Abstract
Turmeric dietary supplement sales, which accounted for US$69 million in spending in 2016, have been increasing exponentially in the USA, making this one of the most popular botanical supplements sold in the USA. Herbal supplement use, which is generally regarded as safe by consumers, is not usually reported to healthcare providers. We reported here on a case of autoimmune hepatitis, occurring in a 71-year-old woman taking turmeric dietary supplements for the maintenance of cardiovascular health, which resolved rapidly following discontinuation of the turmeric supplements. Of particular note, turmeric use was not documented in the patient’s medical records and the potential causative role of the turmeric supplementation was ultimately identified by the patient rather than the healthcare providers. To our knowledge, this is the first documented report of turmeric supplement-induced autoimmune hepatitis.
Keywords: complementary medicine, contraindications and precautions, gastrointestinal system, hepatitis other, healthcare improvement and patient safety
Background
Non-vitamin, non-mineral natural product (NVNM) dietary supplements (DS), the most popular complementary medicine modality in the USA, are used by almost 20% of US adults.1 Of these, turmeric (Curcuma longa L) is one of the top selling DS, with exponentially increasing sales reaching US$69 million in 2016.2 NVNM DS use is even more prevalent in certain high-risk populations (eg, >60 years old, with multiple chronic diseases, and/or taking >3 medications),3 4 with use of turmeric DS, specifically, recently reported by one-third of US adults with rheumatoid arthritis (RA).5 Given the increased vulnerability of certain populations to possible side effects associated with NVNM DS use (eg, reports of diarrhoea, altered liver function and iron metabolism, and worsening heart conduction defects associated with turmeric DS consumption6), it is a particular concern that their use is frequently not reported to health professionals.7 8 Because federal oversight of supplement safety in the USA is primarily limited to a postmarketing assessment of reported adverse events,9–12 recognition of supplement-related injuries is important for both individual and population-based safety outcomes. We discuss here a case of hepatotoxicity associated with turmeric supplement use, compatible with a diagnosis of drug-induced autoimmune hepatitis (DI-AIH). Importantly, the connection between turmeric supplement use and hepatotoxicity was hypothesised by the patient and not the treating clinicians; subsequent cessation of the turmeric supplement, which was not available for analysis, resulted in normalisation of the liver transaminases.
Case presentation
A 71-year-old woman presented to a gastroenterologist for the evaluation of asymptomatic transaminitis of unknown aetiology detected on a routine laboratory evaluation by her primary care physician. Her medical history was significant for hypothyroidism (likely Hashimoto’s), Raynaud’s syndrome, osteoarthritis, hypertension, dyslipidaemia, irritable bowel syndrome and diverticulosis. The patient denied alcohol use. Her family history was remarkable for a possible autoimmune disorder in a sibling diagnosed at 40 years of age with segmental mediolytic arteritis or polyarteritis nodosa. Current doses and types of medications and supplements (n=20), as documented in the medical record, had been unchanged for 1–2 years prior to the onset of transaminitis, remained stable through the period of transaminitis and subsequent recovery, and included amlodipine, metoprolol, atenolol, benazepril, levothyroxine, meloxicam, estradiol, loratadine, diphenhydramine, aspirin, calcium, vitamin D, a multi-vitamin, fish oil, a proprietary formulation of alpha-galactosidase and invertase, lysine, alfalfa powder, glucosamine with chondroitin, a proprietary high fibre supplement and a proprietary formulation containing vitamins, minerals and grape seed extract. While lengthy, this list was notable for the absence of a ‘low cost’ turmeric DS that the patient had purchased at a local DS retail store and begun using, as per labelled recommendations, 8 months prior to transaminitis testing with the goal of maintaining cardiovascular health after reading a news report of beneficial turmeric effects in stroke.13 Prior to the onset of transaminitis, the patient’s laboratory testing had been remarkable for a slight isolated elevation in alanine transaminase (ALT 58, with normal <40) attributed to use of statin-containing red yeast rice, which had been discontinued. She also had dyslipidaemia characterised by hypercholesterolaemia and elevated low-density lipoprotein (LDL), although these laboratory values would later normalise during the 15-month period of transaminitis.
