irie F, Sairenchi T, Ohtaka E, Doi M, Izumi Y, Ohta H. Low-density lipoprotein cholesterol concentrations and death due to intraparenchymal hemorrhage: The Ibaraki Prefectural Health Study. Circulation, 2009; 119: 2136-2145 [DOI] [PubMed] [Google Scholar]
- 41). Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y, MEGA Study Group Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA study): A prospective randomised controlled trial. Lancet, 2006; 368: 1155-1163 [DOI] [PubMed] [Google Scholar]
- 42). The Kyusyu Lipid Intervention Study Group, Pravastatin use and risk of coronary events and cerebral infarction in Japanese men with moderate hypercholesterolemia: The Kyushu Lipid Intervention Study. J Atheroscler Thromb, 2000; 7: 110-121 [DOI] [PubMed] [Google Scholar]
- 43). Ito H, Ouchi Y, Ohashi Y, Saito Y, Ishikawa T, Nakamura H, Orimo H. A comparison of low versus standard dose pravastatin therapy for the prevention of cardiovascular events in the elderly: The Pravastatin Anti-atherosclerosis Trial in the Elderly (PATE). J Atheroscler Thromb, 2001; 8: 33-44 [DOI] [PubMed] [Google Scholar]
- 44). Chikamori T, Sugimoto K, Hamada T, Kitaoka H, Furuno T, Seo H, Doi Y. Efficacy of cholesterol-lowering treatment in Japanese elderly patients with coronary artery disease and normal cholesterol level using 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor. J Cardiol, 2000; 35: 95-101 [PubMed] [Google Scholar]
- 45). Saito I, Iso H, Kokubo Y, Inoue M, Tsugane S. Metabolic syndrome and all-cause and cardiovascular disease mortality: Japan Public Health Center-based prospective (JPHC) study. Circ J, 2009; 73: 878-884 [DOI] [PubMed] [Google Scholar]
- 46). Noda H, Iso H, Saito I, Konishi M, Inoue M, Tsugane S, JPHC Study Group The impact of the metabolic syndrome and its components on the incidence of ischemic heart disease and stroke: The Japan Public Health Center-based study. Hypertens Res, 2009; 32: 289-298 [DOI] [PubMed] [Google Scholar]
- 47). Tsukinoki R, Okamura T, Watanabe M, Kokubo Y, Higashiyama A, Nishimura K, Takegami M, Murakami Y, Okayama A, Miyamoto Y. Blood pressure, low-density lipoprotein cholesterol, and incidences of coronary artery disease and ischemic stroke in Japanese: The Suita study. Am J Hypertens, 2014; 27: 1362-1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Kodama K, Sasaki H, Shimizu Y. Trend of coronary heart disease and its relationship to risk factors in a Japanese population: A 26-year follow-up, Hiroshima/Nagasaki study. Jpn Circ J, 1990; 54: 414-421 [DOI] [PubMed] [Google Scholar]
- 49). Konishi M, Iso H, Iida M, Naito Y, Sato S, Komachi Y, Shimamoto T, Doi M, Ito M. Trends for coronary heart disease and its risk factors in Japan: epidemiologic and pathologic studies. Jpn Circ J, 1990; 54: 428-435 [DOI] [PubMed] [Google Scholar]
- 50). Okamura T, Tanaka H, Miyamatsu N, Hayakawa T, Kadowaki T, Kita Y, Nakamura Y, Okayama A, Ueshima H. The relationship between serum total cholesterol and all-cause or cause-specific mortality in a 17.3-year study of a Japanese cohort. Atherosclerosis, 2007; 190: 216-223 [DOI] [PubMed] [Google Scholar]
- 51). Tanabe N, Iso H, Okada K, Nakamura Y, Harada A, Ohashi Y, Ando T, Ueshima H, Japan Arteriosclerosis L Serum total and non-high-density lipoprotein cholesterol and the risk prediction of cardiovascular events - the JALS-ECC. Circ J, 2010; 74: 1346-1356 [DOI] [PubMed] [Google Scholar]
- 52). Wakugami K, Iseki K, Kimura Y, Okumura K, Ikemiya Y, Muratani H, Fukiyama K. Relationship between serum cholesterol and the risk of acute myocardial infarction in a screened cohort in Okinawa, Japan. Jpn Circ J, 1998; 62: 7-14 [DOI] [PubMed] [Google Scholar]
- 53). Kitamura A, Iso H, Naito Y, Iida M, Konishi M, Folsom AR, Sato S, Kiyama M, Nakamura M, Sankai T, Shimamoto T, Komachi Y. High-density lipoprotein cholesterol and premature coronary heart disease in urban Japanese men. Circulation, 1994; 89: 2533-2539 [DOI] [PubMed] [Google Scholar]
- 54). Sugiyama D, Okamura T, Watanabe M, Higashiyama A, Okuda N, Nakamura Y, Hozawa A, Kita Y, Kadota A, Murakami Y, Miyamatsu N, Ohkubo T, Hayakawa T, Miyamoto Y, Miura K, Okayama A, Ueshima H. Risk of hypercholesterolemia for cardiovascular disease and the population attributable fraction in a 24-year Japanese cohort study. J Atheroscler Thromb, 2015; 22: 95-107 [DOI] [PubMed] [Google Scholar]
- 55). Nagasawa SY, Okamura T, Iso H, Tamakoshi A, Yamada M, Watanabe M, Murakami Y, Miura K, Ueshima H. Relation between serum total cholesterol level and cardiovascular disease stratified by sex and age group: A pooled analysis of 65 594 individuals from 10 cohort studies in Japan. J Am Heart Assoc, 2012; 1: e001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Okumura K, Iseki K, Wakugami K, Kimura Y, Muratani H, Ikemiya Y, Fukiyama K. Low serum cholesterol as a risk factor for hemorrhagic stroke in men: A community-based mass screening in Okinawa, Japan. Jpn Circ J, 1999; 63: 53-58 [DOI] [PubMed] [Google Scholar]
- 57). Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Kondo T, Watanabe Y, Koizumi A, Inaba Y, Tamakoshi A. Serum total cholesterol levels and risk of mortality from stroke and coronary heart disease in Japanese: The JACC study. Atherosclerosis, 2007; 194: 415-420 [DOI] [PubMed] [Google Scholar]
- 58). Tanizaki Y, Kiyohara Y, Kato I, Iwamoto H, Nakayama K, Shinohara N, Arima H, Tanaka K, Ibayashi S, Fujishima M. Incidence and risk factors for subtypes of cerebral infarction in a general population: The Hisayama study. Stroke, 2000; 31: 2616-2622 [DOI] [PubMed] [Google Scholar]
- 59). Cui R, Iso H, Yamagishi K, Saito I, Kokubo Y, Inoue M, Tsugane S. High serum total cholesterol levels is a risk factor of ischemic stroke for general Japanese population: The JPHC study. Atherosclerosis, 2012; 221: 565-569 [DOI] [PubMed] [Google Scholar]
- 60). Satoh M, Ohkubo T, Asayama K, Murakami Y, Sakurai M, Nakagawa H, Iso H, Okayama A, Miura K, Imai Y, Ueshima H, Okamura T. Combined effect of blood pressure and total cholesterol levels on long-term risks of subtypes of cardiovascular death: Evidence for Cardiovascular Prevention from Observational Cohorts in Japan. Hypertension, 2015; 65: 517-524 [DOI] [PubMed] [Google Scholar]
- 61). Cui Y, Blumenthal RS, Flaws JA, Whiteman MK, Langenberg P, Bachorik PS, Bush TL. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med, 2001; 161: 1413-1419 [DOI] [PubMed] [Google Scholar]
- 62). Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation, 2005; 112: 3375-3383 [DOI] [PubMed] [Google Scholar]
- 63). Kitamura A, Noda H, Nakamura M, Kiyama M, Okada T, Imano H, Ohira T, Sato S, Yamagishi K, Iso H. Association between non-high-density lipoprotein cholesterol levels and the incidence of coronary heart disease among Japanese: The Circulatory Risk in Communities Study (CIRCS). J Atheroscler Thromb, 2011; 18: 454-463 [DOI] [PubMed] [Google Scholar]
- 64). Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Miyamoto Y, Yoshimasa Y, Okayama A. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort study: The Suita study. Atherosclerosis, 2009; 203: 587-592 [DOI] [PubMed] [Google Scholar]
- 65). Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Ono Y, Miyamoto Y, Yoshimasa Y, Okayama A. Triglycerides and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort: The Suita study. Atherosclerosis, 2010; 209: 290-294 [DOI] [PubMed] [Google Scholar]
- 66). Noda H, Iso H, Irie F, Sairenchi T, Ohtaka E, Ohta H. Association between non-high-density lipoprotein cholesterol concentrations and mortality from coronary heart disease among Japanese men and women: The Ibaraki Prefectural Health Study. J Atheroscler Thromb, 2010; 17: 30-36 [DOI] [PubMed] [Google Scholar]
- 67). Imamura T, Doi Y, Ninomiya T, Hata J, Nagata M, Ikeda F, Mukai N, Hirakawa Y, Yoshida D, Fukuhara M, Kitazono T, Kiyohara Y. Non-high-density lipoprotein cholesterol and the development of coronary heart disease and stroke subtypes in a general Japanese population: The Hisayama study. Atherosclerosis, 2014; 233: 343-348 [DOI] [PubMed] [Google Scholar]
- 68). Takeuchi T, Nemoto K, Takahashi O, Urayama KY, Deshpande GA, Izumo H. Comparison of cardiovascular disease risk associated with 3 lipid measures in Japanese adults. J Clin Lipidol, 2014; 8: 501-509 [DOI] [PubMed] [Google Scholar]
- 69). Tanaka F, Makita S, Onoda T, Tanno K, Ohsawa M, Itai K, Sakata K, Omama S, Yoshida Y, Ogasawara K, Ogawa A, Ishibashi Y, Kuribayashi T, Okayama A, Nakamura M. Predictive value of lipoprotein indices for residual risk of acute myocardial infarction and sudden death in men with low-density lipoprotein cholesterol levels < 120 mg/dl. Am J Cardiol, 2013; 112: 1063-1068 [DOI] [PubMed] [Google Scholar]
- 70). Kakehi E, Kotani K, Ishikawa S, Gotoh T, Kayaba K, Nakamura Y, Kajii E. Serum non-high-density lipoprotein cholesterol levels and the incidence of ischemic stroke in a Japanese population: The Jichi Medical School Cohort Study. Asia Pac J Public Health, 2015; 27: NP535-543 [DOI] [PubMed] [Google Scholar]
- 71). Kitamura A, Noda H, Nakamura M, Kiyama M, Okada T, Imano H, Ohira T, Sato S, Yamagishi K, Iso H. Association between non-high-density lipoprotein cholesterol levels and the incidence of coronary heart disease among Japanese: The Circulatory Risk in Communities Study (CIRCS). J Atheroscler Thromb, 2011; 18: 454-463 [DOI] [PubMed] [Google Scholar]
- 72). Tanaka F, Makita S, Onoda T, Tanno K, Ohsawa M, Itai K, Sakata K, Omama S, Yoshida Y, Ogasawara K, Ogawa A, Ishibashi Y, Kuribayashi T, Okayama A, Nakamura M. Predictive value of lipoprotein indices for residual risk of acute myocardial infarction and sudden death in men with low-density lipoprotein cholesterol levels < 120 mg/dl. Am J Cardiol, 2013; 112: 1063-1068 [DOI] [PubMed] [Google Scholar]
- 73). Noda H, Iso H, Irie F, Sairenchi T, Ohtaka E, Ohta H. Association between non-high-density lipoprotein cholesterol concentrations and mortality from coronary heart disease among Japanese men and women: The Ibaraki Prefectural Health Study. J Atheroscler Thromb, 2010; 17: 30-36 [DOI] [PubMed] [Google Scholar]
- 74). Shimano H, Arai H, Harada-Shiba M, Ueshima H, Ohta T, Yamashita S, Gotoda T, Kiyohara Y, Hayashi T, Kobayashi J, Shimamoto K, Bujo H, Ishibashi S, Shirai K, Oikawa S, Saito Y, Yamada N. Proposed guidelines for hypertriglyceridemia in Japan with non-HDL cholesterol as the second target. J Atheroscler Thromb, 2008; 15: 116-121 [DOI] [PubMed] [Google Scholar]
- 75). Sugimoto K, Isobe K, Kawakami Y, Yamada N. The relationship between non-HDL cholesterol and other lipid parameters in Japanese subjects. J Atheroscler Thromb, 2005; 12: 107-110 [DOI] [PubMed] [Google Scholar]
- 76). Chei CL, Yamagishi K, Kitamura A, Kiyama M, Imano H, Ohira T, Cui RZ, Tanigawa T, Sankai T, Ishikawa Y, Sato S, Hitsumoto S, Iso H. High-density lipoprotein subclasses and risk of stroke and its subtypes in Japanese population: the Circulatory Risk in Communities Study. Stroke, 2013; 44: 327-333 [DOI] [PubMed] [Google Scholar]
- 77). Soyama Y, Miura K, Morikawa Y, Nishijo M, Nakanishi Y, Naruse Y, Kagamimori S, Nakagawa H. High-density lipoprotein cholesterol and risk of stroke in Japanese men and women: The Oyabe Study. Stroke, 2003; 34: 863-868 [DOI] [PubMed] [Google Scholar]
- 78). Iso H, Sato S, Kitamura A, Imano H, Kiyama M, Yamagishi K, Cui R, Tanigawa T, Shimamoto T. Metabolic syndrome and the risk of ischemic heart disease and stroke among Japanese men and women. Stroke, 2007; 38: 1744-1751 [DOI] [PubMed] [Google Scholar]
- 79). Satoh H, Tomita K, Fujii S, Kishi R, Tsutsui H. Lower high-density lipoprotein cholesterol is a significant and independent risk for coronary artery disease in Japanese men. J Atheroscler Thromb, 2009; 16: 792-798 [DOI] [PubMed] [Google Scholar]
- 80). Okamura T, Hayakawa T, Kadowaki T, Kita Y, Okayama A, Ueshima H, NIPPON DATA90 Research Group The inverse relationship between serum high-density lipoprotein cholesterol level and all-cause mortality in a 9.6-year follow-up study in the Japanese general population. Atherosclerosis, 2006; 184: 143-150 [DOI] [PubMed] [Google Scholar]
- 81). Matsuzaki M, Kita T, Mabuchi H, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K, Itakura H. Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simvastatin therapy in Japanese patients with hypercholesterolemia. Circ J, 2002; 66: 1087-1095 [DOI] [PubMed] [Google Scholar]
- 82). Mabuchi H, Kita T, Matsuzaki M, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K, Itakura H. Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simvastatin therapy in Japanese patients with hypercholesterolemia and coronary heart disease: Secondary prevention cohort study of the Japan Lipid Intervention Trial (J-LIT). Circ J, 2002; 66: 1096-1100 [DOI] [PubMed] [Google Scholar]
- 83). Huxley RR, Barzi F, Lam TH, Czernichow S, Fang X, Welborn T, Shaw J, Ueshima H, Zimmet P, Jee SH, Patel JV, Caterson I, Perkovic V, Woodward M. Isolated low levels of high-density lipoprotein cholesterol are associated with an increased risk of coronary heart disease: An individual participant data meta-analysis of 23 studies in the Asia-Pacific region. Circulation, 2011; 124: 2056-2064 [DOI] [PubMed] [Google Scholar]
- 84). Hirata T, Sugiyama D, Nagasawa SY, Murakami Y, Saitoh S, Okayama A, Iso H, Irie F, Sairenchi T, Miyamoto Y, Yamada M, Ishikawa S, Miura K, Ueshima H, Okamura T. A pooled analysis of the association of isolated low levels of high-density lipoprotein cholesterol with cardiovascular mortality in Japan. Eur J Epidemiol, 2016; 32: 547-557 [DOI] [PubMed] [Google Scholar]
- 85). Arai H, Yamamoto A, Matsuzawa Y, Saito Y, Yamada N, Oikawa S, Mabuchi H, Teramoto T, Sasaki J, Nakaya N, Itakura H, Ishikawa Y, Ouchi Y, Horibe H, Kita T. Serum lipid survey and its recent trend in the general Japanese population in 2000. J Atheroscler Thromb, 2005; 12: 98-106 [DOI] [PubMed] [Google Scholar]
- 86). Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 western prospective studies. Circulation, 2007; 115: 450-458 [DOI] [PubMed] [Google Scholar]
- 87). Patel A, Barzi F, Jamrozik K, Lam TH, Ueshima H, Whitlock G, Woodward M. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific region. Circulation, 2004; 110: 2678-2686 [DOI] [PubMed] [Google Scholar]
- 88). Iso H, Naito Y, Sato S, Kitamura A, Okamura T, Sankai T, Shimamoto T, Iida M, Komachi Y. Serum triglycerides and risk of coronary heart disease among Japanese men and women. Am J Epidemiol, 2001; 153: 490-499 [DOI] [PubMed] [Google Scholar]
- 89). Satoh H, Nishino T, Tomita K, Tsutsui H. Fasting triglyceride is a significant risk factor for coronary artery disease in middle-aged Japanese men. Circ J, 2006; 70: 227-231 [DOI] [PubMed] [Google Scholar]
- 90). Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Ono Y, Nishimura K, Okayama A, Miyamoto Y. A revised definition of the metabolic syndrome predicts coronary artery disease and ischemic stroke after adjusting for low density lipoprotein cholesterol in a 13-year cohort study of Japanese: The Suita study. Atherosclerosis, 2011; 217: 201-206 [DOI] [PubMed] [Google Scholar]
- 91). Iso H, Imano H, Yamagishi K, Ohira T, Cui R, Noda H, Sato S, Kiyama M, Okada T, Hitsumoto S, Tanigawa T, Kitamura A. Fasting and non-fasting triglycerides and risk of ischemic cardiovascular disease in Japanese men and women: The Circulatory Risk in Communities Study (CIRCS). Atherosclerosis, 2014; 237: 361-368 [DOI] [PubMed] [Google Scholar]
- 92). Castelli WP. Lipids, risk factors and ischaemic heart disease. Atherosclerosis, 1996; 124 Suppl: S1-9 [DOI] [PubMed] [Google Scholar]
- 93). Iso H, Imano H, Yamagishi K, Ohira T, Cui R, Noda H, Sato S, Kiyama M, Okada T, Hitsumoto S, Tanigawa T, Kitamura A. Fasting and non-fasting triglycerides and risk of ischemic cardiovascular disease in Japanese men and women: The Circulatory Risk in Communities Study (CIRCS). Atherosclerosis, 2014; 237: 361-368 [DOI] [PubMed] [Google Scholar]
- 94). Labreuche J, Touboul PJ, Amarenco P. Plasma triglyceride levels and risk of stroke and carotid atherosclerosis: A systematic review of the epidemiological studies. Atherosclerosis, 2009; 203: 331-345 [DOI] [PubMed] [Google Scholar]
- 95). Antonios N, Angiolillo DJ, Silliman S. Hypertriglyceridemia and ischemic stroke. Eur Neurol, 2008; 60: 269-278 [DOI] [PubMed] [Google Scholar]
- 96). Saito I, Folsom AR, Aono H, Ozawa H, Ikebe T, Yamashita T. Comparison of fatal coronary heart disease occurrence based on population surveys in Japan and the USA. Int J Epidemiol, 2000; 29: 837-844 [DOI] [PubMed] [Google Scholar]
- 97). Sekikawa A, Miyamoto Y, Miura K, Nishimura K, Willcox BJ, Masaki KH, Rodriguez B, Tracy RP, Okamura T, Kuller LH. Continuous decline in mortality from coronary heart disease in Japan despite a continuous and marked rise in total cholesterol: Japanese experience after the Seven Countries Study. Int J Epidemiol, 2015; 44: 1614-1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98). Kitamura A, Sato S, Kiyama M, Imano H, Iso H, Okada T, Ohira T, Tanigawa T, Yamagishi K, Nakamura M, Konishi M, Shimamoto T, Iida M, Komachi Y. Trends in the incidence of coronary heart disease and stroke and their risk factors in Japan, 1964 to 2003: The Akita-Osaka study. J Am Coll Cardiol, 2008; 52: 71-79 [DOI] [PubMed] [Google Scholar]
- 99). Takii T, Yasuda S, Takahashi J, Ito K, Shiba N, Shirato K, Shimokawa H. Trends in acute myocardial infarction incidence and mortality over 30 years in Japan: Report from the Miyagi-AMI Registry Study. Circ J, 2010; 74: 93-100 [DOI] [PubMed] [Google Scholar]
- 100). Miida T, Nishimura K, Okamura T, Hirayama S, Ohmura H, Yoshida H, Miyashita Y, Ai M, Tanaka A, Sumino H, Murakami M, Inoue I, Kayamori Y, Nakamura M, Nobori T, Miyazawa Y, Teramoto T, Yokoyama S. A multicenter study on the precision and accuracy of homogeneous assays for LDL-cholesterol: Comparison with a beta-quantification method using fresh serum obtained from non-diseased and diseased subjects. Atherosclerosis, 2012; 225: 208-215 [DOI] [PubMed] [Google Scholar]
- 101). Miida T, Nishimura K, Hirayama S, Miyamoto Y, Nakamura M, Masuda D, Yamashita S, Ushiyama M, Komori T, Fujita N, Yokoyama S, Teramoto T. Homogeneous assays for LDL-C and HDL-C are reliable in both the postprandial and fasting state. J Atheroscler Thromb, 2017; 24: 583-599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102). National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Atlanta (GA). Centers for Disease Control and Prevention (US). Chapter 8. The health consequences of smoking-50 years of progress: A report of the Surgeon General, 2014: 411-457 [PubMed] [Google Scholar]
- 103). Kono S, Ikeda M, Tokudome S, Nishizumi M, Kuratsune M. Smoking and mortalities from cancer, coronary heart disease and stroke in male Japanese physicians. J Cancer Res Clin Oncol, 1985; 110: 161-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104). Irie F, Sairenchi T, Iso H, Shimamoto T. Prediction of mortality from findings of annual health checkups utility for health care programs. Nihon Koshu Eisei Zasshi, 2001; 48: 95-108 (in Japanese) [PubMed] [Google Scholar]
- 105). Yamagishi K, Iso H, Kitamura A, Sankai T, Tanigawa T, Naito Y, Sato S, Imano H, Ohira T, Shimamoto T. Smoking raises the risk of total and ischemic strokes in hypertensive men. Hypertens Res, 2003; 26: 209-217 [DOI] [PubMed] [Google Scholar]
- 106). Ueshima H, Choudhury SR, Okayama A, Hayakawa T, Kita Y, Kadowaki T, Okamura T, Minowa M, Iimura O. Cigarette smoking as a risk factor for stroke death in Japan: NIPPON DATA80. Stroke, 2004; 35: 1836-1841 [DOI] [PubMed] [Google Scholar]
- 107). Iso H, Date C, Yamamoto A, Toyoshima H, Watanabe Y, Kikuchi S, Koizumi A, Wada Y, Kondo T, Inaba Y, Tamakoshi A, JACC Study Group Smoking cessation and mortality from cardiovascular disease among Japanese men and women: The JACC Study. Am J Epidemiol, 2005; 161: 170-179 [DOI] [PubMed] [Google Scholar]
- 108). Higashiyama A, Okamura T, Ono Y, Watanabe M, Kokubo Y, Okayama A. Risk of smoking and metabolic syndrome for incidence of cardiovascular disease--comparison of relative contribution in urban Japanese population: The Suita study. Circ J, 2009; 73: 2258-2263 [DOI] [PubMed] [Google Scholar]
- 109). Hata J, Doi Y, Ninomiya T, Fukuhara M, Ikeda F, Mukai N, Hirakawa Y, Kitazono T, Kiyohara Y. Combined effects of smoking and hypercholesterolemia on the risk of stroke and coronary heart disease in Japanese: The Hisayama study. Cerebrovasc Dis, 2011; 31: 477-484 [DOI] [PubMed] [Google Scholar]
- 110). Kondo T, Osugi S, Shimokata K, Honjo H, Morita Y, Maeda K, Yamashita K, Muramatsu T, Shintani S, Matsushita K, Murohara T. Smoking and smoking cessation in relation to all-cause mortality and cardiovascular events in 25,464 healthy male Japanese workers. Circ J, 2011; 75: 2885-2892 [DOI] [PubMed] [Google Scholar]
- 111). Eshak ES, Iso H, Yamagishi K, Kokubo Y, Saito I, Yatsuya H, Sawada N, Inoue M, Tsugane S, JPHC Study Group Modification of the excess risk of coronary heart disease due to smoking by seafood/fish intake. Am J Epidemiol, 2014; 179: 1173-1181 [DOI] [PubMed] [Google Scholar]
- 112). Honjo K, Katanoda K, Horiga K. General. Health and Labour Sciences Research Grant. Research project against circulatory organ diseases, diabetes and other lifestyle habit diseases. Research on comprehensive assessment of the health and economic impacts of the tobacco initiative http://mhlw-grants.niph.go.jp/niph/search/NIDD00.do?resrchNum=201508017A 2016. (in Japanese)
- 113). Katanoda K, Marugame T, Saika K, Satoh H, Tajima K, Suzuki T, Tamakoshi A, Tsugane S, Sobue T. Population attributable fraction of mortality associated with tobacco smoking in Japan: A pooled analysis of three large-scale cohort studies. J Epidemiol, 2008; 18: 251-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114). Cui R, Iso H, Yamagishi K, Tanigawa T, Imano H, Ohira T, Kitamura A, Sato S, Shimamoto T. Relationship of smoking and smoking cessation with ankle-toarm blood pressure index in elderly Japanese men. Eur J Cardiovasc Prev Rehabil, 2006; 13: 243-248 [DOI] [PubMed] [Google Scholar]
- 115). Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation, 2005; 111: 2684-2698 [DOI] [PubMed] [Google Scholar]
- 116). Oono IP, Mackay DF, Pell JP. Meta-analysis of the association between secondhand smoke exposure and stroke. J Public Health (Oxf), 2011; 33: 496-502 [DOI] [PubMed] [Google Scholar]
- 117). Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol, 2015; 3: 958-967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118). Ishizaka N, Ishizaka Y, Toda E, Hashimoto H, Nagai R, Yamakado M. Association between cigarette smoking, metabolic syndrome, and carotid arteriosclerosis in Japanese individuals. Atherosclerosis, 2005; 181: 381-388 [DOI] [PubMed] [Google Scholar]
- 119). Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: An analysis of published data. BMJ, 1989; 298: 784-788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120). Ministry of Health, Labour and Welfare of Japan. Review conference on the health impacts of smoking. Segment 5 health impacts of smoke-free tobacco and electronic cigarettes. http://www.mhlw.go.jp/file/05-Shingikai-10901000-Kenkoukyoku-Soumuka/0000172687.pdf
- 121). Uchiyama S, Senoo Y, Hayashida H, Inaba Y, Nakagome H, Kunugita N. Determination of chemical compounds generated from second-generation e-cigarettes using a sorbent cartridge followed by a two-step elution method. Anal Sci, 2016; 32: 549-555 [DOI] [PubMed] [Google Scholar]
- 122). Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S, Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res, 2014; 37: 253-392 [DOI] [PubMed] [Google Scholar]
- 123). Fujiyoshi A, Ohkubo T, Miura K, Murakami Y, Nagasawa SY, Okamura T, Ueshima H. Blood pressure categories and long-term risk of cardiovascular disease according to age group in Japanese men and women. Hypertens Res, 2012; 35: 947-953 [DOI] [PubMed] [Google Scholar]
- 124). Sasaki J, Kita T, Mabuchi H, Matsuzaki M, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Shimamoto K, Kono S, Itakura H, J-LIT Study Group Gender difference in coronary events in relation to risk factors in Japanese hypercholesterolemic patients treated with low-dose simvastatin. Circ J, 2006; 70: 810-814 [DOI] [PubMed] [Google Scholar]
- 125). Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: The Framingham study. Diabetes Care, 1979; 2: 120-126 [DOI] [PubMed] [Google Scholar]
- 126). Vaccaro O, Stamler J, Neaton JD. Sixteen-year coronary mortality in black and white men with diabetes screened for the Multiple Risk Factor Intervention Trial (MRFIT). Int J Epidemiol, 1998; 27: 636-641 [DOI] [PubMed] [Google Scholar]
- 127). Kadowaki S, Okamura T, Hozawa A, Kadowaki T, Kadota A, Murakami Y, Nakamura K, Saitoh S, Nakamura Y, Hayakawa T, Kita Y, Okayama A, Ueshima H. Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and allcause mortality in a representative sample of the Japanese population. NIPPON DATA80. Diabetologia, 2008; 51: 575-582 [DOI] [PubMed] [Google Scholar]
- 128). Fujishima M, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, Ohmori S, Yoshitake T. Diabetes and cardiovascular disease in a prospective population survey in Japan: The Hisayama study. Diabetes, 1996; 45 Suppl 3: S14-16 [DOI] [PubMed] [Google Scholar]
- 129). Iso H, Imano H, Kitamura A, Sato S, Naito Y, Tanigawa T, Ohira T, Yamagishi K, Iida M, Shimamoto T. Type 2 diabetes and risk of non-embolic ischaemic stroke in Japanese men and women. Diabetologia, 2004; 47: 2137-2144 [DOI] [PubMed] [Google Scholar]
- 130). Yokoyama H, Matsushima M, Kawai K, Hirao K, Oishi M, Sugimoto H, Takeda H, Minami M, Kobayashi M, Sone H. Low incidence of cardiovascular events in Japanese patients with type 2 diabetes in primary care settings: A prospective cohort study (JDDM 20). Diabet Med, 2011; 28: 1221-1228 [DOI] [PubMed] [Google Scholar]
- 131). Nakajima K, Yamasaki Y, Kusuoka H, Izumi T, Kashiwagi A, Kawamori R, Shimamoto K, Yamada N, Nishimura T. Cardiovascular events in Japanese asymptomatic patients with type 2 diabetes: A 1-year interim report of a J-ACCESS 2 investigation using myocardial perfusion imaging. Eur J Nucl Med Mol Imaging, 2009; 36: 2049-2057 [DOI] [PubMed] [Google Scholar]
- 132). Nesto RW, Phillips RT, Kett KG, Hill T, Perper E, Young E, Leland OS., Jr Angina and exertional myocardial ischemia in diabetic and nondiabetic patients: Assessment by exercise thallium scintigraphy. Ann Intern Med, 1988; 108: 170-175 [DOI] [PubMed] [Google Scholar]
- 133). Goraya TY, Leibson CL, Palumbo PJ, Weston SA, Killian JM, Pfeifer EA, Jacobsen SJ, Frye RL, Roger VL. Coronary atherosclerosis in diabetes mellitus: A population-based autopsy study. J Am Coll Cardiol, 2002; 40: 946-953 [DOI] [PubMed] [Google Scholar]
- 134). Kataoka Y, Yasuda S, Morii I, Otsuka Y, Kawamura A, Miyazaki S. Quantitative coronary angiographic studies of patients with angina pectoris and impaired glucose tolerance. Diabetes Care, 2005; 28: 2217-2222 [DOI] [PubMed] [Google Scholar]
- 135). Hoff JA, Quinn L, Sevrukov A, Lipton RB, Daviglus M, Garside DB, Ajmere NK, Gandhi S, Kondos GT. The prevalence of coronary artery calcium among diabetic individuals without known coronary artery disease. J Am Coll Cardiol, 2003; 41: 1008-1012 [DOI] [PubMed] [Google Scholar]
- 136). Cui R, Iso H, Yamagishi K, Saito I, Kokubo Y, Inoue M, Tsugane S. Diabetes mellitus and risk of stroke and its subtypes among Japanese: The Japan public health center study. Stroke, 2011; 42: 2611-2614 [DOI] [PubMed] [Google Scholar]
- 137). Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA, 2007; 298: 765-775 [DOI] [PubMed] [Google Scholar]
- 138). Takara A, Ogawa H, Endoh Y, Mori F, Yamaguchi J, Takagi A, Koyanagi R, Shiga T, Kasanuki H, Hagiwara N. Long-term prognosis of diabetic patients with acute myocardial infarction in the era of acute revascularization. Cardiovasc Diabetol, 2010; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139). Kuramitsu S, Yokoi H, Domei T, Nomura A, Watanabe H, Yamaji K, Soga Y, Arita T, Kondo K, Shirai S, Ando K, Sakai K, Iwabuchi M, Nosaka H, Nobuyoshi M. Impact of post-challenge hyperglycemia on clinical outcomes in Japanese patients with stable angina undergoing percutaneous coronary intervention. Cardiovasc Diabetol, 2013; 12: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140). Hillen T, Coshall C, Tilling K, Rudd AG, McGovern R, Wolfe CD. Cause of stroke recurrence is multifactorial: Patterns, risk factors, and outcomes of stroke recurrence in the south london stroke register. Stroke, 2003; 34: 1457-1463 [DOI] [PubMed] [Google Scholar]
- 141). Shinohara Y, Gotoh F, Tohgi H, Hirai S, Terashi A, Fukuuchi Y, Otomo E, Itoh E, Matsuda T, Sawada T, Yamaguchi T, Nishimaru K, Ohashi Y. Antiplatelet cilostazol is beneficial in diabetic and/or hypertensive ischemic stroke patients. Subgroup analysis of the cilostazol stroke prevention study. Cerebrovasc Dis, 2008; 26: 63-70 [DOI] [PubMed] [Google Scholar]
- 142). Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg, 2007; 45 Suppl S: S5-67 [DOI] [PubMed] [Google Scholar]
- 143). Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta-analysis: Glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med, 2004; 141: 421-431 [DOI] [PubMed] [Google Scholar]
- 144). Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care, 1999; 22: 920-924 [DOI] [PubMed] [Google Scholar]
- 145). Ikeda F, Doi Y, Ninomiya T, Hirakawa Y, Mukai N, Hata J, Shikata K, Yoshida D, Matsumoto T, Kitazono T, Kiyohara Y. Haemoglobin a1c even within non-diabetic level is a predictor of cardiovascular disease in a general Japanese population: The Hisayama study. Cardiovasc Diabetol, 2013; 12: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146). Saito I, Kokubo Y, Yamagishi K, Iso H, Inoue M, Tsugane S. Diabetes and the risk of coronary heart disease in the general Japanese population: The Japan public health center-based prospective (JPHC) study. Atherosclerosis, 2011; 216: 187-191 [DOI] [PubMed] [Google Scholar]
- 147). Nakagami T. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia, 2004; 47: 385-394 [DOI] [PubMed] [Google Scholar]
- 148). DECODE Study Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med, 2001; 161: 397-405 [DOI] [PubMed] [Google Scholar]
- 149). Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: A systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia, 2014; 57: 1542-1551 [DOI] [PubMed] [Google Scholar]
- 150). Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet, 2014; 383: 1973-1980 [DOI] [PubMed] [Google Scholar]
- 151). Fujihara K, Igarashi R, Yamamoto M, Ishizawa M, Matsubayasi Y, Matsunaga S, Kato K, Ito C, Koishi M, Yamanaka N, Kodama S, Sone H. Impact of glucose tolerance status on the development of coronary artery disease among working-age men. Diabetes Metab, 2017; 43: 261-264 [DOI] [PubMed] [Google Scholar]
