Abstract
For the past two decades, echinocandins have shown prophylactic and therapeutic efficacy in patients with Pneumocystis jirovecii pneumonia (PJP), due to their ability to inhibit the synthesis of β-1, 3-glucan, a major component of the cell wall of P. jirovecii. The present study reported two cases of human immunodeficiency virus (HIV)-negative patients who received echinocandins as a salvage therapy at Peking Union Medical College Hospital (Beijing, China), both of whom exhibited good responses to treatment. In both cases, polymerase chain reaction of sputum or bronchoalveolar lavage specimens became negative following treatment. The present study also performed a literature search to identify non-HIV patients with PJP who previously received echinocandins. The results of the present study suggested that echinocandins maybe promising therapeutic agents in the treatment of non-HIV patients with PJP, particularly in combination with trimethoprim-sulfamethoxazole. Therefore, the results warrant a randomized controlled trial.
Keywords: echinocandins, Pneumocystis jirovecii pneumonia, non-human immunodeficiency viruspatients
Introduction
As an opportunistic infection, Pneumocystis jirovecii pneumonia (PJP) is a severe and life-threatening complication experienced by immunocompromised patients (1). With the advent of highly active antiretroviral therapy and prophylaxis strategies, the incidence of PJP has decreased markedly among patients with human immunodeficiency virus (HIV), with a mortality rate <10% (2). By contrast, non-HIV patients with PJP are characterized by advanced age, increased comorbidities, non-classical clinical symptoms, rapid deterioration and poor prognosis (3–7). Once acute respiratory failure (ARF) develops, non-HIV patients with PJP most likely require intensive care and ventilatory support and have a mortality rate of up to 75.6% (5). Despite the aforementioned differences, treatment regimens remain the same for HIV and non-HIV patients with PJP; for example, the administration of trimethoprim/sulfamethoxazole (TMP-SMZ) as the first-line drug, then an adjunctive steroid (standard dosage) for severe ARF, followed by the second-line drug (8–15). Therefore, other treatment options need to be investigated.
Due to the ability to inhibit the synthesis of β-1, 3-glucan, a major component of the cell wall of P. jirovecii, echinocandins could be used in the treatment of PJP (15–20). During the past two decades, the prophylactic and therapeutic efficacies of echinocandins have been investigated in animal models and clinical studies (21,22). A cohort study demonstrated a high success rate (8/10) with caspofungin salvage treatment in HIV-patients with PJP; however, data regarding the treatment of PJP with echinocandins in non-HIV patients remain limited and controversial (15,20).
The current report described two HIV-negative cases of PJP in which echinocandins were used for salvage treatment at the Medical Intensive Care Unit, Peking Union Medical College Hospital (Beijing, China). Both patients provided written informed consent. A review of previous reports involving the use of echinocandins for the treatment of PJP in non-HIV patients was also conducted.
Case report
Case 1
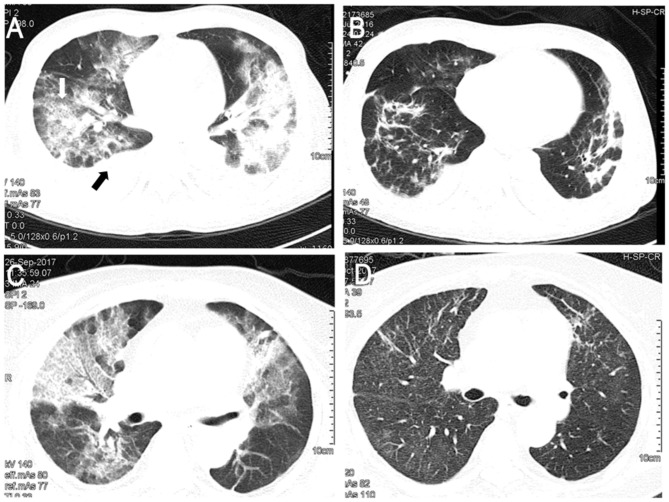
A 71-year-old male presented to the Emergency Department of the Peking Union Medical College Hospital (Beijing, China) in October 2017 with a 1-week history of fever, non-productive cough and progressive dyspnea. The patient had been diagnosed with IgG4-related disease 5 months prior to admission. Prednisone (Shandong Luoxin Pharmaceutical Group Stock Co., Ltd., Linyi, China) was administered and the dosage was gradually reduced to 20 mg/day after achieving remission. The patient also had chronic renal failure with regular dialysis treatment. Examination at the emergency room disclosed severe hypoxemia; chest computed tomography (CT) exhibited diffuse bilateral ground-glass opacities (GGO) of lung fields with pleural effusion (Fig. 1A). The patient