Investigations
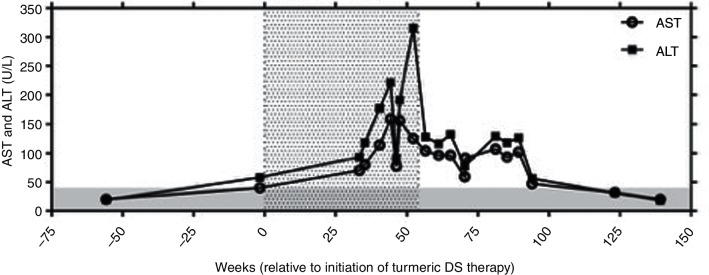
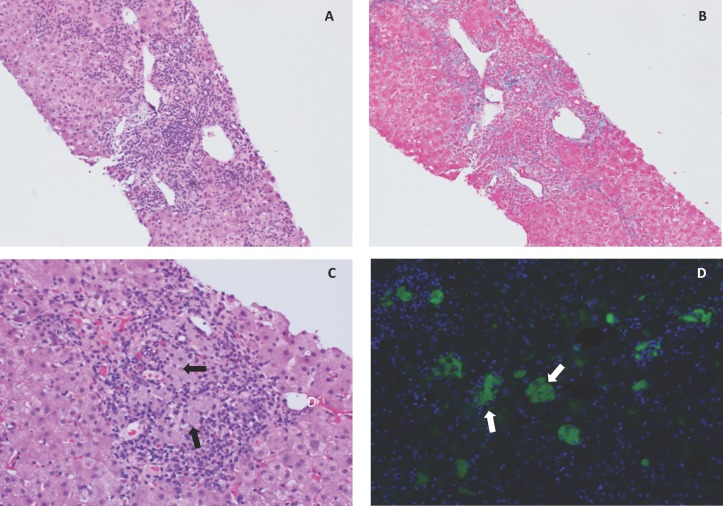
ALT and aspartate aminotransferase (AST) levels (figure 1) were first assayed and noted to be elevated on routine testing 33 weeks after start of the turmeric DS, and peaked at eight or four times the upper limit of normal (ULN), respectively, 44–52 weeks after the initiation of turmeric use. Lactate dehydrogenase (LDH) levels were slightly elevated during this period (243–251 U/L, ULN 214 U/L). A diagnostic evaluation initiated 35–40 weeks after the start of turmeric DS revealed elevated titres of perinuclear anti-neutrophil cytoplasmic antibody (P-ANCA) with an atypical pattern (1:320 and 1:160, with normal <1:20) and actin smooth-muscle antibody (63 U and 67 U, with normal 0–19). Antinuclear antibody (ANA), myeloperoxidase antibody (MPO), proteinase 3 antibody (PR-3), c-ANCA, RA factor, cyclic citrullinated peptide antibody (CCP) and anti-mitochondrial antibody (AMA) titers were negative and levels of alpha-1-antitrypsin and ceruloplasmin were normal. IgG and IgE levels were 1.3-fold and 8.1-fold higher than the ULN, respectively, without evidence of a monoclonal or polyclonal gammopathy on serum or urine protein electrophoresis. Viral serological tests (hepatitis A IgM antibody, hepatitis B surface antigen, hepatitis B core IgM antibody and hepatitis C antibody) were negative or non-reactive. Complete blood count, thyroid stimulating hormone, alkaline phosphatase, gamma-glutamyl transferase and total bilirubin values remained normal. An abdominal CT (performed 42 weeks after initiation of turmeric DS and 9 weeks after documentation of transaminitis) was non-diagnostic. A percutaneous core needle biopsy of the right lobe of the liver performed 3 weeks later revealed acute (neutrophils) and chronic (lymphocytes and plasma cells) moderate to marked portal inflammation with sparse inflammatory cells in the liver lobules (figure 2A), and mild portal fibrosis with sparse focal bridging (figure 2B). Focal piecemeal portal necrosis was present, with focal ballooning hepatocellular degeneration adjacent to the portal inflammation. Focal admixed histiocytes in areas of portal inflammation appeared on H&E staining to contain a greyish pigment of unknown aetiology (figure 2C). These laboratory and biopsy findings led to a diagnosis of autoimmune hepatitis (AIH) and expectant monitoring without treatment by clinicians managing the patient.
Figure 1.
Aspartate aminotransferase (AST) and alanine transaminase (ALT) levels (U/L) plotted by week relative to initiation of turmeric therapy. The grey-shaded area represents an average upper limit of normal for ALT and AST values, which were assayed by various clinical laboratories. The stippled area represents the time period of turmeric use. DS, dietary supplements.
Figure 2.
Photomicrographs of H&E (A; 10×) and trichrome (B; 10×) stained core liver biopsies, demonstrating areas of moderate to marked acute and chronic portal hepatitis associated with piecemeal necrosis and mild portal fibrosis with focal sparse bridging. Within areas of portal inflammation were focal areas of admixed grey-pigment containing histiocytes (C, arrows; H&E, 20×), which was autofluorescent (D; green autofluorescence (excitation 470 nm; emission 475–550 nm), with overlay of blue 4′,6-diamidino-2-phenylindole (DAPI) nuclear counterstain).
Turmeric DS use is first mentioned in the medical records 3 months after the liver biopsy (>1 year after start of turmeric) when it was noted that the patient had recently discontinued turmeric supplementation after she had identified turmeric DS use as possibly being causative of her liver problems, based on information available on the internet. A decrease in transaminases was noted <1 month after stopping turmeric DS (figure 1).
Subsequent reassessment of the liver biopsy by the authors revealed autofluorescent inclusions in the pigment-laden histiocytes (figure 2D), with an excitation/emission spectrum consistent with curcumin,14 15 a turmeric-derived polyphenol present in turmeric DS,6 or possibly lipofuscin.16–19 Histiocyte fluorescence, which was not noted in liver biopsy specimens from patients with unrelated disorders (data not shown), was quenchable by treatment with Sudan Black B (SBB), as has been reported for lipofuscin.18 However, because the authors also documented complete SBB quenching of curcumin autofluorescence in fixed cultured cells specifically loaded with curcuminoids (data not shown), it cannot be ascertained with certainty whether the histiocyte inclusions here were composed of lipofuscin, a lysosomal degradation product and/or curcuminoids derived from the turmeric DS that the patient was still consuming at the time of the biopsy.