- 152). Nakamura J, Kamiya H, Haneda M, Inagaki N, Tanizawa Y, Araki E, Ueki K, Nakayama T. Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001–2010: Report of the committee on causes of death in diabetes mellitus. J Diabetes Investig, 2017; 8: 397-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153). Japanese Society of Nephrology (Ed). Evidence-based Clinical Practice Guideline for CKD 2013. Tokyo Igaku Sha, Tokyo, 2013. (in Japanese) [Google Scholar]
- 154). Japanese Society of Nephrology. Health guidance for the examinees of kidney medical checkup and suggestions to the referral criteria to medical institutions. 2017; 59: 38-42 (in Japanese) [Google Scholar]
- 155). Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y, Fujimoto S, Konta T, Yokoyama H, Makino H, Hishida A, Matsuo S. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol, 2009; 13: 621-630 [DOI] [PubMed] [Google Scholar]
- 156). Joint Committee for Comprehensive Risk Management Chart for the Prevention of Cerebro-Cardiovascular Diseases, Comprehensive risk management for the prevention of cerebro-cardiovascular diseases in Japan. J Atheroscler Thromb, 2017; 24: 749-764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157). Tonelli M, Muntner P, Lloyd A, Manns B, Klarenbach S, Pannu N, James M, Hemmelgarn B, Alberta Kidney Disease Network Association between LDL-C and risk of myocardial infarction in CKD. J Am Soc Nephrol, 2013; 24: 979-986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158). Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E, German Diabetes and Dialysis Study Investigators Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med, 2005; 353: 238-248 [DOI] [PubMed] [Google Scholar]
- 159). Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F, AURORA Study Group Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med, 2009; 360: 1395-1407 [DOI] [PubMed] [Google Scholar]
- 160). Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation, 1998; 97: 1837-1847 [DOI] [PubMed] [Google Scholar]
- 161). NIPPON DATA80 Research Group. Risk assessment chart for death from cardiovascular disease based on a 19-year follow-up study of a Japanese representative population. Circ J, 2006; 70: 1249-1255 [DOI] [PubMed] [Google Scholar]
- 162). Nishimura K, Okamura T, Watanabe M, Nakai M, Takegami M, Higashiyama A, Kokubo Y, Okayama A, Miyamoto Y. Predicting coronary heart disease using risk factor categories for a Japanese urban population, and comparison with the Framingham risk score: The Suita study. J Atheroscler Thromb, 2014; 21: 784-798 [DOI] [PubMed] [Google Scholar]
- 163). Rumana N, Kita Y, Turin TC, Murakami Y, Sugihara H, Morita Y, Tomioka N, Okayama A, Nakamura Y, Abbott RD, Ueshima H. Trend of increase in the incidence of acute myocardial infarction in a Japanese population: Takashima AMI registry, 1990–2001. Am J Epidemiol, 2008; 167: 1358-1364 [DOI] [PubMed] [Google Scholar]
- 164). Ministry of Health Labour and Welfare. Vital statistics of Japan. 2014. http://www.e-stat.go.jp/SG1/estat/List.do?lid=000001137965 (in Japanese)
- 165). Silberberg JS, Wlodarczyk J, Fryer J, Robertson R, Hensley MJ. Risk associated with various definitions of family history of coronary heart disease. The Newcastle Family History Study Ⅱ. Am J Epidemiol, 1998; 147: 1133-1139 [DOI] [PubMed] [Google Scholar]
- 166). Li R, Bensen JT, Hutchinson RG, Province MA, Hertz-Picciotto I, Sprafka JM, Tyroler HA. Family risk score of coronary heart disease (CHD) as a predictor of CHD: The Atherosclerosis Risk in Communities (ARIC) study and the NHLBI family heart study. Genet Epidemiol, 2000; 18: 236-250 [DOI] [PubMed] [Google Scholar]
- 167). Williams RR, Hunt SC, Heiss G, Province MA, Bensen JT, Higgins M, Chamberlain RM, Ware J, Hopkins PN. Usefulness of cardiovascular family history data for population-based preventive medicine and medical research (the Health Family Tree Study and the NHLBI Family Heart Study). Am J Cardiol, 2001; 87: 129-135 [DOI] [PubMed] [Google Scholar]
- 168). Lloyd-Jones DM, Nam BH, D'Agostino RB, Levy D, Murabito JM, Wang TJ, Wilson PW, O'Donnell CJ. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: A prospective study of parents and offspring. JAMA, 2004; 291: 2204-2211 [DOI] [PubMed] [Google Scholar]
- 169). Myers RH, Kiely DK, Cupples LA, Kannel WB. Parental history is an independent risk factor for coronary artery disease: The Framingham study. Am Heart J, 1990; 120: 963-969 [DOI] [PubMed] [Google Scholar]
- 170). Watkins H, Farrall M. Genetic susceptibility to coronary artery disease: From promise to progress. Nat Rev Genet, 2006; 7: 163-173 [DOI] [PubMed] [Google Scholar]
- 171). Inazu A, Brown ML, Hesler CB, Agellon LB, Koizumi J, Takata K, Maruhama Y, Mabuchi H, Tall AR. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N Engl J Med, 1990; 323: 1234-1238 [DOI] [PubMed] [Google Scholar]
- 172). Inazu A, Jiang XC, Haraki T, Yagi K, Kamon N, Koizumi J, Mabuchi H, Takeda R, Takata K, Moriyama Y. Genetic cholesteryl ester transfer protein deficiency caused by two prevalent mutations as a major determinant of increased levels of high density lipoprotein cholesterol. J Clin Invest, 1994; 94: 1872-1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173). Mabuchi H, Kita T, Matsuzaki M, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K, Itakura H, the J-LIT Study Group Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simvastatin therapy in Japanese patients with hypercholesterolemia and coronary heart disease: Secondary prevention cohort study of the Japan Lipid Intervention Trial (J-LIT). Circ J, 2002; 66: 1096-1100 [DOI] [PubMed] [Google Scholar]
- 174). Furukawa Y, Ehara N, Taniguchi R, Haruna Y, Ozasa N, Saito N, Doi T, Hoshino K, Tamura T, Shizuta S, Abe M, Toma M, Morimoto T, Teramukai S, Fukushima M, Kita T, Kimura T, CREDO-Kyoto Investigators Coronary risk factor profile and prognostic factors for young Japanese patients undergoing coronary revascularization. Circ J, 2009; 73: 1459-1465 [DOI] [PubMed] [Google Scholar]
- 175). Hirano K, Yamashita S, Nakajima N, Arai T, Maruyama T, Yoshida Y, Ishigami M, Sakai N, Kameda-Takemura K, Matsuzawa Y. Genetic cholesteryl ester transfer protein deficiency is extremely frequent in the omagari area of Japan. Marked hyperalphalipoproteinemia caused by CETP gene mutation is not associated with longevity. Arterioscler Thromb Vasc Biol, 1997; 17: 1053-1059 [DOI] [PubMed] [Google Scholar]
- 176). Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA, 2012; 308: 788-795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177). Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R, Cholesterol Treatment Trialists' (CTT) Collaboration Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet, 2010; 376: 1670-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178). Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C, Cholesterol Treatment Trialists' (CTT) Collaboration The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet, 2012; 380: 581-590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179). Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A, Cholesterol Treatment Trialists' (CTT) Collaboration Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet, 2015; 385: 1397-1405 [DOI] [PubMed] [Google Scholar]
- 180). Matsuzaki M, Kita T, Mabuchi H, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K, Itakura H, the J-LIT Study Group Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simvastatin therapy in Japanese patients with hypercholesterolemia: Primary prevention cohort study of the Japan Lipid Intervention Trial (J-LIT). Circ J, 2002; 66: 1087-1095 [DOI] [PubMed] [Google Scholar]
- 181). Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, for the Japan EPA lipid intervention study (JELIS) Investigators Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised openlabel, blinded endpoint analysis. Lancet, 2007; 369: 1090-1098 [DOI] [PubMed] [Google Scholar]
- 182). The Japanese Coronary Artery Disease (JCAD) Study Investigators. Current status of the background of patients with coronary artery disease in Japan. Circ J, 2006; 70: 1256-1262 [DOI] [PubMed] [Google Scholar]
- 183). Furukawa Y, Taniguchi R, Ehara N, Ozasa N, Haruna Y, Saito N, Doi T, Hoshino K, Shizuta S, Morimoto T, Imai Y, Teramukai S, Fukushima M, Kita T, Kimura T, CREDO-Kyoto Investigators Better survival with statin administration after revascularization therapy in Japanese patients with coronary artery disease: Perspectives from the CREDO-Kyoto registry. Circ J, 2008; 72: 1937-1945 [DOI] [PubMed] [Google Scholar]
- 184). Uchiyama S, Goto S, Matsumoto M, Nagai R, Origasa H, Yamazaki T, Shigematsu H, Shimada K, Yamada N, Bhatt DL, Steg PG, Ikeda Y, REduction of Atherothrombosis for Continued Health Registry Investigators Cardiovascular event rates in patients with cerebrovascular disease and atherothrombosis at other vascular locations: Results from 1-year outcomes in the Japanese REACH Registry. J Neurol Sci, 2009; 287: 45-51 [DOI] [PubMed] [Google Scholar]
- 185). Goto S, Ikeda Y, Shimada K, Uchiyama S, Origasa H, Kobayashi H, J-TRACE Investigators One-year cardiovascular event rates in Japanese outpatients with myocardial infarction, stroke, and atrial fibrillation. -results from the Japan Thrombosis Registry for Atrial Fibrillation, Coronary, or Cerebrovascular Events (J-TRACE). Circ J, 2011; 75: 2598-2604 [DOI] [PubMed] [Google Scholar]
- 186). Kitagawa K, Hougaku H, Yamagami H, Hashimoto H, Itoh T, Shimizu Y, Takahashi D, Murata S, Seike Y, Kondo K, Hoshi T, Furukado S, Abe Y, Yagita Y, Sakaguchi M, Tagaya M, Etani H, Fukunaga R, Nagai Y, Matsumoto M, Hori M, OSACA2 Study Group Carotid intima-media thickness and risk of cardiovascular events in high-risk patients. Results of the osaka follow-up study for carotid atherosclerosis 2 (OSACA2 Study). Cerebrovasc Dis, 2007; 24: 35-42 [DOI] [PubMed] [Google Scholar]
- 187). Irie Y, Katakami N, Kaneto H, Kasami R, Sumitsuji S, Yamasaki K, Tachibana K, Kuroda T, Sakamoto K, Umayahara Y, Ueda Y, Kosugi K, Shimomura I. Maximum carotid intima-media thickness improves the prediction ability of coronary artery stenosis in type 2 diabetic patients without history of coronary artery disease. Atherosclerosis, 2012; 221: 438-444 [DOI] [PubMed] [Google Scholar]
- 188). Hirano M, Nakamura T, Kitta Y, Takishima I, Deyama J, Kobayashi T, Fujioka D, Saito Y, Watanabe K, Watanabe Y, Kawabata K, Obata JE, Kugiyama K. Short-term progression of maximum intima-media thickness of carotid plaque is associated with future coronary events in patients with coronary artery disease. Atherosclerosis, 2011; 215: 507-512 [DOI] [PubMed] [Google Scholar]
- 189). Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Volzke H, Tuomainen TP, Sander D, Plichart M, Catapano AL, Robertson CM, Kiechl S, Rundek T, Desvarieux M, Lind L, Schmid C, DasMahapatra P, Gao L, Ziegelbauer K, Bots ML, Thompson SG, PROG-IMT Study Group Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): A meta-analysis of individual participant data. Lancet, 2012; 379: 2053-2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190). Olin JW, Allie DE, Belkin M, Bonow RO, Casey DE, Jr, Creager MA, Gerber TC, Hirsch AT, Jaff MR, Kaufman JA, Lewis CA, Martin ET, Martin LG, Sheehan P, Stewart KJ, Treat-Jacobson D, White CJ, Zheng ZJ, American Association of Cardiovascular and Pulmonary Rehabilitation; American Diabetes Association; Society for Atherosclerosis Imaging and Prevention; Society for Cardiovascular Magnetic Resonance; Society of Cardiovascular Computed Tomography; PAD Coalition; American Academy of Podiatric Practice Management; ACCF/AHA Task Force on Performance Measures. Masoudi FA, Bonow RO, DeLong E, Erwin JP, 3rd, Goff DC, Jr, Grady K, Green LA, Heidenreich PA, Jenkins KJ, Loth AR, Peterson ED, Shahian DM. ACCF/AHA/ACR/SCAI/SIR/SVM/SVN/SVS 2010 performance measures for adults with peripheral artery disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures, the American College of Radiology, the Society for Cardiac Angiography and Interventions, the Society for Interventional Radiology, the Society for Vascular Medicine, the Society for Vascular Nursing, and the Society for Vascular Surgery (Writing Committee to Develop Clinical Performance Measures for Peripheral Artery Disease). Vasc Med, 2010; 15: 481-512 [DOI] [PubMed] [Google Scholar]
- 191). Kojima I, Ninomiya T, Hata J, Fukuhara M, Hirakawa Y, Mukai N, Yoshida D, Kitazono T, Kiyohara Y. A low ankle brachial index is associated with an increased risk of cardiovascular disease: The Hisayama study. J Atheroscler Thromb, 2014; 21: 966-973 [DOI] [PubMed] [Google Scholar]
- 192). Cui R, Yamagishi K, Imano H, Ohira T, Tanigawa T, Hitsumoto S, Kiyama M, Okada T, Kitamura A, Iso H, CIRCS investigators Relationship between the anklebrachial index and the risk of coronary heart disease and stroke: The circulatory risk in communities study. J Atheroscler Thromb, 2014; 21: 1283-1289 [DOI] [PubMed] [Google Scholar]
- 193). Shigematsu H, Nishibe T, Obitsu Y, Matsuzaki K, Ishida A, Miyata T, Shindo S, Hida K, Ohta T, Ando M, Kawasaki T, Yasugi T, Matsumoto T. Three-year cardiovascular events and disease progress in patients with peripheral arterial disease: Results from the Japan medication therapy for peripheral arterial disease (J-METHOD). Int Angiol, 2010; 29: 2-13 [PubMed] [Google Scholar]
- 194). Hobbs SD, Claridge MW, Quick CR, Day NE, Bradbury AW, Wilmink AB. LDL cholesterol is associated with small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg, 2003; 26: 618-622 [DOI] [PubMed] [Google Scholar]
- 195). Tornwall ME, Virtamo J, Haukka JK, Albanes D, Huttunen JK. Life-style factors and risk for abdominal aortic aneurysm in a cohort of Finnish male smokers. Epidemiology, 2001; 12: 94-100 [DOI] [PubMed] [Google Scholar]
- 196). Bhak RH, Wininger M, Johnson GR, Lederle FA, Messina LM, Ballard DJ, Wilson SE, Aneurysm Detection and Management (ADAM) Study Group Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg, 2015; 150: 44-50 [DOI] [PubMed] [Google Scholar]
- 197). Akai A, Watanabe Y, Hoshina K, Obitsu Y, Deguchi J, Sato O, Shigematsu K, Miyata T. Family history of aortic aneurysm is an independent risk factor for more rapid growth of small abdominal aortic aneurysms in Japan. J Vasc Surg, 2015; 61: 287-290 [DOI] [PubMed] [Google Scholar]
- 198). Hollier LH, Plate G, O'Brien PC, Kazmier FJ, Gloviczki P, Pairolero PC, Cherry KJ. Late survival after abdominal aortic aneurysm repair: Influence of coronary artery disease. J Vasc Surg, 1984; 1: 290-299 [PubMed] [Google Scholar]
- 199). Kioka Y, Tanabe A, Kotani Y, Yamada N, Nakahama M, Ueda T, Seitou T, Maruyama M. Review of coronary artery disease in patients with infrarenal abdominal aortic aneurysm. Circ J, 2002; 66: 1110-1112 [DOI] [PubMed] [Google Scholar]
- 200). Hirose K, Chikamori T, Hida S, Tanaka H, Igarashi Y, Watanabe Y, Koizumi N, Kawaguchi S, Obitsu Y, Shigematsu H, Yamashina A. Prevalence of coronary heart disease in patients with aortic aneurysm and/or peripheral artery disease. Am J Cardiol, 2009; 103: 1215-1220 [DOI] [PubMed] [Google Scholar]
- 201). Tollefson DF, Ernst CB. Natural history of atherosclerotic renal artery stenosis associated with aortic disease. J Vasc Surg, 1991; 14: 327-331 [PubMed] [Google Scholar]
- 202). Zierler RE, Bergelin RO, Davidson RC, Cantwell-Gab K, Polissar NL, Strandness DE. A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. Am J Hypertens, 1996; 9: 1055-1061 [DOI] [PubMed] [Google Scholar]
- 203). White CJ, Jaff MR, Haskal ZJ, Jones DJ, Olin JW, Rocha-Singh KJ, Rosenfield KA, Rundback JH, Linas SL, American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Radiology and Intervention; American Heart Association Council on Kidney in Cardiovascular Disease Indications for renal arteriography at the time of coronary arteriography: A science advisory from the American Heart Association committee on diagnostic and interventional cardiac catheterization, council on clinical cardiology, and the councils on cardiovascular radiology and intervention and on kidney in cardiovascular disease. Circulation, 2006; 114: 1892-1895 [DOI] [PubMed] [Google Scholar]
- 204). Conlon PJ, Athirakul K, Kovalik E, Schwab SJ, Crowley J, Stack R, McCants CB, Mark DB, Bashore TM, Albers F. Survival in renal vascular disease. J Am Soc Nephrol, 1998; 9: 252-256 [DOI] [PubMed] [Google Scholar]
- 205). Yamanaka H, Japanese Society of Gout and Nucleic Acid Metabolism Japanese guideline for the management of hyperuricemia and gout: Second edition. Nucleosides Nucleotides Nucleic Acids, 2011; 30: 1018-1029 [DOI] [PubMed] [Google Scholar]
- 206). Wang J, Qin T, Chen J, Li Y, Wang L, Huang H, Li J. Hyperuricemia and risk of incident hypertension: A systematic review and meta-analysis of observational studies. PLoS One, 2014; 9: e114259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207). Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and risk of stroke: A systematic review and meta-analysis. Arthritis Rheum, 2009; 61: 885-892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208). Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: A systematic review and meta-analysis. Arthritis Care Res (Hoboken), 2010; 62: 170-180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209). Zhang W, Iso H, Murakami Y, Miura K, Nagai M, Sugiyama D, Ueshima H, Okamura T, EPOCH-JAPAN GROUP Serum uric acid and mortality form cardiovascular disease: EPOCH-JAPAN Study. J Atheroscler Thromb, 2016; 23: 692-703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210). Agarwal V, Hans N, Messerli FH. Effect of allopurinol on blood pressure: A systematic review and meta-analysis. J Clin Hypertens (Greenwich), 2013; 15: 435-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211). MacIsaac RL, Salatzki J, Higgins P, Walters MR, Padmanabhan S, Dominiczak AF, Touyz RM, Dawson J. Allopurinol and cardiovascular outcomes in adults with hypertension. Hypertension, 2016; 67: 535-540 [DOI] [PubMed] [Google Scholar]
- 212). Wang J, Yu W, Gao M, Zhang F, Gu C, Yu Y, Wei Y. Impact of obstructive sleep apnea syndrome on endothelial function, arterial stiffening, and serum inflammatory markers: An updated meta-analysis and metaregression of 18 studies. J Am Heart Assoc, 2015; 4: e002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213). Zhou M, Guo B, Wang Y, Yan D, Lin C, Shi Z. The association between obstructive sleep apnea and carotid intima-media thickness: A systematic review and metaanalysis. Angiology, 2017; 68: 575-583 [DOI] [PubMed] [Google Scholar]
- 214). Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med, 2014; 190: 218-225 [DOI] [PubMed] [Google Scholar]
- 215). Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, Barbe F, Vicente E, Wei Y, Nieto FJ, Jelic S. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA, 2012; 307: 2169-2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216). Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet, 2005; 365: 1046-1053 [DOI] [PubMed] [Google Scholar]
- 217). Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, Soler-Cataluña JJ, Almeida-Gonzalez C, De la Cruz Morón I, Durán-Cantolla J, Montserrat JM. Cardiovascular mortality in obstructive sleep apnea in the elderly: Role of long-term continuous positive airway pressure treatment: A prospective observational study. Am J Respir Crit Care Med, 2012; 186: 909-916 [DOI] [PubMed] [Google Scholar]
- 218). Joint study group 2008–2009. Guidelines for Diagnosis and Treatment of Sleep Disordered Breathing in Cardiovascular Disease (JCS 2010) http://www.j-circ.or.jp/guideline/pdf/JCS2010,momom ura.h.pdf (in Japanese)
- 219). Barbé F, Duran-Cantolla J, Sánchez-de-la-Torre M, Martínez-Alonso M, Carmona C, Barceló A, Chiner E, Masa JF, Gonzalez M, Marín JM, Garcia-Rio F, Diaz de Atauri J, Terán J, Mayos M, de la Peña M, Monasterio C, del Campo F, Montserrat JM, Spanish Sleep And Breathing Network Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: A randomized controlled trial. JAMA, 2012; 307: 2161-2168 [DOI] [PubMed] [Google Scholar]
- 220). McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS, SAVE Investigators and Coordinators CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med, 2016; 375: 919-931 [DOI] [PubMed] [Google Scholar]
- 221). McLean JW, Tomlinson JE, Kuang WJ, Eaton DL, Chen EY, Fless GM, Scanu AM, Lawn RM. cDNA sequence of human apolipoprotein (a) is homologous to plasminogen. Nature, 1987; 330: 132-137 [DOI] [PubMed] [Google Scholar]
- 222). Kronenberg F, Utermann G. Lipoprotein (a): Resurrected by genetics. J Intern Med, 2013; 273: 6-30 [DOI] [PubMed] [Google Scholar]
- 223). Dubé JB, Boffa MB, Hegele RA, Koschinsky ML. Lipoprotein (a): More interesting than ever after 50 years. Curr Opin Lipidol, 2012; 23: 133-140 [DOI] [PubMed] [Google Scholar]
- 224). Schaefer EJ, Lamon-Fava S, Jenner JL, McNamara JR, Ordovas JM, Davis CE, Abolafia JM, Lippel K, Levy RI. Lipoprotein (a) levels and risk of coronary heart disease in men. The lipid research clinics coronary primary prevention trial. JAMA, 1994; 271: 999-1003 [DOI] [PubMed] [Google Scholar]
- 225). Bennet A, Di Angelantonio E, Erqou S, Eiriksdottir G, Sigurdsson G, Woodward M, Rumley A, Lowe GD, Danesh J, Gudnason V. Lipoprotein (a) levels and risk of future coronary heart disease: Large-scale prospective data. Arch Intern Med, 2008; 168: 598-608 [DOI] [PubMed] [Google Scholar]
- 226). Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J, Emerging Risk Factors Collaboration Lipoprotein (a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA, 2009; 302: 412-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227). Marcovina SM, Koschinsky ML. Evaluation of lipoprotein (a) as a prothrombotic factor: Progress from bench to bedside. Curr Opin Lipidol, 2003; 14: 361-366 [DOI] [PubMed] [Google Scholar]
- 228). Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D, Silveira A, Malarstig A, Green FR, Lathrop M, Gigante B, Leander K, de Faire U, Seedorf U, Hamsten A, Collins R, Watkins H, Farrall M, Consortium P. Genetic variants associated with lp (a) lipoprotein level and coronary disease. N Engl J Med, 2009; 361: 2518-2528 [DOI] [PubMed] [Google Scholar]
- 229). Boffa MB, Koschinsky ML. Lipoprotein (a): Truly a direct prothrombotic factor in cardiovascular disease? J Lipid Res, 2016; 57: 745-757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230). Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med, 2005; 353: 46-57 [DOI] [PubMed] [Google Scholar]
- 231). Tsimikas S, Witztum JL. The role of oxidized phospholipids in mediating lipoprotein (a) atherogenicity. Curr Opin Lipidol, 2008; 19: 369-377 [DOI] [PubMed] [Google Scholar]
- 232). Nielsen LB. Atherogenecity of lipoprotein (a) and oxidized low density lipoprotein: Insight from in vivo studies of arterial wall influx, degradation and efflux. Atherosclerosis, 1999; 143: 229-243 [DOI] [PubMed] [Google Scholar]
- 233). Hajjar KA, Nachman RL. The role of lipoprotein (a) in atherogenesis and thrombosis. Annu Rev Med, 1996; 47: 423-442 [DOI] [PubMed] [Google Scholar]
- 234). Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol, 2010; 30: 2311-2316 [DOI] [PubMed] [Google Scholar]
- 235). Yoshida H, Kisugi R. Mechanisms of LDL oxidation. Clin Chim Acta, 2010; 411: 1875-1882 [DOI] [PubMed] [Google Scholar]
- 236). Kotani K, Tashiro J, Yamazaki K, Nakamura Y, Miyazaki A, Bujo H, Saito Y, Kanno T, Maekawa M. Investigation of MDA-LDL (malondialdehyde-modified low-density lipoprotein) as a prognostic marker for coronary artery disease in patients with type 2 diabetes mellitus. Clin Chim Acta, 2015; 450: 145-150 [DOI] [PubMed] [Google Scholar]
- 237). Ito T, Fujita H, Tani T, Ohte N. Malondialdehyde-modified low-density lipoprotein is a predictor of cardiac events in patients with stable angina on lipid-lowering therapy after percutaneous coronary intervention using drug-eluting stent. Atherosclerosis, 2015; 239: 311-317 [DOI] [PubMed] [Google Scholar]
- 238). Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circ Res, 2016; 118: 547-563 [DOI] [PubMed] [Google Scholar]
- 239). Kugiyama K, Doi H, Takazoe K, Kawano H, Soejima H, Mizuno Y, Tsunoda R, Sakamoto T, Nakano T, Nakajima K, Ogawa H, Sugiyama S, Yoshimura M, Yasue H. Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation, 1999; 99: 2858-2860 [DOI] [PubMed] [Google Scholar]
- 240). Nakamura T, Obata JE, Hirano M, Kitta Y, Fujioka D, Saito Y, Kawabata K, Watanabe K, Watanabe Y, Mishina H, Kugiyama K. Predictive value of remnant lipoprotein for cardiovascular events in patients with coronary artery disease after achievement of LDL-cholesterol goals. Atherosclerosis, 2011; 218: 163-167 [DOI] [PubMed] [Google Scholar]
- 241). Nguyen SV, Nakamura T, Kugiyama K. High remnant lipoprotein predicts recurrent cardiovascular events on statin treatment after acute coronary syndrome. Circ J, 2014; 78: 2492-2500 [DOI] [PubMed] [Google Scholar]
- 242). Nguyen SV, Nakamura T, Uematsu M, Fujioka D, Watanabe K, Watanabe Y, Obata JE, Nakamura K, Kugiyama K. Remnant lipoproteinemia predicts cardiovascular events in patients with type 2 diabetes and chronic kidney disease. J Cardiol, 2017; 69: 529-535 [DOI] [PubMed] [Google Scholar]
- 243). Jepsen AM, Langsted A, Varbo A, Bang LE, Kamstrup PR, Nordestgaard BG. Increased remnant cholesterol explains part of residual risk of all-cause mortality in 5414 patients with ischemic heart disease. Clin Chem, 2016; 62: 593-604 [DOI] [PubMed] [Google Scholar]
- 244). Zilversmit DB. Atherogenesis: A postprandial phenomenon. Circulation, 1979; 60: 473-485 [DOI] [PubMed] [Google Scholar]
- 245). Havel RJ. Remnant lipoproteins as therapeutic targets. Curr Opin Lipidol, 2000; 11: 615-620 [DOI] [PubMed] [Google Scholar]
- 246). Eberly LE, Stamler J, Neaton JD, Multiple Risk Factor Intervention Trial Research Group Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med, 2003; 163: 1077-1083 [DOI] [PubMed] [Google Scholar]
- 247). Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, Watts GF, Sypniewska G, Wiklund O, Boren J, Chapman MJ, Cobbaert C, Descamps OS, von Eckardstein A, Kamstrup PR, Pulkki K, Kronenberg F, Remaley AT, Rifai N, Ros E, Langlois M, European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Joint Consensus Initiative Author information. Fasting is not routinely required for determination of a lipid profile: Clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J, 2016; 37: 1944-1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248). Driver SL, Martin SS, Gluckman TJ, Clary JM, Blumenthal RS, Stone NJ. Fasting or nonfasting lipid measurements: It depends on the question. J Am Coll Cardiol, 2016; 67: 1227-1234 [DOI] [PubMed] [Google Scholar]
- 249). Masuda D, Sakai N, Sugimoto T, Kitazume-Taneike R, Yamashita T, Kawase R, Nakaoka H, Inagaki M, Nakatani K, Yuasa-Kawase M, Tsubakio-Yamamoto K, Ohama T, Nakagawa-Toyama Y, Nishida M, Ishigami M, Masuda Y, Matsuyama A, Komuro I, Yamashita S. Fasting serum apolipoprotein b-48 can be a marker of postprandial hyperlipidemia. J Atheroscler Thromb, 2011; 18: 1062-1070 [DOI] [PubMed] [Google Scholar]
- 250). Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA, 1988; 260: 1917-1921 [PubMed] [Google Scholar]
- 251). Krauss, RM. Low-density lipoprotein subclass and risk of coronary disease. Curr opin Lipidol, 1991; 4: 248-252 [Google Scholar]
- 252). St-Pierre AC, Cantin B, Dagenais GR, Mauriege P, Bernard PM, Despres JP, Lamarche B. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular study. Arterioscler Thromb Vasc Biol, 2005; 25: 553-559 [DOI] [PubMed] [Google Scholar]
- 253). Arsenault BJ, Lemieux I, Despres JP, Wareham NJ, Luben R, Kastelein JJ, Khaw KT, Boekholdt SM. Cholesterol levels in small LDL particles predict the risk of coronary heart disease in the epic-norfolk prospective population study. Eur Heart J, 2007; 28: 2770-2777 [DOI] [PubMed] [Google Scholar]
- 254). El Harchaoui K, van der Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JJ, Khaw KT, Boekholdt SM. Value of lowdensity lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: The EPIC Norfolk prospective population study. J Am Coll Cardiol, 2007; 49: 547-553 [DOI] [PubMed] [Google Scholar]
- 255). Austin MA, Rodriguez BL, McKnight B, McNeely MJ, Edwards KL, Curb JD, Sharp DS. Low-density lipoprotein particle size, triglycerides, and high-density lipoprotein cholesterol as risk factors for coronary heart disease in older Japanese-American men. Am J Cardiol, 2000; 86: 412-416 [DOI] [PubMed] [Google Scholar]
- 256). Koba S, Hirano T, Ito Y, Tsunoda F, Yokota Y, Ban Y, Iso Y, Suzuki H, Katagiri T. Significance of small dense lowdensity lipoprotein-cholesterol concentrations in relation to the severity of coronary heart diseases. Atherosclerosis, 2006; 189: 206-214 [DOI] [PubMed] [Google Scholar]
- 257). Rizzo M, Pernice V, Frasheri A, Berneis K. Atherogenic lipoprotein phenotype and LDL size and subclasses in patients with peripheral arterial disease. Atherosclerosis, 2008; 197: 237-241 [DOI] [PubMed] [Google Scholar]
- 258). Rizzo M, Krayenbuhl PA, Pernice V, Frasheri A, Battista Rini G, Berneis K. LDL size and subclasses in patients with abdominal aortic aneurysm. Int J Cardiol, 2009; 134: 406-408 [DOI] [PubMed] [Google Scholar]
- 259). Hirano T, Ito Y, Koba S, Toyoda M, Ikejiri A, Saegusa H, Yamazaki J, Yoshino G. Clinical significance of small dense low-density lipoprotein cholesterol levels determined by the simple precipitation method. Arterioscler Thromb Vasc Biol, 2004; 24: 558-563 [DOI] [PubMed] [Google Scholar]
- 260). Koba S, Yokota Y, Hirano T, Ito Y, Ban Y, Tsunoda F, Sato T, Shoji M, Suzuki H, Geshi E, Kobayashi Y, Katagiri T. Small LDL-cholesterol is superior to LDL-cholesterol for determining severe coronary atherosclerosis. J Atheroscler Thromb, 2008; 15: 250-260 [DOI] [PubMed] [Google Scholar]
- 261). Nishikura T, Koba S, Yokota Y, Hirano T, Tsunoda F, Shoji M, Hamazaki Y, Suzuki H, Itoh Y, Katagiri T, Kobayashi Y. Elevated small dense low-density lipoprotein cholesterol as a predictor for future cardiovascular events in patients with stable coronary artery disease. J Atheroscler Thromb, 2014; 21: 755-767 [DOI] [PubMed] [Google Scholar]
- 262). de Graaf J, Hak-Lemmers HL, Hectors MP, Demacker PN, Hendriks JC, Stalenhoef AF. Enhanced susceptibility to in vitro oxidation of the dense low density lipoprotein subfraction in healthy subjects. Arterioscler Thromb, 1991; 11: 298-306 [DOI] [PubMed] [Google Scholar]
- 263). Galeano NF, Al-Haideri M, Keyserman F, Rumsey SC, Deckelbaum RJ. Small dense low density lipoprotein has increased affinity for LDL receptor-independent cell surface binding sites: A potential mechanism for increased atherogenicity. J Lipid Res, 1998; 39: 1263-1273 [PubMed] [Google Scholar]
