Abstract
Glutathione S‐transferase M (GSTM) family is concerned with oxidative stress, which is associated with breast carcinogenesis and chemotherapy response. The null polymorphism of GSTM1 gene results in a thorough absence of the enzyme function. Our study was to evaluate the association between GSTM1 null/present polymorphism and chemotherapy treatment outcome in breast cancer patients. A total of unrelated 714 patients with a histologically confirmed breast cancer were randomly selected from two independent cancer centers. Polymerase chain reaction was performed to analyze null/present genotypes of GSTM1 in our study. Our study found that the present genotype of GSTM1 was associated with a better relapse‐free survival (RFS) (P = .03) with adjusted hazard ratio (HR) [95% confidence interval (CI)] of 0.63 (95% CI: 0.42‐0.93). The present genotype of GSTM1 was significantly correlated with a better RFS compared with the null genotype in the nonchemotherapy group (HR = 0.17, 95% CI: 0.06‐0.50; P = 0.001), but no effect was observed in the chemotherapy group (HR = 0.81, 95% CI: 0.52‐1.26; P = 0.35). Moreover, the interaction between the GSTM1‐null/present genotype and adjuvant chemotherapy was significant (P = 0.04) in further analysis. Our study suggests that the GSTM1 polymorphism plays a complex role in influencing the chemotherapy response and breast cancer survival. It is suggested that the GSTM1‐present genotype might prevent progression in breast cancer patients. In the meanwhile, it could damage the benefit of adjuvant chemotherapy as well in certain ways.
Keywords: adjuvant chemotherapy, breast cancer, GSTM1, polymorphism
1. INTRODUCTION
It is well‐known that internal estrogens and their metabolites have a tight association with carcinogenesis of the breast in human beings. Estrogen‐quinones and their generated oxidative stress play an important role in this process.1 Quinone oxidoreductases and glutathione S‐transferases (GSTs) can decrease the content of quinines or semi quinones in tissues.2
Glutathione S‐transferases are effective protection against reactive oxygen species (ROS) via conjugating with glutathione.3 GSTM family belongs to the predominant enzymes in the breast tissue. The genes of the GSTM family are arranged in an alignment of the 5′‐GSTM4‐M2‐M1‐M5‐M3‐3′ sequence.4 The GSTM1 gene is localized on chromosome 1p13, and the GSTM1‐null genotype seems to be susceptible to many cancers as breast, lung, and colon cancers. The GSTM1‐null polymorphism results in a complete absence of GSTM1 enzyme function which draws us special attention. In many aspects, genetic changes have been demonstrated to be correlated with cancer prognosis, while their effects are still not fully understood up to now.5, 6, 7
Breast cancer is the most prevalent malignant disease in women all over the world, and chemotherapy is undoubtedly important in the treatment of locally advanced solid tumors like breast cancers. Here we hypothesize that GSTM1‐null/present polymorphism may have an influence on breast cancer progression and chemotherapy treatment response. To our knowledge, many previous studies have reported the association between GSTM1 and survival of patients with breast cancer, whereas these studies have come to discrepant conclusions.8, 9, 10, 11, 12, 13 The underlying relationship between oxidative stress and breast cancer prognosis is sort of complicated in the current understanding. Generally speaking, the high level of oxidative stress would increase the response to chemotherapy for the reason that chemotherapy carries out its cytotoxic effects via reactive oxygen species and concomitant oxidative stress. On the other hand, some evidence has showed that oxidative stress is involved in tumor invasion and metastasis.13, 14, 15, 16 Therefore, to some extent, the factor whether a patient undergoes chemotherapy or not may cause controversial influences of GSTM1 polymorphism on breast cancer progression and prognosis.
In this study, we evaluated the role of GSTM1‐null/present polymorphism in the treatment outcome of patients with breast cancer, and we also investigated whether this effect would be influenced by adjuvant chemotherapy or not.