Differential diagnosis
Highly specific (97%) and sensitive (95%) diagnostic criteria for AIH, established in 1993 and revised in 1999 by an international panel,20 were met in this case (table 1).
Table 1.
Revised original scoring system of the International Autoimmune Hepatitis (AIH) Group*
| Points | Case report | ||
| Sex | Female | 2 | X |
| ALP:AST (or ALT) ratio | >3 | −2 | |
| <1.5 | 2 | X | |
| IgG (or gamma globulin) level above normal | >2 | 3 | |
| 1.5–2 | 2 | ||
| 1–1.5 | 1 | X | |
| <1 | 0 | ||
| ANA, SMA or anti-LKM1 titres | >1:80 | 3 | X |
| 1:80 | 2 | ||
| 1:40 | 1 | ||
| <1:40 | 0 | ||
| AMA | Positive | −4 | |
| Viral markers | Positive | −3 | |
| Negative | 3 | X | |
| Drugs | Yes | −4 | |
| No | 1 | X | |
| Alcohol | <25 g/day | 2 | X |
| >60 g/day | −2 | ||
| HLA | DR3 or DR4 | 1 | |
| Immune disease | Thyroiditis, colitis, others | 2 | X |
| Other markers | Anti-SLA, anti-actin, anti-LC1, pANCA | 2 | X |
| Histological features | Interface hepatitis | 3 | X |
| Plasmacytic | 1 | ||
| Rosettes | 1 | ||
| None of above | −5 | ||
| Biliary changes | −3 | ||
| Other features | −3 | ||
| Treatment response | Complete | 2 | X |
| Relapse | 3 | ||
| Total | 23†‡ |
*Adapted from Manns et al.20
†Pretreatment aggregate score: >15, definite diagnosis; 10–15, probable diagnosis.
‡If assume turmeric is possible drug cause, total score is 18, definite AIH.
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; LC1, liver cytosol antibody; LKM1, liver kidney microsomes-1 antibody; SMA, smooth-muscle antibody; SLA, soluble liver antigen antibody.
If the temporal association with turmeric DS use is ignored, as occurred during the patient’s initial evaluation, a pretreatment aggregate score of 23 was compatible with a definite diagnosis of AIH (table 1), as was a revised score of 18 when the association with turmeric DS use was included. A simplified AIH diagnostic scoring system proposed by Hennes et al,21 which also exhibits high sensitivity and specificity,20 was also consistent with definite AIH (table 2, score >7).
Table 2.
Simplified diagnostic criteria for autoimmune hepatitis (AIH)*
| Points | Case report | ||
| Autoantibodies | ANA or SMA ≥1:80 or LKM ≥1:40 or SLA positive | 2 | X |
| ANA or SMA ≥1:40 | 1 | ||
| IgG/gamma globulins | >1.10 times normal limit | 2 | X |
| >Upper normal limit | 1 | ||
| Liver histology | Typical for AIH | 2 | |
| Compatible with AIH | 1 | X | |
| Atypical for autoimmune | 0 | ||
| Absence of viral hepatitis | Yes | 2 | X |
| No | 0 | ||
| Total | 7† |
Use of the Roussel Uclaf Causality Assessment Method (RUCAM), a tool established by a consensus group in 1993 to assess drug-induced liver injury (DILI), including herb-induced liver injury (HILI),22 identified a hepatocellular injury pattern with a total RUCAM score of 7 (given prior evidence of hepatotoxic turmeric effects),23–30 that was consistent with probable DILI/HILI (table 3). Because a diagnosis of DI-AIH requires a definite temporal association between drug initiation and subsequent AIH with exclusion of other causes,31 32 as occurred here, the combination of probable DILI/HILI by RUCAM and the onset of definite AIH after initiation of turmeric DS use by two scoring systems is suggestive of probable DI-AIH attributable to turmeric DS use.
Table 3.
