Abstract
Gastric cancer (GC) affects the health of 1,000,000 people per year worldwide; however, the biological basis of GC remains largely unknown. The current study aimed to investigate the aberrant expression of miR-744 in GC for the effective treatment of patients with GC. Tumor and adjacent tissues were obtained from 30 patients who underwent tumor resection surgery at Dongying People's Hospital. The results of reverse transcription-quantitative polymerase chain reaction indicated that the expression of miR-744 was significantly decreased in tumor tissues compared with the levels in adjacent tissues. Human gastric cancer cell line SGC-7901 was then randomly divided into three different groups, including the control, miR-negative control (NC) and miR-744 mimic groups. A Cell Counting Kit-8 assay demonstrated that there was a significant decrease in the proliferation rate of SGC-7901 cells in the miR-744 mimics group compared with that observed in the control and miR-NC mimics groups. In addition, flow cytometry demonstrated that apoptosis was significantly increased in the miR-744 mimics group compared with that observed in the control and miR-NC mimics groups. Western blotting indicated that the expression of B cell lymphoma 2 (Bcl-2), B cell lymphoma-2-associated X protein and caspase-3 protein was significantly increased, while the expression of Bcl-2 was significantly decreased in the miR-744 mimics group compared with the levels observed in the control and miR-NC mimics groups. A dual-luciferase assay verified that miR-744 directly targeted the 3′-untranslated region of Bcl-2. Taken together, the present study suggested that miR-744 serves a tumor suppressive role in GC by targeting Bcl-2.
Keywords: gastric cancer, microRNA-744, B-cell lymphoma 2
Introduction
Gastric cancer (GC) is common in developing countries (1). In 2012, there were 700,000 mortalities, making GC the third leading cause of cancer-associated mortality after lung and liver cancer (2). Worldwide, ~42% of male and ~19% of female patients with GC are from China (3). Risk factors for GC include stress, a diet of charred or salty foods, alcohol consumption, smoking, obesity and physical inactivity (4). Current treatments for GC include surgery (5), chemotherapy and/or radiation therapy (6). The outcomes of patients with locally advanced GC are sub-optimal due to the likelihood of relapse (hazard ratio=1.52) (7) and chemotherapy with external beam radiotherapy is the standard adjuvant treatment (8). Radio-resistance makes the successful treatment of GC challenging (9). Target therapy is a novel strategy currently being studied in clinical trials (10). Thus, the identification of novel molecular targets is required to fulfill unmet clinical needs.
B-cell lymphoma 2 (Bcl-2) is an anti-apoptotic member of the Bcl-2 family, which regulates cell apoptosis (11). Deregulation of apoptosis is strongly associated with various diseases, such as schizophrenia, which is thought to result from the decreased expression of Bcl-2 and increased expression of caspase-3 (12).
MicroRNA (miRNA or miR) are a class of non-coding RNA that are ~22 nucleotides long, which act as endogenous suppressors of gene expression by binding to the 3′-untranslated region (3′-UTR) of target mRNA (13,14). miRNA serve a role in cell proliferation and apoptosis, which are associated with tumorigenesis (15). The regulation of Bcl-2 by miRNA and its role in GC progression remain unknown and further investigation is required to identify the pathogenic mechanism of GC.
The present study aimed to investigate the function of miR-744 in GC and the possible molecular mechanisms. RT-qPCR analyses were performed on tumor and adjacent tissues obtained from 30 patients to explore the differencential expression of miR-744. SGC-7901 cells were randomly divided into control, miR-negative control (NC) and miR-744 mimic groups. Cell Counting Kit-8 (CCK-8) assay and flow cytometry were performed to determine the cell proliferation and cell apoptosis of SGC-7901 cells, respectively. Western blotting was performed to evaluate the expression of Bcl-2, B cell lymphoma-2-associated X protein (Bax) and caspase-3. A dual-luciferase assay was used for verification of the interaction between miR-744 and Bcl-2. It was found that miR-744 serves a tumor suppressive role in GC by targeting Bcl-2.
