Abstract
Recently, attention has been focused on methotrexate-induced lymphoproliferative disease (MTX-LPD), and atypical phenotypes are occasionally documented. We encountered two patients with rheumatoid arthritis (RA) who were diagnosed with non-specific LPD (LPD-nos). Biopsy samples were not obtained during the initial examination when the LPD development was discovered, and the patients achieved a complete response after MTX cessation (case 1) or steroid pulse therapy (case 2). However, the tumors flared up 1.5 years later, and LPD-nos was determined following biopsies of the lymph node (LN, case 1) and liver (case 2). Prednisolone was subsequently administered instead of chemotherapy; however, multiple masses, including in the spine (case 1), and severe icterus with liver dysfunction (case 2) were exacerbated within a few months. Although the re-biopsy of LN proved the presence of HL and radiation followed by aggressive chemotherapy rescued the patient (case 1), the superficially accessible biopsy site was not found, and autopsy finally revealed HL (case 2). In both cases, the underlying pathogenesis along with the B symptoms and laboratory abnormalities suggested MTX-LPD, HL in particular. Therefore, even if the pathological diagnosis does not confirm the specific LPD subtype, the administration of aggressive chemotherapy should be considered if the LPD activity flares severely.
Keywords: Hodgkin lymphoma, methotrexate, lymphoproliferative disorder, rheumatoid arthritis, non-specific
INTRODUCTION
The category of “other iatrogenic immunodeficiency-associated lymphoproliferative diseases” (OIIA-LPD) is defined by the 4th edition of the World Health Organization (WHO) classification.1 Methotrexate (MTX) is one of the most potent drugs used for treating rheumatoid arthritis (RA) and is currently regarded as the first-line medication.2-4 OIIA-LPD mainly develops in patients with RA who receive MTX, and is described as MTX-LPD in RA.5-11 The specific features of MTX-LPD include the occurrence of several LPD phenotypes including Hodgkin lymphoma (HL). In our experience with over 90 cases of MTX-LPD, non-specific LDP (LDP-nos) cases, which do not fall into the specific LPD subtypes, are occasionally seen. However, the details of these pathological findings are not well described. In this case-report, we presented the clinical course of two patients who were diagnosed as having LPD-nos.
CASE REPORT
Case 1:
A 60-year-old female patient with a 15-year history of RA had been administered several medications such as nonsteroidal anti-inflammatory drugs (NSAIDs) and disease-modifying antirheumatic drugs (DMARDs). MTX was one of the most effective drugs this patient used, and it was therefore administered for 11 years in combination with other medications such as FK506. Although these medications resulted in a good control of the RA activity, subfever with cystitis symptoms appeared. An antibiotic drug was administered, but the symptoms did not improve. Computer tomo-graphy (CT) demonstrated multiple lymphadenopathies along with elevated serum C-reactive protein (CRP, 4.4 mg/dL) and soluble interleukin-2 receptor (sIL2R, 3320 U/mL), suggesting the development of MTX-LPD. As superficial lymphadenopathy was not found, MTX withdrawal was initially attempted, which caused the regression of the lymphadenopathy within 2 months. The RA activity was well controlled with DMARDs and prednisolone (PSL) for 1.5 years after this episode; however, sustained and fluctuating elevation of the serum CRP (2-8 mg/dL) and sIL-2R (5000-7500 U/mL) was observed. The subsequent CT demonstrated the development of cervical and abdominal lymphadenopathies with splenomegaly (Figure 1A and B) and, therefore, a biopsy of the cervical lymph node (LN) was performed. The features of the specific LPD type, including lymphomas, polymorphic LPD, lymphoplasmacytic LPD, and reactive pattern, were not evident in this sample, and the diagnosis of non-specific LPD was made (Figure 2A). She had B symptoms with simultaneous fever and night sweats. Then, dose-escalated PSL (10-30 mg/day) was administered, but the laboratory data did not improve sufficiently. In addition, she experienced severe, progressive back pain, and multiple lymphadenopathies with a mass involving the L1 and L3 spinal regions was detected using CT/magnetic resonance (MR) (Figure 1C and D). To confirm the pathogenesis, a re-biopsy of the cervical LN was performed. The pathological analysis indicated a CD15+CD30+CD20-PAX-5+OCT-2+BOB-1+ Epstein-Barr virus-encoded RNA (EBER) - lymphoma cell invasion and decreased lymphocytes, leading to the diagnosis of HL-lymphocyte depleted (HL-LD, Figure 2B). As she suddenly developed paralysis of both legs, irradiation was initially preceded with dexamethasone therapy, followed by 6 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) therapy. She achieved a complete response with these therapies and has been in good health without any complications for 6 years.
