Abstract
Sitosterolemia is a rare inherited disease characterized by increased levels of plant sterols, such as sitosterol. The cause of this disease is ATP-binding cassette (ABC) subfamily G member 5 or member 8 (ABCG5 or ABCG8, respectively) gene mutations. Recent advances in genetics have revealed that the prevalence of subjects with deleterious mutations in ABCG5 and/or ABCG8 genes could be more than 1 in ∼200,000 individuals among the general population. Furthermore, accumulated evidence, including infantile cases exhibiting progression/regression of systemic xanthomas associated with LDL cholesterol levels, have shown that the elevation of LDL cholesterol seems to be the major cause of development of atherosclerosis and not the elevation of sitosterol. Regarding therapies, LDL apheresis, as well as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, could be useful for sitosterolemia, in addition to ezetimibe and/or colestimide. In this study, we provide the current understanding and future perspectives of sitosterolemia, which is currently considered an extremely rare disorder but is expected to be much more prevalent in clinical settings.
Keywords: Sitosterolemia, ABCG5, ABCG8, Familial hypercholesterolemia
Introduction
Familial hypercholesterolemia (FH) is a common inherited disorder of plasma lipoprotein metabolism and is characterized by an elevated level of LDL cholesterol, tendon xanthomas, and premature coronary artery disease1). The monogenic causes of FH involve gene mutations such as LDL receptor, apolipoprotein B-100, and proprotein convertase subtilisin/kexin type 9 (PCSK9)2). On the other hand, sitosterolemia (OMIM #210250) is a rare, inherited, autosomal recessive disorder of lipid metabolism characterized by increased absorption and decreased biliary excretion of plant sterols and cholesterol, thus resulting in prominently elevated serum concentrations of plant sterols, such as sitosterol and campesterol3). Subjects suffering from sitosterolemia primarily present with tendinous and tuberous xanthomas and premature coronary atherosclerosis, such as FH4–7). LDL cholesterol levels are more variable in sitosterolemia than in other genetic hyperlipidemias but can be extremely elevated in some patients, particularly in infants; the reason for this phenomenon still remains unclear4). This disease is caused by mutations in either ATP-binding cassette (ABC) subfamily G member 5 or member 8 (ABCG5 and ABCG8, respectively). In this paper, we provide the current understanding and future perspectives of sitosterolemia, which is currently considered an extremely rare disorder but is expected to be much more prevalent in clinical settings.
Epidemiology
Sitosterolemia is now considered an extremely rare disorder. Approximately ∼100 patients have been reported to have sitosterolemia thus far. However, according to The Exome Aggregation Consortium (ExAC) exome browser, 1 in ∼220 individuals have loss of function (LOF) mutations in ABCG5 or ABCG8 genes8); therefore, the rough estimation of the number of patients with sitosterolemia is 1 in ∼200,000 individuals (Fig. 1). In addition to LOF mutations, many missense mutations have also been shown to cause this situation. Moreover, we have already experienced more than 10 Japanese cases thus far4–7). Accordingly, we firmly believe that we have overlooked many sitosterolemic cases because serum sitosterol measurement is not available in most clinics in the world, including Japan. Regarding this important point, Japan Atherosclerosis Society is now investigating the serum sitosterol levels in the general population and the prevalence of sitosterolemia. We anticipate that the measurements of serum sitosterol will be covered by health insurance in Japan in the near future, and such a situation will give us more chances to diagnose this disease accurately.
Fig. 1.
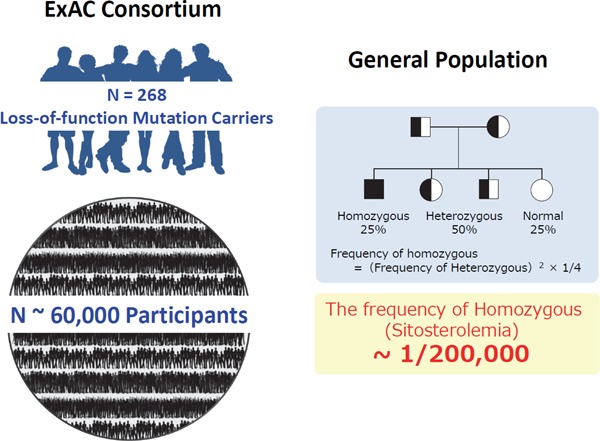
Assumption of prevalence of sitosterolemia
Left Panel: Among ∼60,000 individuals, 268 individuals have LOF mutation(s) in ABCG5 gene or ABCG8 gene.