Materials and methods
Patients
GC tumor tissues and adjacent normal tissues from 30 patients (14 males and 16 females; mean age, 47.30±9.82 years) who underwent surgery on the stomach or esophageal junction at the Dongying People's Hospital (Dongying, China) were collected between November 2014 and November 2016 for the current study. The adjacent tissues were removed at a distance of ≥5 cm away from the tumor tissues from the antrum and corpus mucosa, which were stripped along the side of the lesser curvature. Prior informed and written consent was obtained from each patient. Patients who underwent chemotherapy or radiotherapy prior to surgery were excluded from the current study. The present study was approved by the Ethics Committee of Dongying People's Hospital.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
miRNA from patient tissues were extracted and lysed using an miRNeasy Mini kit (Qiagen China Co., Ltd., Shanghai, China). RT for hsa-miR-744 was performed using a Transcriptor First Strand cDNA Synthesis kit (Roche Diagnostics, Basel, Switzerland) at 65°C for 1 h. Subsequently, cDNA was used for qPCR, which was performed using a SYBR-Green Master mix (ROX; Roche Diagnostics) in a PCR Thermal Cycler Dice (Takara Bio, Inc., Otsu, Japan) at 95°C for 10 min, followed by 50 cycles of 95°C for 10 sec, 55°C for 10 sec, 72°C for 5 sec, 99°C for 1 sec, 59°C for 15 sec, 95°C for 1 sec and final cooling to 40°C. Relative expression of miR-744 was normalized against U6 with the 2−ΔΔCq method (16). Primer sequences were as follows: miR-744 forward, 5′-AATGCGGGGCTAGGGCTA-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′; and U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′ (17).
Cell culture
Human GC cell line SGC-7901 (American Type Culture Collection, Manassas, VA, USA) was cultured in RPMI-1640/F12 medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.), streptomycin (100 mg/ml) and penicillin (100 U/ml) at 37°C in a 95% humidified chamber containing 5% CO2.
Cell transfection
SGC-7901 cells (1×105) were first seeded onto 24-well plates and cultured for 24 h at 37°C. Cells were divided into the following groups: The control group consisting of untreated SGC-7901 cells; the miR-NC mimics group consisting of SGC-7901 cells transfected with miR-NC mimics, and the miR-744 mimic group consisting of SGC-7901 cells transfected with miR-744 mimics. The miR-744 mimics and miR-NC mimics were purchased from GeneCopoeia, Inc. (Rockville, MD, USA). Cell transfection of 30 µM miR-744 mimics or miR-NC mimics was performed using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) and cells were incubated for 48 h at 37°C in accordance with the manufacturer's protocols prior to the performance of subsequent experimentation. The sequences used were as follows: miR-744 mimics forward, 5′-UGCGGGGCUAGGGCUAACAGCA-3′ and reverse, 5′-UGCUGUUAGCCCUAGCCCCGCA-3′; and miR-NC mimics forward, 5′-AAUUCUCCGAACGUGUCACTT-3′ and reverse, 5′-GUGACACGUUCGGAGAAUUTT-3′, according to a previous study (18).
Western blot analysis
SGC-7901 cells were first washed with PBS three times, 5 min each time, and lysed using lysis buffer containing protease inhibitors (Beyotime Institute of Biotechnology, Haimen, China). The concentration of each protein sample was normalized using the bicinchoninic acid method. Equal amounts of proteins from all the obtained samples (15 µg/lane) were loaded and then separated by 8–10% SDS-PAGE. Samples were then transferred on to nitrocellulose membranes. Membranes were blocked using 5% (v/v) skimmed milk for 1 h at room temperature and then incubated and probed with the following primary antibodies: Bax (cat. no. 2772), Bcl-2 (cat. no. 3498), caspase-3 (cat. no. 9662) and GAPDH (cat. no. 5174; all 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight. Membranes were then incubated with bovine anti-rabbit horseradish peroxidase-conjugated secondary antibodies (cat. no. 2370; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature for 2 h. Bands were developed using an enhanced chemiluminescent kit (Beyotime Institute of Biotechnology, Shanghai, China) with GAPDH acting as an endogenous loading control.
