Abstract
For optimizing CD34+ cell collection, appropriately timing peripheral blood stem cell harvest (PBSCH) initiation is crucial. Automatic cell analyzers with the immature myeloid information channel provide hematopoietic progenitor cell (HPC) count, a surrogate marker of CD34+ cells, which can be obtained within a few minutes without requiring monoclonal antibodies. The final decision on PBSCH initiation can be made using the HPC count obtained on the morning of the harvest day. Herein, we evaluated the impact of the HPC count as an indicator for the optimal timing of PBSCH in clinical practice over 9 years.
One hundred and eighteen aphereses from 72 cases had a definite number of CD34+ cells/kg in the PBSC yield. A correlation was found between the HPC count in the PB and the CD34+ cell count (R = 0.563, p < 0.001), whereas no correlation existed between the white blood cell and CD34+ cell counts (R = 0.0418, p = 0.65). We defined >2.0 × 106/kg of CD34+ cells in a single apheresis as good mobilization. Multivariate analysis demonstrated that an HPC count of > 21/μL, myeloblast count of > 12/μL, and age at PBSCH of < 50 years were independently associated with good mobilization (p = 0.001, p < 0.001, and p = 0.005, respectively).
Our findings suggest that the HPC count is a good indicator for the optimal timing of PBSCH.
Keywords: hematopoietic progenitor cell, peripheral blood stem cell harvest, healthy donor, malignant lymphoma, plasma cell neoplasm
INTRODUCTION
Peripheral blood stem cell harvest (PBSCH) is a crucial procedure for allogeneic or autologous stem cell transplantation (SCT) in patients with hematological diseases. For safe engraftment, an infusion of more than 2.0 × 106 CD34+ cells/kg of the recipient’s weight is needed.1 To collect an adequate number of CD34+ cells, it is important to appropriately time the initiation of PBSCH, which is optimized when CD34+ cells are effectively mobilized from the bone marrow to the peripheral blood (PB), several days after the administration of granulocyte colony-stimulating factor (G-CSF) with or without myelosuppressive chemotherapy. For many Japanese institutes, the timing of PBSCH initiation is decided only in reference to the schedule of myelosuppressive chemotherapy or administration of G-CSF. Therefore, PBSCH may be needlessly performed on a day of poor mobilization and not performed on a day of good mobilization because of differences in individual mobilization timing.
Flow cytometry analysis can directly evaluate the number of CD34+ cells in the PB; however, this procedure is complex, expensive, and time consuming to obtain the final results and monoclonal antibodies are needed for the staining process. Automatic cell analyzers with the immature myeloid information (IMI) channel provide the hematopoietic progenitor cell (HPC) count, which is a surrogate marker of CD34+ cells, and can be obtained within only a few minutes and do not require monoclonal antibodies.2-4 Several reports have demonstrated that the HPC count is correlated with CD34+ cell count and is a useful indicator of the optimal timing of PBSCH.5-10 The final decision on PBSCH initiation can be made using the HPC count obtained on the morning of the harvest day.
Herein, we evaluated the impact of the HPC count as a reliable indicator for the optimal timing of PBSCH in clinical practice over 9 years.
MATERIALS AND METHODS
1. Study design
This was a retrospective study on the HPC count as an indicator of the optimal timing of PBSCH at a single institute. This study was approved by the Asahi General Hospital ethics committee and the in-hospital ethical review board prior to the publication of these research results.
2. Patients
All consecutive patients with hematological neoplasms and healthy donors who underwent PBSCH at Asahi General Hospital from November 2006 to June 2015 were included in this study. Data collected included sex, age (on day of harvest), body weight, disease [no disease (healthy donor), malignant lymphoma, plasma cell neoplasm], date of PBSCH, apheresis modality, relevant laboratory data (on day of harvest), and the number of CD34+ cells in the PBSC yields.
3. PBSC mobilization and harvest
The conditioning regimen of PBSCH was generally salvage chemotherapies in patients with malignant lymphoma and high-dose cyclophosphamide therapy in patients with multiple myeloma. Healthy donors and some patients with a plasma cell neoplasm underwent PBSCH with only G-CSF administration. Although the schedule for G-CSF administration and the timing of PBSCH initiation were decided by the individual attending physicians, in many patients with hematological neoplasms, the 17th day of myelosuppressive chemotherapy was planned as the day of PBSCH and the administration of G-CSF was initiated on the 13th day. In many healthy donors, the administration of G-CSF was initiated from 4 days before the scheduled day of PBSCH. PBSCH was performed with COBE Spectra® (Terumo BCT, Tokyo, Japan) from November 2006 to March 2013, and with Spectra Optia® (Terumo BCT) from March 2013 until the study’s end. Blood access was generally via a central venous catheter in patients with hematological disease and cutaneous vein in the cubital region of healthy donors.
4. Measurement of HPC
Measurement of HPCs was performed using XE2100 (Sysmex Corporation, Hyogo, Japan) from 2006 to 2011, and XE5000 (Sysmex Corporation) from 2011 onward. HPCs were identified in the IMI-HPC area, within the IMI channel, of these cell analyzers on the basis of their resistance to the lysing reagent, volume (direct current), and internal structure (radio frequency).4 HPCs are resistant to lysing reagents and are located within a specific gated area of the scattergram.
5. Measurement of CD34+ cells
After lysis of red blood cells in ammonium chloride lysis solution and washing with 1% fetal calf serum, 100 µL of cell suspension was stained with CD45 + PerCP (PR701, Dako, Glostrup, Denmark) and CD34 + FITC (8G12, Becton Dickinson, CA, USA) for 30 min at 4°C in the dark, together with the appropriate immunoglobulin isotype-matched control. Then, the cells were washed four times, resuspended, and examined with FACSCanto II (Becton Dickinson). A total of 1.0 × 104 cells were analyzed by FACSDiva Software (Becton Dickinson).
