Abstract
Endometriosis (EM) is a common benign gynecological disorder. The present study aimed to investigate the potential role of propofol, a commonly used intravenous anesthetic agent, in the pathogenesis of EM. The EM cell line CRL-7566 was used in the present study. CRL-7566 cells were first treated with various concentrations of propofol (0, 1, 5 or 10 µg/ml) for specific duration, and the cell viability and apoptotic rate were determined by performing an MTT and a flow cytometric cell apoptosis assay, respectively. The protein and mRNA levels of cell proliferation- and apoptosis-associated genes were detected by western blot and reverse-transcription quantitative polymerase chain reaction, respectively. The results demonstrated that propofol inhibited CRL-7566 cell proliferation in a dose- and time-dependent manner. CRL-7566 cell apoptosis was dose-dependently induced by propofol treatment. In addition, propofol treatment significantly increased the levels of forkhead box (FOX)O1, FOXO3, Bim, pro-caspase-3, active caspase-3, p53 and p21. In conclusion, the present study suggested that propofol inhibited the proliferation and induced apoptosis of EM cells via inducing the expression/activation of multiple associated genes/proteins, indicating a protective role of propofol in EM.
Keywords: endometriosis, CRL-7566 cell line, propofol, cell proliferation, apoptosis
Introduction
Endometriosis (EM), caused by the presence of the active endometrium at the outer side of the uterine cavity, is a common gynecological disease (1,2). Most of EM lesions are located in the pelvic genital organs and the organs adjacent to the peritoneal surface; this is referred to as pelvic EM, of which ovarian EM is the most common type. The pathological process of EM comprises periodic bleeding, migration of uterine cells and their attachment to other organs. In general, EM is only seen in women of childbearing age, mostly at the age of 25–45 years (3,4). The major symptoms of the disease are dysmenorrhea, chronic pelvic pain and infertility that seriously affect the quality of life of young women (5). The incidence of EM is 10–15%, and a significant increase has been observed in recent years (2,6). Although EM is a benign lesion, it has a similar biological behavior to that of malignant tumors, including invasion, distant metastasis and spread (7,8). To date, the pathogenesis of EM has remained to be fully elucidated. In recent years, research has focused on the association between EM and apoptosis. An increasing number of studies have demonstrated that spontaneous apoptosis of endometrial cells is a key factor in maintaining the normal structure and function of endometrial tissue, while ectopic endometrial cells may settle in the uterine cavity and continue to survive, which is due to a low rate of spontaneous apoptosis and sensitivity to apoptotic signals as well as other changes in apoptotic characteristics (9). Thus, excessive proliferation and apoptosis-evading properties of EM cells are considered to participate in the development of EM (10,11).
Propofol is a commonly used intravenous anesthetic agent, which is widely used in various types of surgery due to its short-term effects and rapid recovery. However, increasing evidence has demonstrated that propofol has numerous non-anesthetic effects (12). In recent years, studies have confirmed the anti-cancer properties of propofol, including the repression of cell metastasis, adhesion and proliferation as well as induction of cell apoptosis (13–15). Thus, propofol has an important role in maintaining the balance of cell proliferation and apoptosis, the dysregulation of which is implicated in EM-associated processes.
To the best of our knowledge, the role of propofol in the development of EM has not been previously reported. Therefore, the present study aimed to investigate the role of propofol in EM by exploring the effects of propofol on the biological behavior of EM cells, as well as to elucidate the underlying molecular mechanisms.
Materials and methods
Cell culture
The EM cell line CRL-7566 was obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were grown in Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin solution at 37°C in a humidified atmosphere containing 5% CO2.
Cell treatment
Propofol was obtained from Corden Pharma Caponago S.P.A. (Milan, Italy) and dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The day before the cell treatments, the CRL-7566 cells were seeded on 96-well plates and then incubated under standard conditions for 12–18 h. The cells were then treated with various concentrations of propofol (0, 1, 5 or 10 µg/ml) for specific durations. The cells in the control group were treated with DMSO only and the final DMSO concentration was 0.2%. The cell suspension was then harvested for subsequent analyses.
MTT assay
To detect the cell viability, an MTT assay was applied in the present study. In brief, CRL-7566 cells were collected, re-suspended and then re-seeded in 96-well plates (1×104 cells/well). The cells were then treated with different concentrations of propofol (0, 1, 5, 10 µg/ml) for 24, 48 or 72 h. Subsequently, 10 µl MTT (Sigma-Aldrich; Merck KGaA) was added to each well, followed by incubation for 4 h at 37°C. The medium in each well was then replaced with 150 µl DMSO. At the end of this experiment, the optical density was read at 490 nm by using a microplate reader. All tests were performed in triplicate.