Figure 1.
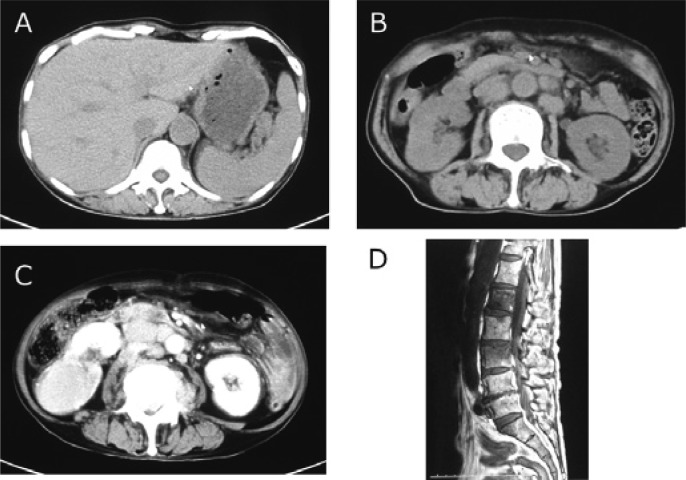
Radiological findings of case 1
Computer tomography (CT) at the time of first (A and B) and second (C and D) of biopsies in case 1. At the time of the first biopsy, CT demonstrated multiple lymphadenopathies, mild splenomegaly, and hydronephrosis of the right kidney (A and B). At the time of the second biopsy, CT similarly revealed multiple lymphadenopathies and splenomegaly (C), and magnetic resonance (MR) imaging indicated a mass involving L1 and L3 spinal regions along with compressed fracture (D).
Figure 2.
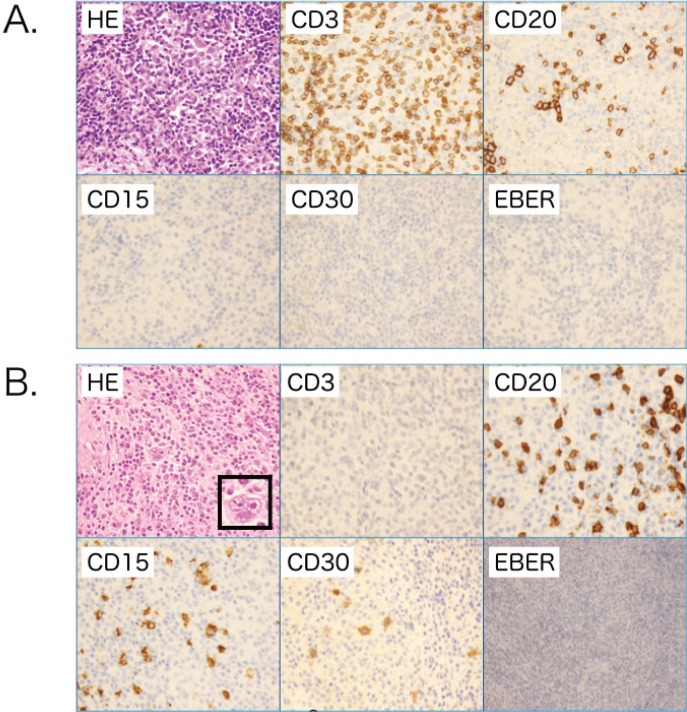
Morphology of case 1
The pathological findings demonstrated the need to biopsy the lymph node (LN) (A) first biopsy and (B) re-biopsy. (A) Hematoxylin and eosin (HE) staining of the LN sample from the first biopsy demonstrated fibrotic change and polymorphic appearance including small lymphocytes, histiocytes, plasma cells, and epithelioid cells. Although the immunostaining sample exhibited some large cells indicating CD15- CD20- CD30+ Epstein-Barr virus-encoded RNA (EBER)-, the features of this sample did not confirm any specific lymphoma category such as Hodgkin lymphoma (HL). (B) In contrast, the re-biopsied sample exhibited typical features of HL including the presence of CD15+CD30+CD20- Reed-Stenberg (RS) cells and decreased lymphocytes, which enabled the diagnosis of classical HL-lymphocyte depleted (LP) to be made; original magnification, ×400. RS cells indicated in the HE frame, x800.