6. Statistics
Pearson’s correlation test was used to assess the significance of the correlation between CD34+ cell count in the PBSC yields and several other parameters. Fisher’s exact probability test was applied to assess the differences between two groups in the univariate analysis of the categorical data. Logistic regression analysis was performed to investigate the influences of some factors in the multivariate analysis. A p-value of less than 0.05 was considered to be significant. All statistical analyses were performed using EZR (Saitama Medical Centre, Jichi Medical University, Japan; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html; Kanda, 2012), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria; version 2.13.0). More precisely, EZR is a modified version of R Commander (version 1.6–3) that was designed to add frequently used statistical functions in biostatistics.11
RESULTS
1. Patient and donor characteristics, and apheresis results
The patient and donor characteristics on the day of harvest are shown in Table 1. A total of 72 cases underwent PBSCH from November 2006 to June 2015 at Asahi General Hospital. Nine cases underwent a second course of PBSCH, of which eight were due to insufficient PBSC yield and one was due to the development of another malignant lymphoma 8 years after the first auto-PBSCT. The median age of the patients and donors at the first apheresis was 54 years (range, 21-68 years), and 41 (56.9%) patients were male. There were 22 (30.6%) healthy donors and 35 patients with malignant lymphoma, including 19 with diffuse large B-cell lymphoma, three with follicular lymphoma, three with peripheral T-cell lymphoma, two with Hodgkin’s lymphoma, two with T-cell lymphoblastic lymphoma, two with Burkitt lymphoma, and one each of either mantle cell lymphoma, extranodal NK/T-cell lymphoma, nasal type, angioimmunoblastic T-cell lymphoma, or anaplastic large cell lymphoma. Fifteen patients had a plasma cell neoplasm, including 14 with multiple myeloma and one with POEMS syndrome. The number of apheresis days required for a course of PBSCH were 1 day (30 patients), 2 days (45 patients), or 3 days (six patients). For PBSC mobilization apheresis, 7–10 µg/kg of lenograstim was administered to 21 healthy donors and 400 µg/m2 of filgrastim in 1 healthy donor. The dose of G-CSF was reduced in nine donors after the second day among 17 donors who had the defined daily dose of G-CSF after the second day. In these nine donors, the median count of white blood cells (WBC) at the time of reduction in G-CSF was 39.9×103/µL (range, 23.8-52.6×103/µL). PBSCH was performed on days 4 and 5 after G-CSF initiation for three donors, days 5 and 6 for 14 donors, only day 5 for two donors, days 4-6 for one donor, and days 5-7 for one donor. For patients with malignant lymphoma, the conditioning regimen for PBSCH was CHASE therapy (etoposide, cyclophosphamide, cytarabine, cyclophosphamide, and dexamethasone) with or without rituximab for 22 patients, ESHAP therapy (etoposide, cisplatin, and cytarabine) with or without rituximab for four patients, high-dose etoposide for five patients, high-dose methotrexate and cytarabine therapy with or without rituximab for four patients, hyper CVAD therapy (cyclophosphamide, adriamycin, vincristine, and dexamethasone) with or without rituximab for two patients, high-dose cyclophosphamide therapy for one patient, and no data for two patients. In patients with plasma cell neoplasms, the conditioning regimen of PBSCH was high-dose cyclophosphamide therapy for 14 patients, VAD therapy (adriamycin, vincristine, and dexamethasone) for one patient, no conditioning regimen (G-CSF alone) for one patient, and no data for one patient.
Table 1. Patient and donor characteristics and apheresis results.
| Patient and donor characteristics (n = 72) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age at PBSCH (median, range) | 54 (21–68) years | ||||||||
| Male/female | 41/31 | ||||||||
| Healthy donor | 22 | ||||||||
| Malignant lymphoma | 35 | ||||||||
| Plasma cell neoplasm | 15 | ||||||||
| Apheresis results (n = 118) | |||||||||
| From healthy donor | 36 | ||||||||
| patient with malignant lymphoma | 53 | ||||||||
| patient with plasma cell neoplasm | 29 | ||||||||
| Course of PBSCH First course | 102 | ||||||||
| Second course | 16 | ||||||||
| PBSCH days Day 1 | 70 | ||||||||
| Day 2 | 43 | ||||||||
| Day 3 | 5 | ||||||||
| Median number (range) of CD34+ cells/kg | |||||||||
| All case | Healthy donors | Patients with ML and PCN | |||||||
| CD34+ cells/kg collected via apheresis (× 106/kg) (n=118) | 1.29 (0.03–22.80) | 1.30 (0.13–11.3) | 1.21 (0.03–22.8) | ||||||
| Total CD34+ cells/kg in a course of PBSCH (× 106/kg) (n=72) | 2.53 (0.13–22.8) | 2.63 (0.75–12.4) | 2.50 (0.13–22.8) | ||||||
| Median counts (range) for the laboratory data in the PB on harvest day | |||||||||
| All case | Healthy donors | Patients with ML and PCN | |||||||
| WBC (× 103/µL) | 19.25 (5–64.0) | 43.4 (22.4–64.0) | 10.6 (5–47.8) | ||||||
| Myeloblast (/µL) | 0 (0–860) | 0 (0–191.5) | 0 (0–860) | ||||||
| Platelets (× 104/µL) | 9.8 (1.6–30.9) | 18.5 (9.3–30.9) | 6.7 (1.6–24.7) | ||||||
| HPC (/µL) | 25 (0–899) | 29 (0-162) | 23.5 (0–899) | ||||||
ML, malignant lymphoma; PCN, plasma cell neoplasm; PBSCH, peripheral blood stem cell harvest; WBC, white blood cell; HPC, hematopoietic progenitor cell.