Flow cytometric analysis
In the present study, fluorescence-assisted cell sorting (FACS) following fluorescein isothiocyanate (FITC)-Annexin V and propidium iodide (PI) double labeling was performed to determine cell apoptosis in different groups by using an Annexin V-FITC/PI Apoptosis Detection Kit (cat. no. 6592; Cell Signaling Technology, Inc., Danvers, MA, USA). In brief, at 24 h after treatment with propofol, the CRL-7566 cells were collected with trypsin and then re-suspended in a binding buffer. The cells were then labeled with Annexin V-FITC and PI according to the manufacturer's instructions, and then incubated for 15 min without light at room temperature. Subsequently, FACS (BD Biosciences, Franklin Lakes, NJ, USA) was performed for flow cytometric analysis and the percentage of apoptotic cells was calculated. All tests were performed in triplicate.
Reverse-transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA from the EM cells was extracted by using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (1 µg) was reversely transcribed into complementary (c)DNA by using a Maxima First Strand cDNA Synthesis kit (cat. no. K1641; Fermentas, Vilnius, Lithuania) according to the manufacturer's recommended protocol. qPCR was performed using a 2X Maxima SYBR-Green/ROX qPCR Master Mix kit (cat. no. K0221; Fermentas). The amplification program was initial denaturation for 2 min at 95°C, followed by 37 cycles of 30 sec at 95°C and 60 sec at 60°C. GAPDH was used as an internal control. The primer sequences (Genscript, Nanjing, China) were as following: FOXO1 forward, 5′-TCGTCATAATCTGTCCCTACACA-3′ and reverse, 5′-CGGCTTCGGCTCTTAGCAAA-3′; FOXO3 forward, 5′-CGGACAAACGGCTCACTCT-3′ and reverse, 5′-GGACCCGCATGAATCGACTAT-3′; Bim forward, 5′-CATATAACCCCGTCAACGCAG-3′ and reverse, 5′-GCAGCCGCCACAAACATAC-3′; p21 forward, 5′-TAGCAGCGGAACAAGGAG-3′ and reverse, 5′-AAACGGGAACCAGGACAC-3′; p53 forward, 5′-CCACCATCCACTACAACTAC-3′ and reverse, 5′-AAACACGCACCTCAAAGC-3′; and GAPDH forward, 5′-GAAGGTGAAGGTCGGAGTC-3 and reverse, 5′-GAAGATGGTGATGGGATTTC-3′. Relative gene expression was calculated by using the 2−ΔΔCq method (16).
Western blot analysis
After incubation for 24 h, CRL-7566 cells were harvested using trypsin, centrifuged and then lysed in radioimmunoprecipitation assay buffer. The extracted total protein was measured by using a bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific, Inc.). Protein samples (30 µg/lane) were resolved by 12% SDS-PAGE and then transferred to a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). Membranes were then washed with PBS and blocked with 5% non-fat milk for 1.5 h. Subsequently, the membranes were incubated overnight at 4°C with the following primary antibodies: Forkhead box (FOX)O1 (cat no. 2880), FOXO3 (cat no. 2497), Bim (cat no. 2933), pro-caspase-3 (cat no. 9665), active caspase-3 (cat no. 9664), p53 (cat no. 2527) and p21 (cat no. 2947; all dilution, 1:1,000; Cell Signaling Technology, Inc.), followed by incubation with anti-rabbit immunoglobulin G horseradish peroxidase-coupled secondary antibodies (cat no. 7074; 1:1,000; Cell Signaling Technology, Inc.) at room temperature for 2 h. The protein bands were finally visualized by using enhanced chemiluminescent reagent (EMD Millipore, Billerica, MA, USA) and imaged by the ChemiDoc XRS+ System (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance followed by Tukey's test or Student's t-test was used to assess the statistical significance of differences between groups. All values are expressed as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
Propofol inhibits CRL-7566 cell proliferation
The EM cell line CRL-7566 was treated with various concentrations of propofol (0, 1, 5 or 10 µg/ml) for 24, 48 or 72 h and the cell viability was analyzed by an MTT assay for determination of the inhibition rate. As presented in Fig. 1, the proliferation of CRL-7566 cells was significantly inhibited by propofol in a dose- and time-dependent manner.