Case 2:
A 56-year-old male patient was diagnosed with RA 30 years ago. He was administered MTX for 20 years in combination with other medications such as NSAIDs, DMARDs, and PSL. Although these combined medications resulted in a good control of the RA activity, he gradually developed B symptoms. CT demonstrated massive splenomegaly and multiple masses in the lung with an elevated serum LDH, CRP, and sIL-2R, suggesting LPD development. His poor general health condition did not permit a biopsy of the lung, spleen, or both to make a diagnosis. In addition, his consciousness was impaired, and he was drowsy and epileptic. Therefore, the patient was initially administered methyl PSL steroid pulse therapy (1 g/day) for 3 days. Lumbar puncture was negative, and EBV was not detected in the serum or cervical nerve system liquid. With intensive care, including respiratory support, the patient recovered in a month without any severe disability. For personal reasons, watchful treatment was instituted, and no additional events, such as fever or data exacerbation, were observed. Based on the pathogenesis, other causes, such as systemic lupus erythematosus, were considered; however, rapid recovery from aggressive activity with the administration of steroid pulse therapy suggested the presence of MTX-LPD along with the laboratory data including anti-nuclear antibody and ds-DNA antibody (x40 and less than 10 U/mL, respectively).
Arthralgia gradually flared up due to RA activity 1.5 years after the steroid pulse therapy. Etanercept plus PSL was administered because this combination was previously effective, resulting in a relief of the RA activity including arthralgia. However, the B symptoms appeared 4 months after the administration of the anti RA drugs. CT demonstrated gall bladder stones along with elevated white blood cell (WBC) count, as well as serum alkaline phosphatase (ALP) and CRP, and cholecystitis was therefore tentatively suspected. The administration of antibiotics appeared to be effective, but sustained liver dysfunction and exacerbated icterus were observed. The subsequent CT demonstrated developing interstitial pneumonitis, multiple lymphadenopathies, and splenomegaly (Figures 3A and B). An underlying MTX-LPD pathogenesis other than the disease, such as an infection, was suggested, and a liver biopsy was then performed. However, it failed to detect any specific lymphomas (Figure 4A), and a diagnosis of non-specific LPD was made. A real-time polymerase chain reaction (PCR) analysis demonstrated high levels of serum EBV (2.5 × 104 copies/µL). Although the definite pathogenesis was not revealed, PSL at 1 mg/kg/day was administered to relieve his condition. As a result, the liver dysfunction and B symptoms were improved, but the icterus was persistent and progressive. Massive plural effusion and ascites were observed in the following CT (Figures 3C and D). Cyclophosphamide (500 mg/day, intravenous for 1 day) was additionally administered, but the patient died 1 week after cyclophosphamide was administered. An autopsy was permitted, which revealed massive infiltration of CD15+CD30+CD20-PAX-5+OCT-2-BOB- 1-EBER+Reed–Sternberg (RS) cells in multiple organs such as the liver, lungs, and bone marrow. The patient was finally diagnosed as having HL-LD (Figure 4B).
Figure 3.