was transferred to the medical intensive care unit (MICU) for noninvasive positive pressure ventilation and continuous renal replacement therapy. Based on the radiographic findings, the patient was empirically treated with TMP-SMZ (15 mg/kg/day TMP; Shandong Xinhua Pharmaceutical Co., Ltd., Zibo, China) along with methylprednisolone (40 mg, twice daily; Pfizer, Inc., New York, NY, USA) for PJP, and moxifloxacin (400 mg/day; Bayer AG, Leverkusen, Germany) for possible infections with atypical pathogens. The patient's symptoms did not improve and required increased respiratory support. Therefore, a regimen of clindamycin (600 mg/12 h; Hubei Shishun Biotechnology Co., Ltd., Hubei, China) and primaquine (30 mg/day; Shanghai Zhongxi Pharmaceutical Co., Ltd., Shanghai, China) was initiated as salvage therapy on day 4 of the ICU stay, the day PJP was confirmed by positive PJ DNA and Gomorimethenamine silver staining in the bronchoalveolar lavage (BAL) specimen. Despite this treatment, the patient's condition further deteriorated and a repeated chest high-resolution CT scan revealed aggravation of the bilateral diffuse GGO combined with consolidation. Caspofungin (Merck & Co., Inc., Whitehouse Station, NJ, USA) was administered at 70 mg on day 8 in the ICU, followed by 50 mg daily. Treatment with moxifloxacin was discontinued on the same day due to no evidence of atypical pathogens. The patient's respiratory condition improved markedly and respiratory support was gradually decreased following treatment with caspofungin. The patient was weaned off non-invasive positive pressure ventilation on day 19 in the ICU. A chest high-resolution CT demonstrated that the majority of the patches were absorbed and the pulmonary infiltrations were reduced after 14 days of treatment with caspofungin (Fig. 1B). The patient was transferred to the general ward on after 22 days in the ICU and eventually recovered, and was discharged on day 54.
Figure 1.
Results of chest computed tomography scans. (A) Chest computed tomography revealed GGO (white arrow) of lung fields with pleural effusion (black arrow) in Case 1. (B) GGO dissapearedfollowingtreatment of case 1 with caspofungin. (C) GGO were found in both lungs in Case 2. (D) GGO of case 2 were absorbed followingtreatment with caspofungin. GGO, ground-glass opacity.
Case 2
A 68-year-old female with systemic lupus erythematosus (SLE) was admitted to the Department of Rheumatology of the Peking Union Medical College Hospital due to lupus disease activity in August 2017. Prior to admission, the patient was treated with methylprednisolone for nearly 3 months due to hemolytic anemia associated SLE. During this hospitalization period, the patient received methylprednisolone 0 mg/12 h; Pfizer, Inc.) in addition to cyclophosphamide (4 mg/kg/day; Sinopharm Shantou Jinshi Pharmaceutical Co., Ltd., Shantou, China). On day 11 after admission, the patient developed a fever of 38.5°C and shortness of breath. A chest CT exhibited diffuse bilateral GGO (Fig. 1C). Polymerase chain reaction (PCR) revealed that P. jirovecii was detected within induced sputum. Treatment with TMP-SMZ was initiated (15 mg/kg/day TMP component, Shandong Xinhua Pharmaceutical Co., Ltd.). Administration of immunosuppressive agents to the patient was discontinued. Despite this treatment, the patient remained febrile and exhibited increasing dyspnea requiring a reservoir face mask. Given the severity of the condition, the patient was transferred to the MICU to start high-flow nasal cannula (HFNC) oxygen therapy (flow of 60 l/min and 60% FiO2) on day 16; caspofungin (Merck & Co., Inc.) was administered as salvage therapy at a loading dose of 70 mg, followed by a maintenance dose of 50 mg daily. After 4 days of treatment with caspofungin, fever alleviated, respiratory conditions improved and the HFNC was replaced by conventional nasal prongs. The patient was transferred to the ICU ward on day 7. A follow-up chest CT scan demonstrated a partially normal appearance of the lung fields (Fig. 1D).
