Abstract
Primary intracranial leiomyosarcoma (PIL) is a rare non-infectious aetiology of focal mass lesions among HIV-infected individuals. With only 16 published cases worldwide, information on its pathophysiology, risk factors, clinical course and management options is limited. We report two cases of PIL in HIV-infected Filipino men who presented with 1–3 months history of persistent headache, progressing in severity. Both had cranial MRI revealing intracranial mass diagnosed as leiomyosarcoma by excision biopsy and immunohistochemical staining. Both patients underwent adjuvant cranial radiotherapy and chemotherapy. Biologics were initiated in one patient. Both patients were alive with evidence of the disease.
Keywords: Hiv / Aids, neurooncology
Background
Despite the availability of highly active antiretroviral therapy (ART), intracranial lesions that are considered AIDS-defining illnesses remain common in low/middle-income nations.1 2 The complex interaction of HIV and the host immune system diversifies the differential diagnoses when confronted with a patient with HIV presenting with an intracranial lesion.3 4 Primary intracranial leiomyosarcoma (PIL) is a rare malignant neoplasm of smooth muscle origin that has only been reported among 16 patients with HIV.5–10 The extremely low incidence of PIL makes its diagnosis and management challenging. We report the first two cases of HIV-associated PIL in the Philippines and documented the dilemma encountered throughout their management to contribute to the better understanding of this rare malignancy.
Case presentation
Case 1
A 29-year-old man with no known chronic medical illness presented with 1-month history of intermittent frontal headache. The patient initially thought the headache as ‘migraine attacks’ and self-medicated with ibuprofen with partial relief. The worsening severity of headache and the development of periorbital numbness and slurred speech prompted admission to a tertiary hospital.
Case 2
A 36-year-old man with known HIV presented with a 3-month history of intermittent nape pain and temporal headache. At the time of presentation, his cluster of differentiation 4 (CD4) count was 144 cells/mm3 and was on ART regimen of efavirenz 600 mg/lamivudine 300 mg/tenofovir 300 mg tablet.
The patient was diagnosed with HIV infection 2 years prior to the occurrence of headache. He also received 6-month treatment for pulmonary tuberculosis (TB) on initial HIV diagnosis. Persistence of headache prompted consult to a neurologist.
Investigations
Case 1
On admission, a contrast-enhanced cranial CT scan showed a non-homogeneous slightly hyperdense ovoid mass at the left parietal lobe measuring 3.36×2.64×2.0 cm (figure 1). The patient was managed as a case of brain abscess with intravenous ceftriaxone and metronidazole. Cotrimoxazole 800/160 mg tablet two tablets every 8 hours were empirically started to cover for cerebral toxoplasmosis. Medical decompression with mannitol was also initiated. Admission laboratory results were unremarkable except for leucopenia (3.9 g/L, 60% lymphocytes) and slightly elevated serum lactate dehydrogenase (235 μ/L). On the 7th day of antibiotic treatment, cranial MRI showed no change in the size of the parietal mass (figure 2); hence, excision biopsy was performed. Histopathology showed spindle-like cells in fascicles. There is marked cytological atypia with more than 10 mitotic figures per 10 high power field (hpf) (figure 3). Immunohistochemistry staining demonstrated that the tissue was markedly positive for smooth muscle antigen (SMA) but negative for epithelial membrane antigen (EMA) and CD34 (figure 4). Abdominal and chest CT scan with contrast did not show other lesions or possible sites of malignancy. A diagnosis of PIL was made. HIV infection was confirmed by western blot. CD4 count was 7 cells/mm3. Serum toxoplasma IgG was negative. Cotrimoxazole dose was reduced to prophylaxis dosage (one 800/160 mg tablet once daily). Due to financial constraints experienced by the patient, tissue in situ hybridisation for Epstein-Barr virus (EBV)-encoded RNA was not performed. The limited funds were allocated for treatment.
Figure 1.
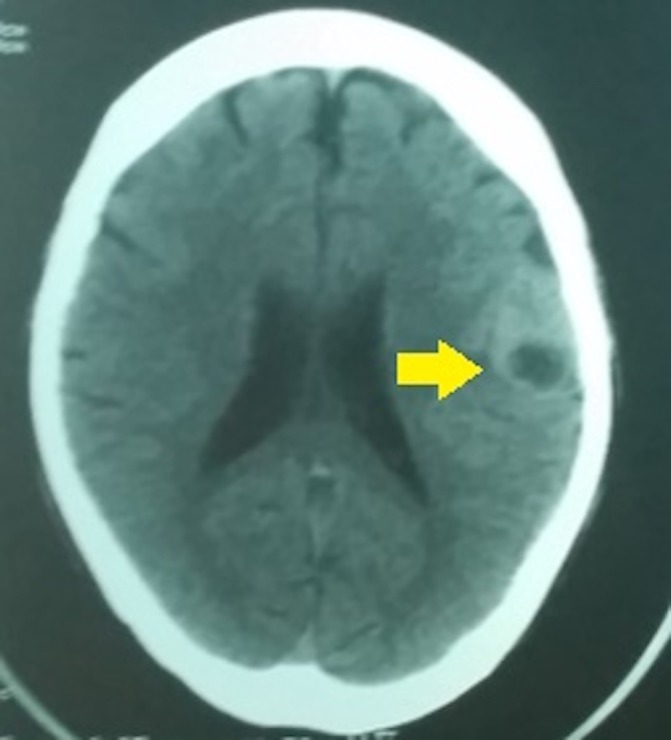
A contrast-enhanced cranial CT scan showing an enhancement of the hyperdense mass in the left frontoparietal region measuring 3.36×2.64×2.0 cm with areas of central necrosis (as pointed by an arrow).