Right panel: According to the frequency of LOF mutation(s) in ABCG5/8 genes, the frequency of sitosterolemia could be estimated around at least 1 in 200,000.
ABCG5 and ABCG8 Mutations
Most sitosterolemic cases in Asians have been shown to be caused by mutations in the ABCG5 gene, whereas those in Caucasian are believed to be caused by mutations in the ABCG8 gene9, 10). However, recent studies, including our previous study, have revealed the causative mutations in the ABCG8 gene from Asian sitosterolemic patients7). Accordingly, ABCG8 and ABCG5 gene mutations should be examined if sitosterolemia is suspected regardless of ethnicity. Furthermore, the ABCG5 and ABCG8 proteins form heterodimers and act in coordination as a complex11). We recently observed a sitosterolemia case caused by combinations of ABCG5 and ABCG8 gene mutations (data not shown). Moreover, we recently published a paper regarding the prevalence of oligogenic FH with deleterious mutations in FH genes and the ABCG5/8 genes12). We found that additional mutation(s) in the ABCG5/8 genes significantly affected LDL cholesterol level and coronary artery disease prevalence. These findings and a recent study revealed that ABCG5/8 genetic mutations significantly affected LDL cholesterol levels in the general population; this information supports the notion that there are many heterozygous carriers of this disease among patients with hypercholesterolemia13). The mutations that could cause sitosterolemia and polymorphisms are illustrated in Fig. 2.
Fig. 2.
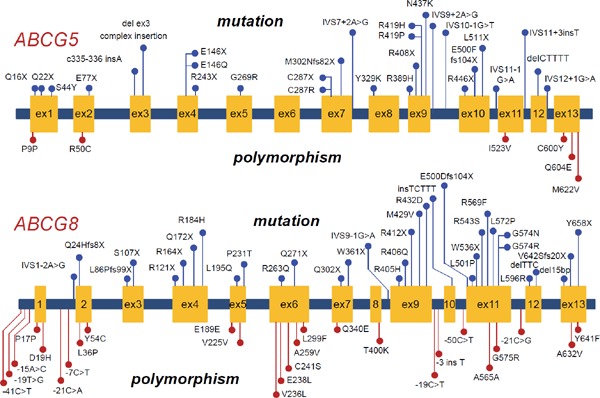
Mutations and polymorphisms in the ABCG5 and ABCG8 genes
Mutations are illustrated as blue. Polymorphisms are illustrated as red.
Upper panel: Mutations and polymorphisms in the ABCG5 gene.
Lower panel: Mutations and polymorphisms in the ABCG8 gene.
Clinical Manifestations
The variations in phenotypic severities of this disease have been reported. We previously described a case with sitosterolemia in a 26-year old lady exhibiting premature myocardial infarction7). Other studies have showed sitosterolemic cases without any apparent atherosclerosis14). Regarding this information, several infantile cases, including ours, have exhibited severe hyper LDL cholesterolemia during breastfeeding4, 10). Moreover, LDL cholesterol levels in adult sitosterolemic patients are more variable than that in other genetic hyperlipidemias15). These facts suggest that sitosterolemic patients are vulnerable to diet-induced hyperlipidemia. Thus, we speculated that LDL cholesterol associated with dietary habits significantly affects their clinical manifestations and conducted a single arm, nonrandomized, open-label, uncontrolled trial to determine if postprandial remnant-like particle (RLP) fractions were elevated in this disease16) We found that postprandial RLP lipoproteins were significantly impaired compared to heterozygous FH17). Accordingly, we believe that RLP and LDL cholesterol associated with their dietary habits are quite important for the prevention of atherosclerosis in this disease.
Heterozygous Mutation Carriers
Although sitosterolemia is now considered a “recessive” disorder, recent studies have revealed the fact that heterozygous mutation carriers also exhibit milder manifestations18, 19). We previously showed that a portion of hypercholesterolemia other than FH could be explained by a deleterious mutation in the ABCG8 gene20). We also showed that single deleterious mutations affected LDL cholesterol levels by 18 mg/dL among individuals exhibiting severe hyper LDL cholesterolemia12). Another group showed that a portion of hypercholesterolemia other than FH could be explained by a deleterious mutation in ABCG5 or ABCG8 gene14). These results collectively suggested that heterozygous mutation carriers in the ABCG5/8 genes could be one of the major genetic causes of hyper LDL cholesterolemia.
