Abstract
The present study aimed to determine the in vitro activities of sulbactam and sitafloxacin against extensively-drug resistant Acinetobacter baumannii (XDR-A. baumannii). A total of 50 strains of XDR-A. baumannii were isolated from clinical specimens. Broth microdilution assay was applied to determine the minimum inhibitory concentration (MIC) for sulbactam and sitafloxacin. Microdilution checkerboard method was used to determine the in vitro activity of this antimicrobial combination. Accordingly, the fractional inhibitory concentration (FIC) and FIC index (FICI) were calculated. Time-kill study was also carried out for four strains with different susceptibilities to determine the bactericidal activities of individual or combined use of sitafloxacin and sulbactam. Isolates with MICs of sitafloxacin ≤2 mg/l were considered to be susceptible to sitafloxacin. The susceptibility rate for sitafloxacin was 92% originally. When combined with sulbactam, this rate increased to 96%. Microdilution checkerboard results indicated that, when tested in combination, sulbactam/sitafloxacin exhibited marked synergistic and partial synergistic effects on 16 and 50% of the 50 strains, respectively. Time-kill assay suggested that sulbactam enhanced the bactericidal activity of sitafloxacin and the combination induced a synergistic effect. For strains that were not susceptible to sitafloxacin, the bactericidal activities of the combination of sitafloxacin and sulbactam at a sub-MIC concentration were impaired. However, this impairment could be overcome with the increase of the concentration to 1X MIC. The present study demonstrated that sulbactam enhanced the in vitro antimicrobial activity of sitafloxacin against XDR-A. baumannii.
Keywords: extensively-drug resistant-Acinetobacter baumannii, sitafloxacin, sulbactam, fractional inhibitory concentration index, time-kill curve
Introduction
Acinetobacter baumannii is an opportunistic pathogen that can cause a broad array of infections including pneumonia, skin and soft tissue infection, meningitis, urinary tract infection and blood stream infection, especially in immunocompromised patients (1). This ubiquitous organism can be detected in a wide range of environments, including hospitals and other care facilities, and can survive for a prolonged period of time on both biotic and abiotic surfaces (2). A. baumannii has been designated as a ‘red alert’ human pathogen due to its extensive antimicrobial resistance (3). Bacterial strains can be classified as multidrug-resistant (MDR), extensively drug-resistant (XDR) and pandrug-resistant (PDR). XDR is defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (4). Therefore, treatment options for XDR-A. baumannii infections are limited. When one agent is routinely used in clinical practice, the susceptibility of A. baumannii to this drug may markedly decrease (5,6). Given unpredictable or suboptimal pharmacodynamics and concerns of resistance emerging along with therapy, antimicrobial combinations may provide improved treatment options (7). Therefore, a search for novel agents and their efficient combinations is required. In the present study, the in vitro antimicrobial activity of sitafloxacin, a new fluoroquinolone, and the combined effect of sitafloxacin and sulbactam were detected against clinical isolates of XDR-A. baumannii.
Materials and methods
Bacterial isolates
XDR-A. baumannii strains were isolated from clinical specimens collected in three tertiary hospitals affiliated to Shandong University (Qilu Hospital, Jinan Central Hospital and Shandong Provincial Qianfoshan Hospital; all in Jinan, China) from November 2014 to December 2015. For patients from whom A. baumannii strains were isolated more than once, only one strain from each patient was included. VITEK® 2 microbial analysis instruments were used to identify the XDR-A. baumannii isolates (bioMérieux, Inc., Marcy l'Etoile, France). The Kirby-Bauer method (8) was applied to re-evaluate the strains to meet the criteria for XDR-A. baumannii (4). As a result, a total of 50 strains were included, of which 36 strains were from sputum, 5 from lavages, 3 from blood, 3 from skin wounds or surgical incisions of skin, 2 from cerebrospinal fluid and 1 from urine. ATCC 25922 and ATCC 27853 were used as quality controls (American Type Culture Collection, Manassas, VA, USA). The Ethics Committee of Qilu Hospital of Shandong University approved the present study (approval no. KYLL-2017-612). All patients provided written informed consent.