Figure 1.
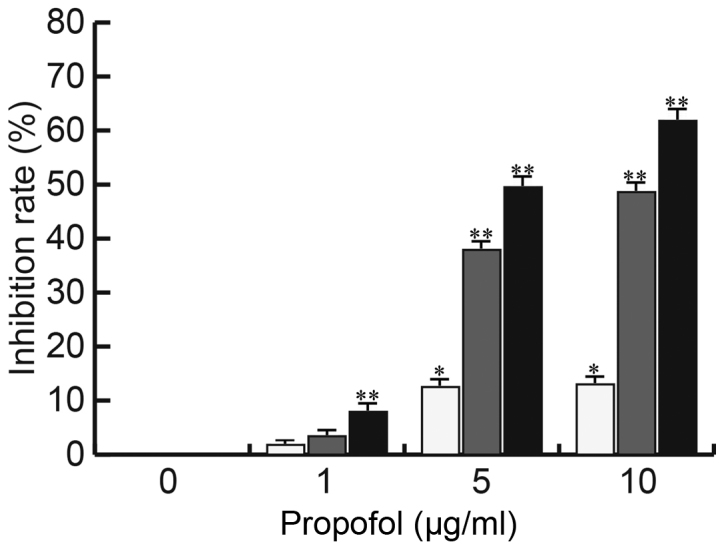
Effect of propofol on CRL-7566 cell proliferation. CRL-7566 cells were treated with various concentrations of propofol (0, 1, 5 or 10 µg/ml) for 24, 48 or 72 h. The cell viability of cells from different groups was then assessed by an MTT assay. Values are expressed as the mean ± standard deviation. *P<0.05, **P<0.01 vs. control group.
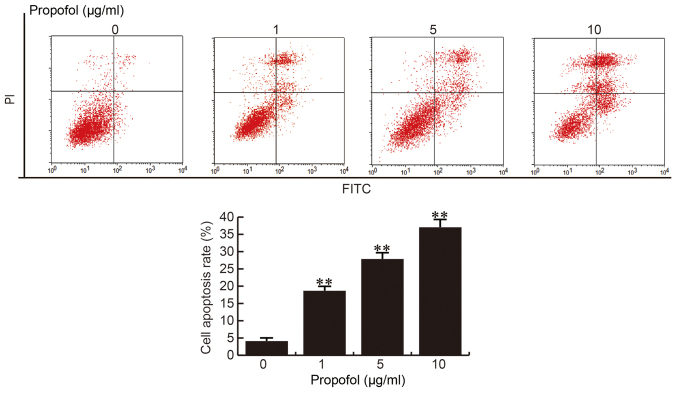
Propofol induces CRL-7566 cell apoptosis
Following 24 h of treatment with different concentrations of propofol (0, 1, 5 or 10 µg/ml), CRL-7566 cells were collected and subjected to cell apoptosis analysis. The results of the cell apoptosis assay demonstrated that compared with the control group, the apoptotic rate of CRL-7566 cells was significantly increased in the propofol treatment groups, and the apoptotic rate was enhanced with the increase of the propofol concentration (Fig. 2). These results indicted that propofol promotes CRL-7566 cell apoptosis in a dose-dependent manner.
Figure 2.
Effect of propofol on CRL-7566 cell apoptosis. After 24 h of treatment with various concentrations of propofol (0, 1, 5 or 10 µg/ml), percentages of apoptotic cells in the different groups were measured by Annexin V/FITC and PI staining followed by flow cytometric analysis. Values are expressed as the mean ± standard deviation. **P<0.01 vs. control group. PI, propidium iodide; FITC, fluorescein isothiocyanate.