2. MATERIALS AND METHODS
2.1. Patients
This study was approved by the Ethical Committee of the Liaoning Cancer Hospital and the Shanghai Cancer Center, and each participant signed an informed consent document.
A peripheral blood sample, and clinicopathologic information and treatment documents were collected for each patient. The survival outcome was followed up for at least 6 months. We totally recruited 730 independent patients who were pathologically confirmed primary breast cancer diagnosis in the Liaoning Cancer Hospital or the Shanghai Cancer Center from June 2007 to January 2009. Patients who were fulfilled with these following criteria were included in our further study: (1) female; (2) unilateral invasive breast cancer; (3) postoperation patients without any evidence of metastasis; (4) patients with complete adjuvant systemic therapy. Among these 730 patients, 16 cases were excluded because of the failure in GSTM1 genotyping, and the final 714 cases constituted the final analysis group.
It was because tumor grade and KI67 index were not accessible in many cases that we did not include these variables, while other patients' essential characteristics were collected. The status of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor‐2 (HER2) determined by immunohistochemistry (IHC) staining was confirmed by two independent pathologists in the Department of Pathology of the Liaoning Cancer Hospital or the Shanghai Cancer Center. Patients with indeterminate HER2 protein expression took a fluorescent in situ hybridization test for gene amplification examination.
Postoperative recurrence risk in our present study was mainly categorized according to the St. Gallen consensus 2007 for breast cancer treatment. The choice of applying adjuvant chemotherapy to patients depended on the risk category: patients with moderate recurrence risk would undergo FAC/FEC regimen; patients with high risk would receive taxane‐containing regimens including AC‐P, CAF‐T, and TAC. All of the ER/PR‐positive patients were recommended to take tamoxifen or aromatase inhibitors for at least 5 years after surgery.
2.2. DNA/RNA preparation, PCR, and PCR‐based allele genotyping
Genomic DNA was gathered from the patients' peripheral blood leukocytes using Gentra's PureGene DNA Purification kit (Gentra Systems, MN, USA) according to the manufacturer's protocol and was kept at −20°C for storage. The null polymorphism of the GSTM1 gene was analyzed using PCR in accordance with previously described methods.13 The GAPDH gene was chosen for an internal control in the experiment. The designed PCR primers of GSTM1 were showed as following:
sense GAACTCCCTGAAAAGCTAAAGC
anti‐sense GTTGGGCTCAAATATACGGTGG
2.3. Survival analysis and statistics
The relapse‐free survival (RFS) in our study was defined as the time from the surgery performed to the first recurrence of disease or the diagnosis of contralateral breast cancer. Patients with loss of follow‐up or study end date were considered to be censored in the survival comparison analysis. Survival outcomes were estimated using the Kaplan‐Meier method, and differences were tested using the log‐rank test (univariate analysis). Hazard ratio (HR) and 95% confidence intervals (95% CIs) were determined by the Cox risk proportion model. The Cox risk proportion model (method: enter) was used to carry out a further multivariate analysis. Significant analysis was carried out by Pearson's χ2 test. Student's t test was used to compare continuous variables between the two cohorts. All P‐values were two‐sided, and a P‐value of less than .05 was considered statistically significant. All statistical analysis was computerized on SPSS 17.0 (IBM institute, IL, USA) and Stata/SE 14.0 (College Station, TX, USA) software.
3. RESULTS
3.1. Association of GSTM1‐null/present polymorphism with RFS
Basic characteristics of breast cancer patients and distributions of GSTM1‐null/present genotypes are shown in Table 1. The four independent clinicopathological characters, including age, lymph node, tumor size, and IHC‐based subtype, presented no significant association with the GSTM1 null genotype to the present genotype.
Table 1.
