Abstract
In the clinical settings, patients often develop opioid-induced hyperalgesia (OIH) after utilization of high dose intra-operative remifentanil. Systemic α2 agonists, including dexmedetomidine, are believed to reduce pain and opioid requirements after surgery, thus decreasing the incidence of hyperalgesia. The present study aimed to investigate the effect of dexmedetomidine on remifentanil-induced hyperalgesia and explored the sex differences. A total of 48 patients (24 male, 24 female) with an American Society of Anesthesiologists physical status of I–II that were undergoing thyroidectomy were randomly assigned to one of the following six groups: Male controlled group (MC) and female controlled group (FC) (group MC, n=8 and group FC, n=8), which received a preoperative placebo of 0.2 µg.kg−1 normal saline and intraoperative remifentanil 0.2 µg.kg−1.min−1; male and female group with low-dose dexmedetomidine (group MD1, n=8 and group FD1, n=8), which received preoperative dexmedetomidine 0.2 µg.kg−1 and intraoperative remifentanil 0.2 µg.kg−1.min−1; and male and female groups with high-dose dexmedetomidine (group MD2, n=8 and group FD2, n=8), which received dexmedetomidine 0.6 µg.kg−1 and intraoperative remifentanil 0.2 µg.kg−1.min−1. Result indicated that the visual analog scale (VAS) scores and morphine dosing frequency were significantly higher in MC and FC groups compared with the other same sex groups. Furthermore, the mechanical hyperalgesia threshold and patients' analgesia satisfaction score after surgery were significantly lower in MC and FC groups. Notably, the frequency of post-operative chills, nausea and vomiting were significantly lower in groups MD1, MD2, FD1 and FD2. The present findings indicated that low- and high-dose dexmedetomidine injection significantly decreased the patient's risk of enhanced pain intensity and increased postoperative morphine dosing caused by remifentanil-induced hyperalgesia. These findings suggest that the influence of dexmedetomidine displayed minimal significant differences between sex. Trial registration no., IRB2018-YX-001 (Name of registry: Institutional Medical Ethics Committee of Tianjin Medical University General Hospital; date of registration: February 1, 2016).
Keywords: dexmedetomidine, remifentanil, opioid induced hyperalgesia, sex difference
Introduction
Remifentanil is a potent, short-acting synthetic opioid analgesic drug which has been widely used in clinical practice for pain management during perioperative period. Its unique pharmacokinetic features such as achieving the therapeutic effect in a short time and extremely rapid clearance rate makes it a favourable analgesic drug for medical procedures (1). However, both clinical and experimental studies indicated that remifentanil had the potential to increase pain sensitivity, distribution or intensity associated with irritation of pain receptors or sensory nerves (2,3). Remifentanil-induced hyperalgesia (RIH) has become more important as the patients received prolonged or escalating dosages of remifentanil, which in turn results in increased postoperative pain and higher opioid consumption (4). One study investigated postoperative analgesia and recovery in patients after abdominal hysterectomy indicated that postoperative pain is one of the key causes of prolonged recovery following abdominal surgery. Also, anesthesia management has the potential to reduce surgery-induced pain (5).
Therefore, it is necessary to investigate relevant strategies to avoid or attenuate the influence of RIH after infusion of remifentanil post-operatively. Many experimental studies have been published in this field. Some studies suggested that N-methyl-D-aspartate (NMDA) receptor antagonists such as ketamine may play a role as a useful adjuvant to reduce RIH (6–8). COX-2 inhibitor parecoxib which were also investigated as nonsteroidal anti-inflammatory drugs were often administered as premedication before surgery (9,10). Whereas other researches focused on modulating the administration of remifentanil as a different approach to avoid the side effects of adjuvants. Comelon demonstrated that gradual withdrawal of remifentanil infusion may prevent RIH compared with abrupt withdrawal (11). While in clinical cases where patients do not respond to them or cannot be treated with regional analgesic techniques, systemic α2 agonists could be utilized (4,12).
Dexmedetomidine is a highly selective α2 adrenergic agonist with sedative effects and analgesic-sparing properties, it also has the advantage of causing less respiratory depression comparing with other commonly used sedatives (13,14). A systematic review has been demonstrated that α2 adrenergic receptor agonists such as dexmedetomidine may provide benefit in the treatment of RIH by decrease postoperative pain intensity and morphine consumption (15). Furthermore, animal studies on α2 adrenergic receptor agonists have been shown to produce significant synergy with opioids in mice (16). However, the precise molecular mechanism of opioid-induced hyperalgesia (OIH) is currently unknown, there are no specific preventive measures for the high incidence of RIH (17).
Consequently, this study aimed to provide a theoretical basis for the clinical application of dexmedetomidine on prevention of RIH after operation; we also evaluate the gender differences on RIH by comparing the effect of using remifentanil alone or combined with high-dose or low-dose dexmedetomidine in patients undergoing thyroidectomy.