- 264). Ip S, Lichtenstein AH, Chung M, Lau J, Balk EM. Systematic review: Association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann Intern Med, 2009; 150: 474-484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265). Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res, 2002; 43: 1363-1379 [DOI] [PubMed] [Google Scholar]
- 266). Reaven GM, Chen YD, Jeppesen J, Maheux P, Krauss RM. Insulin resistance and hyperinsulinemia in individuals with small, dense low density lipoprotein particles. J Clin Invest, 1993; 92: 141-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267). Austin MA, Edwards KL. Small, dense low density lipoproteins, the insulin resistance syndrome and noninsulin-dependent diabetes. Curr Opin Lipidol, 1996; 7: 167-171 [DOI] [PubMed] [Google Scholar]
- 268). Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA, 2005; 294: 326-333 [DOI] [PubMed] [Google Scholar]
- 269). Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, Furberg CD. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein b as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes, 2011; 4: 337-345 [DOI] [PubMed] [Google Scholar]
- 270). Pencina MJ, D'Agostino RB, Zdrojewski T, Williams K, Thanassoulis G, Furberg CD, Peterson ED, Vasan RS, Sniderman AD. Apolipoprotein B improves risk assessment of future coronary heart disease in the Framingham Heart Study beyond LDL-C and non-HDL-C. Eur J Prev Cardiol, 2015; 22: 1321-1327 [DOI] [PubMed] [Google Scholar]
- 271). Thanassoulis G, Williams K, Ye K, Brook R, Couture P, Lawler PR, de Graaf J, Furberg CD, Sniderman A. Relations of change in plasma levels of LDL-C, non-HDL-C and apoB with risk reduction from statin therapy: A meta-analysis of randomized trials. J Am Heart Assoc, 2014; 3: e000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272). Robinson JG, Wang S, Jacobson TA. Meta-analysis of comparison of effectiveness of lowering apolipoprotein B versus low-density lipoprotein cholesterol and nonhighdensity lipoprotein cholesterol for cardiovascular risk reduction in randomized trials. Am J Cardiol, 2012; 110: 1468-1476 [DOI] [PubMed] [Google Scholar]
- 273). McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, Kazmi K, Yusuf S. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART Study): A case-control study. Lancet, 2008; 372: 224-233 [DOI] [PubMed] [Google Scholar]
- 274). Kastelein JJ, van der Steeg WA, Holme I, Gaffney M, Cater NB, Barter P, Deedwania P, Olsson AG, Boekholdt SM, Demicco DA, Szarek M, LaRosa JC, Pedersen TR, Grundy SM, TNT Study Group; IDEAL Study Group Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation, 2008; 117: 3002-3009 [DOI] [PubMed] [Google Scholar]
- 275). Ray KK, Cannon CP, Cairns R, Morrow DA, Ridker PM, Braunwald E. Prognostic utility of apoB/AI, total cholesterol/HDL, non-HDL cholesterol, or hs-CRP as predictors of clinical risk in patients receiving statin therapy after acute coronary syndromes: Results from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol, 2009; 29: 424-430 [DOI] [PubMed] [Google Scholar]
- 276). Hong LF, Yan XN, Fan Y, Wu Q, Luo SH, Yang B, Li JJ. Is the ratio of apoB/apoA-1 the best predictor for the severity of coronary artery lesions in chinese diabetics with stable angina pectoris? An assessment based on gensini scores. J Geriatr Cardiol, 2015; 12: 402-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277). Hisamatsu T, Fujiyoshi A, Miura K, Ohkubo T, Kadota A, Kadowaki S, Kadowaki T, Yamamoto T, Miyagawa N, Zaid M, Torii S, Takashima N, Murakami Y, Okamura T, Horie M, Ueshima H, SESSA Research Group Lipoprotein particle profiles compared with standard lipids in association with coronary artery calcification in the general Japanese population. Atherosclerosis, 2014; 236: 237-243 [DOI] [PubMed] [Google Scholar]
- 278). Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett-Connor E, Benjamin EJ, Bjorkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D'Agostino RB, Dankner R, Davey-Smith G, Deeg D, Dekker JM, Engstrom G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, Jorgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil-Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J, Emerging Risk Factors Collaboration C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med, 2012; 367: 1310-1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279). Kawase Ishihara K, Kokubo Y, Yokota C, Hida E, Miyata T, Toyoda K, Matsumoto M, Minematsu K, Miyamoto Y. Effect of plasma fibrinogen, high-sensitive c-reactive protein, and cigarette smoking on carotid atherosclerosis: The Suita Study. J Stroke Cerebrovasc Dis, 2015; 24: 2385-2389 [DOI] [PubMed] [Google Scholar]
- 280). Chei CL, Yamagishi K, Kitamura A, Kiyama M, Imano H, Ohira T, Cui R, Tanigawa T, Sankai T, Ishikawa Y, Sato S, Iso H. C-reactive protein levels and risk of stroke and its subtype in Japanese: The circulatory risk in communities study (CIRCS). Atherosclerosis, 2011; 217: 187-193 [DOI] [PubMed] [Google Scholar]
- 281). Iso H, Noda H, Ikeda A, Yamagishi K, Inoue M, Iwasaki M, Tsugane S. The impact of c-reactive protein on risk of stroke, stroke subtypes, and ischemic heart disease in middle-aged Japanese: The Japan public health centerbased study. J Atheroscler Thromb, 2012; 19: 756-766 [PubMed] [Google Scholar]
- 282). Wensley F, Gao P, Burgess S, Kaptoge S, Di Angelantonio E, Shah T, Engert JC, Clarke R, Davey-Smith G, Nordestgaard BG, Saleheen D, Samani NJ, Sandhu M, Anand S, Pepys MB, Smeeth L, Whittaker J, Casas JP, Thompson SG, Hingorani AD, Danesh J, C Reactive Protein Coronary Heart Disease Genetics Collaboration (CCGC) Association between c reactive protein and coronary heart disease: Mendelian randomisation analysis based on individual participant data. BMJ, 2011; 342: d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283). Matsuura Y, Hatakeyama K, Imamura T, Tsuruda T, Shibata Y, Kodama T, Kitamura K, Asada Y. Different distribution of pentraxin 3 and c-reactive protein in coronary atherosclerotic plaques. J Atheroscler Thromb, 2012; 19: 837-845 [DOI] [PubMed] [Google Scholar]
- 284). Iwata A, Miura S, Tanaka T, Ike A, Sugihara M, Nishikawa H, Kawamura A, Saku K. Plasma pentraxin-3 levels are associated with coronary plaque vulnerability and are decreased by statin. Coron Artery Dis, 2012; 23: 315-321 [DOI] [PubMed] [Google Scholar]
- 285). Hollan I, Nebuloni M, Bottazzi B, Mikkelsen K, Forre OT, Almdahl SM, Mantovani A, Fagerland MW, Aukrust P, Meroni PL, Feiring Heart Biopsy Study Group Pentraxin 3, a novel cardiovascular biomarker, is expressed in aortic specimens of patients with coronary artery disease with and without rheumatoid arthritis. Cardiovasc Pathol, 2013; 22: 324-331 [DOI] [PubMed] [Google Scholar]
- 286). Yasunaga T, Ikeda S, Koga S, Nakata T, Yoshida T, Masuda N, Kohno S, Maemura K. Plasma pentraxin 3 is a more potent predictor of endothelial dysfunction than high-sensitive c-reactive protein. Int Heart J, 2014; 55: 160-164 [DOI] [PubMed] [Google Scholar]
- 287). Gerhard GT, Duell PB. Homocysteine and atherosclerosis. Curr Opin Lipidol, 1999; 10: 417-428 [DOI] [PubMed] [Google Scholar]
- 288). Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: A metaanalysis. JAMA, 2002; 288: 2015-2022 [DOI] [PubMed] [Google Scholar]
- 289). Verhoef P, Stampfer MJ. Prospective studies of homocysteine and cardiovascular disease. Nutr Rev, 1995; 53: 283-288 [DOI] [PubMed] [Google Scholar]
- 290). de Ruijter W, Westendorp RG, Assendelft WJ, den Elzen WP, de Craen AJ, le Cessie S, Gussekloo J. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: Population based observational cohort study. BMJ, 2009; 338: a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291). Clarke R, Halsey J, Bennett D, Lewington S. Homocysteine and vascular disease: Review of published results of the homocysteine-lowering trials. J Inherit Metab Dis, 2011; 34: 83-91 [DOI] [PubMed] [Google Scholar]
- 292). Lewis SJ, Ebrahim S, Davey Smith G. Meta-analysis of mthfr 677c->t polymorphism and coronary heart disease: Does totality of evidence support causal role for homocysteine and preventive potential of folate? BMJ, 2005; 331: 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293). Martí-Carvajal AJ, Sola I, Lathyris D. Homocysteinelowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev, 2015; 1: CD006612. [DOI] [PubMed] [Google Scholar]
- 294). Fuster V, Lewis A. Conner memorial lecture. Mechanisms leading to myocardial infarction: Insights from studies of vascular biology. Circulation, 1994; 90: 2126-2146 [DOI] [PubMed] [Google Scholar]
- 295). Hou X, Chen X, Shi J. Genetic polymorphism of MTHFR C677T and premature coronary artery disease susceptibility: A meta-analysis. Gene, 2015; 565: 39-44 [DOI] [PubMed] [Google Scholar]
- 296). van Meurs JB, Pare G, Schwartz SM, Hazra A, Tanaka T, Vermeulen SH, Cotlarciuc I, Yuan X, Malarstig A, Bandinelli S, Bis JC, Blom H, Brown MJ, Chen C, Chen YD, Clarke RJ, Dehghan A, Erdmann J, Ferrucci L, Hamsten A, Hofman A, Hunter DJ, Goel A, Johnson AD, Kathiresan S, Kampman E, Kiel DP, Kiemeney LA, Chambers JC, Kraft P, Lindemans J, McKnight B, Nelson CP, O'Donnell CJ, Psaty BM, Ridker PM, Rivadeneira F, Rose LM, Seedorf U, Siscovick DS, Schunkert H, Selhub J, Ueland PM, Vollenweider P, Waeber G, Waterworth DM, Watkins H, Witteman JC, den Heijer M, Jacques P, Uitterlinden AG, Kooner JS, Rader DJ, Reilly MP, Mooser V, Chasman DI, Samani NJ, Ahmadi KR. Common genetic loci influencing plasma homocysteine concentrations and their effect on risk of coronary artery disease. Am J Clin Nutr, 2013; 98: 668-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297). Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, Goldbourt U, Willeit J, Kiechl S, Yarnell JW, Sweetnam PM, Elwood PC, Cushman M, Psaty BM, Tracy RP, Tybjaerg-Hansen A, Haverkate F, de Maat MP, Fowkes FG, Lee AJ, Smith FB, Salomaa V, Harald K, Rasi R, Vahtera E, Jousilahti P, Pekkanen J, D'Agostino R, Kannel WB, Wilson PW, Tofler G, Arocha-Pinango CL, Rodriguez-Larralde A, Nagy E, Mijares M, Espinosa R, Rodriquez-Roa E, Ryder E, Diez-Ewald MP, Campos G, Fernandez V, Torres E, Marchioli R, Valagussa F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Cremer P, Nagel D, Curb JD, Rodriguez B, Yano K, Salonen JT, Nyyssonen K, Tuomainen TP, Hedblad B, Lind P, Loewel H, Koenig W, Meade TW, Cooper JA, De Stavola B, Knottenbelt C, Miller GJ, Bauer KA, Rosenberg RD, Sato S, Kitamura A, Naito Y, Palosuo T, Ducimetiere P, Amouyel P, Arveiler D, Evans AE, Ferrieres J, Juhan-Vague I, Bingham A, Schulte H, Assmann G, Cantin B, Lamarche B, Despres JP, Dagenais GR, Tunstall-Pedoe H, Woodward M, Ben-Shlomo Y, Davey Smith G, Palmieri V, Yeh JL, Rudnicka A, Ridker P, Rodeghiero F, Tosetto A, Shepherd J, Ford I, Robertson M, Brunner E, Shipley M, Feskens EJ, Kromhout D, Dickinson A, Ireland B, Juzwishin K, Kaptoge S, Memon A, Sarwar N, Walker M, Wheeler J, White I, Wood A, Fibrinogen Studies Collaboration Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: An individual participant meta-analysis. JAMA, 2005; 294: 1799-1809 [DOI] [PubMed] [Google Scholar]
- 298). Kunutsor SK, Kurl S, Zaccardi F, Laukkanen JA. Baseline and long-term fibrinogen levels and risk of sudden cardiac death: A new prospective study and meta-analysis. Atherosclerosis, 2016; 245: 171-180 [DOI] [PubMed] [Google Scholar]
- 299). Tabakc? MM, Gerin F, Sunbul M, Toprak C, Durmuş H, Demir S, Arslantaş U, Cerşit S, Batgerel U, Karg?n R. Relation of plasma fibrinogen level with the presence, severity, and complexity of coronary artery disease. Clin Appl Thromb Hemost, 2017; 23: 638-644 [DOI] [PubMed] [Google Scholar]
- 300). Nagasawa SY, Ohkubo T, Masaki K, Barinas-Mitchell E, Miura K, Seto T, El-Saed A, Kadowaki T, Willcox BJ, Edmundowicz D, Kadota A, Evans RW, Kadowaki S, Fujiyoshi A, Hisamatsu T, Bertolet MH, Okamura T, Nakamura Y, Kuller LH, Ueshima H, Sekikawa A. Associations between inflammatory markers and subclinical atherosclerosis in middle-aged white, Japanese-American and Japanese men: The ERA-JUMP study. J Atheroscler Thromb, 2015; 22: 590-598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301). Wang NC, Matthews KA, Barinas-Mitchell EJ, Chang CC, El Khoudary SR. Inflammatory/hemostatic biomarkers and coronary artery calcium progression in women at midlife (from the Study of Women's Health Across the Nation, Heart Study). Am J Cardiol, 2016; 118: 311-318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302). Sakamoto T, Yasue H, Ogawa H, Misumi I, Masuda T. Association of patency of the infarct-related coronary artery with plasma levels of plasminogen activator inhibitor activity in acute myocardial infarction. Am J Cardiol, 1992; 70: 271-276 [DOI] [PubMed] [Google Scholar]
- 303). Hoekstra T, Geleijnse JM, Schouten EG, Kluft C. Plasminogen activator inhibitor-type 1: Its plasma determinants and relation with cardiovascular risk. Thromb Haemost, 2004; 91: 861-872 [DOI] [PubMed] [Google Scholar]
- 304). Nikolopoulos GK, Bagos PG, Tsangaris I, Tsiara CG, Kopterides P, Vaiopoulos A, Kapsimali V, Bonovas S, Tsantes AE. The association between plasminogen activator inhibitor type 1 (PAI-1) levels, PAI-1 4g/5g polymorphism, and myocardial infarction: A mendelian randomization meta-analysis. Clin Chem Lab Med, 2014; 52: 937-950 [DOI] [PubMed] [Google Scholar]
- 305). Tsantes AE, Nikolopoulos GK, Bagos PG, Tsiara CG, Kapsimali V, Travlou A, Vaiopoulos G. Plasminogen activator inhibitor-1 4g/5g polymorphism and risk of ischemic stroke: A meta-analysis. Blood Coagul Fibrinolysis, 2007; 18: 497-504 [DOI] [PubMed] [Google Scholar]
- 306). Ministry of Health, Labour and Welfare. National health and nutrition survey in Japan, 2014. 2016. https://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h26-houkoku.pdf (in Japanese)
- 307). Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes, 1988; 37: 1595-1607 [DOI] [PubMed] [Google Scholar]
- 308). Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med, 1989; 149: 1514-1520 [DOI] [PubMed] [Google Scholar]
- 309). Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism, 1987; 36: 54-59 [DOI] [PubMed] [Google Scholar]
- 310). DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for niddm, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care, 1991; 14: 173-194 [DOI] [PubMed] [Google Scholar]
- 311). World Health Organization Department of Noncommunicable Disease Surveillance. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1 Diagnosis and Classification of Diabetes Mellitus, 1999. http://apps.who.int/iris/bitstream/handle/10665/66040/WHO_NCD_99.2.pdf [Google Scholar]
- 312). Committee on Diagnostic Criteria for Metabolic Syndrome: Definition and diagnostic criteria of metabolic syndrome. J Jpn Soc Intern Med, 2005; 94: 794-809 (in Japanese) [PubMed] [Google Scholar]
- 313). Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group The metabolic syndrome--a new worldwide definition. Lancet, 2005; 366: 1059-1062 [DOI] [PubMed] [Google Scholar]
- 314). Nakamura T, Tsubono Y, Kameda-Takemura K, Funahashi T, Yamashita S, Hisamichi S, Kita T, Yamamura T, Matsuzawa Y, Group of the Research for the Association between Host Origin and Atherosclerotic Diseases under the Preventive Measure for Work-related Diseases of the Japanese Labor Ministry Magnitude of sustained multiple risk factors for ischemic heart disease in Japanese employees: A case-control study. Jpn Circ J, 2001; 65: 11-17 [DOI] [PubMed] [Google Scholar]
- 315). The 1994 Report of the Group of the Research for the Association between Host Origin and Atherosclerotic Diseases under the Preventive Measure for Work-related Diseases of the Japanese Labor Ministry: Significance of host factors contributing to the development of atherosclerosis (in Japanese) [Google Scholar]
- 316). Nakamura Y, Yamamoto T, Okamura T, Kadowaki T, Hayakawa T, Kita Y, Saitoh S, Okayama A, Ueshima H, NIPPON DATA 80 Research Group Combined cardiovascular risk factors and outcome: NIPPON DATA80, 1980–1994. Circ J, 2006; 70: 960-964 [DOI] [PubMed] [Google Scholar]
- 317). Okubo K, Kiyohara Y. Frequency of the metabolic syndrome in the general inhabitant. Rinsho to Kenkyu, 2004; 81: 1736-1740 (in Japanese) [Google Scholar]
- 318). Matsuzawa Y. Multicenter follow-up study of insulin resistance and the lifestyle basis in patients at high risk of diabetes mellitus-establishment of significance of abdominal obesity for intervention. Health Science Research Project, Ministry of Health, Labour and Welfare, 2001. (in Japanese) [Google Scholar]
- 319). Matsuzawa, Y, Inoue S, Ikeda Y, Sakata T, Saitou Y, Satoh H, Shirai A, Oono J, Miyazaki S, Tokunaga M, Fukagawa H, Yamanouchi K, Nakamura M. New evaluation of obesity and diagnostic criteria of obesity. Journal of Japan Society for the Study of Obesity, 2000; 6: 18-28 (in Japanese) [Google Scholar]
- 320). Japan Society for the Study of Obesity. Guidelines for the management of obesity disease 2016. (in Japanese) [Google Scholar]
- 321). Nakamura T, Tokunaga K, Shimomura I, Nishida M, Yoshida S, Kotani K, Islam AH, Keno Y, Kobatake T, Nagai Y. Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis, 1994; 107: 239-246 [DOI] [PubMed] [Google Scholar]
- 322). Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, Shofer JB, Wahl PW. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the seattle Japanese-American community diabetes study. Diabetes Care, 1999; 22: 1808-1812 [DOI] [PubMed] [Google Scholar]
- 323). Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation, 2004; 110: 1245-1250 [DOI] [PubMed] [Google Scholar]
- 324). Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP, San Antonio Heart Study National cholesterol education program versus world health organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation, 2004; 110: 1251-1257 [DOI] [PubMed] [Google Scholar]
- 325). Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care, 2001; 24: 683-689 [DOI] [PubMed] [Google Scholar]
- 326). Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA, 2002; 288: 2709-2716 [DOI] [PubMed] [Google Scholar]
- 327). Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol, 2010; 56: 1113-1132 [DOI] [PubMed] [Google Scholar]
- 328). Takeuchi H, Saitoh S, Takagi S, Ohnishi H, Ohhata J, Isobe T, Shimamoto K. Metabolic syndrome and cardiac disease in Japanese men: Applicability of the concept of metabolic syndrome defined by the National Cholesterol Education Program-Adult Treatment Panel III to Japanese men--the Tanno and Sobetsu study. Hypertens Res, 2005; 28: 203-208 [DOI] [PubMed] [Google Scholar]
- 329). Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation, 2009; 120: 1640-1645 [DOI] [PubMed] [Google Scholar]
- 330). Hiuge-Shimizu A, Kishida K, Funahashi T, Ishizaka Y, Oka R, Okada M, Suzuki S, Takaya N, Nakagawa T, Fukui T, Fukuda H, Watanabe N, Yoshizumi T, Nakamura T, Matsuzawa Y, Yamakado M, Shimomura I. Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (the VACATION-J study). Ann Med, 2012; 44: 82-92 [DOI] [PubMed] [Google Scholar]
- 331). D'Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation, 2008; 117: 743-753 [DOI] [PubMed] [Google Scholar]
- 332). Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetiere P, Jousilahti P, Keil U, Njolstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM, SCORE project group Estimation of ten-year risk of fatal cardiovascular disease in Europe: The score project. Eur Heart J, 2003; 24: 987-1003 [DOI] [PubMed] [Google Scholar]
- 333). Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Sorlie P, Stone NJ, Wilson PW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American college of cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol, 2014; 63: 2935-2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334). Arima H, Yonemoto K, Doi Y, Ninomiya T, Hata J, Tanizaki Y, Fukuhara M, Matsumura K, Iida M, Kiyohara Y. Development and validation of a cardiovascular risk prediction model for Japanese: The Hisayama study. Hypertens Res, 2009; 32: 1119-1122 [DOI] [PubMed] [Google Scholar]
- 335). Matsumoto M, Ishikawa S, Kayaba K, Gotoh T, Nago N, Tsutsumi A, Kajii E, Jichi Medical School (JMS) Cohort Study Group Risk charts illustrating the 10-year risk of myocardial infarction among residents of Japanese rural communities: The JMS cohort study. J Epidemiol, 2009; 19: 94-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336). Ishikawa S, Matsumoto M, Kayaba K, Gotoh T, Nago N, Tsutsumi A, Kajii E, Jichi Medical School (JMS) Cohort Study Group Risk charts illustrating the 10-year risk of stroke among residents of Japanese rural communities: The JMS cohort study. J Epidemiol, 2009; 19: 101-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337). Tanabe N, Iso H, Okada K, Nakamura Y, Harada A, Ohashi Y, Ando T, Ueshima H, Japan Arteriosclerosis Longitudinal Study Group Serum total and non-highdensity lipoprotein cholesterol and the risk prediction of cardiovascular events - the JALS-ECC -. Circ J, 2010; 74: 1346-1356 [DOI] [PubMed] [Google Scholar]
- 338). Noda H, Iso H, Sairenchi T, Irie F, Fukasawa N, Toriyama Y, Ota H, Nose T. Prediction of stroke, coronary heart disease, cardiovascular disease, cancer, and total death based on results of annual health checkups. Nihon Koshu Eisei Zasshi, 2006: 265-276 (in Japanese) [PubMed] [Google Scholar]
- 339). Yatsuya H, Iso H, Yamagishi K, Kokubo Y, Saito I, Suzuki K, Sawada N, Inoue M, Tsugane S. Development of a point-based prediction model for the incidence of total stroke: Japan public health center study. Stroke, 2013; 44: 1295-1302 [DOI] [PubMed] [Google Scholar]
- 340). Yatsuya H, Iso H, Li Y, Yamagishi K, Kokubo Y, Saito I, Sawada N, Inoue M, Tsugane S. Development of a risk equation for the incidence of coronary artery disease and ischemic stroke for middle-aged Japanese - Japan Public Health Center-Based Prospective Study. Circ J, 2016; 80: 1386-1395 [DOI] [PubMed] [Google Scholar]
- 341). Nakai M, Miyamoto Y, Higashiyama A, Murakami Y, Nishimura K, Yatsuya H, Saitoh S, Sakata K, Iso H, Miura K, Ueshima H, Okamura T, EPOCH-JAPAN Research Group Calibration between the estimated probability of the risk assessment chart of Japan atherosclerosis society and actual mortality using external population: Evidence for cardiovascular prevention from observational cohorts in Japan (EPOCH-JAPAN). J Atheroscler Thromb, 2016; 23: 176-195 [DOI] [PubMed] [Google Scholar]
- 342). Tanaka T, Okamura T. Blood cholesterol level and risk of stroke in community-based or worksite cohort studies: A review of Japanese cohort studies in the past 20 years. Keio J Med, 2012; 61: 79-88 [DOI] [PubMed] [Google Scholar]
- 343). Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American college of cardiology/American Heart Association task force on practice guidelines. Circulation, 2014; 129: S1-45 [DOI] [PubMed] [Google Scholar]
- 344). Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL. 2016 ESC/EAS guidelines for the management of dyslipidaemias: The task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis, 2016; 253: 281-344 [DOI] [PubMed] [Google Scholar]
- 345). Asayama K, Satoh M, Murakami Y, Ohkubo T, Nagasawa SY, Tsuji I, Nakayama T, Okayama A, Miura K, Imai Y, Ueshima H, Okamura T, Evidence for Cardiovascular Prevention From Observational Cohorts in Japan (EPOCH-JAPAN) Research Group Cardiovascular risk with and without antihypertensive drug treatment in the Japanese general population: Participantlevel meta-analysis. Hypertension, 2014; 63: 1189-1197 [DOI] [PubMed] [Google Scholar]
- 346). Okamura T, Sugiyama D, Tanaka T, Dohi S. Worksite wellness for the primary and secondary prevention of cardiovascular disease in Japan: The current delivery system and future directions. Prog Cardiovasc Dis, 2014; 56: 515-521 [DOI] [PubMed] [Google Scholar]
- 347). Fager G, Wiklund O. Cholesterol reduction and clinical benefit. Are there limits to our expectations? Arterioscler Thromb Vasc Biol, 1997; 17: 3527-3533 [DOI] [PubMed] [Google Scholar]
- 348). Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists' (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet, 2005; 366: 1267-1278 [DOI] [PubMed] [Google Scholar]
- 349). Saiki Y, Otsuka T, Kato K, Kawada T. A proposal for the optimal management target for serum non-high-density lipoprotein cholesterol level in low-risk Japanese workers. J Atheroscler Thromb, 2016; 23: 422-430 [DOI] [PubMed] [Google Scholar]
- 350). Kuwabara K, Harada S, Sugiyama D, Kurihara A, Kubota Y, Higashiyama A, Hirata T, Nishida Y, Kawasaki M, Takebayashi T, Okamura T. Relationship between non-high-density lipoprotein cholesterol and low-density lipoprotein cholesterol in the general population. J Atheroscler Thromb, 2016; 23: 477-490 [DOI] [PubMed] [Google Scholar]
- 351). Hirata T, Sugiyama D, Nagasawa SY, Murakami Y, Saitoh S, Okayama A, Iso H, Irie F, Sairenchi T, Miyamoto Y, Yamada M, Ishikawa S, Miura K, Ueshima H, Okamura T, Evidence for Cardiovascular Prevention from Observational Cohorts in Japan (EPOCH-JAPAN) Research Group A pooled analysis of the association of isolated low levels of high-density lipoprotein cholesterol with cardiovascular mortality in Japan. Eur J Epidemiol, 2017: 32: 547-557 [DOI] [PubMed] [Google Scholar]
- 352). Tan CE, Glantz SA. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation, 2012; 126: 2177-2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353). Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev, 2013: CD000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354). US Department of Health and Human Services: Treating Tobacco Use and Dependence: 2008 Update. https://www.ncbi.nlm.nih.gov/books/NBK63952/.pdf
- 355). The Japanese Circulation Society, The Japan Lung Cancer Society, Japanese Cancer Association, The Japanese Respiratory Society. Standard procedure for smoking cessation treatment. vol. 6, 2014. (in Japanese) [Google Scholar]
- 356). Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev, 2012; 11: CD000146. [DOI] [PubMed] [Google Scholar]
- 357). Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev, 2016: CD006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358). Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: metaanalysis. BMJ, 2012; 345: e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359). Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J, 2011; 161: 145-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360). Komiyama M, Shimada S, Wada H, Yamakage H, Satoh-Asahara N, Shimatsu A, Akao M, Morimoto T, Takahashi Y, Hasegawa K. Time-dependent changes of atherosclerotic LDL complexes after smoking cessation. J Atheroscler Thromb, 2016; 23: 1270-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361). Clair C, Rigotti NA, Porneala B, Fox CS, D'Agostino RB, Pencina MJ, Meigs JB. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA, 2013; 309: 1014-1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362). Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: A 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed), 1984; 289: 1257-1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363). Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed), 1984; 288: 1401-1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364). Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Shofer JB, Fish BE, Knopp RH, Kahn SE. Intra-abdominal fat is a major determinant of the national cholesterol education program adult treatment panel Ⅲ criteria for the metabolic syndrome. Diabetes, 2004; 53: 2087-2094 [DOI] [PubMed] [Google Scholar]
- 365). Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS Lett, 2006; 580: 2917-2921 [DOI] [PubMed] [Google Scholar]
- 366). Japan Society for the Study of Obesity, ed.: 2011 Diagnostic guidelines for obesity. J Jpn Soc Study of Obesity, 2011; 17: 1-78 (in Japanese) [Google Scholar]
- 367). The Examination Committee of Criteria for Obesity Disease in Japan (2002) Japan Society for the Study of Obesity: New criteria for obesity disease in Japan. Circ J, 2002; 66: 987-992 [DOI] [PubMed] [Google Scholar]
- 368). Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH, American Heart Association Council on Nutrition Physical Activity, and Metabolism Clinical implications of obesity with specific focus on cardiovascular disease: A statement for professionals from the American Heart Association council on nutrition, physical activity, and metabolism: Endorsed by the American college of cardiology foundation. Circulation, 2004; 110: 2952-2967 [DOI] [PubMed] [Google Scholar]
- 369). Muramoto A, Matsushita M, Kato A, Yamamoto N, Koike G, Nakamura M, Numata T, Tamakoshi A, Tsushita K. Three percent weight reduction is the minimum requirement to improve health hazards in obese and overweight people in Japan. Obes Res Clin Pract., 2014; 8: e466-475 [DOI] [PubMed] [Google Scholar]
- 370). Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Tomaselli GF, AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation, 2014; 129: S102-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371). The Japan Diabetes Society. Chapter 3. Dietary therapy. Practice Guidelines for the Treatment for Diabetes in Japan 2016. (in Japanese) [Google Scholar]
- 372). Tsushita K. Study of the effect on health indicator and affordable medical care by preventing and managing lifestyle-related diseases. 2011. (in Japanese) http://mhlw-grants.niph.go.jp/niph/search/NIDD00.do?resrchNum=201021041A [Google Scholar]
- 373). Svendsen OL, Hassager C, Christiansen C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med, 1993; 95: 131-140 [DOI] [PubMed] [Google Scholar]
- 74). Kuller LH, Simkin-Silverman LR, Wing RR, Meilahn EN, Ives DG. Women's healthy lifestyle project: A randomized clinical trial: Results at 54 months. Circulation, 2001; 103: 32-37 [DOI] [PubMed] [Google Scholar]
- 375). Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet, 2015; 115: 1447-1463 [DOI] [PubMed] [Google Scholar]
- 376). Schwingshackl L, Hoffmann G. Comparison of effects of long-term low-fat vs high-fat diets on blood lipid levels in overweight or obese patients: a systematic review and meta-analysis. J Acad Nutr Diet, 2013; 113: 1640-1661 [DOI] [PubMed] [Google Scholar]
- 377). Naude CE, Schoonees A, Senekal M, Young T, Garner P, Volmink J. Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: a systematic review and meta-analysis. PLoS One, 2014; 9: e100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378). Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS, Brehm BJ, Bucher HC. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med, 2006; 166: 285-293 [DOI] [PubMed] [Google Scholar]