Roussel Uclaf Causality Assessment Method for drug-induced liver injury/herb-induced liver injury hepatocellular injury*
| Points | Case report | ||
| Time to onset from beginning of drug/herb | 5–90 days (re-challenge: 1–15 days) | 2 | |
| <5 or >90 days (re-challenge: >15 days) | 1 | X | |
| Time to onset from cessation of drug/herb | ≤15 days (except for slowly metabolised chemicals >15 days) | 1 | |
| Course of ALT after cessation of drug/herb (% difference between ALT peak and N) |
Decrease ≥50% within 8 days | 3 | |
| Decrease ≥50% within 30 days | 2 | X | |
| No information or continued drug use | 0 | ||
| Decrease ≥50% after the 30th day | 0 | ||
| Decrease <50% after the 30th day or recurrent increase | −2 | ||
| Risk factors | Alcohol use—presence | 1 | |
| Alcohol use—absence | 0 | X | |
| Age ≥55 years | 1 | X | |
| Age <55 years | 0 | ||
| Concomitant drug(s)/herb(s) | None or no information | 0 | |
| Concomitant drug/herb with incompatible time to onset | 0 | X | |
| Concomitant drug/herb with compatible or suggestive time to onset | −1 | ||
| Concomitant drug/herb known as hepatotoxin and with compatible or suggestive time to onset | −2 | ||
| Concomitant drug/herb with evidence for its role in this case (positive rechallenge or validated test) | −3 | ||
| Search for alternative causes† | All causes reasonably ruled out (group I and II) | 2 | X |
| Six causes of group I ruled out | 1 | ||
| 4–5 causes of group I ruled out | 0 | ||
| <4 causes of group I ruled out | −2 | ||
| Alternative cause highly probable | −3 | ||
| Previous hepatotoxicity of drug/herb | Reaction labelled in the product characteristics | 2 | |
| Reaction published but unlabelled | 1 | X | |
| Reaction unknown | 0 | ||
| Response to unintended re-exposure | Doubling of ALT with drug/herb alone, (if ALT <5N before re-exposure) | 3 | |
| Doubling of ALT with drug/herb already given at time of first reaction | 1 | ||
| Increase of ALT but <5N in the same conditions as for first administration | −2 | ||
| Other situations | 0 | ||
| Total | 7‡ |
*Adapted from Danan and Benichou.22
†Group I causes: HAV, HBV, HCV, biliary obstruction (imaging), alcoholism, acute recent hypotension.
Group II causes: complications of underlying disease (eg, sepsis), CMV, EBV, HSV.
‡≤0, excluded causality; 1 – 2, unlikely; 3 – 5, possible; 6 – 8, probable; ≥ 9, highly probable.
ALT, alanine transaminase; CMV, cytomegalovirus; EBV; Epstein Barr virus; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HSV, herpes simplex virus.
Outcome and follow-up
Treatment was limited to withdrawing turmeric DS use. AST and ALT, which significantly decreased within 30 days of discontinuation, normalised by 13 months and have remained normal until the present (3 years) with continued polypharmacy, excluding turmeric DS use (figure 1). Importantly, the patient, not the clinicians, hypothesised that the turmeric DS may have been the cause of the elevated liver transaminases and elected to cease its use. The patient brought her case to the attention of the authors out of a concern for the safety of others when she noted our recruitment efforts for a clinical trial testing turmeric DS in patients with RA, a population at increased risk for AIH.20 33 Unfortunately, because the identity of the specific turmeric DS used was not known, no testing for turmeric content or contaminants could be performed.
Discussion
This is the first report, to our knowledge, to link DI-AIH with the use of a turmeric DS. Because clinical studies have popularised turmeric DS use in certain immune disorders, such as autoimmune thyroiditis, RA and ulcerative colitis, which are associated with an increased risk of AIH,5 20 33 clinicians should take note of a potential link between turmeric DS use and DI-AIH. The prevalence of DI-AIH among patients with AIH is estimated to be 10%–15%, with up to 20% of cases attributable to herbals.31 32 A long duration (23±16 months) of drug use prior to DI-AIH diagnosis has been reported32; whether this relates to a delay in identifying the inciting agent or a latent period of liver injury is unclear, but further underscores the importance of considering herbal supplements in the pathogenesis of DI-AIH.
The possible demonstration of fluorescent curcumin in areas of portal inflammation in this case is suggestive of a possible causal role for these turmeric-derived polyphenols. A review of 35 published turmeric DS trials in various populations25–30 34–62 reveals a 5% overall incidence of abnormal liver function associated with turmeric DS use (including transaminases, LDH, alkaline phosphatase and/or bilirubin) in studies that incorporated screening (n=20 studies, representing 526 treated subjects), with all reported cases occurring in studies of >1 month duration, an observation of potential relevance in the context of chronic consumer use. This low incidence, however, is consistent with the notion that turmeric DS-induced liver injury may be idiosyncratic with no clear relationship between degree of liver injury and dosage. This contrasts with intrinsic DILI, such as that seen with acetaminophen, where the relationship between high doses of the drug and liver injury is predictable.10 Herbal-related DILI has been increasing in frequency over the last decade in the USA, accounting for 20% of DILI cases in 2013,9 and an even higher proportion of cases in countries where herbal use is more common.63 64 Known risk factors for idiosyncratic DILI, including advanced age, female gender, alcohol consumption and the consumption of more than 100 mg/day of the offending agent,10 64 could likely have been met in this case, with the exception of alcohol use, since the majority of US turmeric DS deliver more than 100 mg of curcuminoids.6 In rodents, high doses of turmeric rhizome (3% curcuminoids by weight) or isolated curcuminoids have been reported to cause hepatotoxicity23 and in silico analysis predicts hepatotoxic effects for 64 of 200 compounds isolated from turmeric,24 a relevant finding since one-third of turmeric DS contain additional non-curcuminoid, turmeric-derived components.6
