Abstract
A classification for adult T-cell leukemia-lymphoma (ATL) based on clinical features was proposed in 1991: acute, lymphoma, chronic, and smoldering types, and their median survival times (MSTs) were reported to be 6.2, 10.2, 24.3 months, and not reached, respectively. Several new therapies for ATL have since been developed, i.e. dose-intensity multi-agent chemotherapies, allogeneic hematopoietic stem cell transplantation (allo-HSCT), monoclonal antibodies, and anti-viral therapy. The monoclonal antibody to CCR4, mogamulizumab, clearly improved response rates in patients with treatment-naïve and relapsed aggressive ATL, and has the potential to provide a survival advantage. The outcomes of allo-HSCT have been reported since the early 2000s. High treatment-related mortality was initially the crucial issue associated with this treatment approach; however, reduced intensity conditioning regimens have decreased the risk of treatment-related mortality. The introduction of allo-HSCT has had a positive impact on the prognosis of and potential curability with treatments for ATL. A meta-analysis of a treatment with interferon-α and zidovudine (IFN/AZT) revealed a survival benefit in patients with the leukemic subtype. A phase 3 study comparing IFN/AZT with watchful waiting in patients with indolent ATL is ongoing in Japan. Several clinical trials on novel agents are currently being conducted, such as the histone deacetylase inhibitors, alemtuzumab, brentuximab vedotin, nivolumab, and an EZH1/2 dual inhibitor.
Keywords: adult T-cell leukemia-lymphoma, dose-intensity multi-agent chemotherapy, allogeneic hematopoietic stem cell transplantation
INTRODUCTION
Human T-cell lymphotropic virus type I (HTLV-1) causes latent infection in T-lymphocytes following its transmission by breast feeding, blood transfusions, and sexual contact. The number of HTLV-1 carriers is estimated to be 5-20 million worldwide,1,2 and HTLV-1 is endemic in southwest Japan, Intertropical Africa, the Caribbean, the Middle East, and South America.3 HTLV-1 carriers develop HTLV-1-associated myelopathy-tropical spastic paraparesis, chronic inflammatory diseases, and adult T-cell leukemia-lymphoma (ATL). Between 5 and 7% of men and 2 and 4% of women develop ATL in their lifetime,4 and ATL occurs in most individuals older than 50 years of age after infection via breast feeding. A classification for ATL patients based on clinical information was proposed in 1991: the acute, lymphoma, chronic, and smoldering types, and their median survival times (MSTs) were 6.2, 10.2, 24.3 months, and not reached, respectively.5 Furthermore, patients with chronic ATL may be divided into unfavorable and favorable chronic types based on values for serum lactate dehydrogenase (LDH), blood urea nitrogen (BUN), and serum albumin.6 Patients with the acute, lymphoma, and unfavorable chronic types, and those with the favorable chronic and smoldering types are categorized as aggressive and indolent ATL, respectively. This classification has been used as a tool for the selection of therapeutic interventions as well as the identification of patients at different risks of disease progression for more than two decades.
We herein assessed the impact of treatment advances for ATL such as dose-intensity multi-agent chemotherapy, allogeneic hematopoietic stem cell transplantation (allo-HSCT), a monoclonal antibody to CC chemokine receptor 4 (CCR4), and anti-viral therapy on prognoses based on findings obtained in this field for more than two decades.
OVERVIEW OF CLINICAL FEATURES OF ATL
ATL is characterized by the presence of leukemic cells showing flower-like nuclei in the blood, lymphadenopathy, skin lesions, high serum LDH levels, hypercalcemia, and organ involvement including the lung, gastrointestinal tract, liver, spleen, central nervous system, and bone marrow. Among patients with these clinical features, the diagnosis of ATL is confirmed by a positive serological test for the anti-HTLV-1 antibody, and histologically or cytologically proven peripheral T-cell malignancy. The monoclonal integration of HTLV-1 is confirmed by Southern blotting or linker-mediated polymerase chain reaction in uncertain cases. We conducted a nationwide survey on patients with ATL newly diagnosed between 2000 and 2009, and collected clinical data with the aim of elucidating their outcomes and establishing a prognostic index (the ATL-PI project). According to the dataset obtained, the presence of hypercalcemia, an Eastern Cooperative Oncology Group performance status from 2-4, and B symptoms were present in 41%, 50%, and 32%, and 17%, 35%, and 30% of patients with acute and lymphoma ATL, respectively, implying that these patients were unable to continue normal activities or were in a critical condition at the diagnosis of ATL. ATL patients were frequently compromised by opportunistic infections due to the impairment of cellular immunity. The rates of death from infection without disease progression were 14%, 12%, 15%, and 9% for acute, lymphoma, chronic, and smoldering ATL, respectively.7 Opportunistic infections often interfere with treatments including chemotherapies and allo-HSCT, resulting in the poor prognosis associated with aggressive ATL.