Broth microdilution assay
Mueller-Hinton (MH) powder was purchased from Thermo Fisher Scientific, Inc. and dissolved according to the manufacturer's protocol. Isolated colonies of A. baumannii strains were maintained in 10 ml fresh MH broth and shaken in a thermo-incubator at 37°C overnight. Suspensions with a turbidity that matched the 0.5 McFarland standard [1.5×108 colony-forming unit (CFU)/ml] were further diluted to obtain the final bacterial counts of 2–8×105 CFU/ml. Sitafloxacin and sulbactam were obtained from Beijing Biodee Biotechnology Co., Ltd. (Beijing, China). To determine minimum inhibitory concentration (MIC) values, broth microdilution method was carried out as described in Clinical and Laboratory Standards Institute (CLSI) guidelines (9). Susceptibility was also determined for each isolate using these criteria. The serially diluted drugs were at concentrations of 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125 and 0 µg/ml. When MICs were higher than 128 µg/ml, drug arrays of 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25 and 0 µg/ml were further prepared. Following the addition of bacterial suspensions, 96-well plates were incubated overnight in ambient atmosphere at 37°C. MIC values were determined by the concentrations of drugs at which bacterial growth was visibly inhibited.
Microdilution checkerboard assay
Following determination of MICs of sulbactam and sitafloxacin for each strain, another set of dilution series was prepared for the two agents: 8X MIC, 4X MIC, 2X MIC, 1X MIC, 0.5X MIC, 0.25X MIC, 0.125X MIC and 0 µg/ml. Sitafloxacin was added by column, while sulbactam was added by row in 96-well plates. Bacterial suspensions at concentrations of 2–8×105 CFU/ml were subsequently added and incubated overnight at 37°C. Fractional inhibitory concentration index (FICI) values were calculated as follows: FICI=MIC value of sulbactam combined with sitafloxacin/MIC value of sulbactam applied alone + MIC value of sitafloxacin combined with sulbactam/MIC value of sitafloxacin applied alone. FICI values were interpreted as follows: ≤0.5, synergy; >0.5 to <1, partial synergy; 1, addition; >1 to <4, indifference; and ≥4, antagonism (10). The above steps were performed in triplicate and average values were used as the final results. The software we used in this study was Excel 2010 (Microsoft Corporation, Redmond, WA, USA).
Time-kill assay
Time-kill assay was conducted for four representative strains with different susceptibilities to sitafloxacin and sulbactam. Bacterial suspensions were prepared following the aforementioned steps and turbidity was adjusted respectively. Thereafter, drugs alone or in combination were added into the suspensions and incubated at 37°C. Time-kill curves for individual or combined sulbactam and sitafloxacin were plotted at 0.5X MIC and 1X MIC, respectively. Drug concentrations were selected according to MIC levels instead of a fixed concentration and the four representative strains were different from each other in their susceptibilities towards the two agents. 1X MIC were chosen to guarantee the antimicrobial activity, while 0.5X MIC were chosen to see whether synergistic effects could be achieved at sub-MIC levels. Samples were removed at 0, 1, 2, 4, 6, 12 and 24 h. Aliquots (100 µl) were serially diluted with cold and sterile PBS. Bacterial counts were determined by plating three spots of 10 µl of appropriate dilutions (1:100) on MH agar plates and incubation at 35°C for 18–24 h. Time-kill curves were subsequently constructed by plotting the mean colony counts (log10 CFU/ml) vs time. The bactericidal activities of drug combination were defined as a 3 log10 CFU/ml (99.9%) reduction compared with the most efficient drug at 24 h. Synergy was defined as a 2 log10 CFU/ml decrease between the combination and the most active agent alone at 24 h (11). The drug combination was considered to be antagonistic with a ≥2 log10 increase in counts. In addition, the combination was considered to be indifferent if there was a <2 log10 increase or decrease in colony count compared with the most active drug alone. The experiment was performed in duplicate to ensure reproducibility. The experiment was continued for >24 h.
Results
Combined use of sitafloxacin and sulbactam exhibits an increased inhibitory effect on XDR-A. baumannii strains compared with the individual use of either
To test the effects of sitafloxacin and sulbactam against the XDR-A. baumannii strains, MICs and susceptibility rates were determined. Given that CLSI breakpoints are not reported for sitafloxacin, CLSI breakpoints for other fluoroquinolones were used to assess the susceptibility of pathogens to sitafloxacin. Isolates with MICs of sitafloxacin ≤2 mg/l were provisionally considered to be susceptible to sitafloxacin (12). CLSI breakpoints were not available for the use of sulbactam alone, and, therefore, breakpoints of ampicilin/sulbactam against Acinetobacter spp (susceptible, ≤8/4 µg/ml; intermediate, 16/8 µg/ml; and resistant, ≥32/16 µg/ml) were used as interpretation criteria instead (13). The data indicated that the lowest concentration of the antibiotic at which 50% of the isolates were inhibited (MIC50) and the lowest concentration of the antibiotic at which 90% of the isolates were inhibited (MIC90) for sitafloxacin decreased and the susceptibility rates increased when the drug was combined with sulbactam. Similarly, the MIC50 and MIC90 for sulbactam decreased and the susceptibility rates increased when it was combined with sitafloxacin (Table I). MIC values were determined by the concentrations of drugs at which bacterial growth was completely inhibited. The results suggested that the combined use of sitafloxacin and sulbactam exhibited a greater inhibitory effect on XDR-A. baumannii strains compared with the individual use of either.