Figure 2.
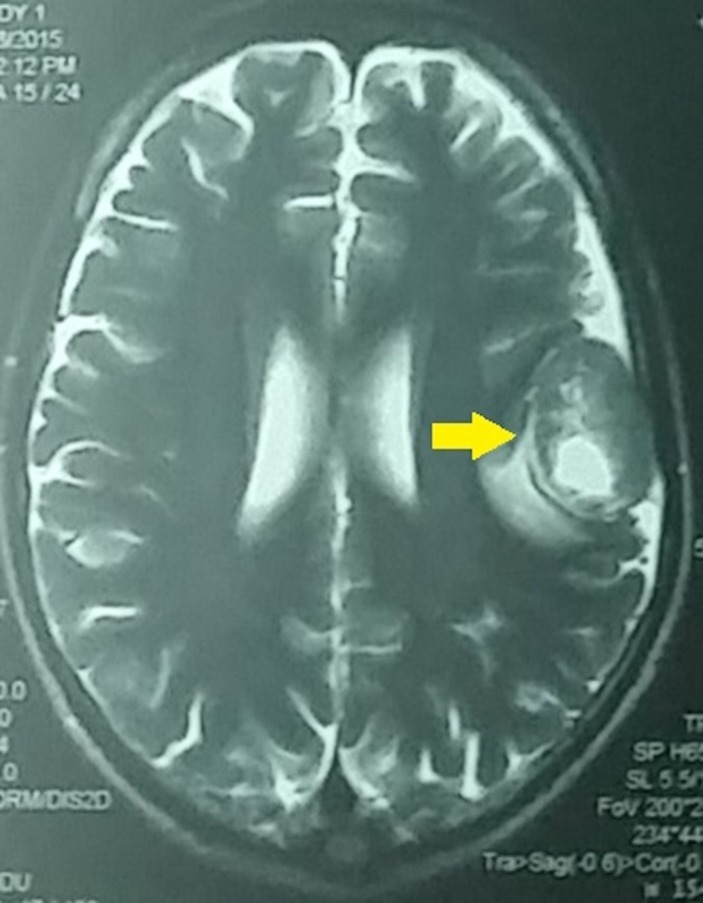
Cranial MRI with gadolinium showing a well-defined, extra-axial, heterogeneously enhancing solid mass in the left parietal area with central necrosis measuring 3.4×2.6×3.7 cm (as pointed by an arrow).
Figure 3.
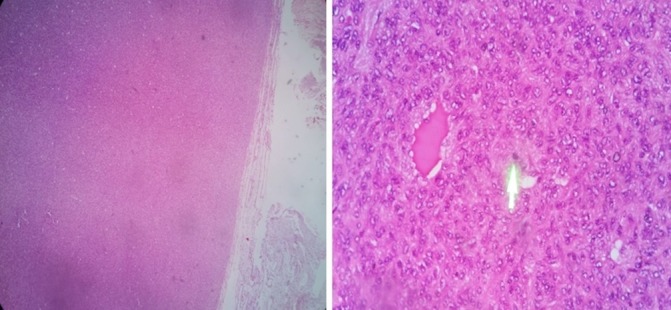
H&E stain of the left parietal mass showing a well-defined, eosinophilic staining tissue with interspersed blood vessels. Green arrow pointing to an area of a mitotically active nucleus.
Figure 4.
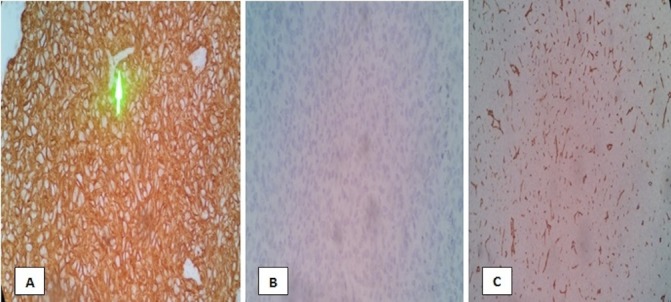
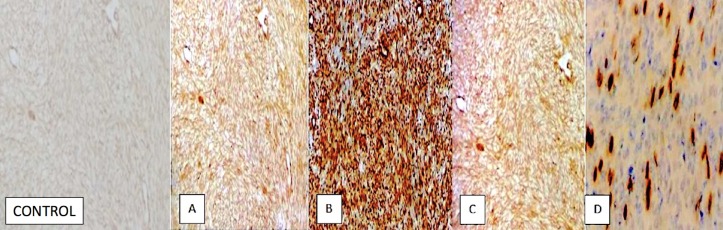
Immunohistochemistry staining of the left parietal mass revealed the following: (A) smooth muscle antigen—positive tumour cells (B) epithelial membrane antigen—negative tumour cells and (C) CD34 stain—negative tumour cells. Green arrow pointing to an area of tumor’s vascular supply.