Cell proliferation assay
SGC-7901 cell proliferation rate was assessed using CCK-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) according to the manufacturer's protocol. Briefly, SGC-7901 cells were seeded in each well of a 96-well plate at a concentration of 5×103 cells/well and cultured at 37°C for 1–5 days. A total of 10 µl CCK-8 solution was then added into each well and incubated for 1 h at 37°C. The optical density value from each sample was read at a wavelength of 450 nm using a microplate reader.
Cell apoptosis analysis
SGC-7901 cell apoptosis was evaluated using an Annexin V-fluorescein isothiocyanate/propidium iodide (FITC/PI) double-staining apoptosis detection kit (Beyotime Institute of Biotechnology) in accordance with the manufacturer's protocols. FITC specifically binds to the phosphatidyl serine residues on the cell membrane, while PI binds to DNA when the cell membrane is permeable (19). SGC-7901 cells were collected at 48 h following transfection and resuspended in 400 µl of 90% RPMI-1640 medium with 10% FBS to the final concentration of 1×106 cells/ml. A total of 5 µl Annexin V-FITC was then added to the suspension and SGC-7901 cells were incubated at room temperature in the dark for 15 min prior to the addition of 5 µl PI. After 5 min, SGC-7901 cells were analyzed using a flow cytometer and quantified using FlowJo software version 7.6.2 (FlowJo LLC, Ashland, OR, USA).
Luciferase reporter assay
Wild-type (WT) or mutant Bcl-2 3′-UTR was cloned into a pmirGLO luciferase reporter vector (Promega Corporation, Madison, WI, USA). Following the incubation of SGC-7901 cells at 37°C for 24 h, SGC-7901 cells were co-transfected with miR-744 mimics and pmir-Bcl-2WT-3′-UTR or pmir-Bcl-2mut-3′-UTR using Lipofectamine® 2000, according to the manufacturer's protocol. SGC-7901 cells were then seeded in 24-well plates at a concentration of 5×104 cells/well and cultured at 37°C for 18 h. Subsequently, SGC-7901 cells were transfected with firefly luciferase vector (500 ng/µl) and mixed with Vigofect transfection reagent (Vigorous Biotechnology, Beijing, China), according to the manufacturer's protocol. Following continuous exposure for 48 h, the luciferase activities from firefly and Renilla were measured with a Dual-luciferase reporter assay system (Promega Corporation), with Renilla activity acting as a normalized parameter.
Statistical analysis
All data are expressed as the mean ± standard deviation and all experiments were repeated three times. GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA) and a Student's two-sample unpaired t-test were used for the analyses of data, while data among three groups were compared by one-way analysis of variance followed by a Tukey's post hoc test. A Pearson's correlation test was used to perform correlation analysis between miR-744 expression and GC progression in Table I. P<0.05 was considered to indicate a statistically significant difference.
Table I.
Clinicopathological characteristics and expression of miR-774 in tissues from patients with gastric cancer.
| Characteristics | ||||
|---|---|---|---|---|
| Patient number | Sex | Age, years | TNM stage | Relative miR-774 level |
| 1 | F | 57 | IV | 0.40 |
| 2 | F | 30 | I A | 1.21 |
| 3 | M | 38 | III A | 0.93 |
| 4 | F | 46 | II | 1.01 |
| 5 | M | 39 | I A | 1.20 |
| 6 | F | 54 | II | 1.10 |
| 7 | M | 62 | III A | 0.97 |
| 8 | M | 37 | IV | 0.52 |
| 9 | F | 60 | III B | 0.88 |
| 10 | M | 58 | III B | 0.64 |
| 11 | M | 39 | III B | 0.78 |
| 12 | M | 34 | III B | 0.84 |
| 13 | F | 45 | IV | 0.45 |
| 14 | F | 28 | I A | 1.23 |
| 15 | M | 32 | I A | 1.32 |
| 16 | M | 45 | III A | 0.91 |
| 17 | F | 51 | III B | 0.74 |
| 18 | M | 36 | IV | 0.62 |
| 19 | F | 42 | IV | 0.60 |
| 20 | F | 52 | IV | 0.47 |
| 21 | F | 54 | III A | 0.98 |
| 22 | F | 46 | I B | 1.21 |
| 23 | M | 49 | III B | 0.78 |
| 24 | F | 52 | III B | 0.75 |
| 25 | M | 57 | IV | 0.56 |
| 26 | M | 55 | III B | 0.67 |
| 27 | F | 53 | III B | 0.85 |
| 28 | M | 47 | III A | 0.95 |
| 29 | F | 59 | IV | 0.48 |
| 30 | F | 62 | II | 1.04 |
The relative mRNA level of each patient was compared to that in healthy people. M, male; F, female; TNM, tumor, node and metastasis; miR, microRNA.