Table I.
MIC values of sitafloxacin and sulbactam against XDR-A. baumannii isolates.
| Antimicrobial agent | MIC range (µg/ml) | MIC50 (µg/ml) | MIC90 (µg/ml) | Rate of susceptibility (%) |
|---|---|---|---|---|
| Sitafloxacin | ||||
| Alone | 0.125–16 | 1 | 2 | 92 |
| Combined | 0.016–8 | 0.5 | 2 | 96 |
| Sulbactam | ||||
| Alone | 2–256 | 32 | 64 | 16 |
| Combined | 0.016–64 | 8 | 32 | 48 |
MIC, minimum inhibitory concentration; MIC50, the lowest concentration of the antibiotic at which 50% of the isolates were inhibited; MIC90, the lowest concentration of the antibiotic at which 90% of the isolates were inhibited.
Sitafloxacin and sulbactam induce a synergistic or partial synergistic effect on the majority of the 50 strains
To further examine the inhibitory effect of sitafloxacin and sulbactam against XDR-A. baumannii strains, FICI was calculated. The data indicated that sitafloxacin and sulbactam induced a synergistic effect in 16% of the 50 strains. In addition, sitafloxacin and sulbactam induced a partial synergistic effect on 50% of the 50 strains. Importantly, sitafloxacin and sulbactam exhibited no antagonistic effect on any strain (Table II). The results indicated that sitafloxacin and sulbactam exhibited synergistic or partial synergistic effects on the majority of the 50 strains.
Table II.
Distribution of FICI values for the combination of sitafloxacin and sulbactam against XDR-A. baumannii isolates.
| Sulbactam/sitafloxacin | Synergy (FICI ≤0.5) | Partial synergy (FICI 0.5–1) | Addition (FICI 1) | Indifference (FICI 1–4) | Antagonism (FICI ≥4) |
|---|---|---|---|---|---|
| Number of isolates | 8 | 25 | 8 | 9 | 0 |
| Rate | 16% | 50% | 16% | 18% | 0 |
FICI, fractional inhibitory concentration index.
Majority of the 50 isolates are not susceptible to sulbactam and are susceptible to sitafloxacin
According to the susceptibility status of the 50 isolates to sulbactam and sitafloxacin, they were divided into the following four groups: i) Susceptible to both sulbactam and sitafloxacin (SS); ii) non-susceptible to sulbactam and susceptible to sitafloxacin (NS); iii) susceptible to sulbactam and non-susceptible to sitafloxacin (SN); and iv) non-susceptible to either sulbactam or sitafloxacin (NN). The data indicated that isolates in the SS group accounted for 14% of all strains, those in the NS group accounted for 78%, those in the SN group accounted for 2% and those in the NN group accounted for 6% (Table III). The results suggested that the majority of the 50 isolates were not susceptible to sulbactam and were susceptible to sitafloxacin.
Table III.
Distribution of strains susceptible and non-susceptible to sitafloxacin and sulbactam.
| Sulbactam | ||
|---|---|---|
| Number of isolates | Susceptible | Non-susceptible |
| Sitafloxacin | ||
| Susceptible | 7 (14%) | 39 (78%) |
| Non-susceptible | 1 (2%) | 3 (6%) |
Combined use of sitafloxacin and sulbactam at the concentration of 1X MIC exhibits bactericidal activity against all four isolates at 24 h, and synergistic effect on the four selected isolates
To conduct the time-kill studies, four representative strains were randomly selected from the four groups shown in Table III, respectively. Sulbactam and sitafloxacin displayed a synergistic effect on the strain selected from the SS group, with a FICI value of 0.375. Sulbactam and sitafloxacin had FICI values of 0.75 and 0.675 for strains selected from the NS and SN groups, respectively. Furthermore, sulbactam and sitafloxacin had an FICI value of 1.5 for the strain selected from the NN group. Time-kill curves for individual or combined use of sulbactam and sitafloxacin were plotted at 0.5X MIC and 1X MIC, respectively. Time-kill assays indicated that sitafloxacin exhibited a rapid bacteriostatic effect at the concentration of 1X MIC, regardless of the susceptibility status of the four isolates. Combined use of sitafloxacin and sulbactam at the concentration of 0.5X MIC exhibited a bactericidal effect for the strains from the SS and NS groups, and synergistic effect for all strains but that from NN group. Furthermore, combined use of sitafloxacin and sulbactam at the concentration of 1X MIC exhibited bactericidal activity, and synergistic effect against all four isolates at 24 h regardless of the susceptibility status (Figs. 1–4; Table IV). Combined use of sitafloxacin and sulbactam at the concentration of 1X MIC completely removed the strains from SS and NS groups with no regrowth after 24 h.
