Abstract
In the present study, the expression of microRNA (miR)-365 and interleukin (IL)-6 in peripheral blood mononuclear cells and serum from patients with sepsis following multiple trauma has been investigated. A total of 26 patients with sepsis following multiple trauma were included as the experimental group, whereas 21 contemporaneous patients without sepsis following multiple trauma were included as the negative control group. The expression of IL-6 mRNA and miR-365 was determined by reverse transcription-quantitative polymerase chain reaction, and western blot analysis was used to measure IL-6 protein expression. ELISA was performed to determine the secretion of IL-6 protein. Following stimulation with lipopolysaccharide (LPS) for 24 h, THP-1 cells were used to examine the expression of miR-365 and the levels of IL-6 protein and mRNA in cells simulating sepsis. A dual luciferase reporter assay revealed that IL-6 mRNA was a direct target of miR-365. Patients with sepsis following multiple trauma exhibited significantly higher IL-6 mRNA and protein levels than patients without sepsis (P<0.05). In addition, miR-365 expression in patients with sepsis following trauma was significantly lower than in patients without sepsis (P<0.05). Similar effects were observed in THP-1 cells treated with LPS. The present study demonstrated that increased expression of IL-6 in patients with sepsis following multiple trauma is associated with decreased expression of miR-365. miR-365 may regulate the occurrence and immune response of sepsis following multiple trauma via IL-6. These results may elucidate agents for clinical diagnosis and treatment of the disease.
Keywords: microRNA-365, sepsis following multiple trauma, interleukin-6, mononuclear cells
Introduction
Multiple trauma is defined as the simultaneous injury of two or more anatomical sites of the body (1). Multiple trauma is often accompanied by major bleeding, shock or severe physiological disorders, all of which can be life threatening (1). Patients with multiple trauma typically exhibit systemic inflammatory response syndrome, sepsis or multiple organ dysfunction syndrome (2). Sepsis is a common complication of severe trauma, burns, shock and major surgery, and one of the main causes of mortality in patients with multiple trauma injuries (2,3).
Sepsis is a serious complication of infection with a number of clinical manifestations and symptoms. Sepsis occurs when there is excessive inflammation in response to an infection, which subsequently damages the host (4). It is commonly accepted that the activation of neutrophils, lymphocytes and mononuclear macrophages and the release of endogenous mediators serve key roles in the pathophysiology of sepsis following trauma (5,6). In 1992, sepsis was defined by the American Thoracic Society and the Society of Critical Care Medicine as an infection and systemic inflammatory response syndrome (7). Sepsis manifests as a systemic inflammatory response syndrome caused by infection, which requires rapid treatment (8).
Interleukin (IL)-6 is an important factor in immune responses that is produced by activated monocytes and macrophages (9). IL-6 promotes the growth and differentiation of primary bone marrow-derived cells by coordinating with colony stimulating factor, and enhances the lysing ability of natural killer cells (10–12). A previous study has reported that microRNA (miRNA or miR)-365 has a negative regulatory effect on IL-6 expression in 293 and HeLa cells (13). However, the regulatory effect of miR-365 on IL-6 in blood monocytes from patients with sepsis following multiple traumas is rarely reported. In the present study, miR-365 expression was measured, as well as the levels of IL-6 mRNA and protein in peripheral blood mononuclear cells (PBMC) and serum from patients with sepsis following multiple trauma, and the mechanism of action of miR-365 in sepsis was investigated.
Patients and methods
Patients
A total of 26 patients with sepsis following multiple trauma who received treatment at the Affiliated Hospital of Nantong University (Nantong, China) between June 2012 and January 2017 were included in the present study as the experimental sepsis group. During the same time period 21 patients without sepsis following multiple trauma were recruited as the negative control (NC) group. Among the patients with sepsis, 11 were male and 15 were female (age range, 18–60 years; median age, 41 years). In the control group, 8 patients were male and 13 were female (age range, 16–62 years; median age, 42 years). None of the participants had diabetes, tumors or other immune diseases. The Ethics Committee of Nantong University approved all procedures and written informed consent was obtained from all patients or their families prior to their inclusion in the present study.
Peripheral blood was collected from all participants and stored at 4°C for 1–2 h. The serum was then separated, centrifuged at a speed of 400 × g for 10 min at 4°C, and aliquoted into Eppendorf tubes (100 µl in each tube) prior to storage at −70°C. To collect PBMCs, anticoagulant venous blood was mixed with an equal volume of serum-free Iscove's Modified Dulbecco's Medium (cat. no. 12440053; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and the mixture was added onto the surface of human lymphocyte separation solution (5 ml; cat. no. 40503ES60; Shanghai Yeasen Biotechnology Co., Ltd., Shanghai, China). Following centrifugation at 400 × g for 30 min at 4°C the middle mist layer was gently aspirated into tubes and mixed with 5× the volume of Hank's balanced salt solution (cat. no. C0218; Beyotime Institute of Biotechnology, Haimen, China), the mixture then underwent centrifugation at 300 × g for 10 min. The cells were washed with Hank's balanced salt solution twice, and then diluted to 3×106 cells/ml and seeded into culture dishes. Following cultivation at 37°C in an atmosphere of 5% CO2 for 2 h, the cells that adhered to the bottom of the culture dish were identified as PBMCs.