Materials and methods
Methods
This randomized double blind controlled trial was approved by Institutional Medical Ethics Committee of Tianjin Medical University General Hospital and formal, written consent from all participants was obtained.
The method of using visual analog scale (VAS) were informed to the participants before the surgery, which is unidimensional measurement instrument of pain intensity. VAS consist of a horizontal straight line which is 100 mm long, with the left end of the line representing no pain and the right end of the line representing pain as bad as it could possibly be. After the operation, participants were instructed to indicate the position on the scale that best describes their perception of pain at that time. Von Frey filaments (Bioseb™, Chaville, France) was used to measure the mechanical hyperalgesia threshold after the operation. This type of anesthesiometer consist of 20 monofilaments with size ranges from 1.65–6.65 and force ranges from 0.008–300 g. When the tip of a fiber is pressed against the skin surface at right angles, the participants were instructed to respond ‘yes’ or ‘no’ if that contact was felt or not felt. The researcher continues to advance the probe and the force of application increases until the elastic column buckled. After the fiber buckles, continued compress creates more bend, but the force contributed by the column is fairly constant. We continued apply a larger filament until the patient gave a positive response. The lowest force (g.mm−2) necessary to bend a Von Frey filament was defined as the mechanical hyperalgesia threshold.
Subjects
We selected forty-eight American Society of Anesthesiologists physical status I–II patients (24 male, 24 female) aged between 18–65 years who were scheduled for thyroidectomy from the general surgery department in the hospital. The operation time is limited between 1–4 h with the necessity of extubation afterwards. All the selected patients were able to communicate verbally during their participation in this clinical research. Exclusion criteria regarding to patient's medical history were allergy to dexmedetomidine or other drugs; long-term use of narcotic analgesics, sedative or non-steroid anti-inflammatory drug (NSAIDS); neuromuscular disease, endocrine system disease or psychiatric conditions. Perioperative exclusion criteria were preoperative heart rate HR <50 bpm, SBP <100 mmHg, abnormal cardiac conduction or rhythm; blood loss >600 ml or re-operative patients; patients participated in clinical trials of other drugs in the past 3 month and patients identified by the researchers as inappropriate to participate in the clinical trials.
Groups
Twenty four male patients were randomly divided in to three groups (n=8 per group) using computer generated random number tables. The male controlled group (MC) received a preoperative placebo of 0.2 µg.kg−1 normal saline and intraoperative remifentanil 0.2 µg.kg−1.min−1 intravenously by infusion pump. The second male (MD1) group received low dose preoperative dexmedetomidine 0.2 µg.kg−1 and intraoperative remifentanil 0.2 µg.kg−1.min−1. The third (MD2) group received higher dose of preoperative dexmedetomidine 0.6 µg.kg−1 and intraoperative remifentanil 0.2 µg.kg−1.min−1. For twenty-four female patients, they were also randomly divided into three groups: FC, FD1 and FD2. The female controlled group (FC) received normal saline. within each group, same methods and dosages of dexmedetomidine and remifentanil for these group were applied.
Anesthesia
Patients were given general anesthesia with tracheal intubation. Induction of anesthesia was commenced with intravenous midazolam 0.06 mg.kg−1, propofol 1–3 mg.kg−1, remifentanil 1 µg.kg−1, rapid tracheal intubation was facilitated by rocuronium to assist ventilation. During operation, propofol 3–5 mg.kg−1.h−1, remifentanil 0.2 µg.kg−1.min−1 and rocuronium bromide were used to maintain the bispectral index (BIS) values between 40–60. Conventional care was given for the prevention of nausea and vomiting by navoban 4 mg. Upon completion of induction, if SBP <80 mmHg, or a decrease of more than 30% of the basic blood pressure, phenylephrine 0.05–0.1 mg was injected intravenously with increased infusion speed. Repeat this procedure when necessary; if SBP >180 mmHg, intravenous nitroglycerin 0.25–0.5 mg or urapidil 5–10 mg was given with slow infusion, repeat the procedure if necessary; when patient's HR <50 bpm, intravenous atropine 0.2–0.5 mg was injected, repeat when necessary; when patient's HR >110 bpm, esmolol 0.5 mg.kg−1 was given intravenously, repeat when necessary.
Data collection
Postoperative care was given in the Post Anesthesia Care Unit (PACU) for 2 h. During the observation period patient's VAS was maintained ≥3 with morphine 0.1 mg.kg−1 intravenous injection. The incidence of postoperative adverse reaction within 24 h such as nausea, vomiting, chills and respiratory depression were recorded. Other observation index includes: preoperative 30 min, 1, 2, 4, 8, 24 h VAS; Ramsay score 10 min after extubation; time to first postoperative analgesic requirement and analgesic consumption in the PACU stay and patient's mechanical pain threshold at 30 min, 1, 2, 4, 8, 24 h postoperatively. In addition, patient satisfaction score on analgesic effect was also documented.