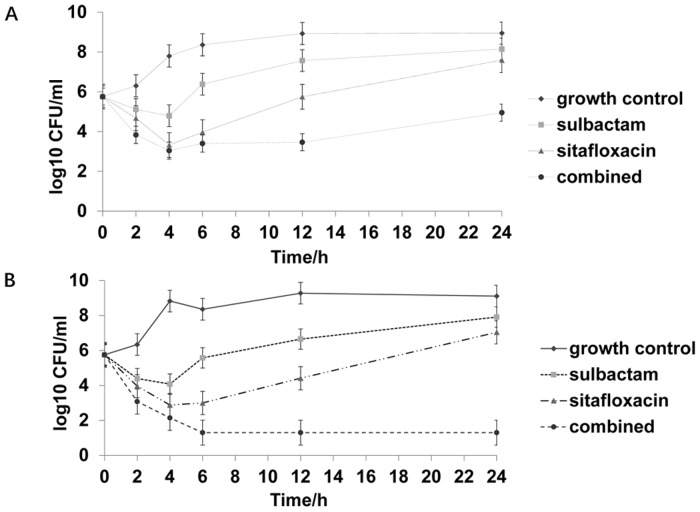
Figure 1.
Time-kill curve of one strain susceptible to both sulbactam and sitafloxacin. (A) 0.5X MIC sulbactam, 0.5X MIC sitafloxacin, and the combination of 0.5X MIC sulbactam and 0.5X MIC sitafloxacin. (B) 1X MIC sulbactam, 1X MIC sitafloxacin, and the combination of 1X MIC sulbactam and 1X MIC sitafloxacin. MIC, minimum inhibitory concentration; CFU, colony-forming unit.
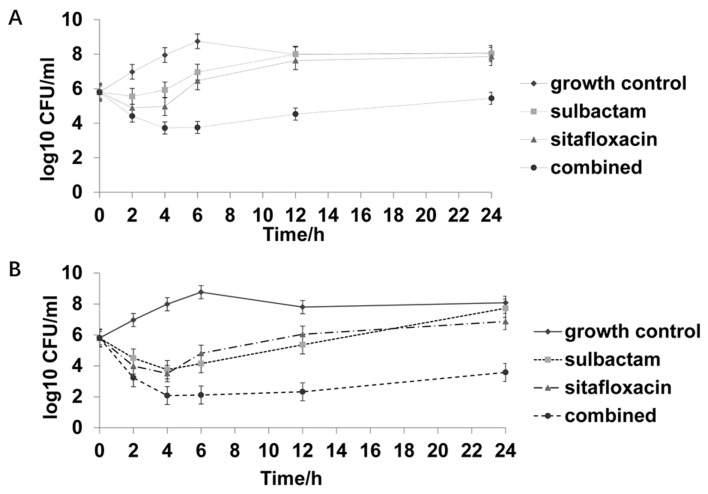
Figure 4.
Time-kill curve of one strain not susceptible to either sulbactam or sitafloxacin. (A) 0.5X MIC sulbactam, 0.5X MIC sitafloxacin, and the combination of 0.5X MIC sulbactam and 0.5X MIC sitafloxacin. (B) 1X MIC sulbactam, 1X MIC sitafloxacin, and the combination of 1X MIC sulbactam and 1X MIC sitafloxacin. MIC, minimum inhibitory concentration; CFU, colony-forming unit.
Table IV.
Results of time-kill assay for the four strains under different drug concentrations.
| Results | Strains under a drug concentration of 0.5X MIC | Strains under a drug concentration of 1X MIC |
|---|---|---|
| Bactericidal activities | SS, NS, SN, NN | |
| Synergy | SS, NS, SN | |
| Indifferent | NN |
SS, susceptible to sulbactam and sitafloxacin; NS, non-susceptible to sulbactam and susceptible to sitafloxacin; SN, susceptible to sulbactam and non-susceptible to sitafloxacin; NN, non-susceptible to sulbactam and sitafloxacin.