Quantitative polymerase chain reaction (qPCR)
DNA was extracted using a commercial kit (QIAamp DNA Mini kit; Qiagen GmbH, Hilden, Germany) following the manufacturers protocol, but with elution using 30 µl Tris-EDTA buffer solution (Beijing Leagene Biotech Co., Ltd., Beijing, China). Purified DNA was then used as a template to amplify the part of the mitochondrial gene, which encodes the large subunit of rRNA. PCR was performed using the following primers: pAZ102-E forward, 5′-GATGGCTGTTTCCAAGCCCA-3′ and reverse, 5′-GTGTACGTTGCAAAGTACTC-3′. Albumin forward, 5′-TGGTGAAATGGCTGACTGCT-3′ and reverse, 5′-CTCTGGTCTCACCAATCGGG-3′. PCR was performed with a total reaction volume of 20 µl, comprising: Template DNA 2 µl (10–100 ng), 0.4 µM of forward primer 0.4 µl, 0.4 Μm of reverse primer 0.4 µl, ddH2O 6.8 µl and the SYBR Green Mix (2×) 10 µl (SYBRGreen qPCR Master Mix, MedChemExpress USA, Monmouth Junction, NJ, USA). The thermocycling conditions were as follows: Pre-amplification denaturation at 95°C for 5 min; 40 cycles of 95°C for 15 sec, 60°C for 30 sec The relative amount of gene expression was normalized using Albumin and was calculated using the 2−ΔΔCq formula as previously described (23). PCR mixtures were prepared in a laminar-flow cabinet and several controls were implemented. All amplifications were performed in parallel with a negative control (ultrapure distilled water) and a positive control (BAL samples of patients with definite Pneumocystis pneumonia).
Gomorimethenamine silver staining
Slides were prepared using cytocentrifugation (13,800 × g, 15 min, 4°C), cut to a thickness of 2–3 µm and microwaved in a 10% chromic acid (Hubei XinRunde Chemical Co., Ltd., Hubei, China) solution at 65°C for 40 sec. Samples were then washed with water and cleared using 1% sodium metabisulfite for 30 sec. After the slides were washed with distilled water, they were placed in a Coplin jar containing 50 ml of methenamine working solution and microwaved at 65°C for a further 65 sec. The slides were rinsed again with distilled water and treated with 1% gold chloride for 2–5 sec. Following further rinsing with distilled water, samples were exposed to 5% sodium thiosulfate for 1 min, counterstained with a light green working solution (Beijing Leagene Biotech Co., Ltd.) and cleared using xylene. Samples were then covered with cover slips and examined using routine light microscopy at a magnification of ×70.
Through a literature review, the present study identified 22 HIV-negative patients with PJP treated with echinocandins (8–20). The demographic and clinical characteristics of these cases, and the two patients included in the present study, are summarized in Table I. Among these patients, the mean age was 49.8 years. Underlying conditions varied between the included cases, with solid organ transplant most commonly reported (11/24; 45.8%). Only three patients were given primary prophylaxis. All enrolled cases exhibited concurrent bilateral pulmonary infiltrations and were clinically treated for pneumonia. P. jirovecii was the predominant pathogen that was confirmed by methenamine silver staining or PCR. Of all patients, 8 and 16 were treated with echinocandins as initial and salvage regimens, respectively, and the mean duration of treatment with echinocandins was 19 days. All included patients but one (11) were treated with caspofungin at 70 mg on the first day with a maintenance dose of 50 mg/day. Associations between the type of echinocandin treatment strategy, adjunctive corticosteroids and the underlying disease are described in Table II.
Table I.