Statistical analysis
Categorical variables were described as number while numeric variables were expressed as mean ± standard deviation. Comparison of six groups and following parameters were accomplished by one-way ANOVA followed by Tukey's test using Statistical Package for Social Sciences Statistics (SPSS) software, v19.0 (SPSS, Inc., Chicago, IL, USA): Age, body weight, duration of operation, anesthesia time, recovery time, time to extubation after surgery, mechanical hyperalgesia threshold, 10mins after extubation Ramsay score, patient's analgesia satisfaction score, time to first postoperative analgesic requirement and analgesic consumption. Chi-square tests were used to analyze analgesic-related adverse effects such as PONV, chills and respiratory depression. P<0.05 was considered to indicate a statistically significant difference.
Results
Demographic data and anesthesia-related information
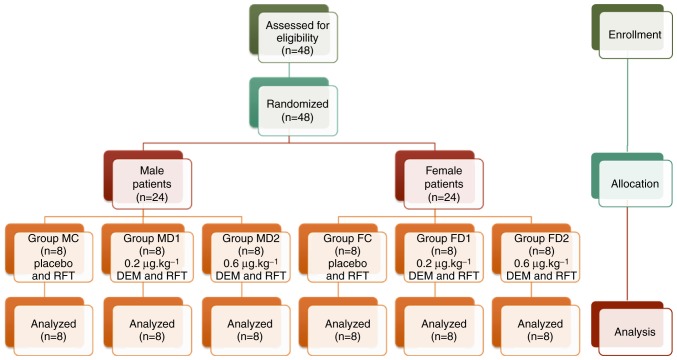
A total of 48 patients undergoing thyroidectomy under remifentanil-based anesthesia were assessed for eligibility and divided into three male groups and three female groups after randomization. No patient was dropped during the study (Fig. 1).
Figure 1.
Consolidated standards of reporting trials flowchart. DEM, dexmedetomidine, RFT, remifentanil.
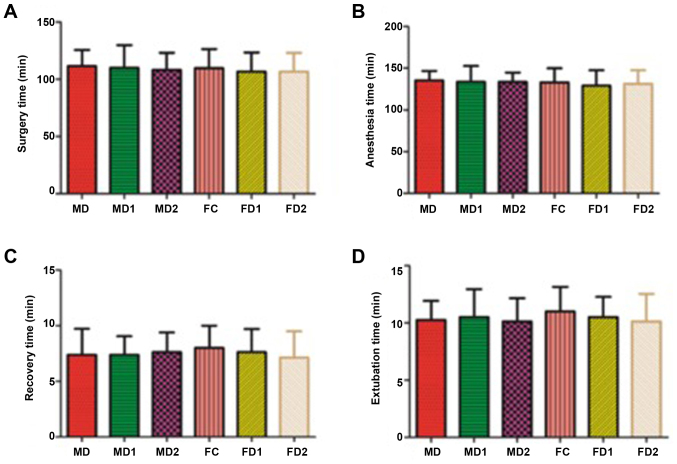
There was no significant difference among the six groups with respect to anesthetic parameters such as age, body weight, duration of anesthesia or time to extubation after surgery. Recovery time and extubation time were slightly higher in the female controlled group (FC) than other groups (Fig. 2).
Figure 2.
Surgery-related parameters. (A) Surgery time, (B) Anesthesia time, (C) Recovery time and (D) Extubation time. Values are expressed as mean ± standard deviation. Group MC/FC, male/female controlled group received placebo + remifentanil (0.2 µg.kg−1.min−1); Group MD1/FD1, male/female group, received low-dose dexmedetomidine (0.2 µg.kg−1) + remifentanil (0.2 µg.kg−1.min−1); Group MD2/FD2, male/female group received high-dose dexmedetomidine (0.6 µg.kg−1) + remifentanil (0.2 µg.kg−1.min−1). MC, male controlled group; FC, female controlled group.