Discussion
A. baumannii is one of the most common causes of nosocomial infection in Asia (8). The risk factors of A. baumannii infection include long stay in hospital or intensive care unit, mechanical ventilation, invasive operation, antibiotic exposure, multiple infections, diabetes and COPD (14). A. baumannii can form biofilms, which can survive in various implants (catheterization, endotracheal intubation and deep vein catheterization) for a long time (15). Patients often merge with basic diseases, multiple infections or invasive operations. Their immunity is weak and requires longer treatment time compared with other infectious diseases. Therefore, the clinical treatment of A. baumannii infection is usually longer. Chinese experts recommend combination therapy for the treatment of drug resistant A. baumannii (16). This pathogen harbors multiple resistance mechanisms and reduces therapeutic choices to a limited set of active antibiotics (16). With the rise of MDR, XDR and PDR strains globally (17–20), there is a requirement for the development of safe and effective therapeutic strategies. Fluoroquinolones have a broad-spectrum antimicrobial activity and are commonly used in clinical practice (21). At present, the majority of nosocomial isolates of A. baumannii are resistant to fluoroquinolones (22–24), and, therefore, fluoroquinolones are not ideal for the empirical treatment of A. baumannii-associated infections. A new fluoroquinolone antibiotic, sitafloxacin, has demonstrated a good in vitro activity against pathogens that are resistant to other fluoroquinolones (25,26). Sitafloxacin has been reported to exhibit acceptable antimicrobial effects against carbapenem-resistant A. baumannii and the respective susceptibility rates were 91.4 and 58.9% according to two separate reports (12,27). Sulbactam exhibits affinities for penicillin-binding proteins and inhibits bacterial cell wall synthesis (28,29). Sulbactam is active against A. baumannii and has been clinically used for infections caused by this organism (30). In addition, sulbactam exhibits synergistic effects with other antibiotics (31).
In the present study, sitafloxacin induced a promising antimicrobial activity against XDR-A. baumannii with a susceptibility rate of 92%. Furthermore, combined use of sitafloxacin with sulbactam resulted in a susceptibility rate of 96%. Sitafloxacin and sulbactam exhibited synergistic effects in 16% of the 50 strains. In addition, sitafloxacin and sulbactam have shown partial synergistic effect on 50% of strains. None of the strains exhibited antagonistic effects. However, Odds (32) proposed alternative criteria for interpretation of FICI values. The authors suggested that researchers submitting research articles containing FICI data should interpret synergy as FICI ≤0.5, antagonism as FICI >4.0 and no interaction as FICI >0.5–4.0. Additionally, these authors argued that their proposed criteria would encourage conservative interpretation of results (32). According to the suggestions by Odds (32), sitafloxacin and sulbactam exhibited no interaction in 84% of XDR-A. baumannii strains included in the present study. The time-kill assay was performed to further investigate the bactericidal activity of the two drugs and their combination (33,34).
Time-kill assays performed in the present study indicated that sitafloxacin induced a rapid bacteriostatic effect at the concentration of 1X MIC, regardless of the susceptibility status of the four isolates. Combined use of sitafloxacin and sulbactam at the concentration of 0.5X MIC only exhibited bactericidal effects for the strains susceptible to sitafloxacin. In addition, combined use of sitafloxacin and sulbactam at the concentration of 1X MIC exhibited bactericidal activity against all four isolates at 24 h. The strains from the SS and NS groups were completely removed with no regrowth after 24 h when sitafloxacin and sulbactam are combined at the concentration of 1X MIC. Combined use of sitafloxacin and sulbactam at the concentration of 0.5X MIC exhibited a synergistic effect for all strains except for the NN group strain. In addition, synergistic effect was achieved for all four isolates, regardless of the susceptibility status, when both drugs are used at the concentration of 1X MIC. The results of the present study indicated that the combination of sitafloxacin and sulbactam was more efficient at eliminating isolates that were susceptible to sitafloxacin compared with isolates that were non-susceptible to sitafloxacin.
Sitafloxacin and sulbactam combination could be a promising alternative treatment for XDR- A. baumannii infection. However, in vitro experimental results do not necessarily correspond with clinical efficacy (35), which may be the result of the metabolism of the agents and the discordant redistribution of different agents in target tissues (24). Still, in vitro experiments provide a convenient way to screen compounds to propose combinations that could be synergistic in vivo.