Results
miR-744 expression levels are decreased in GC tissues
RT-qPCR was used to identify the role of miR-744 in GC progression by determining the differential expression of miR-744 in GC tissues and adjacent tissues. The results demonstrated that the expression of miR-744 was significantly decreased in tumor tissues compared with the level in adjacent normal tissues (Fig. 1).
Figure 1.
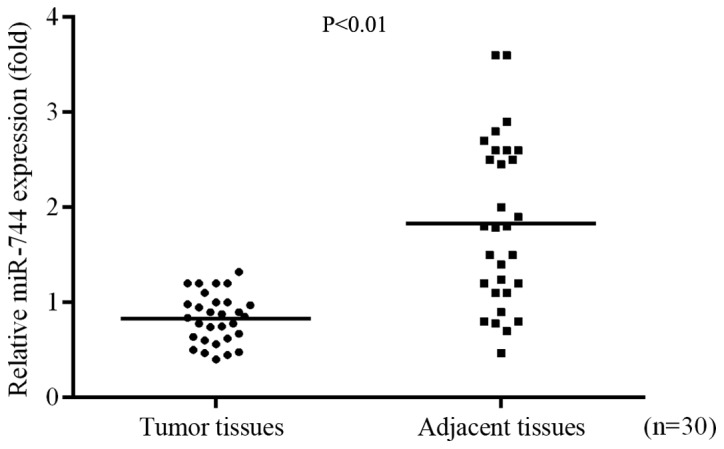
miR-744 expression is decreased in tumor tissues compared with the level in adjacent tissues from patients with gastric cancer.
Bcl-2 is a direct target of miR-744 in GC
A previous study indicated that miR-744 was involved in cervical cancer through targeting Bcl-2 (16). Therefore, the current study aimed to determine whether miR-744 regulated Bcl-2 in GC using a luciferase target assay for the detection of an association between miR-744 and Bcl-2 in SGC-7901 cells. The results indicated that miR-744 significantly decreased the relative luciferase activity of the WT Bcl-2 3′-UTR compared with that observed in the NC group, while the luciferase activity of the mutant Bcl-2 3′-UTR was not significantly different between the NC and miR-744 groups (Fig. 2). These results suggest that Bcl-2 was a direct target of miR-744 in SGC-7901 cells.
Figure 2.
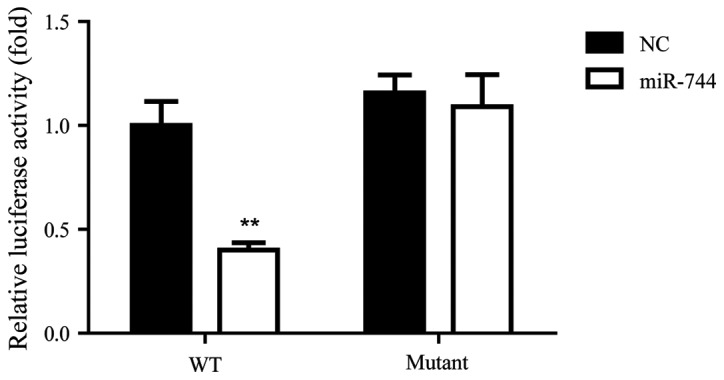
miR-744 directly targets Bcl-2 in SGC-7901 cells. Relative luciferase activity of the WT Bcl-2 3′-UTR and mutant Bcl-2 3′-UTR in the NC and miR-744 groups indicated that miR-744 directly targets Bcl-2 in SGC-7901 cells. The experiments were replicated 3 times and data are expressed as the mean ± standard deviation. **P<0.01 vs. NC. Bcl-2, B-cell lymphoma 2; WT, wild-type; UTR, untranslated region; NC, negative control.