Cells
THP-1 cells (Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China) were cultured in RPMI-1640 complete medium (cat. no. 11875093; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (cat. no. SH30084.03; Hyclone; Thermo Fisher Scientific, Inc.) at 37°C and 5% CO2 for 48 h. The cells were treated with 1 µg/ml lipopolysaccharide (LPS) for 24 h to simulate a sepsis environment (14) and then compared with THP-1 cells that were not treated with LPS. The expression of miR-365 and IL-6 in the cells was measured.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 100 µl blood or 2×106 PBMCs (2×107/ml) using TRIzol reagent (Thermo Fisher Scientific, Inc.) following the manufacturer's protocol (15). The same procedure was used as for patient samples. The concentration and quality of the RNA was measured using a Nanodrop ND2000 ultraviolet spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE, USA). Subsequently, complementary DNA was obtained by RT from 1 µg RNA and stored at −20°C. RT of mRNA was performed using a TIANScript II cDNA First Strand Synthesis kit, and RT of miRNA was performed using an miRcute miRNA cDNA First Strand Synthesis kit (both Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturers protocol.
PCR was performed using a SuperReal PreMix (SYBR Green) RT-qPCR kit (Tiangen Biotech Co., Ltd.) to detect mRNA expression of IL-6; GAPDH was used as an internal reference. The PCR primer sequences used were as follows: IL-6 forward, 5′-GGCACTGGCAGAAAACAACC-3′ and reverse, 5′-GCAAGTCTCCTCATTGAATCC-3′; and GAPDH forward, 5′-GGGAAACTGCGGCGTGAT-3′ and reverse, 5′-AAAGGTGGAGGAGTGGGT-3′. The reaction system (25 µl) was composed of 12.5 µl SYBR Premix EXTaq, 0.5 µl upstream primer, 0.5 µl downstream primer, 1 µl cDNA and 10.5 µl double distilled (dd) H2O. The PCR conditions were an initial denaturation at 95°C for 30 sec, followed by 45 cycles of denaturation at 95°C for 5 sec and annealing at 57°C for 30 sec; with final extension at 72°C for 1 min (iQ5 Multicolor Real-Time PCR Detection System; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The 2−ΔΔCq method (16) was used to calculate the relative expression of IL-6 mRNA against GAPDH. Each sample had a minimum of three repeats.
The expression of miR-365 was determined using U6 as an internal reference. The PCR primer sequences used were as follows: miR-365 forward, 5′-GCGTAATGCCCCTAAAAATCC-3′, the reverse primer was universal and provided by the kit. The forward and reverse PCR primer sequences used for U6 were 5′-AACGCTTCACGAATTTGCGT-3′ and 5′-CTCGCTTCGGCAGCACA-3′, respectively. The reaction system (20 µl) contained 10 µl RT-qPCR-Mix, 0.5 µl upstream primer, 0.5 µl downstream universal primer, 2 µl cDNA and 7 µl ddH2O. The reaction protocol was: Initial denaturation at 90°C for 30 sec; 40 cycles of denaturation at 95°C for 5 sec and annealing at 60°C for 34 sec; with final extension at 72°C for 1 min. The 2−ΔΔCq method was used to calculate the relative expression of miR-365 against U6. Each sample had a minimum of three repeats.
Western blot analysis
A precooled radio-immunoprecipitation assay lysis buffer (600 µl) composed of 50 mM Tris-base, 1 mM EDTA, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% Triton-X-100 and 1% sodium deoxycholate (all Beyotime Institute of Biotechnology) was used to lyse the samples (100 µl blood or 2×106 cells). Following lysis for 50 min on ice, the mixture was centrifuged at 12,000 × g at 4°C for 5 min. The supernatant was used to determine the protein concentration using a bicinchoninic acid protein concentration determination kit [RTP7102, Real-Times (Beijing) Biotechnology Co., Ltd., Beijing, China]. Protein samples (20 µg) were mixed with a sodium dodecyl sulfate loading buffer (cat. no. P0015; Beyotime, Institute of Biotechnology) prior to denaturation in a boiling water bath for 5 min. The samples (20 µg/lane) were then subjected to 10% SDS-PAGE gel electrophoresis. The resolved proteins were transferred to polyvinylidene difluoride membranes on ice (100 V, 2 h) and blocked with 5% skimmed milk at room temperature for 1 h. The membranes were subsequently incubated with rabbit anti-human primary antibodies against IL-6 (1:1,000; ab6672) or β-actin (1:5,000; ab129348; both Abcam, Cambridge, UK) at 4°C overnight. Following washing with PBS with Tween-20 three times for 15 min, the membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:3,000; ab6721; Abcam) for 1 h at room temperature. Following this they were washed again with PBS with Tween-20 three times for 15 min. The membranes were then developed using an ECL Western Blotting Substrate kit (ab65623; Abcam) for imaging. Image Lab version 3.0 software (Bio-Rad Laboratories, Inc.) was used to acquire and analyze the imaging signals. The relative content of IL-6 protein was expressed as the IL-6/β-actin ratio.