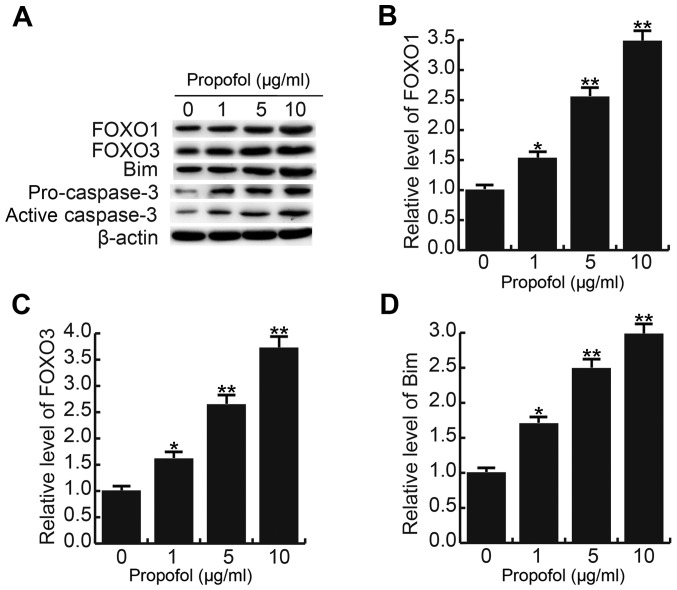
Propofol enhances FOXO1, FOXO3, Bim, pro-caspase-3 and active caspase-3 levels in CRL-7566 cells
After 24 h of treatment with different concentrations of propofol (0, 1, 5 or 10 µg/ml), the levels of cell proliferation- and apoptosis-associated proteins/genes were detected by western blot and RT-qPCR analysis, respectively. It was observed that treatment with propofol increased the protein levels of FOXO1, FOXO3, Bim, pro-caspase-3 and active caspase-3 in CRL-7566 cells in a dose-dependent manner (Fig. 3A). The mRNA levels of FOXO1, FOXO3 and Bim were also dose-dependently increased by propofol treatment (Fig. 3B-D).
Figure 3.
Effect of propofol on FOXO1, FOXO3, Bim, pro-caspase-3 and active caspase-3 levels in CRL-7566 cells. After 24 h of treatment with various concentrations of propofol (0, 1, 5 or 10 µg/ml), (A) the protein levels of FOXO1, FOXO3, Bim, pro-caspase-3 and active caspase-3 in CRL-7566 cells were determined by western blot analysis, and (B-D) the mRNA levels of (B) FOXO1, (C) FOXO3 and (D) Bim were determined by reverse-transcription quantitative polymerase chain reaction. Values are expressed as the mean ± standard deviation. *P<0.05, **P<0.01 vs. control group. FOXO, forkhead box O.
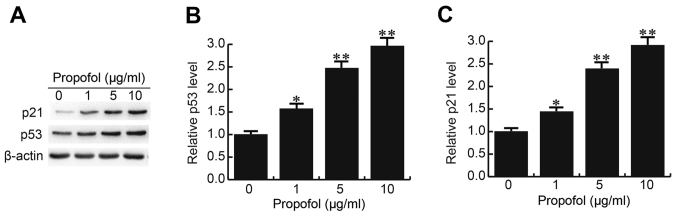
Propofol enhances p53 and p21 expression in CRL-7566 cells
p53 and p21, which were previously reported to be downregulated in EM cells and to have critical roles in cell growth regulation (17,18), were also assessed in the present study. As expected, propofol increased the mRNA and protein expression levels of p53 and p21 in CRL-7566 cells in a dose-dependent manner (Fig. 4).
Figure 4.
Effect of propofol on p53 and p21 expression in CRL-7566 cells. After 24 h of treatment with various concentrations of propofol (0, 1, 5 or 10 µg/ml), the (A) protein and (B and C) mRNA and expression levels of p53 and p21 in CRL-7566 cells were determined by western blot and reverse-transcription quantitative polymerase chain reaction analysis, respectively. Values are expressed as the mean ± standard deviation. *P<0.05, **P<0.01 vs. control group.
Discussion
EM, a prevalent and complex gynecological disease, affects the health and life quality of ~10% of women of reproductive age (19). Multiple factors may contribute to the development and progression of EM, including environmental factors, the immune response and hormones (20–22). EM is benign; however, it has the characteristics of malignant tumors, including metastasis, infiltration and recurrence (7,8). To date, the treatment outcomes of EM have remained unsatisfactory, and the pathogenesis of EM has not been fully elucidated (23). The association between apoptosis and EM has attracted the attention of numerous researchers (9–11). Cell apoptosis, also known as programmed cell death, is required for the maintenance of tissue homeostasis, and is a type of cell death of independent order that is controlled by specific genes (24). Cell apoptosis has an important role in the upstate and steady-state maintenance of normal tissue as well as the prevention and treatment of diseases (25). Following its initiation, the occurrence of cell apoptosis is achieved by transduction of a variety of cell apoptosis signals and is controlled by apoptosis-associated genes (26). In recent years, the association between EM and apoptosis has received increasing attention from researchers (27).