Of note, however, the association of turmeric DS use with liver injury in this case does not necessarily imply a causal role for the turmeric content of the DS or even for the turmeric DS itself. Given the polypharmacy associated with this case, while the onset and resolution of hepatic injury were associated with turmeric DS use, it is possible that pharmacokinetic or pharmacodynamic interactions between the turmeric DS and other concurrent medications, including those previously associated with cholestatic or hepatocellular liver injury (amlodipine, metoprolol, atenolol, benazepril, meloxicam, estradiol and loratadine65), were causative. Similarly, given the chemical complexity of botanical DS, as well as their frequent contamination with heavy metals or pesticides associated with idiosyncratic liver injury, such as lead, mercury, cadmium and arsenic,66 the turmeric content of the DS used here may not necessarily have been causative. For example, lead contamination of turmeric DS has been reported, particularly for turmeric root-containing products,6 67 and up to one-half of turmeric DS are formulated to contain additional botanicals.6 Of particular note, one quarter of US turmeric DS, contain piperine,6 a black pepper-derived product thought to enhance curcumin bioavailability via the suppression of hepatic drug-metabolising enzymes,68 69 an effect that could also adversely alter the metabolism of concurrent medications. While the absence of an identifiable product in this case precludes determination of any associations between product content and outcome, attribution of causation under the best of circumstances could be difficult given the complexity of turmeric DS composition.6 Nonetheless, while the lack of an identifiable herbal product is an extremely common occurrence in cases of suspected DS-induced adverse events,70 given the chemical complexity of DS and challenges associated with their regulation, such reports have formed the basis for previous consumer warnings (eg, black cohosh DS-associated hepatotoxicity).70
The possible role of polypharmacy in turmeric DS-associated AIH in this case also highlights the importance of discussing DS use in particular healthcare settings, such as with elderly patients, where polypharmacy is seen in 20%–40% and carries an increased risk of adverse drug/supplement interactions that can be compounded by age-related reductions in hepatic metabolism and/or alterations in end-organ responsiveness.71 Approximately one-third of older patients are estimated to be at risk for adverse interactions between NVNM DS and prescription medicines,71 and while 64% of surveyed adults over the age of 65 use NVNM DS,72 73 only 35% have this usage documented in their medical records.72 This is particularly salient when discussing DI-AIH, given that 23% of patients with AIH are >60 years of age.20 Because a lack of physician inquiry or knowledge about CAM use is a reason frequently cited by US adults for non-disclosed use of NVNM DS or other CAM modalities,7 greater vigilance on the part of healthcare providers is needed, particularly when caring for at-risk populations with a high prevalence of NVNM use.
Learning points.
Turmeric dietary supplements can cause idiosyncratic drug-induced autoimmune hepatitis.
The precise causative agent in this case is not known (plant constituent [eg, curcuminoids] versus other constituent/contaminant in dietary supplement, and/or possible pharmacodynamic interaction with concurrent medications).
While not common, autoimmune hepatitis occurs more commonly in elderly women, a demographic that is also a top user of dietary supplements.
Hepatotoxicity has been reported in approximately 5% of patients in turmeric dietary supplement clinical trials conducted in various populations, particularly when treatment duration was >1 month. Patients with abnormal immune function (autoimmune diseases or immunosuppression) concurrently taking medications with possible hepatotoxic effects may be at increased risk.
A causative role for botanical dietary supplements in drug-induced toxicities can be missed due to lack of effective communication about dietary supplement use, as well as a lack of knowledge or consideration of possible side effects associated with dietary supplement use on the part of both patients and healthcare providers.
Acknowledgments
The authors would like to thank Jennifer B Frye and Andrew Kunihiro for their assistance in assessing quenchable curcumin autofluorescence.
Footnotes
Contributors: AL, SM, CA and JF contributed to data acquisition, and AL and JF contributed to data analysis and preparation of the manuscript, which was approved by all authors.
Funding: This research was supported by the National Center for Complementary and Integrative Health at the National Institutes of Health (NIH-NCCIH, R34AT007837).