ELISA
Serum levels of IL-6 were tested using a human IL-6 ELISA kit (ab178013; Abcam) according to the manufacturer's protocol. In 96-well microplates, 50 µl samples (10 µl sample liquid and 40 µl diluent) and blank (diluent) were set into predefined wells. Horseradish peroxidase-labelled conjugates (100 µl) were added to all wells prior to sealing the plates for incubation at 37°C for 1 h. Following washing of the plates five times with washing liquid provided by the kit, substrates A (50 µl) and B (50 µl; provided by the kit) were added into each well. Following incubation at 37°C for 15 min, a stop solution (50 µl) was added into each well, and the absorbance of each well was measured at 450 nm within 15 min.
Dual luciferase reporter assay
Bioinformatics prediction is a powerful tool for the study of the functions of miRNAs. To understand the regulatory mechanism of IL-6, several different bioinformatics tools were used to predict miRNA molecules that may regulate IL-6. These included miRanda (microrna.org/microrna/home.do), TargetScan (targetscan.org), PiTa (genie.weizmann.ac.il/pubs/mir07/mir07_data.html), RNAhybrid (bibiserv.techfak.uni-bielefeld.de/rnahybrid/) and PicTar (pictar.mdc-berlin.de). Wild-type (WT) and mutant seed regions of miR-365 in the 3′-untranslated region (UTR) of the IL-6 gene were chemically synthesized in vitro, Spe-1 and HindIII restriction sites were added, and then cloned into pMIR-REPORT luciferase reporter plasmids (Ambion; Thermo Fisher Scientific, Inc.). Plasmids (0.8 µg) with WT or mutant 3′-UTR DNA sequences were co-transfected with agomiR-365 (100 nM; Sangon Biotech Co., Ltd., Shanghai, China) into 293 T cells (Type Culture Collection of the Chinese Academy of Sciences). Following cultivation for 24 h the cells were lysed using Dual-luciferase Reporter Assay System (Promega Corporation, Madison, WI, USA) according to the manufacturer's protocol, and the fluorescence intensity was measured using a GloMax 20/20 luminometer (Promega Corporation). Using Renilla fluorescence activity as the internal reference, the fluorescence values of each group of cells were measured.
Statistical analysis
The results were analyzed using SPSS version 18.0 statistical software (SPSS, Inc., Chicago, IL, USA). The results are expressed as the mean ± standard deviation. Data were tested for normality. Multigroup measurement data were analyzed using one-way analysis of variance. In case of homogeneity of variance, least significant difference and Student-Newman-Keuls methods were used; in case of heterogeneity of variance, Tamhane's T2 or Dunnett's T3 method was used. P<0.05 was considered to indicate a statistically significant difference.
Results
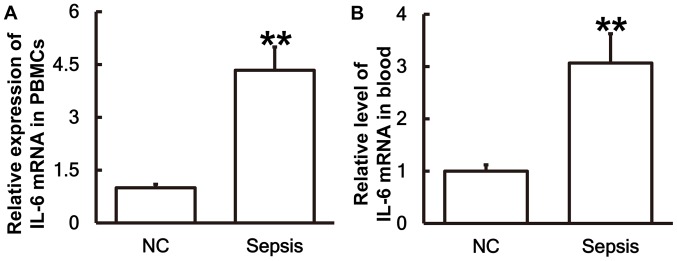
Patients with sepsis following multiple traumas exhibit higher IL-6 mRNA levels than patients without sepsis
To measure the expression of IL-6 mRNA in different samples, RT-qPCR was performed. The results demonstrated that the levels of IL-6 mRNA in PBMCs (Fig. 1A) and blood (Fig. 1B) from patients with sepsis were significantly higher than those in the control group (P<0.01).
Figure 1.