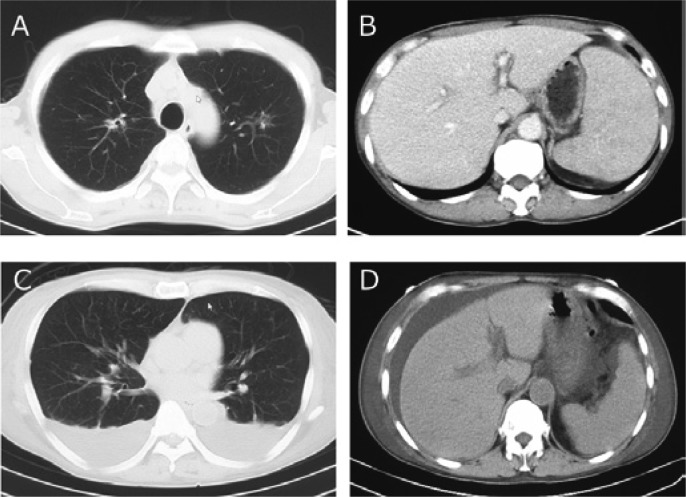
Radiological findings of case 2
Computer tomography (CT) at the time of liver biopsy (A and B) and the subsequent CT just before death (C and D) of case 1. At the time of liver biopsy, CT demonstrated interstitial pneumonitis, lymphadenopathies, splenomegaly, and gallbladder stones (A and B). Massive pleural effusion and ascites were observed in the subsequent CT (C and D).
Figure 4.
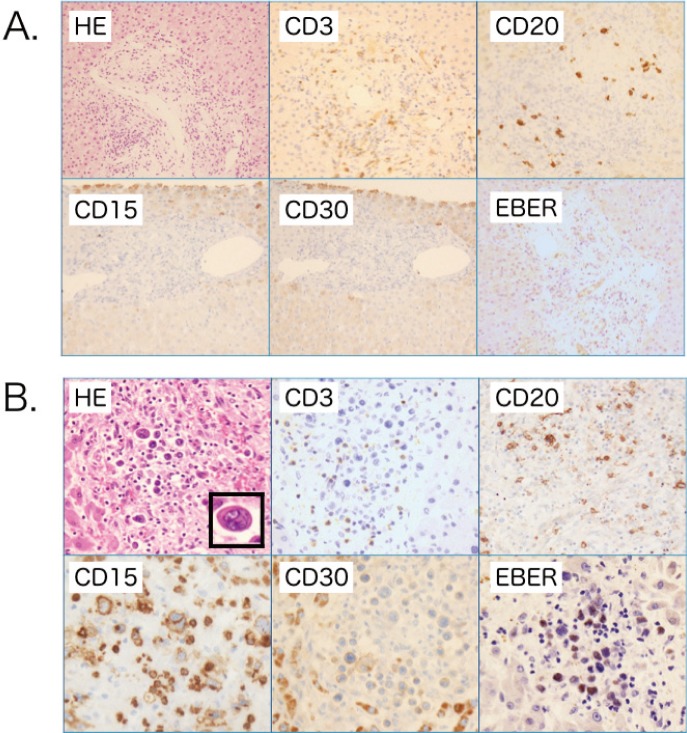
Morphology of case 2
The pathological findings from the biopsy of (A) the liver and (B) the liver sample from the autopsy. The liver biopsy exhibited slight fibrosis, small lymphocyte infiltration around the portal vein, and cholestasis. (A) Although some epithelioid cell granuloma was evident, other specific features suggesting lymphoproliferative disease were not detected with immunostaining including CD15, 30, 20, and EBER. (B) In contrast, the liver biopsy sample exhibited massive RS cell infiltration with EBER-positivity, and RS cells were seen in multiple organs such as the bone marrow, spleen, multiple lymph nodes, and lungs. As lymphocytes were decreased in these tissues, the diagnosis was suggested to be classical HL-LP; original magnification, X400. RS cells indicated in the HE frame, x800.
DISCUSSION
Here, we described the cases of two patients with RA with HL, who were initially diagnosed as having LPD-nos using biopsies along with the development of the suspected symptoms and laboratory data suggestive of LPD such as the elevation of CRP, LDH, and sIL-2R. In both cases, aggressive chemotherapy was not subsequently administered because we were unable to obtain the specific LPD subtype in the first biopsy attempt. Fortunately, the patient in case 1 was rescued using ABVD therapy based on the second biopsy, which finally led to the diagnosis of HL. However, the patient in case 2 died without the administration of any aggressive chemotherapy.