Clinical characteristics of the reported cases of echinocandins use for Pneumocystis jirovecii pneumonia in patients.
| Author, year | Patient no | Age/gender | Underlying Disease | Initial treatment | Cause of EC use | Salvage regimen | Time to use EC (days) | Steroid used? | Duration of EC (days) | End | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang et al, 2006 | 1 | 93/M | COPD | TMP-SMZ | Adverse reaction | CA | 32 | No | 42 | S | (8) |
| Annaloro et al, 2006 | 2 | 45/M | For HSCT | TMP-SMZ | Treatment failure | CA+ TMP-SMZ | 11 | Yes | 45 | S | (9) |
| Beltz et al, 2006 | 3 | 5/M | ALL | CA+ TMP-SMZ | Empirical use | None used | NR | Yes | 22 | S | (10) |
| Kamboj et al, 2006 | 4 | 13/M | HSCT | CA | Antifungal | Pentamidine | NR | No | 18 | D | (11) |
| Kamboj et al, 2006 | 5 | 42/F | ALL | TMP-SMZ | Antifungal | MI + Others | 9 | No | 30 | D | (11) |
| Takeda et al, 2009 | 6 | 47/M | Liver TP | MI+ TMP-SMZ | Empirical use | MI+ TMP-SMZ | NR | No | N/A | S | (12) |
| Zhang et al, 2012 | 7 | 58/M | Lung Cancer | N/A | Treatment failure | CA+ TMP-SMZ + CLI+PRI | NR | Yes | N/A | D | (13) |
| Li, 2016 | 8 | 46/M | CKD | TMP-SMZ | Adverse reaction | CA+CLI | NR | Yes | 33 | S | (14) |
| Kim et al, 2013 | 9 | 1/M | SCID | TMP-SMZ | Treatment failure | CA+ TMP-SMZ +AT+PRO (CLI+PRI) | 26 | No | 26 | D | (15) |
| Kim et al, 2013 | 10 | 63/M | Liver TP | TMP-SMZ | Treatment failure | CA+ TMP-SMZ | 9 | No | 5 | D | (15) |
| Kim et al, 2013 | 11 | 57/M | Kidney TP | TMP-SMZ | Treatment failure | TMP-SMZ + PRI+CLI (CA) | 18 | No | 11 | D | (15) |
| Kim et al, 2013 | 12 | 46/F | Liver TP | TMP-SMZ | Treatment failure | CA+ TMP-SMZ (CLI+PRI) | 2 | No | 7 | S | (15) |
| Tu et al, 2013 | 13 | 61/M | Kidney TP | TMP-SMZ | Adverse reaction | CA+ TMP-SMZ | >10 | Yes | 14 | D | (16) |
| Tu et al, 2013 | 14 | 35/M | Kidney TP | TMP-SMZ | Adverse reaction | CA+ TMP-SMZ | 10 | Yes | 14 | S | (16) |
| Tu et al, 2013 | 15 | 43/M | Kidney TP | CA+ TMP-SMZ | Empirical use | None used | 7 | No | 14 | S | (16) |
| Jiang et al, 2013 | 16 | 46/M | LBC-L | CA | Adverse reaction | None used | 5 | No | NA | S | (17) |
| Mu et al, 2009 | 17 | 76/M | CML | CA | Adverse reaction | CA+ TMP-SMZ | 9 | Yes | 21 | S | (18) |
| Hof and Schnülle, 2008 | 18 | 60/M | WG | TMP-SMZ | Treatment failure | CA | 9 | No | 21 | S | (19) |
| Utili et al, 2007 | 19 | 57/F | Kidney TP | CA | Antifungal | CA+ TMP-SMZ | 1 | No | 14 | S | (20) |
| Utili et al, 2007 | 20 | 28/M | Kidney TP | TMP-SMZ | Treatment failure | CA+ TMP-SMZ | 7 | Yes | 16 | S | (20) |
| Utili et al, 2007 | 21 | 59/M | Heart TP | TMP-SMZ | Treatment failure | CA+ TMP-SMZ | 6 | Yes | 7 | S | (20) |
| Utili et al, 2007 | 22 | 58/F | Heart TP | CA+ TMP-SMZ | Empirical use | None used | 1 | Yes | 14 | S | (20) |
| Present study | 23 | 71/M | IgG4 | TMP-SMZ | Treatment failure | CA+ TMP-SMZ | 14 | Yes | 21 | S | – |
| Present study | 24 | 68/F | SLE | TMP-SMZ | Treatment failure | CA+ TMP-SMZ | NR | Yes | 7 | S | – |
ALL, acute lymphocytic leukaemia; AT, atovaquone; CA, caspofungin; CKD, chronic kidney dysfunction; COPD, chronic obstructive pulmonary disease; CLI, clindamycin; CML, chronic myelocytic leukaemia; D, mortality; DM, diagnosis method; EC, echinocandins; F, female; HSCT, hematopoietic stem cell transplant; LBC-L, large-B-cell lymphoma; M, male; MI, micafungin; N/A, not available; NR, no report; PRO, proguanil; PRI, primaquine; Pt, patient; Refs., reference; S, survived; SCID, severe combined immune deficiency; TP, transplant.