Patients with sepsis exhibit significantly higher levels of IL-6 mRNA compared with the NC group. IL-6 mRNA expression in (A) PBMCs and (B) blood was measured using reverse transcription-quantitative polymerase chain reaction. **P<0.01 vs. the NC group. IL, interleukin; NC, negative control; PBMC, peripheral blood mononuclear cell.
IL-6 protein expression in PBMCs is upregulated in patients with sepsis following multiple trauma
To determine the expression of the IL-6 protein in PBMCs, western blot analysis was performed. The results revealed that IL-6 protein expression in PBMCs from patients with sepsis was significantly elevated compared with the NC group (P<0.05; Fig. 2A and B).
Figure 2.
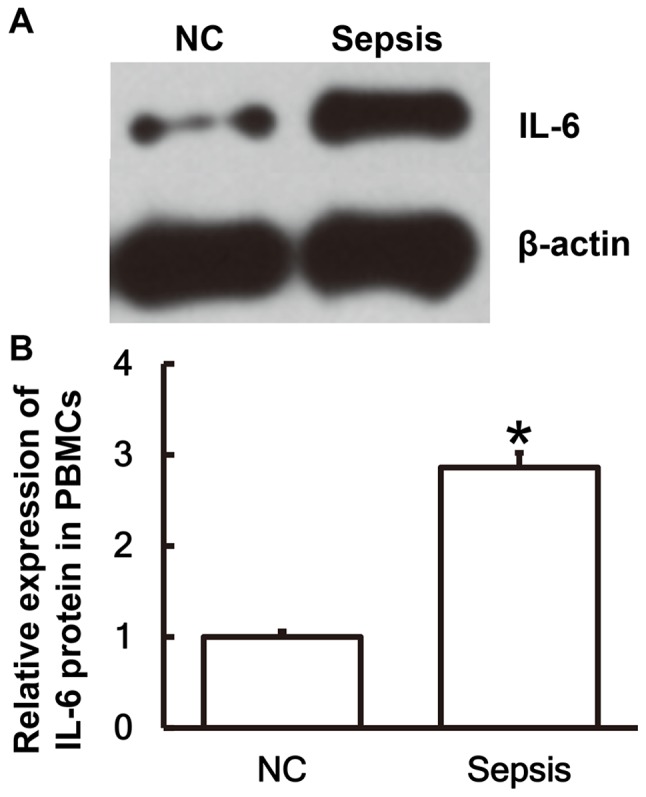
IL-6 protein expression is significantly higher in PBMCs from patients with sepsis following multiple trauma compared with the NC group. (A) Western blot analysis was performed to measure the protein expression of IL-6 in PBMCs. (B) This was then quantified using Image Lab version 3.0 software. *P<0.05 vs. the NC group. IL, interleukin; NC, negative control; PBMC, peripheral blood mononuclear cell.
Serum from patients with sepsis following multiple trauma contains a higher IL-6 content compared with serum from patients without sepsis
To examine the secretion of IL-6 by PBMCs, ELISA was performed. The results demonstrated that the IL-6 content in the serum of patients with sepsis was significantly higher than in the control group (P<0.01; Fig. 3).
Figure 3.
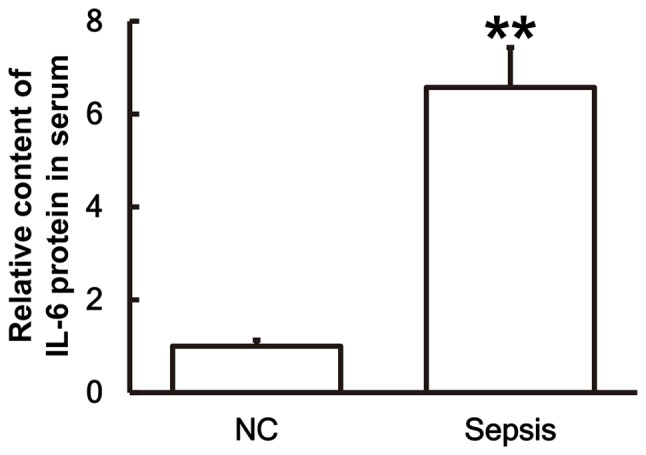
IL-6 protein levels in the serum of patients with sepsis are significantly higher than in the NC group. ELISA was used to measure the protein content of IL-6 in serum for patients with sepsis and the NC group. **P<0.01 vs. the NC group. NC, negative control; IL, interleukin.