Postoperative VAS evaluation
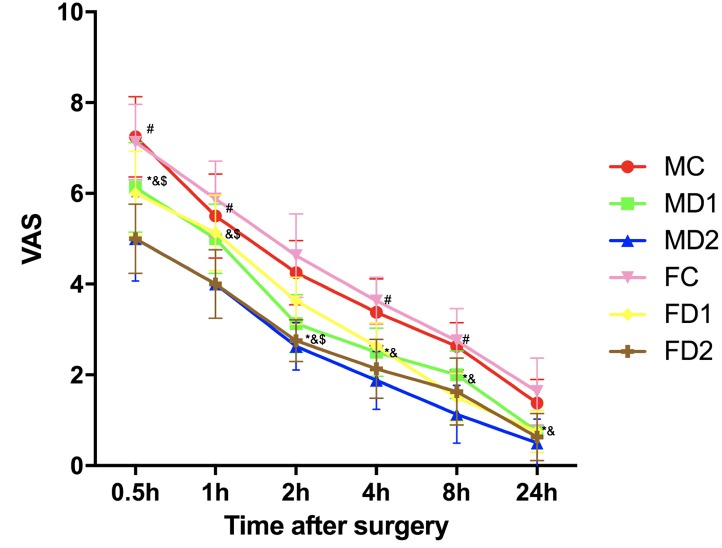
Postoperative mechanical pain threshold measured by VAS was significantly lower in group MD1 at 30 min, 2, 4, 8 and 24 h postoperative time points, respectively (P<0.05). For group MD2, significantly different VAS scores was shown at all of the time points compared with group MC (P<0.05). For female groups, the mechanical pain threshold in group FD1 at all the post-operative time points were all significantly lower compared with those of group FC except at 1 h. At the same time, group FD2 has shown significant difference at all the post-operative time points compared with group FC (P<0.05). In addition, the mechanical pain threshold at the operation site was not significantly different among the different gender difference groups (Fig. 3).
Figure 3.
Postoperative mechanical pain threshold measured with VAS. Group MC/FC, male/female controlled group received placebo + remifentanil (0.2 µg.kg−1.min−1); Group MD1/FD1, male/female group, received low-dose dexmedetomidine (0.2 µg.kg−1) + remifentanil (0.2 µg.kg−1.min−1); Group MD2/FD2, male/female group received high-dose dexmedetomidine (0.6 µg.kg−1) + remifentanil (0.2 µg.kg−1.min−1). VAS was measured at 30-min, 1, 2, 4, 8 and 24-h time points after surgery. Values were expressed as median and the error bars indicate the interquartile range. *P<0.05 vs. Group MC, #P<0.05 vs. Group MD1, &P<0.05 vs. Group FC and $P<0.05 vs. Group FD1. VAS, visual analog scale. MC, male controlled group; FC, female controlled group.
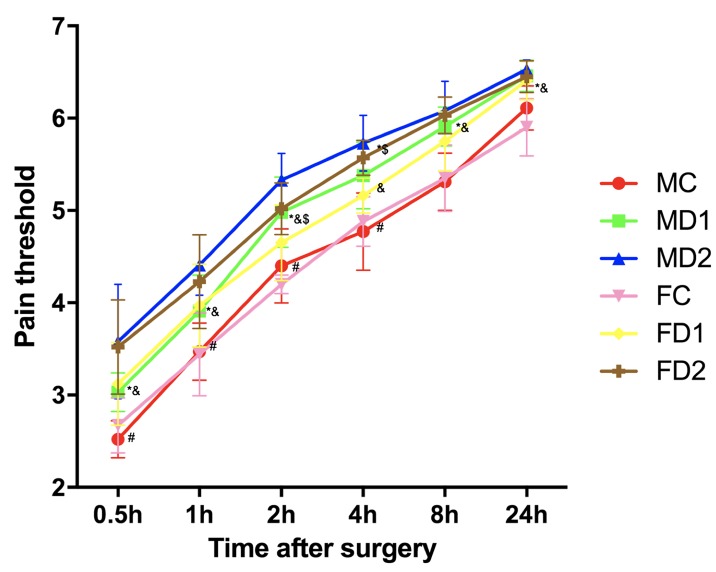
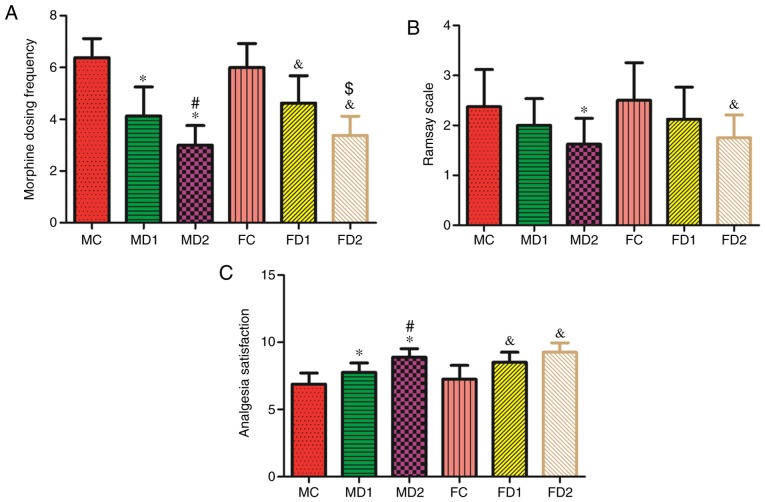
The post-operative mechanical hyperalgesia threshold at all the time points was significantly lower in group MC compared with other male groups. For female groups, same results were obtained (P<0.05; Fig. 4). Post-operative morphine dosing frequency was significantly higher in both group MC and FC (P<0.05). Furthermore, high-dose dexmedetomidine groups MD2 and FD2 showed significantly lower morphine dosing frequency compared with low-dose groups (MD1 and FD1) (Fig. 5).
