Abstract
Aim: To analyze associations among the serum endostatin level, renal function, and carotid atherosclerosis of healthy residents of Japan.
Methods: Among 1,057 Japanese residents who attended free public physical examinations between 2010 and 2011, we evaluated the data of 648 healthy residents (200 men and 448 women, age 24 to 84 years) for whom the serum endostatin level and common carotid intima-media thickness (IMT) were measured. Renal function was assessed by estimated glomerular filtration rate (eGFR). Multiple linear regression analysis was done to determine the association of eGFR and serum endostatin level after adjustment for known covariates. Mediation analysis was done using Baron and Kenny's regression approach.
Results: The median endostatin level was 63.7 ng/mL (interquartile range: 49.7–93.2). The mean eGFR was 78.4 ± 14.8 mL/min/1.73m2. Univariate analysis showed that age (r = −0.37, P < 0.01), non current smoking (85.8 ± 13.0 vs. 77.5 ± 14.8 mL/min/1.73 m2, P < 0.01), hemoglobin A1c (r = −0.08, P = 0.05), low-density lipoprotein-cholesterol (r = −0.13, P < 0.01), uric acid (r = −0.15, P < 0.01), carotid IMT (r = −0.11, P < 0.01), and log-transformed endostatin (r = −0.36, P < 0.01) were significantly associated with eGFR. In multiple linear regression analysis, log-transformed endostatin was significantly associated with eGFR (beta = −0.24, P < 0.01). While, carotid IMT was no longer significant. Mediation analysis showed serum endostatin level to be a mediator in the association between carotid IMT and eGFR.
Conclusions: The association between carotid IMT and eGFR is mediated by the serum endostatin level of healthy individuals.
Keywords: Renal function, Endostatin, Carotid intima-media thickness, Epidemiology
1. Introduction
Reduction in the glomerular filtration rate (GFR) is associated with an increased risk of cardiovascular disease1–3), end-stage renal disease, and all-cause mortality4). GFR is also used to diagnose, classify, and monitor chronic kidney disease (CKD)5).
Endostatin is a cleavage of the C-terminal domain of collagen XVIII, a component of the extra-cellular matrix6), and a potent endogenous angiogenesis inhibitor in vivo and in vitro7–9). Collagen XVIII is a major component of the basal membranes, is highly expressed in kidney, and is found in the Bowman's capsule, as well as in the glomerular and tubular basal membranes10, 11). Cleavage of collagen XVIII during extracellular matrix (ECM) remodeling gives rise to the circulating endostatin level12). Over the past few years, several studies have reported an association between the circulating endostatin level and the renal function of CKD patients13–16), the elderly population12, 17, 18), and diabetes patients19). Thus, it is well documented that serum endostatin is associated with renal function and can be a useful marker of CKD. However, the association between the serum endostatin level and the renal function of healthy individuals, including younger persons, is unclear.
Carotid intima-media thickness (IMT), a marker of subclinical atherosclerosis, is a predictor of the incidence of CKD20, 21). We previously showed a significant relation between serum endostatin and carotid atherosclerosis22). On the other hand, no studies have been done that have shown association of the serum endostatin level, CKD, and carotid IMT. This may be a basic information for using the serum endostatin level as a clinical marker. In this study, we did exploratory cross-sectional observational research about the impact of the serum endostatin level on the association between carotid atherosclerosis and the renal function in a healthy Japanese population.
2. Patients and Methods
2.1. Study Participants
This study is part of the Kyushu and Okinawa Population Study (KOPS) survey of vascular events associated with lifestyle-related diseases23, 24). For the present study, we used the same study population as in our previous KOPS report22). Study eligible participants were 1,057 residents who took part in free public physical examinations between 2010 and 2011. The following were excluded from analysis: 1) 28 because of insufficient data; 2) 44 who did not agree to undergo carotid ultrasonographic measurement; 3) 77 who had a history of cardiovascular disease, malignancy, or a chronic inflammatory disease (collagen disease, inflammatory bowel disease); 4) 260 receiving treatment for hypertension, diabetes, or dyslipidemia. After exclusions, the data of 648 subjects (200 men and 448 women) were available for analysis22). The age of the subjects ranged from 24 to 84 years (mean ± SD: 56.3 ± 10.6 years). The study design was approved by the Kyushu University Hospital Ethics Committee (permission number: 590-00). Written informed consent was obtained from each participant prior to the examination. The study was conducted in accordance with the principles of the Helsinki Declaration of 1975, as revised in 2000.