A total of 118 aphereses had a definite number of CD34+ cells/kg in the PBSC yield. There were 36 aphereses from healthy donors, 53 from patients with malignant lymphoma, and 29 from patients with a plasma cell neoplasm. During the first course of PBSCH, 62 aphereses were performed on day 1, 37 on day 2, and 3 on day 3; during the second course, 8 were performed on day 1, 6 on day 2, and 2 on day 3. The median number of CD34+ cells/kg in the PBSC yields collected via apheresis was 1.29 × 106/kg (range, 0.03-22.80 × 106/kg). More than 2.0 × 106/kg of CD34+ cells was collected in 41 aphereses (34.7%). The median number of total CD34+ cells/kg in a course of PBSCH (n = 72) was 2.53 × 106/kg (range, 0.13–22.8 × 106/kg). In total, 44 courses (61.1%) collected more than 2.0 × 106/kg of CD34+ cells.
Regarding the laboratory data, the median counts in the PB on the day of harvest were: HPC count, 25 (range, 0-899)/µL; WBC count, 19.25 (range, 5–64) × 103/µL; myeloblast count, 0 (range, 0-860)/µL; and platelet count, 9.8 (range, 1.6-30.9) × 104/µL. The myeloblast counts were 0/µL in all healthy donors except one.
2. Correlation between CD34+ cell count and HPC, myeloblast, WBC, and platelet counts
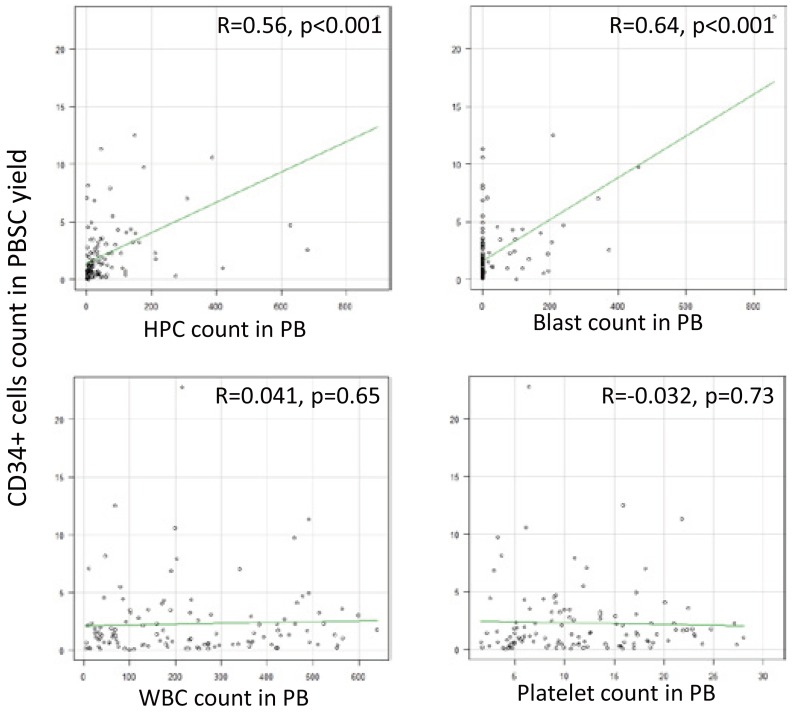
For all samples with confirmed data, a correlation was found between the CD34+ cell count in the PBSC yield and the HPC count in the PB on the day of harvest (R = 0.56, p < 0.001, 95% confidence interval [CI] 0.42 to 0.68), as well as between the CD34+ cell count in the PBSC yield and the myeloblast count in the PB (R = 0.64, p < 0.001, 95% CI 0.52 to 0.74). No correlations were found between the CD34+ cell count in the PBSC yield and the WBC count in the PB (R = 0.041, p = 0.65, 95% CI –0.14 to 0.22) or between the CD34+ cell count in the PBSC yield and the platelet count in the PB (R = –0.032, p = 0.73, 95% CI –0.21 to 0.15) (Figure 1).
Fig. 1.
Correlation of the CD34+ cell count with the HPC, myeloblast, WBC, and platelet counts
PBSC, peripheral blood stem cell; PB, peripheral blood; WBC, white blood cell; HPC, hematopoietic progenitor cell.
