Abstract
Background
There are several therapeutic strategies for the management of resectable stage IIIA/N2 non‐small‐cell lung cancer (NSCLC) patients. However, the role of radiotherapy as a preoperative adjuvant therapy is unclear.
Methods
We retrospectively analyzed the data of stage IIIA/N2 NSCLC patients who either underwent preoperative radiation (PrORT), or did not undergo preoperative radiation, collected from the Surveillance, Epidemiology and End Results (SEER) database, between 2004 and 2013. The primary endpoints were cancer‐specific survival (CSS) and overall survival (OS).
Results
Ultimately, 493 patients treated with preoperative radiation and 2675 patients treated who were not treated with preoperative radiation, were included in the analysis. Overall, preoperative radiation was associated with a better CSS (HR: 1.427 [1.297‐1.572], P = 0.014) and OS (HR: 1.220 [1.131‐1.493], P = 0.002) than that observed in patients who did not undergo preoperative radiation. After PSM, preoperative radiation still showed advantage in both CSS and OS. Only age, T stage, and preoperative radiation remained independent prognostic factors for both OS and CSS. In the subgroup analysis, the advantages of preoperative radiotherapy were more pronounced in patients with stage T3 tumors and highly differentiated tumors.
Conclusions
Preoperative radiation may improve the outcomes of resectable IIIA/N2 NSCLC patients. In IIIA/N2 NSCLC patients, particularly with T3 and highly differentiated tumors, clinicians should boldly apply preoperative radiotherapy to improve the patients' survival.
Keywords: IIIA/N2, non‐small cell lung cancer, preoperative radiation, prognosis, propensity score matching
1. INTRODUCTION
The treatment for potentially resectable stage IIIA/N2 non‐small‐cell lung carcinoma (NSCLC) remains controversial. Currently, there are several combined modality approaches that are preferred, including surgery after induction chemotherapy, surgery after accelerated radiotherapy following chemotherapy, palliative radiotherapy, surgery after induced chemoradiation, and chemotherapy after induction of synchronous surgery.1, 2, 3, 4 However, it is unclear which of these is the optimal modality as the 5‐year survival rates of patients receiving any of these treatments are 20%‐45%.5 It is even more difficult to determine the best treatment regimen because stage III NSCLC patients form a very broad population, particularly patients with stage IIIA/N2 tumors.6 This is because the tumor size, lymph node involvement, and comorbidities vary widely. At this point, it appears unwise to decide on a universal treatment plan.
Surgery is often the first choice for such patients, but other preoperative neoadjuvant treatment options are available.7 The utilization of radiation before surgery is always debated and many studies report that preoperative chemotherapy alone is sufficient prior to surgery in IIIA‐N2 patients.8, 9, 10, 11 On the other hand, some studies have confirmed that preoperative radiotherapy, at a high or standard dose, can significantly improve survival.12, 13, 14, 15 In short, the role of radiotherapy as a preoperative adjuvant therapy is unclear. In this study, we sought to answer the question of whether preoperative radiation should be performed in patients with resectable stage IIIA/N2 NSCLC.
2. MATERIALS AND METHODS
2.1. Patient selection
This retrospective study was conducted by acquiring data from the Surveillance Epidemiology and End Results (SEER) database.15, 16 Data were obtained by SEER*STAT 8.3.2, in October 2017. Using this software, we screened NSCLC patients between 2004 and 2013. To be included in the study, patients had to meet the following criteria: diagnosis confirmed microscopically, age recorded, active follow‐up, no distant metastasis, and the presence of only one primary tumor. Patients with incomplete staging, of unknown age, unknown cause of death, unknown survival period, and death within 30 days after surgery were excluded.
2.2. Ethics statement
This study was mainly based on the SEER database and was conducted in compliance with the Declaration of Helsinki. We obtained permission to access the files of SEER program research data (reference number 11561‐Nov 2016). Informed consent was not required because patients were not personally identified. This study was approved by the Ethics Review Committee of the Shandong Cancer Hospital, affiliated with the Shandong University.