Propofol is an intravenous sedative-hypnotic agent, which is employed in the clinic to induce and sustain anesthesia. Various studies have suggested that propofol prevents cancer procession through directly and indirectly inhibiting cancer cell viability and proliferation by facilitating cell apoptosis (28–30). Therefore, propofol has critical roles in regulating the balance of cell proliferation and apoptosis. To date, the effect of propofol on EM has remained elusive. Thus, the present study investigated the potential role of propofol in the pathogenesis of EM. It was revealed that propofol inhibited the proliferation of the EM cell line CRL-7566 in a dose- and time-dependent manner, and cell apoptosis was dose-dependently induced by propofol treatment. These results indicated that propofol may have a protective role in EM.
To further explore the underlying mechanism of the inhibition of cell proliferation and promotion of cell apoptosis caused by propofol, the levels of FOXO1, FOXO3, Bim, pro-caspase-3, active caspase-3, p53 and p21 in CRL-7566 cells were determined. FOXO participates in the growth and apoptosis of cells through directly promoting the expression of FOXO3a-dependent apoptotic protein Bim (31,32) and the activation of caspase family proteins (33). In addition, p53 and p21, which have important roles in the regulation of cell apoptosis and have been reported to be downregulated in EM (17,18), were analyzed in the present study. The results demonstrated that propofol treatment significantly increased the levels of FOXO1, FOXO3, Bim, pro-caspase-3, active caspase-3, p53 and p21, indicating that propofol exerts its roles in EM via affecting the expression levels and activation of cell growth- and apoptosis-associated genes/proteins.
In conclusion, to the best of our knowledge, the present study was the first to reveal that propofol had a protective role in EM though inhibiting cell proliferation and inducing cell apoptosis via regulating the expression/activation of multiple cell proliferation- and apoptosis-associated genes/proteins in EM cells. The results provide a scientific basis for the development of novel clinical treatments for EM.
Acknowledgements
The authors would like to thank Professor Xiuping Lv and Professor Zhaori Chen (Department of Gynecology, Affiliated Hospital of Weifang Medical University, Weifang, China), and Professor Kechang Huang (Department of Anesthesiology, Affiliated Hospital of Weifang Medical University, Weifang, China) for their guidance and assistance.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Authors' contributions
SF and YS collaborated to design the study, access and analysis the data, interpret the results, and write the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nyholt DR, Low SK, Anderson CA, Painter JN, Uno S, Morris AP, MacGregor S, Gordon SD, Henders AK, Martin NG, et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet. 2012;44:1355–1359. doi: 10.1038/ng.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann NY Acad Sci. 2002;955:11–22; discussion 34–36, 396–406. doi: 10.1111/j.1749-6632.2002.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 3.Benagiano G, Habiba M, Brosens I. The pathophysiology of uterine adenomyosis: An update. Fertil Steril. 2012;98:572–579. doi: 10.1016/j.fertnstert.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Maheshwari A, Gurunath S, Fatima F, Bhattacharya S. Adenomyosis and subfertility: A systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update. 2012;18:374–392. doi: 10.1093/humupd/dms006. [DOI] [PubMed] [Google Scholar]
- 5.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 6.Tandrasasmita OM, Sutanto AM, Arifn PF, Tjandrawinata RR. Anti-inflammatory, antiangiogenic, and apoptosis-inducing activity of DLBS1442, a bioactive fraction of Phaleria macrocarpa, in a RL95-2 cell line as a molecular model of endometriosis. Int J Womens Health. 2015;7:161–169. doi: 10.2147/IJWH.S74552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kodaman PH. Current strategies for endometriosis management. Obstet Gynecol Clin North Am. 2015;42:87–101. doi: 10.1016/j.ogc.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Grimstad FW, Carey E. Periclitoral endometriosis: The dilemma of a chronic disease invading a rare location. J Minim Invasive Gynecol. 2015;22:684–686. doi: 10.1016/j.jmig.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Agic A, Djalali S, Diedrich K, Hornung D. Apoptosis in endometriosis. Gynecol Obstet Invest. 2009;68:217–223. doi: 10.1159/000235871. [DOI] [PubMed] [Google Scholar]