Pseudo Familial Hypercholesterolemia
Sitosterolemia has been described as “Pseudo FH,” particularly infant cases, on the basis of phenotype, such as hyper LDL cholesterolemia, as well as tendon xanthomas21) (Fig. 3). Previous studies have shown multiple infant cases of sitosterolemia with extreme hyper LDL cholesterolemia associated with breastfeeding4, 10). Interestingly, weaning can reduce their LDL cholesterol level substantially but will not affect their sitosterol level (Fig. 4). The detailed mechanism of this extreme situation is still unclear; however, the disturbance of postprandial remnant lipoprotein fractions seems to be one of the factors that contribute to this situation17).
Fig. 3.
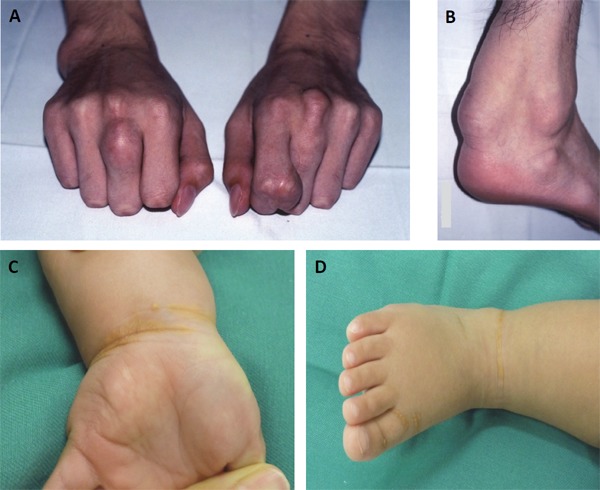
Physical xanthomas in sitosterolemia
Tendious xantomas of the case with sitosterolemia (16-year-old male, Ref. 6)
Achilles' tendon xanthomas with sitosterolemia (16-year-male, Ref. 6)
Xanthomas at the wrist (1-year-old female, Ref. 4)
Xanthomas at the ankle (1-year-old female, Ref. 4)
Fig. 4.
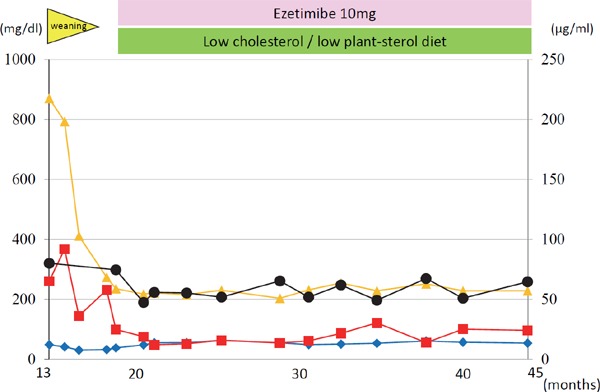
Representative infantile case with breastfed sitosterolemia
The clinical course of the infantile case with sitosterolemia (13-month-old girl). Closed black circle indicates sitosterol, and closed orange triangle indicates LDL cholesterol. Closed red square indicates triglyceride. Closed blue rhombus indicates high-density lipoprotein cholesterol.
Sitosterol or Cholesterol?
Sitosterolemia is known to show elevated sitosterol and LDL cholesterol levels. In contrast to LDL cholesterol, the pathogenicity of sitosterol on the development of atherosclerosis still remains unclear. Regarding this point, only a few studies have systematically investigated this issue, and serum sitosterol level itself does not seem to be the causal factor22, 23). Further studies with larger sample sizes are needed to elucidate this issue. On the other hand, a recent clinical trial has shown that serum sitosterol level could be a good biomarker of ezetimibe efficacy24). Serum sitosterol has been shown as a surrogate marker of cholesterol absorption25). This result may reflect the fact that ezetimibe could be more effective in patients with increased cholesterol absorption due to primary or secondary causes. Accordingly, serum sitosterol measurements could be used not only for the clinical diagnosis of sitosterolemia but also for the prediction of ezetimibe efficacy.