Figure 4.
Post-operative mechanical hyperalgesia threshold. Group MC/FC, male/female controlled group received placebo + remifentanil (0.2 µg.kg−1.min−1); Group MD1/FD1, male/female group, received low-dose dexmedetomidine (0.2 µg.kg−1) + remifentanil (0.2 µg.kg−1.min−1); Group MD2/FD2, male/female group received high-dose dexmedetomidine (0.6 µg.kg−1) + remifentanil (0.2 µg.kg−1.min−1). Pain threshold were measured at 30-min, 1, 2, 4, 8 and 24-h time points after surgery. Values were expressed as medians and the error bars indicate the interquartile range. *P<0.05 vs. Group MC, #P<0.05 vs. Group MD1, &P<0.05 vs. Group FC and $P<0.05 vs. Group FD1. MC, male controlled group; FC, female controlled group.
Figure 5.
Postoperative associated parameters. (A) Bar chart of post-operative morphine dosing frequency. (B) Post-operative sedation level of the patients were assessed by Ramsay scale. (C) Patients' analgesia satisfaction score after operation. Values are expressed as mean ± standard deviation.*P<0.05 vs. Group MC, #P<0.05 vs. Group MD1, &P<0.05 vs. Group FC and $P<0.05 vs. Group FD1. MC, male controlled group; FC, female controlled group.
Postoperative Ramsay scale
Post-operative sedation level of the patients were assessed by Ramsay scale, both MD2 group and FD2 had a significant lower score compared with the other groups (P<0.05). Patient's analgesia satisfaction score were significantly higher in both gender groups with dexmedetomidine as an adjuvant to analgesia intra-operatively (P<0.05; Fig. 5).
Postoperative adverse effects
The incidence of Post-Operative Nausea and Vomiting (PONV) was significantly higher in group MC and FC. Post-anesthetic chills was significantly lower in group MD2 and FD2. None of the patient developed respiratory depression during recovery period (Table I).
Table I.
Postoperative side effects.
| Group MC (n=8) | Group MD1 (n=8) | Group MD2 (n=8) | Group FC (n=8) | Group FD1 (n=8) | Group FD2 (n=8) | |
|---|---|---|---|---|---|---|
| Nausea and vomiting | 5 | 3 | 0 | 4 | 2 | 1 |
| Chills | 3 | 1 | 0 | 2 | 1 | 0 |
| Respiratory depression | 0 | 0 | 0 | 0 | 0 | 0 |
Group MC/FC, male/female controlled group received placebo + remifentanil (0.2 µg.kg−1.min−1); Group MD1/FD1, male/female group, received low-dose dexmedetomidine (0.2 µg.kg−1) + remifentanil (0.2 µg.kg−1.min−1); Group MD2/FD2, male/female group received high-dose dexmedetomidine (0.6 µg.kg−1) + remifentanil (0.2 µg.kg−1.min−1)
Discussion
In the present study, RIH was significantly attenuated by intraoperative infusion of low-dose (0.2 µg.kg−1) or high-dose (0.6 µg kg−1) dexmedetomidine in patients undergoing thyroidectomy. However, the protective effects against remifentanil-induced hyperalgesia of dexmedetomidine showed minimal significant differences between two gender groups.
In the present study, RIH was revealed as enhanced pain intensity, reduced mechanical hyperalgesia threshold and increased morphine consumption obtained from high dose intraoperative remifentanil. Compared with patients in controlled groups, the patients in groups with dexmedetomidine infusion had lower VAS scores, greater mechanical pain threshold and less postoperative morphine consumption.
Postoperative nausea and vomiting (PONV) was a common complication of general anesthesia and spinal anesthesia. Recently, the effect of dexmedetomidine on PONV has been the focus of clinical researches. As an α2 adrenergic receptor agonist, it decreases sympathetic activity by reducing level of catecholamine and/or its opioid-sparing effect (18). Two meta-analysis suggested that administration of dexmedetomidine decrease the incidence of PONV (15,19). This finding was consistent with our study as both MC and FC group has had the higher number of PONV patients, and the number of patients decreased as the dosage of dexmedetomidine increased.
Dexmedetomidine was proved to be effective in suppressing the post anesthetic shivering in patients who underwent general anesthesia. It reduces shivering by lowering shivering thresholds and vasoconstriction (20). The result of the present study support the fact that dexmedetomidine have antihyperalgesic and antishivering effects. In addition, none of the patient reported respiration depression during recovery. These results were consistent with other studies regarding to hemodynamic effects of α2 adrenergic receptor agonists (15,21).