2.2. Anthropometric Measurement and Questionnaire
Anthropometric measurements were performed with each participant wearing indoor clothing and without shoes. Body mass index (BMI) was calculated as weight [kg] divided by height [m] squared. Systolic and diastolic blood pressures (SBP and DBP) were measured on the right arm, in the sitting position, with an automated sphygmomanometer (HEM-780, Omron Healthcare, Kyoto, Japan) after a five-minute rest.
Each participant completed a self-administrated questionnaire to gather information about personal medical history, family medical history, use of drugs, smoking status (current or non-current), and alcohol consumption (habitual or non-habitual). The questionnaire was checked for unfilled or inconsistent answers, first by nurses and again by our staff physicians22).
2.3. Laboratory Measurements
As part of a free public physical examination, blood samples were collected after an 8-hour overnight fast to determine the serum levels of creatinine, hemoglobin A1c (HbA1c), low-density lipoprotein (LDL) cholesterol, and high sensitive C-reactive protein (hs-CRP)22). The serum level of high-density lipoprotein (HDL) cholesterol (Determiner L HDL-C and T, Kyowa Medex Co., Ltd, Tokyo, Japan), triglycerides (Determiner L TG II, Kyowa Medex Co., Ltd, Tokyo, Japan), and uric acid (L-Type UA F, Wako Pure Chemical Industries Co., Ltd, Osaka, Japan) were determined by automated, standardized enzymatic analysis. Aliquots of whole blood and fresh plasma and serum samples after separation were stored at 4°C in refrigerated containers and sent to a commercial laboratory (SRL, Inc., Tokyo, Japan). The estimated GFR (eGFR) was calculated using the modification of diet in renal disease study equation modified for Japanese subjects: eGFR (mL/min/1.73m2) = 194 × age−0.287 × serum creatinine (mg/dL)−1.094 (if female × 0.739)25). All remaining fasting serum samples were immediately frozen and stored at −80°C until assayed. The serum endostatin level (range: 16–500 ng/mL) was measured from defrosted samples using a commercially available enzyme-linked immunosorbent assay kit (R&D Systems Inc., Minneapolis, USA). Assessment of reproducibility testing showed good results, the recovery rate for spiked samples was 91%–108%, and there was no influence of interfering substances at normal levels. The intra- and inter-assay coefficients of variation were 5.0% and 6.4%, respectively22).
2.4. Ultrasonographic Measurement
Carotid IMT was assessed by ultrasound22). Carotid artery lesions were measured using high-resolution B-mode ultrasonography with a 7.5 MHz linear array probe (UF-4300R®, Fukuda Denshi Co., Ltd, Tokyo, Japan) by the well-trained physicians of our department. Images were obtained 20 mm proximal to the origin of the carotid bulb at the far wall by the IMT measurement software, Intimascope (Media Cross Co., Ltd, Tokyo, Japan)26). The mean value of the bilateral average mean-IMT level was used as the mean carotid IMT level.
2.5. Statistical Analysis
Data are presented as the means ± standard deviation (SD) or percentage. Because the distributions of the serum endostatin and hs-CRP levels were highly skewed22), they were log-transformed before the statistical analysis and expressed as the median (interquartile ranges). The univariate analysis for associations between eGFR and clinical variables was done using Pearson's correlation coefficient analysis (categorical variables were compared with the difference between groups). For comparisons of participants with an above/below median serum endostatin level, unpaired Student's t-test was used to compare mean values and the chi-square test was used to evaluate differences in prevalence rates. Multivariate linear regression was used to examine the association of eGFR and serum endostatin level after adjustment for the previously mentioned covariates. Mediation analysis was done using Baron and Kenny's regression approach27) to investigate the mediating effect of serum endostatin level in the association between carotid IMT and eGFR. A stepwise approach was followed in which four conditions must be met: 1) an established association between the carotid IMT and eGFR; 2) an established relationship between carotid IMT and serum endostatin level; 3) an association between serum endostatin level and eGFR; and 4) after adjustment for serum endostatin, the association between carotid IMT and eGFR is no longer significant. The statistical analysis was performed using JMP Pro ver. 11 (SAS Institute Inc., Cary, NC, USA). A two-tailed P value < 0.05 was considered statistically significant.
