Abstract
Aim: Fibroblast growth factor-21 (FGF-21) is a metabolic regulator with beneficial effects on glucolipid metabolism. Since FGF-21 has lipid-lowering, anti-inflammatory and anti-oxidant properties, it may play a protective role against atherosclerosis. However, blood FGF-21 levels in coronary artery disease (CAD) or peripheral artery disease (PAD) have not been elucidated.
Methods: We measured plasma FGF-21 levels in 417 patients undergoing coronary angiography, who also had ankle-brachial index test for PAD screening.
Results: CAD was found in 224 patients (1-vessel [1-VD], n = 92; 2-vessel [2-VD], n = 65; 3-vessel disease [3-VD], n = 67). No significant difference was found in the FGF-21 levels between 224 patients with CAD and 193 without CAD (median 26.0 vs. 25.9 pg/mL). FGF-21 levels in 4 groups of CAD(−), 1-VD, 2-VD, and 3-VD were 25.9, 37.2, 19.4, and 0.0 pg/mL. FGF-21 tended to be highest in 1-VD and lowest in 3-VD, but the difference did not reach statistical significance. PAD was found in 38 patients. Compared to the 379 patients without PAD, 38 with PAD had CAD more often (87% vs. 50%), especially 3-VD (P < 0.001). FGF-21 levels were lower in patients with PAD than in those without PAD (0.0 vs. 30.7 pg/mL, P < 0.02). In multivariate analysis, the FGF-21 level was an independent factor for PAD, but not for CAD. Odds ratio for PAD was 2.13 (95%CI= 1.01–4.49) for a low FGF-21 level (< 15.6 pg/mL).
Conclusion: No significant difference was found in the FGF-21 levels between patients with and without CAD. However, FGF-21 levels were low in patients with PAD, and were a factor for PAD independent of atherosclerotic risk factors.
Keywords: Coronary artery disease, FGF-21, Peripheral artery disease
Introduction
Fibroblast growth factor (FGF)-21 has been recognized as a metabolic regulator with multiple beneficial effects on glucose and lipid metabolism and on insulin sensitivity1, 2). FGF-21 is secreted from the liver, adipose tissues, and skeletal muscle2). Transgenic mice overexpressing FGF-21 were shown to be resistant to diet-induced obesity1), and the administration of FGF-21 reduced blood glucose and triglyceride levels in animal models1, 3). However, in humans, the blood levels of FGF-21 were reported to be high in patients with obesity, metabolic syndrome, and type 2 diabetes mellitus (DM)4–6). It is hypothesized that this paradoxical increase of FGF-21 may reflect a compensatory response4, 7).
Recently, FGF-21 was suggested to play a protective role in atherosclerosis8). FGF-21 was demonstrated to have lipid-lowering, anti-inflammatory and anti-oxidant properties1, 8–11). In apolipoprotein E-deficient mice, FGF-21 deficiency caused marked atherosclerotic plaque formation9), and the administration of FGF-21 ameliorated plaque formation12). With regard to the blood FGF-21 levels in patients with atherosclerotic diseases, Chow et al.13) investigated serum FGF-21 levels in 670 subjects in whom carotid intima-media thickness (IMT) was measured. They reported that FGF-21 levels positively correlated with carotid IMT in women but not in men. An et al.14) also measured serum FGF-21 levels in 213 subjects undergoing carotid ultrasound and reported that FGF-21 levels were higher in subjects with carotid plaques than in those without carotid plaques. Increased level of FGF-21 in patients with carotid atherosclerosis is thus considered to be a protective response to the development of atherosclerosis. However, blood FGF-21 levels in patients with coronary artery disease (CAD) remain controversial. Two studies15, 16) reported that serum FGF-21 levels were higher in patients with CAD than in those without CAD, whereas another study17) reported that FGF-21 levels were not high in patients with CAD. Moreover, FGF-21 levels in patients with peripheral artery disease (PAD) have not been elucidated. Thus, our cross-sectional study was performed to elucidate the associations between plasma FGF-21 levels and CAD and PAD in 417 patients undergoing coronary angiography, who also had an ankle-brachial index (ABI) test to screen for PAD.