Currently, opioid-induced hyperalgesia remains as a clinical challenge in different pain settings due to lack of clear diagnostic criteria, limited alternative options of opioid analgesics and risk of opioid withdrawal with opioid reduction (22). The exact molecular mechanism of OIH is not yet understood. One of the proposed mechanisms of acute opioid hyperalgesia was glutamate-associated activation of N-methyl-D-aspartate (NMDA) receptors caused spinal neuron sensitization. NMDA receptors are composed of different types of subunits, the NMDA receptor 2B (NR2B) subunit plays the most important role in spinal dorsal horn sensory pathways (23). Moreover, the capability of NMDA receptor antagonists to suppress OIH provides further evidence that NMDA receptors are involved in hyperalgesia states (23–25). While other studies have suggested that a splice variant of the mu opioid receptor known as MOR-1K contributes to the development of OIH. Human genetic and cell signaling studies supported the evidence that MOR-1K produces increased cellular activity via Gs signaling while decrease opioid analgesic responses (26,27). Other mechanisms suggested were increased excitatory peptide neurotransmitters such as cholecystokinin (CCK), caused by the release of endogenous opioid spinal dynorphin which activates the kappa opioid receptor and NMDA receptors (28), genetic influence of the Catechol-O-methyltransferase (COMT) gene which was an enzyme that breakdown catecholamines and regulates the metabolism of dopamine/noradrenaline (29), and decreased reuptake of neurotransmitters such as substance P and gultamate from the primary afferent fibers along will increase the responsiveness of the spinal neurons to the transmitters after chronic opioid intake (30). In this study, we found that intraoperative high-dose remifentanil was associated with increased pain intensity. Therefore, leading to greater morphine consumption, decreased mechanical hyperalgesia threshold and higher Ramsay score. In addition, both controlled male and female group (MD & FD) had a significant lower analgesia experience satisfaction score compared with the other two experimental groups. This was studied from patient's perspective.
Dexmedetomidine was first introduced into clinical use as a short-term sedative because it is a rapidly-metabolized drug with a short plasmatic half-time of 2–2.5 h (11). It is chemically related to clonidine, but has a much greater affinity for α2 receptors over α1 receptors. In the dorsal horn of the spinal cord, the presence of dexmedetomidine at α2 adrenergic receptors modulates the release of substance P and produceds its analgesic effects (31). Its molecular mechanism has been investigated by animal models, Yuan et al (32) reported that dexmedetomidine could prevent RIH via regulating spinal NMDA receptor trafficking as well as protein kinase C and calmodulin-dependent protein kinase II pathway. This result was consistent with other animal studies that suggested combination of α2 adrenergic receptor agonist with an opioid can increase analgesic potency to some extent (33,34). Moreover, by effectively suppressing glial cell proliferation and activate the adjacent nerve cells involved in peripheral and central sensitization, dexmedetomidine has been shown effectively decreasing hyperalgesia by increasing acetylcholine levels in the spinal cord (35). Several clinical trials and case reports have confirmed that dexmedetomidine as an α2 adrenergic agonist has the potential to synergize with opioids (4,15,35). Therefore, dexmedetomidine was set out as an adjuvant to reduce RIH and improving pain control in patient undergoing thyroidectomy. In our study, both low dose and high dose dexmedetomidine groups has significant reduction in morphine dosing frequency after operation, in particular, high-dose dexmedetomidine was more effective than low-dose dexmedetomidine in male and female group. Furthermore, VAS scores were significantly lower in groups with either high or low dose of dexmedetomidine compared with the groups receiving placebo. Our data correspond with other supporting evidence indicated that dexmedetomidine infusion efficiently alleviated RIH symptoms (35,36).
In conclusion, we found that both low dose and high dose dexmedetomidine intraoperative infusion significantly decreased the patient's risk of enhanced pain intensity and increased postoperative morphine consumption caused by remifentanil induced hyperalgesia. Patient reported higher analgesia satisfaction when dexmedetomidine was utilized as an adjuvant in pain management. The protective effects against remifentanil-induced hyperalgesia of dexmedetomidine showed minimal significant differences between two gender groups. Additional studies are required to determine the rational regimens and to clarify the adverse effect of dexmedetomidine. Although the research has reached its aims, there were some unavoidable limitations. Due to time limit and limited resources, this research was conducted only on a small size of population. Therefore, to generalize the results for larger groups, the study should have involved more participants at different levels. Second, multivariate analysis was not performed in this study. A univariate model is less comprehensive compared to multivariate models and are unable to show relationships between different factors. Multivariate analysis should be considered in the further studies.
Acknowledgements
Not applicable.
Funding
The present study was funded by Tianjin Medical University International Medical School.