2.3. Statistical analysis
For all patients, the following variables were analyzed as follows: age, race, sex, AJCC stage, pathological grade, and history of preoperative radiation. The primary endpoints of this study were cancer‐specific survival (CSS) and overall survival (OS), which were extracted from the SEER database. Baseline characteristics of different groups were compared using chi‐square tests. Survival curves were generated with the use of Kaplan‐Meier estimates. The differences between the curves were analyzed through the Log Rank test. Propensity score matching analysis was used for the matching of patients. Univariate and multivariate COX proportional hazards regression models were utilized to evaluate risk of mortality and conduct subgroup analyses. All statistical tests were two‐sided and results were considered statistically significant when a test of a P < 0.05 was obtained. The statistical software SPSS 18.0 (SPSS, IL, Chicago) was used for all data analyses.
3. RESULTS
3.1. Patient demographics
In total, 493 patients who underwent preoperative radiotherapy (PrORT) and 2675 who did not undergo preoperative radiotherapy (no‐PrORT) were included in this retrospective cohort study. Obvious differences in age, gender, degree of differentiation, pathological type, and T stage, were noted between the two groups (Table 1). Specifically, the PrORT group had a higher proportion of elderly patients, male patients, and grade III‐IV, squamous, T3 tumors. This indicated that the baseline characteristics of the two groups were not balanced. After the 1:1 Propensity Score Matching (PSM), a total of 465 pairs were matched successfully (Table 2). In the final analysis model, baseline characteristics, including age, race, sex, grade of differentiation, pathological findings, and T stage were all balanced.
Table 1.
Baseline characteristics for patients with PrORT/no‐PrORT before PSM
| Variables | Radiation (%) | None (%) | P |
|---|---|---|---|
| Age | <0.001 | ||
| <65 | 306 (62.1) | 1078 (40.3) | |
| ≥65 | 187 (37.9) | 1597 (59.7) | |
| Race | 0.457 | ||
| White | 408 (82.8) | 2203 (82.4) | |
| Black | 53 (10.8) | 260 (9.7) | |
| Other | 32 (6.5) | 212 (7.9) | |
| Sex | 0.022 | ||
| Male | 272 (55.2) | 1326 (49.6) | |
| Female | 221 (44.8) | 1349 (50.4) | |
| Grade | <0.001 | ||
| I‐II | 171 (34.7) | 1293 (48.3) | |
| III‐IV | 322 (65.3) | 1382 (51.7) | |
| Pathology | 0.003 | ||
| Squamous | 149 (30.2) | 658 (24.6) | |
| Adenocarcinoma | 232 (47.1) | 1478 (55.3) | |
| Others | 112 (22.7) | 539 (20.1) | |
| T stage | <0.001 | ||
| T1 | 99 (20.1) | 775 (29.0) | |
| T2 | 302 (61.3) | 1695 (63.4) | |
| T3 | 92 (18.7) | 205 (7.7) | |
Table 2.
Baseline characteristics for patients with PrORT/no‐PrORT after PSM
| Variables | Radiation | None | P |
|---|---|---|---|
| Age | 1.00 | ||
| <65 | 283 (60.9) | 283 (60.9) | |
| ≥65 | 182 (39.1) | 182 (39.1) | |
| Race | 1.00 | ||
| White | 389 (83.7) | 389 (83.7) | |
| Black | 48 (10.3) | 48 (10.3) | |
| Other | 28 (6.0) | 28 (6.0) | |
| Sex | 1.00 | ||
| Male | 258 (55.5) | 258 (55.5) | |
| Female | 207 (44.5) | 207 (44.5) | |
| Grade | 1.00 | ||
| I‐II | 161 (34.6) | 161 (34.6) | |
| III‐IV | 304 (65.4) | 304 (65.4) | |
| Pathology | 1.00 | ||
| Squamous | 141 (30.3) | 141 (30.3) | |
| Adenocarcinoma | 220 (47.3) | 220 (47.3) | |
| Others | 104 (22.4) | 104 (22.4) | |
| T stage | 1.00 | ||
| T1 | 95 (20.4) | 95 (20.4) | |
| T2 | 297 (63.9) | 297 (63.9) | |
| T3 | 73 (15.7) | 73 (15.7) | |
3.2. Outcomes of patients before and after PSM
Before PSM, the PrORT group was superior to the no‐PrORT group in both CSS (HR: 1.427 [1.297‐1.572], P = 0.014) and OS (HR: 1.220 [1.131‐1.493], P = 0.002; Figure 1). The 5‐year survival rates of the PrORT and no‐PrORT groups were 40.9% and 33.8%, respectively (P = 0.018). After PSM, OS and CSS for the PrORT group and the no‐PrORT group were similar (Figure 2). A 5‐year survival rate of 43.6% was found in the PrORT group, and 35.2% in the no‐PrORT group (P = 0.009).