Case 2
On admission, neurological and physical examination were unremarkable except for an enlarged bilateral neck mass. Cranial CT scan revealed a 2.0×1.7×1.6 cm hyperdense focus on the left occipital lobe and a 1.2×1.1×0.9 cm in the right uncal region (figure 5). Biopsy of the neck masses and the occipital mass was pursued. Histopathology of the neck mass tissue showed Langhans giant cells. Molecular testing with GeneXpert revealed rifampicin-resistant Mycobacterium tuberculosis (MTB). However, mycobacterial culture failed to isolate MTB. The patient was started on TB treatment (rifampicin 150 mg/isoniazid 75 mg/pyrazinamide 400 mg/ethambutol 275 mg fixed-dose combination tablet) plus levofloxacin 750 mg tablet. Excision biopsy of the left occipital mass showed interlacing fascicles of spindle-like cells with moderate pleiomorphism. Abnormal mitosis was noted with more than 10 mitotic figures per 10 hpf (figure 6). Immunohistochemistry results were consistent with leiomyosarcoma (positive for SMA, caldesmon and vimentin, and negative for s100, EMA, CD56 and CD31) (figure 7).
Figure 5.
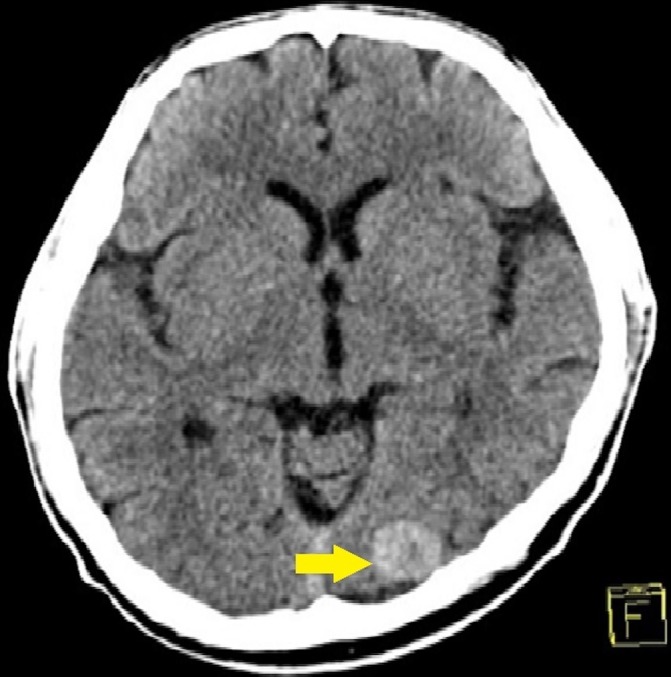
A contrast-enhanced cranial CT scan showing a 2.0×1.7×1.6 cm hyperdense focus on the left occipital lobe (as pointed by an arrow).
Figure 6.
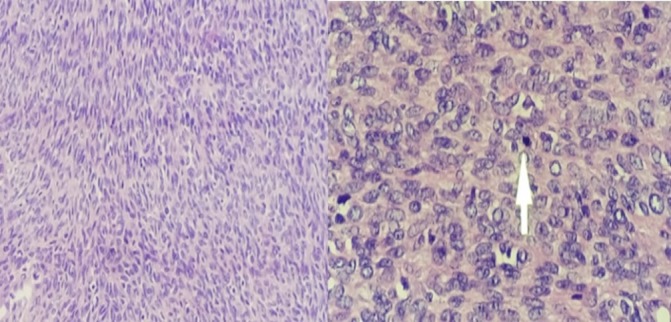
H&E stain demonstrating interlacing fascicles of spindle cells with mild to moderate pleiomorphism. Arrow pointing to a mitotically active cell nucleus.
Figure 7.
Immunohistochemical stains showing: (A) vimentin, (B) caldesmon, (C) smooth muscle antigen—positive in majority of the tumour cells, (D) desmin—focal, strong positive.