3. Results
3.1. Clinical Characteristics
The median endostatin level was 63.7 ng/mL (interquartile range: 49.7–93.2 ng/mL). The mean eGFR was 78.4 ± 14.8 mL/min/1.73m2, and 54 participants (8.3%) had renal dysfunction (eGFR < 60.0 mL/min/1.73m2). Furthermore, the median endostatin level of subjects with (without) renal dysfunction was 101.8 (62.7) ng/mL [interquartile range: 70.3–127.9 (49.3–90.4) ng/mL]. The clinical characteristics of subjects with above (≥ 63.7 ng/mL) and below (< 63.7 ng/mL) median endostatin levels are presented in Table 1. Subjects with above median endostatin had significantly lower eGFR (74.6 ± 13.3 vs. 82.3 ± 15.2 mL/min/1.73m2, P < 0.001) and higher carotid IMT (0.71 ± 0.14 vs. 0.65 ± 0.09 mm, P < 0.001) than those with below median endostatin. Age, sex, habitual drinking, SBP, DBP, HbA1c, LDL and HDL cholesterol level, and uric acid level were also significantly different between the subjects with above and below median endostatin levels.
Table 1. Clinical characteristics by serum endostatin level.
| Variable | Below median endostatin | Above median endostatin | P-value |
|---|---|---|---|
| (< 63.7 ng/mL, n = 324) | (≥ 63.7 ng/mL, n = 324) | ||
| Serum endostatin (ng/mL) | 49.7 (43.1–56.3) | 93.2 (77.1–110.2) | - |
| Age (years) | 52.4 ± 9.3 | 60.1 ± 10.4 | < 0.001 |
| Men (%) | 24.1 | 37.7 | < 0.001 |
| Body mass index (kg/m2) | 22.5 ± 3.2 | 22.5 ± 2.9 | 0.997 |
| Habitual drinker, n (%) | 42 (13.0) | 80 (24.7) | < 0.001 |
| Current smoker, n (%) | 41 (12.8) | 33 (10.2) | 0.324 |
| Systolic blood pressure (mmHg) | 127.4 ± 18.7 | 122.4 ± 17.0 | 0.004 |
| Diastolic blood pressure (mmHg) | 75.9 ± 12.7 | 73.4 ± 11.9 | 0.035 |
| HbA1c (%) | 5.4 ± 0.5 | 5.5 ± 0.4 | < 0.001 |
| LDL-cholesterol (mmol/L) | 3.2 ± 0.8 | 3.1 ± 0.8 | 0.009 |
| HDL-cholesterol (mmol/L) | 1.8 ± 0.4 | 1.7 ± 0.4 | < 0.001 |
| Triglycerides (mmol/L) | 1.1 ± 0.7 | 1.2 ± 0.8 | 0.713 |
| Uric acid (mg/dL) | 4.5 ± 1.1 | 5.0 ± 1.3 | < 0.001 |
| hs-CRP (mg/L) | 0.26 (0.11–0.58) | 0.28 (0.13–0.64) | 0.089 |
| Mean carotid IMT (mm) | 0.65 ± 0.09 | 0.71 ± 0.14 | < 0.001 |
| eGFR (mL/min/1.73m2) | 82.3 ± 15.2 | 74.6 ± 13.3 | < 0.001 |
Data are presented as the mean ± standard deviation, median (interquartile ranges), or number of subjects (percent) for categorical variables. Overall P-values were calculated by unpaired t-test or chi-square test.
HbA1c: hemoglobin A1c, LDL: low density lipoprotein, HDL: high density lipoprotein, hs-CRP: high sensitive C-reactive protein, IMT: intima-media thickness, eGFR: estimated glomerular filtration rate
3.2. Association between eGFR, Endostatin, and Carotid IMT
Univariate analysis showed age (r = −0.37, P < 0.001), non current smoking (85.8 ± 13.0 vs. 77.5 ± 14.8 mL/min/1.73 m2, P < 0.001), HbA1c (r = −0.08, P = 0.045), LDL-cholesterol (r = −0.13, P < 0.001), uric acid (r = −0.15, P < 0.001), carotid IMT (r = −0.11, P = 0.003), and log-transformed endostatin (r = −0.36, P < 0.001) to be significantly associated with eGFR (Fig. 1). In multiple linear regression, the log-transformed serum endostatin level was significantly correlated to eGFR (Table 2). Although carotid IMT and eGFR were related in univariate analysis, carotid IMT was not significantly associated with eGFR change after casting serum endostatin into a multiple linear regression. From this, the association between carotid IMT and eGFR may be mediated through serum end-ostatin level. To verify this, we did a Baron and Kenny analysis to determine if the requirements to demonstrate mediation were met27). eGFR (dependent variable) showed a positive association with carotid IMT (independent variable) [Model 1: beta = −0.11, P = 0.003]. The association between carotid IMT and log-transformed serum endostatin level (mediator) was significant [Model 2: beta = 0.26, P < 0.001]. Furthermore, there was a significant association between log-transformed serum endostatin level and eGFR [Model 3: beta = −0.36, P < 0.001]. After adjustment for log-transformed serum endostatin level, the association between carotid IMT and eGFR did not remain significant [Model 4: beta = −0.02, P = 0.568]. Additionally, the beta coefficient for eGFR in model 4 was lower than in model 1. Mediation analysis showed that the serum endostatin level could be considered as a (complete) mediator in the association between carotid IMT and eGFR.