In conclusion, the present study indicated that sulbactam enhanced the bactericidal activity of sitafloxacin and this combination revealed synergistic or partial synergistic effect in the majority of cases. For strains that were non-susceptible to sitafloxacin, the bactericidal activities of the combination of sitafloxacin and sulbactam were impaired at a concentration that is lower than its MIC. However, this impairment was overcome by increasing the concentration to 1X MIC. In future investigations, insights should be gained into the clinical impact of the combination of sitafloxacin and sulbactam, and the possible benefits associated with the application of this combination.
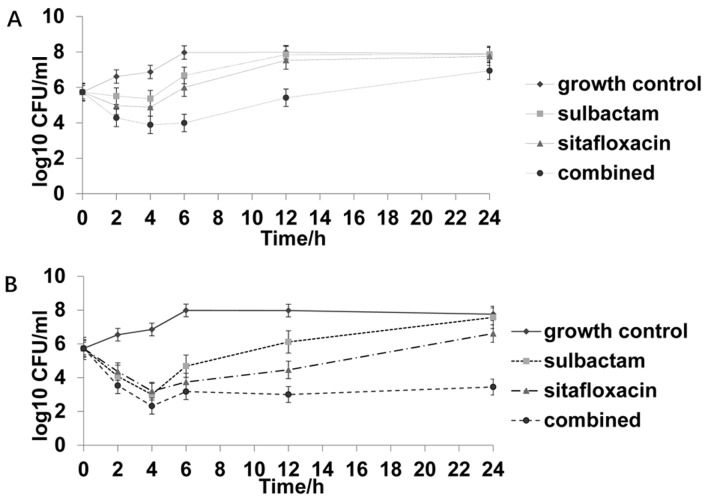
Figure 2.
Time-kill curve of one strain not susceptible to sulbactam and susceptible to sitafloxacin. (A) 0.5X MIC sulbactam, 0.5X MIC sitafloxacin, and the combination of 0.5X MIC sulbactam and 0.5X MIC sitafloxacin. (B) 1X MIC sulbactam, 1X MIC sitafloxacin, and the combination of 1X MIC sulbactam and 1X MIC sitafloxacin. MIC, minimum inhibitory concentration; CFU, colony-forming unit.
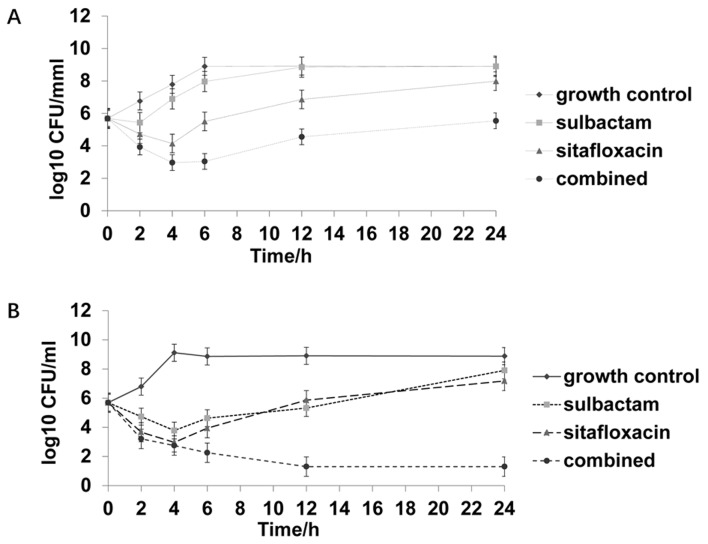
Figure 3.
Time-kill curve of one strain not susceptible to sitafloxacin and susceptible to sulbactam. (A) 0.5X MIC sulbactam, 0.5X MIC sitafloxacin, and the combination of 0.5X MIC sulbactam and 0.5X MIC sitafloxacin. (B) 1X MIC sulbactam, 1X MIC sitafloxacin, and the combination of 1X MIC sulbactam and 1X MIC sitafloxacin. MIC, minimum inhibitory concentration; CFU, colony-forming unit.
Acknowledgements
The authors wish to thank their department and research team for their help and dedication.
Funding
The present study was supported by the 2015 Scientific Medicine Development Fund of Shandong Province (grant no. 2015WS0290).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
The final version of the manuscript has been read and approved by all authors, and each author believes that the manuscript represents honest work. NX, GW and QX collaborated to design the study. NX, GW, YL, XD and FC were responsible for performing experiments. NX, GW and YL analyzed the data. All authors collaborated to interpret results and develop the manuscript.