Table II.
Mortality of patients with Pneumocystis jirovecii pneumonia treated with echinocandins.
| Author, year | Characteristic | Mortality (%) | (Refs.) |
|---|---|---|---|
| Zhang et al, 2006; Hof and Schnülle, 2008 | Used EC as monotherapyin salvage regimen | 0/2 (0) | (8,19) |
| Kamboj et al, 2006 | EC as initial regimen | 1/8 (12.5) | (11) |
| Kamnoj et al, 2006; Zhang et al, 2012; Kim et al, 2013; Tu et al, 2013 | Total mortality | 7/24 (29.2) | (11,13,15,16) |
| Kamnoj et al, 2006; Zhang et al, 2012; Kim et al, 2013; Tu et al, 2013 | Used EC as combination therapy in salvage regimen | 5/14 (35.7) | (11,13,15,16) |
| Kamnoj et al, 2006; Zhang et al, 2012; Kim et al, 2013; Tu et al, 2013 | EC as salvage regimen | 5/16 (31.5) | (11,13,15,16) |
| Kamboj et al, 2006; Kim et al, 2013 | Treatment without steroid regimen | 5/12 (41.7) | (11,15) |
| Zhang et al, 2012; Kim et al, 2013 | EC+(TMP-SMZ+PRI+CLI) | 2/3 (66.7) | (13,15) |
| Zhang et al, 2012; Tu et al, 2013 | Treatment with steroid regimen | 2/12 (16.7) | (13,16) |
| Kim et al, 2013 | EC+(AT+PRO)/(PRI+CLI)a | 1/1 (100) | (15) |
| Kim et al, 2013; Tu et al, 2013 | EC+TMP-SMZin salvage regimen | 2/9 (22.2) | (15,16) |
| Kamnoj et al, 2006 | Used EC as monotherapyin initial regimen | 1/2 (50.0) | (11) |
| – | Used ECplusTMP-SMZin initial regimen | 0/6 (0) | – |
| – | EC+(TMP-SMZ+CLI) | 0/1 (0) | – |
Following treatment for 2 weeks, regimen of AT+PRO shifted to regimen of PRI+CLI. EC, echinocandins; TMP-SMZ, trimethoprim/sulfamethoxazole; CLI, clindamycin; AT, atovaquone; PRO, proguanil; PRI, primaquine.
Discussion
The present study described two successful cases of treatment with echinocandins for non-HIV patients with PJP. In addition to the two cases in the present study, 24 non-HIV PJP cases treated with echinocandins were identified in the literature (8–20). This revealed that, although a rationale exists for the use of echinocandins in the treatment of PJP, the current clinical use appears to be low. Potential reasons for this may include that fact that the use of echinocandins to treat PJP has only appeared in recent years, the lack of convincing efficacy data and that the use of echinocandins for PJP treatment is currently off-label (11,19,20).