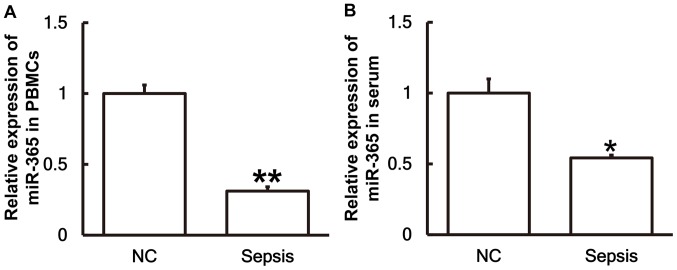
Patients with sepsis following multiple trauma exhibit lower levels of miR-365 than patients without sepsis
To investigate the levels of miR-365 in the serum and PBMCs, RT-qPCR was performed. The results revealed that the levels of miR-365 in PBMCs (Fig. 4A) and serum (Fig. 4B) from patients with sepsis were significantly lower than those in the control group (P<0.01 and P<0.05, respectively).
Figure 4.
Expression of miR-365 is significantly lower in patients with sepsis compared with the NC group. The expression of miR-365 in (A) PBMCs and (B) serum of patients with sepsis and the NC group were measured using reverse transcription-quantitative polymerase chain reaction. *P<0.05 and **P<0.01 vs. the NC group. miR, microRNA; NC, negative control; PBMC, peripheral blood mononuclear cells.
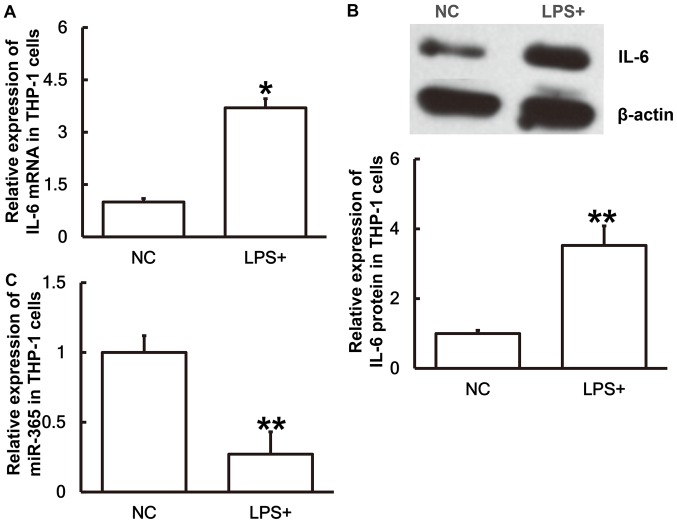
IL-6 expression is upregulated whereas miR-365 expression is downregulated in THP-1 cells stimulated with LPS
To simulate sepsis at a cellular level THP-1 cells were treated with LPS for 24 h prior to the levels of miR-365 and IL-6 being measured. The results revealed that IL-6 mRNA (Fig. 5A) and protein expression (Fig. 5B) in THP-1 cells treated with LPS were significantly higher than those in the NC group (P<0.05 and P<0.01, respectively). Conversely, the miR-365 expression in cells treated with LPS was significantly lower than the negative control group (P<0.01; Fig. 5C). The results suggest that IL-6 expression is upregulated and miR-365 expression is downregulated in THP-1 cells stimulated with LPS.
Figure 5.
Expression levels of IL-6 and miR-365 in THP-1 cells. To replicate a sepsis environment at a cellular level, THP-1 cells were treated with LPS for 24 h. Then the levels of (A) IL-6 mRNA, (B) IL-6 protein and (C) miR-365 in THP-1 cells was measured. Western blot analysis was used to measure the protein expression and reverse transcription-quantitative polymerase chain reaction was used to measure the mRNA and miR levels. *P<0.05 and **P<0.01 vs. the NC group. IL, interleukin; miR, microRNA; LPS, lipopolysaccharide; NC, negative control.
miR-365 binds with the 3′-UTR seed region of IL-6 mRNA to regulate its expression
To identify interactions between miR-365 and the 3′-UTR of IL-6 mRNA, a dual luciferase reporter assay was performed. Bioinformatics results identified that miR-365 was potentially able to regulate IL-6 (Fig. 6A). The fluorescence value of cells co-transfected with miR-365 mimics and WT luciferase reporter plasmids was significantly lower than that in the NC group (P<0.01). By contrast, the fluorescence value of cells co-transfected with miR-365 mimics and mutant luciferase reporter plasmids was not significantly different from that in the NC group (Fig. 6B). These results indicates that miR-365 binds with the 3′-UTR seed region of IL-6 mRNA to regulate its expression.
Figure 6.