Figure 1.
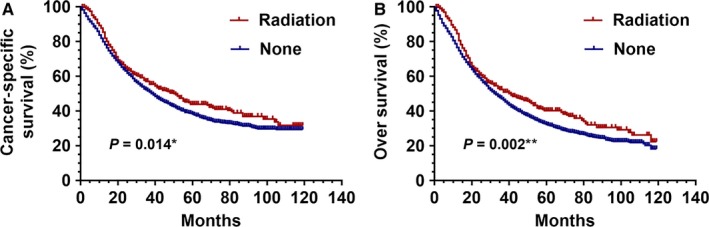
Kaplan‐Meier curves for CSS and OS before PSM. Cancer‐specific survival (A) and overall survival difference (B) between preoperative radiation and no preoperative radiation groups, before 1:1 Propensity Score Matching analysis. *P = 0.014 for PrORT group compared to no‐PrORT group for CSS; **P = 0.002 for PrORT group compared to no‐PrORT group for OS
Figure 2.
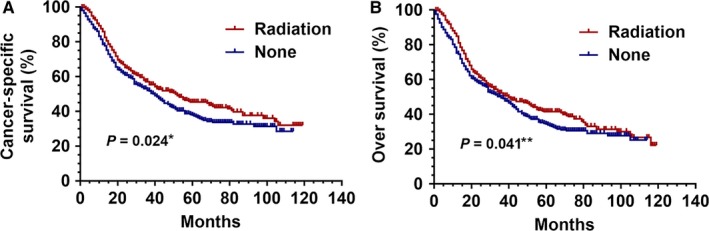
Kaplan‐Meier curves for CSS and OS after PSM. Cancer‐specific survival (A) and overall survival difference (B) between preoperative radiation and no preoperative radiation groups, after 1:1 Propensity Score Matching analysis. *P = 0.024 for PrORT group compared to no‐PrORT group for CSS; **P = 0.041 for PrORT group compared to no‐PrORT group for OS
3.3. Univariate and multivariate analysis
In the COX hazard analysis, after testing the proportional hazards assumptions, we selected multiple variables, including age, race, sex, grade, pathology, and T stage. According to the result of univariate COX regression analysis, age, race, sex, grade, pathology, T stage, and radiation were associated with a shorter OS and CSS, after PSM. In the multivariate analysis of CSS and OS, age, T stage, and preoperative radiotherapy were statistically significant (P < 0.05). Results of the univariate and multivariate COX regression of prognostic factors for CSS and OS in stage IIIA (cN2) NSCLC patients are shown in Table 3.
Table 3.
CSS univariate and multivariate Cox regression after PSM
| Variables | CSS | ||
|---|---|---|---|
| Univariate | Multivariate | ||
| P | HR (95% CI) | P | |
| Age | 0.002 | 0.001 | |
| <65 | Reference | ||
| ≥65 | 1.388 (1.147‐1.680) | 0.001 | |
| Race | 0.096 | Not entered | |
| White | |||
| Black | |||
| Other | |||
| Sex | 0.025 | 0.065 | |
| Female | Reference | ||
| Male | 1.196 (0.989‐1.447) | 0.065 | |
| Grade | 0.121 | Not entered | |
| I | |||
| II | |||
| III | |||
| IV | |||
| Pathology | 0.046 | 0.080 | |
| Adenocarcinoma | Reference | ||
| Squamous | 1.101 (0.878‐1.380) | 0.403 | |
| Others | 1.304 (1.035‐1.643) | 0.024 | |
| T stage | 0.001 | 0.001 | |
| T1 | Reference | ||
| T2 | 1.189 (0.930‐1.522) | 0.168 | |
| T3 | 1.798 (1.314‐2.460) | <0.001 | |
| Radiation | 0.020 | 0.010 | |
| No | Reference | ||
| Yes | 0.783 (0.649‐0.944) | 0.010 | |
3.4. Subgroup analysis for OS and CSS after PSM
In the subgroup analysis following PSM, the OS and CSS of patients who underwent PrORT were higher than those of patients in the no‐PrORT group. All subgroups analysis‐derived CSS and OS were in favor of PrORT, as seen in the overall study population (Figures 3 and 4). Age < 65 (HR: 0.763 [0.597‐0.795], P = 0.03), males (HR: 0.781 [0.61‐0.999], P = 0.047), tumor grade III‐IV (HR: 0.748 [0.594‐0.942], P = 0.013), and T3 tumor (HR: 0.515 [0.33‐0.803], P = 0.01), were statistically in favor of PrORT in terms of CSS. On the other hand, males (HR: 0.789 [0.627‐0. 992], P = 0.04), tumor grade III‐IV (HR: 0.769 [0.62‐0.954], P = 0.015) and T3 tumor (HR: 0.586 [0.388‐0.884], P = 0.008) were statistically in favor of PrORT, in terms of OS (Table 4).