- 379). Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, Leboff MS, Rood JC, de Jonge L, Greenway FL, Loria CM, Obarzanek E, Williamson DA. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med, 2009; 360: 859-873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380). Schwingshackl L, Hoffmann G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: A systematic review and meta-analysis. Nutr J, 2013; 12: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381). Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, Moore H, Davey Smith G. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev, 2011: CD002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382). Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev, 2015: CD011737. [DOI] [PubMed] [Google Scholar]
- 383). Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med, 2010; 7: e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384). Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F, Giampaoli S, Jansen A. Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: the Seven Countries Study. Prev Med, 1995; 24: 308-315 [DOI] [PubMed] [Google Scholar]
- 385). Tanasescu M, Cho E, Manson JE, Hu FB. Dietary fat and cholesterol and the risk of cardiovascular disease among women with type 2 diabetes. Am J Clin Nutr, 2004; 79: 999-1005 [DOI] [PubMed] [Google Scholar]
- 386). Jakobsen MU, O'Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, Pietinen P, Spiegelman D, Stevens J, Virtamo J, Willett WC, Ascherio A. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr, 2009; 89: 1425-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387). Guasch-Ferré M, Babio N, Martínez-González MA, Corella D, Ros E, Martín-Peláez S, Estruch R, Arós F, Gómez-Gracia E, Fiol M, Santos-Lozano JM, Serra-Majem L, Bulló M, Toledo E, Barragán R, Fitó M, Gea A, Salas-Salvadó J, PREDIMED Study Investigators Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr, 2015; 102: 1563-1573 [DOI] [PubMed] [Google Scholar]
- 388). Blekkenhorst LC, Prince RL, Hodgson JM, Lim WH, Zhu K, Devine A, Thompson PL, Lewis JR. Dietary saturated fat intake and atherosclerotic vascular disease mortality in elderly women: a prospective cohort study. Am J Clin Nutr, 2015; 101: 1263-1268 [DOI] [PubMed] [Google Scholar]
- 389). Puaschitz NG, Strand E, Norekvål TM, Dierkes J, Dahl L, Svingen GF, Assmus J, Schartum-Hansen H, Øyen J, Pedersen EK, Drevon CA, Tell GS, Nygård O. Dietary intake of saturated fat is not associated with risk of coronary events or mortality in patients with established coronary artery disease. J Nutr, 2015; 145: 299-305 [DOI] [PubMed] [Google Scholar]
- 390). Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr, 2010; 91: 535-546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391). Iso H, Stampfer MJ, Manson JE, Rexrode K, Hu F, Hennekens CH, Colditz GA, Speizer FE, Willett WC. Prospective study of fat and protein intake and risk of intraparenchymal hemorrhage in women. Circulation, 2001; 103: 856-863 [DOI] [PubMed] [Google Scholar]
- 392). Iso H, Sato S, Kitamura A, Naito Y, Shimamoto T, Komachi Y. Fat and protein intakes and risk of intraparenchymal hemorrhage among middle-aged Japanese. Am J Epidemiol, 2003; 157: 32-39 [DOI] [PubMed] [Google Scholar]
- 393). Yamagishi K, Iso H, Yatsuya H, Tanabe N, Date C, Kikuchi S, Yamamoto A, Inaba Y, Tamakoshi A, JACC Study Group Dietary intake of saturated fatty acids and mortality from cardiovascular disease in Japanese: The Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) study. Am J Clin Nutr, 2010; 92: 759-765 [DOI] [PubMed] [Google Scholar]
- 394). Yamagishi K, Iso H, Kokubo Y, Saito I, Yatsuya H, Ishihara J, Inoue M, Tsugane S, JPHC Study Group Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: The JPHC study. Eur Heart J, 2013; 34: 1225-1232 [DOI] [PubMed] [Google Scholar]
- 395). Fattore E, Bosetti C, Brighenti F, Agostoni C, Fattore G. Palm oil and blood lipid-related markers of cardiovascular disease: a systematic review and meta-analysis of dietary intervention trials. Am J Clin Nutr, 2014; 99: 1331-1350 [DOI] [PubMed] [Google Scholar]
- 396). Engel S, Tholstrup T. Butter increased total and LDL cholesterol compared with olive oil but resulted in higher HDL cholesterol compared with a habitual diet. Am J Clin Nutr, 2015; 102: 309-315 [DOI] [PubMed] [Google Scholar]
- 397). Vafeiadou K, Weech M, Altowaijri H, Todd S, Yaqoob P, Jackson KG, Lovegrove JA. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: Results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am J Clin Nutr, 2015; 102: 40-48 [DOI] [PubMed] [Google Scholar]
- 398). Ginsberg HN, Kris-Etherton P, Dennis B, Elmer PJ, Ershow A, Lefevre M, Pearson T, Roheim P, Ramakrishnan R, Reed R, Stewart K, Stewart P, Phillips K, Anderson N. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: the DELTA study, protocol 1. Arterioscler Thromb Vasc Biol, 1998; 18: 441-449 [DOI] [PubMed] [Google Scholar]
- 399). Barr SL, Ramakrishnan R, Johnson C, Holleran S, Dell RB, Ginsberg HN. Reducing total dietary fat without reducing saturated fatty acids does not significantly lower total plasma cholesterol concentrations in normal males. Am J Clin Nutr, 1992; 55: 675-681 [DOI] [PubMed] [Google Scholar]
- 400). Wardlaw GM, Snook JT. Effect of diets high in butter, corn oil, or high-oleic acid sunflower oil on serum lipids and apolipoproteins in men. Am J Clin Nutr, 1990; 51: 815-821 [DOI] [PubMed] [Google Scholar]
- 401). Temme EH, Mensink RP, Hornstra G. Comparison of the effects of diets enriched in lauric, palmitic, or oleic acids on serum lipids and lipoproteins in healthy women and men. Am J Clin Nutr, 1996; 63: 897-903 [DOI] [PubMed] [Google Scholar]
- 402). Nakamura Y, Okuda N, Turin TC, Fujiyoshi A, Okamura T, Hayakawa T, Yoshita K, Miura K, Ueshima H, NIPPON DATA80/90 Research Group Fatty acids intakes and serum lipid profiles: NIPPON DATA90 and the national nutrition monitoring. J Epidemiol, 2010; 20 Suppl 3: S544-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403). Guo Z, Miura K, Turin TC, Hozawa A, Okuda N, Okamura T, Saitoh S, Sakata K, Nakagawa H, Okayama A, Yoshita K, Kadowaki T, Choudhury SR, Nakamura Y, Rodriguez BL, Curb DJ, Elliott P, Stamler J, Ueshima H. Relationship of the polyunsaturated to saturated fatty acid ratio to cardiovascular risk factors and metabolic syndrome in Japanese: The INTERLIPID study. J Atheroscler Thromb, 2010; 17: 777-784 [DOI] [PubMed] [Google Scholar]
- 404). Kotwal S, Jun M, Sullivan D, Perkovic V, Neal B. Omega 3 fatty acids and cardiovascular outcomes: systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes, 2012; 5: 808-818 [DOI] [PubMed] [Google Scholar]
- 405). Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, Kokubo Y, Tsugane S, JPHC Study Group Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: The Japan Public Health Center-Based (JPHC) study cohort I. Circulation, 2006; 113: 195-202 [DOI] [PubMed] [Google Scholar]
- 406). Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, Inaba Y, Tanabe N, Tamakoshi A, Japan Collaborative Cohort Study for Evaluation of Cancer Risk Study Group Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) study. J Am Coll Cardiol, 2008; 52: 988-996 [DOI] [PubMed] [Google Scholar]
- 407). Miyagawa N, Miura K, Okuda N, Kadowaki T, Takashima N, Nagasawa SY, Nakamura Y, Matsumura Y, Hozawa A, Fujiyoshi A, Hisamatsu T, Yoshita K, Sekikawa A, Ohkubo T, Abbott RD, Okamura T, Okayama A, Ueshima H, NIPPON DATA80 Research Group Long-chain n-3 polyunsaturated fatty acids intake and cardiovascular disease mortality risk in Japanese: a 24-year follow-up of NIPPON DATA80. Atherosclerosis, 2014; 232: 384-389 [DOI] [PubMed] [Google Scholar]
- 408). Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, Hunter D, Manson JE. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA, 2002; 287: 1815-1821 [DOI] [PubMed] [Google Scholar]
- 409). Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA, 2001; 285: 304-312 [DOI] [PubMed] [Google Scholar]
- 410). Strom M, Halldorsson TI, Mortensen EL, Torp-Pedersen C, Olsen SF. Fish, n-3 fatty acids, and cardiovascular diseases in women of reproductive age: a prospective study in a large national cohort. Hypertension, 2012; 59: 36-43 [DOI] [PubMed] [Google Scholar]
- 411). de Goede J, Geleijnse JM, Boer JM, Kromhout D, Verschuren WM. Marine (n-3) fatty acids, fish consumption, and the 10-year risk of fatal and nonfatal coronary heart disease in a large population of dutch adults with low fish intake. J Nutr, 2010; 140: 1023-1028 [DOI] [PubMed] [Google Scholar]
- 412). Ascherio A, Rimm EB, Stampfer MJ, Giovannucci EL, Willett WC. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N Engl J Med, 1995; 332: 977-982 [DOI] [PubMed] [Google Scholar]
- 413). Manger MS, Strand E, Ebbing M, Seifert R, Refsum H, Nordrehaug JE, Nilsen DW, Drevon CA, Tell GS, Bleie O, Vollset SE, Pedersen ER, Nygard O. Dietary intake of n-3 long-chain polyunsaturated fatty acids and coronary events in Norwegian patients with coronary artery disease. Am J Clin Nutr, 2010; 92: 244-251 [DOI] [PubMed] [Google Scholar]
- 414). Streppel MT, Ocke MC, Boshuizen HC, Kok FJ, Kromhout D. Long-term fish consumption and n-3 fatty acid intake in relation to (sudden) coronary heart disease death: The Zutphen study. Eur Heart J, 2008; 29: 2024-2030 [DOI] [PubMed] [Google Scholar]
- 415). Hooper L, Thompson RL, Harrison RA, Summerbell CD, Moore H, Worthington HV, Durrington PN, Ness AR, Capps NE, Davey Smith G, Riemersma RA, Ebrahim SB. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst Rev, 2004: CD003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416). Musa-Veloso K, Binns MA, Kocenas A, Chung C, Rice H, Oppedal-Olsen H, Lloyd H, Lemke S. Impact of low v. Moderate intakes of long-chain n-3 fatty acids on risk of coronary heart disease. Br J Nutr, 2011; 106: 1129-1141 [DOI] [PubMed] [Google Scholar]
- 417). de Oliveira Otto MC, Wu JH, Baylin A, Vaidya D, Rich SS, Tsai MY, Jacobs DR, Mozaffarian D. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc, 2013; 2: e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418). Leslie MA, Cohen DJ, Liddle DM, Robinson LE, Ma DW. A review of the effect of omega-3 polyunsaturated fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis, 2015; 14: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419). Eslick GD, Howe PR, Smith C, Priest R, Bensoussan A. Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta-analysis. Int J Cardiol, 2009; 136: 4-16 [DOI] [PubMed] [Google Scholar]
- 420). Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis, 2006; 189: 19-30 [DOI] [PubMed] [Google Scholar]
- 421). Agren JJ, Hanninen O, Julkunen A, Fogelholm L, Vidgren H, Schwab U, Pynnönen O, Uusitupa M. Fish diet, fish oil and docosahexaenoic acid rich oil lower fasting and postprandial plasma lipid levels. Eur J Clin Nutr, 1996; 50: 765-771 [PubMed] [Google Scholar]
- 422). Mori TA, Bao DQ, Burke V, Puddey IB, Watts GF, Beilin LJ. Dietary fish as a major component of a weightloss diet: effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am J Clin Nutr, 1999; 70: 817-825 [DOI] [PubMed] [Google Scholar]
- 423). Vedtofte MS, Jakobsen MU, Lauritzen L, O'Reilly EJ, Virtamo J, Knekt P, Colditz G, Hallmans G, Buring J, Steffen LM, Robien K, Rimm EB, Heitmann BL. Association between the intake of α-linolenic acid and the risk of CHD. Br J Nutr, 2014; 112: 735-743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424). Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, Mozaffarian D, Hu FB. A-linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr, 2012; 96: 1262-1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425). Fleming JA, Kris-Etherton PM. The evidence for α-linolenic acid and cardiovascular disease benefits: comparisons with eicosapentaenoic acid and docosahexaenoic acid. Adv Nutr, 2014; 5: 863S-876S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426). Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, Khaw KT, Mozaffarian D, Danesh J, Di Angelantonio E. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med, 2014; 160: 398-406 [DOI] [PubMed] [Google Scholar]
- 427). Wu JH, Lemaitre RN, King IB, Song X, Psaty BM, Siscovick DS, Mozaffarian D. Circulating omega-6 polyunsaturated fatty acids and total and cause-specific mortality: the Cardiovascular Health Study. Circulation, 2014; 130: 1245-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428). Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, Willett WC, Hu FB. Dietary linoleic acid and risk of coronary heart disease: a systematic review and metaanalysis of prospective cohort studies. Circulation, 2014; 130: 1568-1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429). Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez-González MA, the PREDIMED Study Investigators Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med, 2013; 368: 1279-1290 [DOI] [PubMed] [Google Scholar]
- 430). Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis, 2014; 13: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431). Foley M, Ball M, Chisholm A, Duncan A, Spears G, Mann J. Should mono- or poly-unsaturated fats replace saturated fat in the diet? Eur J Clin Nutr, 1992; 46: 429-436 [PubMed] [Google Scholar]
- 432). Berglund L, Lefevre M, Ginsberg HN, Kris-Etherton PM, Elmer PJ, Stewart PW, Ershow A, Pearson TA, Dennis BH, Roheim PS, Ramakrishnan R, Reed R, Stewart K, Phillips KM, DELTA Investigators Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: Studies in the fasting and postprandial states. Am J Clin Nutr, 2007; 86: 1611-1620 [DOI] [PubMed] [Google Scholar]
- 433). Ginsberg HN, Barr SL, Gilbert A, Karmally W, Deckelbaum R, Kaplan K, Ramakrishnan R, Holleran S, Dell RB. Reduction of plasma cholesterol levels in normal men on an American Heart Association Step 1 diet or a Step 1 diet with added monounsaturated fat. N Engl J Med, 1990; 322: 574-579 [DOI] [PubMed] [Google Scholar]
- 434). Thijssen MA, Mensink RP. Small differences in the effects of stearic acid, oleic acid, and linoleic acid on the serum lipoprotein profile of humans. Am J Clin Nutr, 2005; 82: 510-516 [DOI] [PubMed] [Google Scholar]
- 435). Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb, 1992; 12: 911-919 [DOI] [PubMed] [Google Scholar]
- 436). Schwingshackl L, Strasser B, Hoffmann G. Effects of monounsaturated fatty acids on cardiovascular risk factors: a systematic review and meta-analysis. Ann Nutr Metab, 2011; 59: 176-186 [DOI] [PubMed] [Google Scholar]
- 437). Nestel P, Noakes M, Belling B, McArthur R, Clifton P, Janus E, Abbey M. Plasma lipoprotein lipid and Lp (a) changes with substitution of elaidic acid for oleic acid in the diet. J Lipid Res, 1992; 33: 1029-1036 [PubMed] [Google Scholar]
- 438). Mozaffarian D, Aro A, Willett WC. Health effects of trans-fatty acids: experimental and observational evidence. Eur J Clin Nutr. 2009; 63 Suppl 2: S5-21 [DOI] [PubMed] [Google Scholar]
- 439). de Roos B, Wanders AJ, Wood S, Horgan G, Rucklige G, Reid M, Siebelink E, Brouwer IA. A high intake of industrial or ruminant trans fatty acids does not affect the plasma proteome in healthy men. Proteomics, 2011; 11: 3928-3934 [DOI] [PubMed] [Google Scholar]
- 440). Vega-López S, Ausman LM, Jalbert SM, Erkkilä AT, Lichtenstein AH. Palm and partially hydrogenated soybean oils adversely alter lipoprotein profiles compared with soybean and canola oils in moderately hyperlipidemic subjects. Am J Clin Nutr, 2006; 84: 54-62 [DOI] [PubMed] [Google Scholar]
- 441). Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA. Trans-palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr, 2013; 97: 854-861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442). Aro A, Jauhiainen M, Partanen R, Salminen I, Mutanen M. Stearic acid, trans fatty acids, and dairy fat: effects on serum and lipoprotein lipids, apolipoproteins, lipoprotein (a), and lipid transfer proteins in healthy subjects. Am J Clin Nutr, 1997; 65: 1419-1426 [DOI] [PubMed] [Google Scholar]
- 443). Mensink RP, Zock PL, Katan MB, Hornstra G. Effect of dietary cis and trans fatty acids on serum lipoprotein (a) levels in humans. J Lipid Res, 1992; 33: 1493-1501 [PubMed] [Google Scholar]
- 444). Wanders AJ, Brouwer IA, Siebelink E, Katan MB. Effect of a high intake of conjugated linoleic acid on lipoprotein levels in healthy human subjects. PLoS One, 2010; 5: e9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445). de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schunemann H, Beyene J, Anand SS. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ, 2015; 351: h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446). Pietinen P, Ascherio A, Korhonen P, Hartman AM, Willett WC, Albanes D, Virtamo J. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Epidemiol, 1997; 145: 876-887 [DOI] [PubMed] [Google Scholar]
- 447). Oomen CM, Ocké MC, Feskens EJ, van Erp-Baart MA, Kok FJ, Kromhout D. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective populationbased study. Lancet, 2001; 357: 746-751 [DOI] [PubMed] [Google Scholar]
- 448). Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr, 2009; 63 Suppl 2: S22-33 [DOI] [PubMed] [Google Scholar]
- 449). Mori K, Ishida T, Yasuda T, Hasokawa M, Monguchi T, Sasaki M, Kondo K, Nakajima H, Shinohara M, Shinke T, Irino Y, Toh R, Nishimura K, Hirata K. Serum transfatty acid concentration is elevated in young patients with coronary artery disease in Japan. Circ J, 2015; 79: 2017-2025 [DOI] [PubMed] [Google Scholar]
- 450). Lacroix E, Charest A, Cyr A, Baril-Gravel L, Lebeuf Y, Paquin P, Chouinard PY, Couture P, Lamarche B. Randomized controlled study of the effect of a butter naturally enriched in trans fatty acids on blood lipids in healthy women. Am J Clin Nutr, 2012; 95: 318-325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451). Gayet-Boyer C, Tenenhaus-Aziza F, Prunet C, Marmonier C, Malpuech-Brugère C, Lamarche B, Chardigny JM. Is there a linear relationship between the dose of ruminant trans-fatty acids and cardiovascular risk markers in healthy subjects: results from a systematic review and meta-regression of randomised clinical trials. Br J Nutr, 2014; 112: 1914-1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452). Ministry of Agriculture, Forestry and Fisheries of Japan. The cite for understanding tras fatty acids: http://www.maff.go.jp/j/syouan/seisaku/trans_fat/t_wakaru/ (in Japanese)
- 453). Joint WHO/FAO Expert Consultation. Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser, 2003; 916: i-viii, 1–149 [PubMed] [Google Scholar]
- 454). Uauy R, Aro A, Clarke R, Ghafoorunissa, L'Abbé MR, Mozaffarian D, Skeaff CM, Stender S, Tavella M. WHO scientific update on trans fatty acids: summary and conclusions. Eur J Clin Nutr, 2009; 63: S68-S75 [Google Scholar]
- 455). McGee D, Reed D, Stemmerman G, Rhoads G, Yano K, Feinleib M. The relationship of dietary fat and cholesterol to mortality in 10 years: the Honolulu Heart Program. Int J Epidemiol, 1985; 14: 97-105 [DOI] [PubMed] [Google Scholar]
- 456). Posner BM, Cobb JL, Belanger AJ, Cupples LA, D'Agostino RB, Stokes J. Dietary lipid predictors of coronary heart disease in men. The Framingham Study. Arch Intern Med, 1991; 151: 1181-1187 [PubMed] [Google Scholar]
- 457). Xu J, Eilat-Adar S, Loria C, Goldbourt U, Howard BV, Fabsitz RR, Zephier EM, Mattil C, Lee ET. Dietary fat intake and risk of coronary heart disease: the Strong Heart Study. Am J Clin Nutr, 2006; 84: 894-902 [DOI] [PubMed] [Google Scholar]
- 458). Millen BE, Franz MM, Quatromoni PA, Gagnon DR, Sonnenberg LM, Ordovas JM, Wilson PW, Schaefer EJ, Cupples LA. Diet and plasma lipids in women. I. Macronutrients and plasma total and low-density lipoprotein cholesterol in women: the Framingham nutrition studies. J Clin Epidemiol, 1996; 49: 657-663 [DOI] [PubMed] [Google Scholar]
- 459). Johnson C, Greenland P. Effects of exercise, dietary cholesterol, and dietary fat on blood lipids. Arch Intern Med, 1990; 150: 137-141 [PubMed] [Google Scholar]
- 460). Fielding CJ, Havel RJ, Todd KM, Yeo KE, Schloetter MC, Weinberg V, Frost PH. Effects of dietary cholesterol and fat saturation on plasma lipoproteins in an ethnically diverse population of healthy young men. J Clin Invest, 1995; 95: 611-618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461). Berger S, Raman G, Vishwanathan R, Jacques PF, Johnson EJ. Dietary cholesterol and cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr, 2015; 102: 276-294 [DOI] [PubMed] [Google Scholar]
- 462). Nicklas BJ, Katzel LI, Bunyard LB, Dennis KE, Goldberg AP. Effects of an American Heart Association diet and weight loss on lipoprotein lipids in obese, postmenopausal women. Am J Clin Nutr, 1997; 66: 853-859 [DOI] [PubMed] [Google Scholar]
- 463). Dengel JL, Katzel LI, Goldberg AP. Effect of an American Heart Association diet, with or without weight loss, on lipids in obese middle-aged and older men. Am J Clin Nutr, 1995; 62: 715-721 [DOI] [PubMed] [Google Scholar]
- 464). Lichtenstein AH, Ausman LM, Jalbert SM, Vilella-Bach M, Jauhiainen M, McGladdery S, Erkkila AT, Ehnholm C, Frohlich J, Schaefer EJ. Efficacy of a Therapeutic Lifestyle Change/Step 2 diet in moderately hypercholesterolemic middle-aged and elderly female and male subjects. J Lipid Res, 2002; 43: 264-273 [PubMed] [Google Scholar]
- 465). Rivellese AA, Auletta P, Marotta G, Saldalamacchia G, Giacco A, Mastrilli V, Vaccaro O, Riccardi G. Long term metabolic effects of two dietary methods of treating hyperlipidaemia. BMJ, 1994; 308: 227-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466). Katan MB, Beynen AC, de Vries JH, Nobels A. Existence of consistent hypo- and hyperresponders to dietary cholesterol in man. Am J Epidemiol, 1986; 123: 221-234 [DOI] [PubMed] [Google Scholar]
- 467). Djoussé L, Gaziano JM. Dietary cholesterol and coronary artery disease: a systematic review. Curr Atheroscler Rep, 2009; 11: 418-422 [DOI] [PubMed] [Google Scholar]
- 468). Clifton PM, Kestin M, Abbey M, Drysdale M, Nestel PJ. Relationship between sensitivity to dietary fat and dietary cholesterol. Arteriosclerosis, 1990; 10: 394-401 [DOI] [PubMed] [Google Scholar]
- 469). Ginsberg HN, Karmally W, Siddiqui M, Holleran S, Tall AR, Rumsey SC, Deckelbaum RJ, Blaner WS, Ramakrishnan R. A dose-response study of the effects of dietary cholesterol on fasting and postprandial lipid and lipoprotein metabolism in healthy young men. Arterioscler Thromb, 1994; 14: 576-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470). Chakrabarty G, Manjunatha S, Bijlani RL, Ray RB, Mahapatra SC, Mehta N, Lakshmy R, Vashisht S, Manchanda SC. The effect of ingestion of egg on the serum lipid profile of healthy young indians. Indian J Physiol Pharmacol, 2004; 48: 286-292 [PubMed] [Google Scholar]
- 471). Herron KL, Vega-Lopez S, Conde K, Ramjiganesh T, Shachter NS, Fernandez ML. Men classified as hypo- or hyperresponders to dietary cholesterol feeding exhibit differences in lipoprotein metabolism. J Nutr, 2003; 133: 1036-1042 [DOI] [PubMed] [Google Scholar]
- 472). Flaim E, Ferreri LF, Thye FW, Hill JE, Ritchey SJ. Plasma lipid and lipoprotein cholesterol concentrations in adult males consuming normal and high cholesterol diets under controlled conditions. Am J Clin Nutr, 1981; 34: 1103-1108 [DOI] [PubMed] [Google Scholar]
- 473). Sacks FM, Salazar J, Miller L, Foster JM, Sutherland M, Samonds KW, Albers JJ, Kass EH. Ingestion of egg raises plasma low density lipoproteins in free-living subjects. Lancet, 1984; 1: 647-649 [DOI] [PubMed] [Google Scholar]
- 474). Roberts SL, McMurry MP, Connor WE. Does egg feeding (i.e., dietary cholesterol) affect plasma cholesterol levels in humans? The results of a double-blind study. Am J Clin Nutr, 1981; 34: 2092-2099 [DOI] [PubMed] [Google Scholar]
- 475). Knopp RH, Retzlaff BM, Walden CE, Dowdy AA, Tsunehara CH, Austin MA, Nguyen T. A double-blind, randomized, controlled trial of the effects of two eggs per day in moderately hypercholesterolemic and combined hyperlipidemic subjects taught the NCEP step I diet. J Am Coll Nutr, 1997; 16: 551-561 [PubMed] [Google Scholar]
- 476). Severins N, Mensink RP, Plat J. Effects of lutein-enriched egg yolk in buttermilk or skimmed milk on serum lipids & lipoproteins of mildly hypercholesterolemic subjects. Nutr Metab Cardiovasc Dis, 2015; 25: 210-217 [DOI] [PubMed] [Google Scholar]
- 477). Baumgartner S, Kelly ER, van der Made S, Berendschot TT, Husche C, Lutjohann D, Plat J. The influence of consuming an egg or an egg-yolk buttermilk drink for 12 wk on serum lipids, inflammation, and liver function markers in human volunteers. Nutrition, 2013; 29: 1237-1244 [DOI] [PubMed] [Google Scholar]
- 478). Flynn MA, Nolph GB, Flynn TC, Kahrs R, Krause G. Effect of dietary egg on human serum cholesterol and triglycerides. Am J Clin Nutr, 1979; 32: 1051-1057 [DOI] [PubMed] [Google Scholar]
- 479). Fuller NR, Caterson ID, Sainsbury A, Denyer G, Fong M, Gerofi J, Baqleh K, Williams KH, Lau NS, Markovic TP. The effect of a high-egg diet on cardiovascular risk factors in people with type 2 diabetes: the Diabetes and Egg (DIABEGG) study-a 3-mo randomized controlled trial. Am J Clin Nutr, 2015; 101: 705-713 [DOI] [PubMed] [Google Scholar]
- 480). Katz DL, Gnanaraj J, Treu JA, Ma Y, Kavak Y, Njike VY. Effects of egg ingestion on endothelial function in adults with coronary artery disease: a randomized, controlled, crossover trial. Am Heart J, 2015; 169: 162-169 [DOI] [PubMed] [Google Scholar]
- 481). Blesso CN, Andersen CJ, Barona J, Volek JS, Fernandez ML. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolkfree egg substitute in individuals with metabolic syndrome. Metabolism, 2013; 62: 400-410 [DOI] [PubMed] [Google Scholar]
- 482). Pearce KL, Clifton PM, Noakes M. Egg consumption as part of an energy-restricted high-protein diet improves blood lipid and blood glucose profiles in individuals with type 2 diabetes. Br J Nutr, 2011; 105: 584-592 [DOI] [PubMed] [Google Scholar]
- 483). Weggemans RM, Zock PL, Katan MB. Dietary cholesterol from eggs increases the ratio of total cholesterol to high-density lipoprotein cholesterol in humans: a metaanalysis. Am J Clin Nutr, 2001; 73: 885-891 [DOI] [PubMed] [Google Scholar]
- 484). Hu FB, Stampfer MJ, Rimm EB, Manson JE, Ascherio A, Colditz GA, Rosner BA, Spiegelman D, Speizer FE, Sacks FM, Hennekens CH, Willett WC. A prospective study of egg consumption and risk of cardiovascular disease in men and women. JAMA, 1999; 281: 1387-1394 [DOI] [PubMed] [Google Scholar]
- 485). Qureshi AI, Suri FK, Ahmed S, Nasar A, Divani AA, Kirmani JF. Regular egg consumption does not increase the risk of stroke and cardiovascular diseases. Med Sci Monit, 2007; 13: CR1-8 [PubMed] [Google Scholar]
- 486). Nakamura Y, Okamura T, Tamaki S, Kadowaki T, Hayakawa T, Kita Y, Okayama A, Ueshima H, NIPPON DATA80 Research Group Egg consumption, serum cholesterol, and cause-specific and all-cause mortality: the National Integrated Project for Prospective Observation of Non-communicable Disease and Its Trends in the Aged, 1980 (NIPPON DATA80). Am J Clin Nutr, 2004; 80: 58-63 [DOI] [PubMed] [Google Scholar]
- 487). Houston DK, Ding J, Lee JS, Garcia M, Kanaya AM, Tylavsky FA, Newman AB, Visser M, Kritchevsky SB, Study HA. Dietary fat and cholesterol and risk of cardiovascular disease in older adults: the Health ABC study. Nutr Metab Cardiovasc Dis, 2011; 21: 430-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488). Shin JY, Xun P, Nakamura Y, He K. Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis. Am J Clin Nutr, 2013; 98: 146-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489). Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ, 2014; 349: g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490). Zhan J, Liu YJ, Cai LB, Xu FR, Xie T, He QQ. Fruit and vegetable consumption and risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr, 2015: 2017; 57: 1650-1663 [DOI] [PubMed] [Google Scholar]
- 491). Law MR, Morris JK. By how much does fruit and vegetable consumption reduce the risk of ischaemic heart disease? Eur J Clin Nutr, 1998; 52: 549-556 [DOI] [PubMed] [Google Scholar]
- 492). Gan Y, Tong X, Li L, Cao S, Yin X, Gao C, Herath C, Li W, Jin Z, Chen Y, Lu Z. Consumption of fruit and vegetable and risk of coronary heart disease: A metaanalysis of prospective cohort studies. Int J Cardiol, 2015; 183: 129-137 [DOI] [PubMed] [Google Scholar]
- 493). Dauchet L, Amouyel P, Dallongeville J. Fruit and vegetable consumption and risk of stroke: A meta-analysis of cohort studies. Neurology, 2005; 65: 1193-1197 [DOI] [PubMed] [Google Scholar]
- 494). He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: Meta-analysis of cohort studies. Lancet, 2006; 367: 320-326 [DOI] [PubMed] [Google Scholar]
- 495). Hu D, Huang J, Wang Y, Zhang D, Qu Y. Fruits and vegetables consumption and risk of stroke: A meta-analysis of prospective cohort studies. Stroke, 2014; 45: 1613-1619 [DOI] [PubMed] [Google Scholar]
- 496). Sherzai A, Heim LT, Boothby C, Sherzai AD. Stroke, food groups, and dietary patterns: A systematic review. Nutr Rev, 2012; 70: 423-435 [DOI] [PubMed] [Google Scholar]
- 497). Okuda N, Miura K, Okayama A, Okamura T, Abbott RD, Nishi N, Fujiyoshi A, Kita Y, Nakamura Y, Miyagawa N, Hayakawa T, Ohkubo T, Kiyohara Y, Ueshima H, NIPPON DATA80 Research Group Fruit and vegetable intake and mortality from cardiovascular disease in Japan: A 24-year follow-up of the NIPPON DATA80 study. Eur J Clin Nutr, 2015; 69: 482-488 [DOI] [PubMed] [Google Scholar]
- 498). Nakamura K, Nagata C, Oba S, Takatsuka N, Shimizu H. Fruit and vegetable intake and mortality from cardiovascular disease are inversely associated in Japanese women but not in men. J Nutr, 2008; 138: 1129-1134 [DOI] [PubMed] [Google Scholar]
- 499). Sauvaget C, Nagano J, Allen N, Kodama K. Vegetable and fruit intake and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Stroke, 2003; 34: 2355-2360 [DOI] [PubMed] [Google Scholar]
- 500). Nagura J, Iso H, Watanabe Y, Maruyama K, Date C, Toyoshima H, Yamamoto A, Kikuchi S, Koizumi A, Kondo T, Wada Y, Inaba Y, Tamakoshi A, JACC Study Group Fruit, vegetable and bean intake and mortality from cardiovascular disease among Japanese men and women: The JACC Study. Br J Nutr, 2009; 102: 285-292 [DOI] [PubMed] [Google Scholar]
- 501). Mano R, Ishida A, Ohya Y, Todoriki H, Takishita S. Dietary intervention with Okinawan vegetables increased circulating endothelial progenitor cells in healthy young women. Atherosclerosis, 2009; 204: 544-548 [DOI] [PubMed] [Google Scholar]
- 502). Tuekpe MK, Todoriki H, Sasaki S, Zheng KC, Ariizumi M. Potassium excretion in healthy Japanese women was increased by a dietary intervention utilizing home-parcel delivery of Okinawan vegetables. Hypertens Res, 2006; 29: 389-396 [DOI] [PubMed] [Google Scholar]