Characteristics of breast cancer patients and distribution of GSTM1‐null/present genotype
| Phenotype | Number of patients | GSTM1‐null/present genotype | P a | |||
|---|---|---|---|---|---|---|
| Null | Present | |||||
| N | % | N | % | |||
| Age | ||||||
| ≤45 y | 248 | 139 | 56 | 109 | 44 | .94 |
| >45 y | 466 | 259 | 56 | 207 | 44 | |
| Lymph node | ||||||
| Negative | 405 | 228 | 56 | 177 | 44 | .73 |
| Positive | 309 | 170 | 55 | 139 | 45 | |
| Size | ||||||
| ≤2 cm | 399 | 229 | 57 | 170 | 43 | .32 |
| >2 cm | 315 | 169 | 54 | 146 | 46 | |
| IHC‐based subtype | ||||||
| HR+HER2− | 415 | 228 | 55 | 187 | 45 | .49 |
| HR+HER2+ | 85 | 54 | 64 | 31 | 36 | |
| HR‐HER2− | 134 | 72 | 54 | 62 | 46 | |
| HR‐HER2+ | 80 | 44 | 55 | 36 | 45 | |
| Chemotherapy | ||||||
| No | 213 | 119 | 56 | 94 | 44 | .96 |
| Yes | 501 | 279 | 56 | 222 | 44 | |
HR, hormone receptor; IHC, immunohistochemistry.
χ2 tested P values for heterogeneity.
We studied the association of GSTM1‐null/present polymorphisms with RFS (Table 2) and found GSTM1‐null/present polymorphism had a significant association with RFS (P = .03) in univariate analysis. A further multivariate analysis demonstrated that lymph node status (P < .001), tumor size (P < .001), IHC‐based subtype (P < .001), chemotherapy or not (P < .001), and GSTM1‐null/present polymorphism (P = .02) were significant independent factors for RFS.
Table 2.
Univariate and multivariate analysis of risk factor for relapse‐free survival
| Log‐rank P | Adjusted HRa (95% CI) | Adjusted P | |
|---|---|---|---|
| Age | |||
| ≤45 y | .35 | Ref. | .33 |
| >45 y | 1.21 (0.82‐1.79) | ||
| Lymph node | |||
| Negative | <.001 | Ref. | <.001 |
| Positive | 3.25 (2.03‐5.18) | ||
| Size | |||
| ≤2 cm | <.001 | Ref. | <.001 |
| >2 cm | 2.50 (1.64‐3.82) | ||
| IHC‐based subtype | |||
| HR+HER2− | <.001 | Ref. | <.001 |
| HR+HER2+ | 2.52 (1.44‐4.42) | ||
| HR‐HER2− | 3.28 (1.99‐5.40) | ||
| HR‐HER2+ | 3.79 (2.27‐6.33) | ||
| Chemotherapy | |||
| No | .06 | Ref. | <.001 |
| Yes | 0.32 (0.18‐0.57) | ||
| GSTM1 | |||
| Null | .03 | Ref. | .02 |
| Present | 0.63 (0.42‐0.93) | ||
CI, confidence interval; HR, hazard ratio.
Adjusted for age, lymph node status, tumor size, immunohistochemistry‐based subtype, chemotherapy and GSTM1‐null/present polymorphism. HR with its 95% CI is calculated by the Cox risk proportion model.
3.2. Association of GSTM1‐null/present polymorphism with DFS is modified by adjuvant chemotherapy
We further stratified the patients by age, lymph node status, tumor size, and adjuvant chemotherapy (Table 3). Interestingly, in patients without adjuvant chemotherapy, the unadjusted survival curves showed a statistically significant result (P = .015, Table 3 , Figure 1B). By contrast, in those patients who were treated with adjuvant chemotherapy, the GSTM1‐null/present polymorphism had no effect on RFS (P = .32, Table 3, Figure 1C). The findings presented above strongly suggested the presence of an interaction. After adjustment, the present of GSTM1 was significantly correlated with better RFS compared with the null genotype in the nonchemotherapy group (HR = 0.17, 95% CI: 0.06‐0.50, P = .001), but this effect was not preserved in the adjuvant chemotherapy group (HR = 0.81, 95% CI: 0.52‐1.26, P = .35), with a significant P‐value of .04 for interaction between the GSTM1 genotype and adjuvant chemotherapy.