Figure 3.
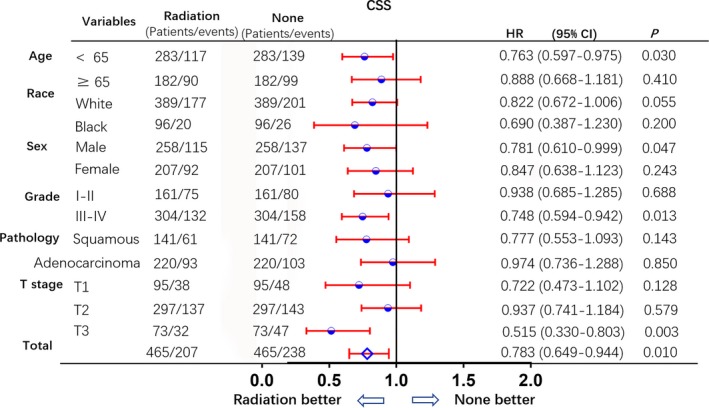
Subgroup analysis for CSS after PSM. Subgroup analysis of cancer‐specific survival between preoperative radiation and no preoperative radiation groups, after 1:1 Propensity Score Matching analysis. All subgroups derived CSS benefit in favor of preoperative radiation group. Age < 65, male, grade III‐IV and T3 were statistically in favor of preoperative radiation group
Figure 4.
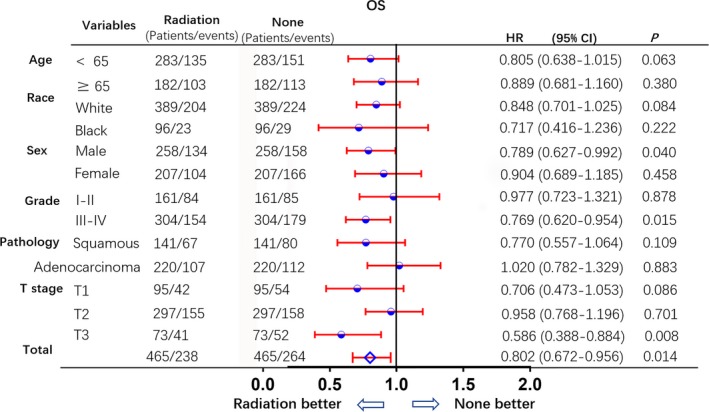
Subgroup analysis for OS after PSM. Subgroup analysis of overall survival between preoperative radiation and no preoperative radiation groups, after 1:1 Propensity Score Matching analysis. All subgroups derived OS benefit statistically in favor of preoperative radiation group, particularly, males, with grade III‐IV and T3 tumors
Table 4.