The present study analyzed the application strategy of caspofungin in non-HIV patients with PJP. The results reported in previous studies suggested that echinocandins were most frequently considered as a salvage regimen (16/24), with a mortality rate of 31.5% (5/16 patients) (8–9,11,13–16,19,20). As of yet, no sufficient data for the outcome of echinocandins as a salvage regimen in non-HIV patients with PJP have been reported. In fact, for HIV patients who fail to respond to initial treatment, several traditional salvage regimens have shown high therapeutic effectiveness, including the combination of clindamycin and primaquine [42-44/48 (88–92%)] or atovaquone [4/5 (80%)] (24). However, regarding non-HIV patients with PJP, the data of these traditional salvage regimens is limited. In a previous study, clindamycin-primaquine or pentamidine were used as salvage regimens following treatment failure of the first-line regimen in 12 non-HIV patients with PJP and the mortality rate was 66.7% (8/12) (25). Therefore, the present results indicated that use of echinocandins(caspofungin starting with a loading dosage of 70 mg followed by 50 mg/day) as salvage therapy resulted in favorable and comparative rates of mortality in non-HIV patients compared with previous salvage regimens; however a statistical comparison was not possible. This suggests that an echinocandin-based salvage regimen maybe an option for the treatment of PJP in non-HIV patients.
There were 8 cases from previously published studies that used an echinocandin-based regimen for the initial treatment (10–12,16–18,20) and exhibited a good response with survival rates of 87.5%. When only the 6/8 cases that used the combination regimen of echinocandins and TMP-SMZ were considered, an excellent response was observed as all 6 patients had survived the infection. When the cases that used the combined regimen of echinocandins and TMP-SMZ in salvage therapy were also included, the mortality rate was 13.3% (2/15). These results appeared to favor the combination of echinocandins and TMP-SMZ.
The combined regimen of echinocandins and TMP-SMZ has been associated with high effectiveness in clearing the invading P. jirovecii (26). P. jirovecii is a fungus that exists in either trophic or cyst forms (trophic/cyst 9:1) (27). The primary component of the cyst cell wall is β-1, 3-glucan, which is poorly expressed in the trophic forms. Therefore, echinocandins mainly act on cyst forms by inhibiting the β-1, 3-glucan synthaseenzyme and disturbing the integrity of the cell wall (21,22). Previous experiments suggested that the use of caspofungin alone was associated with a 90% decrease in cyst forms after 4 days and a 66% reduction in trophic forms after 21 days of treatment. Furthermore, in an animal model of PJP, the administration of low doses of caspofungin with TMP-SMX may provide an improved clearance of Pneumocystis infection (28). On the other hand, echinocandins exhibit reduced activity against the trophic forms of P. jirovecii and the use of echinocandin alone may not completely eradicate the infection. Therefore, the co-administration of echinocandins and TMP/SMZ, which is primarily active against trophic forms, may exert synergistic activity against P. jirovecii by fully inhibiting the life cycle of the organism. However, clinical data regarding this combined therapy regimen were confined to case reports only (13,16,25).
In conclusion, positive clinical effects of echinocandins were observed in the present study, which suggested that the addition of echinocandins to TMP/SMZ was active against trophic forms and may demonstrate activity against P. jirovecii by inhibiting the organism's life cycle. However, the present analysis was based exclusively on case reports. It is possible that patients with favorable outcomes from the use of echinocandins may have been selectively reported in these case reports. Therefore, a large sample, well-designed randomized trials, particularly in the use of echinocandins combined with TMP-SMZ for the treatment of PJP are warranted to verify these results.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- ARF
acute respiratory failure
- CT
computed tomography
- GGO
ground-glass opacity
- HFNC
high flow nasal cannula
- HIV
human immunodeficiency virus
- MICU
medical intensive care unit
- PJP
Pneumocystis jirovecii pneumonia
- SLE
systemic lupus erythematosus
- TMP-SMZ
trimethoprim/sulfamethoxazole
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
HBH, JMP and BD contributed to the conception, design and data interpretation. All authors read and approved the manuscript.
Ethics approval and consent to participate
Patients provided written informed consent to participate.