Clinical Phenotypes of Sitosterolemia Other Than Atherosclerotic Disease
It has been shown that some patients with sitosterolemia suffer from hematologic abnormalities, including abnormal erythrocyte shape, thrombocytopenia, and arthritis26, 27). This phenomenon is now being investigated because of the accumulation of elevated plant sterols. However, we previously showed a unique case complicated by arthritis due to a combination disease of sitosterolemia and familial Mediterranean fever, whose typical manifestation includes arthritis5). This case shows a recessive disorder that is usually associated with consanguineous marriage; hence, another recessive disorder could occur with these other phenotypes. Comprehensive genetic analysis for such cases should be quite useful for uncovering the potential cause of those situations.
Managements for Sitosterolemia
Several medications for reducing LDL cholesterol and sitosterol have been introduced for sitosterolemic patients28, 29). Among these medications, ezetimibe and cholestimide have been described as appropriate therapies9). It is true that ezetimibe and colestimide are effective in reducing cholesterol and sitosterol, and HMG-CoA reductase inhibitors (statins) are effective in reducing LDL cholesterol in sitosterolemic patients28, 30). In addition to those established approaches, we experienced a sitosterolemia case wherein PCSK9 inhibition had great effect for reducing LDL cholesterol (data not shown). As stated above, the efficacy of reducing sitosterol levels for the prevention of atherosclerosis is still controversial. PCSK9 inhibitors and statins with robust evidence for the reduction of atherosclerotic events could also be considered for patients with sitosterolemia31). For patients with advanced atherosclerotic regions, LDL apheresis could be considered if applicable6).
Conclusions and Perspectives
It is true that sitosterolemia as a monogenic disorder is rather rare; however, the prevalence of this disorder is currently underestimated. Furthermore, relatives of sitosterolemia patients or those with heterozygous mutation carriers usually exhibit elevated sitosterol and LDL cholesterol levels. Moreover, such mutations affect the phenotype of FH. Considering the potential mechanism of elevation of such sterols in this situation, including heterozygous mutation carriers, ezetimibe should be the best treatment; however, statins and PCSK9 inhibitors could be considered if the LDL cholesterol level is very high. The evidence suggests that LDL cholesterol rather than sitosterol should be treated despite the name of this disease. The accurate prevalence data of this disease and clinical data from clinical diagnoses supported by health insurance and comprehensive genetic analyses are needed to further elucidate the clinical importance of this disease.
Acknowledgments and Notice of Grant Support
None
Conflicts of Interest
None
References
- 1). Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In The Metabolic and Molecular Bases of Inherited Diseases. 8th edition Scriver CR, Beaudet AL, Sly WS, Valle D. editors. McGraw-Hill, New York: 2001; 2863-2913 [Google Scholar]
- 2). Soutar AK, Naoumova RP. Mechanisms of Disease: genetic causes of familial hypercholesterolemia. Nat Pract Cardiovasc Med. 2007; 4: 214-225 [DOI] [PubMed] [Google Scholar]
- 3). Escolà-Gil JC, Quesada H, Julve J, Martín-Campos JM, Cedó L, Blanco-Vaca F. Sitosterolemia: diagnosis, investigation, and management. Curr Atheroscler Rep. 2014; 16: 424. [DOI] [PubMed] [Google Scholar]