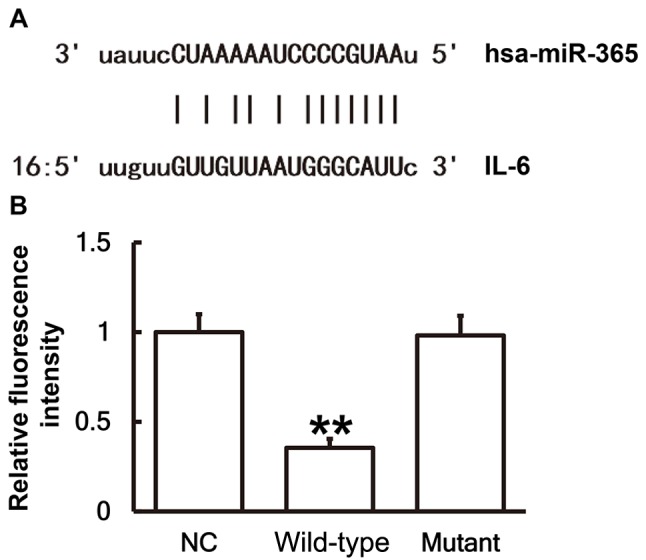
There are direct interactions between miR-365 and IL-6. (A) miR-365 was potentially able to regulate IL-6 through direct interactions. miR, microRNA; IL, interleukin. (B) Plasmids (0.8 µg) with WT or mutant 3′-untranslated region DNA sequences were co-transfected with miR-365 mimics into 293T cells. Following cultivation for 24 h the cells were lysed using a dual luciferase reporter assay kit, and fluorescence intensity was measured. Using Renilla fluorescence activity as internal reference, the fluorescence values of each group of cells were measured. **P<0.01 vs. the NC group. IL, interleukin; miR, microRNA; WT, wild-type.
Discussion
Sepsis is a systemic inflammatory response syndrome caused by infection, which can lead to acute organ dysfunction or septic shock (17). In clinical practice, septic shock caused by sepsis is the most common cause of mortality in intensive care patients, and the mortality rate is 20–80% (18). Previous studies have demonstrated that cytokines serve key roles in the pathophysiology of post-traumatic sepsis, and the upregulation of tumor necrosis factor (TNF)-α, IL-6 and IL-8 have been identified as associated with post traumatic infection (19,20). IL-6 is a pleiotropic cytokine synthesized by mononuclear cells, macrophages, T cells, B cells, vascular endothelial cells and fibroblasts in response to IL-1 and TNF-α (21). Under normal circumstances, IL-6 contents in the blood, serum and body are minimal, but it has a high biological activity and exerts an effect via paracrine and autocrine signaling pathways (22). IL-6 induces the production of C-reactive protein and fibrinogen in inflammation and promotes thrombosis (23). Increased levels of IL-6 in the body can cause inflammatory diseases by binding to the IL-6 receptor (24). The IL-6 receptor is primarily expressed on the surface of liver cells, neutrophils, macrophages or lymphocytes. In rheumatoid arthritis IL-6 stimulates the secretion of inflammatory mediators by T lymphocytes and B lymphocytes, and promotes the maturation and differentiation of B lymphocytes (25). In inflammatory responses IL-6 is chemotactic to other inflammatory cells, including neutrophils and mononuclear macrophages (26). Riedemann et al (27) previously reported that IL-6 enhances the proinflammatory activity of secondary inflammatory mediator complement component 5a. Pritts et al (28) previously reported that nuclear factor-κB and activator protein-1 are associated with the regulation of IL-6 gene activation and synthesis in effector cells. In the present study, it was identified that IL-6 mRNA and protein expression are elevated in PBMCs and serum from patients with sepsis following multiple trauma, which suggests that septic infection following multiple trauma may activate mononuclear cells and lymphocytes, which secrete abundant IL-6 to produce a large number of antigen immune responses.
miRNA is widely associated with various pathophysiological processes, including the proliferation, invasion and migration of tumor cells, hypertension, diabetes mellitus and atherosclerosis (29,30). It has been reported that the expression of miR-365 in breast cancer and endometriosis tissues is disordered (31). In NIH3T3 cells, ultraviolet irradiation significantly increases the expression of miR-365, which suggests that miR-365 has certain anti-tumor effects (32). Overexpression of tumor-suppressor gene NGX6 leads to the decreased expression of miR-365, suggesting that miR-365 may also have oncogene activity (33). The expression of miR-365 is downregulated in senescent lung fibroblasts, which indicates that miR-365 may be associated with the regulation of cell proliferation (34). In addition, miR-365 has negative regulation on IL-6 expression in 293 and HeLa cells (13). In the present study it has been identified that miR-365 is downregulated and IL-6 is upregulated in PBMCs and serum from patients with sepsis following multiple trauma. These results reveal that the levels of miR-365 and IL-6 in the serum reflect inflammatory responses and tissue injuries. In addition, THP-1 cells treated with LPS to simulate a sepsis environment achieved similar results. The dual luciferase reporter assay demonstrated that IL-6 is a direct target gene of miR-365.