Methods
Study Patients
We investigated plasma FGF-21 levels in 417 consecutive patients undergoing elective coronary angiography for suspected CAD at Tokyo Medical Center, and who also had an ABI test to screen for PAD from July 2008 to April 2015. The ABI was measured in a supine position after 5 minutes of rest using the VaSera VS-1000 instrument (Fukuda Denshi, Tokyo), and PAD was defined as an ABI of < 0.918). The severity of PAD was classified from stage I to stage IV according to the Fontaine's classification. Any of the patients with a history of percutaneous coronary intervention or cardiac surgery were excluded. Patients with acute myocardial infarction (AMI) were also excluded, because FGF-21 levels were reported to be elevated after AMI19). Hypertension was defined as blood pressures of ≥ 140/90 mmHg or on drugs. Among the 417 patients, hypertension was present in 301 (72%) patients, and 253 (61%) patients were taking anti-hypertensive drugs. Hyperlipidemia was defined as a LDL-cholesterol level of > 140 mg/dl or on drugs. Hyperlipidemia was present in 206 (49%) patients, and 147 (35%) patients were taking statins. DM (fasting plasma glucose level of ≥ 126 mg/dl or on treatment) was present in 103 (25%) patients, and 76 (18%) patients were taking anti-diabetic drugs or were undergoing insulin treatment. Current smoking (≥ 1 cigarette/day) was found in 117 (28%) patients. Our study was approved by the ethics committee of our hospital (R07-054/R15-056) in June 2008. After written informed consent was obtained from the study patients, overnight-fasting blood samples were taken on the morning of the day when coronary angiography was performed.
Measurement of Plasma FGF-21 Levels
Blood samples were collected in EDTA-containing tubes. The plasma was stored at −80°C. Plasma FGF-21 levels were measured by an enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (Human Fibroblast Growth Factor-21 ELISA Kit; Phoenix Pharmaceuticals, Inc., Belmont, USA) at Ochanomizu University, in accordance with the manufacturer's instructions. The minimum level detected by this assay was 15.6 pg/mL, and the level below the limit of detection (< 15.6 pg/mL) was described as 0.0 pg/mL. The range of values detected by this assay was 15.6 to 1000 pg/mL. The intra-assay and inter-assay coefficients of variation were < 10% and < 15%, respectively.
Coronary Angiography
Coronary angiograms were recorded on a cineangiogram system (Philips Electronics Japan, Tokyo, Japan). CAD was defined as at least one coronary artery having < 50% luminal diameter stenosis. The severity of coronary atherosclerosis was represented as the number of > 50% stenotic vessels, the number of > 50% stenotic segments, and the severity score of stenosis. The degree of coronary artery stenosis in each segment was scored from 0 to 4 points (0, ≤ 25%; 1, 26–50%; 2, 51–75%; 3, 76–90%; 4, < 90% stenosis), and the severity score of stenosis was defined as the sum of the scores of all segments. Coronary artery segments were defined as 29 segments according to the Coronary Artery Surgery Study classification. All angiograms were evaluated by a single cardiologist (Y.M.), blinded to clinical and laboratory data.
Statistical Analysis
Differences between 2 groups were evaluated by the unpaired t-test for parametric variables, the Mann-Whitney U test for nonparametric variables, and the chi-squared test for categorical variables. Differences among ≥ 3 groups were evaluated by ANOVA with Scheffe's test for parametric variables, by Kruskal-Wallis test for nonparametric variables, and by chi-squared test for categorical variables. Correlations between FGF-21 levels and the severity of coronary atherosclerosis were evaluated using Spearman's rank correlation test. A multiple logistic regression analysis was used to determine independent factors associated with PAD or CAD, including FGF-21 and conventional risk factors. A P value of < 0.05 was considered statistically significant. Results are presented as the mean ± SD or the median value.
Results
Among the 417 study patients, CAD was found in 224 patients (1-vessel disease [1-VD], n = 92; 2-vessel disease [2-VD], n = 65; and 3-vessel disease [3-VD], n = 67). In comparison to the 193 patients without CAD, 224 patients with CAD were older and had a male predominance and higher prevalence of hypertension, DM and hyperlipidemia (Table 1). However, no significant difference was found in plasma FGF-21 levels between patients with and without CAD (median 26.0 pg/mL vs. 25.9 pg/mL, P = 0.38). FGF-21 levels in the 4 groups of CAD(−), 1-VD, 2-VD, and 3-VD were median 25.9, 37.2, 19.4, and 0.0 pg/mL, respectively. Among these 4 groups, FGF-21 levels tended to be highest in patients with 1-VD and lowest in those with 3-VD; however, these differences did not reach statistical significance (Fig. 1). Spearman's rank correlation test revealed no significant correlations between FGF-21 levels and the number of > 50% stenotic coronary segments (r = 0.08, P = 0.11) or the severity score of stenosis (r = 0.09, P = 0.08).