Prognosis of aggressive ATL
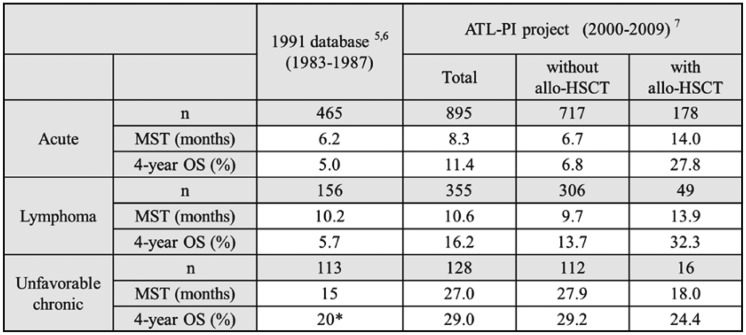
Aggressive ATL consists of acute, lymphoma, and unfavorable chronic types. According to an analysis of patients with newly diagnosed ATL between 1983 and 1987, MSTs were 6.2, 10.2, and 15 months, while overall survival rates (OS) at 4 years were 5.0%, 5.7%, and approximately 20% in patients with acute, lymphoma and unfavorable chronic ATL, respectively5,6 (Table 1). The patients registered in that analysis were treated with various conventional combination chemotherapies, but not allo-HSCT. According to the dataset of the ATL-PI project, MSTs were 8.3, 10.6, and 27.0 months, while OSs at 4 years were 11.4%, 16.2%, and 29% in patients with acute, lymphoma, and unfavorable chronic ATL, respectively.7 Among them, 20% of patients underwent allo-HSCT. In terms of the prognoses of patients treated with allo-HSCT, MSTs were 14.0, 13.9, and 18.0 months and OSs at 4 years were 27.8%, 32.3%, and 24.4% in patients with acute, lymphoma, and unfavorable chronic ATL, respectively. In a comparison of prognoses between the dataset of the ATL-PI project to those of the study conducted in 1991, allo-HSCT appears to contribute to improving the survival of patients with acute and lymphoma-type ATL. Among 128 patients with unfavorable chronic ATL, 16 were treated with allo-HSCT and their prognoses were worse than those without allo-HSCT. Since patients with unfavorable chronic type present various prognoses, the patients who had allo-HSCT could potentially have aggressive disease.
Table 1. A comparison of prognoses of aggressive ATL between the 1991 database and ATL-PI project.
*: data not shown, estimated from graphics.
Abbreviations: ATL-PI project, prognostic index project for adult T-cell leukemia-lymphoma; allo-HSCT, allogeneic-hematopoietic stem cell transplantation; MST, median survival time; OS, overall survival
Prognosis of indolent ATL
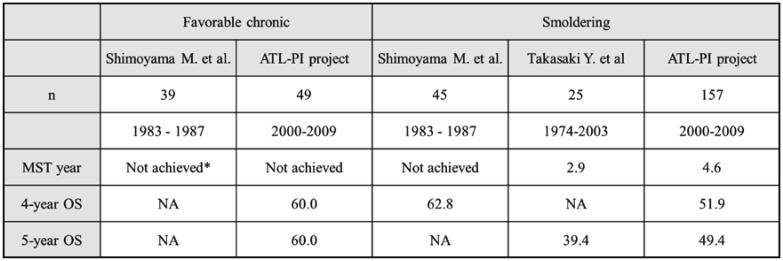
The analysis of patients who were diagnosed with ATL between 1983 and 1987 showed that MSTs were not reached in 39 patients with favorable chronic-type ATL5,6 (Table 2). The prognoses of 49 patients from the dataset of the ATL-PI project were similar to the results described above, MST and OS at 4 years were not achieved and 60.0%. In terms of the smoldering type, differences were observed in prognoses between several studies. Shimoyama M. et al. reported that MST and OS at 4 years were not achieved and 62.8% in 45 patients.5 On the other hand, prognoses in recent studies were worse than those reported by Shimoyama M. et al.; MSTs were 2.9 and 4.6 years in the study by Takasaki et al.8 and the ATL-PI project, respectively. The most common cause of death was progressive disease, at rates of 76%, whereas the rates of death from infection without disease progression were 9% for smoldering type according to the ATL-PI project. We compared available clinical data between the study conducted in 1991 and ours; however, the reasons for the different prognoses were unclear. Further studies are needed in order to reveal the accurate prognosis of patients with smoldering ATL.
Table 2. A comparison of prognoses of indolent ATL.
*: data not shown, estimated from graphics.