Disclaimer: Views expressed here do not necessarily represent those of the NIH or NCCIH.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wu CH, Wang CC, Tsai MT, et al. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 national health interview surveys. Evid Based Complement Alternat Med 2014;2014:872320 10.1155/2014/872320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith T, Kawa K, Eckl V, et al. Herbal Supplement Sales in US Increase 7.7% in 2016. Consumer preferences shifting toward ingredients with general wellness benefits, driving growth of adaptogens and digestive health products. Herbalgram 2017;115:56–65. [Google Scholar]
- 3.Farina EK, Austin KG, Lieberman HR. Concomitant dietary supplement and prescription medication use is prevalent among US adults with doctor-informed medical conditions. J Acad Nutr Diet 2014;114:1784–90. 10.1016/j.jand.2014.01.016 [DOI] [PubMed] [Google Scholar]
- 4.Falci L, Shi Z, Greenlee H. Multiple chronic conditions and use of complementary and alternative medicine among US adults: results from the 2012 national health interview survey. Prev Chronic Dis 2016;13:E61 10.5888/pcd13.150501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groff R, Strom M, Hopkins L, et al. Dietary supplements and nutritional approaches used for rheumatoid arthritis self-management. Faseb J 2017;31(Suppl 1):lb396. [Google Scholar]
- 6.Skiba MB, Luis PB, Alfarara C, et al. Curcuminoid content and safety-related markers of quality of turmeric dietary supplements sold in an urban retail marketplace in the United States. Mol Nutr Food Res 2018;29:e1800143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jou J, Johnson PJ. Nondisclosure of complementary and alternative medicine use to primary care physicians: findings from the 2012 National Health Interview Survey. JAMA Intern Med 2016;176:545–6. 10.1001/jamainternmed.2015.8593 [DOI] [PubMed] [Google Scholar]
- 8.Wu CH, Wang CC, Kennedy J. Changes in herb and dietary supplement use in the U.S. adult population: a comparison of the 2002 and 2007 National Health Interview Surveys. Clin Ther 2011;33:1749–58. 10.1016/j.clinthera.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 9.Avigan MI, Mozersky RP, Seeff LB. Scientific and regulatory perspectives in herbal and dietary supplement associated hepatotoxicity in the United States. Int J Mol Sci 2016;17:331 10.3390/ijms17030331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014;109:950–66. 10.1038/ajg.2014.131 [DOI] [PubMed] [Google Scholar]
- 11.Sax JK. Dietary supplements are not all safe and not all food: how the low cost of dietary supplements preys on the consumer. Am J Law Med 2015;41:374–94. 10.1177/0098858815591523 [DOI] [PubMed] [Google Scholar]
- 12.Downing NS, Shah ND, Aminawung JA, et al. Postmarket safety events among novel therapeutics approved by the us food and drug administration between 2001 and 2010. JAMA 2017;317:1854–63. 10.1001/jama.2017.5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson W. Turmeric studied as stroke preventer. Arizona Daily Star. 2011. http://tucson.com/news/science/turmeric-studied-as-stroke-preventer/article_6b776950-2e1e-56b6-ac9d-aad1bb35ac5c.html (cited 20 Mar 2017).
- 14.Kunwar A, Barik A, Mishra B, et al. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim Biophys Acta 2008;1780:673–9. 10.1016/j.bbagen.2007.11.016 [DOI] [PubMed] [Google Scholar]
- 15.Midura-Kiela MT, Radhakrishnan VM, Larmonier CB, et al. Curcumin inhibits interferon-γ signaling in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 2012;302:G85–96. 10.1152/ajpgi.00275.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marmorstein AD, Marmorstein LY, Sakaguchi H, et al. Spectral profiling of autofluorescence associated with lipofuscin, Bruch’s Membrane, and sub-RPE deposits in normal and AMD eyes. Invest Ophthalmol Vis Sci 2002;43:2435–41. [PubMed] [Google Scholar]
- 17.Croce AC, De Simone U, Freitas I, et al. Human liver autofluorescence: an intrinsic tissue parameter discriminating normal and diseased conditions. Lasers Surg Med 2010;42:371–8. 10.1002/lsm.20923 [DOI] [PubMed] [Google Scholar]
- 18.Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem 1999;47:719–30. 10.1177/002215549904700601 [DOI] [PubMed] [Google Scholar]
- 19.Ivy GO, Kanai S, Ohta M, et al. Leupeptin causes an accumulation of lipofuscin-like substances in liver cells of young rats. Mech Ageing Dev 1991;57:213–31. 10.1016/0047-6374(91)90048-5 [DOI] [PubMed] [Google Scholar]
- 20.Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology 2010;51:2193–213. 10.1002/hep.23584 [DOI] [PubMed] [Google Scholar]
- 21.Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169–76. 10.1002/hep.22322 [DOI] [PubMed] [Google Scholar]
- 22.Danan G, Benichou C. Causality assessment of adverse reactions to drugs–I. A novel method based on the conclusions of international consensus meetings : application to drug-induced liver injuries. J Clin Epidemiol 1993;46:1323–30. 10.1016/0895-4356(93)90101-6 [DOI] [PubMed] [Google Scholar]