Table 1. Clinical characteristics and plasma FGF-21 levels of patients with and without CAD.
| CAD(−) | P value | CAD | 1-VD | 2-VD | 3-VD | P value | |
|---|---|---|---|---|---|---|---|
| (n = 193) | CAD(−) vs. CAD | (n = 224) | (n = 92) | (n = 65) | (n = 67) | Among 4 groups | |
| Age (years) | 66 ± 11 | < 0.01 | 70 ± 10 | 69 ± 10 | 69 ± 10 | 73 ± 7 | < 0.01 |
| Gender (male) | 120 (62%) | < 0.01 | 171 (76%) | 70 (76%) | 46 (71%) | 55 (82%) | 0.02 |
| BMI (kg/m2) | 23.6 ± 4.5 | 0.06 | 23.7 ± 3.3 | 23.9 ± 3.3 | 23.7 ± 3.2 | 23.3 ± 3.3 | 0.85 |
| Hypertension | 126 (65%) | < 0.01 | 175 (78%) | 71 (77%) | 49 (75%) | 55 (82%) | 0.03 |
| SBP (mmHg) | 131 ± 21 | 0.88 | 131 ± 23 | 129 ± 23 | 137 ± 21 | 128 ± 23 | 0.07 |
| Diabetes mellitus | 26 (13%) | < 0.01 | 77 (34%) | 27 (29%) | 24 (37%) | 26 (39%) | < 0.01 |
| Smoking | 49 (25%) | 0.20 | 68 (30%) | 32 (35%) | 17 (26%) | 19 (28%) | 0.50 |
| Hyperlipidemia | 74 (38%) | < 0.01 | 132 (59%) | 52 (57%) | 40 (62%) | 40 (60%) | < 0.01 |
| Statin | 46 (24%) | < 0.01 | 101 (45%) | 40 (43%) | 28 (43%) | 33 (49%) | < 0.01 |
| LDL-C (mg/dL) | 111 ± 29 | 0.18 | 115 ± 32 | 112 ± 33 | 118 ± 32 | 115 ± 30 | 0.33 |
| HDL-C (mg/dL) | 58 ± 15 | < 0.01 | 51 ± 13 | 53 ± 14 | 49 ± 11 | 49 ± 12 | < 0.01 |
| FGF-21 level (pg/mL) | 25.9 [0.0, 105.3] | 0.38 | 26.0 [0.0, 86.1] | 37.2 [0.0, 88.6] | 19.4 [0.0, 77.6] | 0.0 [0.0, 63.4] | 0.21 |
| FGF-21 < 15.6 pg/mL | 81 (42%) | 0.50 | 104 (46%) | 36 (39%) | 32 (49%) | 36 (54%) | 0.30 |
| PAD (ABI < 0.9) | 5 (3%) | < 0.01 | 33 (15%) | 9 (10%) | 6 (9%) | 18 (27%) | < 0.01 |
| Antiplatelet drugs | 62 (32%) | < 0.01 | 134 (60%) | 50 (54%) | 46 (71%) | 38 (57%) | < 0.01 |
Data represent the mean ± SD or the number (%) of patients, with the exception of FGF-21 level which is presented as the median value and interquartile range. BMI indicates body mass index; SBP, systolic blood pressure; LDL-C, low-density lipoprotein cholesterol; and HDL-C, high-density lipoprotein cholesterol.
Fig. 1.
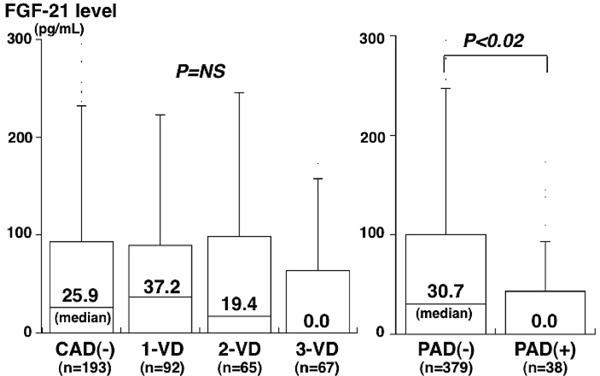
Plasma FGF-21 levels and the number of stenotic coronary vessels or the presence of PAD
FGF-21 levels tended to be highest in 1-VD and lowest in 3-VD, but there was no significant difference among the 4 groups (P = NS) (left). However, FGF-21 levels in PAD were significantly lower than those in PAD(−) (P < 0.02) (right). The central line represents the median, and the boxes span from the 25th to 75th percentiles. 1-VD, 1-vessel disease; 2-VD, 2-vessel disease; 3-VD, 3-vessel disease; PAD, peripheral artery disease.