Abbreviations: ATL-PI project, prognostic index project for adult T-cell leukemia-lymphoma; MST, median survival time; OS, overall survival; NA, not available
PROGNOSTIC INDEX FOR ATL
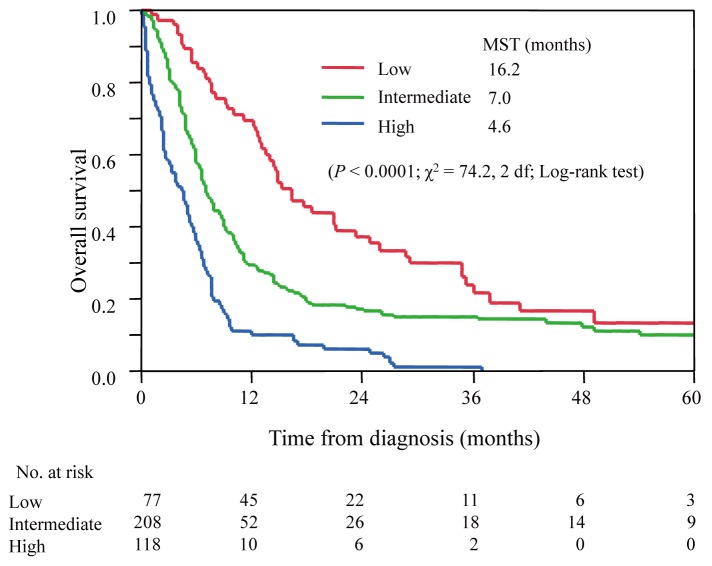
The clinical subtypes based on Shimoyama’s classification have been used to select treatment strategies for patients with ATL as well as a prognostic indicator. However, clinical courses and prognoses vary, even in the same clinical subtypes. Therefore, we elucidated prognostic factors using the clinical information of 807 patients with acute and lymphoma ATL who did not receive allo-HSCT, and 248 patients with chronic and smoldering ATL gathered by the ATL-PI project, and subsequently developed prognostic indexes. Datasets were randomly split into either a training sample for developing prognostic indexes or a validation sample for independently evaluating the prognostic indexes obtained for acute and lymphoma ATL as well as chronic and smoldering ATL. The Ann Arbor stage (I–II vs. III–IV), performance status (0–1 vs. 2–4), age, serum albumin, and soluble interleukin-2 receptor (sIL-2R) were identified as independent prognostic factors in patients with acute and lymphoma ATL. Using these variables, a prognostic model was devised to identify different levels of risk. The ATL prognostic index (ATL-PI) was established as follows: prognostic score = 2 (if the stage = III or IV); + 1 (if the ECOG performance status > 1); + 1 (if age > 70); + 1 (if albumin < 3.5 g/dL; + 1 (if sIL2R > 20,000 U/mL). Scores from 0 to 2 were categorized as low risk, 3 to 4 as intermediate risk, and 5 to 6 as high risk. In validation samples, MSTs were 16.2, 7.0, and 4.6 months, and OSs at 2 years were 37, 17, and 6% for patients at low, intermediate, and high risks, respectively (P < .0001, figure 1).9
Fig. 1.
Survival curves for acute and lymphoma types according to ATL-PI.
The prognostic score = 2 (if the stage = III or IV); + 1 (if the ECOG performance status > 1); + 1 (if age > 70); + 1 (if albumin < 3.5 g/dL; + 1 (if sIL2R > 20,000 U/mL). Scores from 0 to 2 were categorized as low risk, 3 to 4 as intermediate risk, and 5 to 6 as high risk.
In terms of chronic and smoldering ATL, only sIL-2R was identified as an independent prognostic factor. According to trichotomizing sIL-2R at 1,000 and 6,000 U/mL using a quartile point, patients with more than 6,000 U/mL of sIL-2R were categorized as high risk, less than and equal to 1,000 U/mL as low risk, and the others as intermediate risk. MSTs were not reached, 5.5, and 1.6 years and OSs at 4 years were 77.6, 55.6, and 26.2% for patients at low, intermediate, and high risks, respectively (P < .0001).10 These prognostic indexes have potential as novel tools for risk-adopted therapeutic approaches.