- 503). Anderson CA, Appel LJ, Okuda N, Brown IJ, Chan Q, Zhao L, Ueshima H, Kesteloot H, Miura K, Curb JD, Yoshita K, Elliott P, Yamamoto ME, Stamler J. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: The INTERMAP study. J Am Diet Assoc, 2010; 110: 736-745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504). Miura K, Okuda N, Turin TC, Takashima N, Nakagawa H, Nakamura K, Yoshita K, Okayama A, Ueshima H, NIPPON DATA80/90 Research Group Dietary salt intake and blood pressure in a representative Japanese population: Baseline analyses of NIPPON DATA80. J Epidemiol, 2010; 20 Suppl 3: S524-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K. Treatment a) lifestyle modification: Executive summary of the Japan atherosclerosis society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan--2012 version. J Atheroscler Thromb, 2013; 20: 835-849 [DOI] [PubMed] [Google Scholar]
- 506). Iso H, Kubota Y. Nutrition and disease in the Japan collaborative cohort study for evaluation of cancer (JACC). Asian Pac J Cancer Prev, 2007; 8 Suppl: 35-80 [PubMed] [Google Scholar]
- 507).Inspection and Safety Division, Department of Food Safety, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare. Q&A regarding arsenic in Hijiki 2004. http://www.mhlw.go.jp/topics/2004/07/tp0730-1.html (in Japanese)
- 508). Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med, 2009; 169: 659-669 [DOI] [PubMed] [Google Scholar]
- 509). Li M, Fan Y, Zhang X, Hou W, Tang Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: Metaanalysis of prospective cohort studies. BMJ Open, 2014; 4: e005497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510). Yamada T, Hayasaka S, Shibata Y, Ojima T, Saegusa T, Gotoh T, Ishikawa S, Nakamura Y, Kayaba K, Jichi Medical School Cohort Study Group Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: The Jichi Medical School cohort study. J Epidemiol, 2011; 21: 169-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511). Takachi R, Inoue M, Ishihara J, Kurahashi N, Iwasaki M, Sasazuki S, Iso H, Tsubono Y, Tsugane S, JPHC Study Group Fruit and vegetable intake and risk of total cancer and cardiovascular disease: Japan public health center-based prospective study. Am J Epidemiol, 2008; 167: 59-70 [DOI] [PubMed] [Google Scholar]
- 512). Tsubota-Utsugi M, Ohkubo T, Kikuya M, Metoki H, Kurimoto A, Suzuki K, Fukushima N, Hara A, Asayama K, Satoh H, Tsubono Y, Imai Y. High fruit intake is associated with a lower risk of future hypertension determined by home blood pressure measurement: The OHASAMA study. J Hum Hypertens, 2011; 25: 164-171 [DOI] [PubMed] [Google Scholar]
- 513). Gorinstein S, Caspi A, Libman I, Lerner HT, Huang D, Leontowicz H, Leontowicz M, Tashma Z, Katrich E, Feng S, Trakhtenberg S. Red grapefruit positively influences serum triglyceride level in patients suffering from coronary atherosclerosis: Studies in vitro and in humans. J Agric Food Chem, 2006; 54: 1887-1892 [DOI] [PubMed] [Google Scholar]
- 514). Gorinstein S, Caspi A, Libman I, Katrich E, Lerner HT, Trakhtenberg S. Preventive effects of diets supplemented with sweetie fruits in hypercholesterolemic patients suffering from coronary artery disease. Prev Med, 2004; 38: 841-847 [DOI] [PubMed] [Google Scholar]
- 515). Gammon CS, Kruger R, Conlon CA, von Hurst PR, Jones B, Stonehouse W. Inflammatory status modulates plasma lipid and inflammatory marker responses to kiwifruit consumption in hypercholesterolaemic men. Nutr Metab Cardiovasc Dis, 2014; 24: 91-99 [DOI] [PubMed] [Google Scholar]
- 516). Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, Mattila P, Jula A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr, 2008; 87: 323-331 [DOI] [PubMed] [Google Scholar]
- 517). Liu K, Xing A, Chen K, Wang B, Zhou R, Chen S, Xu H, Mi M. Effect of fruit juice on cholesterol and blood pressure in adults: A meta-analysis of 19 randomized controlled trials. PLoS One, 2013; 8: e61420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518). Gorinstein S, Caspi A, Libman I, Katrich E, Lerner HT, Trakhtenberg S. Fresh israeli jaffa sweetie juice consumption improves lipid metabolism and increases antioxidant capacity in hypercholesterolemic patients suffering from coronary artery disease: Studies in vitro and in humans and positive changes in albumin and fibrinogen fractions. J Agric Food Chem, 2004; 52: 5215-5222 [DOI] [PubMed] [Google Scholar]
- 519). Kurowska EM, Spence JD, Jordan J, Wetmore S, Freeman DJ, Piche LA, Serratore P. HDL-cholesterol-raising effect of orange juice in subjects with hypercholesterolemia. Am J Clin Nutr, 2000; 72: 1095-1100 [DOI] [PubMed] [Google Scholar]
- 520). Keller A, Heitmann BL, Olsen N. Sugar-sweetened beverages, vascular risk factors and events: A systematic literature review. Public Health Nutr, 2015; 18: 1145-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521). Tasevska N, Park Y, Jiao L, Hollenbeck A, Subar AF, Potischman N. Sugars and risk of mortality in the NIHAARP diet and health study. Am J Clin Nutr, 2014; 99: 1077-1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522). Eshak ES, Iso H, Kokubo Y, Saito I, Yamagishi K, Inoue M, Tsugane S. Soft drink intake in relation to incident ischemic heart disease, stroke, and stroke subtypes in Japanese men and women: The Japan public health centre-based study cohort I. Am J Clin Nutr, 2012; 96: 1390-1397 [DOI] [PubMed] [Google Scholar]
- 523). Chiavaroli L, de Souza RJ, Ha V, Cozma AI, Mirrahimi A, Wang DD, Yu M, Carleton AJ, Di Buono M, Jenkins AL, Leiter LA, Wolever TM, Beyene J, Kendall CW, Jenkins DJ, Sievenpiper JL. Effect of fructose on established lipid targets: A systematic review and meta-analysis of controlled feeding trials. J Am Heart Assoc, 2015; 4: e001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524). Kelishadi R, Mansourian M, Heidari-Beni M. Association of fructose consumption and components of metabolic syndrome in human studies: A systematic review and meta-analysis. Nutrition, 2014; 30: 503-510 [DOI] [PubMed] [Google Scholar]
- 525). Lowndes J, Sinnett S, Yu Z, Rippe J. The effects of fructose-containing sugars on weight, body composition and cardiometabolic risk factors when consumed at up to the 90th percentile population consumption level for fructose. Nutrients, 2014; 6: 3153-3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526). Lowndes J, Sinnett S, Pardo S, Nguyen VT, Melanson KJ, Yu Z, Lowther BE, Rippe JM. The effect of normally consumed amounts of sucrose or high fructose corn syrup on lipid profiles, body composition and related parameters in overweight/obese subjects. Nutrients, 2014; 6: 1128-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527). Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S, JPHC Study Group Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: The Japan Public Health Center-based (JPHC) study cohort I. Circulation, 2007; 116: 2553-2562 [DOI] [PubMed] [Google Scholar]
- 528). Yamasaki K, Kayaba K, Ishikawa S. Soy and soy products intake, all-cause mortality, and cause-specific mortality in Japan: The Jichi medical school cohort study. Asia Pac J Public Health, 2015; 27: 531-541 [DOI] [PubMed] [Google Scholar]
- 529). Ashton EL, Dalais FS, Ball MJ. Effect of meat replacement by tofu on CHD risk factors including copper induced LDL oxidation. J Am Coll Nutr, 2000; 19: 761-767 [DOI] [PubMed] [Google Scholar]
- 530). Ashton E, Ball M. Effects of soy as tofu vs meat on lipoprotein concentrations. Eur J Clin Nutr, 2000; 54: 14-19 [DOI] [PubMed] [Google Scholar]
- 531). Jenkins DJ, Kendall CW, Garsetti M, Rosenberg-Zand RS, Jackson CJ, Agarwal S, Rao AV, Diamandis EP, Parker T, Faulkner D, Vuksan V, Vidgen E. Effect of soy protein foods on low-density lipoprotein oxidation and ex vivo sex hormone receptor activity--a controlled crossover trial. Metabolism, 2000; 49: 537-543 [DOI] [PubMed] [Google Scholar]
- 532). Gardner CD, Messina M, Kiazand A, Morris JL, Franke AA. Effect of two types of soy milk and dairy milk on plasma lipids in hypercholesterolemic adults: A randomized trial. J Am Coll Nutr, 2007; 26: 669-677 [DOI] [PubMed] [Google Scholar]
- 533). Welty FK, Lee KS, Lew NS, Zhou JR. Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch Intern Med, 2007; 167: 1060-1067 [DOI] [PubMed] [Google Scholar]
- 534). Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Padyab M, Hu FB, Willett WC. Soy inclusion in the diet improves features of the metabolic syndrome: A randomized crossover study in postmenopausal women. Am J Clin Nutr, 2007; 85: 735-741 [DOI] [PubMed] [Google Scholar]
- 535). Matthan NR, Jalbert SM, Ausman LM, Kuvin JT, Karas RH, Lichtenstein AH. Effect of soy protein from differently processed products on cardiovascular disease risk factors and vascular endothelial function in hypercholesterolemic subjects. Am J Clin Nutr, 2007; 85: 960-966 [DOI] [PubMed] [Google Scholar]
- 536). Harland JI, Haffner TA. Systematic review, meta-analysis and regression of randomised controlled trials reporting an association between an intake of circa 25 g soya protein per day and blood cholesterol. Atherosclerosis, 2008; 200: 13-27 [DOI] [PubMed] [Google Scholar]
- 537). Oldewage-Theron W, Egal A. The effect of consumption of soy foods on the blood lipid profile of women: A pilot study from Qwa-Qwa. J Nutr Sci Vitaminol (Tokyo), 2013; 59: 431-436 [DOI] [PubMed] [Google Scholar]
- 538). Sapbamrer R, Visavarungroj N, Suttajit M. Effects of dietary traditional fermented soybean on reproductive hormones, lipids, and glucose among postmenopausal women in northern Thailand. Asia Pac J Clin Nutr, 2013; 22: 222-228 [DOI] [PubMed] [Google Scholar]
- 539). van Nielen M, Feskens EJ, Rietman A, Siebelink E, Mensink M. Partly replacing meat protein with soy pro tein alters insulin resistance and blood lipids in postmenopausal women with abdominal obesity. J Nutr, 2014; 144: 1423-1429 [DOI] [PubMed] [Google Scholar]
- 540). Bakhtiary A, Yassin Z, Hanachi P, Rahmat A, Ahmad Z, Jalali F. Effects of soy on metabolic biomarkers of cardiovascular disease in elderly women with metabolic syndrome. Arch Iran Med, 2012; 15: 462-468 [PubMed] [Google Scholar]
- 541). Shidfar F, Ehramphosh E, Heydari I, Haghighi L, Hosseini S, Shidfar S. Effects of soy bean on serum paraoxonase 1 activity and lipoproteins in hyperlipidemic postmenopausal women. Int J Food Sci Nutr, 2009; 60: 195-205 [DOI] [PubMed] [Google Scholar]
- 542). Sirtori CR, Bosisio R, Pazzucconi F, Bondioli A, Gatti E, Lovati MR, Murphy P. Soy milk with a high glycitein content does not reduce low-density lipoprotein cholesterolemia in type II hypercholesterolemic patients. Ann Nutr Metab, 2002; 46: 88-92 [DOI] [PubMed] [Google Scholar]
- 543). Chiechi LM, Secreto G, Vimercati A, Greco P, Venturelli E, Pansini F, Fanelli M, Loizzi P, Selvaggi L. The effects of a soy rich diet on serum lipids: The menfis randomized trial. Maturitas, 2002; 41: 97-104 [DOI] [PubMed] [Google Scholar]
- 544). Meyer BJ, Larkin TA, Owen AJ, Astheimer LB, Tapsell LC, Howe PR. Limited lipid-lowering effects of regular consumption of whole soybean foods. Ann Nutr Metab, 2004; 48: 67-78 [DOI] [PubMed] [Google Scholar]
- 545). Zittermann A, Geppert J, Baier S, Zehn N, Gouni-Berthold I, Berthold HK, Reinsberg J, Stehle P. Short-term effects of high soy supplementation on sex hormones, bone markers, and lipid parameters in young female adults. Eur J Nutr, 2004; 43: 100-108 [DOI] [PubMed] [Google Scholar]
- 546). Anderson JW, Fuller J, Patterson K, Blair R, Tabor A. Soy compared to casein meal replacement shakes with energy-restricted diets for obese women: Randomized controlled trial. Metabolism, 2007; 56: 280-288 [DOI] [PubMed] [Google Scholar]
- 547). St-Onge MP, Claps N, Wolper C, Heymsfield SB. Supplementation with soy-protein-rich foods does not enhance weight loss. J Am Diet Assoc, 2007; 107: 500-505 [DOI] [PubMed] [Google Scholar]
- 548). Bertipaglia de Santana M, Mandarino MG, Cardoso JR, Dichi I, Dichi JB, Camargo AE, Fabris BA, Rodrigues RJ, Fatel EC, Nixdorf SL, Simao AN, Cecchini R, Barbosa DS. Association between soy and green tea (Camellia sinensis) diminishes hypercholesterolemia and increases total plasma antioxidant potential in dyslipidemic subjects. Nutrition, 2008; 24: 562-568 [DOI] [PubMed] [Google Scholar]
- 549). Beavers KM, Serra MC, Beavers DP, Hudson GM, Willoughby DS. The lipid-lowering effects of 4 weeks of daily soymilk or dairy milk ingestion in a postmenopausal female population. J Med Food, 2010; 13: 650-656 [DOI] [PubMed] [Google Scholar]
- 550). Simão AN, Lozovoy MA, Dichi I. Effect of soy product kinako and fish oil on serum lipids and glucose metabolism in women with metabolic syndrome. Nutrition, 2014; 30: 112-115 [DOI] [PubMed] [Google Scholar]
- 551). Moghaddam AS, Entezari MH, Iraj B, Askari GR, Maracy MR. The effects of consumption of bread fortified with soy bean flour on metabolic profile in type 2 diabetic women: A cross-over randomized controlled clinical trial. Int J Prev Med, 2014; 5: 1529-1536 [PMC free article] [PubMed] [Google Scholar]
- 552). Padhi EM, Blewett HJ, Duncan AM, Guzman RP, Hawke A, Seetharaman K, Tsao R, Wolever TM, Ramdath DD. Whole soy flour incorporated into a muffin and consumed at 2 doses of soy protein does not lower LDL cholesterol in a randomized, double-blind controlled trial of hypercholesterolemic adults. J Nutr, 2015; 145: 2665-2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553). Acharjee S, Zhou JR, Elajami TK, Welty FK. Effect of soy nuts and equol status on blood pressure, lipids and inflammation in postmenopausal women stratified by metabolic syndrome status. Metabolism, 2015; 64: 236-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554). Takatsuka N, Nagata C, Kurisu Y, Inaba S, Kawakami N, Shimizu H. Hypocholesterolemic effect of soymilk supplementation with usual diet in premenopausal normolipidemic Japanese women. Prev Med, 2000; 31: 308-314 [DOI] [PubMed] [Google Scholar]
- 555). Rivas M, Garay RP, Escanero JF, Cia P, Alda JO. Soy milk lowers blood pressure in men and women with mild to moderate essential hypertension. J Nutr, 2002; 132: 1900-1902 [DOI] [PubMed] [Google Scholar]
- 556). Nasca MM, Zhou JR, Welty FK. Effect of soy nuts on adhesion molecules and markers of inflammation in hypertensive and normotensive postmenopausal women. Am J Cardiol, 2008; 102: 84-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557). Husain D, Khanna K, Puri S, Haghighizadeh M. Supplementation of soy isoflavones improved sex hormones, blood pressure, and postmenopausal symptoms. J Am Coll Nutr, 2015; 34: 42-48 [DOI] [PubMed] [Google Scholar]
- 558). Zhan S, Ho SC. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr, 2005; 81: 397-408 [DOI] [PubMed] [Google Scholar]
- 559). Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: New insights. Circulation, 2011; 123: 2870-2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560). Kromhout D, Keys A, Aravanis C, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, Pekkarinen M. Food consumption patterns in the 1960s in seven countries. Am J Clin Nutr, 1989; 49: 889-894 [DOI] [PubMed] [Google Scholar]
- 561).National Institute of Health and Nutrition. Current nutrition intake in Japan. http://www0.nih.go.jp/eiken/chosa/kokumin_eiyou/ (in Japanese)
- 562). Shimazu T, Kuriyama S, Hozawa A, Ohmori K, Sato Y, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Dietary patterns and cardiovascular disease mortality in Japan: A prospective cohort study. Int J Epidemiol, 2007; 36: 600-609 [DOI] [PubMed] [Google Scholar]
- 563). Maruyama K, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, Inaba Y, Tamakoshi A, JACC Study Group Dietary patterns and risk of cardiovascular deaths among middle-aged Japanese: JACC Study. Nutr Metab Cardiovasc Dis, 2013; 23: 519-527 [DOI] [PubMed] [Google Scholar]
- 564). Nakamura Y, Ueshima H, Okamura T, Kadowaki T, Hayakawa T, Kita Y, Abbott RD, Okayama A, National Integrated Project for Prospective Observation of Non-Communicable Diseases and its Trends in the Aged RG A Japanese diet and 19-year mortality: National integrated project for prospective observation of non-communicable diseases and its trends in the aged, 1980. Br J Nutr, 2009; 101: 1696-1705 [DOI] [PubMed] [Google Scholar]
- 565). Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ, 2016; 353: i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566). Tada N, Maruyama C, Koba S, Tanaka H, Birou S, Teramoto T, Sasaki J. Japanese dietary lifestyle and cardiovascular disease. J Atheroscler Thromb, 2011; 18: 723-734 [DOI] [PubMed] [Google Scholar]
- 567).Ministry of Health, Labour and Welfare. The National Health and Nutrition Survey in Japan, 2015. http://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h27-houkoku.pdf (in Japanese)
- 568). Ikehara S, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Kondo T, Watanabe Y, Koizumi A, Wada Y, Inaba Y, Tamakoshi A, Japan Collaborative Cohort Study Group Alcohol consumption and mortality from stroke and coronary heart disease among Japanese men and women: The Japan collaborative cohort study. Stroke, 2008; 39: 2936-2942 [DOI] [PubMed] [Google Scholar]
- 569). Shirai K, Kobayashi J, Inadera H, Ohkubo Y, Mori S, Saito Y, Yoshida S. Type I hyperlipoproteinemia caused by lipoprotein lipase defect in lipid-interface recognition was relieved by administration of medium-chain triglyceride. Metabolism, 1992; 41: 1161-1164 [DOI] [PubMed] [Google Scholar]
- 570). Rouis M, Dugi KA, Previato L, Patterson AP, Brunzell JD, Brewer HB, Santamarina-Fojo S. Therapeutic response to medium-chain triglycerides and omega-3 fatty acids in a patient with the familial chylomicronemia syndrome. Arterioscler Thromb Vasc Biol, 1997; 17: 1400-1406 [DOI] [PubMed] [Google Scholar]
- 571). Sacks FM, Carey VJ, Anderson CA, Miller ER, Copeland T, Charleston J, Harshfield BJ, Laranjo N, McCarron P, Swain J, White K, Yee K, Appel LJ. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: The omnicarb randomized clinical trial. JAMA, 2014; 312: 2531-2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572). Kristo AS, Matthan NR, Lichtenstein AH. Effect of diets differing in glycemic index and glycemic load on cardiovascular risk factors: Review of randomized controlled-feeding trials. Nutrients, 2013; 5: 1071-1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573). Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk--a meta-analysis of observational studies. Am J Clin Nutr, 2008; 87: 627-637 [DOI] [PubMed] [Google Scholar]
- 574). Fan J, Song Y, Wang Y, Hui R, Zhang W. Dietary glycemic index, glycemic load, and risk of coronary heart disease, stroke, and stroke mortality: A systematic review with meta-analysis. PLoS One, 2012; 7: e52182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575). Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med, 2011; 364: 1218-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576). Imai S, Matsuda M, Hasegawa G, Fukui M, Obayashi H, Ozasa N, Kajiyama S. A simple meal plan of ‘eating vegetables before carbohydrate’ was more effective for achieving glycemic control than an exchange-based meal plan in Japanese patients with type 2 diabetes. Asia Pac J Clin Nutr, 2011; 20: 161-168 [PubMed] [Google Scholar]
- 577). Koba S, Tanaka H, Maruyama C, Tada N, Birou S, Teramoto T, Sasaki J. Physical activity in the Japan population: Association with blood lipid levels and effects in reducing cardiovascular and all-cause mortality. J Atheroscler Thromb, 2011; 18: 833-845 [DOI] [PubMed] [Google Scholar]
- 578). Brown T, Avenell A, Edmunds LD, Moore H, Whittaker V, Avery L, Summerbell C. Systematic review of longterm lifestyle interventions to prevent weight gain and morbidity in adults. Obes Rev, 2009; 10: 627-638 [DOI] [PubMed] [Google Scholar]
- 579). Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur J Epidemiol, 2015; 30: 529-542 [DOI] [PubMed] [Google Scholar]
- 580). Hsieh SD, Yoshinaga H, Muto T, Sakurai Y. Regular physical activity and coronary risk factors in Japanese men. Circulation, 1998; 97: 661-665 [DOI] [PubMed] [Google Scholar]
- 581). Pandey A, Garg S, Khunger M, Darden D, Ayers C, Kumbhani DJ, Mayo HG, de Lemos JA, Berry JD. Dose-response relationship between physical activity and risk of heart failure: A meta-analysis. Circulation, 2015; 132: 1786-1794 [DOI] [PubMed] [Google Scholar]
- 582). Lee IM, Paffenbarger RS. Preventing coronary heart disease: The role of physical activity. Phys Sportsmed, 2001; 29: 37-52 [DOI] [PubMed] [Google Scholar]
- 583). Oguma Y, Shinoda-Tagawa T. Physical activity decreases cardiovascular disease risk in women: Review and metaanalysis. Am J Prev Med, 2004; 26: 407-418 [DOI] [PubMed] [Google Scholar]
- 584). Froelicher VF, Myers JN. Exercise and the heart (4th ed.) W. B. Sanders Company, 2000 [Google Scholar]
- 585). Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: A meta-analysis. Stroke, 2003; 34: 2475-2481 [DOI] [PubMed] [Google Scholar]
- 586). Wendel-Vos GC, Schuit AJ, Feskens EJ, Boshuizen HC, Verschuren WM, Saris WH, Kromhout D. Physical activity and stroke. A meta-analysis of observational data. Int J Epidemiol, 2004; 33: 787-798 [DOI] [PubMed] [Google Scholar]
- 587). Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, Lancet Physical Activity Series Working Group Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet, 2012; 380: 219-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588). Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996-2011. Am J Prev Med, 2011; 41: 207-215 [DOI] [PubMed] [Google Scholar]
- 589). Lee PH, Wong FK. The association between time spent in sedentary behaviors and blood pressure: A systematic review and meta-analysis. Sports Med, 2015; 45: 867-880 [DOI] [PubMed] [Google Scholar]
- 590). Kikuchi H, Inoue S, Odagiri Y, Inoue M, Sawada N, Tsugane S, Japan Public Health Centre (JPHC) study group Occupational sitting time and risk of all-cause mortality among Japanese workers. Scand J Work Environ Health, 2015; 41: 519-528 [DOI] [PubMed] [Google Scholar]
- 591). Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann Intern Med, 2002; 136: 493-503 [DOI] [PubMed] [Google Scholar]
- 592). Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, Regensteiner JG, Rubin RR, Sigal RJ, American College of Sports Medicine; American Diabetes Association. Exercise and type 2 diabetes : American college of sports medicine and the American diabetes association: Joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exerc, 2010; 42: 2303. [DOI] [PubMed] [Google Scholar]
- 593). Lee KW, Lip GY. Effects of lifestyle on hemostasis, fibrinolysis, and platelet reactivity: A systematic review. Arch Intern Med, 2003; 163: 2368-2392 [DOI] [PubMed] [Google Scholar]
- 594). Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev, 2015: CD005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595). Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev, 2008: CD005381. [DOI] [PubMed] [Google Scholar]
- 596). Paffenbarger RS, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med, 1986; 314: 605-613 [DOI] [PubMed] [Google Scholar]
- 597). Kujala UM, Kaprio J, Sarna S, Koskenvuo M. Relationship of leisure-time physical activity and mortality: The Finnish twin cohort. JAMA, 1998; 279: 440-444 [DOI] [PubMed] [Google Scholar]
- 598). Leon AS, Myers MJ, Connett J. Leisure time physical activity and the 16-year risks of mortality from coronary heart disease and all-causes in the Multiple Risk Factor Intervention Trial (MRFIT). Int J Sports Med, 1997; 18 Suppl 3: S208-215 [DOI] [PubMed] [Google Scholar]
- 599). Echouffo-Tcheugui JB, Butler J, Yancy CW, Fonarow GC. Association of physical activity or fitness with incident heart failure: A systematic review and meta-analysis. Circ Heart Fail, 2015; 8: 853-861 [DOI] [PubMed] [Google Scholar]
- 600). Lakka TA, Venalainen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med, 1994; 330: 1549-1554 [DOI] [PubMed] [Google Scholar]
- 601). Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA, 1989; 262: 2395-2401 [DOI] [PubMed] [Google Scholar]
- 602). Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med, 2002; 346: 793-801 [DOI] [PubMed] [Google Scholar]
- 603). Katzmarzyk PT, Church TS, Blair SN. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med, 2004; 164: 1092-1097 [DOI] [PubMed] [Google Scholar]
- 604). Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care, 2004; 27: 83-88 [DOI] [PubMed] [Google Scholar]
- 605). Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, Karasik P, Greenberg M, Papademetriou V, Singh S. Exercise capacity and mortality in black and white men. Circulation, 2008; 117: 614-622 [DOI] [PubMed] [Google Scholar]
- 606). Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA, 2009; 301: 2024-2035 [DOI] [PubMed] [Google Scholar]
- 607). Durstine JL, Grandjean PW, Davis PG, Ferguson MA, Alderson NL, DuBose KD. Blood lipid and lipoprotein adaptations to exercise: A quantitative analysis. Sports Med, 2001; 31: 1033-1062 [DOI] [PubMed] [Google Scholar]
- 608). Leon AS, Sanchez OA. Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc, 2001; 33: S502-515; discussion S528–509 [DOI] [PubMed] [Google Scholar]
- 609). Kelley GA, Kelley KS, Tran ZV. Walking, lipids, and lipoproteins: A meta-analysis of randomized controlled trials. Prev Med, 2004; 38: 651-661 [DOI] [PubMed] [Google Scholar]
- 610). Kelley GA, Kelley KS, Tran ZV. Aerobic exercise and lipids and lipoproteins in women: A meta-analysis of randomized controlled trials. J Womens Health (Larchmt), 2004; 13: 1148-1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611). Kelley GA, Kelley KS, Tran ZV. Exercise, lipids, and lipoproteins in older adults: A meta-analysis. Prev Cardiol, 2005; 8: 206-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612). Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, Suzuki E, Shimano H, Yamamoto S, Kondo K, Ohashi Y, Yamada N, Sone H. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: A meta-analysis. Arch Intern Med, 2007; 167: 999-1008 [DOI] [PubMed] [Google Scholar]
- 613). Kuhle CL, Steffen MW, Anderson PJ, Murad MH. Effect of exercise on anthropometric measures and serum lipids in older individuals: A systematic review and meta-analysis. BMJ Open, 2014; 4: e005283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614). Kelley GA, Kelley KS. Aerobic exercise and lipids and lipoproteins in men: A meta-analysis of randomized controlled trials. J Mens Health Gend, 2006; 3: 61-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615). Kelley GA, Kelley KS. Impact of progressive resistance training on lipids and lipoproteins in adults: Another look at a meta-analysis using prediction intervals. Prev Med, 2009; 49: 473-475 [DOI] [PubMed] [Google Scholar]
- 616).Ministry of Health, Labour and Welfare. Review conference on the revision of exercising guidelines and indices Guidelines for physical activities for health-building. 2013. http://www.mhlw.go.jp/stf/houdou/2r9852000002xpleatt/2r9852000002xpqt.pdf (in Japanese)
- 617). Hootman JM, Macera CA, Ainsworth BE, Addy CL, Martin M, Blair SN. Epidemiology of musculoskeletal injuries among sedentary and physically active adults. Med Sci Sports Exerc, 2002; 34: 838-844 [DOI] [PubMed] [Google Scholar]
- 618). Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, Limacher M, Pina IL, Stein RA, Williams M, Bazzarre T. AHA science advisory. Resistance exercise in individuals with and without cardiovascular disease: Benefits, rationale, safety, and prescription: An advisory from the committee on exercise, rehabilitation, and prevention, council on clinical cardiology, American Heart Association; position paper endorsed by the American college of sports medicine. Circulation, 2000; 101: 828-833 [DOI] [PubMed] [Google Scholar]
- 619). Hosomi N, Nagai Y, Kohriyama T, Ohtsuki T, Aoki S, Nezu T, Maruyama H, Sunami N, Yokota C, Kitagawa K, Terayama Y, Takagi M, Ibayashi S, Nakamura M, Origasa H, Fukushima M, Mori E, Minematsu K, Uchiyama S, Shinohara Y, Yamaguchi T, Matsumoto M, J-STARS Collaborators The Japan statin treatment against recurrent stroke (J-STARS): A multicenter, randomized, open-label, parallel-group study. EBioMedicine, 2015; 2: 1071-1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620). Mizuno K, Nakaya N, Ohashi Y, Tajima N, Kushiro T, Teramoto T, Uchiyama S, Nakamura H, MEGA Study Group Usefulness of pravastatin in primary prevention of cardiovascular events in women: Analysis of the management of elevated cholesterol in the primary prevention group of adult Japanese (MEGA study). Circulation, 2008; 117: 494-502 [DOI] [PubMed] [Google Scholar]
- 621). Uchiyama S, Nakaya N, Mizuno K, Ohashi Y, Tajima N, Kushiro T, Teramoto T, Nakamura H, MEGA Study Group Risk factors for stroke and lipid-lowering effect of pravastatin on the risk of stroke in Japanese patients with hypercholesterolemia: Analysis of data from the mega study, a large randomized controlled trial. J Neurol Sci, 2009; 284: 72-76 [DOI] [PubMed] [Google Scholar]
- 622). Tanaka K, Ishikawa Y, Yokoyama M, Origasa H, Matsuzaki M, Saito Y, Matsuzawa Y, Sasaki J, Oikawa S, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, JELIS Investigators Japan Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: Subanalysis of the JELIS trial. Stroke, 2008; 39: 2052-2058 [DOI] [PubMed] [Google Scholar]
- 623). Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, Lopez-Jaramillo P, Leiter LA, Dans A, Avezum A, Piegas LS, Parkhomenko A, Keltai K, Keltai M, Sliwa K, Peters RJ, Held C, Chazova I, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Sanchez-Vallejo G, McKelvie R, Pogue J, Jung H, Gao P, Diaz R, Lonn E, HOPE-3 Investigators Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med, 2016; 374: 2021-2031 [DOI] [PubMed] [Google Scholar]
- 624). Daida H, Teramoto T, Kitagawa Y, Matsushita Y, Sugihara M. The relationship between low-density lipoprotein cholesterol levels and the incidence of cardiovascular disease in high-risk patients treated with pravastatin: Main results of the APPROACH-J study. Int Heart J, 2014; 55: 39-47 [DOI] [PubMed] [Google Scholar]
- 625). Oikawa S, Kita T, Mabuchi H, Matsuzaki M, Matsuzawa Y, Nakaya N, Saito Y, Sasaki J, Shimamoto K, Itakura H, J-LIT Study Group Risk of coronary events in Japanese patients with both hypercholesterolemia and type 2 diabetes mellitus on low-dose simvastatin therapy: Implication from Japan Lipid Intervention Trial (J-LIT). Atherosclerosis, 2007; 191: 440-446 [DOI] [PubMed] [Google Scholar]
- 626). Teramoto T, Uno K, Miyoshi I, Khan I, Gorcyca K, Sanchez RJ, Yoshida S, Mawatari K, Masaki T, Arai H, Yamashita S. Low-density lipoprotein cholesterol levels and lipid-modifying therapy prescription patterns in the real world: An analysis of more than 33,000 high cardiovascular risk patients in Japan. Atherosclerosis, 2016; 251: 248-254 [DOI] [PubMed] [Google Scholar]