The 417 study patients were divided into 2 groups according to the presence or absence of DM. However, between 103 patients with DM and 314 without DM, no significant difference was found in FGF-21 levels (median 27.4 pg/mL vs. 25.6 pg/mL, P = 0.82). Among the 314 patients without DM, FGF-21 levels did not differ between patients with and those without CAD (median 18.5 pg/mL vs. 29.6 pg/mL, P = 0.25). FGF-21 levels tended to be highest in patients with 1-VD and lowest in those with 3-VD; however, there were no significant differences among CAD(−), 1-VD, 2-VD, and 3-VD groups (median 29.6, 36.3, 0.0, and 0.0 pg/mL, respectively). Moreover, among the 103 patients with DM, FGF-21 levels did not differ between patients with and without CAD (median 29.8 pg/mL vs. 18.8 pg/mL, P = 0.96) or among CAD(−), 1-VD, 2-VD and 3-VD groups (median 18.8, 38.2, 31.3, and 6.7 pg/mL).
Among the 417 study patients, PAD (ABI < 0.9) was found in 38 (9%) patients (5 out of the 193 [3%] patients without CAD and 33 out of the 224 [15%] with CAD). Compared with 379 patients without PAD, 38 patients with PAD were older and had a higher prevalence of hypertension and smoking (Table 2). Notably, FGF-21 levels in patients with PAD were significantly lower than those in patients without PAD (median 0.0 pg/mL vs. 30.7 pg/mL, P = 0.02) (Fig. 1). FGF-21 levels in patients with PAD were more often below the limit of detection (< 15.6 pg/mL) than in those without PAD (63% vs. 43%, P = 0.03) (Table 2). Of the 38 patients with PAD, 22 and 16 patients were found to have stage I and II PAD, respectively. FGF-21 levels in patients with stage II PAD tended to be lower (median 0.0 pg/mL vs. 6.4 pg/mL) and to be more often < 15.6 pg/mL (69% vs. 59%) than in those with stage I PAD. However, these differences did not reach statistical significance. Notably, patients with PAD more often had CAD (87% vs. 50%), especially 3-VD (47% vs. 13%), than those without PAD (P < 0.01) (Table 2). FGF-21 levels in PAD patients with CAD(−), 1-VD, 2-VD, and 3-VD were median 12.8, 18.5, 0.0, and 0.0 pg/mL, respectively.
Table 2. Clinical characteristics and plasma FGF-21 levels of patients with and without PAD.
| PAD(−) | PAD | P value | |
|---|---|---|---|
| (n = 379) | (n = 38) | ||
| Age (years) | 67 ± 10 | 75 ± 6 | < 0.01 |
| Gender (male) | 263 (69%) | 28 (74%) | 0.80 |
| BMI (kg/m2) | 23.7 ± 4.0 | 22.7 ± 3.2 | 0.12 |
| Hypertension | 267 (70%) | 34 (89%) | 0.03 |
| Systolic blood pressure (mmHg) | 131 ± 20 | 131 ± 30 | 0.92 |
| Diabetes mellitus | 89 (23%) | 14 (37%) | 0.70 |
| Smoking | 103 (27%) | 14 (37%) | 0.30 |
| Hyperlipidemia | 182 (48%) | 24 (63%) | 0.70 |
| Statin | 125 (33%) | 22 (58%) | < 0.01 |
| LDL-cholesterol (mg/dL) | 113 ± 31 | 113 ± 29 | 0.92 |
| HDL-cholesterol (mg/dL) | 55 ± 14 | 49 ± 11 | 0.01 |
| FGF-21 level (pg/mL) | 30.7 [0.0, 100.5] | 0.0 [0.0, 39.4] | 0.02 |
| FGF-21 < 15.6 pg/mL | 163 (43%) | 24 (63%) | 0.03 |
| CAD | 191 (50%) | 33 (87%) | < 0.01 |
| 1-VD | 83 (22%) | 9 (24%) | 0.95 |
| 2-VD | 59 (16%) | 6 (6%) | 0.90 |
| 3-VD | 49 (13%) | 18 (47%) | < 0.01 |
| Antiplatelet drugs | 171 (45%) | 25 (66%) | 0.03 |
Data represent the mean ± SD or the number (%) of patients, with the exception of FGF-21 level which is presented as the median value and interquartile range.
Between patients with and without PAD, there was no significant difference in the prevalence of DM (37% vs. 23%). Among the 314 patients without DM, FGF-21 levels tended to be lower in patients with PAD than in those without PAD (median 0.0 pg/mL vs. 29.4 pg/mL). Among the 103 patients with DM, FGF-21 levels also tended to be lower in patients with PAD than in those without PAD (median 0.0 pg/mL vs. 31.3 pg/mL).