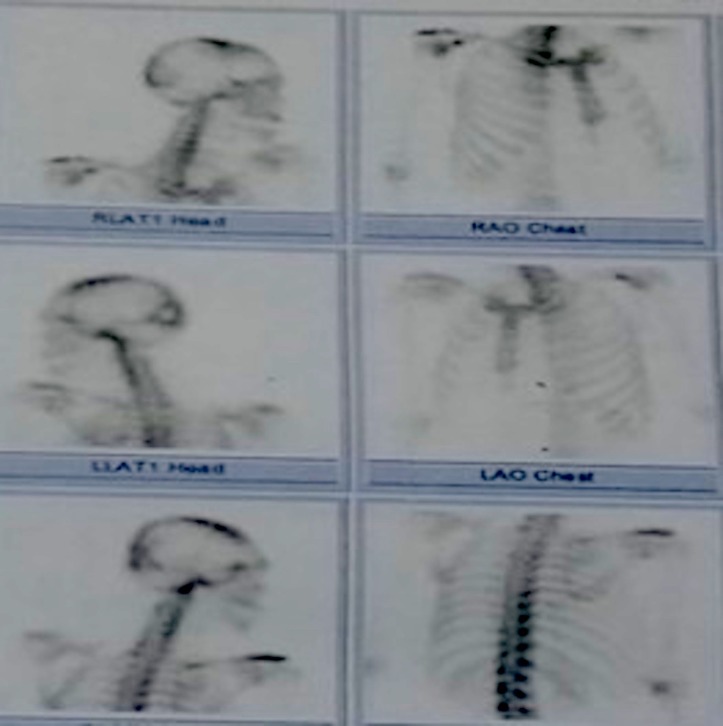
Two months after discharge, the patient experienced bilateral knee and ankle pain with associated lower extremity cramping. Neuropathic pain was considered. Electromyography and nerve conduction studies of all extremities were unremarkable but small muscle fibre disease cannot be ruled out. Abdominal and chest CT scan showed lytic foci in the 9th to 10th thoracic and 1st to 3rd lumbar vertebral bodies. Bone scintigraphy scan revealed a mildly increased osteoblastic activity in the right lambdoid suture and occipital bone. There are no metastatic foci noted in the thoracic and lumbar vertebral bodies (figure 8). Other workups included a negative serum toxoplasma IgG (0 UI/mL) and an elevated EBV IgG (5.37) and IgM (0.49). EBV-encoded RNA in situ hybridisation of the excised occipital mass was not done. The high cost of the test prevented us in documenting tissue EBV infection.
Figure 8.
Bone scintigraphy revealed a mildly increased osteoblastic activity in the right lambdoid suture and occipital bone.
Differential diagnosis
In areas with high burden of infectious diseases, opportunistic infections should always be considered as aetiologies of focal brain mass lesions. Bacterial brain abscess, TB and toxoplasmosis are common in patients with AIDS.11 Immune reconstitution inflammatory syndrome (IRIS), a paradoxical worsening or unmasking of an inadequately treated opportunistic infection, can also present as brain mass lesions. One of the most common non-infectious aetiologies include primary central nervous system lymphoma (PCNSL), a known AIDS-defining condition.11 Other CNS masses include round cell neoplasms such as gliomas that may have spindle-like cell morphology similar to leiomyosarcoma, therefore, immunohistochemical stains are needed for differentiation.
Treatment
Case 1
The patient underwent adjuvant cranial radiotherapy with daily dose of 200 cGy for 30 days (total dose of 6000 cGy). Temozolomide 120 mg (75 mg/m2 body surface area, BSA) was given 1 hour before radiotherapy. Temozolomide was maintained for the next 3 months together with nimotuzumab 200 mg intravenous every 14 days for six cycles. Nimotuzumab was shifted to cyclophosphamide intravenous (1 mg/m2 BSA) after new brain lesions developed.
Case 2
Adjuvant whole brain radiotherapy using partial boost (100 cGy/dose for 12 days; total dose of 2400 cGy) was started. On discovery of the metastatic thoracic foci, radiotherapy of the thoracic spine (T6–T11) was instituted (daily dose of 300 cGy for 10 days; total dose of 3000 cGy). The radiotherapy of the spine was discontinued after ruling out vertebral foci on bone scintigraphy. Medications for pain included pregabalin 75 mg tablet, carbamazepine 200 mg tablet and morphine 15 mg tablet.
Outcome and follow-up
Case 1
Three months after initial presentation, the patient had worsening headache, blurring of vision, complete ophthalmoplegia and ptosis of the left eye. Cranial MRI revealed new lesions at the left occipital and middle aspect of the right middle cranial fossa. Another lesion was seen in the middle aspect of the left middle cranial fossa measuring 0.7×0.5×0.6 cm (figure 9), which compresses the cavernous segment of the abducens, oculomotor and trochlear nerve.
Figure 9.
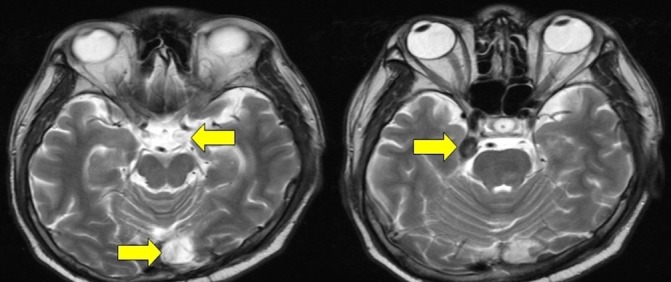
Cranial MRI showing increase in the size of the masses in the left occipital and middle aspect of the right middle cranial fossa. A new lesion was seen in the middle aspect of the left middle cranial fossa in the left parasellar region measuring 0.7×0.5×0.6 cm (as pointed by an arrow).
The patient remained on ART and prophylactic medications for opportunistic infections. Repeat CD4 count was 34 cells/mm3. Stereotactic radiosurgery of the new brain lesions was performed with continuation of cyclophosphamide treatment. One month after stereotactic radiosurgery, the patient improved with resolution of ophthalmoplegia and ptosis.