- 627). Hasegawa K, Tsukamoto K, Kunimi M, Asahi K, Iseki K, Moriyama T, Yamagata K, Tsuruya K, Fujimoto S, Narita I, Konta T, Kondo M, Kimura K, Ohashi Y, Watanabe T. Control status of atherosclerotic cardiovascular risk factors among Japanese high-risk subjects: Analyses of a Japanese health check database from 2008 to 2011. J Atheroscler Thromb, 2016; 23: 991-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628). Ford I, Murray H, McCowan C, Packard CJ. Long-term safety and efficacy of lowering low-density lipoprotein cholesterol with statin therapy: 20-year follow-up of West of Scotland Coronary Prevention Study. Circulation, 2016; 133: 1073-1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629). Bulbulia R, Bowman L, Wallendszus K, Parish S, Armitage J, Peto R, Collins R, Heart Protection Study Collaborative Group Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: A randomised controlled trial. Lancet, 2011; 378: 2013-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630). Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, Kahn J, Afonso L, Williams KA, Flack JM. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: A Mendelian randomization analysis. J Am Coll Cardiol, 2012; 60: 2631-2639 [DOI] [PubMed] [Google Scholar]
- 631). Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol, 2006; 48: 438-445 [DOI] [PubMed] [Google Scholar]
- 632). Naito R, Miyauchi K, Konishi H, Tsuboi S, Ogita M, Dohi T, Kasai T, Tamura H, Okazaki S, Isoda K, Daida H. Appropriate level of low-density lipoprotein cholesterol for secondary prevention of coronary artery disease. J Atheroscler Thromb, 2016; 23: 413-421 [DOI] [PubMed] [Google Scholar]
- 633). Natsuaki M, Furukawa Y, Morimoto T, Nakagawa Y, Ono K, Kaburagi S, Inada T, Mitsuoka H, Taniguchi R, Nakano A, Kita T, Sakata R, Kimura T, CREDO-Kyoto PCI/CABG registry cohort-2 investigators Intensity of statin therapy, achieved low-density lipoprotein cholesterol levels and cardiovascular outcomes in Japanese patients after coronary revascularization. Perspectives from the CREDO-Kyoto registry cohort-2. Circ J, 2012; 76: 1369-1379 [DOI] [PubMed] [Google Scholar]
- 634). Ota T, Ishii H, Suzuki S, Shibata Y, Tatami Y, Harata S, Shimbo Y, Takayama Y, Tanaka A, Kawamura Y, Osugi N, Maeda K, Kondo T, Murohara T. Impact of the statin escape phenomenon on long-term clinical outcomes in patients with acute myocardial infarction: Subgroup analysis of the nagoya acute myocardial infarction study (NAMIS). Atherosclerosis, 2015; 242: 155-160 [DOI] [PubMed] [Google Scholar]
- 635). Bangalore S, Fayyad R, Kastelein JJ, Laskey R, Amarenco P, DeMicco DA, Waters DD. 2013 cholesterol guidelines revisited: Percent LDL cholesterol reduction or attained LDL cholesterol level or both for prognosis? Am J Med, 2016; 129: 384-391 [DOI] [PubMed] [Google Scholar]
- 636). Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, DeLucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM, IMPROVE-IT Investigators Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med, 2015; 372: 2387-2397 [DOI] [PubMed] [Google Scholar]
- 637). Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ, ODYSSEY LONG TERM Investigators Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med, 2015; 372: 1489-1499 [DOI] [PubMed] [Google Scholar]
- 638). Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA, Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med, 2015; 372: 1500-1509 [DOI] [PubMed] [Google Scholar]
- 639). Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR, FOURIER Steering Committee and Investigators Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med, 2017; 376: 1713-1722 [DOI] [PubMed] [Google Scholar]
- 640). Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD, DePalma SM, Minissian MB, Orringer CE, Smith SC, Committee W. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: A report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol, 2016; 68: 92-125 [DOI] [PubMed] [Google Scholar]
- 641). Nicholls SJ, Hsu A, Wolski K, Hu B, Bayturan O, Lavoie A, Uno K, Tuzcu EM, Nissen SE. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol, 2010; 55: 2399-2407 [DOI] [PubMed] [Google Scholar]
- 642). Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, Daida H. Early statin treatment in patients with acute coronary syndrome: Demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: The establish study. Circulation, 2004; 110: 1061-1068 [DOI] [PubMed] [Google Scholar]
- 643). Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M, JAPAN-ACS Investigators Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: A multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol, 2009; 54: 293-302 [DOI] [PubMed] [Google Scholar]
- 644). Nakajima N, Miyauchi K, Yokoyama T, Ogita M, Miyazaki T, Tamura H, Nishino A, Yokoyama K, Okazaki S, Kurata T, Suwa S, Daida H. Effect of combination of ezetimibe and a statin on coronary plaque regression in patients with acute coronary syndrome: Zeus trial (Ezetimibe Ultrasound Study). IJC Metab Endocr, 2014; 3:8-13 [Google Scholar]
- 645). Tsujita K, Sugiyama S, Sumida H, Shimomura H, Yamashita T, Yamanaga K, Komura N, Sakamoto K, Oka H, Nakao K, Nakamura S, Ishihara M, Matsui K, Sakaino N, Nakamura N, Yamamoto N, Koide S, Matsumura T, Fujimoto K, Tsunoda R, Morikami Y, Matsuyama K, Oshima S, Kaikita K, Hokimoto S, Ogawa H, PRECISE-IVUS Investigators Impact of dual lipidlowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: The multicenter randomized controlled precise-ivus trial. J Am Coll Cardiol, 2015; 66: 495-507 [DOI] [PubMed] [Google Scholar]
- 646). Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, LaRosa JC, Waters DD, DeMicco DA, Simes RJ, Keech AC, Colquhoun D, Hitman GA, Betteridge DJ, Clearfield MB, Downs JR, Colhoun HM, Gotto AM, Jr., Ridker PM, Grundy SM, Kastelein JJ. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: A meta-analysis of statin trials. J Am Coll Cardiol, 2014; 64: 485-494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647). Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans affairs high-density lipoprotein cholesterol intervention trial study group. N Engl J Med, 1999; 341: 410-418 [DOI] [PubMed] [Google Scholar]
- 648). Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V, Helsinki Heart Study : Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med, 1987; 317: 1237-1245 [DOI] [PubMed] [Google Scholar]
- 649). Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesaniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M, FIELD study investigators Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet, 2005; 366: 1849-1861 [DOI] [PubMed] [Google Scholar]
- 650). Ginsberg HN, Elam MB, Lovato LC, Crouse JR, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC, Cushman WC, Simons-Morton DG, Byington RP, ACCORD Study Group Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med, 2010; 362: 1563-1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651). Jakob T, Nordmann AJ, Schandelmaier S, Ferreira-Gonzalez I, Briel M. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev, 2016; 11: CD009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652). Wang D, Liu B, Tao W, Hao Z, Liu M. Fibrates for secondary prevention of cardiovascular disease and stroke. Cochrane Database Syst Rev, 2015: CD009580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653). Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W, AIM-HIGH Investigators Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med, 2011; 365: 2255-2267 [DOI] [PubMed] [Google Scholar]
- 654). Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J, HPS2-THRIVE Collaborative Group Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med, 2014; 371: 203-212 [DOI] [PubMed] [Google Scholar]
- 655). Murphy MJ, Sheng X, MacDonald TM, Wei L. Hypertriglyceridemia and acute pancreatitis. JAMA Intern Med, 2013; 173: 162-164 [DOI] [PubMed] [Google Scholar]
- 656). Mabuchi H, Haba T, Tatami R, Miyamoto S, Sakai Y, Wakasugi T, Watanabe A, Koizumi J, Takeda R. Effect of an inhibitor of 3-hydroxy-3-methyglutaryl coenzyme a reductase on serum lipoproteins and ubiquinone-10-levels in patients with familial hypercholesterolemia. N Engl J Med, 1981; 305: 478-482 [DOI] [PubMed] [Google Scholar]
- 657). Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res, 1992; 33: 1569-1582 [PubMed] [Google Scholar]
- 658). Bilheimer DW, Grundy SM, Brown MS, Goldstein JL. Mevinolin and colestipol stimulate receptor-mediated clearance of low density lipoprotein from plasma in familial hypercholesterolemia heterozygotes. Proc Natl Acad Sci USA, 1983; 80: 4124-4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659). Bakker-Arkema RG, Davidson MH, Goldstein RJ, Davignon J, Isaacsohn JL, Weiss SR, Keilson LM, Brown WV, Miller VT, Shurzinske LJ, Black DM. Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. JAMA, 1996; 275: 128-133 [PubMed] [Google Scholar]
- 660). Pharmaceuticals and Medical Devices Agency Revisions to the “Precautions of use” of HMG-CoA reducatase inhibitor-containing pharmaceutical drugs. 2016. https://www.pmda.go.jp/files/000214541.pdf (in Japanese)
- 661). Mammen AL. Statin-associated autoimmune myopathy. N Engl J Med, 2016; 374: 664-669 [DOI] [PubMed] [Google Scholar]
- 662). Edison RJ, Muenke M. Central nervous system and limb anomalies in case reports of first-trimester statin exposure. N Engl J Med, 2004; 350: 1579-1582 [DOI] [PubMed] [Google Scholar]
- 663). Gagne C, Gaudet D, Bruckert E, Ezetimibe Study Group Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation, 2002; 105: 2469-2475 [DOI] [PubMed] [Google Scholar]
- 664). Brown G, Albers JJ, Fisher LD, Schaefer SM, Lin JT, Kaplan C, Zhao XQ, Bisson BD, Fitzpatrick VF, Dodge HT. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med, 1990; 323: 1289-1298 [DOI] [PubMed] [Google Scholar]
- 665). Mabuchi H, Koizumi J, Kajinami K, Takeda M, Nitta Y, Matsubara T, Misawa K, Oota M, Sumitani T, Kametani T, Takekoshi N, Hifumi S, Yagi K, Kitamura H, Sanada H, Ohka T, Kanaya H, Uno Y, Miyamoto S, Yoshimura A, Ueda K, Fujita H, Takegoshi T, Wakasugi T, Mizuno S, Ohsato K, Murakami T, Konishi K, Arai Y. Effects of MCI-196 combied with pravastatin on hypercholesterolemia (I). Study in familial hypercholesterolemia (FH). Rinshoiyaku, 1996; 12: 1435-1462 (in Japanese) [Google Scholar]
- 666). Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Sun S, LeBeaut AP, Sager PT, Veltri EP, Ezetimibe Study Group Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: A prospective, randomized, double-blind trial. Circulation, 2003; 107: 2409-2415 [DOI] [PubMed] [Google Scholar]
- 667). Thomopoulos C, Skalis G, Michalopoulou H, Tsioufis C, Makris T. Effect of low-density lipoprotein cholesterol lowering by ezetimibe/simvastatin on outcome incidence: Overview, meta-analyses, and meta-regression analyses of randomized trials. Clin Cardiol, 2015; 38: 763-769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668). Takada T, Yamanashi Y, Konishi K, Yamamoto T, Toyoda Y, Masuo Y, Yamamoto H, Suzuki H. NPC1L1 is a key regulator of intestinal vitamin k absorption and a modulator of warfarin therapy. Sci Transl Med, 2015; 7: 275ra223. [DOI] [PubMed] [Google Scholar]
- 669).The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA, 1984; 251: 351-364 [DOI] [PubMed] [Google Scholar]
- 670).The lipid research clinics coronary primary prevention trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA, 1984; 251: 365-374 [PubMed] [Google Scholar]
- 671). Rudling MJ, Reihner E, Einarsson K, Ewerth S, Angelin B. Low density lipoprotein receptor-binding activity in human tissues: Quantitative importance of hepatic receptors and evidence for regulation of their expression in vivo. Proc Natl Acad Sci USA, 1990; 87: 3469-3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672). Kodama T, Reddy P, Kishimoto C, Krieger M. Purification and characterization of a bovine acetyl low density lipoprotein receptor. Proc Natl Acad Sci USA, 1988; 85: 9238-9242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673). Kita T, Ishii K, Yokode M, Kume N, Nagano Y, Arai H, Kawai C. The role of oxidized low density lipoprotein in the pathogenesis of atherosclerosis. Eur Heart J, 1990; 11 Suppl E: 122-127 [DOI] [PubMed] [Google Scholar]
- 674). Itabe H, Takeshima E, Iwasaki H, Kimura J, Yoshida Y, Imanaka T, Takano T. A monoclonal antibody against oxidized lipoprotein recognizes foam cells in atherosclerotic lesions. Complex formation of oxidized phosphatidylcholines and polypeptides. J Biol Chem, 1994; 269: 15274-15279 [PubMed] [Google Scholar]
- 675). Itabe H, Yamamoto H, Suzuki M, Kawai Y, Nakagawa Y, Suzuki A, Imanaka T, Takano T. Oxidized phosphatidylcholines that modify proteins. Analysis by monoclonal antibody against oxidized low density lipoprotein. J Biol Chem, 1996; 271: 33208-33217 [DOI] [PubMed] [Google Scholar]
- 676). Tardif JC, Cote G, Lesperance J, Bourassa M, Lambert J, Doucet S, Bilodeau L, Nattel S, deGuise P. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and probucol study group. N Engl J Med, 1997; 337: 365-372 [DOI] [PubMed] [Google Scholar]
- 677). Yokoi H, Daida H, Kuwabara Y, Nishikawa H, Takatsu F, Tomihara H, Nakata Y, Kutsumi Y, Ohshima S, Nishiyama S, Seki A, Kato K, Nishimura S, Kanoh T, Yamaguchi H. Effectiveness of an antioxidant in preventing restenosis after percutaneous transluminal coronary angioplasty: The probucol angioplasty restenosis trial. J Am Coll Cardiol, 1997; 30: 855-862 [DOI] [PubMed] [Google Scholar]
- 678). Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, Kashiwagi S, Hayashi J. Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST). J Am Coll Cardiol, 2002; 39: 610-616 [DOI] [PubMed] [Google Scholar]
- 679). Yamashita S, Bujo H, Arai H, Harada-Shiba M, Matsui S, Fukushima M, Saito Y, Kita T, Matsuzawa Y. Longterm probucol treatment prevents secondary cardiovascular events: A cohort study of patients with heterozygous familial hypercholesterolemia in Japan. J Atheroscler Thromb, 2008; 15: 292-303 [DOI] [PubMed] [Google Scholar]
- 680). Walldius G, Erikson U, Olsson AG, Bergstrand L, Hadell K, Johansson J, Kaijser L, Lassvik C, Molgaard J, Nilsson S. The effect of probucol on femoral atherosclerosis: The Probucol Quantitative Regression Swedish Trial (PQRST). Am J Cardiol, 1994; 74: 875-883 [DOI] [PubMed] [Google Scholar]
- 681). Ni YG, Condra JH, Orsatti L, Shen X, Di Marco S, Pandit S, Bottomley MJ, Ruggeri L, Cummings RT, Cubbon RM, Santoro JC, Ehrhardt A, Lewis D, Fisher TS, Ha S, Njimoluh L, Wood DD, Hammond HA, Wisniewski D, Volpari C, Noto A, Lo Surdo P, Hubbard B, Carfi A, Sitlani A. A proprotein convertase subtilisinlike/kexin type 9 (PCSK9) c-terminal domain antibody antigen-binding fragment inhibits PCSK9 internalization and restores low density lipoprotein uptake. J Biol Chem, 2010; 285: 12882-12891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682). Kiyosue A, Honarpour N, Kurtz C, Xue A, Wasserman SM, Hirayama A. A phase 3 study of evolocumab (AMG 145) in statin-treated Japanese patients at high cardiovascular risk. Am J Cardiol, 2016; 117: 40-47 [DOI] [PubMed] [Google Scholar]
- 683). Teramoto T, Kobayashi M, Tasaki H, Yagyu H, Higashikata T, Takagi Y, Uno K, Baccara-Dinet MT, Nohara A. Efficacy and safety of alirocumab in Japanese patients with heterozygous familial hypercholesterolemia or at high cardiovascular risk with hypercholesterolemia not adequately controlled with statins - ODESSEY JAPAN Randomized Controlled Trial. Circ J, 2016; 80: 1980-1987 [DOI] [PubMed] [Google Scholar]
- 684). Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, Ikewaki K, Siegelman ES, Gregg RE, Rader DJ. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med, 2007; 356: 148-156 [DOI] [PubMed] [Google Scholar]
- 685). Samaha FF, McKenney J, Bloedon LT, Sasiela WJ, Rader DJ. Inhibition of microsomal triglyceride transfer protein alone or with ezetimibe in patients with moderate hypercholesterolemia. Nat Clin Pract Cardiovasc Med, 2008; 5: 497-505 [DOI] [PubMed] [Google Scholar]
- 686). Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res, 1996; 37: 907-925 [PubMed] [Google Scholar]
- 687). Fruchart JC, Brewer HB, Leitersdorf E. Consensus for the use of fibrates in the treatment of dyslipoproteinemia and coronary heart disease. Fibrate consensus group. Am J Cardiol, 1998; 81: 912-917 [DOI] [PubMed] [Google Scholar]
- 688). Alderman JD, Pasternak RC, Sacks FM, Smith HS, Monrad ES, Grossman W. Effect of a modified, well-tolerated niacin regimen on serum total cholesterol, high density lipoprotein cholesterol and the cholesterol to high density lipoprotein ratio. Am J Cardiol, 1989; 64: 725-729 [DOI] [PubMed] [Google Scholar]
- 689). Teramoto T, Yamada N, Shimano H, Oka Y, Itakura H, Saito Y, Morisaki N, Shirai K, Ishikawa T, Tada N, Ito H, Yamanouchi T, Matsushima T, Kawakami M, Murase T, Okubo M, Totsuka Y, Kikuchi M. Dose-dependent effect of niceritrol on plasma lipoprotein-a. Scand J Clin Lab Invest, 1996; 56: 359-365 [DOI] [PubMed] [Google Scholar]
- 690). Carlson LA, Hamsten A, Asplund A. Pronounced lowering of serum levels of lipoprotein Lp (a) in hyperlipidaemic subjects treated with nicotinic acid. J Intern Med, 1989; 226: 271-276 [DOI] [PubMed] [Google Scholar]
- 691). Matsunaga A, Handa K, Mori T, Moriyama K, Hidaka K, Yuki M, Sasaki J, Arakawa K. Effects of niceritrol on levels of serum lipids, lipoprotein (a), and fibrinogen in patients with primary hypercholesterolemia. Atherosclerosis, 1992; 94: 241-248 [DOI] [PubMed] [Google Scholar]
- 692). Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, Japan EPA lipid intervention study (JELIS) Investigators Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet, 2007; 369: 1090-1098 [DOI] [PubMed] [Google Scholar]
- 693). Kromhout D, Giltay EJ, Geleijnse JM, Alpha Omega Trial Group N-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med, 2010; 363: 2015-2026 [DOI] [PubMed] [Google Scholar]
- 694). Bosch J, Gerstein HC, Dagenais GR, Diaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, Riddle MC, Ryden LE, Yusuf S, ORIGIN Trial Investigators N-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med, 2012; 367: 309-318 [DOI] [PubMed] [Google Scholar]
- 695). Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellstrom B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Gronhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R, SHARP Investigators The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet, 2011; 377: 2181-2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696). Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K, JELIS Investigators, Japan Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: Sub-analysis of primary prevention cases from the Japan EPA lipid intervention study (JELIS). Atherosclerosis, 2008; 200: 135-140 [DOI] [PubMed] [Google Scholar]
- 697). ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC, Jr., Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med, 2010; 362: 1563-1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698). AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med, 2011; 365: 2255-2267 [DOI] [PubMed] [Google Scholar]
- 699). The HPS2-THRIVE Collaborative Group. Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med, 2014; 371: 203-212 [DOI] [PubMed] [Google Scholar]
- 700). Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, Krumholz HM. Risks associated with statin therapy: A systematic overview of randomized clinical trials. Circulation, 2006; 114: 2788-2797 [DOI] [PubMed] [Google Scholar]
- 701). Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA, 2003; 289: 1681-1690 [DOI] [PubMed] [Google Scholar]
- 702). Joy TR, Hegele RA. Narrative review: Statin-related myopathy. Ann Intern Med, 2009; 150: 858-868 [DOI] [PubMed] [Google Scholar]
- 703). Bays H. Statin safety: An overview and assessment of the data--2005. Am J Cardiol, 2006; 97: 6C-26C [DOI] [PubMed] [Google Scholar]
- 704). Law M, Rudnicka AR. Statin safety: A systematic review. Am J Cardiol, 2006; 97: 52C-60C [DOI] [PubMed] [Google Scholar]
- 705). Katz DH, Intwala SS, Stone NJ. Addressing statin adverse effects in the clinic: The 5 Ms. J Cardiovasc Pharmacol Ther, 2014; 19: 533-542 [DOI] [PubMed] [Google Scholar]
- 706). Jacobson TA. Toward “Pain-free” Statin prescribing: Clinical algorithm for diagnosis and management of myalgia. Mayo Clin Proc, 2008; 83: 687-700 [DOI] [PubMed] [Google Scholar]
- 707). Charles EC, Olson KL, Sandhoff BG, McClure DL, Merenich JA. Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization. Am J Med, 2005; 118: 618-624 [DOI] [PubMed] [Google Scholar]
- 708). Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet, 2010; 375: 735-742 [DOI] [PubMed] [Google Scholar]
- 709). Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. JAMA, 2011; 305: 2556-2564 [DOI] [PubMed] [Google Scholar]
- 710). Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: A meta-analysis. Diabetes Care, 2009; 32: 1924-1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711). Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. Lancet, 2012; 380: 565-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712). Wiklund O, Pirazzi C, Romeo S. Monitoring of lipids, enzymes, and creatine kinase in patients on lipid-lowering drug therapy. Curr Cardiol Rep, 2013; 15: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713). Hirota T, Ieiri I. Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol, 2015; 11: 1435-1447 [DOI] [PubMed] [Google Scholar]
- 714). Omar MA, Wilson JP, Cox TS. Rhabdomyolysis and HMG-CoA reductase inhibitors. Ann Pharmacother, 2001; 35: 1096-1107 [DOI] [PubMed] [Google Scholar]
- 715). Enajat M, Teerenstra S, van Kuilenburg JT, van Sorge-Greve AH, Albers-Akkers MT, Verheugt FW, Pop GA. Safety of the combination of intensive cholesterol-lowering therapy with oral anticoagulation medication in elderly patients with atrial fibrillation: A randomized, double-blind, placebo-controlled study. Drugs Aging, 2009; 26: 585-593 [DOI] [PubMed] [Google Scholar]
- 716). Neutel JM, Bestermann WH, Dyess EM, Graff A, Kursun A, Sutradhar S, Yunis C. The use of a single-pill calcium channel blocker/statin combination in the management of hypertension and dyslipidemia: A randomized, placebo-controlled, multicenter study. Journal of clinical hypertension (Greenwich, Conn.), 2009; 11: 22-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 717). Patel BV, Leslie RS, Thiebaud P, Nichol MB, Tang SS, Solomon H, Honda D, Foody JM. Adherence with single-pill amlodipine/atorvastatin vs a two-pill regimen. Vasc Health Risk Manag, 2008; 4: 673-681 [PMC free article] [PubMed] [Google Scholar]
- 718). Hussein MA, Chapman RH, Benner JS, Tang SS, Solomon HA, Joyce A, Foody JM. Does a single-pill antihypertensive/lipid-lowering regimen improve adherence in us managed care enrolees? A non-randomized, observational, retrospective study. Am J Cardiovasc Drugs, 2010; 10: 193-202 [DOI] [PubMed] [Google Scholar]
- 719). Mann DM, Woodward M, Muntner P, Falzon L, Kronish I. Predictors of nonadherence to statins: A systematic review and meta-analysis. Ann Pharmacother, 2010; 44: 1410-1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720). Wiegand P, McCombs JS, Wang JJ. Factors of hyperlipidemia medication adherence in a nationwide health plan. Am J Manag Care, 2012; 18: 193-199 [PubMed] [Google Scholar]
- 721). Shalev V, Chodick G, Silber H, Kokia E, Jan J, Heymann AD. Continuation of statin treatment and allcause mortality: A population-based cohort study. Arch Intern Med, 2009; 169: 260-268 [DOI] [PubMed] [Google Scholar]
- 722). Origasa H, Yokoyama M, Matsuzaki M, Saito Y, Matsuzawa Y, JELIS Investigators Clinical importance of adherence to treatment with eicosapentaenoic acid by patients with hypercholesterolemia. Circ J, 2010; 74: 510-517 [DOI] [PubMed] [Google Scholar]
- 723). Evans CD, Eurich DT, Taylor JG, Blackburn DF. The Collaborative Cardiovascular Risk Reduction in Primary Care (CCARP) study. Pharmacotherapy, 2010; 30: 766-775 [DOI] [PubMed] [Google Scholar]
- 724). Helin-Salmivaara A, Lavikainen P, Korhonen MJ, Halava H, Junnila SY, Kettunen R, Neuvonen PJ, Martikainen JE, Ruokoniemi P, Saastamoinen LK, Virta L, Huupponen R. Long-term persistence with statin therapy: A nationwide register study in Finland. Clin Ther, 2008; 30 Pt 2: 2228-2240 [DOI] [PubMed] [Google Scholar]
- 725). Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther, 2001; 23: 1296-1310 [DOI] [PubMed] [Google Scholar]
- 726). Osterberg L, Blaschke T. Adherence to medication. N Engl J Med, 2005; 353: 487-497 [DOI] [PubMed] [Google Scholar]
- 727). Gagne JJ, Choudhry NK, Kesselheim AS, Polinski JM, Hutchins D, Matlin OS, Brennan TA, Avorn J, Shrank WH. Comparative effectiveness of generic and brandname statins on patient outcomes: A cohort study. Ann Intern Med, 2014; 161: 400-407 [DOI] [PubMed] [Google Scholar]
- 728). Benner JS, Tierce JC, Ballantyne CM, Prasad C, Bullano MF, Willey VJ, Erbey J, Sugano DS. Follow-up lipid tests and physician visits are associated with improved adherence to statin therapy. Pharmacoeconomics, 2004; 22 Suppl 3: 13-23 [DOI] [PubMed] [Google Scholar]
- 729). Donnelly LA, Doney AS, Morris AD, Palmer CN, Donnan PT. Long-term adherence to statin treatment in diabetes. Diabet Med, 2008; 25: 850-855 [DOI] [PubMed] [Google Scholar]
- 730). Caspard H, Chan AK, Walker AM. Compliance with a statin treatment in a usual-care setting: Retrospective database analysis over 3 years after treatment initiation in health maintenance organization enrollees with dyslipidemia. Clin Ther, 2005; 27: 1639-1646 [DOI] [PubMed] [Google Scholar]
- 731). Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA, 2002; 288: 455-461 [DOI] [PubMed] [Google Scholar]
- 732). Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Boren J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjarg-Hansen A, Panel EASC. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European Atherosclerosis Society. Eur Heart J, 2013; 34: 3478-3490a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 733). Neil A, Cooper J, Betteridge J, Capps N, McDowell I, Durrington P, Seed M, Humphries SE. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: A prospective registry study. European heart journal, 2008; 29: 2625-2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734). Harada-Shiba M, Sugisawa T, Makino H, Abe M, Tsushima M, Yoshimasa Y, Yamashita T, Miyamoto Y, Yamamoto A, Tomoike H, Yokoyama S. Impact of statin treatment on the clinical fate of heterozygous familial hypercholesterolemia. J Atheroscler Thromb, 2010; 17: 667-674 [DOI] [PubMed] [Google Scholar]
- 735). De Backer G, Besseling J, Chapman J, Hovingh GK, Kastelein JJ, Kotseva K, Ray K, Reiner Ž, Wood D, De Bacquer D, EUROASPIRE Investigators Prevalence and management of familial hypercholesterolaemia in coronary patients: An analysis of EUROASPIRE IV, a study of the European Society of Cardiology. Atherosclerosis, 2015; 241: 169-175 [DOI] [PubMed] [Google Scholar]
- 736). Rallidis LS, Triantafyllis AS, Tsirebolos G, Katsaras D, Rallidi M, Moutsatsou P, Lekakis J. Prevalence of heterozygous familial hypercholesterolaemia and its impact on long-term prognosis in patients with very early st-segment elevation myocardial infarction in the era of statins. Atherosclerosis, 2016; 249: 17-21 [DOI] [PubMed] [Google Scholar]
- 737). Nanchen D, Gencer B, Muller O, Auer R, Aghlmandi S, Heg D, Klingenberg R, Raber L, Carballo D, Carballo S, Matter CM, Luscher TF, Windecker S, Mach F, Rodondi N. Prognosis of patients with familial hypercholesterolemia after acute coronary syndromes. Circulation, 2016; 134: 698-709 [DOI] [PubMed] [Google Scholar]
- 738). Santos RD, Gidding SS, Hegele RA, Cuchel MA, Barter PJ, Watts GF, Baum SJ, Catapano AL, Chapman MJ, Defesche JC, Folco E, Freiberger T, Genest J, Hovingh GK, Harada-Shiba M, Humphries SE, Jackson AS, Mata P, Moriarty PM, Raal FJ, Al-Rasadi K, Ray KK, Reiner Z, Sijbrands EJ, Yamashita S, International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel Defining severe familial hypercholesterolaemia and the implications for clinical management: A consensus statement from the international atherosclerosis society severe familial hypercholesterolemia panel. Lancet Diabetes Endocrinol, 2016; 4: 850-861 [DOI] [PubMed] [Google Scholar]
- 739). Sato H, Kinjo K, Ito H, Hirayama A, Nanto S, Fukunami M, Nishino M, Lim YJ, Kijima Y, Koretsune Y, Nakatani D, Mizuno H, Shimizu M, Osaka Acute Coronary Insufficiency Study (OACIS)-LIPID Study Investigators Effect of early use of low-dose pravastatin on major adverse cardiac events in patients with acute myocardial infarction: The OACIS-LIPID study. Circ J, 2008; 72: 17-22 [DOI] [PubMed] [Google Scholar]
- 740). Daida H, Miyauchi K, Ogawa H, Yokoi H, Matsumoto M, Kitakaze M, Kimura T, Matsubara T, Ikari Y, Kimura K, Tsukahara K, Origasa H, Morino Y, Tsutsui H, Kobayashi M, Isshiki T, PACIFIC investigators Management and two-year long-term clinical outcome of acute coronary syndrome in Japan: Prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ J, 2013; 77: 934-943 [DOI] [PubMed] [Google Scholar]
- 741). Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T, Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: The MIRACL study: A randomized controlled trial. JAMA, 2001; 285: 1711-1718 [DOI] [PubMed] [Google Scholar]
- 742). Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM, Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med, 2004; 350: 1495-1504 [DOI] [PubMed] [Google Scholar]
- 743). Hulten E, Jackson JL, Douglas K, George S, Villines TC. The effect of early, intensive statin therapy on acute coronary syndrome: A meta-analysis of randomized controlled trials. Arch Intern Med, 2006; 166: 1814-1821 [DOI] [PubMed] [Google Scholar]
- 744). Briel M, Schwartz GG, Thompson PL, de Lemos JA, Blazing MA, van Es GA, Kayikcioglu M, Arntz HR, den Hartog FR, Veeger NJ, Colivicchi F, Dupuis J, Okazaki S, Wright RS, Bucher HC, Nordmann AJ. Effects of early treatment with statins on short-term clinical outcomes in acute coronary syndromes: A meta-analysis of randomized controlled trials. JAMA, 2006; 295: 2046-2056 [DOI] [PubMed] [Google Scholar]
- 745). Dohi T, Miyauchi K, Okazaki S, Yokoyama T, Yanagisawa N, Tamura H, Kojima T, Yokoyama K, Kurata T, Daida H. Early intensive statin treatment for six months improves long-term clinical outcomes in patients with acute coronary syndrome (Extended-ESTABLISH trial): A follow-up study. Atherosclerosis, 2010; 210: 497-502 [DOI] [PubMed] [Google Scholar]
- 746). Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW, PROSPECT Investigators A prospective naturalhistory study of coronary atherosclerosis. N Engl J Med, 2011; 364: 226-235 [DOI] [PubMed] [Google Scholar]
- 747). Cheng JM, Garcia-Garcia HM, de Boer SP, Kardys I, Heo JH, Akkerhuis KM, Oemrawsingh RM, van Domburg RT, Ligthart J, Witberg KT, Regar E, Serruys PW, van Geuns RJ, Boersma E. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: Results of the ATHEROREMO-IVUS study. Eur Heart J, 2014; 35: 639-647 [DOI] [PubMed] [Google Scholar]