To elucidate the independent associations between FGF-21 levels and CAD or PAD, variables (age, gender, hypertension, hyperlipidemia, statin use, DM, smoking, HDL-cholesterol, and FGF-21 levels) were entered into a multiple logistic regression model. The FGF-21 level was found to be a significant factor for PAD independent of atherosclerotic risk factors, but not for CAD or 3-VD (Table 3) (model 1). The odds ratio for PAD in patients with a low FGF-21 level (< 15.6 pg/mL) was 2.13 (95%CI = 1.01–4.49, P < 0.05). In the model including CAD as well as atherosclerotic risk factors and FGF-21 levels, FGF-21 level and CAD were independent factors associated with PAD (model 2). However, in the model including 3-VD as well as atherosclerotic risk factors and FGF-21 levels, 3-VD was a significant factor associated with PAD (model 3).
Table 3. Factors associated with PAD and CAD.
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| PAD | ||||||
| Age (per 10 yrs) | 2.33 (1.44–3.77) | < 0.01 | 2.27 (1.39–3.70) | < 0.01 | 2.33 (1.40–3.87) | < 0.01 |
| Gender (male) | --- | NS | --- | NS | --- | NS |
| Hypertension | --- | NS | --- | NS | --- | NS |
| Hyperlipidemia | --- | NS | --- | NS | --- | NS |
| Statin | 2.27 (1.10–4.66) | 0.03 | --- | NS | --- | NS |
| HDL-C (per 1 mg/dL) | 0.96 (0.93–0.99) | 0.02 | --- | NS | --- | NS |
| Smoking | --- | NS | --- | NS | --- | NS |
| Diabetes mellitus | --- | NS | --- | NS | --- | NS |
| Anti-platelet drugs | --- | NS | --- | NS | --- | NS |
| FGF-21(< 15.6 pg/mL) | 2.10 (1.01–4.40) | 0.04 | 2.15 (1.02–4.49) | 0.04 | --- | NS |
| CAD | 5.23 (1.96–13.9) | < 0.01 | ||||
| 3-vessel disease | 4.86 (2.34–10.1) | < 0.01 | ||||
| CAD | ||||||
| Age (per 10 yrs) | 1.50 (1.20–1.88) | < 0.01 | 1.43 (1.14–1.79) | < 0.01 | ||
| Gender (male) | 2.22 (1.30–3.79) | < 0.01 | 2.21 (1.29–3.80) | < 0.01 | ||
| Hypertension | --- | NS | --- | NS | ||
| Hyperlipidemia | 2.53 (1.57–4.09) | < 0.01 | 2.48 (1.53–4.01) | < 0.01 | ||
| Statin | --- | NS | --- | NS | ||
| HDL-C (per 1 mg/dL) | 0.97 (0.95–0.99) | < 0.01 | 0.97 (0.95–0.99) | < 0.01 | ||
| Smoking | --- | NS | --- | NS | ||
| Diabetes mellitus | 2.14 (1.23–3.69) | < 0.01 | 2.13 (1.23–3.69) | < 0.01 | ||
| Anti-platelet drugs | 2.58 (1.64–4.05) | < 0.01 | 2.51 (1.59–3.96) | < 0.01 | ||
| FGF-21(< 15.6 pg/mL) | --- | NS | --- | NS | ||
| PAD | 3.37 (1.20–9.45) | 0.02 | ||||
| 3-vessel disease | ||||||
| Age (per 10 yrs) | 1.93 (1.39–2.70) | < 0.01 | 1.82 (1.29–2.56) | < 0.01 | ||
| Gender (male) | 2.54 (1.21–5.31) | 0.01 | 2.23 (1.07–4.64) | 0.03 | ||
| Hypertension | --- | NS | --- | NS | ||
| Hyperlipidemia | --- | NS | --- | NS | ||
| Statin | 1.94 (1.09–3.45) | 0.02 | --- | NS | ||
| HDL-C (per 1 mg/dL) | 0.97 (0.95–0.99) | 0.02 | 0.97 (0.95–0.99) | 0.02 | ||
| Smoking | --- | NS | --- | NS | ||
| Diabetes mellitus | --- | NS | --- | NS | ||
| Anti-platelet drugs | --- | NS | --- | NS | ||
| FGF-21(< 15.6 pg/mL) | --- | NS | --- | NS | ||
| PAD | 4.14 (1.96–8.74) | < 0.01 | ||||
OR indicates odds ratio; 95%CI, 95% confidence interval.
Discussion
In the present study, there was no significant difference in plasma FGF-21 levels between patients with and without CAD. No significant correlation was found between FGF-21 levels and the severity of coronary atherosclerosis, defined as the numbers of stenotic vessels and segments. However, the present study showed that FGF-21 levels were lower in patients with PAD than in those without PAD and that they were a significant factor associated with PAD independent of atherosclerotic risk factors.