Ethics approval and consent to participate
All procedures performed in the current study were approved by the Ethics Committee of Shandong University (Jinan, China). Written informed consent was obtained from all patients or their families.
Patient consent for publication
Written informed consents for publication of any associated data and accompanying images were obtained from all patients or their parents, guardians or next of kin.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lee K, Yong D, Jeong SH, Chong Y. Multidrug-resistant acinetobacter spp.: Increasingly problematic nosocomial pathogens. Yonsei Med J. 2011;52:879–891. doi: 10.3349/ymj.2011.52.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: Emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerqueira GM, Peleg AY. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life. 2011;63:1055–1060. doi: 10.1002/iub.533. [DOI] [PubMed] [Google Scholar]
- 4.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 5.Corbella X, Montero A, Pujol M, Domínguez MA, Ayats J, Argerich MJ, Garrigosa F, Ariza J, Gudiol F. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J Clin Microbiol. 2000;38:4086–4095. doi: 10.1128/jcm.38.11.4086-4095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viehman JA, Nguyen MH, Doi Y. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs. 2014;74:1315–1333. doi: 10.1007/s40265-014-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Q, Li X, Zhou H, Jiang Y, Chen Y, Hua X, Yu Y. Decreased susceptibility to tigecycline in Acinetobacter baumannii mediated by a mutation in trm encoding sam-dependent methyltransferase. J Antimicrob Chemother. 2014;69:72–76. doi: 10.1093/jac/dkt319. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute, corp-author. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute; Wayne, PA: 2015. [Google Scholar]
- 9.Sader HS, Flamm RK, Jones RN. Antimicrobial activity of daptomycin tested against gram-positive pathogens collected in europe, latin america, and selected countries in the asia-pacific region (2011) Diagn Microbiol Infect Dis. 2013;75:417–422. doi: 10.1016/j.diagmicrobio.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Ji J, Du X, Chen Y, Fu Y, Wang H, Yu Y. In vitro activity of sulbactam in combination with imipenem, meropenem, panipenem or cefoperazone against clinical isolates of Acinetobacter baumannii. Int J Antimicrob Agents. 2013;41:400–401. doi: 10.1016/j.ijantimicag.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Vidaillac C, Benichou L, Duval RE. In vitro synergy of colistin combinations against colistin-resistant Acinetobacter baumanniiPseudomonas aeruginosa, and klebsiella pneumoniae isolates. Antimicrob Agents Chemother. 2012;56:4856–4861. doi: 10.1128/AAC.05996-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YS, Wang JT, Sheng WH, Chuang YC, Chang SC. Comparative in vitro activity of sitafloxacin against bacteremic isolates of carbapenem resistant Acinetobacter baumannii complex. J Microbiol Immunol Infect. 2015;48:545–551. doi: 10.1016/j.jmii.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Chung DR, Song JH, Kim SH, Thamlikitkul V, Huang SG, Wang H, So TM, Yasin RM, Hsueh PR, Carlos CC, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in asia. Am J Respir Crit Care Med. 2011;184:1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 14.Munoz-price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358:1271–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 15.Djeribi R, Bouchloukh W, Jouenne T, Menaa B. Characterization of bacterial biofilms formed on urinary catheters. Am J Infect Control. 2012;40:854–859. doi: 10.1016/j.ajic.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Doi Y, Murray GL, Peleg AY. Acinetobacter baumannii: Evolution of antimicrobial resistance - treatment options. Semin Respir Crit Care Med. 2015;36:85–98. doi: 10.1055/s-0034-1398388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 18.Potron A, Poirel L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii Mechanisms and epidemiology. Int J Antimicrob Agents. 2015;45:568–585. doi: 10.1016/j.ijantimicag.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Göttig S, Gruber TM, Higgins PG, Wachsmuth M, Seifert H, Kempf VA. Detection of pan drug-resistant Acinetobacter baumannii in germany. J Antimicrob Chemother. 2014;69:2578–2579. doi: 10.1093/jac/dku170. [DOI] [PubMed] [Google Scholar]