In conclusion, the present study demonstrates that increased expression of IL-6 in patients with sepsis following multiple trauma is caused by decreased expression of miR-365. In addition, miR-365 may regulate the occurrence and immune response of sepsis following multiple trauma via IL-6. These results may elucidate agents for clinical diagnosis and treatment of the disease.
Acknowledgements
The authors would like to thank Dr Zhongwei Huang of Department of Emergency Surgery, Affiliated Hospital of Nantong University (Nantong, China) for his kind assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
DZ conceived and designed the present study. HG performed the experiments and wrote the manuscript. XS and JX collected the samples. All authors collaborated to interpret results and develop the manuscript. The final version of the manuscript has been read and approved by all authors, and each author believes that the manuscript represents honest work.
Ethics approval and consent to participate
The Ethics Committee of Nantong University approved all procedures and written informed consent was obtained from all patients or their families prior to their inclusion in the present study.
Patient consent for publication
Patients provided consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hilbert-Carius P, Wurmb T, Lier H, Fischer M, Helm M, Lott C, Böttiger BW, Bernhard M. Care for severely injured persons: Update of the 2016 S3 guideline for the treatment of polytrauma and the severely injured. Anaesthesist. 2017;66:195–206. doi: 10.1007/s00101-017-0265-9. (In German) [DOI] [PubMed] [Google Scholar]
- 2.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372:1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 3.Jensen KO, Held L, Kraus A, Hildebrand F, Mommsen P, Mica L, Wanner GA, Steiger P, Moos RM, Simmen HP, Sprengel K. The impact of mild induced hypothermia on the rate of transfusion and the mortality in severely injured patients: A retrospective multi-centre study. Eur J Med Res. 2016;21:37. doi: 10.1186/s40001-016-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Y, Yang Y, He X, Dong S, Wang W, Wang D, Zhang P. Esmolol reduces apoptosis and inflammation in early sepsis rats with abdominal infection. Am J Emerg Med. 2017;35:1480–1484. doi: 10.1016/j.ajem.2017.04.056. [DOI] [PubMed] [Google Scholar]
- 5.Patrick AL, Grin PM, Kraus N, Gold M, Berardocco M, Liaw PC, Fox-Robichaud AE, Canadian Critical Care Translational Biology Group Resuscitation fluid composition affects hepatic inflammation in a murine model of early sepsis. Intensive Care Med Exp. 2017;5:5. doi: 10.1186/s40635-017-0118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin JB, Badeaux JE. Interpreting laboratory tests in infection: Making sense of biomarkers in sepsis and systemic inflammatory response syndrome for intensive care unit patients. Crit Care Nurs Clin North Am. 2017;29:119–130. doi: 10.1016/j.cnc.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 7.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. doi: 10.1097/00003246-199206000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Warren HS. Strategies for the treatment of sepsis. N Engl J Med. 1997;336:952–953. doi: 10.1056/NEJM199703273361311. [DOI] [PubMed] [Google Scholar]
- 9.Huang M, Yang D, Xiang M, Wang J. Role of interleukin-6 in regulation of immune responses to remodeling after myocardial infarction. Heart Fail Rev. 2015;20:25–38. doi: 10.1007/s10741-014-9431-1. [DOI] [PubMed] [Google Scholar]
- 10.Vila N, Reverter JC, Yague J, Chamorro A. Interaction between interleukin-6 and the natural anticoagulant system in acute stroke. J Interferon Cytokine Res. 2000;20:325–329. doi: 10.1089/107999000312478. [DOI] [PubMed] [Google Scholar]
- 11.Tezono K, Sarker KP, Kikuchi H, Nasu M, Kitajima I, Maruyama I. Bioactivity of the vascular endothelial growth factor trapped in fibrin clots: Production of IL-6 and IL-8 in monocytes by fibrin clots. Haemostasis. 2001;31:71–79. doi: 10.1159/000048047. [DOI] [PubMed] [Google Scholar]