FGF-21 is recognized as a metabolic regulator with beneficial effects on glucolipid metabolism and insulin sensitivity1, 2). Blood FGF-21 levels were reported to be high in patients with obesity, metabolic syndrome and DM4–6). This paradoxical increase in FGF-21 levels has been suggested to be a compensatory response4, 7). Because FGF-21 has lipid-lowering, anti-inflammatory and anti-oxidant properties1, 8–11), FGF-21 is considered to play a protective role against atherosclerosis8). In apoE-deficient mice, FGF-21 deficiency caused marked atherosclerosis9), whereas FGF-21 administration ameliorated atherosclerosis12). However, blood FGF-21 levels in CAD patients remain controversial. Lin et al.15) reported that serum FGF-21 levels in 135 patients with CAD were higher than those in 61 controls; however, the study included patients with AMI. In patients with AMI, FGF-21 levels are shown to be elevated and to remain high for 7 days after AMI19). Kim et al.20) measured serum FGF-21 levels in 120 patients undergoing coronary angiography (30 CAD[−] DM[−], 30 CAD[+] DM[−], 30 CAD[−] DM[+] and 30 CAD[+] DM[+] patients). They excluded AMI patients and reported that FGF-21 levels were higher in patients with CAD than in those without CAD, but only among DM(−) patients. In contrast, Lee et al.17) investigated serum FGF-21 levels in 189 patients undergoing coronary computed tomography. They showed that FGF-21 levels in 60 patients with CAD were not higher than those in 129 patients without CAD. Our study investigated plasma FGF-21 levels in 417 patients undergoing elective coronary angiography. AMI patients were excluded. However, FGF-21 levels in 224 patients with CAD were not higher than those in 193 patients without CAD and were not a significant factor for CAD, regardless of the presence or absence of DM.
Recently, Li et al.21) investigated the association between FGF-21 levels and mortality in 1668 patients with CAD. They showed that both the highest and lowest FGF-21 levels were associated with an increased risk of cardiovascular mortality, suggesting a U-shaped association between FGF-21 levels and mortality. Moreover, Jung et al.22) recently reported the U-shaped association between FGF-21 levels and diabetic retinopathy in 227 patients with DM. Both highest and lowest FGF-21 levels were associated with diabetic retinopathy, but not with nephropathy or neuropathy. They suggested that a very low FGF-21 level itself may be associated with diabetic retinopathy and also a relatively elevated FGF-21 level may be a compensatory increase to protect against microvascular complication. In our study, FGF-21 levels in CAD(−), 1-VD, 2-VD, and 3-VD groups were 25.9, 37.2, 19.4, and 0.0 pg/mL, respectively, and tended to be highest in patients with 1-VD and lowest in those with 3-VD, suggesting an inverted U-shaped association. Because FGF-21 levels were shown to be high in subjects with carotid plaques14), patients with mild CAD, such as 1-VD, may have high FGF-21 levels, which reflect a protective response to CAD progression. In contrast, patients with severe CAD, such as 3-VD, may have low FGF-21 levels, thereby leading to the development of severe CAD. However, to elucidate the role of FGF-21 in CAD progression, further studies are needed to show the association between FGF-21 levels and the progression of CAD in a prospective manner.
Atherosclerosis is a progressive disease that affects multiple vascular beds, and patients with CAD are known to be often complicated by PAD18, 23). In our study, PAD was found in 5 of the 193 patients without CAD (3%) and 33 of the 224 with CAD (15%). This prevalence of PAD was similar to that reported by Lee et al.18) (5 of 119 without CAD (4%) and 385 of 2424 with CAD (16%) among 2543 patients undergoing coronary angiography). Regarding blood FGF-21 levels in patients with PAD, only one study investigated the association between FGF-21 levels and PAD in 504 Chinese patients with type 2 DM24). They reported that FGF-21 levels were higher in female patients with PAD than in those without PAD, but FGF-21 levels tended to be lower in male patients with PAD than in those without PAD. Because patients with PAD were reported to have skeletal muscle mitochondrial dysfunction25) and because mitochondrial dysfunction enhances FGF-21 expression26), patients with PAD may have an increased FGF-21 expression associated with muscle mitochondrial dysfunction. However, our study demonstrated that plasma FGF-21 levels were significantly lower in patients with PAD than in those without PAD, and that FGF-21 levels in patients with PAD were often very low (below the limit of detection). The low FGF-21 level was found to be a significant factor for PAD, independent of atherosclerotic risk factors. Notably, patients with PAD often had CAD, especially 3-VD. In multivariate analysis, 3-VD was significantly associated with PAD. FGF-21 levels in PAD patients with CAD(−), 1-VD, 2-VD and 3-VD were 12.8, 18.5, 0.0 and 0.0 pg/mL, respectively. Our findings suggest that patients with PAD, especially those with 3-VD, have very low FGF-21 levels, which thereby lead to the development of severe atherosclerosis.