Fig. 1.
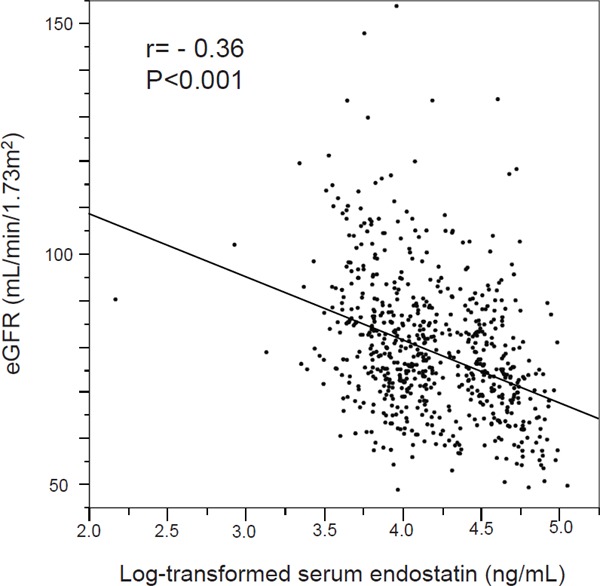
Scatterplot of the log-transformed serum endostatin level and eGFR eGFR: estimated glomerular filtration rate
Table 2. Multiple linear regression analysis of the association between eGFR and serum endostatin.
| Variable | Beta | P-value | F | Adjusted R2 | |
|---|---|---|---|---|---|
| Model 1 | Mean carotid IMT (mm) | 0.05 | 0.166 | 27.5 | 0.224 |
| Log-transformed serum endostatin (ng/mL) | −0.24 | < 0.001 | |||
| Model 2 | Mean carotid IMT (mm) | 0.04 | 0.340 | 10.1 | 0.237 |
| Log-transformed serum endostatin (ng/mL) | −0.20 | < 0.001 | |||
| Model 3 | Mean carotid IMT (mm) | 0.04 | 0.272 | 24.0 | 0.221 |
| Log-transformed serum endostatin (ng/mL) | −0.26 | < 0.001 | |||
Beta coefficient and P-value were calculated by multiple linear regression.
Model 1: Adjusted for significant risk factors found in the univariate analysis (age, current smoking, HbA1c, low-density lipoprotein cholesterol, and uric acid).
Model 2: Adjusted for the known covariates sex, body mass index, systolic blood pressure, diastolic blood pressure, high-density lipoprotein cholesterol, and triglycerides in addition to the adjustments made for model 1.
Model 3: Adjusted for the covariates age, sex, HbA1c, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and uric acid selected by stepwise method from significant parameters with serum endostatin level in Table 1.