Availability of data and material
All data and materials generated and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HQ wrote the manuscript and revised it critically for important intellectual content. ZS made substantial contributions to conception and design. FS, RA, PC, GP and ZM made substantial contributions to the acquisition of data, analysis and interpretation of data. YY provided final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Ethics approval and consent to participate
This randomized double blind controlled trial was approved by Institutional Medical Ethics Committee of Tianjin Medical University General Hospital and obtained formal written consent from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Egan TD. Remifentanil pharmacokinetics and pharmacodynamics. A preliminary appraisal. Clin Pharmacokinet. 1995;29:80–94. doi: 10.2165/00003088-199529020-00003. [DOI] [PubMed] [Google Scholar]
- 2.Yu EH, Tran DH, Lam SW, Irwin MG. Remifentanil tolerance and hyperalgesia: Short-term gain, long-term pain? Anaesthesia. 2016;71:1347–1362. doi: 10.1111/anae.13602. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Stoicea N, Soghomonyan S, Bergese SD. Remifentanil-acute opioid tolerance and opioid-induced hyperalgesia: A systematic review. Am J Ther. 2015;22:e62–e74. doi: 10.1097/MJT.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 4.Belgrade M, Hall S. Dexmedetomidine infusion for the management of opioid-induced hyperalgesia. Pain Med. 2010;11:1819–1826. doi: 10.1111/j.1526-4637.2010.00973.x. [DOI] [PubMed] [Google Scholar]
- 5.Ge DJ, Qi B, Tang G, Li JY. Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal hysterectomy: A double-blind, randomized clinical trial. Sci Rep. 2016;6:21514. doi: 10.1038/srep21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joly V, Richebe P, Guignard B, Fletcher D, Maurette P, Sessler DI, Chauvin M. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103:147–155. doi: 10.1097/00000542-200507000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Yalcin N, Uzun ST, Reisli R, Borazan H, Otelcioglu S. A comparison of ketamine and paracetamol for preventing remifentanil induced hyperalgesia in patients undergoing total abdominal hysterectomy. Int J Med Sci. 2012;9:327–333. doi: 10.7150/ijms.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi E, Lee H, Park HS, Lee GY, Kim YJ, Baik HJ. Effect of intraoperative infusion of ketamine on remifentanil-induced hyperalgesia. Korean J Anesthesiol. 2015;68:476–480. doi: 10.4097/kjae.2015.68.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenz H, Raeder J, Draegni T, Heyerdahl F, Schmelz M, Stubhaug A. Effects of COX inhibition on experimental pain and hyperalgesia during and after remifentanil infusion in humans. Pain. 2011;152:1289–1297. doi: 10.1016/j.pain.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Tröster A, Sittl R, Singler B, Schmelz M, Schüttler J, Koppert W. Modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by parecoxib in humans. Anesthesiology. 2006;105:1016–1023. doi: 10.1097/00000542-200611000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Comelon M, Raeder J, Stubhaug A, Nielsen CS, Draegni T, Lenz H. Gradual withdrawal of remifentanil infusion may prevent opioid-induced hyperalgesia. Br J Anaesth. 2016;116:524–530. doi: 10.1093/bja/aev547. [DOI] [PubMed] [Google Scholar]
- 12.Sheehy KA, Finkel JC, Darbari DS, Guerrera MF, Quezado ZM. Dexmedetomidine as an adjuvant to analgesic strategy during vaso-occlusive episodes in adolescents with sickle-cell disease. Pain Pract. 2015;15:E90–E97. doi: 10.1111/papr.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamibayashi T, Maze M. Clinical uses of alpha2-adrenergic agonists. Anesthesiology. 2000;93:1345–1349. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Bai Z. New therapeutic uses for an alpha2 adrenergic receptor agonist-dexmedetomidine in pain management. Neurosci Lett. 2014;561:7–12. doi: 10.1016/j.neulet.2013.12.039. [DOI] [PubMed] [Google Scholar]
- 15.Blaudszun G, Lysakowski C, Elia N, Tramèr MR. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: Systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116:1312–1322. doi: 10.1097/ALN.0b013e31825681cb. [DOI] [PubMed] [Google Scholar]
- 16.Gold MS, Dastmalchi S, Levine JD. Alpha 2 adrenergic receptor subtypes in rat dorsal root and superior cervical ganglion neurons. Pain. 1997;69:179–190. doi: 10.1016/S0304-3959(96)03218-6. [DOI] [PubMed] [Google Scholar]
- 17.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145–161. [PubMed] [Google Scholar]