- 4). Tada H, Kawashiri MA, Takata M, Matsunami K, Imamura A, Matsuyama M, Sawada H, Nunoi H, Konno T, Hayashi K, Nohara A, Inazu A, Kobayashi J, Mabuchi H, Yamagishi M. Infantile Cases of Sitosterolaemia with Novel Mutations in the ABCG5 Gene: Extreme Hypercholesterolaemia is Exacerbated by Breastfeeding. JIMD Rep. 2015; 21: 115-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Tada H, Kawashiri MA, Okada H, Endo S, Toyoshima Y, Konno T, Nohara A, Inazu A, Takao A, Mabuchi H, Yamagishi M, Hayashi K. A Rare Coincidence of Sitosterolemia and Familial Mediterranean Fever Identified by Whole Exome Sequencing. J Atheroscler Thromb. 2016; 23: 884-890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Sakuma N, Tada H, Mabuchi H, Hibino T, Kasuga H. Lipoprotein Apheresis for Sitosterolemia. Ann Intern Med. 2017; 167: 896-899 [DOI] [PubMed] [Google Scholar]
- 7). Kawamura R, Saiki H, Tada H, Hara A. Acute myocardial infarction in a 25-year-old woman with sitosterolemia. J Clin Lipidol. 2018; 12: 246-249 [DOI] [PubMed] [Google Scholar]
- 8). Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O'Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG, Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016; 536: 285-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Tsubakio-Yamamoto K, Nishida M, Nakagawa-Toyama Y, Masuda D, Ohama T, Yamashita S. Current therapy for patients with sitosterolemia--effect of ezetimibe on plant sterol metabolism. Atheroscler Thromb. 2010; 17: 891-900 [DOI] [PubMed] [Google Scholar]
- 10). Park JH, Chung IH, Kim DH, Choi MH, Garg A, Yoo EG. Sitosterolemia presenting with severe hypercholesterolemia and intertriginous xanthomas in a breastfed infant: case report and brief review. J Clin Endocrinol Metab. 2014; 99: 1512-1518 [DOI] [PubMed] [Google Scholar]
- 11). Yu XH, Qian K, Jiang N, Zheng XL, Cayabyab FS, Tang CK. ABCG5/ABCG8 in cholesterol excretion and atherosclerosis. Clin Chim Acta. 2014; 428: 82-88 [DOI] [PubMed] [Google Scholar]
- 12). Tada H, Kawashiri MA, Nomura A, Teramoto R, Hosomichi K, Nohara A, Inazu A, Mabuchi H, Tajima A, Yamagishi M. Oligogenic Familial Hypercholesterolemia, LDL Cholesterol, and Coronary Artery Disease. Circulation. 2017; 136: A11731. [DOI] [PubMed] [Google Scholar]
- 13). Lamiquiz-Moneo I, Baila-Rueda L, Bea AM, Mateo-Gallego R, Pérez-Calahorra S, Marco-Benedí V, Martín-Navarro A, Ros E, Cofán M, Rodríguez-Rey JC, Pocovi M, Cenarro A, Civeira F. ABCG5/G8 gene is associated with hypercholesterolemias without mutation in candidate genes and noncholesterol sterols. J Clin Lipidol. 2017; 11: 1432-1440 [DOI] [PubMed] [Google Scholar]
- 14). Hansel B, Carrié A, Brun-Druc N, Leclert G, Chantepie S, Coiffard AS, Kahn JF, Chapman MJ, Bruckert E. Premature atherosclerosis is not systematic in phytosterolemic patients: severe hypercholesterolemia as a confounding factor in five subjects. Atherosclerosis. 2014; 234: 162-168 [DOI] [PubMed] [Google Scholar]
- 15). Wang J, Joy T, Mymin D, Frohlich J, Hegele RA. Phenotypic heterogeneity of sitosterolemia. J Lipid Res. 2004; 45: 2361-2367 [DOI] [PubMed] [Google Scholar]
- 16). Nomura A, Tada H, Nohara A, Kawashiri MA, Yamagishi M. Oral Fat Tolerance Test for Sitosterolemia and Familial Hypercholesterolemia: A Study Protocol. J Atheroscler Thromb. 2018: 25; 741-746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Tada H, Nomura A, Nohara A, Inazu A, Mabuchi H, Yamagishi M, Kawashiri M. Post-prandial Remnant Lipoprotein Metabolism in Sitosterolemia. J Atheroscler Thromb. 2018. July 12. 10.5551/jat.44768. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Hidaka H, Nakamura T, Aoki T, Kojima H, Nakajima Y, Kosugi K, Hatanaka I, Harada M, Kobayashi M, Tamura A, Fujii T, Shigeta Y. Increased plasma plant sterol levels in heterozygotes with sitosterolemia and xanthomatosi. J Lipid Res. 1990; 31: 881-888 [PubMed] [Google Scholar]
- 19). Bardawil T, Rebeiz A, Chaabouni M, El Halabi J, Kambris Z, Abbas O, Abou Hassan O, Hamie L, Bitar F, Ghani Kibbi A, Nemer G, Kurban M. Mutations in the ABCG8 gene are associated with sitosterolaemia in the homozygous form and xanthelasmas in the heterozygous form. Eur J Dermatol. 2017; 27: 519-523 [DOI] [PubMed] [Google Scholar]