eGFR: estimated glomerular filtration rate, IMT: intima-media thickness, HbA1c: hemoglobin A1c
4. Discussion
The main finding of the present study is that the association between carotid IMT and eGFR is mediated through the serum endostatin level of healthy individuals. Additionally, a high serum endostatin level was significantly associated with a decrease of eGFR in multiple linear regression adjusted for known covariates, including carotid IMT and traditional risk factors for atherosclerosis, such as blood pressure, blood glucose, and blood lipids, as reported in the data of a population-based study28). Recent observational studies have shown that the circulating endostatin level is associated with lower eGFR and higher albuminuria12), the severity of kidney dysfunction13), the CKD and CKD stage14, 15), and hypertensive target-organ damage including urinary albumin/creatinine ratio17) and that it is a predictor of future mortality18, 19) and cardiovascular events16). However, these studies were limited in CKD patients13–16), an elderly population12, 17, 18), or diabetes patients19). In the present study, we extended the field to a healthy population that included younger persons who ranged from 24 to 84 years (mean ± SD: 56.3 ± 10.6 years), which is epidemiologically and clinically important. Our findings suggest the possibility that serum endostatin would be a useful marker for the management of CKD, cardiovascular disease, and the mortality of healthy individuals, including younger persons. Although GFR has some problems as a surrogate marker of CKD, it is generally used to diagnose, classify, and monitor CKD5) and is considered to be an independent risk factor of cardiovascular disease1–3), end-stage renal disease, and all-cause mortality4). GFR may be a clinically useful surrogate endpoint.
The direct mechanisms related to the elevation of serum endostatin level and renal dysfunction remain unclear. In this regard, we have previously shown that the serum endostatin level is independently associated with carotid IMT with vascular endothelial dysfunction and ECM remodeling22). Because atherosclerosis is a risk factor for the development of CKD, it is possible that the association between serum endostatin and renal function reflects the influence of an endostatin- producing increase derived from sclerotic blood vessels. On the other hand, it has been reported that circulating endostatin partly reflects renal ECM remodeling12). Elevation of the endostatin level may represent renal microvascular rarefaction and fibrosis in ECM remodeling in kidney29). We also referred to the possibility that an increase in the serum endostatin level is a useful marker for the early detection of kidney injury in our previous study of 161 residents with prediabetes30). The serum endostatin level may reflect not only a production increase derived from sclerotic blood vessels but also production derived from ischemia and fibrosis in kidney. Furthermore, it is necessary to consider the influence of renal clearance of circulating endostatin as well as the endostatin-producing increase. Urinary excretion was the major elimination route of endostatin in an animal experiment31). Elevation of serum endostatin level may be due to decreased renal clearance, similar to that of cystatin C. Both cystatin C and endostatin are of similar size and can pass through the glomerular barrier12). These findings suggest that serum endostatin strongly reflects ECM remodeling in blood vessels and the kidney, especially in healthy individuals or early renal injury patients and may better reflect decreased renal clearance in patients with severe renal dysfunction.
This study has some limitations. First, the cross-sectional observational design makes it difficult to draw concrete inferences regarding causality between the serum endostatin level, carotid IMT, and eGFR. Second, we used the results of a single time measurement for our evaluation of serum endostatin. Third, the impact of age and sex on renal function might be stronger because eGFR was used to evaluate renal function. Finally, we did not evaluate urinary albumin. In spite of these limitations, this study is the first to show an association between the serum endostatin level, renal function, and subclinical atherosclerosis in a population of healthy residents of Japan. We believe that our findings will contribute the clarification of the usefulness of serum endostatin measurement in the management of CKD.
We found that the association between carotid IMT and eGFR is mediated by the serum endostatin level of healthy Japanese residents. Future, longitudinal studies will be necessary to assess the clinical usefulness of the circulating endostatin level as an indicator of future CKD, especially for healthy individuals or those with early renal impairment.
Acknowledgment
We are grateful to Drs. Mosaburo Kainuma, Eiichi Ogawa, Kazuhiro Toyoda, Takeo Hayashi, Takeshi Ihara, Kazuya Ura, Ayaka Komori, Eri Kumade, and Masaru Sakiyama from our department for their assistance.