Table 3.
Impact of GSTM1‐null/present genotype on relapse‐free survival by stratification
| GSTM1 genotype | Log‐rank P | Adjusted HRa (95% CI) | Adjusted P | Interaction P | |
|---|---|---|---|---|---|
| Age | |||||
| ≤45 y | Null | .51 | Ref. | .414 | >.05 |
| Present | 0.78 (0.43‐1.41) | ||||
| >45 y | Null | .03 | Ref. | .013 | |
| Present | 0.52 (0.31‐0.87) | ||||
| Lymph node | |||||
| Negative | Null | .01 | Ref. | .013 | >.05 |
| Present | 0.40 (0.19‐0.82) | ||||
| Positive | Null | .50 | Ref. | .232 | |
| Present | 0.75 (0.46‐1.20) | ||||
| Size | |||||
| ≤2 cm | Null | .02 | Ref. | .019 | >.05 |
| Present | 0.45 (0.23‐0.88) | ||||
| >2 cm | Null | .32 | Ref. | .247 | |
| Present | 0.75 (0.46‐1.22) | ||||
| Chemotherapy | |||||
| No | Null | .02 | Ref. | .001 | .04 |
| Present | 0.17 (0.06‐0.50) | ||||
| Yes | Null | .32 | Ref. | .35 | |
| Present | 0.81 (0.52‐1.26) | ||||
CI, confidence interval; HR, hazard ratio.
Adjusted for age, lymph node status, tumor size, immunohistochemistry‐based subtype, and chemotherapy. HR with its 95% CI is calculated by the Cox risk proportion model.
Figure 1.
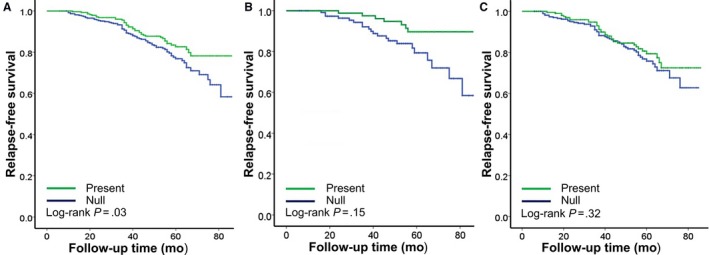
Effects of GSTM1‐null/present genotype on relapse‐free survival according to adjuvant chemotherapy in primary breast cancer. A, overall population; B, no chemotherapy; C, with chemotherapy. P‐value tested by log‐rank test
4. DISCUSSION
The GST super‐family of enzymes play a vital role in the metabolism of xenobiotics and drugs, including chemotherapeutic agents in breast cancer treatment, like doxorubicin/epirubicin and paclitaxel/docetaxel. The role of GSTM1 in chemotherapy efficacy and cancer prognosis draws much attention. However, the association between breast cancer disease outcomes and genotypes of GSTM1 in prior studies still remains inconsistent results.8, 9, 10, 11, 12, 13 In the present study, we mainly investigate the association between RFS and GSTM1‐null/present polymorphisms in breast cancer patients. Interestingly, after the adjustment of clinical phenotypes, we find GSTM1‐null/present genotypes to be an independent prognostic factor in patients without adjuvant chemotherapy, but not in those with adjuvant chemotherapy.
In previous literature, some observational studies concluded controversial results of the association between the GSTM1 genotype and treatment outcome in breast cancer patients. Petros et al17 reported the median overall survival for the patients with GSTM1‐null genotype was significantly longer when comparing to the patients with GSTM1‐present genotype. Ambrosone et al18 came to a parallel conclusion that women with null genotypes for GSTM1 and GSTT1 had reduced hazard of death in relation to those with alleles present. However, Gor et al19 observed similar outcomes between GSTM1‐null and GSTM1‐present patients in their multivariable model. Yang et al20 and Duggan et al10 also reached a negative result with respect to GSTM1 genotypes. Our results indicate that GSTM1‐present genotype plays protective roles in disease progression if no chemotherapy is administered. However, this effect fades away when the chemotherapy was administrated; that is to say, patients harboring GSTM1‐present genotype gain limited benefit from chemotherapy.