Expression profile of miR-744 in tissues from patients with GC
There were 14 male and 16 female patients included in the current study. The mean age of patients was 47.30±9.82 years and 16 patients were aged <50 years old, while 14 patients were aged ≥50 years old. The tumor, node and metastasis (TNM) staging system according to seventh edition of the American Joint Committee on Cancer TNM Classification was used for this study (20). There were 5 patients at histological stage I (4 IA and 1 IB), 3 patients (the lowest rate) at histological stage II, 14 patients (the highest rate) at histological stage III (5 IIIA and 9 IIIB) and 8 patients (the highest rate) at histological stage IV. The expression profile of miR-744 was also determined, and it was demonstrated that the more serious the TNM stage of patients the lower the miR-744 mRNA level, suggesting that miR-744 was negatively associated with the progression of GC (Table I). This association requires further study and verification in a larger sample size.
miR-744 inhibits SGC-7901 cell proliferation
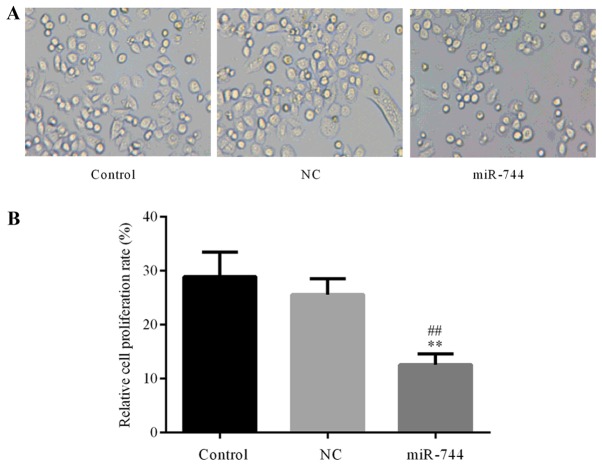
The role of miR-744 in GC cell proliferation was further confirmed using a CCK-8 assay to determine SGC-7901 cell proliferation in the different groups. The results demonstrated that the proliferation of SGC-7901 cells was significantly decreased in the miR-744 mimics group compared with that observed in the control and miR-NC mimics groups (Fig. 3). These results suggest that miR-744 inhibited cell proliferation in GC cells.
Figure 3.
miR-744 inhibits SGC-7901 cell proliferation. (A) Representative images indicating cell proliferation in the control, NC and miR-744 groups. (B) Quantification of SGC-7901 cell proliferation in the different groups using a Cell Counting Kit-8 assay. The experiments were replicated 3 times and data are expressed as the mean ± standard deviation. ##P<0.01 vs. the control group. **P<0.01 vs. the NC group. NC, negative control mimics group; miR-744, miR-744 mimics group.
miR-744 induces SGC-7901 cell apoptosis
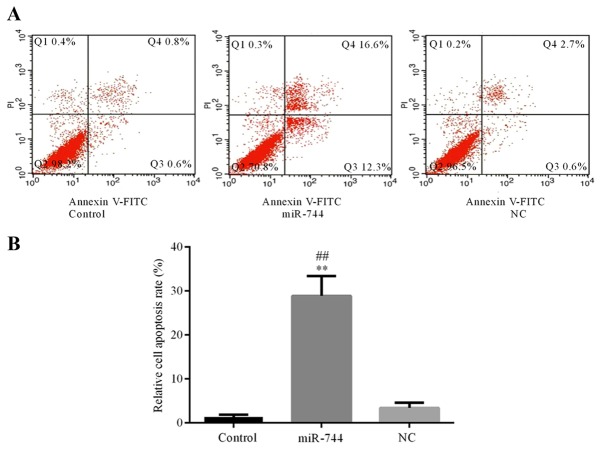
Flow cytometry was used to determine the apoptosis rate of SGC-7901 cells in the different groups. The results demonstrated that cell apoptosis was significantly increased in the miR-744 mimics group compared with that observed in the control and miR-NC mimics groups (Fig. 4). These results suggest that miR-744 promotes cell apoptosis in GC cells.