3. Cut-off levels of the HPC and myeloblast counts in the PB for good mobilization
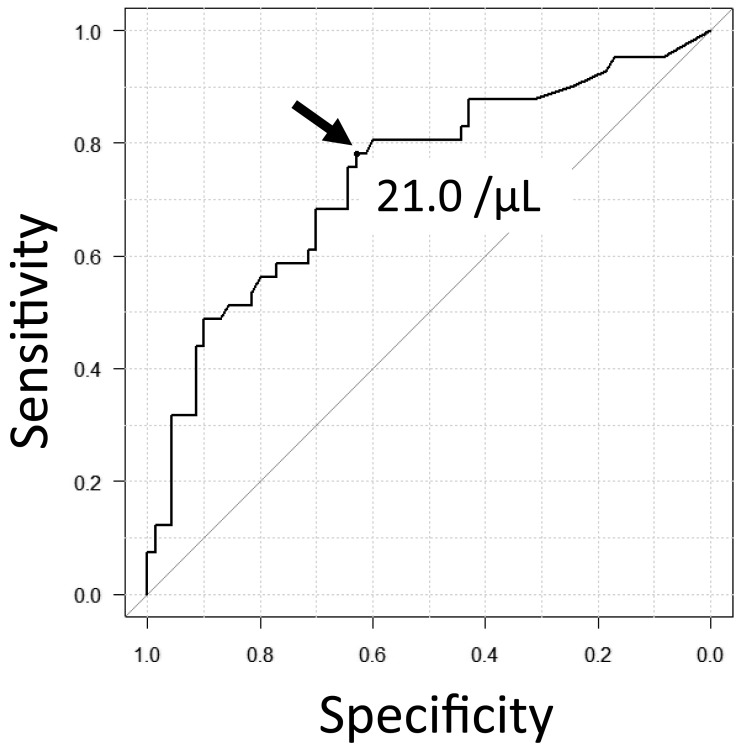
We defined more than 2.0 × 106/kg of CD34+ cells in a single apheresis as good mobilization. We performed receiver operating characteristic (ROC) curve analysis to obtain the optimal cut-off levels of the HPC and myeloblast counts in the PB for good mobilization. The cut-off level of the HPC count was established as 21.0/µL, with a sensitivity of 0.78, specificity of 0.63, and area under the curve (AUC) of 0.74 (Figure 2); furthermore, the cut-off level of the myeloblast count was established as 12.0/µL, with a sensitivity of 0.45, specificity of 0.88, and AUC of 0.67.
Fig. 2.
Receiver operating characteristic curve to assess the optimal cut-off level of the HPC count
The cut-off level of the HPC count for >2.0 × 106/kg of CD34+ cells in a single apheresis was established as 21.0/µL, with a sensitivity of 0.78, specificity of 0.63, and area under the curve of 0.74.
HPC, hematopoietic progenitor cell.
4. Positive predictive value (PPV) and negative predictive value (NPV) at different cut-off levels
We calculated the PPV and NPV at different cut-off levels of the HPC count (Table 2a). When the target number of CD34+ cells was set at >2.0 × 106/kg, the PPV and NPV of an HPC count of more than 20/µL were 0.55 and 0.83, respectively. When the target was set at >1.0 × 106/kg, the PPV of an HPC count of more than 20/µL was 0.71, and when the target was set at >0.5 ×106/kg, the NPV of an HPC count of more than 1/µL was 0.71. Among the patients with malignant lymphomas and plasma cell neoplasms, we calculated PPV and NPV at different myeloblast count cut-off levels (Table 2b). When the target number of CD34+ cells was set at >2.0 × 106/kg, PPV and NPV of a myeloblast count of more than 10/µL were 0.65 and 0.80, respectively. When the target was set at >1.0 × 106/kg, PPV of a myeloblast count of more than 10/µL was 0.81, and when the target was set at >0.5 ×106/kg, NPV of an HPC count of more than 1/µL was 0.40.
Table 2a. PPV and NPV of HPC count in the PB for different targets of CD34+ cell count in the PBSC yield.
| Cut-off value of HPC (/µL) | Target CD34+ cells in the PBSC yield | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| >0.5 × 106/kg | >1.0 × 106/kg | >2.0 × 106/kg | |||||||||
| NPV | PPV | NPV | PPV | NPV | PPV | ||||||
| >1 | 0.71 (0.42–0.92) | 0.79 (0.70–0.87) | 0.86 (0.57–0.98) | 0.64 (0.54–0.73) | 0.86 (0.57–0.98) | 0.40 (0.30–0.51) | |||||
| >5 | 0.52 (0.33–0.71) | 0.82 (0.72–0.89) | 0.72 (0.53–0.87) | 0.68 (0.54–0.73) | 0.83 (0.64–0.94) | 0.44 (0.33–0.55) | |||||
| >10 | 0.49 (0.31–0.66) | 0.83 (0.73–0.91) | 0.66 (0.48–0.81) | 0.68 (0.57–0.79) | 0.86 (0.70–0.95) | 0.47 (0.36–0.59) | |||||
| >20 | 0.36 (0.23–0.50) | 0.81 (0.69–0.90) | 0.57 (0.42–0.70) | 0.71 (0.57–0.82) | 0.83 (0.70–0.92) | 0.55 (0.42–0.68) | |||||
| >50 | 0.33 (0.23–0.44) | 0.88 (0.71–0.97) | 0.51 (0.39–0.62) | 0.78 (0.60–0.91) | 0.74 (0.64–0.84) | 0.65 (0.47–0.81) | |||||
| >100 | 0.31 (0.22–0.41) | 0.90 (0.68–0.99) | 0.46 (0.36–0.57) | 0.75 (0.51–0.91) | 0.70 (0.60–0.80) | 0.70 (0.46–0.88) | |||||
PB, peripheral blood; PBSC, peripheral blood stem cell; HPC, hematopoietic progenitor cell; NPV, negative predictive value; PPV, positive predictive value.
Table 2b. PPV and NPV of myeloblast count in the PB for different targets of CD34+ cell count in the PBSC yield among the other than the healthy donors.
| Cut-off value of myeloblast (/µL) | Target CD34+ cells in the PBSC yield | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| >0.5 × 106/kg | >1.0 × 106/kg | >2.0 × 106/kg | |||||||||
| NPV | PPV | NPV | PPV | NPV | PPV | ||||||
| >1 | 0.40 (0.27–0.54) | 0.96 (0.81–1.0) | 0.55 (0.41–0.68) | 0.78 (0.58–0.91) | 0.80 (0.67–0.90) | 0.62 (0.42–0.81) | |||||
| >10 | 0.39 (0.27–0.53) | 0.96 (0.77–1.0) | 0.55 (0.42–0.69) | 0.81 (0.61–0.93) | 0.80 (0.68–0.90) | 0.65 (0.44–0.83) | |||||
| >50 | 0.35 (0.24–0.49) | 0.95 (0.75–1.0) | 0.50 (0.37–0.63) | 0.75 (0.51–0.91) | 0.77 (0.65–0.87) | 0.70 (0.46–0.88) | |||||
PB, peripheral blood; PBSC, peripheral blood stem cell; NPV, negative predictive value; PPV, positive predictive value.