OS univariate and multivariate Cox regression after PSM
| Variables | OS | ||
|---|---|---|---|
| Univariate | Multivariate | ||
| P | HR (95% CI) | P | |
| Age | <0.001 | <0.001 | |
| <65 | Reference | ||
| ≥65 | 1.460 (1.215‐1.753) | <0.001 | |
| Race | 0.017 | 0.026 | |
| White | Reference | ||
| Black | 1.183 (0.877‐1.596) | 0.270 | |
| Other | 0.592 (0.384‐0.913) | 0.018 | |
| Sex | 0.003 | 0.006 | |
| Female | Reference | ||
| Male | 1.288 (1.074‐1.545) | 0.006 | |
| Grade | 0.030 | 0.008 | |
| I | Reference | ||
| II | 1.526(0.931‐2.501) | 0.094 | |
| III | 1.472 (0.909‐2.383) | 0.116 | |
| IV | 3.697(1.731‐7.896) | 0.001 | |
| Pathology | 0.047 | 0.013 | |
| Adenocarcinoma | Reference | ||
| Squamous | 1.028 (0.827‐1.278) | 0.805 | |
| Others | 1.378 (1.103‐1.721) | 0.005 | |
| T stage | <0.001 | <0.001 | |
| T1 | Reference | ||
| T2 | 1.230 (0.973‐1.555) | 0.083 | |
| T3 | 1.973 (1.467‐2.655) | <0.001 | |
| Radiation | 0.035 | 0.014 | |
| No | Reference | ||
| Yes | 0.802 (0.672‐0.956) | 0.014 | |
4. DISCUSSION
More than one‐third of NSCLC patients presented with locally advanced tumors, without distant metastatic disease, which could not be primarily resected.16 Radiotherapy is usually used as the primary local treatment but has associated unsatisfying survival rates.17, 18, 19, 20 Locally recurrent tumors and their complications account for about 75% of the deaths in patients with epidermoid lung cancer, and approximately 40% of the deaths in patients with other histologic subtypes of NSCLC.21, 22 To have an impact on the overall outcome of the management of patients with localized NSCLC, one should make an attempt to decrease the probability of local failure as well as distant metastasis.
The rationale for preoperative radiation is to increase the resectability of the tumor, reduce the number of cells capable of implantation at the time of surgery, and to sterilize the microscopic disease.23 Several studies24, 25, 26 have demonstrated high resectability rates, ranging from 50% to 72%, with the usage of preoperative radiation. Although the first preoperative trial of radiotherapy in NSCLC by Bromley et al27 failed to show an improvement in survival, it did demonstrate that 47% of the patients had no histologically demonstrable tumor in the surgical specimen. Depending on the total dose of radiation delivered preoperatively, other authors24, 26, 27, 28, 29, 30, 31 have reported somewhere between 20% and 54% of patients having no persistent tumor or only having microscopic disease.
The results of a phase III, multicenter, randomized clinical trial by the Japan Western Thoracic Cancer Cooperative Group (WJTOC) suggested that the addition of radiotherapy to neoadjuvant treatment may improve the rate of efficacy and stage decline.15 In this study, 60 stage III operable NSCLC patients, with a pathological stage of pN2, were randomly divided into neoadjuvant chemotherapy group and neoadjuvant chemoradiation group. The results showed that preoperative neoadjuvant chemoradiotherapy group showed a significant decrease in stage reduction rate (40% and 21%). Median progression‐free survival (PFS, 55.0 months vs 9.4 months) and median OS (63.3 months vs 29.5 months) were significantly greater in patients with mediastinal lymph node stage declining after treatment than in undecreased patients. Therefore, the WJTOC9033 study suggested that adding radiotherapy to induction therapy can improve the local control rate of the tumor without increasing the treatment‐related adverse reactions.
Several prospective randomized studies have failed to demonstrate ant advantages in delivering preoperative radiation, in patients with N2 NSCLC.26, 30 The reasons for failure in these trials may be that these studies have often included patients with all operable stages of NSCLC, irrespective of the local extent of the tumor, as opposed to the patients in our study, in whom preoperative radiation was used on IIIA/N2 tumors. It is possible that the benefit of preoperative radiation is seen only in patients with locally advanced disease, which does not extend to all other NSCLC patients.
The neoadjuvant treatment of NSCLC is a vast subject of interest. The debate on radiotherapy and surgery is based on deciding between preoperative and postoperative radiotherapy. So far, most studies have focused on the value of postoperative radiotherapy and less on preoperative radiotherapy. Due to the fact that preoperative radiotherapy usually takes 4 weeks, and a further recover period of 2‐3 weeks, it is difficult for patients and surgeons to accept such a long wait for surgery.32 However, preoperative radiotherapy leads to reduced lesions and surgical complications. Our study retrospectively compared the effect of preoperative radiotherapy on the prognosis of patients with stage III N2 NSCLC, to that of those who had not undergone radiotherapy. To obtain a clearer picture, we adopted the PSM method to process the data. The results showed that both before and after PSM, PrORT was superior in the management of stage IIIA/N2 NSCLC tumors. In the COX regression analysis, we found that preoperative radiotherapy was an independent prognostic factor for both CSS and OS. In the subgroup analysis, the advantages of preoperative radiotherapy are more pronounced in patients with T3 tumors and highly differentiated tumors.