Figure 4.
miR-744 induces SGC-7901 cell apoptosis. Flow cytometry was used to determine the rate of apoptosis in SGC-7901 cells in the miR-744, NC and control groups. (A) Flow cytometry of apoptosis in the different groups. (B) Relative apoptosis rate in the different groups. The experiments were replicated 3 times and data are expressed as the mean ± standard deviation. ##P<0.01 vs. the control group. **P<0.01 vs. the NC group. NC, microRNA negative control mimics group; miR-744, miR-744 mimics group; FITC, fluorescein isothiocyanate; PI, propidium iodide.
miR-744 decreases the expression of anti-apoptosis protein Bcl-2 and increases the expression of pro-apoptosis proteins Bax and caspase-3 in SGC-7901 cells
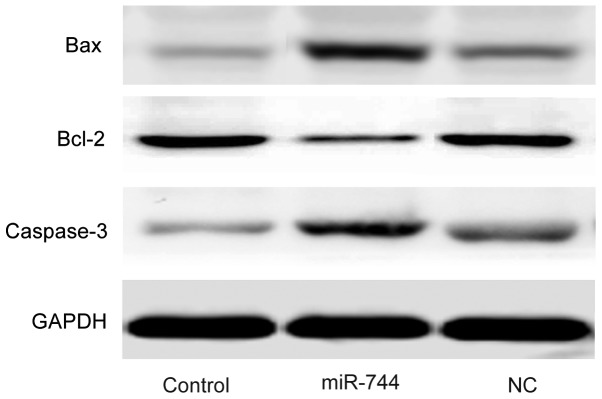
Western blot analysis was used to determine the expression levels of Bcl-2, Bax and caspase-3 in SGC-7901 cells. It was demonstrated that the expression levels of Bax and caspase-3 were markedly increased while the expression of Bcl-2 was decreased in the miR-744 mimics group compared with the levels observed in the control and miR-NC mimics groups (Fig. 5).
Figure 5.
Western blot analysis of the expression of Bax, Bcl-2 and caspase-3 in the control, miR-744 and NC groups. Compared with control group and miR-NC mimics group, there was an increased protein expression level of Bax and caspase-3, and a decreased protein expression level of Bcl-2 in the miR-744 mimics treatment group in SGC-7901 cells. Bax, B-cell lymphoma 2-associated X protein; Bcl-2, B-cell lymphoma 2; NC, microRNA negative control mimics group; miR-744, miR-744 mimics group.
Discussion
GC is common in developing countries (1). Targeted therapy is a novel treatment strategy for GC, which is being studied in clinical trials (10). However, due to the heterogeneity of GC, the identification of potential targets is required to fulfill the unmet clinical need.
miRNA are endogenous suppressors of gene expression, which bind to mRNA 3′-UTRs (13,14) and are associated with cell proliferation and apoptosis (15). Apoptosis has been demonstrated to modulate schizophrenia, which may be caused by decreased expression of Bcl-2 and increased expression of caspase-3 (12). miRNA that may regulate Bcl-2 were investigated in the present study. Bcl-2 is an integral protein of the external mitochondrial membrane, which reduces cell apoptosis (21). Upregulation of the expression of Bcl-2 by secretory leukocyte protease inhibitor has been demonstrated to promote the proliferation of GC cells (22).
A previous study indicated that miR-744 targets Bcl-2 (23), thus it was chosen as the focus of the current study. The differential expression of miR-744 between tumor tissues and adjacent normal tissues was investigated in the present study. RT-qPCR demonstrated that the expression level of miR-744 was significantly decreased in tumor tissues compared with the level in adjacent normal tissues. These results suggest that miR-744 may be a tumor suppressor gene, which is consistent with a previous study that indicated the decreased expression of miR-744 in human hepatocellular carcinoma (17). Notably, miR-744 has been suggested to inhibit various types of cancer, including colon cancer, breast cancer and GC (24). Furthermore, the relationship between Bcl-2 and miR-744 was demonstrated using a dual-luciferase assay in SGC-7901 cells in the current study, which indicated that miR-744 significantly reduced the relative luciferase activity of the WT Bcl-2 3′-UTR, while the luciferase activity of the mutant Bcl-2 3′-UTR was not significantly changed.