Patient consent for publication
Consent for publication was obtained from the patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Li MC, Lee NY, Lee CC, Lee HC, Chang CM, Ko WC. Pneumocystis jiroveci pneumonia in immunocompromised patients: Delayed diagnosis and poor outcomes in 2014,non-HIV-infected individuals. J Microbiol Immunol Infect. 2014;47:42–47. doi: 10.1016/j.jmii.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Mansharamani NG, Garland R, Delaney D, Koziel H. Management and outcome patterns for adult pneumocystis carinii pneumonia, 1985 to 1995: Comparison of HIV-associated cases to other immunocompromised states. Chest. 2000;118:704–711. doi: 10.1378/chest.118.3.704. [DOI] [PubMed] [Google Scholar]
- 3.Miller RF, Allen E, Copas A, Singer M, Edwards SG. Improved survival for HIV infected patients with severe pneumocystis jirovecii pneumonia is independent of highly active antiretroviral therapy. Thorax. 2006;61:716–721. doi: 10.1136/thx.2005.055905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: Associated illness and prior corticosteroid therapy. Mayo Clin Proc. 1996;71:5–13. doi: 10.4065/71.1.5. [DOI] [PubMed] [Google Scholar]
- 5.Weng L, Huang X, Chen L, Feng LQ, Jiang W, Hu XY, Peng JM, Wang CY, Zhan QY, Du B. Prognostic factors for severe Pneumocystis jirovecipneumonia of non-HIV patients in intensive care unit: A bicentric retrospective study. BMC Infectious Dis. 2016;16:528. doi: 10.1186/s12879-016-1855-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monnet X, Vidal-Petiot E, Osman D, Hamzaoui O, Durrbach A, Goujard C, Miceli C, Bourée P, Richard C. Critical care management and outcome of severe Pneumocystis pneumonia in patients with and without HIV infection. Crit Care. 2008;12:R28. doi: 10.1186/cc6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boonsarngsuk V, Sirilak S, Kiatboonsri S. Acute respiratory failure due to pneumocystis pneumonia: Outcome and prognostic factors. Int J Infect Dis. 2008;13:59–66. doi: 10.1016/j.ijid.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Zhang JC, Dai JY, Fan J, Wu XP. The treatment of pneumocystis Carinii pneumonia with caspofungin in elderly patients: A case report and literature review. Zhonghua Jie He He Hu Xi Za Zhi. 2006;29:463. (In Chinese) [PubMed] [Google Scholar]
- 9.Annaloro C, Volpe AD, Usardi P, Lambertenghi Deliliers G. Caspofungin treatment of Pneumocystis pneumonia during conditioning for bone marrow transplantation. Eur J Clin Microbiol Infect Dis. 2006;25:52–54. doi: 10.1007/s10096-005-0065-z. [DOI] [PubMed] [Google Scholar]
- 10.Beltz K, Kramm CM, Laws HJ, Schroten H, Wessalowski R, Göbel U. Combined trimethoprim and caspofungin treatment for severe pneumocystis jiroveci pneumonia in a five year old boy with acute lymphoblastic leukemia. Klin Pädiatr. 2006;218:177–179. doi: 10.1055/s-2006-933433. [DOI] [PubMed] [Google Scholar]
- 11.Kamboj M, Weinstock D, Sepkowitz KA. Progression of pneumocystis jiroveci pneumonia in patients receiving echinocandin therapy. Clin Infect Dis. 2006;43:e92–e94. doi: 10.1086/508282. [DOI] [PubMed] [Google Scholar]
- 12.Takeda K, Morioka D, Kumamoto T, Matsuo K, Tanaka K, Endo I, Togo S, Shimada H. A survival case of ABO-incompatible liver transplantation complicated with severe preoperative infection and subsequent overwhelming postsplenectomy infection. Transplant Proc. 2009;41:3941–3944. doi: 10.1016/j.transproceed.2009.02.094. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Wang GF, Nie LG, et al. Clinical characteristics of Pneumocystis pneumonia during chemotherapy and radiotherapy in lung cancer patients. J Prac Oncol. 2012;27:175–179. [Google Scholar]