- 12.Anderson AE, Pratt AG, Sedhom MA, Doran JP, Routledge C, Hargreaves B, Brown PM, Lê Cao KA, Isaacs JD, Thomas R. IL-6-driven STAT signalling in circulating CD4+ lymphocytes is a marker for early anticitrullinated peptide antibody-negative rheumatoid arthritis. Ann Rheum Dis. 2016;75:466–473. doi: 10.1136/annrheumdis-2014-205850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Xiao SB, Xu P, Xie Q, Cao L, Wang D, Luo R, Zhong Y, Chen HC, Fang LR. miR-365, a novel negative regulator of interleukin-6 gene expression, is cooperatively regulated by Sp1 and NF-kappaB. J Biol Chem. 2011;286:21401–21412. doi: 10.1074/jbc.M110.198630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rump K, Brendt P, Frey UH, Schäfer ST, Siffert W, Peters J, Adamzik M. Aquaporin 1 and 5 expression evoked by the β2 adrenoreceptor agonist terbutaline and lipopolysaccharide in mice and in the human monocytic cell line THP-1 is differentially regulated. Shock. 2013;40:430–436. doi: 10.1097/SHK.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 15.Rio DC, Ares M Jr, Hannon GJ, Nilsen TW. Purification of RNA using TRIzol (TRI reagent) Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5439. pdb.prot5439. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heumann D, Glauser MP, Calandra T. Molecular basis of host-pathogen interaction in septic shock. Curr Opin Microbiol. 1998;1:49–55. doi: 10.1016/S1369-5274(98)80142-2. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Li C. Systemic inflammatory response syndrome, compensatory anti-inflammatory response syndrome and multiple organ dysfunction syndrome. Chin J Crit Care Med. 2003:1–40. [Google Scholar]
- 20.Mimasaka S, Hashiyada M, Nata M, Funayama M. Correlation between serum IL-6 levels and death: Usefulness in diagnosis of ‘traumatic shock’? Tohoku J Exp Med. 2001;193:319–324. doi: 10.1620/tjem.193.319. [DOI] [PubMed] [Google Scholar]
- 21.DeLong WG, Jr, Born CT. Cytokines in patients with polytrauma. Clin Orthop Relat Res. 2004:1–65. doi: 10.1097/01.blo.0000130840.64528.1e. [DOI] [PubMed] [Google Scholar]
- 22.Stover JF, Sakowitz OW, Schoning B, Rupprecht S, Kroppenstedt SN, Thomale UW, Woiciechowsky C, Unterberg AW. Norepinephrine infusion increases interleukin-6 in plasma and cerebrospinal fluid of brain-injured rats. Med Sci Monit. 2003;9:BR382–BR388. [PubMed] [Google Scholar]
- 23.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 24.Tone M, Powell MJ, Tone Y, Thompson SA, Waldmann H. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J Immunol. 2000;165:286–291. doi: 10.4049/jimmunol.165.1.286. [DOI] [PubMed] [Google Scholar]
- 25.Hu F, Liu H, Xu L, Li Y, Liu X, Shi L, Su Y, Qiu X, Zhang X, Yang Y, et al. Hypoxia-inducible factor-1α perpetuates synovial fibroblast interactions with T cells and B cells in rheumatoid arthritis. Eur J Immunol. 2016;46:742–751. doi: 10.1002/eji.201545784. [DOI] [PubMed] [Google Scholar]
- 26.Luo Q, Ma X, Wahl SM, Bieker JJ, Crossley M, Montaner LJ. Activation and repression of interleukin-12 p40 transcription by erythroid Kruppel-like factor in macrophages. J Biol Chem. 2004;279:18451–18456. doi: 10.1074/jbc.M400320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riedemann NC, Neff TA, Guo RF, Bernacki KD, Laudes IJ, Sarma JV, Lambris JD, Ward PA. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J Immunol. 2003;170:503–507. doi: 10.4049/jimmunol.170.1.503. [DOI] [PubMed] [Google Scholar]
- 28.Pritts T, Hungness E, Wang Q, Robb B, Hershko D, Hasselgren PO. Mucosal and enterocyte IL-6 production during sepsis and endotoxemia-role of transcription factors and regulation by the stress response. Am J Surg. 2002;183:372–383. doi: 10.1016/S0002-9610(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 29.Varshney J, Subramanian S. MicroRNAs as potential target in human bone and soft tissue sarcoma therapeutics. Front Mol Biosci. 2015;2:31. doi: 10.3389/fmolb.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta. 2016;1859:169–176. doi: 10.1016/j.bbagrm.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG, Hull LM. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol. 2009;23:265–275. doi: 10.1210/me.2008-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo L, Huang ZX, Chen XW, Deng QK, Yan W, Zhou MJ, Ou CS, Ding ZH. Differential expression profiles of microRNAs in NIH3T3 cells in response to UVB irradiation. Photochem Photobiol. 2009;85:765–773. doi: 10.1111/j.1751-1097.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang XY, Wu MH, Liu F, Li Y, Li N, Li GY, Shen SR. Differential miRNA expression and their target genes between NGX6-positive and negative colon cancer cells. Mol Cell Biochem. 2010;345:283–290. doi: 10.1007/s11010-010-0582-7. [DOI] [PubMed] [Google Scholar]
- 34.Maes OC, Sarojini H, Wang E. Stepwise up-regulation of microRNA expression levels from replicating to reversible and irreversible growth arrest states in WI-38 human fibroblasts. J Cell Physiol. 2009;221:109–119. doi: 10.1002/jcp.21834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.