5. Influencing factors for good mobilization
In the univariate analysis, an HPC count of more than 21/µL, myeloblast count of more than 12/µL, the number of courses of PBSCH, and being younger than 50 years of age at first PBSCH were significantly associated with good mobilization (p < 0.001, p < 0.001, p = 0.01, and p = 0.002, respectively). In addition, WBC and platelet count were not associated with good mobilization (p = 0.70 and p = 0.57, respectively). Multivariate analysis with these factors demonstrated that an HPC count of more than 21/µL, myeloblast count of more than 12/µL, and being younger than 50 years of age at PBSCH were independently associated with good mobilization (p = 0.001, p < 0.001, and p = 0.005, respectively) (Table 3).
Table 3. Influencing factors associated with good mobilization.
| Good mobilization | The other | P value (univariate) | P value (multivariate) | ||
|---|---|---|---|---|---|
| Sex | Male | 27 | 40 | 0.17 | |
| Female | 14 | 37 | |||
| Age at PBSCH | <50 years | 21 | 14 | <0.001 | <0.01 |
| >50 years | 20 | 63 | |||
| Disease | Healthy donor | 13 | 23 | 0.30 | |
| Malignant lymphoma | 15 | 38 | |||
| Plasma cell neoplasm | 13 | 16 | |||
| Donor/no donor | Donor | 13 | 23 | 0.84 | |
| No donor | 28 | 54 | |||
| Body weight | <55 kg | 17 | 31 | 1 | |
| >55 kg | 24 | 46 | |||
| Date of PBSCH | Before June 2011 | 14 | 34 | 0.33 | |
| After June 2011 | 27 | 43 | |||
| PBSCH modality | Spectra Optia | 16 | 24 | 0.42 | |
| COBE Spectra | 25 | 52 | |||
| Course of PBSCH | |||||
| First course | 40 | 62 | 0.01 | ||
| Second course | 1 | 15 | |||
| PBSCH days | Day 1 | 24 | 46 | 0.24 | |
| Day 2 | 17 | 26 | |||
| Day 3 | 0 | 5 | |||
| WBC count | <20,000/µL | 20 | 41 | 0.70 | |
| >20,000/µL | 21 | 35 | |||
| Myeloblast count | <12/µL | 22 | 66 | <0.001 | <0.001 |
| >12/µL | 18 | 9 | |||
| Platelet count | <100,000/µL | 18 | 38 | 0.57 | |
| >100,000/µL | 23 | 38 | |||
| HPC count | <21/µL | 9 | 44 | <0.0001 | <0.01 |
| >21/µL | 32 | 26 |
Collection of more than 2.0 × 106/kg of CD34+ cells in a single apheresis was defined as good mobilization; PBSCH, peripheral blood stem cell harvest; WBC, white blood cells; HPC, hematopoietic progenitor cell.
6. Analysis of the healthy donors and patients
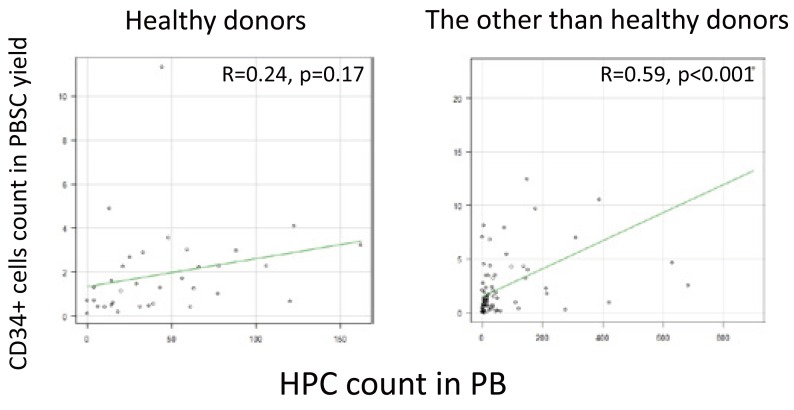
Among the aphereses from the healthy donors (n = 36), no correlations were found between the CD34+ cell count in the PBSC yield and the HPC count in the PB on the day of harvest (R = 0.24, p = 0.17, 95% CI –0.10 to 0.54). Further-more, among the aphereses from patients with malignant lymphoma and plasma cell neoplasms (n = 82), correlations were found between the CD34+ cell count in the PBSC yield and the HPC count in the PB on the harvest day (R = 0.59, p < 0.001, 95% CI: 0.42–0.72) (Figure 3).
Fig. 3.
Correlation of the CD34+ cell count with the HPC count among the healthy donors and patients
PBSC, peripheral blood stem cell; PB, peripheral blood; HPC, hematopoietic progenitor cell.