Case 2
Five months after the initial headache presentation, the patient developed bilateral lower extremity weakness and preferential gaze to the right. Follow-up cranial MRI showed new heterogeneous enhancing mass located in the proximal body of the corpus callosum, measuring 2.3×4.4×3.8 cm in its widest dimensions. Smaller lesions were also noted in the right distal body of the corpus callosum and the dorsal left aspect of the pons. Chemotherapy with etoposide (100 mg/m2 BSA) and cisplatin (20 mg/m2 BSA) was started. Cyclophosphamide (1 mg/m2 BSA) was given 21 days after the initial chemotherapy.
Discussion
In developed countries, PCNSL and cerebral toxoplasmosis account for most focal brain lesions.11 In resource-limited settings, other infectious aetiologies remain common and should be considered. Depending on the level of immunosuppression, infectious agents may have varying presentations on imaging and can mimic a neoplasm.12–14 Single-photon emission CT or positron emission tomography scan is the recommended screening imaging of choice for intracranial masses,15 but the high cost of these tests limited the management of our patients. Empiric antibiotics for the first patient and anti-TB medications for the second patient were started while waiting for final histological diagnosis. TB IRIS of the CNS was also considered due to the concomitant TB adenitis in the second case.
Diagnosis of leiomyosarcoma requires histological confirmation. Leiomyosarcoma and its benign counterpart, leiomyoma are both positive for actin and desmin stains.5–10 16 However, the presence of cellular atypia, coagulative necrosis and high mitotic index (10 or more mitosis per 10 hpf) favour a diagnosis of leiomyosarcoma.17 Most cases of leiomyosarcomas are metastatic from other primary sites.18 Our patients were diagnosed with PIL when no other primary sites were identified on whole-body imaging. PIL represents 0.1% of all intracranial tumours and its exact pathogenesis is poorly understood.19 20 Some authors believe that immunosuppression is a prerequisite for its occurrence.19 20 However, PIL has also been reported among immunocompetent individuals.21–23
We identified 16 published cases of PIL among HIV-infected individuals and compared our patients with the existing literature (table 1). From the 18 cases, mean age at diagnosis was 34±11 years with no sex predilection. Most cases presented with single mass lesion (61%), while the rest were multifocal. The mean CD4 count on PIL diagnosis was 40±52 cells/mm3. The time from initial HIV diagnosis to brain lesion discovery was a mean of 3.5±4 years. It can be hypothesised that chronic HIV infection is necessary to induce neoplastic changes. Although the diagnosis of HIV in our first patient was only made on current hospitalisation, it is likely that he has been chronically infected with HIV given the low CD4 count.
Table 1.
Profile of all cases of primary intracranial leiomyosarcoma among HIV-infected population
| Author | Age/sex | Location | CD4 count (cells/mm3) |
Time from HIV diagnosis (years) | ART | EBV | Treatment | Outcome (months) |
| Litofsky et al32 | 50/M | Occipital | 27 | 6 | NR | EBER-1 (+) | CR | NED 8 mos |
| Bejjani et al39 | 38/M | Lateral sphenoid | NR | 0 | NR | EBV LMP (−) | CR | NED 12 mos |
| Brown et al25 | 34/F | Pontine cistern | 177 | 2 | − | EBER-1 (+) | PR+Rx | AWD 12 mos |
| Blumentha et al5 | 43/M | Cavernous sinus | 23 | 12 | NR | EBER (+) EBV LMP (−) |
Ch, Bx/excision |
AWD 24 mos |
| Ritter et al40 | 5/F | Cavernous sinus | NR | 4 | NR | EBNA 2 (+) | PR | NR |
| Citow et al41 | 31/F | Parasellar | NR | 0.5 | NR | EBV IHC (+) | PR | NR |
| Lerdlum et al27 | NR | NR | 20–160 | NR | NR | NR | Sx+Rx | NR |
| Zevallos-Giampietri et al26 | 29/M | Parasellar | NR | 3 | + | EBER (+) EBV LMP (−) |
PR+Rx | AWD 6 mos |
| Suankratay et al36 | 43/F | Multifocal | 26 | 4 | + | EBER-1 (+) | CR+Rx | DOC 4 mos |
| 49/F | Multifocal | 26 | 4 | + | EBER-1 (+) | PR+Rx | AWD 10 mos | |
| 34/F | Tentorium cerebelli | 7 | 5 | + | EBER-1 (+) | CR+Rx | NED 8 mos | |
| 31/F | Multifocal | 3 | 1 | − | EBER-1 (+) | PR+Rx | AWD 5 mos | |
| 35/M | Multifocal | 20 | 0 | + | EBER-1 (+) | CR+Rx | AWD 4 mos | |
| Gupta et al31 | 17/F | Paracentral | 22 | 14 | − | EBER (+) | Bx+Rx+Ch | AWD 15 mos |
| Sivendran et al8 | 43/M | Frontal | 14 | NR | + | EBER (+) | CR | NED 20 mos |
| Muengtawee pongsa10 | 33/M | Cavernous sinus | NR | 1.5 | NR | NR | PR | NR |
| Francisco et al (2017) | 29/M | Multifocal | 7 | 0 | + | NA | CR+Rx+Ch | AWD 23 mos |
| Francisco et al (2017) | 36/M | Multifocal | 144 | 1 | + | NA | CR+Rx+Ch | AWD 27 mos |
AWD, alive with disease; Bx, biopsy; Ch, chemotherapy; CR, complete resection; DOC, dead of other causes; DOD, dead of disease; EBER, Epstein-Barr Early RNA; EBNA, EBV nuclear antigen; EBV, Epstein-Barr virus; F, female; M, male; mos, months; NA, not applicable; NED, No evidence of disease; NR, not reported; PR, partial resection; Rx, radiotherapy; Sx, unspecified surgery.