- 20). Miwa K, Inazu A, Kobayashi J, Higashikata T, Nohara A, Kawashiri M, Katsuda S, Takata M, Koizumi J, Mabuchi H. ATP-binding cassette transporter G8 M429V polymorphism as a novel genetic marker of higher cholesterol absorption in hypercholesterolaemic Japanese subjects. Clin Sci (Lond). 2005; 109: 183-188 [DOI] [PubMed] [Google Scholar]
- 21). Yoshida A, Naito M, Miyazaki K. Japanese sisters associated with pseudohomozygous familial hypercholesterolemia and sitosterolemia. J Atheroscler Thromb. 2000; 7: 33-38 [DOI] [PubMed] [Google Scholar]
- 22). Genser B, Silbernagel G, De Backer G, Bruckert E, Carmena R, Chapman MJ, Deanfield J, Descamps OS, Rietzschel ER, Dias KC, März W. Plant sterols and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2012; 33: 444-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Silbernagel G, Chapman MJ, Genser B, Kleber ME, Fauler G, Scharnagl H, Grammer TB, Boehm BO, Mäkelä KM, Kähönen M, Carmena R, Rietzschel ER, Bruckert E, Deanfield JE, Miettinen TA, Raitakari OT, Lehtimäki T, März W. High intestinal cholesterol absorption is associated with cardiovascular disease and risk alleles in ABCG8 and ABO: evidence from the LURIC and YFS cohorts and from a meta-analysis. J Am Coll Cardiol. 2013; 62: 291-299 [DOI] [PubMed] [Google Scholar]
- 24). Hagiwara N, Kawada-Watanabe E, Koyanagi R, Arashi H, Yamaguchi J, Nakao K, Tobaru T, Tanaka H, Oka T, Endoh Y, Saito K, Uchida T, Matsui K, Ogawa H. Low-density lipoprotein cholesterol targeting with pitavastatin + ezeti mibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ-PROPER study, a prospective, openlabel, randomized trial. Eur Heart J. 2017; 38: 2264-2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Dayspring TD, Varvel SA, Ghaedi L, Thiselton DL, Bruton J, McConnell JP. Biomarkers of cholesterol homeostasis in a clinical laboratory database sample comprising 667,718 patients. J Clin Lipidol. 2015; 9: 807-881 [DOI] [PubMed] [Google Scholar]
- 26). Su Y, Wang Z, Yang H, Cao L, Liu F, Bai X, Ruan C. Clinical and molecular genetic analysis of a family with sitosterolemia and co-existing erythrocyte and platelet abnormalities. Haematologica. 2006; 91: 1392-1395 [PubMed] [Google Scholar]
- 27). Bastida JM, Benito R, Janusz K, Díez-Campelo M, Hernández-Sánchez JM, Marcellini S, Girós M, Rivera J, Lozano ML, Hortal A, Hernández-Rivas JM, González-Porras JR. Two novel variants of the ABCG5 gene cause xanthelasmas and macrothrombocytopenia: a brief review of hematologic abnormalities of sitosterolemia. J Thromb Haemost. 2017; 15: 1859-1866 [DOI] [PubMed] [Google Scholar]
- 28). Nguyen LB, Cobb M, Shefer S, Salen G, Ness GC, Tint GS. Regulation of cholesterol biosynthesis in sitosterolemia: effects of lovastatin, cholestyramine, and dietary sterol restriction. J Lipid Res. 1991; 32: 1941-1948 [PubMed] [Google Scholar]
- 29). Salen G, von Bergmann K, Lütjohann D, Kwiterovich P, Kane J, Patel SB, Musliner T, Stein P, Musser B, Multicenter Sitosterolemia Study Group Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004; 109: 966-971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Hidaka H, Kojima H, Kawabata T, Nakamura T, Konaka K, Kashiwagi A, Kikkawa R, Shigeta Y. Effects of an HMG-CoA reductase inhibitor, pravastatin, and bile sequestering resin, cholestyramine, on plasma plant sterol levels in hypercholesterolemic subjects. J Atheroscler Thromb. 1995; 2: 60-65 [DOI] [PubMed] [Google Scholar]
- 31). Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR, FOURIER Steering Committee and Investigators Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017; 376: 1713-1722 [DOI] [PubMed] [Google Scholar]