- 10.Nasu K, Yuge A, Tsuno A, Nishida M, Narahara H. Involvement of resistance to apoptosis in the pathogenesis of endometriosis. Histol Histopathol. 2009;24:1181–1192. doi: 10.14670/HH-24.1181. [DOI] [PubMed] [Google Scholar]
- 11.Nishida M, Nasu K, Ueda T, Fukuda J, Takai N, Miyakawa I. Endometriotic cells are resistant to interferon-gamma-induced cell growth inhibition and apoptosis: A possible mechanism involved in the pathogenesis of endometriosis. Mol Hum Reprod. 2005;11:29–34. doi: 10.1093/molehr/gah133. [DOI] [PubMed] [Google Scholar]
- 12.Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, Katsargyris A, Papadimitriou L. Propofol: A review of its non-anaesthetic effects. Eur J Pharmacol. 2009;605:1–8. doi: 10.1016/j.ejphar.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Wang N, Zhou S, Ye W, Jing G, Zhang M. Propofol induces proliferation and invasion of gallbladder cancer cells through activation of Nrf2. J Exp Clin Cancer Res. 2012;31:66. doi: 10.1186/1756-9966-31-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang ZT, Gong HY, Zheng F, Liu DJ, Yue XQ. Propofol suppresses proliferation and invasion of gastric cancer cells via downregulation of microRNA-221 expression. Genet Mol Res. 2015;14:8117–8124. doi: 10.4238/2015.July.17.20. [DOI] [PubMed] [Google Scholar]
- 15.Wang ZT, Gong HY, Zheng F, Liu DJ, Dong TL. Propofol suppresses proliferation and invasion of pancreatic cancer cells by upregulating microRNA-133a expression. Genet Mol Res. 2015;14:7529–7537. doi: 10.4238/2015.July.3.28. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Agui T, McConkey DJ, Tanigawa N. Comparative study of various biological parameters, including expression of survivin, between primary and metastatic human colonic adenocarcinomas. Anticancer Res. 2002;22:1769–1776. [PubMed] [Google Scholar]
- 18.Palazzo JP, Mercer WE, Kovatich AJ, McHugh M. Immunohistochemical localization of p21(WAF1/CIP1) in normal, hyperplastic, and neoplastic uterine tissues. Hum Pathol. 1997;28:60–66. doi: 10.1016/S0046-8177(97)90280-X. [DOI] [PubMed] [Google Scholar]
- 19.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Painter JN, Anderson CA, Nyholt DR, Macgregor S, Lin J, Lee SH, Lambert A, Zhao ZZ, Roseman F, Guo Q, et al. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet. 2011;43:51–54. doi: 10.1038/ng.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson JL, Elias S, Malinak LR, Buttram VC., Jr Heritable aspects of endometriosis. I. Genetic studies. Am J Obstet Gynecol. 1980;137:327–331. doi: 10.1016/0002-9378(80)90917-5. [DOI] [PubMed] [Google Scholar]
- 22.Anger DL, Foster WG. The link between environmental toxicant exposure and endometriosis. Front Biosci. 2008;13:1578–1593. doi: 10.2741/2782. [DOI] [PubMed] [Google Scholar]
- 23.Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: Pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–275. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 24.Tower J. Programmed cell death in aging. Ageing Res Rev. 2015;23:90–100. doi: 10.1016/j.arr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of apoptosis in disease. Aging (Albany NY) 2012;4:330–349. doi: 10.18632/aging.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuchs Y, Steller H. Live to die another way: Modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol. 2015;16:329–344. doi: 10.1038/nrm3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gogacz M, Gałczyński K, Wojtaś M, Winkler I, Adamiak A, Romanek-Piva K, Rechberger T, Kotarski J. Fas-related apoptosis of peritoneal fluid macrophages in endometriosis patients: Understanding the disease. J Immunol Res. 2017;2017:3175394. doi: 10.1155/2017/3175394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun C, Li N, Yang Z, Zhou B, He Y, Weng D, Fang Y, Wu P, Chen P, Yang X, et al. miR-9 regulation of BRCA1 and ovarian cancer sensitivity to cisplatin and PARP inhibition. J Natl Cancer Inst. 2013;105:1750–1758. doi: 10.1093/jnci/djt302. [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Benzonana LL, Zhao H, Watts HR, Perry NJ, Bevan C, Brown R, Ma D. Prostate cancer cell malignancy via modulation of HIF-1α pathway with isoflurane and propofol alone and in combination. Br J Cancer. 2014;111:1338–1349. doi: 10.1038/bjc.2014.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mammoto T, Mukai M, Mammoto A, Yamanaka Y, Hayashi Y, Mashimo T, Kishi Y, Nakamura H. Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Lett. 2002;184:165–170. doi: 10.1016/S0304-3835(02)00210-0. [DOI] [PubMed] [Google Scholar]
- 31.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 33.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.