There are several limitations to our study. Firstly, it is a retrospective study which carries a greater chance of bias in comparison with a prospective study. Secondly, we could not separate “Intentional selected” and “Compromised” from the SEER database. Details on pre‐and postoperative lung function, comorbidities, costs, details of induction therapy, major morbidity, the radicality of surgery and in‐hospital duration were unavailable. The details of concurrent chemotherapy regimens were also unavailable for further analysis.
5. CONCLUSION
Preoperative radiation may improve the outcomes of resectable IIIA/N2 NSCLC patients. In IIIA/N2 NSCLC patients, particularly patients with T3 and highly differentiated tumors, clinicians should boldly apply preoperative radiotherapy, to improve the patients' survival.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
This study was funded by a grant from the National Health and Family Planning Commission of China (201402011), Shandong Provincial Natural Science Foundation (ZR2015HZ004) and supported by a grant from the National Natural Science Foundation of China (81472812).
Chen D, Wang H, Song X, Yue J, Yu J. Preoperative radiation may improve the outcomes of resectable IIIA/N2 non‐small‐cell lung cancer patients: A propensity score matching‐based analysis from surveillance, epidemiology, and end results database. Cancer Med. 2018;7:4354–4360. 10.1002/cam4.1701
Dawei Chen and Haiyong Wang are contributed equally to this work.
REFERENCES
- 1. Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non‐small‐cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol. 2017;35(25):2960‐2974. [DOI] [PubMed] [Google Scholar]
- 2. Roth JA, Atkinson EN, Fossella F, et al. Long‐term follow‐up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non‐small‐cell lung cancer. Lung Cancer. 1998;21:1‐6. [DOI] [PubMed] [Google Scholar]
- 3. Rosell R, Gomez‐Codina J, Camps C, et al. Preresectional chemotherapy in stage IIIA non‐small‐cell lung cancer: a 7‐year assessment of a randomized controlled trial. Lung Cancer. 1999;26:7‐14. [DOI] [PubMed] [Google Scholar]
- 4. Betticher DC, Hsu‐Schmitz SF, Tötsch T, et al. Mediastinal lymph node clearance after docetaxel‐cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non‐small‐cell lung cancer: a multicenter phase II trial. J Clin Oncol 2003;21:1752‐1759. [DOI] [PubMed] [Google Scholar]
- 5. Farray D, Mirkovic N, Albain KS. Multimodality therapy for stage III non‐small‐cell lung cancer. J Clin Oncol. 2005;23(14):3257‐3269. [DOI] [PubMed] [Google Scholar]
- 6. Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non‐small‐cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol. 2006;24(19):2998‐3006. [DOI] [PubMed] [Google Scholar]
- 7. Eberhardt WE, Pöttgen C, Gauler TC, et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non‐small‐cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J Clin Oncol. 2015;33(35):4194‐4201. [DOI] [PubMed] [Google Scholar]
- 8. Sauvaget J, Rebischung J, Vannetzel J. Phase III study of neoadjuvant MVP versus MVP plus chemoradiotherapy in stage III NSCLC. Proc Am Soc Clin Oncol. 2000;19:495a. [Google Scholar]
- 9. Thomas M, Rübe C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non‐small‐cell lung cancer. Lancet Oncol. 2008;9:636‐648. [DOI] [PubMed] [Google Scholar]
- 10. Pless M, Stupp R, Ris H, et al. Neoadjuvant chemotherapy with or without preoperative irradiation in stage IIIA/N2 non‐small cell lung cancer (NSCLC): a randomized phase III trial by the Swiss Group for Clinical Cancer Research (SAKK trial 16/00) [Abstract]. Proc ASCO 2013:A7503.