Financial Support
This study was funded by the Japan Multi-institutional Collaborative Cohort Study (J-MICC Study), a Grant-in-Aid for Scientific Research on Priority Areas of Cancer [No. 17015018] and Innovative Areas [No. 221S0001], and by a Grant-in-Aid for Scientific Research (A) [JPSP KAKENHI Grant Number JP 16H06277] from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1). Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA, 2007; 298: 2038-2047 [DOI] [PubMed] [Google Scholar]
- 2). Holzmann MJ, Aastveit A, Hammar N, Jungner I, Walldius G, Holme I: Renal dysfunction increases the risk of ischemic and hemorrhagic stroke in the general population. Ann Med, 2012; 44: 607-615 [DOI] [PubMed] [Google Scholar]
- 3). Shlipak MG, Katz R, Kestenbaum B, Fried LF, Siscovick D, Sarnak MJ: Clinical and subclinical cardiovascular disease and kidney function decline in the elderly. Atherosclerosis, 2009; 204: 298-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, Kleefstra N, Naimark D, Roderick P, Tonelli M, Wetzels JF, Astor BC, Gansevoort RT, Levin A, Wen CP, Coresh J: Age and association of kidney measures with mortality and end-stage renal disease. JAMA, 2012; 308: 2349-2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Stevens PE, Levin A: Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med, 2013; 158: 825-830 [DOI] [PubMed] [Google Scholar]
- 6). Seppinen L, Pihlajaniemi T: The multiple functions of collagen XVIII in development and disease. Matrix Biol, 2011; 30: 83-92 [DOI] [PubMed] [Google Scholar]
- 7). O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J: Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell, 1997; 88: 277-285 [DOI] [PubMed] [Google Scholar]
- 8). Skovseth DK, Veuger MJ, Sorensen DR, De Angelis PM, Haraldsen G: Endostatin dramatically inhibits endothelial cell migration, vascular morphogenesis, and perivascular cell recruitment in vivo. Blood, 2005; 105: 1044-1051 [DOI] [PubMed] [Google Scholar]
- 9). Schuch G, Heymach JV, Nomi M, Machluf M, Force J, Atala A, Eder JP, Jr, Folkman J, Soker S: Endostatin inhibits the vascular endothelial growth factor-induced mobilization of endothelial progenitor cells. Cancer Res, 2003; 63: 8345-8350 [PubMed] [Google Scholar]
- 10). Saarela J, Ylikärppä R, Rehn M, Purmonen S, Pihlajaniemi T: Complete primary structure of two variant forms of human type ⅩⅧ collagen and tissue-specific differences in the expression of the corresponding transcripts. Matrix Biol, 1998; 16: 319-328 [DOI] [PubMed] [Google Scholar]
- 11). Tomono Y, Naito I, Ando K, Yonezawa T, Sado Y, Hirakawa S, Arata J, Okigaki T, Ninomiya Y: Epitope-defined monoclonal antibodies against multiplexin collagens demonstrate that type XV and XVIII collagens are expressed in specialized basement membranes. Cell Struct Funct, 2002; 27: 9-20 [DOI] [PubMed] [Google Scholar]
- 12). Ruge T, Carlsson AC, Larsson TE, Carrero JJ, Larsson A, Lind L, Ärnlöv J: Endostatin level is associated with kidney injury in the elderly: findings from two community-based cohorts. Am J Nephrol, 2014; 40: 417-424 [DOI] [PubMed] [Google Scholar]
- 13). Wątorek E, Paprocka M, Duś D, Kopeć W, Klinger M: Endostatin and vascular endothelial growth factor: potential regulators of endothelial progenitor cell number in chronic kidney disease. Pol Arch Med Wewn, 2011; 121: 296-301 [PubMed] [Google Scholar]
- 14). Chen J, Hamm LL, Kleinpeter MA, Husserl F, Khan IE, Chen CS, Liu Y, Mills KT, He C, Rifai N, Simon EE, He J: Elevated plasma levels of endostatin are associated with chronic kidney disease. Am J Nephrol, 2012; 35: 335-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Charytan DM, Padera R, Helfand AM, Zeisberg M, Xu X, Liu X, Himmelfarb J, Cinelli A, Kalluri R, Zeisberg EM: Increased concentration of circulating angiogenesis and nitric oxide inhibitors induces endothelial to mesenchymal transition and myocardial fibrosis in patients with chronic kidney disease. Int J Cardiol, 2014; 176: 99-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Kanbay M, Afsar B, Siriopol D, Unal HU, Karaman M, Saglam M, Gezer M, Taş A, Eyileten T, Guler AK, Aydin İ, Oguz Y, Tarim K, Covic A, Yilmaz MI: Endostatin in chronic kidney disease: Associations with inflammation, vascular abnormalities, cardiovascular events and survival. Eur J Intern Med, 2016; 33: 81-87 [DOI] [PubMed] [Google Scholar]