- 23.Deshpande SS, Lalitha VS, Ingle AD, et al. Subchronic oral toxicity of turmeric and ethanolic turmeric extract in female mice and rats. Toxicol Lett 1998;95:183–93. 10.1016/S0378-4274(98)00035-6 [DOI] [PubMed] [Google Scholar]
- 24.Balaji S, Chempakam B. Toxicity prediction of compounds from turmeric (Curcuma longa L). Food Chem Toxicol 2010;48:2951–9. 10.1016/j.fct.2010.07.032 [DOI] [PubMed] [Google Scholar]
- 25.Chainani-Wu N, Madden E, Lozada-Nur F, et al. High-dose curcuminoids are efficacious in the reduction in symptoms and signs of oral lichen planus. J Am Acad Dermatol 2012;66:752–60. 10.1016/j.jaad.2011.04.022 [DOI] [PubMed] [Google Scholar]
- 26.Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol 2006;4:1502–6. 10.1016/j.cgh.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 27.Kanai M, Yoshimura K, Asada M, et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol 2011;68:157–64. 10.1007/s00280-010-1470-2 [DOI] [PubMed] [Google Scholar]
- 28.Kurd SK, Smith N, VanVoorhees A, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: A prospective clinical trial. J Am Acad Dermatol 2008;58:625–31. 10.1016/j.jaad.2007.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoskes D, Lapierre C, Cruz-Correa M, et al. Beneficial effects of the bioflavonoids curcumin and quercetin on early function in cadaveric renal transplantation: a randomized placebo controlled trial. Transplantation 2005;80:1556–9. 10.1097/01.tp.0000183290.64309.21 [DOI] [PubMed] [Google Scholar]
- 30.Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 2004;10:6847–54. 10.1158/1078-0432.CCR-04-0744 [DOI] [PubMed] [Google Scholar]
- 31.Yeong TT, Lim KH, Goubet S, et al. Natural history and outcomes in drug-induced autoimmune hepatitis. Hepatol Res 2016;46:E79–E88. 10.1111/hepr.12532 [DOI] [PubMed] [Google Scholar]
- 32.Björnsson E, Talwalkar J, Treeprasertsuk S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology 2010;51:2040–8. 10.1002/hep.23588 [DOI] [PubMed] [Google Scholar]
- 33.Bright JJ. Curcumin and autoimmune disease. Adv Exp Med Biol 2007;595:425–51. 10.1007/978-0-387-46401-5_19 [DOI] [PubMed] [Google Scholar]
- 34.Baum L, Lam CW, Cheung SK, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol 2008;28:110–3. 10.1097/jcp.0b013e318160862c [DOI] [PubMed] [Google Scholar]
- 35.Bayet-Robert M, Kwiatkowski F, Leheurteur M, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther 2010;9:8–14. 10.4161/cbt.9.1.10392 [DOI] [PubMed] [Google Scholar]
- 36.Carroll RE, Benya RV, Turgeon DK, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res 2011;4:354–64. 10.1158/1940-6207.CAPR-10-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chainani-Wu N, Silverman S, Reingold A, et al. A randomized, placebo-controlled, double-blind clinical trial of curcuminoids in oral lichen planus. Phytomedicine 2007;14:437–46. 10.1016/j.phymed.2007.05.003 [DOI] [PubMed] [Google Scholar]
- 38.Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, et al. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012;35:2121–7. 10.2337/dc12-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruz-Correa M, Shoskes DA, Sanchez P, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol 2006;4:1035–8. 10.1016/j.cgh.2006.03.020 [DOI] [PubMed] [Google Scholar]
- 40.Deodhar SD, Sethi R, Srimal RC. Preliminary study of antirheumatic activity of curcumin (diferuloyl methane). Indian J Med Res 1980;4:1035–8. [PubMed] [Google Scholar]
- 41.Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res 2008;14:4491–9. 10.1158/1078-0432.CCR-08-0024 [DOI] [PubMed] [Google Scholar]
- 42.Garcea G, Berry DP, Jones DJ, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev 2005;14:120–5. [PubMed] [Google Scholar]
- 43.Kuptniratsaikul V, Thanakhumtorn S, Chinswangwatanakul P, et al. Efficacy and safety of Curcuma domestica extracts in patients with knee osteoarthritis. J Altern Complement Med 2009;15:891–7. 10.1089/acm.2008.0186 [DOI] [PubMed] [Google Scholar]
- 44.Pungcharoenkul K, Thongnopnua P. Effect of different curcuminoid supplement dosages on total in vivo antioxidant capacity and cholesterol levels of healthy human subjects. Phytother Res 2011;25:1721–6. 10.1002/ptr.3608 [DOI] [PubMed] [Google Scholar]
- 45.Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol 1986;24:651–4. [PubMed] [Google Scholar]
- 46.Suskind DL, Wahbeh G, Burpee T, et al. Tolerability of curcumin in pediatric inflammatory bowel disease: a forced-dose titration study. J Pediatr Gastroenterol Nutr 2013;56:277–9. 10.1097/MPG.0b013e318276977d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wongcharoen W, Jai-Aue S, Phrommintikul A, et al. Effects of curcuminoids on frequency of acute myocardial infarction after coronary artery bypass grafting. Am J Cardiol 2012;110:40–4. 10.1016/j.amjcard.2012.02.043 [DOI] [PubMed] [Google Scholar]
- 48.Agarwal KA, Tripathi CD, Agarwal BB, et al. Efficacy of turmeric (curcumin) in pain and postoperative fatigue after laparoscopic cholecystectomy: a double-blind, randomized placebo-controlled study. Surg Endosc 2011;25:3805–10. 10.1007/s00464-011-1793-z [DOI] [PubMed] [Google Scholar]