There are three discussion points to note when considering the treatment strategy. The first is the clinical implication of the diagnosis of LPD-nos. Our records reveal that approximately 10% of patients with MTX-LPD were diagnosed as having LPD-nos. The LPDs of these patients were not as aggressive as those of the presented cases, and no additional treatment other than MTX withdrawal was required. Although the category of LPD-nos in this disease entity has not been well analyzed, and it is unclear whether LPD-nos is one of the subtypes of MTX-LPD, the presented two cases suggested that specific LPD, including HL, may be an underlying factor in some patients with LPD-nos.
Second, is the possibility of sampling error in these cases. Although the first biopsy sample of the lymph node in patient 1 was enough to evaluate the pathological diagnosis along with the flow cytometry and karyotype analysis, the liver biopsy in patient 2 was a needle sample, thus sampling error may have occurred. Indeed, live autopsy samples indicated the presence of HL within 1 month after the live biopsy. As the patient’s condition was poor, a second liver biopsy or opened live biopsy would have been difficult. If a definite diagnosis of the biopsy is not obtained, a sampling error may be considered.
Third, is the choice of the upfront administration of chemotherapy even though we were unable to obtain the diagnosis of specific LPD. To rescue patients, especially those with conditions similar to that of case 2, the upfront administration of chemotherapy should be considered. We previously reported three specific clinical patterns in MTX-LPD manifestations: MTX-regressive-LPD (LPD regression occurred after the withdrawal of MTX), MTX-persistent-LPD (LPD persisted after MTX withdrawal), and other-mediated-LPD (LPD developed only after MTX was discontinued).5 The features of other-mediated-LPD are: 1) a history of MTX administration, 2) elevated LDH, CRP, and sIL-2R in serum, 3) disappearance of suggestive LPD after MTX withdrawal, 4) common development of the HL phenotype during the administration of anti-RA drugs other than MTX, and 5) relapse within a few years after MTX regression.5,15 Based on these facts, case 1 eventually fell within the category of the other-mediated-LPD group and mostly suggested HL phenotype. In contrast, case 2 was difficult to define and distinguish among the three subtypes because the steroid pulse therapy masked the LPD regression after the withdrawal of MTX. On the other hand, EBV was detected in the pathological sample and serum. It has been well documented that EBV has an influence and is suggested as one of the pathogenic factors of MTX-LPD.1,5,7,9,11-15 In addition, we previously demonstrated that the viral load of serum EBV is related with the disease activity of HL.5 Furthermore, the frequency of EBV positivity of HL is greater than 80%. Considering these facts collectively, the pathogenesis of case 2 may have suggested HL even without any definite pathological diagnosis of HL. To exclude the possibility of T-cell lymphoma in these cases, we performed rearrangement of TCR β and γ with the samples diagnosed as HL in both cases. The result of case 1 was negative, and case 2 indicated a poor technical study using autopsy tissues.