- 17). Carlsson AC, Ruge T, Sundström J, Ingelsson E, Larsson A, Lind L, Arnlöv J: Association between circulating endostatin, hypertension duration, and hypertensive target-organ damage. Hypertension, 2013; 62: 1146-1151 [DOI] [PubMed] [Google Scholar]
- 18). Ärnlöv J, Ruge T, Ingelsson E, Larsson A, Sundström J, Lind L: Serum endostatin and risk of mortality in the elderly: findings from 2 community-based cohorts. Arterioscler Thromb Vasc Biol, 2013; 33: 2689-2695 [DOI] [PubMed] [Google Scholar]
- 19). Carlsson AC, Östgren CJ, Länne T, Larsson A, Nystrom FH, Ärnlöv J: The association between endostatin and kidney disease and mortality in patients with type 2 diabetes. Diabetes Metab, 2016; 42: 351-357 [DOI] [PubMed] [Google Scholar]
- 20). Zhang L, Zhao F, Yang Y, Qi L, Zhang B, Wang F, Wang S, Liu L, Wang H: Association between carotid artery intima-media thickness and early-stage CKD in a Chinese population. Am J Kidney Dis, 2007; 49: 786-792 [DOI] [PubMed] [Google Scholar]
- 21). Shimizu M, Furusyo N, Mitsumoto F, Takayama K, Ura K, Hiramine S, Ikezaki H, Ihara T, Mukae H, Ogawa E, Toyoda K, Kainuma M, Murata M, Hayashi J: Subclinical carotid atherosclerosis and triglycerides predict the incidence of chronic kidney disease in the Japanese general population: Results from the Kyushu and Okinawa Population Study (KOPS). Atherosclerosis, 2015; 238: 207-212 [DOI] [PubMed] [Google Scholar]
- 22). Kato Y, Furusyo N, Tanaka Y, Ueyama T, Yamasaki S, Murata M, Hayashi J: The relation between serum endostatin level and carotid atherosclerosis in healthy residents of Japan: Results from the Kyushu and Okinawa Population Study (KOPS). J Atheroscler Thromb, 2017; 24: 1023-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Furusyo N, Koga T, Ai M, Otokozawa S, Kohzuma T, Ikezaki H, Schaefer EJ, Hayashi J: Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS). Diabetologia, 2011; 54: 3028-3036 [DOI] [PubMed] [Google Scholar]
- 24). Furusyo N, Koga T, Ai M, Otokozawa S, Kohzuma T, Ikezaki H, Schaefer EJ, Hayashi J: Plasma glycated albumin level and atherosclerosis: results from the Kyushu and Okinawa Population Study (KOPS). Int J Cardiol, 2013; 167: 2066-2072 [DOI] [PubMed] [Google Scholar]
- 25). Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A: Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis, 2009; 53: 982-992 [DOI] [PubMed] [Google Scholar]
- 26). Yanase T, Nasu S, Mukuta Y, Shimizu Y, Nishihara T, Okabe T, Nomura M, Inoguchi T, Nawata H: Evaluation of a new carotid intima-media thickness measurement by B-mode ultrasonography using an innovative measurement software, intimascope. Am J Hypertens, 2006; 19: 1206-1212 [DOI] [PubMed] [Google Scholar]
- 27). Baron RM, Kenny DA: The moderator-mediator variable distinction in social psychological research:conceptual, strategic, and statistical considerations. J Pers Soc Psychol, 1986; 51: 1173-1182 [DOI] [PubMed] [Google Scholar]
- 28). Qin G, Luo L, Lv L, Xiao Y, Tu J, Tao L, Wu J, Tang X, Pan W: Decision tree analysis of traditional risk factors of carotid atherosclerosis and a cutpoint-based prevention strategy. PLoS One, 2014; 9: e111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Lin CH, Chen J, Ziman B, Marshall S, Maizel J, Goligorsky MS: Endostatin and kidney fibrosis in aging: a case for antagonistic pleiotropy? Am J Physiol Heart Circ Physiol, 2014; 306: 1692-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Shimizu M, Furusyo N, Tanaka Y, Kato Y, Mitsumoto-Kaseida F, Takayama K, Ura K, Hiramine S, Hayashi T, Ikezaki H, Ihara T, Mukae H, Ogawa E, Toyoda K, Kainuma M, Murata M, Hayashi J: The relation of postprandial plasma glucose and serum endostatin to the urinary albumin excretion of residents with prediabetes: results from the Kyushu and Okinawa Population Study (KOPS). Int Urol Nephrol, 2016; 48: 851-857 [DOI] [PubMed] [Google Scholar]
- 31). Yang XX, Hu ZP, Chan E, Duan W, Zhou S: Pharmacokinetics of recombinant human endostatin in rats. Curr Drug Metab, 2006; 7: 565-576 [DOI] [PubMed] [Google Scholar]