- 49.Burns J, Joseph PD, Rose KJ, et al. Effect of oral curcumin on Déjérine-Sottas disease. Pediatr Neurol 2009;41:305–8. 10.1016/j.pediatrneurol.2009.04.030 [DOI] [PubMed] [Google Scholar]
- 50.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 2001;2:2895–900. [PubMed] [Google Scholar]
- 51.Conteas CN, Panossian AM, Tran TT, et al. Treatment of HIV-associated diarrhea with curcumin. Dig Dis Sci 2009;54:2188–91. 10.1007/s10620-008-0597-z [DOI] [PubMed] [Google Scholar]
- 52.Golombick T, Diamond TH, Badmaev V, et al. The potential role of curcumin in patients with monoclonal gammopathy of undefined significance–its effect on paraproteinemia and the urinary N-telopeptide of type I collagen bone turnover marker. Clin Cancer Res 2009;15:5917–22. 10.1158/1078-0432.CCR-08-2217 [DOI] [PubMed] [Google Scholar]
- 53.Golombick T, Diamond TH, Manoharan A, et al. Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: a randomized, double-blind placebo-controlled cross-over 4g study and an open-label 8g extension study. Am J Hematol 2012;87:455–60. 10.1002/ajh.23159 [DOI] [PubMed] [Google Scholar]
- 54.Irving GR, Howells LM, Sale S, et al. Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration–a clinical pilot study including assessment of patient acceptability. Cancer Prev Res 2013;6:119–28. 10.1158/1940-6207.CAPR-12-0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res 1999;13:318–22. [DOI] [PubMed] [Google Scholar]
- 56.Lao CD, Ruffin MT, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 2006;6:10 10.1186/1472-6882-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreillon JJ, Bowden RG, Deike E, et al. The use of an anti-inflammatory supplement in patients with chronic kidney disease. J Complement Integr Med 2013;10:143–52. 10.1515/jcim-2012-0011 [DOI] [PubMed] [Google Scholar]
- 58.Pinsornsak P, Niempoog S. The efficacy of Curcuma Longa L. extract as an adjuvant therapy in primary knee osteoarthritis: a randomized control trial. J Med Assoc Thai 2012;95(Suppl 1):S51–8. [PubMed] [Google Scholar]
- 59.Ponnurangam S, Mondalek FG, Govind J, et al. Urine and serum analysis of consumed curcuminoids using an IkappaB-luciferase surrogate marker assay. In Vivo 2010;24:861–4. [PMC free article] [PubMed] [Google Scholar]
- 60.Ryan JL, Heckler CE, Ling M, et al. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res 2013;180:34–43. 10.1667/RR3255.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soni KB, Kuttan R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J Physiol Pharmacol 1992;36:273–5. [PubMed] [Google Scholar]
- 62.Vareed SK, Kakarala M, Ruffin MT, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev 2008;17:1411–7. 10.1158/1055-9965.EPI-07-2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarges P, Steinberg JM, Lewis JH. Drug-Induced Liver Injury: Highlights from a Review of the 2015 Literature. Drug Saf 2016;39:801–21. 10.1007/s40264-016-0427-8 [DOI] [PubMed] [Google Scholar]
- 64.Brown AC. Liver toxicity related to herbs and dietary supplements: online table of case reports. Part 2 of 5 series. Food Chem Toxicol 2017;107(Pt A):472–501. 10.1016/j.fct.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 65.LiverTox. National institute of diabetes and digestive and kidney disease, NIDDK. 2017. https://livertox.nlm.nih.gov/index.html (cited 10 March 2017).
- 66.Kutz GD. Herbal dietary supplements: examples of deceptive or questionable marketing practices and potentially dangerous advice: United States Government Accountability Office: GAO-10-662T, 2010. [Google Scholar]
- 67.Cowell W, Ireland T, Vorhees D, et al. Ground turmeric as a source of lead exposure in the United States. Public Health Rep 2017;132:289–93. 10.1177/0033354917700109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shoba G, Joy D, Joseph T, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 1998;64:353–6. 10.1055/s-2006-957450 [DOI] [PubMed] [Google Scholar]
- 69.Suresh D, Srinivasan K. Influence of curcumin, capsaicin, and piperine on the rat liver drug-metabolizing enzyme system in vivo and in vitro. Can J Physiol Pharmacol 2006;84:1259–65. 10.1139/y06-074 [DOI] [PubMed] [Google Scholar]
- 70.Mahady GB, Low Dog T, Barrett ML, et al. United States Pharmacopeia review of the black cohosh case reports of hepatotoxicity. Menopause 2008;15:628–38. 10.1097/gme.0b013e31816054bf [DOI] [PubMed] [Google Scholar]
- 71.Hilmer SN, McLachlan AJ, Le Couteur DG. Clinical pharmacology in the geriatric patient. Fundam Clin Pharmacol 2007;21:217–30. 10.1111/j.1472-8206.2007.00473.x [DOI] [PubMed] [Google Scholar]
- 72.Cohen RJ, Ek K, Pan CX. Complementary and alternative medicine (cam) use by older adults: a comparison of self-report and physician chart documentation. J Gerontol A Biol Sci Med Sci 2002;57:M223–7. 10.1093/gerona/57.4.M223 [DOI] [PubMed] [Google Scholar]
- 73.Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 2016;176:473–82. 10.1001/jamainternmed.2015.8581 [DOI] [PMC free article] [PubMed] [Google Scholar]