Our study has several limitations. First, angiography was used to evaluate coronary atherosclerosis. Angiography cannot visualize plaques and only shows lumen characteristics. Second, PAD was present in 5 out of 193 patients without CAD (3%) and in 33 out of 224 with CAD (15%). Although atherosclerotic plaques in peripheral arteries were reported to be common in patients without any symptoms of PAD, especially in those with CAD27), this prevalence of PAD was similar to that reported by Lee et al.18) (4% of patients without CAD and 16% of those with CAD). However, the small number of patients with PAD (n = 38) was one of the major limitations. Moreover, in our study, an ABI test was used to screen for PAD, and PAD was defined as an ABI of < 0.918). Angiography or computed tomography were not always performed to confirm the diagnosis of PAD. This is another of the study limitations. Third, our study was cross-sectional in nature, and it could not establish causality, since it only showed some associations and proposed some hypotheses. Finally, all four of the previous studies on FGF-21 levels in CAD patients were reported from China and Korea (2 studies from each country)15–17, 20). Since our study was performed in Japanese patients, the results cannot be applicable to other ethnic populations.
In conclusion, plasma FGF-21 levels were not different between patients with and without CAD and were not a significant factor for CAD. However, FGF-21 levels were found to be low in patients with PAD, especially in PAD patients with 3-VD, and to be a significant factor associated with PAD independent of atherosclerotic risk factors. Our results suggest that FGF-21 may play a protective role against the development of PAD.
Funding
This study was supported in part by a grant from Honjo International Scholarship Foundation. Financial funding was provided in part by Bayer Yakuhin Ltd., Daiichi Sankyo Co. and Pfizer Japan Inc.; however, these sponsors had no role in the design, analysis, or interpretation of the study.
Conflict of Interest
Our study has no conflict of interest to disclose.
References
- 1). Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB: FGF-21 as a novel metabolic regulator. J Clin Invest, 2005; 115: 1627-1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Li H, Zhang J, Jia W: Fibroblast growth factor 21: a novel metabolic regulator from pharmacology to physiology. Front Med, 2013; 7: 25-30 [DOI] [PubMed] [Google Scholar]
- 3). Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ: The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology, 2007; 148: 774-781 [DOI] [PubMed] [Google Scholar]
- 4). Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A: Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes, 2008; 57: 1246-1253 [DOI] [PubMed] [Google Scholar]
- 5). Chen C, Cheung BM, Tso AW, Wang Y, Law LS, Ong KL, Wat NM, Xu A, Lam KS: High plasma level of fibroblast growth factor 21 is an Independent predictor of type 2 diabetes: a 5.4-year population-based prospective study in Chinese subjects. Diabetes Care, 2011; 34: 2113-2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D, Matoulek M, Dostalova I, Humenanska V, Haluzik M: Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol, 2009; 71: 369-375 [DOI] [PubMed] [Google Scholar]
- 7). Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E: Obesity Is a Fibroundergoing blast Growth Factor 21-Resistant State. Diabetes, 2010; 59: 2781-2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Kokkinos J, Tang S, Rye KA, Ong KL: The role of fibroblast growth factor 21 in atherosclerosis. Atherosclerosis, 2017; 257: 259-265 [DOI] [PubMed] [Google Scholar]
- 9). Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, Jin L, Lian Q, Huang Y, Ding H, Triggle C, Wang K, Li X, Xu A: Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation, 2015; 131: 1861-1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Yu Y, He J, Li S, Song L, Guo X, Yao W, Zou D, Gao X, Liu Y, Bai F, Ren G, Li D: Fibroblast growth factor 21 inhibits macrophage-mediated inflammation by activating Nrf2 and suppressing NF-kB signaling pathway. Int Immunopharmacol, 2016; 38: 144-152 [DOI] [PubMed] [Google Scholar]
- 11). Wang XM, Song SS, Xiao H, Gao P, Li XJ, Si LY: Fibroblast growth factor 21 protects against high glucose induced cellular damage and dysfunction of endothelial nitric-oxide synthase in endothelial cells. Cell Physiol Biochem, 2014; 34: 658-671 [DOI] [PubMed] [Google Scholar]