- 748). Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudek D, Dudeck D, Falk E, Feldman MD, Fitzgerald P, Garcia-Garcia HM, Garcia H, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CC, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel MA, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Raber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PW, Shimada K, Shinke T, Shite J, Siegel E, Sonoda S, Sonada S, Suter M, Takarada S, Tanaka A, Terashima M, Thim T, Troels T, Uemura S, Ughi GJ, van Beusekom HM, van der Steen AF, van Es GA, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol, 2012; 59: 1058-1072 [DOI] [PubMed] [Google Scholar]
- 749). Levantesi G, Macchia A, Marfisi R, Franzosi MG, Maggioni AP, Nicolosi GL, Schweiger C, Tavazzi L, Tognoni G, Valagussa F, Marchioli R, GISSI-Prevenzione Investigators Metabolic syndrome and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol, 2005; 46: 277-283 [DOI] [PubMed] [Google Scholar]
- 750). Costa J, Borges M, David C, Vaz Carneiro A. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: Meta-analysis of randomised controlled trials. BMJ, 2006; 332: 1115-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751). Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterollowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: A meta-analysis. Lancet, 2008; 371: 117-125 [DOI] [PubMed] [Google Scholar]
- 752). Kohsaka S, Kimura T, Goto M, Lee VV, Elayda M, Furukawa Y, Fukushima M, Komeda M, Sakata R, Willerson JT, Wilson JM, Kita T. Difference in patient profiles and outcomes in Japanese versus American patients undergoing coronary revascularization (collaborative study by CREDO-Kyoto and the Texas Heart Institute Research Database). Am J Cardiol, 2010; 105: 1698-1704 [DOI] [PubMed] [Google Scholar]
- 753). Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Haffner S, Hsia J, Breazna A, LaRosa J, Grundy S, Waters D. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: The Treating to New Targets (TNT) study. Diabetes Care, 2006; 29: 1220-1226 [DOI] [PubMed] [Google Scholar]
- 754). Bayturan O, Kapadia S, Nicholls SJ, Tuzcu EM, Shao M, Uno K, Shreevatsa A, Lavoie AJ, Wolski K, Schoenhagen P, Nissen SE. Clinical predictors of plaque progression despite very low levels of low-density lipoprotein choles terol. Journal of the American College of Cardiology, 2010; 55: 2736-2742 [DOI] [PubMed] [Google Scholar]
- 755). Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M, JAPAN-ACS Investigators Diabetes mellitus is a major negative determinant of coronary plaque regression during statin therapy in patients with acute coronary syndrome--serial intravascular ultrasound observations from the Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome Trial (the JAPAN-ACS Trial). Circ J, 2010; 74: 1165-1174 [DOI] [PubMed] [Google Scholar]
- 756). Arai H, Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M, JAPAN-ACS Investigators More intensive lipid lowering is associated with regression of coronary atherosclerosis in diabetic patients with acute coronary syndrome--sub-analysis of JAPAN-ACS study. J Atheroscler Thromb, 2010; 17: 1096-1107 [DOI] [PubMed] [Google Scholar]
- 757). Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Rother J, Wilson PW, REACH Registry Investigators International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA, 2006; 295: 180-189 [DOI] [PubMed] [Google Scholar]
- 758).Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The scandinavian simvastatin survival study (4S). Lancet, 1994; 344: 1383-1389 [PubMed] [Google Scholar]
- 759).Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) study group. N Engl J Med, 1998; 339: 1349-1357 [DOI] [PubMed] [Google Scholar]
- 760). Plehn JF, Davis BR, Sacks FM, Rouleau JL, Pfeffer MA, Bernstein V, Cuddy TE, Moye LA, Piller LB, Rutherford J, Simpson LM, Braunwald E. Reduction of stroke incidence after myocardial infarction with pravastatin: The cholesterol and recurrent events (CARE) study. The Care Investigators. Circulation, 1999; 99: 216-223 [DOI] [PubMed] [Google Scholar]
- 761). Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, Browner D. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med, 1992; 326: 381-386 [DOI] [PubMed] [Google Scholar]
- 762). Al-Omran M, Lindsay TF. Commentary: One-year cardiovascular event rates in outpatients with atherothrombosis. Steg PG, Bhatt DL, Wilson PW, et al. REACH Registry Investigators JAMA, 2007; 297: 1197-1206. Perspect Vasc Surg Endovasc Ther. 2007; 19: 416-417 [DOI] [PubMed] [Google Scholar]
- 763). Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR, Siscovick D. Anklearm index as a predictor of cardiovascular disease and mortality in the cardiovascular health study. The cardiovascular health study group. Arterioscler Thromb Vasc Biol, 1999; 19: 538-545 [DOI] [PubMed] [Google Scholar]
- 764). McDermott MM, Mandapat AL, Moates A, Albay M, Chiou E, Celic L, Greenland P. Knowledge and attitudes regarding cardiovascular disease risk and prevention in patients with coronary or peripheral arterial disease. Arch Intern Med, 2003; 163: 2157-2162 [DOI] [PubMed] [Google Scholar]
- 765). Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: Morbidity and mortality implications. Circulation, 2006; 114: 688-699 [DOI] [PubMed] [Google Scholar]
- 766). Sprengers RW, Janssen KJ, Moll FL, Verhaar MC, van der Graaf Y, SMART Study Group Prediction rule for cardiovascular events and mortality in peripheral arterial disease patients: Data from the prospective second manifestations of arterial disease (SMART) cohort study. J Vasc Surg, 2009; 50: 1369-1376 [DOI] [PubMed] [Google Scholar]
- 767). Stoekenbroek RM, Boekholdt SM, Fayyad R, Laskey R, Tikkanen MJ, Pedersen TR, Hovingh GK. High-dose atorvastatin is superior to moderate-dose simvastatin in preventing peripheral arterial disease. Heart, 2015; 101: 356-362 [DOI] [PubMed] [Google Scholar]
- 768). Hussein AA, Uno K, Wolski K, Kapadia S, Schoenhagen P, Tuzcu EM, Nissen SE, Nicholls SJ. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol, 2011; 57: 1220-1225 [DOI] [PubMed] [Google Scholar]
- 769). Kasai T, Miyauchi K, Kajimoto K, Kubota N, Dohi T, Tsuruta R, Ogita M, Yokoyama T, Amano A, Daida H. Prognostic significance of glomerular filtration rate estimated by the Japanese equation among patients who underwent complete coronary revascularization. Hypertens Res, 2011; 34: 378-383 [DOI] [PubMed] [Google Scholar]
- 770). Dohi T, Miyauchi K, Okazaki S, Yokoyama T, Tamura H, Kojima T, Yokoyama K, Kurata T, Daida H. Longterm impact of mild chronic kidney disease in patients with acute coronary syndrome undergoing percutaneous coronary interventions. Nephrol Dial Transplant, 2011; 26: 2906-2911 [DOI] [PubMed] [Google Scholar]
- 771). Chonchol M, Cook T, Kjekshus J, Pedersen TR, Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis, 2007; 49: 373-382 [DOI] [PubMed] [Google Scholar]
- 772). Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G, Cholesterol and Recurrent Events (CARE) Trial Investigators Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med, 2003; 138: 98-104 [DOI] [PubMed] [Google Scholar]
- 773). Koren MJ, Davidson MH, Wilson DJ, Fayyad RS, Zuckerman A, Reed DP, ALLIANCE Investigators Focused atorvastatin therapy in managed-care patients with coronary heart disease and CKD. Am J Kidney Dis, 2009; 53: 741-750 [DOI] [PubMed] [Google Scholar]
- 774). Shepherd J, Kastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, Wilson DJ, Zuckerman A, Wenger NK. Intensive lipid lowering with atorvastatin in patients with coronary heart disease and chronic kidney disease: The TNT (Treating to New Targets) study. J Am Coll Cardiol, 2008; 51: 1448-1454 [DOI] [PubMed] [Google Scholar]
- 775). Natsuaki M, Furukawa Y, Morimoto T, Sakata R, Kimura T, CREDO-Kyoto PCI/CABG Registry Cohort-2 Investigators Renal function and effect of statin therapy on cardiovascular outcomes in patients undergoing coronary revascularization (from the CREDO-Kyoto PCI/CABG registry Cohort-2). Am J Cardiol, 2012; 110: 1568-1577 [DOI] [PubMed] [Google Scholar]
- 776). Deedwania P, Barter P, Carmena R, Fruchart JC, Grundy SM, Haffner S, Kastelein JJ, LaRosa JC, Schachner H, Shepherd J, Waters DD. Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: Analysis of the Treating to New Targets study. Lancet, 2006; 368: 919-928 [DOI] [PubMed] [Google Scholar]
- 777). Kasai T, Miyauchi K, Kurata T, Okazaki S, Kajimoto K, Kubota N, Daida H. Impact of metabolic syndrome among patients with and without diabetes mellitus on long-term outcomes after percutaneous coronary intervention. Hypertens Res, 2008; 31: 235-241 [DOI] [PubMed] [Google Scholar]
- 778). Kasai T, Miyauchi K, Kajimoto K, Kubota N, Kurata T, Amano A, Daida H. The impact of pravastatin therapy on long-term outcome in patients with metabolic syndrome undergoing complete coronary revascularization. Circ J, 2009; 73: 2104-2109 [DOI] [PubMed] [Google Scholar]
- 779). Goldenberg I, Jonas M, Tenenbaum A, Boyko V, Matetzky S, Shotan A, Behar S, Reicher-Reiss H, Bezafibrate Infarction Prevention Study Group Current smoking, smoking cessation, and the risk of sudden cardiac death in patients with coronary artery disease. Arch Intern Med, 2003; 163: 2301-2305 [DOI] [PubMed] [Google Scholar]
- 780). Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: A systematic review. JAMA, 2003; 290: 86-97 [DOI] [PubMed] [Google Scholar]
- 781). van Domburg RT, Meeter K, van Berkel DF, Veldkamp RF, van Herwerden LA, Bogers AJ. Smoking cessation reduces mortality after coronary artery bypass surgery: A 20-year follow-up study. J Am Coll Cardiol, 2000; 36: 878-883 [DOI] [PubMed] [Google Scholar]
- 782). Rea TD, Heckbert SR, Kaplan RC, Smith NL, Lemaitre RN, Psaty BM. Smoking status and risk for recurrent coronary events after myocardial infarction. Ann Intern Med, 2002; 137: 494-500 [DOI] [PubMed] [Google Scholar]
- 783). Wilson K, Gibson N, Willan A, Cook D. Effect of smoking cessation on mortality after myocardial infarction: Meta-analysis of cohort studies. Arch Intern Med, 2000; 160: 939-944 [DOI] [PubMed] [Google Scholar]
- 784). Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Röther J, Smith SC, Salette G, Contant CF, Massaro JM, Steg PG, REACH Registry Investigators Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA, 2010; 304: 1350-1357 [DOI] [PubMed] [Google Scholar]
- 785). Kinjo K, Sato H, Sakata Y, Nakatani D, Mizuno H, Shimizu M, Sasaki T, Kijima Y, Nishino M, Uematsu M, Tanouchi J, Nanto S, Otsu K, Hori M. Impact of smoking status on long-term mortality in patients with acute myocardial infarction. Circ J, 2005; 69: 7-12 [DOI] [PubMed] [Google Scholar]
- 786). Baba S, Iso H, Mannami T, Sasaki S, Okada K, Konishi M, Tsugane S, JPHC Study Group Cigarette smoking and risk of coronary heart disease incidence among middle-aged Japanese men and women: The JPHC Study Cohort I. Eur J Cardiovasc Prev Rehabil, 2006; 13: 207-213 [DOI] [PubMed] [Google Scholar]
- 787). Frey P, Waters DD, DeMicco DA, Breazna A, Samuels L, Pipe A, Wun CC, Benowitz NL. Impact of smoking on cardiovascular events in patients with coronary disease receiving contemporary medical therapy (from the Treating to New Targets [TNT] and the Incremental Decrease in End Points Through Aggressive Lipid Lowering [IDEAL] trials). Am J Cardiol, 2011; 107: 145-150 [DOI] [PubMed] [Google Scholar]
- 788). Athyros VG, Tziomalos K, Katsiki N, Gossios TD, Giouleme O, Anagnostis P, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP. The impact of smoking on cardiovascular outcomes and comorbidities in statin-treated patients with coronary artery disease: A post hoc analysis of the GREACE study. Curr Vasc Pharmacol, 2013; 11: 779-784 [DOI] [PubMed] [Google Scholar]
- 789). Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United kingdom prospective diabetes study (UKPDS: 23). BMJ, 1998; 316: 823-828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 790). Sone H, Tanaka S, Iimuro S, Oida K, Yamasaki Y, Oikawa S, Ishibashi S, Katayama S, Ohashi Y, Akanuma Y, Yamada N. Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: Subanalysis of the Japan diabetes complications study (JDCS). J Clin Endocrinol Metab, 2011; 96: 3448-3456 [DOI] [PubMed] [Google Scholar]
- 791). Murakami K, Ishibashi S, Yoshida Y, Yamada N, Akanuma Y. Lipoprotein (a) as a coronary risk factor in Japanese patients with Type Ⅱ (non-insulin-dependent) diabetes mellitus. Relation with apolipoprotein (a) phenotypes. Diabetologia, 1998; 41: 1397-1398 [DOI] [PubMed] [Google Scholar]
- 792). Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA, 2001; 286: 421-426 [DOI] [PubMed] [Google Scholar]
- 793). Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int, 2003; 63: 225-232 [DOI] [PubMed] [Google Scholar]
- 794). Cheung N, Wang JJ, Klein R, Couper DJ, Sharrett AR, Wong TY. Diabetic retinopathy and the risk of coronary heart disease: The atherosclerosis risk in communities study. Diabetes Care, 2007; 30: 1742-1746 [DOI] [PubMed] [Google Scholar]
- 795). Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, Erqou S, Sattar N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: A metaanalysis of randomised controlled trials. Lancet (London, England), 2009; 373: 1765-1772 [DOI] [PubMed] [Google Scholar]
- 796). Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassai B, Erpeldinger S, Wright JM, Gueyffier F, Cornu C. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: Meta-analysis of randomised controlled trials. BMJ, 2011; 343: d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 797). Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, McCarren M, Duckworth WC, Emanuele NV. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med, 2015; 372: 2197-2206 [DOI] [PubMed] [Google Scholar]
- 798). Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, Ellis D, Becker DJ. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the pittsburgh epidemiology of diabetes complications study. Diabetes Care, 2003; 26: 1374-1379 [DOI] [PubMed] [Google Scholar]
- 799). Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med, 2015; 373: 2117-2128 [DOI] [PubMed] [Google Scholar]
- 800). Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med, 2016; 375: 311-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801). Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsboll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med, 2016; 375: 1834-1844 [DOI] [PubMed] [Google Scholar]
- 802). Goto A, Goto M, Terauchi Y, Yamaguchi N, Noda M. Association between severe hypoglycemia and cardiovascular disease risk in Japanese patients with type 2 diabetes. J Am Heart Assoc, 2016; 4: e002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 803). Araki A, Iimuro S, Sakurai T, Umegaki H, Iijima K, Nakano H, Oba K, Yokono K, Sone H, Yamada N, Ako J, Kozaki K, Miura H, Kashiwagi A, Kikkawa R, Yoshimura Y, Nakano T, Ohashi Y, Ito H. Non-high-density lipoprotein cholesterol: An important predictor of stroke and diabetes-related mortality in Japanese elderly diabetic patients. Geriatr Gerontol Int, 2012; 12 Suppl 1: 18-28 [DOI] [PubMed] [Google Scholar]
- 804). Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet (London, England), 2004; 364: 685-696 [DOI] [PubMed] [Google Scholar]
- 805). Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesaniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M. Effects of longterm fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet (London, England), 2005; 366: 1849-1861 [DOI] [PubMed] [Google Scholar]
- 806). Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC, Jr., Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med, 2010; 362: 1563-1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807). Oikawa S, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Suppressive effect of EPA on the incidence of coronary events in hypercholesterolemia with impaired glucose metabolism: Sub-analysis of the Japan EPA lipid intervention study (JELIS). Atherosclerosis, 2009; 206: 535-539 [DOI] [PubMed] [Google Scholar]
- 808). Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med, 2015; 372: 2387-2397 [DOI] [PubMed] [Google Scholar]
- 809). Kengne AP, Patel A, Barzi F, Jamrozik K, Lam TH, Ueshima H, Gu DF, Suh I, Woodward M, Asia Pacific Cohort Studies Collaboration Systolic blood pressure, diabetes and the risk of cardiovascular diseases in the Asia-Pacific region. J Hypertens, 2007; 25: 1205-1213 [DOI] [PubMed] [Google Scholar]
- 810). McBrien K, Rabi DM, Campbell N, Barnieh L, Clement F, Hemmelgarn BR, Tonelli M, Leiter LA, Klarenbach SW, Manns BJ. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: Systematic review and meta-analysis. Arch Intern Med, 2012; 172: 1296-1303 [DOI] [PubMed] [Google Scholar]
- 811). Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: A systematic review and meta-analysis. JAMA, 2015; 313: 603-615 [DOI] [PubMed] [Google Scholar]
- 812). Niskanen L, Hedner T, Hansson L, Lanke J, Niklason A. Reduced cardiovascular morbidity and mortality in hypertensive diabetic patients on first-line therapy with an ACE inhibitor compared with a diuretic/betablocker-based treatment regimen: A subanalysis of the Captopril Prevention Project. Diabetes Care, 2001; 24: 2091-2096 [DOI] [PubMed] [Google Scholar]
- 813). Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet (London, England), 2002; 359: 995-1003 [DOI] [PubMed] [Google Scholar]
- 814). Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT study group. Lancet (London, England), 1998; 351: 1755-1762 [DOI] [PubMed] [Google Scholar]
- 815). Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med, 2003; 348: 383-393 [DOI] [PubMed] [Google Scholar]
- 816). Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med, 2008; 358: 580-591 [DOI] [PubMed] [Google Scholar]
- 817). Nakamura Y, Ueshima H, Okamura T, Kadowaki T, Hayakawa T, Kita Y, Abbott RD, Okayama A. A Japanese diet and 19-year mortality: National integrated project for prospective observation of non-communicable diseases and its trends in the aged, 1980. Br J Nutr, 2009; 101: 1696-1705 [DOI] [PubMed] [Google Scholar]
- 818). Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med, 2002; 347: 716-725 [DOI] [PubMed] [Google Scholar]
- 819). Iso H, Date C, Yamamoto A, Toyoshima H, Watanabe Y, Kikuchi S, Koizumi A, Wada Y, Kondo T, Inaba Y, Tamakoshi A. Smoking cessation and mortality from cardiovascular disease among Japanese men and women: The JACC study. Am J Epidemiol, 2005; 161: 170-179 [DOI] [PubMed] [Google Scholar]
- 820). Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med, 2013; 369: 145-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821). Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: A systematic review and meta-analysis. Ann Intern Med, 2013; 159: 543-551 [DOI] [PubMed] [Google Scholar]
- 822). Sone H, Tanaka S, Iimuro S, Oida K, Yamasaki Y, Oikawa S, Ishibashi S, Katayama S, Yamashita H, Ito H, Yoshimura Y, Ohashi Y, Akanuma Y, Yamada N. Longterm lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: A nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study). Diabetologia, 2010; 53: 419-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 823). Sone H, Tanaka S, Suzuki S, Seino H, Hanyu O, Sato A, Toyonaga T, Okita K, Ishibashi S, Kodama S, Akanuma Y, Yamada N. Leisure-time physical activity is a significant predictor of stroke and total mortality in Japanese patients with type 2 diabetes: Analysis from the Japan Diabetes Complications Study (JDCS). Diabetologia, 2013; 56: 1021-1030 [DOI] [PubMed] [Google Scholar]
- 824). American Diabetes Association Cardiovascular disease and risk management. Diabetes Care, 2016; 39 Suppl 1: S60-71 [DOI] [PubMed] [Google Scholar]
- 825). Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: A randomized controlled trial. JAMA, 2008; 300: 2134-2141 [DOI] [PubMed] [Google Scholar]
- 826). Okada S, Morimoto T, Ogawa H, Sakuma M, Soejima H, Nakayama M, Sugiyama S, Jinnouchi H, Waki M, Doi N, Horii M, Kawata H, Somekawa S, Soeda T, Uemura S, Saito Y. Effect of low-dose aspirin on primary prevention of cardiovascular events in Japanese diabetic patients at high risk. Circ J, 2013; 77: 3023-3028 [DOI] [PubMed] [Google Scholar]
- 827). Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr., Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 2014; 129:S1-45 [DOI] [PubMed] [Google Scholar]
- 828). Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte op, Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur Heart J, 2012; 33: 1635-1701 [DOI] [PubMed] [Google Scholar]
- 829). Arai H, Sasaki J, Teramoto T. Comment on the new guidelines in usa by the JAS guidelines committee. J Atheroscler Thromb, 2014; 21: 79-81 [DOI] [PubMed] [Google Scholar]
- 830). Krempf M, Parhofer KG, Steg PG, Bhatt DL, Ohman EM, Rother J, Goto S, Pasquet B, Wilson PW. Cardiovascular event rates in diabetic and nondiabetic individuals with and without established atherothrombosis (from the reduction of atherothrombosis for continued health [REACH] registry). Am J Cardiol, 2010; 105: 667-671 [DOI] [PubMed] [Google Scholar]
- 831). Yokoyama H, Oishi M, Kawai K, Sone H. Reduced gfr and microalbuminuria are independently associated with prevalent cardiovascular disease in type 2 diabetes: JDDM study 16. Diabet Med, 2008; 25: 1426-1432 [DOI] [PubMed] [Google Scholar]
- 832). Moriya T, Tanaka S, Kawasaki R, Ohashi Y, Akanuma Y, Yamada N, Sone H, Yamashita H, Katayama S. Diabetic retinopathy and microalbuminuria can predict macroalbuminuria and renal function decline in Japanese type 2 diabetic patients: Japan diabetes complications study. Diabetes Care, 2013; 36: 2803-2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833). Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet (London, England), 2012; 380: 1662-1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834). Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS35): Prospective observational study. BMJ, 2000; 321: 405-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835). Ninomiya T, Kubo M, Doi Y, Yonemoto K, Tanizaki Y, Rahman M, Arima H, Tsuryuya K, Iida M, Kiyohara Y. Impact of metabolic syndrome on the development of cardiovascular disease in a general Japanese population: The Hisayama study. Stroke, 2007; 38: 2063-2069 [DOI] [PubMed] [Google Scholar]
- 836). Kadota A, Hozawa A, Okamura T, Kadowak T, Nakmaura K, Murakami Y, Hayakawa T, Kita Y, Okayama A, Nakamura Y, Kashiwagi A, Ueshima H. Relationship between metabolic risk factor clustering and cardiovascular mortality stratified by high blood glucose and obesity: NIPPON DATA90, 1990-2000. Diabetes Care, 2007; 30: 1533-1538 [DOI] [PubMed] [Google Scholar]
- 837). Nakamura Y, Yamamoto T, Okamura T, Kadowaki T, Hayakawa T, Kita Y, Saitoh S, Okayama A, Ueshima H. Combined cardiovascular risk factors and outcome: NIPPON DATA80, 1980-1994. Circ J, 2006; 70: 960-964 [DOI] [PubMed] [Google Scholar]
- 838). Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet (London, England), 2010; 376: 1670-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839). Kasai T, Miyauchi K, Kajimoto K, Kubota N, Kurata T, Daida H. Influence of diabetes on > 10-year outcomes after percutaneous coronary intervention. Heart Vessels, 2008; 23: 149-154 [DOI] [PubMed] [Google Scholar]
- 840). Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M. Diabetes mellitus is a major negative determinant of coronary plaque regression during statin therapy in patients with acute coronary syndrome--serial intravascular ultrasound observations from the Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome Trial (the JAPAN-ACS trial). Circ J, 2010; 74: 1165-1174 [DOI] [PubMed] [Google Scholar]
- 841). Nakatani D, Sakata Y, Suna S, Usami M, Matsumoto S, Shimizu M, Sumitsuji S, Kawano S, Ueda Y, Hamasaki T, Sato H, Nanto S, Hori M, Komuro I. Incidence, predictors, and subsequent mortality risk of recurrent myocardial infarction in patients following discharge for acute myocardial infarction. Circ J, 2013; 77: 439-446 [DOI] [PubMed] [Google Scholar]
- 842). Kobayashi S. Japanese Stroke Data Bank 2015. Nakayama Shoten, 2015. (in Japanese) [Google Scholar]
- 843). Tanaka H, Iso H, Yokoyama T, Yoshiike N, Kokubo Y. Cerebrovascular disease. In: Deteis R, McEwen J, Beaglehole R. (eds). Oxford text book of publich health (4th ed), Vol. 3, Oxford Press, Oxford, 2001; 1193-1226 [Google Scholar]
- 844). Kubo M, Kiyohara Y, Ninomiya T, Tanizaki Y, Yonemoto K, Doi Y, Hata J, Oishi Y, Shikata K, Iida M. Decreasing incidence of lacunar vs other types of cerebral infarction in a Japanese population. Neurology, 2006; 66: 1539-1544 [DOI] [PubMed] [Google Scholar]
- 845). Kimura K, Kazui S, Minematsu K, Yamaguchi T. Analysis of 16,922 patients with acute ischemic stroke and transient ischemic attack in Japan. A hospital-based prospective registration study. Cerebrovasc Dis, 2004; 18: 47-56 [DOI] [PubMed] [Google Scholar]
- 846). Petty GW, Brown RD, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: A population-based study of incidence and risk factors. Stroke, 1999; 30: 2513-2516 [DOI] [PubMed] [Google Scholar]
- 847). Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration Blood cholesterol and vascular mortality by age, sex, and blood pressure: A metaanalysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet, 2007; 370: 1829-1839 [DOI] [PubMed] [Google Scholar]
- 848).Blood pressure, cholesterol, and stroke in eastern Asia. Eastern stroke and coronary heart disease collaborative research group. Lancet, 1998; 352: 1801-1807 [PubMed] [Google Scholar]
- 849). Schneider AT, Kissela B, Woo D, Kleindorfer D, Alwell K, Miller R, Szaflarski J, Gebel J, Khoury J, Shukla R, Moomaw C, Pancioli A, Jauch E, Broderick J. Ischemic stroke subtypes: A population-based study of incidence rates among blacks and whites. Stroke, 2004; 35: 1552-1556 [DOI] [PubMed] [Google Scholar]
- 850). Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: Incidence, recurrence, and long-term survival in ischemic stroke subtypes: A population-based study. Stroke, 2001; 32: 2735-2740 [DOI] [PubMed] [Google Scholar]
- 851). Iso H, Jacobs DR, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med, 1989; 320: 904-910 [DOI] [PubMed] [Google Scholar]
- 852). Leppala JM, Virtamo J, Fogelholm R, Albanes D, Heinonen OP. Different risk factors for different stroke subtypes: Association of blood pressure, cholesterol, and antioxidants. Stroke, 1999; 30: 2535-2540 [DOI] [PubMed] [Google Scholar]
- 853). Zhang X, Patel A, Horibe H, Wu Z, Barzi F, Rodgers A, MacMahon S, Woodward M, Asia Pacific Cohort Studies Collaboration Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Int J Epidemiol, 2003; 32: 563-572 [DOI] [PubMed] [Google Scholar]
- 854). Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: Systematic review and metaanalysis. BMJ, 2003; 326: 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 855). Bots ML, Elwood PC, Nikitin Y, Salonen JT, Freire de Concalves A, Inzitari D, Sivenius J, Benetou V, Tuomilehto J, Koudstaal PJ, Grobbee DE. Total and HDL cholesterol and risk of stroke. EUROSTROKE: A collaborative study among research centres in Europe. J Epidemiol Community Health, 2002; 56 Suppl 1: i19-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 856). Shahar E, Chambless LE, Rosamond WD, Boland LL, Ballantyne CM, McGovern PG, Sharrett AR. Atherosclerosis Risk in Communities Study. Plasma lipid profile and incident ischemic stroke: The Atherosclerosis Risk in Communities (ARIC) study. Stroke, 2003; 34: 623-631 [DOI] [PubMed] [Google Scholar]
- 857). Arima H, Tanizaki Y, Yonemoto K, Doi Y, Ninomiya T, Hata J, Fukuhara M, Matsumura K, Iida M, Kiyohara Y. Impact of blood pressure levels on different types of stroke: The Hisayama study. J Hypertens, 2009; 27: 2437-2443 [DOI] [PubMed] [Google Scholar]
- 858). Sturgeon JD, Folsom AR, Longstreth WT, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke, 2007; 38: 2718-2725 [DOI] [PubMed] [Google Scholar]
- 859). Soyama Y, Miura K, Morikawa Y, Nishijo M, Nakanishi Y, Naruse Y, Kagamimori S, Nakagawa H, Study O. High-density lipoprotein cholesterol and risk of stroke in Japanese men and women: The Oyabe study. Stroke, 2003; 34: 863-868 [DOI] [PubMed] [Google Scholar]
- 860). Lindenstrom E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: The Copenhagen City Heart Study. BMJ, 1994; 309: 11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 861). Wannamethee SG, Shaper AG, Ebrahim S. HDL-cholesterol, total cholesterol, and the risk of stroke in middle-aged British men. Stroke, 2000; 31: 1882-1888 [DOI] [PubMed] [Google Scholar]
- 862). Bowman TS, Sesso HD, Ma J, Kurth T, Kase CS, Stampfer MJ, Gaziano JM. Cholesterol and the risk of ischemic stroke. Stroke, 2003; 34: 2930-2934 [DOI] [PubMed] [Google Scholar]
- 863). Håheim LL, Holme I, Hjermann I, Leren P. Risk factors of stroke incidence and mortality. A 12-year follow-up of the Oslo Study. Stroke, 1993; 24: 1484-1489 [DOI] [PubMed] [Google Scholar]
- 864). Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA, 2008; 300: 2142-2152 [DOI] [PubMed] [Google Scholar]
- 865). Amarenco P, Bogousslavsky J, Callahan A, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA, Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med, 2006; 355: 549-559 [DOI] [PubMed] [Google Scholar]
- 866). Martí-Fàbregas J, Gomis M, Arboix A, Aleu A, Pagonabarraga J, Belvís R, Cocho D, Roquer J, Rodríguez A, García MD, Molina-Porcel L, Díaz-Manera J, Martí-Vilalta JL. Favorable outcome of ischemic stroke in patients pretreated with statins. Stroke, 2004; 35: 1117-1121 [DOI] [PubMed] [Google Scholar]
- 867). Colivicchi F, Bassi A, Santini M, Caltagirone C. Discontinuation of statin therapy and clinical outcome after ischemic stroke. Stroke, 2007; 38: 2652-2657 [DOI] [PubMed] [Google Scholar]
- 868). The Japan Stroke Society Japanese Guidelines for the Management of Stroke 2015. Kyowa Kikaku. 2015. (in Japanese) [Google Scholar]
- 869). Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA, Guidelines for the primary prevention of stroke : A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 2011; 42: 517-584 [DOI] [PubMed] [Google Scholar]
- 870). Catapano AL, Reiner Z, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Perrone Filardi P, Riccardi G, Storey RF, Wood D, ESC/EAS guidelines for the management of dyslipidaemias : The task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis, 2011; 217 Suppl 1: S1-44 [DOI] [PubMed] [Google Scholar]
- 871). The Japan Diabetes Society Guidelines for Diabetes Treatment 2014–2015. Bunkodo, Tokyo; 2014. (in Japanese) [Google Scholar]
- 872). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K. Absolute risk of cardiovascular disease and lipid management targets. J Atheroscler Thromb, 2013; 20: 689-697 [DOI] [PubMed] [Google Scholar]
- 873). Mellen PB, Bleyer AJ, Erlinger TP, Evans GW, Nieto FJ, Wagenknecht LE, Wofford MR, Herrington DM. Serum uric acid predicts incident hypertension in a biethnic cohort: The atherosclerosis risk in communities study. Hypertension, 2006; 48: 1037-1042 [DOI] [PubMed] [Google Scholar]