Among the aphereses from the healthy donors, the optimal cut-off level of the HPC count was established as 44.0/µL, with a sensitivity of 0.69, specificity of 0.76, and AUC of 0.77. In the univariate analysis, an HPC count of more than 44/µL and younger than 45 years of age at PBSCH were significantly associated with good mobilization. Multivariate analysis with these factors demonstrated that these two factors were independently associated with good mobilization (Table 4). Moreover, among the aphereses from patients with malignant lymphoma and plasma cell neoplasms, the optimal cut-off level of the HPC count was established as 24.0 /µL, with a sensitivity of 0.71, specificity of 0.69, and AUC of 0.72. On univariate analysis, an HPC count of more than 24/µL, myeloblast count of more than 12/µL, number of PBSCH courses, and age of less than 50 years at PBSCH were significantly associated with good mobilization. Multivariate analysis using these factors showed that an HPC count of more than 24/µL, myeloblast count of more than 12/µL, and age of less than 50 years at PBSCH were independently associated with good mobilization (Table 5).
Table 4. Influencing factors associated with good mobilization among the healthy donors.
| Good mobilization | The other | P value (univariate) | P value (multivariate) | ||
|---|---|---|---|---|---|
| Sex | Male | 10 | 13 | 0.29 | |
| Female | 3 | 10 | |||
| Age at PBSCH | <45 years | 8 | 4 | 0.01 | 0.01 |
| >45 years | 5 | 19 | |||
| Body weight | <60 kg | 7 | 10 | 0.73 | |
| >60 kg | 6 | 13 | |||
| Date of PBSCH | Until June 2011 | 3 | 12 | 0.16 | |
| Since June 2011 | 10 | 11 | |||
| PBSCH modality | Spectra Optia | 4 | 5 | 0.70 | |
| COBE Spectra | 9 | 17 | |||
| Course of PBSCH | |||||
| First course | 13 | 23 | 1 | ||
| Second course | 0 | 0 | |||
| PBSCH days | Day 1 | 6 | 14 | 0.37 | |
| Day 2 | 7 | 7 | |||
| Day 3 | 0 | 2 | |||
| WBC count | <40,000/µL | 3 | 10 | 0.28 | |
| >40,000/µL | 10 | 12 | |||
| HPC count | <44/µL | 4 | 16 | 0.01 | 0.03 |
| >44/µL | 9 | 5 | |||
| Platelet count | <200,000/µL | 8 | 13 | 1 | |
| >200,000/µL | 5 | 9 |
Collection of more than 2.0 × 106/kg of CD34+ cells in a single apheresis was defined as good mobilization; PBSCH, peripheral blood stem cell harvest; WBC, white blood cell; HPC, hematopoietic progenitor cell.
Table 5. Influencing factors associated with good mobilization among the other than the healthy donors.
| Good mobilization | The other | P value (univariate) | P value (multivariate) | ||
|---|---|---|---|---|---|
| Sex | Male | 17 | 27 | 0.48 | |
| Female | 11 | 27 | 0.48 | ||
| Age at PBSCH | <50 years | 12 | 6 | <0.01 | 0.02 |
| >50 years | 16 | 48 | |||
| Disease | Malignant lymphoma | 15 | 38 | 0.15 | |
| Plasma cell neoplasms | 13 | 16 | |||
| Body weight | <55 kg | 11 | 22 | 1 | |
| >55kg | 17 | 32 | |||
| Date of PBSCH | Until June 2011 | 11 | 22 | 1 | |
| Since June 2011 | 17 | 32 | |||
| PBSCH modality | Spctra Optia | 12 | 19 | 0.63 | |
| COBE Spectra | 16 | 35 | |||
| Course of PBSCH | First course | 27 | 39 | <0.01 | |
| Second course | 1 | 15 | |||
| PBSCH days | Day 1 | 18 | 32 | 0.70 | |
| Day 2 | 10 | 19 | |||
| Day 3 | 0 | 3 | |||
| WBC count | <10000/µL | 9 | 29 | 0.10 | |
| >10,000/µL | 19 | 25 | |||
| Myeloblast count | <12/µL | 11 | 45 | <0.001 | <0.01 |
| >12/µL | 17 | 9 | |||
| Platelet count | <70,000/µL | 9 | 29 | 0.10 | |
| >70,000/µL | 19 | 25 | |||
| HPC count | <24/µL | 8 | 34 | <0.001 | 0.01 |
| >24/µL | 20 | 15 |
Collection of more than 2.0 × 106/kg of CD34+ cells in a single apheresis was defined as good mobilization; PBSCH, peripheral blood stem cell harvest; WBC, white blood cells; HPC, hematopoietic progenitor cell.
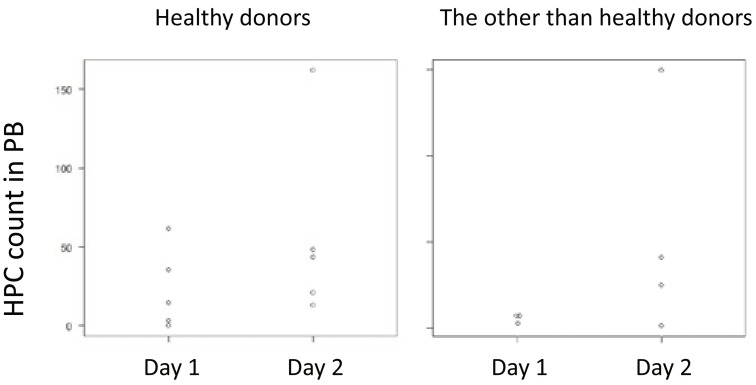
7. Analysis of cases as good mobilization on day 2
Seven healthy donors (Table 4) and 10 patients with malignant lymphoma and plasma cell neoplasms (Table 5) were listed as “good mobilization” on day 2. Among them, in five healthy donors and four cases with malignant lymphoma and plasma cell neoplasm, 2.0 × 106/kg of CD34+ cells in the PBSC yield on day 1 was not achieved. These nine cases were plotted as the HPC count on the vertical axis (Figure 4).