- 12). Wu X, Qi YF, Chang JR, Lu WW, Zhang JS, Wang SP, Cheng SJ, Zhang M, Fan Q, Lv Y, Zhu H, Xin MK, Lv Y, Liu JH: Possible role of fibroblast growth factor 21 on atherosclerosis via amelioration of endoplasmic reticulum stress-mediated apoptosis in apoE(-/-) mice. Heart Vessels, 2015; 30: 657-668 [DOI] [PubMed] [Google Scholar]
- 13). Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH, Tse HF, Chau MT, Cheung BM, Lam KS: Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler Thromb Vasc Biol, 2013; 33: 2454-2459 [DOI] [PubMed] [Google Scholar]
- 14). An SY, Lee MS, Yi SA, Ha ES, Han SJ, Kim HJ, Kim DJ, Lee KW: Serum fibroblast growth factor 21 was elevated in subjects with type 2 diabetes mellitus and was associated with the presence of carotid artery plaques. Diabetes Res Clin Pract, 2012; 96: 196-203 [DOI] [PubMed] [Google Scholar]
- 15). Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S, Xiao J, Wang X, Feng W, Li X: Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS One, 2010; 5: e15534- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Shen Y, Ma X, Zhou J, Pan X, Hao Y, Zhou M, Lu Z, Gao M, Bao Y, Jia W: Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovasc Diabetol, 2013; 12: 124- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Lee Y, Lim S, Hong ES, Kim JH, Moon MK, Chun EJ, Choi SI, Kim YB, Park YJ, Park KS, Jang HC, Choi SH: Serum FGF21 concentration is associated with hypertriglyceridaemia, hyperinsulinaemia and pericardial fat accumulation, independently of obesity but not with current coronary artery status. Clin Endocrinol, 2014; 80: 57-64 [DOI] [PubMed] [Google Scholar]
- 18). Lee JY, Lee SW, Lee WS, Han S, Park YK, Kwon CH, Jang JY, Cho YR, Park GM, Ahn JM, Kim WJ, Park DW, Kang SJ, Kim YH, Lee CW, Park SW, Park SJ: Prevalence and clinical implications of newly revealed, asymptomatic abnormal ankle-brachial index in patients with significant coronary artery disease. JACC Cardiovasc Interv, 2013; 6: 1303-1313 [DOI] [PubMed] [Google Scholar]
- 19). Zhang W, Chu S, Ding W, Wang F: Serum level of fibroblast growth factor 21 is independently associated with acute myocardial infarction. PLoS One, 2015; 10: e0129791- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Kim WJ, Kim SS, Lee HC, Song SH, Bae MJ, Yi YS, Jeon YK, Kim BH, Kim YK, Kim IJ: Association between serum fibroblast growth factor 21 and coronary artery disease in patients with type 2 diabetes. J Korean Med Sci, 2015; 30: 586-590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Li Q, Zhang Y, Ding D, Yang Y, Chen Q, Su D, Chen X, Yang W, Qiu J, Ling W: Association between serum fibroblast growth factor 21 and mortality among patients with coronary artery disease. J Clin Endocrinol Metab, 2016; 101: 4886-4894 [DOI] [PubMed] [Google Scholar]
- 22). Jung CH, Jung SH, Kim BY, Kim CH, Kang SK, Mok JO: The U-shaped relationship between fibroblast growth factor 21 and microvascular complication in type 2 diabetes mellitus. J Diabetes Complications, 2017; 31: 134-140 [DOI] [PubMed] [Google Scholar]
- 23). Zheng ZJ, Sharrett AR, Chambless LE, Rosamond WD, Nieto FJ, Sheps DS, Dobs A, Evans GW, Heiss G: Associations of ankle-brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis, 1997; 131: 115-125 [DOI] [PubMed] [Google Scholar]
- 24). Zhang X, Hu Y, Zeng H, Li L, Zhao J, Zhao J, Liu F, Bao Y, Jia W: Serum fibroblast growth factor 21 levels is associated with lower extremity atherosclerotic disease in Chinese female diabetic patients. Cardiovasc Diabetol, 2015; 14: 32- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Rontoyanni VG, Nunez Lopez O, Fankhauser GT, Cheema ZF, Rasmussen BB, Porter C: Mitochondrial bioenergetics in the metabolic myopathy accompanying peripheral artery disease. Front Physiol, 2017; 8: 141- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Ribas R, Villarroya J, Hondares E, Giralt M, Villarroya F: FGF21 expression and release in muscle cells: involvement of MyoD and regulation by mitochondria-driven signalling. Biochem J, 2014; 463: 191-199 [DOI] [PubMed] [Google Scholar]
- 27). Nakamura E, Sato Y, Iwakiri T, Yamashita A, Moriguchi-Goto S, Maekawa K, Gi T, Asada Y: Asymptomatic plaques of lower peripheral arteries and their association with cardiovascular disease: an autopsy study. J Atheroscler Thromb, 2017; 24: 921-927 [DOI] [PMC free article] [PubMed] [Google Scholar]