- 14.Li H, Huang H, He H. Successful treatment of severe Pneumocystis pneumonia in an immunosuppressed patient using caspofungin combined with clindamycin: A case report and literature review. BMC Pulm Med. 2016;16:144. doi: 10.1186/s12890-016-0307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim T, Hong HL, Lee YM, Sung H, Kim SH, Choi SH, Kim YS, Woo JH, Lee SO. Is caspofungin really an effective treatment for pneumocystis jirovecii pneumonia in immunocompromised patients without human immunodeficiency virus infection? Experiences at a single center and a literature review. Scand J Infect Dis. 2013;45:484–488. doi: 10.3109/00365548.2012.760842. [DOI] [PubMed] [Google Scholar]
- 16.Tu GW, Ju MJ, Xu M, Rong RM, He YZ, Xue ZG, Zhu TY, Luo Z. Combination of caspofungin and low-dose trimethoprim/sulfamethoxazole for the treatment of severe pneumocystis jirovecii pneumonia in renal transplant recipients. Nephrology (Carlton) 2013;18:736–742. doi: 10.1111/nep.12133. [DOI] [PubMed] [Google Scholar]
- 17.Jiang XQ, Fang L, Mei XD, Wang XJ, Bao MH. Pneumocystis jiroveci pneumonia in patients with non-Hodgkin's lymphoma after Rituximab-containing regimen: Two cases of report and literature review. J Thorac Dis. 2013;5:E162–E166. doi: 10.3978/j.issn.2072-1439.2013.08.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mu XD, Que CL, He B, Wang GF, Li HC. Caspofungin in salvage treatment of severe pneumocystis pneumonia: Case report and literature review. Chin Med J (Engl) 2009;122:996–999. [PubMed] [Google Scholar]
- 19.Hof H, Schnülle P. Pneumocystis jiroveci pneumonia in a patient with Wegener's granulomatosis treated efficiently with caspofungin. Mycoses. 2008;1(Suppl):S65–S67. doi: 10.1111/j.1439-0507.2008.01530.x. [DOI] [PubMed] [Google Scholar]
- 20.Utili R, Durante-mangoni E, Basilico C, Mattei A, Ragone E, Grossi P. Efficacy of caspofungin addition to trimethoprim-sulfamethoxazole treatment for severe pneumocystis pneumonia in solid organ transplant recipients. Transplantation. 2007;84:685–688. doi: 10.1097/01.tp.0000280546.91617.6c. [DOI] [PubMed] [Google Scholar]
- 21.Schmatz DM, Romancheck MA, Pittarelli LA, Schwartz RE, Fromtling RA, Nollstadt KH, Vanmiddlesworth FL, Wilson KE, Turner MJ. Treatment of pneumocystis carinii pneumonia with 1,3-beta-glucan synthesis inhibitors. Proc Natl Acad Sci USA. 1990;87:5950–5954. doi: 10.1073/pnas.87.15.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrongjames D, Stebbing J, John L, Murungi A, Bower M, Gazzard B, Nelson M. A trial of caspofungin salvage treatment in PCP pneumonia. Thorax. 2011;66:537–538. doi: 10.1136/thx.2010.135350. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Smego RA, Jr, Nagar S, Maloba B, Popara M. A Meta-analysis of salvage therapy for pneumocystis carinii pneumonia. Arch Intern Med. 2001;161:1529–1533. doi: 10.1001/archinte.161.12.1529. [DOI] [PubMed] [Google Scholar]
- 25.Kim T, Kim SH, Park KH, Cho OH, Sung H, Kim MN, Choi SH, Jeong JY, Woo JH, Kim YS, Lee SO. Clindamycin-primaquine versus pentamidine for the second-line treatment of pneumocystis pneumonia. J Infect Chemother. 2009;15:343–346. doi: 10.1007/s10156-009-0710-Z. [DOI] [PubMed] [Google Scholar]
- 26.Powles MA, Liberator P, Anderson J, Karkhanis Y, Dropinski JF, Bouffard FA, Balkovec JM, Fujioka H, Aikawa M, McFadden D, Schmatz D. Efficacy of MK-991 (L-743,872), a semisynthetic pneumocandin, in murine models of Pneumocystis carinii. Antimicrob Agents Chemother. 1998;42:1985–1989. doi: 10.1128/aac.42.8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas CF, Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298–308. doi: 10.1038/nrmicro1621. [DOI] [PubMed] [Google Scholar]
- 28.Lobo ML, Esteves F, De SB, de Sousa B, Cardoso F, Cushion MT, Antunes F, Matos O. Therapeutic potential of caspofungin combined with trimethoprim-sulfamethoxazole for pneumocystis pneumonia: A pilot study in mice. PLoS One. 2013;8:e70619. doi: 10.1371/journal.pone.0070619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.