The expression of miR-744 in patients with GC at different TNM stages was also examined. The results demonstrated that the more serious the TNM stage of patients the lower the miR-744 mRNA level, the expression of miR-744 was negatively associated with the progression of GC. This association requires further study and verification in a larger sample size. Thus, the results of the current study indicated that miR-744 serves a role in inhibiting the progression of GC in patients, with Bcl-2 as the target gene in GC cells.
The impact of miR-744 on GC cell behavior was further investigated by determining the cell proliferation and apoptosis rates of SGC-7901 cells using a CCK-8 assay and flow cytometry, respectively. The results of the CCK-8 assay demonstrated that the proliferation of SGC-7901 cells was significantly decreased in the miR-744 mimics group compared with the control and miR-NC mimics groups. In addition, flow cytometry analysis indicated that apoptosis of SGC-7901 cells was significantly increased in the miR-744 mimics group compared with the control and miR-NC mimics groups.
However, the molecules regulated by miR-744 remained unclear. Therefore, western blot analysis was performed and the results indicated that the expression levels of Bax and caspase-3 were increased, while the expression of Bcl-2 was decreased in the miR-744 mimics group compared with the levels observed in the control and miR-NC mimics groups in SGC-7901 cells. The differential expression of Bcl-2, Bax and caspase-3 were consistent with the results that were indicated for SGC-7901 cell apoptosis and proliferation. Caspase-3 is an essential indicator of apoptosis (25) and Bax activates cell apoptosis by activating the activity of caspase-3 (26).
In conclusion, the results of the current study demonstrated that miR-744 was downregulated in GC tissues compared with normal gastric tissues; miR-744 decreased the expression of Bcl-2 and SGC-7901 cell proliferation, while it increased the expression of caspase-3, Bax and the SGC-7901 cell apoptosis rate. Thus, miR-744 may serve as a tumor suppressor via the inhibition of cell proliferation and promotion of apoptosis by targeting Bcl-2 in GC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
Authors' contributions
JL designed and conducted the experiments, and analyzed the data. YW and SL conducted the experiments and analyzed the data. YL and HL conducted the experiments. JL analyzed the data. XZ designed the experiments, analyzed the data and wrote the manuscript.
Ethics approval and consent to participate
Prior informed and written consent was obtained from each patient. The present study was approved by the Ethics Committee of Dongying People's Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An JY, Kim KM, Kim YM, Cheong JH, Hyung WJ, Noh SH. Surgical complications in gastric cancer patients preoperatively treated with chemotherapy: Their risk factors and clinical relevance. Ann Surg Oncol. 2012;19:2452–2458. doi: 10.1245/s10434-012-2267-9. [DOI] [PubMed] [Google Scholar]
- 4.Oh DY, Choi KS, Shin HR, Bang YJ. Public awareness of gastric cancer risk factors and disease screening in a high risk region: A population-based study. Cancer Res Treat. 2009;41:59–66. doi: 10.4143/crt.2009.41.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen K, Xu XW, Zhang RC, Pan Y, Wu D, Mou YP. Systematic review and meta-analysis of laparoscopy-assisted and open total gastrectomy for gastric cancer. World J Gastroenterol. 2013;19:5365–5376. doi: 10.3748/wjg.v19.i32.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pretz JL, Wo JY, Mamon HJ, Kachnic LA, Hong TS. Chemoradiation therapy: Localized esophageal, gastric, and pancreatic cancer. Surg Oncol Clin N Am. 2013;22:511–524. doi: 10.1016/j.soc.