- 874). Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or type II diabetes in Japanese male office workers. Eur J Epidemiol, 2003; 18: 523-530 [DOI] [PubMed] [Google Scholar]
- 875). Yamanaka H, Japanese Society of Gout and Nucleic Acid Metabolism Japanese guideline for the management of hyperuricemia and gout: Second edition 2012. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 876). Fujiwara H. Smoking cessation treatment covered by public health insurance: revisions to “Nicotine-addiction management fees” and smoking-cession treatment in the youth. Japanese Journal of Tobacco Control, 2016; 11: 3 (in Japanese) [Google Scholar]
- 877).Health and longevity management handbook. Essence of geriatrics for the general physician. Tokyo: Medical Review; 2012. (in Japanese) [Google Scholar]
- 878). Mabuchi H, Koizumi J, Shimizu M, Takeda R. Development of coronary heart disease in familial hypercholesterolemia. Circulation, 1989; 79: 225-232 [DOI] [PubMed] [Google Scholar]
- 879). Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni-Berthold I, Somaratne R, Bridges I, Wasserman SM, Gaudet D, RUTHERFORD-2 Investigators PCSK9 inhibition with evolocumab (AMG145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): A randomised, double-blind, placebo-controlled trial. Lancet, 2015; 385: 331-340 [DOI] [PubMed] [Google Scholar]
- 880). Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R, Blom D, Civeira F, Krempf M, Lorenzato C, Zhao J, Pordy R, Baccara-Dinet MT, Gipe DA, Geiger MJ, Farnier M, ODYSSEY FH I FH II : 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J, 2015; 36: 2996-3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 881). Uauy R, Vega GL, Grundy SM, Bilheimer DM. Lovastatin therapy in receptor-negative homozygous familial hypercholesterolemia: Lack of effect on low-density lipoprotein concentrations or turnover. J Pediatr, 1988; 113: 387-392 [DOI] [PubMed] [Google Scholar]
- 882). Stein EA, Honarpour N, Wasserman SM, Xu F, Scott R, Raal FJ. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, amg 145, in homozygous familial hypercholesterolemia. Circulation, 2013; 128: 2113-2120 [DOI] [PubMed] [Google Scholar]
- 883). Raal FJ, Pilcher GJ, Panz VR, van Deventer HE, Brice BC, Blom DJ, Marais AD. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation, 2011; 124: 2202-2207 [DOI] [PubMed] [Google Scholar]
- 884). Harada-Shiba M, Ikewaki K, Nohara A, Otsubo Y, Yanagi K, Yoshida M, Chang Q, Foulds P. Efficacy and safety of lomitapide in Japanese patients with homozygous familial hypercholesterolemia. J Atheroscler Thromb, 2017; 24: 402-411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885). Yamamoto A, Matsuzawa Y, Yokoyama S, Funahashi T, Yamamura T, Kishino B. Effects of probucol on xanthomata regression in familial hypercholesterolemia. Am J Cardiol, 1986; 57: 29H-35H [DOI] [PubMed] [Google Scholar]
- 886). Harada-Shiba M, Ohta T, Ohtake A, Ogura M, Dobashi K, Nohara A, Yamashita S, Yokote K. Guidance for pedilesterol familial hypercholesterolemia 2017. J Atheroscler Thromb, 2018; 25: 539-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 887). Goldstein JL, Schrott HG, Hazzard WR, Bierman EL, Motulsky AG. Hyperlipidemia in coronary heart disease. Ii. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest, 1973; 52: 1544-1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 888). Brahm AJ, Hegele RA. Combined hyperlipidemia: Familial but not (usually) monogenic. Curr Opin Lipidol, 2016; 27: 131-140 [DOI] [PubMed] [Google Scholar]
- 889). Iwata F, Okada T, Kuromori Y, Hara M, Harada K. Screening for familial combined hyperlipidemia in children using lipid phenotypes. J Atheroscler Thromb, 2003; 10: 299-303 [DOI] [PubMed] [Google Scholar]
- 890). Austin MA, McKnight B, Edwards KL, Bradley CM, McNeely MJ, Psaty BM, Brunzell JD, Motulsky AG. Cardiovascular disease mortality in familial forms of hypertriglyceridemia: A 20-year prospective study. Circulation, 2000; 101: 2777-2782 [DOI] [PubMed] [Google Scholar]
- 891). Pitsavos C, Skoumas I, Masoura C, Aznaouridis K, Papadimitriou L, Chrysohoou C, Giotsas N, Toutouza M, Stefanadis C. Prevalence and determinants of coronary artery disease in males and females with familial combined hyperlipidaemia. Atherosclerosis, 2008; 199: 402-407 [DOI] [PubMed] [Google Scholar]
- 892). Mabuchi H, Koizumi J: Serum lipid and coronary sclerosis of familial compound type hyperlipidemia judged with family investigation. The 1997 report of Research Committee for Primary Hyperlipidemia, Research on Measures against Intractable Diseases by the Japanese Ministry of Health, Labour and Welfare. 1998: 24-28 (in Japanese) [Google Scholar]
- 893). Mahley RW, Huang Y, Rall SC. Pathogenesis of type Ⅲ hyperlipoproteinemia (dysbetalipoproteinemia). Questions, quandaries, and paradoxes. J Lipid Res, 1999; 40: 1933-1949 [PubMed] [Google Scholar]
- 894). Hopkins PN, Brinton EA, Nanjee MN. Hyperlipoproteinemia type 3: The forgotten phenotype. Curr Atheroscler Rep, 2014; 16: 440. [DOI] [PubMed] [Google Scholar]
- 895). LaRosa JC, Chambless LE, Criqui MH, Frantz ID, Glueck CJ, Heiss G, Morrison JA. Patterns of dyslipoproteinemia in selected north American populations. The lipid research clinics program prevalence study. Circulation, 1986; 73: I12-29 [PubMed] [Google Scholar]
- 896). Hopkins PN, Nanjee MN, Wu LL, McGinty MG, Brinton EA, Hunt SC, Anderson JL. Altered composition of triglyceride-rich lipoproteins and coronary artery disease in a large case-control study. Atherosclerosis, 2009; 207: 559-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 897). Eto M, Saito M, Nakata H, Iwashima Y, Watanabe K, Ikoda A, Kaku K. Type Ⅲ hyperlipoproteinema with apolipoprotein E2/2 genotype in Japan. Clin Genet, 2002; 61: 416-422 [DOI] [PubMed] [Google Scholar]
- 898). Yamamura T, Kazokusei Familial type Ⅲ hyperlipidemia. J Jpn Soc Int Med, 1992; 81: 1772-1777 (in Japanese) [PubMed] [Google Scholar]
- 899). Sniderman A, Tremblay A, Bergeron J, Gagne C, Couture P. Diagnosis of type Ⅲ hyperlipoproteinemia from plasma total cholesterol, triglyceride, and apolipoprotein b. J Clin Lipidol, 2007; 1: 256-263 [DOI] [PubMed] [Google Scholar]
- 900). Murase T, Okubo M, Takeuchi I. Non-HDL-cholesterol/apolipoprotein B ratio: A useful distinguishing feature in the screening for type Ⅲ hyperlipoproteinemia. J Clin Lipidol, 2010; 4: 99-104 [DOI] [PubMed] [Google Scholar]
- 901). Yuasa-Kawase M, Masuda D, Kitazume-Taneike R, Yamashita T, Kawase R, Nakaoka H, Inagaki M, Nakatani K, Tsubakio-Yamamoto K, Ohama T, Toyama-Nakagawa Y, Nishida M, Ishigami M, Saito M, Eto M, Matsuyama A, Komuro I, Yamashita S. Apolipoprotein b-48 to triglyceride ratio is a novel and useful marker for detection of type III hyperlipidemia after antihyperlipidemic intervention. J Atheroscler Thromb, 2012; 19: 862-871 [DOI] [PubMed] [Google Scholar]
- 902). Barrett-Connor E, Suarez L, Khaw K, Criqui MH, Wingard DL. Ischemic heart disease risk factors after age 50. J Chronic Dis, 1984; 37: 903-908 [DOI] [PubMed] [Google Scholar]
- 903). Benfante R, Reed D. Is elevated serum cholesterol level a risk factor for coronary heart disease in the elderly? JAMA, 1990; 263: 393-396 [PubMed] [Google Scholar]
- 904). Corti MC, Guralnik JM, Salive ME, Harris T, Ferrucci L, Glynn RJ, Havlik RJ. Clarifying the direct relation between total cholesterol levels and death from coronary heart disease in older persons. Ann Intern Med, 1997; 126: 753-760 [DOI] [PubMed] [Google Scholar]
- 905). Harris T, Cook EF, Kannel WB, Goldman L. Proportional hazards analysis of risk factors for coronary heart disease in individuals aged 65 or older. The Framingham Heart Study. J Am Geriatr Soc, 1988; 36: 1023-1028 [DOI] [PubMed] [Google Scholar]
- 906). Pacala JT. The relation of serum cholesterol to risk of coronary heart disease: Implications for the elderly. J Am Board Fam Pract, 1990; 3: 271-282 [PubMed] [Google Scholar]
- 907). Rubin SM, Sidney S, Black DM, Browner WS, Hulley SB, Cummings SR. High blood cholesterol in elderly men and the excess risk for coronary heart disease. Ann Intern Med, 1990; 113: 916-920 [DOI] [PubMed] [Google Scholar]
- 908). Kronmal RA, Cain KC, Ye Z, Omenn GS. Total serum cholesterol levels and mortality risk as a function of age. A report based on the Framingham data. Arch Intern Med, 1993; 153: 1065-1073 [PubMed] [Google Scholar]
- 909). Krumholz HM, Seeman TE, Merrill SS, Mendes de Leon CF, Vaccarino V, Silverman DI, Tsukahara R, Ostfeld AM, Berkman LF. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all-cause mortality in persons older than 70 years. JAMA, 1994; 272: 1335-1340 [PubMed] [Google Scholar]
- 910). Weverling-Rijnsburger AW, Blauw GJ, Lagaay AM, Knook DL, Meinders AE, Westendorp RG. Total cholesterol and risk of mortality in the oldest old. Lancet, 1997; 350: 1119-1123 [DOI] [PubMed] [Google Scholar]
- 911). Ito T, Arima H, Fujiyoshi A, Miura K, Takashima N, Ohkubo T, Kadota A, Hayakawa T, Kita Y, Miyagawa N, Okayama A, Okamura T, Ueshima H. Relationship between non-high-density lipoprotein cholesterol and the long-term mortality of cardiovascular diseases: NIPPON DATA90. Int J Cardiol, 2016; 220: 262-267 [DOI] [PubMed] [Google Scholar]
- 912). Prospective Studies C. Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet, 2007; 370: 1829-1839 [DOI] [PubMed] [Google Scholar]
- 913). Zhang X, Patel A, Horibe H, Wu Z, Barzi F, Rodgers A, MacMahon S, Woodward M, Asia Pacific Cohort Studies C Cholesterol, coronary heart disease, and stroke in the Asia Pacific region. Int J Epidemiol, 2003; 32: 563-572 [DOI] [PubMed] [Google Scholar]
- 914). Hayashi T, Araki A, Kawashima S, Sone H, Watanabe H, Ohrui T, Yokote K, Takemoto M, Kubota K, Noda M, Noto H, Ina K, Nomura H, Japan CDM group Metabolic predictors of ischemic heart disease and cerebrovascular attack in elderly diabetic individuals: Difference in risk by age. Cardiovasc Diabetol, 2013; 12: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915). Odden MC, Shlipak MG, Whitson HE, Katz R, Kearney PM, defilippi C, Shastri S, Sarnak MJ, Siscovick DS, Cushman M, Psaty BM, Newman AB. Risk factors for cardiovascular disease across the spectrum of older age: The cardiovascular health study. Atherosclerosis, 2014; 237: 336-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 916). Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG, Pravastatin in elderly individuals at risk of vascular disease (PROSPER) : A randomised controlled trial. Lancet, 2002; 360: 1623-1630 [DOI] [PubMed] [Google Scholar]
- 917). Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R, Cholesterol Treatment Trialists C Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet, 2005; 366: 1267-1278 [DOI] [PubMed] [Google Scholar]
- 918). Cholesterol Treatment Trialists C. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet, 2010; 376: 1670-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 919). Roberts CG, Guallar E, Rodriguez A. Efficacy and safety of statin monotherapy in older adults: A meta-analysis. J Gerontol A Biol Sci Med Sci, 2007; 62: 879-887 [DOI] [PubMed] [Google Scholar]
- 920). Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJ, Eisenberg MJ. Statins for secondary prevention in elderly patients: A hierarchical bayesian meta-analysis. J Am Coll Cardiol, 2008; 51: 37-45 [DOI] [PubMed] [Google Scholar]
- 921). Hosomi N, Nagai Y, Kohriyama T, Ohtsuki T, Aoki S, Nezu T, Maruyama H, Sunami N, Yokota C, Kitagawa K, Terayama Y, Takagi M, Ibayashi S, Nakamura M, Origasa H, Fukushima M, Mori E, Minematsu K, Uchiyama S, Shinohara Y, Yamaguchi T, Matsumoto M, J-STARS Collaborators The Japan statin treatment against recurrent stroke (J-STARS): A multicenter, randomized, open-label, parallel-group study. EBioMedicine, 2015; 2: 1071-1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 922). Nakaya N, Mizuno K, Ohashi Y, Teramoto T, Yokoyama S, Hirahara K, Mizutani M, Nakamura H, MEGA Study Group Low-dose pravastatin and age-related differences in risk factors for cardiovascular disease in hypercholesterolaemic Japanese: Analysis of the management of elevated cholesterol in the primary prevention group of adult Japanese (MEGA STUDY). Drugs Aging, 2011; 28: 681-692 [DOI] [PubMed] [Google Scholar]
- 923). Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, de Craen AJ, Knopp RH, Nakamura H, Ridker P, van Domburg R, Deckers JW. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: Meta-analysis of randomised controlled trials. BMJ, 2009; 338: b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 924). Savarese G, Gotto AM, Jr., Paolillo S, D'Amore C, Losco T, Musella F, Scala O, Marciano C, Ruggiero D, Marsico F, De Luca G, Trimarco B, Perrone-Filardi P. Benefits of statins in elderly subjects without established cardiovascular disease: A meta-analysis. J Am Coll Cardiol, 2013; 62: 2090-2099 [DOI] [PubMed] [Google Scholar]
- 925). Yaffe K, Barrett-Connor E, Lin F, Grady D. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol, 2002; 59: 378-384 [DOI] [PubMed] [Google Scholar]
- 926). Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA, Manuck SB. Effects of lovastatin on cognitive function and psychological well-being. Am J Med, 2000; 108: 538-546 [DOI] [PubMed] [Google Scholar]
- 927). Muldoon MF, Ryan CM, Sereika SM, Flory JD, Manuck SB. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med, 2004; 117: 823-829 [DOI] [PubMed] [Google Scholar]
- 928). McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016: CD003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 929). White HD, Westerhout CM, Alexander KP, Roe MT, Winters KJ, Cyr DD, Fox KA, Prabhakaran D, Hochman JS, Armstrong PW, Ohman EM, TRILOGY ACS investigators Frailty is associated with worse outcomes in non-st-segment elevation acute coronary syndromes: Insights from the targeted platelet inhibition to clarify the optimal strategy to medically manage acute coronary syndromes (TRILOGY ACS) trial. Eur Heart J Acute Cardiovasc Care, 2016; 5: 231-242 [DOI] [PubMed] [Google Scholar]
- 930). Sergi G, Veronese N, Fontana L, De Rui M, Bolzetta F, Zambon S, Corti MC, Baggio G, Toffanello ED, Crepaldi G, Perissinotto E, Manzato E. Pre-frailty and risk of cardiovascular disease in elderly men and women: The Pro.V.A. study. J Am Coll Cardiol, 2015; 65: 976-983 [DOI] [PubMed] [Google Scholar]
- 931). Ohara M, Kohara K, Tabara Y, Igase M, Miki T. Portable indices for sarcopenia are associated with pressure wave reflection and central pulse pressure: The J-SHIPP study. J Hypertens, 2015; 33: 314-322 [DOI] [PubMed] [Google Scholar]
- 932). Scott D, Blizzard L, Fell J, Jones G. Statin therapy, muscle function and falls risk in community-dwelling older adults. QJM, 2009; 102: 625-633 [DOI] [PubMed] [Google Scholar]
- 933). Krishnan GM, Thompson PD. The effects of statins on skeletal muscle strength and exercise performance. Curr Opin Lipidol, 2010; 21: 324-328 [DOI] [PubMed] [Google Scholar]
- 934). Lynch JE, Henderson NR, Ramage L, McMurdo ME, Witham MD. Association between statin medication use and improved outcomes during inpatient rehabilitation in older people. Age Ageing, 2012; 41: 260-262 [DOI] [PubMed] [Google Scholar]
- 935). Kutner JS, Blatchford PJ, Taylor DH, Jr., Ritchie CS, Bull JH, Fairclough DL, Hanson LC, LeBlanc TW, Samsa GP, Wolf S, Aziz NM, Currow DC, Ferrell B, Wagner-Johnston N, Zafar SY, Cleary JF, Dev S, Goode PS, Kamal AH, Kassner C, Kvale EA, McCallum JG, Ogunseitan AB, Pantilat SZ, Portenoy RK, Prince-Paul M, Sloan JA, Swetz KM, Von Gunten CF, Abernethy AP. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: A randomized clinical trial. JAMA Intern Med, 2015; 175: 691-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 936).Ministry of Health, Labour and Welfare. Journal of health and welfare statistics 2015/2016. 2015; 62 (in Japanese) [Google Scholar]
- 937). Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the world health organization monica project. Registration procedures, event rates, and casefatality rates in 38 populations from 21 countries in four continents. Circulation, 1994; 90: 583-612 [DOI] [PubMed] [Google Scholar]
- 938). Ueshima H. Explanation for the Japanese paradox: Prevention of increase in coronary heart disease and reduction in stroke. J Atheroscler Thromb, 2007; 14: 278-286 [DOI] [PubMed] [Google Scholar]
- 939). Fukiyama K, Kimura Y, Wakugami K, Muratani H. Incidence and long-term prognosis of initial stroke and acute myocardial infarction in Okinawa, Japan. Hypertens Res, 2000; 23: 127-135 [DOI] [PubMed] [Google Scholar]
- 940). Kubo M, Kiyohara Y, Kato I, Tanizaki Y, Arima H, Tanaka K, Nakamura H, Okubo K, Iida M. Trends in the incidence, mortality, and survival rate of cardiovascular disease in a Japanese community: The Hisayama study. Stroke, 2003; 34: 2349-2354 [DOI] [PubMed] [Google Scholar]
- 941). Kudenchuk PJ, Maynard C, Martin JS, Wirkus M, Weaver WD. Comparison of presentation, treatment, and outcome of acute myocardial infarction in men versus women (the Myocardial Infarction Triage and iItervention Registry). Am J Cardiol, 1996; 78: 9-14 [DOI] [PubMed] [Google Scholar]
- 942). Chandra NC, Ziegelstein RC, Rogers WJ, Tiefenbrunn AJ, Gore JM, French WJ, Rubison M. Observations of the treatment of women in the United States with myocardial infarction: A report from the National Registry of Myocardial Infarction-I. Arch Intern Med, 1998; 158: 981-988 [DOI] [PubMed] [Google Scholar]
- 943). Vakili BA, Kaplan RC, Brown DL. Sex-based differences in early mortality of patients undergoing primary angioplasty for first acute myocardial infarction. Circulation, 2001; 104: 3034-3038 [DOI] [PubMed] [Google Scholar]
- 944). Marso SP, Gowda M, O'Keefe JH, Coen MM, McCallister BD, Giorgi LV, Huber KC, Laster SB, Johnson WL, Rutherford BD. Improving in-hospital mortality in the setting of an increasing risk profile among patients undergoing catheter-based reperfusion for an acute myocardial infarction without cardiogenic shock. J Invasive Cardiol, 2003; 15: 711-716 [PubMed] [Google Scholar]
- 945). Kimura Y, Takishita S, Muratani H, Kinjo K, Shinzato Y, Muratani A, Fukiyama K. Demographic study of firstever stroke and acute myocardial infarction in Okinawa, Japan. Intern Med, 1998; 37: 736-745 [DOI] [PubMed] [Google Scholar]
- 946). Kosuge M, Kimura K, Kojima S, Sakamoto T, Ishihara M, Asada Y, Tei C, Miyazaki S, Sonoda M, Tsuchihashi K, Yamagishi M, Ikeda Y, Shirai M, Hiraoka H, Inoue T, Saito F, Ogawa H. Sex differences in early mortality of patients undergoing primary stenting for acute myocardial infarction. Circ J, 2006; 70: 217-221 [DOI] [PubMed] [Google Scholar]
- 947). Kubo M, Hata J, Doi Y, Tanizaki Y, Iida M, Kiyohara Y. Secular trends in the incidence of and risk factors for ischemic stroke and its subtypes in Japanese population. Circulation, 2008; 118: 2672-2678 [DOI] [PubMed] [Google Scholar]
- 948). Turin TC, Kita Y, Rumana N, Nakamura Y, Takashima N, Ichikawa M, Sugihara H, Morita Y, Hirose K, Okayama A, Miura K, Ueshima H. Ischemic stroke subtypes in a Japanese population: Takashima Stroke Registry, 1988-2004. Stroke, 2010; 41: 1871-1876 [DOI] [PubMed] [Google Scholar]
- 949). Kita Y, Turin TC, Ichikawa M, Sugihara H, Morita Y, Tomioka N, Rumana N, Okayama A, Nakamura Y, Abbott RD, Ueshima H. Trend of stroke incidence in a Japanese population: Takashima Stroke Registry, 1990–2001. Int J Stroke, 2009; 4: 241-249 [DOI] [PubMed] [Google Scholar]
- 950). Maeda K, Toyoda K, Minematsu K, Kobayashi S, Japan Standard Stroke Registry Study Group Effects of sex difference on clinical features of acute ischemic stroke in Japan. J Stroke Cerebrovasc Dis, 2013; 22: 1070-1075 [DOI] [PubMed] [Google Scholar]
- 951). Nagasawa SY, Okamura T, Iso H, Tamakoshi A, Yamada M, Watanabe M, Murakami Y, Miura K, Ueshima H. Relation between serum total cholesterol level and cardiovascular disease stratified by sex and age group: A pooled analysis of 65 594 individuals from 10 cohort studies in Japan. J Am Heart Assoc, 2012; 1: e001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 952). Honjo K, Iso H, Tsugane S, Tamakoshi A, Satoh H, Tajima K, Suzuki T, Sobue T. The effects of smoking and smoking cessation on mortality from cardiovascular disease among Japanese: Pooled analysis of three largescale cohort studies in Japan. Tob Control, 2010; 19: 50-57 [DOI] [PubMed] [Google Scholar]
- 953). Nakamura K, Nakagawa H, Sakurai M, Murakami Y, Irie F, Fujiyoshi A, Okamura T, Miura K, Ueshima H, EPOCH-JAPAN Research Group Influence of smoking combined with another risk factor on the risk of mortality from coronary heart disease and stroke: Pooled analysis of 10 Japanese cohort studies. Cerebrovasc Dis, 2012; 33: 480-491 [DOI] [PubMed] [Google Scholar]
- 954). Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: A systematic review and meta-analysis of prospective cohort studies. Lancet, 2011; 378: 1297-1305 [DOI] [PubMed] [Google Scholar]
- 955). Kawano H, Soejima H, Kojima S, Kitagawa A, Ogawa H. Sex differences of risk factors for acute myocardial infarction in Japanese patients. Circ J, 2006; 70: 513-517 [DOI] [PubMed] [Google Scholar]
- 956). Nishino Y, Tsuji I, Tanaka H, Nakayama T, Nakatsuka H, Ito H, Suzuki T, Katanoda K, Sobue T, Tominaga S. Stroke mortality associated with environmental tobacco smoke among never-smoking Japanese women: A prospective cohort study. Prev Med, 2014; 67: 41-45 [DOI] [PubMed] [Google Scholar]
- 957). Miura K, Nakagawa H, Ohashi Y, Harada A, Taguri M, Kushiro T, Takahashi A, Nishinaga M, Soejima H, Ueshima H. Four blood pressure indexes and the risk of stroke and myocardial infarction in Japanese men and women: A meta-analysis of 16 cohort studies. Circulation, 2009; 119: 1892-1898 [DOI] [PubMed] [Google Scholar]
- 958). Ikeda A, Iso H, Yamagishi K, Inoue M, Tsugane S. Blood pressure and the risk of stroke, cardiovascular disease, and all-cause mortality among Japanese: The JPHC Study. Am J Hypertens, 2009; 22: 273-280 [DOI] [PubMed] [Google Scholar]
- 959). Takashima N, Ohkubo T, Miura K, Okamura T, Murakami Y, Fujiyoshi A, Nagasawa SY, Kadota A, Kita Y, Miyagawa N, Hisamatsu T, Hayakawa T, Okayama A, Ueshima H. Long-term risk of bp values above normal for cardiovascular mortality: A 24-year observation of Japanese aged 30 to 92 years. J Hypertens, 2012; 30: 2299-2306 [DOI] [PubMed] [Google Scholar]
- 960). Kato M, Noda M, Mizoue T, Goto A, Takahashi Y, Matsushita Y, Nanri A, Iso H, Inoue M, Sawada N, Tsugane S. Diagnosed diabetes and premature death among middle-aged Japanese: Results from a large-scale population-based cohort study in Japan (JPHC study). BMJ Open, 2015; 5: e007736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 961). Doi Y, Ninomiya T, Hata J, Fukuhara M, Yonemoto K, Iwase M, Iida M, Kiyohara Y. Impact of glucose tolerance status on development of ischemic stroke and coronary heart disease in a general Japanese population: The Hisayama study. Stroke, 2010; 41: 203-209 [DOI] [PubMed] [Google Scholar]
- 962). Kokubo Y, Okamura T, Watanabe M, Higashiyama A, Ono Y, Miyamoto Y, Furukawa Y, Kamide K, Kawanishi K, Okayama A, Yoshimasa Y. The combined impact of blood pressure category and glucose abnormality on the incidence of cardiovascular diseases in a Japanese urban cohort: The Suita study. Hypertens Res, 2010; 33: 1238-1243 [DOI] [PubMed] [Google Scholar]
- 963). Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med, 2000; 343: 16-22 [DOI] [PubMed] [Google Scholar]
- 964). Chiuve SE, Fung TT, Rexrode KM, Spiegelman D, Manson JE, Stampfer MJ, Albert CM. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA, 2011; 306: 62-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 965). Chiuve SE, Rexrode KM, Spiegelman D, Logroscino G, Manson JE, Rimm EB. Primary prevention of stroke by healthy lifestyle. Circulation, 2008; 118: 947-954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 966). Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, Rimm EB. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol, 2015; 65: 43-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 967). Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: A systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update, 2011; 17: 589-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 968). Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity c-reactive protein or dyslipidemia: Results from the justification for the use of statins in prevention: An intervention trial evaluating rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation, 2010; 121: 1069-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 969). Edison RJ, Muenke M. Gestational exposure to lovastatin followed by cardiac malformation misclassified as holoprosencephaly. N Engl J Med, 2005; 352: 2759. [DOI] [PubMed] [Google Scholar]
- 970). Bateman BT, Hernandez-Diaz S, Fischer MA, Seely EW, Ecker JL, Franklin JM, Desai RJ, Allen-Coleman C, Mogun H, Avorn J, Huybrechts KF. Statins and congenital malformations: Cohort study. BMJ, 2015; 350: h1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 971). Gutierrez J, Ramirez G, Rundek T, Sacco RL. Statin therapy in the prevention of recurrent cardiovascular events: A sex-based meta-analysis. Arch Intern Med, 2012; 172: 909-919 [DOI] [PubMed] [Google Scholar]
- 972). Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, Wetterslev J. Intensive glycaemic con trol for patients with type 2 diabetes: Systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ, 2011; 343: d6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 973). Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med, 2005; 353: 2643-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 974). Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med, 2008; 359: 1577-1589 [DOI] [PubMed] [Google Scholar]
- 975). Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, Deedwania P, Eckel RH, Ershow AG, Fradkin J, Inzucchi SE, Kosiborod M, Nelson RG, Patel MJ, Pignone M, Quinn L, Schauer PR, Selvin E, Vafiadis DK, American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Quality of Care and Outcomes Research; American Diabetes Association. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence : A scientific statement from the American Heart Association and the American diabetes association. Diabetes Care, 2015; 38: 1777-1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 976). Miura K, Nagai M, Ohkubo T. Epidemiology of hypertension in Japan: Where are we now? Circ J, 2013; 77: 2226-2231 [DOI] [PubMed] [Google Scholar]
- 977). Turnbull F, Woodward M, Neal B, Barzi F, Ninomiya T, Chalmers J, Perkovic V, Li N, MacMahon S. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J, 2008; 29: 2669-2680 [DOI] [PubMed] [Google Scholar]
- 978). Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) research group. JAMA, 1998; 280: 605-613 [DOI] [PubMed] [Google Scholar]
- 979). Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N, HERS Research Group Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA, 2002; 288: 49-57 [DOI] [PubMed] [Google Scholar]
- 980). Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: The women's health initiative: A randomized trial. JAMA, 2003; 289: 2673-2684 [DOI] [PubMed] [Google Scholar]
- 981). Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med, 2003; 349: 523-534 [DOI] [PubMed] [Google Scholar]
- 982). Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J. Effects of conjugated equine estrogen on stroke in the women's health initiative. Circulation, 2006; 113: 2425-2434 [DOI] [PubMed] [Google Scholar]
- 983). Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA, 2007; 297: 1465-1477 [DOI] [PubMed] [Google Scholar]
- 984). Japan Society of Obstetrics and Gynecology, The Japan Society for Menopause and Women's Health Hormone replacement therapy guidelines ver. 2012. (in Japanese) [Google Scholar]
- 985). Lokkegaard E, Andreasen AH, Jacobsen RK, Nielsen LH, Agger C, Lidegaard O. Hormone therapy and risk of myocardial infarction: A national register study. Eur Heart J, 2008; 29: 2660-2668 [DOI] [PubMed] [Google Scholar]
- 986). Japan Society of Obstetrics and Gynecology Interpretations on Hormone replacement therapy. September 2, http://www.jsog.or.jp/kaiin/html/hrt_2sep2002.html (in Japanese) [Google Scholar]
- 987).Tobacco Information News. (from the survey by Japan Tobacco Inc. (JT), former Japan Tobacco and Salt Public Corporation) http://www.health-net.or.jp/tobacco/product/pd090000.html (in Japanese)
- 988). Okada T, Murata M, Yamauchi K, Harada K. New criteria of normal serum lipid levels in Japanese children: The nationwide study. Pediatr Int, 2002; 44: 596-601 [DOI] [PubMed] [Google Scholar]
- 989). Abe Y, Okada T, Sugiura R, Yamauchi K, Murata M. Reference ranges for the non-high-density lipoprotein cholesterol levels in Japanese children and adolescents. J Atheroscler Thromb, 2015; 22: 669-675 [DOI] [PubMed] [Google Scholar]
- 990). Kobayashi Y, Sugihara S, Tanaka Y, Ishihara H, Ohno K, Fujita H, Takizawa N, Dobashi M. A consideration on criteria for blood collection after a meal in medical examination of life-style related diseases in children. Journal of the Japan Pediatric Society, 2011; 115: 1255-64 (in Japanese) [Google Scholar]
- 991). Zeitler P, Fu J, Tandon N, Nadeau K, Urakami T, Barrett T, Maahs D, Diabetes International Society for Pediatric and Adolescent Diabetes ISPAD clinical practice consensus guidelines 2014. Type 2 diabetes in the child and adolescent. Pediatr Diabetes, 2014; 15 Suppl 20: 26-46 [DOI] [PubMed] [Google Scholar]
- 992). The Japan Diabetes Society, The Japanese Society of Pediatric Endocrinology Consensus guideline of diabetes in childhood and adolescents. 2015. (in Japanese) [Google Scholar]
- 993). Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking A preliminary report from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) research group. JAMA, 1990; 264: 3018-3024 [DOI] [PubMed] [Google Scholar]
- 994). Newman WP, Freedman DS, Voors AW, Gard PD, Srinivasan SR, Cresanta JL, Williamson GD, Webber LS, Berenson GS. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N Engl J Med, 1986; 314: 138-144 [DOI] [PubMed] [Google Scholar]
- 995). Amemiya S, Dobashi K, Urakami T, Sugihara S, Ohzeki T, Tajima N. Metabolic syndrome in youths. Pediatr Diabetes, 2007; 8 Suppl 9: 48-54 [DOI] [PubMed] [Google Scholar]
- 996). Asayama K, Ozeki T, Sugihara S, Ito K, Okada T, Tamai H, Takaya R, Hanaki K, Murata M. Criteria for medical intervention in obese children: A new definition of ‘obesity disease’ in Japanese children. Pediatr Int, 2003; 45: 642-646 [DOI] [PubMed] [Google Scholar]
- 997). Ministry of Health, Labour and Welfare Dieary Reference Intakes for Japanese, 2015; http://www.mhlw.go.jp/stf/shingi/0000041824.html (in Japanese)
- 998). Dobashi K. Evaluation of obesity in school-age children. J Atheroscler Thromb, 2016; 23: 32-38 [DOI] [PubMed] [Google Scholar]