- 11. Shah AA, Berry FB, Tzao C, et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg. 2012;93:1807‐1812. [DOI] [PubMed] [Google Scholar]
- 12. Sonett JR, Suntharalingam M, Edelman MJ, et al. Pulmonary resection after curative intent radiotherapy (>59 Gy) and concurrent chemotherapy in non‐small cell lung cancer. Ann Thorac Surg. 2004;78:1200‐1205. [DOI] [PubMed] [Google Scholar]
- 13. Cerfolio RJ, Bryant AS, Spencer SA, et al. Pulmonary resection after high‐dose and low‐dose chest irradiation. Ann Thorac Surg. 2005;80:1224‐1230. [DOI] [PubMed] [Google Scholar]
- 14. Bradley JD, Paulus R, Komaki R, et al. Standard‐dose versus high‐dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non‐small‐cell lung cancer (RTOG 0617): a randomised, two‐by‐two factorial phase 3 study. Lancet Oncol. 2015;16:187‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okamoto I, Sakai K, Morita S, et al. Multiplex genomic profiling of non–small cell lung cancers from the LETS phase III trial of first‐line S‐1/carboplatin versus paclitaxel/carboplatin: results of a West Japan Oncology Group study. Oncotarget. 2014;5(8):2293‐2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 17. Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non‐oat‐cell carcinoma of the lung. Cancer. 1980;45(11):2744‐2753. [DOI] [PubMed] [Google Scholar]
- 18. Petrovich Z, Stanley K, Cox JD, et al. Radiotherapy in the management of locally advanced lung cancer of all cell types. Cancer. 1981;48(6):1335‐1340. [DOI] [PubMed] [Google Scholar]
- 19. Sealy R, Lagakos S, Barkley T, et al. Radiotherapy of regional epidermoid carcinoma of the lung. A study in fractionation. Cancer. 1982;49:1338‐1345. [DOI] [PubMed] [Google Scholar]
- 20. Shah K, Olson MH, Ray P, et al. Comparison of dose‐time‐fractionation schemes in non‐oat cell lung. Cancer. 1981;48:1127‐1132. [DOI] [PubMed] [Google Scholar]
- 21. Cox J, Yesner R. Adenocarcinoma of the lung: results from the veterans administration lung group. Am Rev Respir Dis. 1979;120:1025‐1029. [DOI] [PubMed] [Google Scholar]
- 22. Cox J, Yesner R, Mietlowski W, et al. Influence of cell type on failure pattern after irradiation for locally advanced carcinoma of the lung. Cancer. 1979;44:94‐98. [DOI] [PubMed] [Google Scholar]
- 23. Besse B, Adjei A, Baas P, et al. 2nd ESMO Consensus Conference on Lung Cancer: non‐small‐cell lung cancer first‐line/second and further lines of treatment in advanced disease. Ann Oncol 2014;25(8):1475‐1484. [DOI] [PubMed] [Google Scholar]
- 24. Sherman DM, Neptune W, Weichselbaum R, et al. An aggressive approach to marginally resectable lung cancer. Cancer. 1978;4(1):2040‐2045. [DOI] [PubMed] [Google Scholar]
- 25. Shields TW. Preoperative radiation therapy in the treatment of bronchial carcinoma. Cancer. 1972;30:1388‐1394. [DOI] [PubMed] [Google Scholar]
- 26. Warram J. Preoperative irradiation of cancer of the lung: final report of a therapeutic trial. A collaborative study. Cancer. 1975;36:914‐925. [DOI] [PubMed] [Google Scholar]
- 27. Bromley LL, Szur L. Combined radiotherapy and resection for carcinoma of the bronchus. Experiences with 66 patients. Lancet. 1955;2:937‐941. [DOI] [PubMed] [Google Scholar]
- 28. Bloedorn FG, Crowley A, Cuccia C, et al. Combined therapy: irradiation and surgery in the treatment of bronchogenic carcinoma. Am J Roentgenol. 1961;85:875‐885. [Google Scholar]
- 29. Manfredi F, King R, Behnke R, et al. Preoperative irradiation in bronchogenic carcinoma. Am Rev Respir Dis. 1966;9:1793‐1805. [DOI] [PubMed] [Google Scholar]
- 30. Shields TW, Higgins CA, Lawton R, et al. Preoperative x‐ray therapy as an adjuvant in the treatment of bronchogenic carcinoma. J Thorac Cardiovasc Surg. 1970;59:49‐69. [PubMed] [Google Scholar]
- 31. Widow W. Preoperative irradiation of bronchial carcinoma. Cancer. 1971;28:798‐801. [DOI] [PubMed] [Google Scholar]
- 32. Steven C, Eric V, Candice L, et al. Higher versus standard preoperative radiation in the trimodality treatment of stage IIIa lung cancer. Ann Thorac Surg. 2015;100:207‐214. [DOI] [PubMed] [Google Scholar]