Fig. 4.
Dot plot of nine cases for whom more than 2.0 × 106/kg of CD34+ cells in the PBSC yield could not be collected on day 1, but could be collected on day 2.
DISCUSSION
In the present study, we retrospectively analyzed the impact of the HPC count as an indicator for the optimal timing of PBSCH. This study verified the role of the HPC count in clinical practice in the long term. Our findings confirmed that there is a correlation between the HPC count in the PB on the day of harvest and the CD34+ cell count in the PBSC yields. However, the WBC and platelet count on the harvest day was not correlated with the CD34+ cell count in the PBSC yields. Furthermore, the HPC count, but not WBC and platelet count, was significantly associated with good mobilization. An HPC count over 21/µL, as assessed by ROC curve analysis, was independently associated with good mobilization. However, it is questionable whether the appropriate timing of PBSCH can be judged with a single cut-off level for the HPC count. In previous studies, the optimal cut-off level of the HPC count varied from 5 to 50/µL.5,8,12,13 Jo et al. reported that a rate of HPC increase of more than 2.0/µL/day is a good indicator.10 Furthermore, Letestu et al. reported that when the HPC count is between 0 and 30/µL, direct enumeration of the CD34+ cells is required for decision making.8 In our PPV and NPV data for the different HPC cut-off levels, when the HPC count was more than 100/µL on the day of harvest, the probability that the apheresis obtained on that day collected more than 2.0 × 106/kg of CD34+ cells was 70%. When the HPC count was less than or equal to 1/µL, the probability that the apheresis obtained on that day could not collect more than 0.5 × 106/kg of CD34+ cells was 70%. The final decision on PBSCH should be made using the HPC count on the morning of the harvest day. This can help postpone or advance the scheduled day of PBSCH and avoid a needless PBSCH. The dot plots (Figure 4) of nine cases for whom a sufficient PBSC yield could not be collected on day 1, but could be collected on day 2, suggested that at least in patients with hematological neoplasms, we may be able to omit the collection of day 1 depending on the HPC value.
Our findings revealed that the myeloblast count in the PB had the same efficacy as the HPC count. As the myeloblast count was zero in all healthy donors except for one, we calculated PPV and NPV at different cut-off levels of the myeloblast count among the healthy donors. On comparing PPV and NPV at different HPC count cut-off levels, the PPV at different myeloblast count cut-off levels among the patients with hematological neoplasms seemed to be slightly higher. On the other hand, NPV seemed to be far lower because the myeloblast count was zero in more than half of the patients. Another disadvantage of myeloblast count as an indicator for the optimal timing of PBSCH is that measurement of the myeloblast count requires time consuming staining and numeration by an expert laboratory technician.
Another notable point is that among healthy donors, no statistical correlation was found between the HPC count in the PB and the CD34+ cell count in the PBSC yield. Vogel et al. previously reported the same result.7 There are two potential reasons that may explain this phenomenon. One is that the number of patients in our study was insufficient, and the variability in our data was large. Another reason is that the mechanism of mobilization in healthy donors may be different from that in patients with hematological diseases treated with chemotherapy. The second reason is more likely because the myeloblast count in the PB on the harvest day was zero in all the healthy donors except for one. Previous reports revealed a correlation between hematocrit and CD34+ cells in the PBSC yield in healthy donors.14,15 Among the healthy donors in our study, however, there was no correlation between hemoglobin on the harvest day and the CD34+ cell count in the PBSC yields (data not shown). Here, we analyzed the healthy donors and patients. Our analyses demonstrated that the HPC count may be a reliable indicator for the optimal timing of PBSCH even among healthy donors because an HPC count of more than 44/µL, as evaluated by ROC curve analysis, was one of the factors that was independently associated with good mobilization. In only six of 22 healthy donors, more than 2.0 × 106/kg of CD34+ cells was collected on day 1. This seems to be inefficient. Among 12 donors who were apheresed until 2011, there was no donor from whom more than 2.0 × 106/kg of CD34+ cells was collected on day 1; less than 2.0 × 106/kg of CD34+ cells was collected from nine donors on day 1; and no data of the CD34+ cell count in the PBSC yields was available for three donors. Among 11 donors who were apheresed since 2011, more than 2.0 × 106/kg of CD34+ cells was collected from six donors on day 1; and less than 2.0 × 106/kg of CD34+ cells was collected from five donors on day 1. In many healthy donors, the dose of G-CSF was reduced after the second day. Furthermore, in many of these donors, the WBC count at the time of G-CSF dose reduction was less than 50.0 × 103/µL. Guidelines for PBSC mobilization of healthy donors, which are available on the website of the Japan Society for Hematopoietic Stem Cell Transplantation (http://www.jshct.com/guideline/pdf/08n_pbsc_harvest.pdf), were revised in 2010, and recommend that if the WBC count of the PBSC donor rises above 50.0 × 103/µL, G-CSF administration should be reduced and if the WBC count rises above 75.0 × 103/µL, G-CSF administration should be temporarily terminated. For several years since 2007, when PBSCH was started at our institute, physicians likely reduced the dose of G-CSF based on factors other than WBC count, with excessive emphasis on avoiding adverse events of G-CSF in healthy donors. Due to the insufficiency of G-CSF, PBSC mobilization may be delayed and PBSCH might be required to be performed twice in many healthy donors.