The inconsistent survival outcomes between the chemotherapy and nonchemotherapy groups could be explained from three main aspects. First, it is well‐established that the GSTM1‐present genotype is associated with elevating activity of estrogen‐quinone metabolizing enzymes, reducing ROS levels and inhibiting oxidative stress (OS)‐induced cancer cell proliferation and angiogenesis. However, most chemotherapy agents exert their cytotoxic effects by elevating the OS levels in the breast carcinoma, increasing OS damage to a level that the cancer cells cannot cope with and leading to cell death.13, 15 To this point, the originally protective effect of ROS might cause “resistance” to chemotherapy for the GSTM1‐present patients. Second, because both anthracyclines and cyclophosphamide are metabolized through reactions mediated by GSTMs,21 the presence of GSTM1 could accelerate the inactivation and metabolism mechanisms of these therapeutic agents and also come to lower levels of circulating active drugs. Third, these different findings might attribute to variation between the studies in different sources of patients, cancer stages, sample collecting ways, or other factors.
Frankly speaking, our study has several limitations. First, genetic variants in other OS‐related genes as well as combinations of these genotypes are not included in the present study. Second, the chemotherapy regimens in our retrospective study are not uniform, and different reactions to specific treatment may be neglected. Third, endocrine therapy for ER/PR‐positive and target therapy for HER2‐positive patients are generally recommended in adjuvant treatment, and their effect on survival is not taken into full consideration in our analysis.
In summary, our results suggest that breast cancer patients with GSTM1‐present genotype may gain a better survival outcome when comparing to their wild‐type counterparts, but the benefit probably compromises due to the intervention of adjuvant chemotherapy. The interaction between GSTM1‐null/present polymorphism and adjuvant chemotherapy may lead to potential drug resistance and influence the survival of breast cancer patients. The new understanding of interactions between chemotherapy resistance and host genetic factors might contribute to the future design of individualized cancer treatment for patients with breast cancer.
CONFLICT OF INTEREST
The authors have declared that no competing interests exist.
Li S, Lang G‐T, Zhang Y‐Z, Yu K‐D, Shao Z‐M, Zhang Q. Interaction between glutathione S‐transferase M1‐null/present polymorphism and adjuvant chemotherapy influences the survival of breast cancer. Cancer Med. 2018;7:4202–4207. 10.1002/cam4.1567
Shuang Li, Guan‐tian Lang and Ying‐Zhou Zhang have contributed equally to this work.
Contributor Information
Ke‐Da Yu, Email: yukeda@fudan.edu.cn.
Zhi‐Ming Shao, Email: zhi_ming_shao@163.com.
Qiang Zhang, Email: zhangqiang8220@126.com.