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Eng J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Tian Y, Tang Y, Wang X, Li N, Ren H, Fang H, Feng Y, Wang S, Song Y, et al. A Phase II prospective nonrandomized trial of magnetic resonance imaging-guided hematopoietic bone marrow-sparing radiotherapy for gastric cancerpatients with concurrent chemotherapy. Onco Targets Ther. 2016;9:2701–2707. doi: 10.2147/OTT.S91586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang M, Xiao J, Wang J, Zhou P, Wei T, Zhao T, Wang R. MiR-24 enhances radiosensitivity in nasopharyngeal carcinoma by targeting SP1. Cancer Med. 2016;5:1163–1173. doi: 10.1002/cam4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meza-Junco J, Au HJ, Sawyer MB. Critical appraisal of trastuzumab in treatment of advanced stomach cancer. Cancer Manag Res. 2011;3:57–64. doi: 10.2147/CMAR.S12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azimian H, Bahreyni-Toossi MT, Rezaei AR, Rafatpanah H, Hamzehloei T, Fardid R. Up-regulation of Bcl-2 expression in cultured human lymphocytes after exposure to low doses of gamma radiation. J Med Phys. 2015;40:38–44. doi: 10.4103/0971-6203.152249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr Res. 2006;81:47–63. doi: 10.1016/j.schres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Van Rooij E. The art of microRNA research. Circ Res. 2011;108:219–234. doi: 10.1161/CIRCRESAHA.110.227496. [DOI] [PubMed] [Google Scholar]
- 14.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 15.Munker R, Calin GA. MicroRNA profiling in cancer. Clin Sci (Lond) 2011;121:141–158. doi: 10.1042/CS20110005. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, Qin L, Wu X, Zheng Y, Yang Y, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19:232–243. doi: 10.1016/j.ccr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Lin F, Ding R, Zheng S, Xing D, Hong W, Zhou Z, Shen J. Decrease expression of microRNA-744 promotes cell proliferation by targeting c-Myc in human hepatocellular carcinoma. Cancer Cell Int. 2014;14:58. doi: 10.1186/1475-2867-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding J, Yuan L, Chen G. Aspirin enhances the cytotoxic activity of bortezomib against myeloma cells via suppression of Bcl-2, survivin and phosphorylation of AKT. Oncol Lett. 2017;13:647–654. doi: 10.3892/ol.2016.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edge SB, Compton CC. The American joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 21.Roy M, Chakraborty S, Siddiqi M, Bhattacharya RK. Induction of apoptosis in tumor cells by natural phenolic compounds. Asian Pac J Cancer Prev. 2002;3:1–67. [PubMed] [Google Scholar]
- 22.Du XY, Liu X, Wang ZJ, Wang YY. SLPI promotes the gastric cancer growth and metastasis by regulating the expression of P53, Bcl-2 and caspase-8. Eur Rev Med Pharmacol Sci. 2017;21:1495–1501. [PubMed] [Google Scholar]
- 23.Chen XF, Liu Y. MicroRNA-744 inhibited cervical cancer growth and progression through apoptosis induction by regulating Bcl-2. Biomed Pharmacother. 2016;81:379–387. doi: 10.1016/j.biopha.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Song MY, Pan KF, Su HJ, Zhang L, Ma JL, Li JY, Yuasa Y, Kang D, Kim YS, You WC. Identification of serum microRNAs as novel non-invasive biomarkers for early detection of gastric cancer. PLoS One. 2012;7:e33608. doi: 10.1371/journal.pone.0033608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu S, Rajakaruna S, De Arcangelis A, Zhang L, Georges-Labouesse E, Menko AS. α6 integrin transactivates insulin-like growth factor receptor-1 (IGF-1R) to regulate caspase-3-mediated lens epithelial cell differentiation initiation. J Biol Chem. 2014;289:3842–3855. doi: 10.1074/jbc.M113.515254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Wang Z. Effects of rapamycin on expression of Bcl-2 and Bax in human lens epithelial cells and cell cycle in rats. J Huazhong Univ Sci Technolog Med Sci. 2011;31:555–559. doi: 10.1007/s11596-011-0489-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of data and materials: All data generated or analyzed during this study are included in this published article.