The association of PIL with EBV has been documented in multiple studies.24–26 In our review, majority (93%) of patients had positive tissue EBV-encoded RNA using in situ hybridisation. We hypothesise that the ability of HIV and EBV to infect a wide array of cells and induce latency have synergistic action in tumourigenesis. On the other hand, some published cases did not report documentation of EBV infection in the tumour cells.10 27 Similarly, the cases that we present are also limited by the lack of documentation of EBV tissue infection. In situ hybridisation remains the method of choice in detecting EBV in tissue sections.28 29 The utility of serum EBV antibodies remains controversial. In resource-limited setting, EBV in situ hybridisation is not readily available in most centres and its high cost makes this test underused. Though the documentation of EBV–PIL association strengthens the role of EBV in tumourigenesis, however, it has limited clinical utility.
There are no standard guidelines for the management of PIL among HIV-infected patients.30 Of the 18 patients reported, including our two cases, 94% (n=17) underwent surgical intervention. Adjuvant radiotherapy was instituted in 61% (n=11). Systemic chemotherapy was started in 22% (n=4), two of whom had unresected tumour. Chemotherapy alone was initiated in one patient due to the central location of the lesion.5 In our case series, chemotherapy was started as adjunctive treatment following surgery and radiotherapy. Chemotherapy remains controversial due to poor blood–brain barrier permeability.31 32 Temozolomide, an alkylating agent, was used in the first patient due to good brain penetration and acceptable safety profile.33 It has modest activity in unresectable soft-tissue sarcomas with 8% tumour response rate making it a plausible agent against PIL.9 33 One patient in our series was given nimotuzumab, an epidermal growth factor receptor (EGFR) monoclonal antibody. Soft-tissue sarcomas like leiomyosarcoma express EGFR,34 wherein its blockade can decrease chemoresistance and inactivation of tumour survival pathways. Bevacizumab, a vascular endothelial growth factor receptor monoclonal antibody, has similarly been used in other reports.23 Robust evidence on monoclonal antibody effectiveness against PIL is lacking.23 35 Multimodality approach is applied making surgical resection and radiotherapy as mainstay of treatment.30 31 36 Almost half of the PIL patients were on ART (44%). Immune reconstitution through ART initiation may theoretically improve patient outcomes, but there is limited evidence on the utility of ART in PIL progression, recurrence or cure.37 38
The prognosis of PIL is poor with the longest reported survival at 32 months after diagnosis.29 Review of published cases with a reported mean duration of follow-up of 12 months showed that most patients were alive with the disease (n=9). No radiological evidence of the disease (NED) was reported in four patients with median duration of 10 months. NED cases had complete resection of unifocal tumour. On the other hand, long-term prognosis remains unknown.
Patient’s perspective.
I am very happy that despite the rarity of my condition, my doctors were able to diagnose me promptly. In this time of new diagnostic tests and medications, I remain hopeful that my health will improve continuously. I am now living a symptom free life and I am now enjoying life to its fullest. I hope that many doctors will learn from my case and be able to help other patients with the same condition.
Learning points.
The diagnostic work-up of an HIV-infected patient presenting with headache should include a comprehensive history taking, physical examination and appropriate laboratory and imaging tests.
Primary intracranial leiomyosarcoma (PIL) is an extremely rare non-infectious mass lesion that has emerged among chronically infected HIV patients with very low CD4 count.
The spectrum of differential diagnoses for chronically infected HIV patients presenting with headache and focal mass lesions should be expanded to infectious and non-infectious aetiologies.
The diagnosis of PIL is made after excluding other possible primary sites of malignancy through whole-body imaging.
There are no standard guidelines in the management of PIL, but a multimodal approach using surgery, chemotherapy and radiotherapy seem to be the most effective approach.
Acknowledgments
We would like to thank our colleagues from the department of neurosciences, UP-Philippine General Hospital, Lennie Lyn De Castillo and Julette Marie Batara for helping in the acquisition of patient data. We would also like to thank Louie Mar Gangcuangco for his help in editing the final manuscript.