- 18.Zhong WG, Ge XY, Zhu H, Liang X, Gong HX, Zhong M, Xiao X. Dexmedetomidine for antiemesis in gynecologic surgery: A meta-analysis of randomized controlled trials. Int J Clin Exp Med. 2015;8:14566–14576. [PMC free article] [PubMed] [Google Scholar]
- 19.Jin S, Liang DD, Chen C, Zhang M, Wang J. Dexmedetomidine prevent postoperative nausea and vomiting on patients during general anesthesia: A PRISMA-compliant meta analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e5770. doi: 10.1097/MD.0000000000005770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bajwa SJ, Gupta S, Kaur J, Singh A, Parmar S. Reduction in the incidence of shivering with perioperative dexmedetomidine: A randomized prospective study. J Anaesthesiol Clin Pharmacol. 2012;28:86–91. doi: 10.4103/0970-9185.92452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Shinha C, Kumar A, Kumari P. The effect of intravenous dexmedetomidine compared to propofol on patients hemodynamics as a sedative in brachial plexus block: A comparative study. Anesth Essays Res. 2017;11:201–205. doi: 10.4103/0259-1162.200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher D, Martinez V. How can we prevent opioid induced hyperalgesia in surgical patients? Br J Anaesth. 2016;116:447–449. doi: 10.1093/bja/aew050. [DOI] [PubMed] [Google Scholar]
- 23.Gu X, Wu X, Liu Y, Cui S, Ma Z. Tyrosine phosphorylation of the N-Methyl-D-Aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: The preventive effect of ketamine. Mol Pain. 2009;5:76. doi: 10.1186/1744-8069-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye L, Xiao L, Bai X, Yang SY, Li Y, Chen Y, Cui Y, Chen Y. Spinal mitochondrial-derived ROS contributes to remifentanil-induced postoperative hyperalgesia via modulating NMDA receptor in rats. Neurosci Lett. 2016;634:79–86. doi: 10.1016/j.neulet.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Lin H, He G, Lin W, Yang J. Magnesium sulphate attenuate remifentanil-induced postoperative hyperalgesia via regulating tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord. BMC Anesthesiol. 2017;17:30. doi: 10.1186/s12871-017-0325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the µ-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. doi: 10.1016/S0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 27.Oladosu FA, Conrad MS, O'buckley SC, Rashid NU, Slade GD, Nackley AG. Mu Opioid Splice Variant MOR-1K Contributes to the Development of Opioid-Induced Hyperalgesia. PLoS One. 2015;10:e0135711. doi: 10.1371/journal.pone.0135711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, Zhang ET, Malan TP, Jr, Ossipov MH, Lai J, Porreca F. Dynorphin promotes abnormal pain and spinal opioid antinoceceptive tolerance. J Neurosci. 2000;20:7074–7079. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen KB, Lonsdorf TB, Schalling M, Kosek E, Ingvar M. Increased sensitivity to thermal pain following a single opiate dose is influenced by the COMT val(158)met polymorphism. PLoS One. 2009;4:e6016. doi: 10.1371/journal.pone.0006016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youssef F, Pater A, Shehata M. Opioid-induced hyperalgesia. J Pain Relief. 2015;4:183. [Google Scholar]
- 31.Li A, Yuen VM, Goulay-Dufay S, Kwok PC. Pharmacokinetics and pharmacodynamics of dexmedetomidine. Drug Dev Ind Pharm. 2016;42:1917–1927. doi: 10.1080/03639045.2016.1232727. [DOI] [PubMed] [Google Scholar]
- 32.Yuan Y, Sun Z, Chen Y, Zheng Y, Xie KL, He Y, Wang Z, Wang GL, Yu YH. Prevention of remifentanil induced postoperative hyperalgesia by dexmedetomidine via regulation the trafficking and function of spinal NMDA receptors as well as PKC and CaMKII level in vivo and in vitro. PLoS One. 2017;12:e0171348. doi: 10.1371/journal.pone.0171348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fairbanks CA, Kitto KF, Nguyen HO, Stone LS, Wilcox GL. Clonidine and dexmedetomidine produce antinociceptive synergy in mouse spinal cord. Anesthesiology. 2009;110:638–647. doi: 10.1097/ALN.0b013e318195b51d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fairbanks CA, Stone LS, Kitto KF, Nguyen HO, Posthumus IJ, Wilcox GL. α2c-adrenergic receptors mediate spinal analgesia and adrenergic-opioid synergy. J Pharmacol Exp Ther. 2001;300:282–290. doi: 10.1124/jpet.300.1.282. [DOI] [PubMed] [Google Scholar]
- 35.Lee C, Kim YD, Kim JN. Antihyperalgesic effects of dexmedetomidine on high-dose remifentanil-induced hyperalgesia. Korean J Anesthesiol. 2013;64:301–307. doi: 10.4097/kjae.2013.64.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Z, Wu W, Wu X, Lei H, Gong C, Xu S. Protective effects of dexmedetomidine combined with flurbiprofen axetil on remifentanil-induced hyperalgesia: A randomized controlled trial. Exp Ther Med. 2016;12:2622–2628. doi: 10.3892/etm.2016.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]