- 20.Karah N, Haldorsen B, Hermansen NO, Tveten Y, Ragnhildstveit E, Skutlaberg DH, Tofteland S, Sundsfjord A, Samuelsen Ø. Emergence of OXA-carbapenemase- and 16S rRNA methylase-producing international clones of Acinetobacter baumannii in norway. J Med Microbiol. 2011;60:515–521. doi: 10.1099/jmm.0.028340-0. [DOI] [PubMed] [Google Scholar]
- 21.Sharma PC, Jain A, Jain S. Fluoroquinolone antibacterials: A review on chemistry, microbiology and therapeutic prospects. Acta Pol Pharm. 2009;66:587–604. [PubMed] [Google Scholar]
- 22.Krzyściak P, Chmielarczyk A, Pobiega M, Romaniszyn D, Wójkowska-Mach J. Acinetobacter baumannii isolated from hospital-acquired infection: Biofilm production and drug susceptibility. APMIS. 2017;125:1017–1026. doi: 10.1111/apm.12739. [DOI] [PubMed] [Google Scholar]
- 23.Saroj SD, Clemmer KM, Bonomo RA, Rather PN. Novel mechanism for fluoroquinolone resistance in Acinetobacter baumannii. Antimicrob Agents Chemother. 2012;56:4955–4957. doi: 10.1128/AAC.00739-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grochowalska A, Kozioå-Montewka M, Sobieszczańska A. Analysis of Acinetobacter baumannii resistance patterns in patients with chronic obstructive pulmonary disease (copd) in terms of choice of effective empiric antibiotic therapy. Ann Agric Environ Med. 2017;24:307–311. doi: 10.26444/aaem/74710. [DOI] [PubMed] [Google Scholar]
- 25.Keating GM. Sitafloxacin: In bacterial infections. Drugs. 2011;71:731–744. doi: 10.2165/11207380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Deguchi T, Yasuda M, Kawamura T, Nakano M, Ozeki S, Kanematsu E, Nishino Y, Kawada Y. Improved antimicrobial activity of DU-6859a, a new fluoroquinolone, against quinolone-resistant klebsiella pneumoniae and enterobacter cloacae isolates with alterations in GyrA and ParC proteins. Antimicrob Agents Chemother. 1997;41:2544–2546. doi: 10.1128/aac.41.11.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thamlikitkul V, Tiengrim S. In vitro activity of sitafloxacin against carbapenem-resistant Acinetobacter baumannii. Int J Antimicrob Agents. 2013;42:284–285. doi: 10.1016/j.ijantimicag.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Rafailidis PI, Ioannidou EN, Falagas ME. Ampicillin/sulbactam: Current status in severe bacterial infections. Drugs. 2007;67:1829–1849. doi: 10.2165/00003495-200767130-00003. [DOI] [PubMed] [Google Scholar]
- 29.Penwell WF, Shapiro AB, Giacobbe RA, Gu RF, Gao N, Thresher J, McLaughlin RE, Huband MD, DeJonge BL, Ehmann DE, Miller AA. Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii. Antimicrob Agents Chemother. 2015;59:1680–1689. doi: 10.1128/AAC.04808-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urban C, Go E, Mariano N, Rahal JJ. Interaction of sulbactam, clavulanic acid and tazobactam with penicillin-binding proteins of imipenem-resistant and -susceptible acinetobacter baumannii. FEMS Microbiol Lett. 1995;125:193–197. doi: 10.1111/j.1574-6968.1995.tb07357.x. [DOI] [Google Scholar]
- 31.Poulikakos P, Tansarli GS, Falagas ME. Combination antibiotic treatment versus monotherapy for multidrug-resistant, extensively drug-resistant, and pandrug-resistant acinetobacter infections: A systematic review. Eur J Clin Microbiol Infect Dis. 2014;33:1675–1685. doi: 10.1007/s10096-014-2124-9. [DOI] [PubMed] [Google Scholar]
- 32.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 33.Nageeb W, Metwally L, Kamel M, Zakaria S. In vitro antimicrobial synergy studies of carbapenem-resistant Acinetobacter baumannii isolated from intensive care units of a tertiary care hospital in egypt. J Infect Public Health. 2015;8:593–602. doi: 10.1016/j.jiph.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Phee LM, Betts JW, Bharathan B, Wareham DW. Colistin and fusidic acid, a novel potent synergistic combination for treatment of multidrug-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother. 2015;59:4544–4550. doi: 10.1128/AAC.00753-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simsek F, Gedik H, Yildirmak MT, Iris NE, Türkmen A, Ersoy A, Ersöz M, Gücüyener A. Colistin against colistin-only-susceptible Acinetobacter baumannii-related infections: Monotherapy or combination therapy? Indian J Med Microbiol. 2012;30:448–452. doi: 10.4103/0255-0857.103767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.