Footnotes
Contributors: CNF took part in patient data acquisition, involved in the planning and design of the report, writing of the manuscript, review of related literature, analysis of data and approval of the final manuscript. MA was involved in patient data acquisition, planning in the concept of the report, analysis of data, review of related literature, editing the manuscript and approval of the final manuscript. EMS was involved in patient data acquisition, analysis of data and approval of final manuscript. VMdVA was involved in patient data acquisition, planning and design of the report and approval of the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bhigjee AI, Naidoo K, Patel VB, et al. Intracranial mass lesions in HIV-positive patients--the KwaZulu/natal experience. Neuroscience AIDS research group. S Afr Med J 1999;89:1284–8. [PubMed] [Google Scholar]
- 2.Ciricillo SF, Rosenblum ML. Use of CT and MR imaging to distinguish intracranial lesions and to define the need for biopsy in AIDS patients. J Neurosurg 1990;73:720–4. 10.3171/jns.1990.73.5.0720 [DOI] [PubMed] [Google Scholar]
- 3.Tan IL, Smith BR, von Geldern G, et al. HIV-associated opportunistic infections of the CNS. Lancet Neurol 2012;11:605–17. 10.1016/S1474-4422(12)70098-4 [DOI] [PubMed] [Google Scholar]
- 4.McGuire D, 2013. Neurologic complications of HIV. HIV In site http://hivinsite.ucsf.edu/InSite?page=kb-04-01-02.
- 5.Blumenthal DT, Raizer JJ, Rosenblum MK, et al. Primary intracranial neoplasms in patients with HIV. Neurology 1999;52:1648–51. 10.1212/WNL.52.8.1648 [DOI] [PubMed] [Google Scholar]
- 6.McClain KL, Leach CT, Jenson HB, et al. Association of Epstein-Barr virus with leiomyosarcomas in young people with AIDS. N Engl J Med 1995;332:12–18. 10.1056/NEJM199501053320103 [DOI] [PubMed] [Google Scholar]
- 7.Chaves NJ, Kotsimbos TC, Warren MA, et al. Cranial leiomyosarcoma in an Epstein-Barr Virus (EBV)-mismatched lung transplant recipient. J Heart Lung Transplant 2007;26:753–5. 10.1016/j.healun.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 8.Sivendran S, Vidal CI, Barginear MF, et al. Primary intracranial leiomyosarcoma in an HIV-infected patient. Int J Clin Oncol 2011;16:63–6. 10.1007/s10147-010-0110-5 [DOI] [PubMed] [Google Scholar]
- 9.Purgina B, Rao UN, Miettinen M, et al. AIDS-related EBV-associated smooth muscle tumors: a review of 64 published cases. Patholog Res Int 2011;2011:1–10. Article ID 561548 10.4061/2011/561548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muengtaweepongsa S. Intracranial leiomyosarcoma in an HIV-infected adult. J Neurol Disord 2015;03:1 10.4172/2329-6895.1000i107 [DOI] [Google Scholar]
- 11.Smego RA, Orlovic D, Wadula J, et al. An algorithmic approach to intracranial mass lesions in HIV/AIDS. Int J STD AIDS 2006;17:271–6. 10.1258/095646206776253390 [DOI] [PubMed] [Google Scholar]
- 12.Doraiswamy V, Vaswani RK, Lahiri KR, et al. Neurotoxoplasmosis mimicking intracranial tuberculoma. J Postgrad Med 2010;56:31–4. 10.4103/0022-3859.62432 [DOI] [PubMed] [Google Scholar]
- 13.Nelson CA, Zunt JR. Tuberculosis of the central nervous system in immunocompromised patients: HIV infection and solid organ transplant recipients. Clin Infect Dis 2011;53:915–26. 10.1093/cid/cir508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamid Changal K. Central nervous system manifestations of tuberculosis: a review article. Mycobacterial Diseases 2014;04:146 10.4172/2161-1068.1000146 [DOI] [Google Scholar]
- 15.Foster M, Sherman P, Tharin B, et al. An algorithmic approach to neuroimaging in aids. american academy of neurology. Neurology 1998;50:21–6.9443452 [Google Scholar]
- 16.Omiyale AO. Primary leiomyoma of the liver: a review of a rare tumour. HPB Surg 2014;2014:1–8. Article ID 959202 10.1155/2014/959202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robboy SJ, Bentley RC, Butnor K, et al. Pathology and pathophysiology of uterine smooth-muscle tumors. Environ Health Perspect 2000;108(Suppl 5):779–84. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Dong L, Huang Y, et al. Primary intracranial leiomyosarcoma: review of the literature and presentation of a case. Onkologie 2012;35:609–16. 10.1159/000342676 [DOI] [PubMed] [Google Scholar]
- 19.Niwa J, Hashi K, Minase T, et al. Radiation induced intracranial leiomyosarcoma: its histopathological features. Acta Neurochir 1996;138:1470–1. 10.1007/BF01411129 [DOI] [PubMed] [Google Scholar]
- 20.Eckhardt BP, Brandner S, Zollikofer CL, et al. Primary cerebral leiomyosarcoma in a child. Pediatr Radiol 2004;34:495–8. 10.1007/s00247-003-1123-2 [DOI] [PubMed] [Google Scholar]
- 21.Aeddula NR, Pathireddy S, Samaha T, et al. Primary intracranial leiomyosarcoma in an immunocompetent adult. J Clin Oncol 2011;29:e407–e410. 10.1200/JCO.2010.33.4805 [DOI] [PubMed] [Google Scholar]