REFERENCES
- 1. Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270‐282. [DOI] [PubMed] [Google Scholar]
- 2. Strange RC, Fryer AA. The glutathione S‐transferases: influence of polymorphism on cancer susceptibility. IARC Sci Publ. 1999;148:231‐249. [PubMed] [Google Scholar]
- 3. Thompson PA, Ambrosone C. Molecular epidemiology of genetic polymorphisms in estrogen metabolizing enzymes in human breast cancer. J Natl Cancer Inst Monogr. 2000;27:125‐134. [DOI] [PubMed] [Google Scholar]
- 4. Pearson WR, Vorachek WR, Xu SJ, et al. Identification of class‐mu glutathione transferase genes GSTM1‐GSTM5 on human chromosome 1p13. Am J Hum Genet. 1993;53:220‐233. [PMC free article] [PubMed] [Google Scholar]
- 5. Olivier M, Langerod A, Carrieri P, et al. The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin Cancer Res. 2006;12:1157‐1167. [DOI] [PubMed] [Google Scholar]
- 6. Kalinsky K, Jacks LM, Heguy A, et al. PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res. 2009;15:5049‐5059. [DOI] [PubMed] [Google Scholar]
- 7. Udler M, Maia AT, Cebrian A, et al. Common germline genetic variation in antioxidant defense genes and survival after diagnosis of breast cancer. J Clin Oncol. 2007;25:3015‐3023. [DOI] [PubMed] [Google Scholar]
- 8. Bai YL, Zhou B, Jing XY, et al. Predictive role of GSTs on the prognosis of breast cancer patients with neoadjuvant chemotherapy. Asian Pac J Cancer Prev. 2012;13:5019‐5022. [DOI] [PubMed] [Google Scholar]
- 9. Franco RL, Schenka NG, Schenka AA, Rezende LF, Gurgel MS. Glutathione S‐transferase Pi expression in invasive breast cancer and its relation with the clinical outcome. J BUON. 2012;17:259‐264. [PubMed] [Google Scholar]
- 10. Duggan C, Ballard‐Barbash R, Baumgartner RN, Baumgartner KB, Bernstein L, McTiernan A. Associations between null mutations in GSTT1 and GSTM1, the GSTP1 Ile(105)Val polymorphism, and mortality in breast cancer survivors. Springerplus. 2013;2:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oliveira AL, Oliveira Rodrigues FF, Dos Santos RE, Rozenowicz RL, Barbosa de Melo M. GSTT1, GSTM1, and GSTP1 polymorphisms as a prognostic factor in women with breast cancer. Genet Mol Res. 2014;13:2521‐2530. [DOI] [PubMed] [Google Scholar]
- 12. Wang J, Wang T, Yin GY, Yang L, Wang ZG, Bu XB. Glutathione S‐transferase polymorphisms influence chemotherapy response and treatment outcome in breast cancer. Genet Mol Res. 2015;14:11126‐11132. [DOI] [PubMed] [Google Scholar]
- 13. Yu KD, Huang AJ, Fan L, Li WF, Shao ZM. Genetic variants in oxidative stress‐related genes predict chemoresistance in primary breast cancer: a prospective observational study and validation. Can Res. 2012;72:408‐419. [DOI] [PubMed] [Google Scholar]
- 14. Brown NS, Bicknell R. Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001;3:323‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1‐11. [DOI] [PubMed] [Google Scholar]
- 16. Mongaret C, Alexandre J, Thomas‐Schoemann A, et al. Tumor invasion induced by oxidative stress is dependent on membrane ADAM 9 protein and its secreted form. Int J Cancer. 2011;129:791‐798. [DOI] [PubMed] [Google Scholar]
- 17. Petros WP, Hopkins PJ, Spruill S, et al. Associations between drug metabolism genotype, chemotherapy pharmacokinetics, and overall survival in patients with breast cancer. J Clin Oncol. 2005;23:6117‐6125. [DOI] [PubMed] [Google Scholar]
- 18. Ambrosone CB, Sweeney C, Coles BF, et al. Polymorphisms in glutathione S‐transferases (GSTM1 and GSTT1) and survival after treatment for breast cancer. Can Res. 2001;61:7130‐7135. [PubMed] [Google Scholar]
- 19. Gor PP, Su HI, Gray RJ, et al. Cyclophosphamide‐metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node‐positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12:R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang G, Shu XO, Ruan ZX, et al. Genetic polymorphisms in glutathione‐S‐transferase genes (GSTM1, GSTT1, GSTP1) and survival after chemotherapy for invasive breast carcinoma. Cancer. 2005;103:52‐58. [DOI] [PubMed] [Google Scholar]
- 21. Tan SH, Lee SC, Goh BC, Wong J. Pharmacogenetics in breast cancer therapy. Clin Cancer Res. 2008;14:8027‐8041. [DOI] [PubMed] [Google Scholar]