In summary, we demonstrated the aggressive clinical courses of two patients diagnosed with LPD-nos of MTX-LPD. Although patients with LPD-nos usually indicate a non-aggressive clinical course in our records, it should be emphasized that HL with a sudden flare up of high activity is observed in LPD-nos. Although it is not clear if LPD-nos developed into HL in both cases, the pathogenesis of HL was underlying at the diagnosis of LPD-nos based on the duration of a few months from the first biopsy to the second biopsy or autopsy. Upfront aggressive chemotherapy should be considered if LPD activity severely flares up and threatens the patient’s life even when the specific LPD has not been identified. However, it is necessary to investigate these clinical questions further in an additional population of patients with MTX-LPD.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Swerdlow SH, Harris NL, Jaffe ES, Pileri SA, Stein H, et al.: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th edn. IARC: Lyon, 2008 [Google Scholar]
- 2.Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, et al. : Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med 312: 818-822, 1985. 10.1056/NEJM198503283121303 [DOI] [PubMed] [Google Scholar]
- 3.Hazlewood GS, Barnabe C, Tomlinson G, Marshall D, Devoe D, Bombardier C: Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ 353: i1777, 2016. 10.1136/bmj.i1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulus HE: The use of combinations of disease-modifying antirheumatic agents in rheumatoid arthritis. Arthritis Rheum 33: 113-120, 1990. 10.1002/art.1780330116 [DOI] [PubMed] [Google Scholar]
- 5.Tokuhira M, Watanabe R, Nemoto T, Sagawa M, Tomikawa T, et al. : Clinicopathological analyses in patients with other iatrogenic immunodeficiency-associated lymphoproliferative diseases and rheumatoid arthritis. Leuk Lymphoma 53: 616-623, 2012. 10.3109/10428194.2011.625101 [DOI] [PubMed] [Google Scholar]
- 6.Hoshida Y, Xu JX, Fujita S, Nakamichi I, Ikeda J, et al. : Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol 34: 322-331, 2007 [PubMed] [Google Scholar]
- 7.Ichikawa A, Arakawa F, Kiyasu J, Sato K, Miyoshi H, et al. : Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: histology, Epstein-Barr virus, and clonality are important predictors of disease progression and regression. Eur J Haematol 91: 20-28, 2013. 10.1111/ejh.12116 [DOI] [PubMed] [Google Scholar]
- 8.Yamakawa N, Fujimoto M, Kawabata D, Terao C, Nishikori M, et al. : A clinical, pathological, and genetic characterization of methotrexate-associated lymphoproliferative disorders. J Rheumatol 41: 293-299, 2014. 10.3899/jrheum.130270 [DOI] [PubMed] [Google Scholar]
- 9.Kamel OW, van de Rijn M, Weiss LM, Del Zoppo GJ, Hench PK, et al. : Brief report: reversible lymphomas associated with Epstein-Barr virus occurring during methotrexate therapy for rheumatoid arthritis and dermatomyositis. N Engl J Med 328: 1317-1321, 1993. 10.1056/NEJM199305063281806 [DOI] [PubMed] [Google Scholar]
- 10.Svensson AM, Jacobson ER, Ospina D, Tindle BH: Reversible Epstein-Barr virus-negative lymphadenopathy and bone marrow involved by Hodgkin’s lymphoma in a rheumatoid arthritis patient undergoing long-term treatment with low-dose methotrexate: a case-report and review of the literature. Int J Hematol 83: 47-50, 2006. 10.1532/IJH97.NA0503 [DOI] [PubMed] [Google Scholar]
- 11.Miyazaki T, Fujimaki K, Shirasugi Y, Yoshiba F, Ohsaka M, et al. : Remission of lymphoma after withdrawal of methotrexate in rheumatoid arthritis: relationship with type of latent Epstein-Barr virus infection. Am J Hematol 82: 1106-1109, 2007. 10.1002/ajh.21003 [DOI] [PubMed] [Google Scholar]
- 12.Alspaugh MA, Henle G, Lennette ET, Henle W: Elevated levels of antibodies to Epstein-Barr virus antigens in sera and synovial fluids of patients with rheumatoid arthritis. J Clin Invest 67: 1134-1140, 1981. 10.1172/JCI110127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawada S, Takei M: Epstein-Barr virus etiology in rheumatoid synovitis. Autoimmun Rev 4: 106-110, 2003. 10.1016/j.autrev.2004.08.034 [DOI] [PubMed] [Google Scholar]
- 14.Balandraud N, Meynard JB, Auger I, Sovran H, Mugnier B, et al. : Epstein-Barr virus load in the peripheral blood of patients with rheumatoid arthritis: accurate quantification using real-time polymerase chain reaction. Arthritis Rheum 48: 1223-1228, 2003. 10.1002/art.10933 [DOI] [PubMed] [Google Scholar]
- 15.Kimura Y, Takahashi Y, Tomikawa T, Sawaga M, Anan T, et al. : Impact Of Epstein-Barr Viral Infection In The Regression Of Methotrexate-Induced Lymphoproliferative Diseases In Patients With Rheumatoid Arthritis. Blood 122: 2013 [Google Scholar]