- 22.Gautam S, Meena RK. Primary intracranial leiomyosarcoma presenting with massive peritumoral edema and mass effect: case report and literature review. Surg Neurol Int 2017;8:278 10.4103/sni.sni_219_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallagher SJ, Rosenberg SA, Francis D, et al. Primary intracranial leiomyosarcoma in an immunocompetent patient: case report and review of the literature. Clin Neurol Neurosurg 2018;165:76–80. 10.1016/j.clineuro.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 24.Rogatsch H, Bonatti H, Menet A, et al. Epstein-barr virus-associated multicentric leiomyosarcoma in an adult patient after heart transplantation: case report and review of the literature. Am J Surg Pathol 2000;24:614–21. [DOI] [PubMed] [Google Scholar]
- 25.Brown HG, Burger PC, Olivi A, et al. Intracranial leiomyosarcoma in a patient with AIDS. Neuroradiology 1999;41:35–9. 10.1007/s002340050701 [DOI] [PubMed] [Google Scholar]
- 26.Zevallos-Giampietri EA, Yañes HH, Orrego Puelles J, et al. Primary meningeal epstein-barr virus-related leiomyosarcoma in a man infected with human immunodeficiency virus: Review of literature, emphasizing the differential diagnosis and pathogenesis. Appl Immunohistochem Mol Morphol 2004;12:387–91. [DOI] [PubMed] [Google Scholar]
- 27.Lerdlum S, Lalitanantpong S, Numkarunarunrote N, et al. MR imaging of CNS leiomyosarcoma in AIDS patients. J Med Assoc Thai 2004;87 Suppl 2:152–60. [PubMed] [Google Scholar]
- 28.Delecluse HJ, Feederle R, O’Sullivan B, et al. Epstein Barr virus-associated tumours: an update for the attention of the working pathologist. J Clin Pathol 2007;60:1358–64. 10.1136/jcp.2006.044586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loughrey M, Trivett M, Lade S, et al. Diagnostic application of Epstein-Barr virus-encoded RNA in situ hybridisation. Pathology 2004;36:301–8. 10.1080/0031302042000224584 [DOI] [PubMed] [Google Scholar]
- 30.Hussain S, Nanda A, Fowler M, et al. Primary intracranial leiomyosarcoma: report of a case and review of the literature. Sarcoma 2006;2006:1–3. Article ID 52140 10.1155/SRCM/2006/52140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta S, Havens PL, Southern JF, et al. Epstein-Barr virus-associated intracranial leiomyosarcoma in an HIV-positive adolescent. J Pediatr Hematol Oncol 2010;32:e144–e147. 10.1097/MPH.0b013e3181c80bf3 [DOI] [PubMed] [Google Scholar]
- 32.Litofsky NS, Pihan G, Corvi F, et al. Intracranial leiomyosarcoma: a neuro-oncological consequence of acquired immunodeficiency syndrome. J Neurooncol 1998;40:179–83. 10.1023/A:1006167629968 [DOI] [PubMed] [Google Scholar]
- 33.Ridolfi C, Pasini G, Drudi F, et al. Long lasting clinical response to chemotherapy for advanced uterine leiomyosarcoma: a case report. J Med Case Rep 2013;7:29 10.1186/1752-1947-7-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kersting C, Packeisen J, Leidinger B, et al. Pitfalls in immunohistochemical assessment of EGFR expression in soft tissue sarcomas. J Clin Pathol 2006;59:585–90. 10.1136/jcp.2005.028373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sette G, Salvati V, Memeo L, et al. EGFR inhibition abrogates leiomyosarcoma cell chemoresistance through inactivation of survival pathways and impairment of CSC potential. PLoS One 2012;7:e46891–14. 10.1371/journal.pone.0046891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suankratay C, Shuangshoti S, Mutirangura A, et al. Epstein-Barr virus infection-associated smooth-muscle tumors in patients with AIDS. Clin Infect Dis 2005;40:1521–8. 10.1086/429830 [DOI] [PubMed] [Google Scholar]
- 37.Barbaro G, Barbarini G. HIV infection and cancer in the era of highly active antiretroviral therapy (Review). Oncol Rep 2007;17:1121–6. 10.3892/or.17.5.1121 [DOI] [PubMed] [Google Scholar]
- 38.Leng LK, Pancharoen C, Bunupuradah T, et al. Regression of a cervical spinal mass following highly active antiretroviral therapy (haart) in child with advanced human immunodeficiency virus (hiv) disease. J Med Assoc Thai 2007;90:1937–4. [PubMed] [Google Scholar]
- 39.Bejjani GK, Stopak B, Schwartz A, et al. Primary dural leiomyosarcoma in a patient infected with human immunodeficiency virus: case report. Neurosurgery 1999;44:199–202. 10.1097/00006123-199901000-00119 [DOI] [PubMed] [Google Scholar]
- 40.Ritter AM, Amaker BH, Graham RS, et al. Central nervous system leiomyosarcoma in patients with acquired immunodeficiency syndrome. Report of two cases. J Neurosurg 2000;92:688–92. 10.3171/jns.2000.92.4.0688 [DOI] [PubMed] [Google Scholar]
- 41.Citow JS, Kranzler L. Multicentric intracranial smooth-muscle tumor in a woman with human immunodeficiency virus. Case report. J Neurosurg 2000;93:701–3. 10.3171/jns.2000.93.4.0701 [DOI] [PubMed] [Google Scholar]