There were some limitations in our study. One is that it was a retrospective study over a long-term period. As the conditioning regimen of PBSCH and the schedule of administration of G-CSF were not uniformly defined, variability in the data may be present. The second limitation is that we could not analyze the correlation or comparison between the HPC count in the PB and the CD34+ cell count in the PB. The third limitation is that XE2100 and XE5000, which were used in our study over different time periods, were used in the blood cell analysis. Furthermore, new XN-series automated hematology analyzers (Sysmex Corporation) have already been developed, although the principles for HPC measurements with these hematology analyzers are the same.
In conclusion, we evaluated the impact of the HPC count as an indicator for the optimal timing of PBSCH in clinical practice by designing a retrospective study, conducted at a single institute over a period of nine years. Taken together, our findings suggest that HPC count is a good indicator for the optimal timing of PBSCH. The final decision on PBSCH should be made using the HPC count on the morning of the harvest day.
Footnotes
CONFLICT OF INTEREST: The authors disclose no potential conflicts of interest.
REFERENCES
- 1.Kawamura K, Kikuchi M, Terasako K, Wada H, Yamasaki R, et al. : Comparison of the efficacy of peripheral blood stem cell mobilization using G-CSF alone from healthy donors and patients with hematologic malignancies. Transfus Apheresis Sci 49: 334-340, 2013. 10.1016/j.transci.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 2.Yamane T, Takekawa K, Tatsumi N: Possibility of identification of hematopoietic stem cells using a conventional blood cell counter. Eur J Haematol 55: 207-208, 1995. 10.1111/j.1600-0609.1995.tb00254.x [DOI] [PubMed] [Google Scholar]
- 3.Mougi H, Shinmyozu K, Osame M: Determining the optimal time for peripheral blood stem cell harvest by detecting immature cells (immature leukocytes and immature reticulocytes) using two newly developed automatic cell analyzers. Int J Hematol 66: 303-313, 1997. 10.1016/S0925-5710(97)00040-6 [DOI] [PubMed] [Google Scholar]
- 4.Takekawa K, Yamane T, Suzuki K, Hino M, Tatsumi N: Identification of hematopoietic stem cells by the SE-9000 automated hematology analyzer in peripheral blood stem cell harvest samples. Acta Haematol 98: 54-55, 1997. 10.1159/000203564 [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Leisenring W, Fritschle W, Heimfeld S, Shulman H, et al. : Enumeration of HPC in mobilized peripheral blood with the Sysmex SE9500 predicts final CD34+ cell yield in the apheresis collection. Bone Marrow Transplant 25: 1157-1164, 2000. 10.1038/sj.bmt.1702406 [DOI] [PubMed] [Google Scholar]
- 6.Park KU, Kim SH, Suh C, Kim S, Lee SJ, et al. : Correlation of hematopoietic progenitor cell count determined by the SE-automated hematology analyzer with CD34(+) cell count by flow cytometry in leukapheresis products. Am J Hematol 67: 42-47, 2001. 10.1002/ajh.1074 [DOI] [PubMed] [Google Scholar]
- 7.Vogel W, Kopp HG, Kanz L, Einsele H: Correlations between hematopoietic progenitor cell counts as measured by Sysmex and CD34+ cell harvest yields following mobilization with different regimens. J Cancer Res Clin Oncol 128: 380-384, 2002. 10.1007/s00432-002-0351-4 [DOI] [PubMed] [Google Scholar]
- 8.Letestu R, Marzac C, Audat F, Belhocine R, Tondeur S, et al. : Use of hematopoietic progenitor cell count on the Sysmex XE-2100 for peripheral blood stem cell harvest monitoring. Leuk Lymphoma 48: 89-96, 2007. 10.1080/10428190600886149 [DOI] [PubMed] [Google Scholar]
- 9.Mitani N, Yujiri T, Tanaka Y, Tanaka M, Fujii Y, et al. : Hematopoietic progenitor cell count, but not immature platelet fraction value, predicts successful harvest of autologous peripheral blood stem cells. J Clin Apher 26: 105-110, 2011. 10.1002/jca.20275 [DOI] [PubMed] [Google Scholar]
- 10.Jo JC, Yoon DH, Kim S, Jang S, Park CJ, et al. : Increment of hematopoietic progenitor cell count as an indicator of efficient autologs stem cell harvest in patients with multiple myeloma. J Clin Apher 27: 229-234, 2012. 10.1002/jca.21231 [DOI] [PubMed] [Google Scholar]
- 11.Kanda Y: Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48: 452-458, 2013. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JL, Kim SB, Lee GW, Ryu MH, Kim EK, et al. : Clinical usefulness of the hematopoietic progenitor cell counts in predicting the optimal timing of peripheral blood stem cell harvest. J Korean Med Sci 18: 27-35, 2003. 10.3346/jkms.2003.18.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh C, Kim S, Kim SH, Kim EK, Lee JL, et al. : Initiation of peripheral blood progenitor cell harvest based on peripheral blood hematopoietic progenitor cell counts enumerated by the Sysmex SE9000. Transfusion 44: 1762-1768, 2004. 10.1111/j.0041-1132.2004.04166.x [DOI] [PubMed] [Google Scholar]
- 14.Del Fante C, Perotti C, Viarengo G, Bellotti L, Parisi C, et al. : Clinical impact of a new automated system employed for peripheral blood stem cell collection. J Clin Apher 21: 227-232, 2006. 10.1002/jca.20102 [DOI] [PubMed] [Google Scholar]
- 15.Pornprasertsud N, Niparuck P, Kidkarn R, Puavilai T, Sirachainan N, et al. : The use of hematocrit level for predicting the efficiency of peripheral blood CD34(+) cell collection after G-CSF Mobilization in Healthy Donors. J Clin Apher 30: 329-334, 2015. 10.1002/jca.21383 [DOI] [PubMed] [Google Scholar]