RECENT TREATMENT ADVANCES FOR ATL
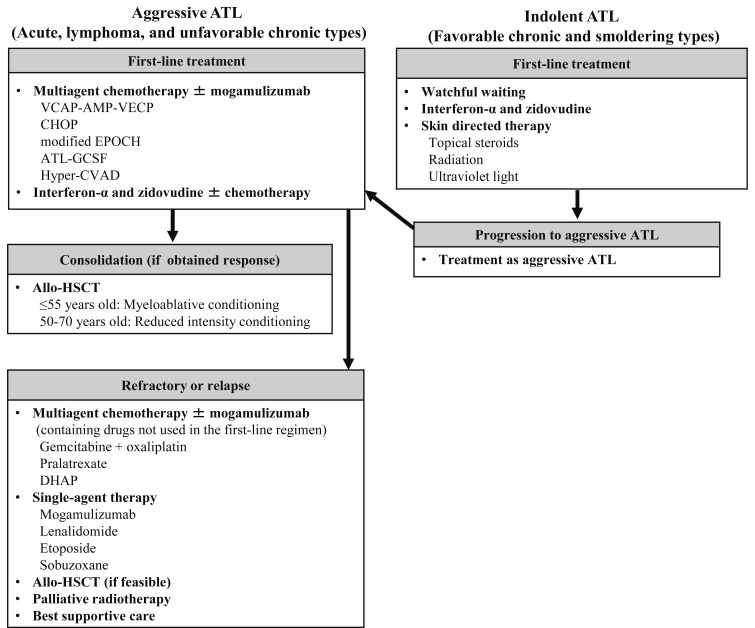
The treatment algorithm is shown in figure 2. An international consensus meeting recommends a first-line treatment with multi-agent chemotherapy for patients with aggressive ATL, such as VCAP-AMP-VECP, which is sequential combination chemotherapy consisting of VCAP (vincristine, cyclophosphamide, doxorubicin, and prednisolone), AMP (doxorubicin, ranimustine, and prednisolone), and VECP (vindesine, etoposide, carboplatin, and prednisolone) with granulocyte colony-stimulating factor (G-CSF) and intrathecal prophylaxis.11,12 If these patients respond to first-line chemotherapy and have a HLA-matched donor, subsequent allo-HSCT is recommended. The potential of the monoclonal antibody to CCR4, mogamulizumab, has been demonstrated in patients with aggressive ATL.13,14 In Japan, mogamulizumab was approved as a treatment for relapsed or resistant ATL patients in 2012, and for newly diagnosed ATL patients in combination with anti-cancer agents in 2014. In terms of the treatment of patients with indolent ATL, watchful waiting including skin-directed therapy for skin lesions is recommended. The treatment with interferon-α in combination of zidovudine (IFN/AZT) are effective for leukemic type of ATL,15 and it is considered to be a therapeutic option outside Japan. However, neither IFN nor AZT has been approved by the national health insurance system in Japan yet.
Fig. 2.
Treatment algorithm for ATL patients.
The multi-agent chemotherapies are recommended for patients with aggressive ATL as first-line treatment, such as VCAP-AMP-VECP and CHOP. If these patients respond to first-line chemotherapy and have a HLA-matched donor, subsequent allo-HSCT is recommended. Mogamulizumab is used for patients whose ATL cells express CC chemokine receptor 4. Patients with refractory or relapsed aggressive ATL are treated with multi-agent chemotherapy containing drugs not used in the prior regimen or single-agent therapies. The combination of gemcitabine and oxaliplatin, pralatrexate, or DHAP were used as investigator’s choice regimens in a prospective study. The combination of interferon-α and zidovudine (IFN-AZT) have been used for the treatment of acute, chronic, and smoldering types outside Japan. The retrospective study showed that low-dose IFN-AZT with chemotherapy is also an option for lymphoma type. Regarding indolent ATL, watchful waiting including skin-directed therapy for skin lesions or IFN-AZT are recommended. Chemotherapy is applied if transformation to an aggressive type occurs.
Abbreviations: allo-HSCT, allogeneic-hematopoietic stem cell transplantation; CHOP, consisting of cyclophosphamide, doxorubicin, vincristine, and prednisolone; VCAP-AMP-VECP, sequential combination chemotherapy consisting of VCAP (vincristine, cyclophosphamide, doxorubicin, and prednisolone), AMP (doxorubicin, ranimustine, and prednisolone), and VECP (vindesine, etoposide, carboplatin, and prednisolone); ATL-GCSG, consisting of vincristine, vindesine, doxorubicin, mitoxantrone, cyclophosphamide, etoposide, ranimustine, and prednisolone with prophylactic support by granulocyte colony-stimulating factor; Modified EPOCH consisting of etoposide, prednisolone, vincristine, carboplatin, and doxorubicin: cyclophosphamide used in original EPOCH was substituted for carboplatin.; DHAP, consisting of dexamethasone, high-dose cytarabine, and cisplatin; Hyper-CVAD, consisting of cyclophosphamide, doxorubicin, vincristine, and dexamethasone.
Multi-agent chemotherapies (Table 3)
Table 3. Outcomes from chemotherapies in aggressive ATL.
*: data not shown, estimated from graphics.
Abbreviations: NA, not available; allo-HSCT, allogeneic-hematopoietic stem cell transplantation; MST, median survival time; OS, overall survival; VEPA, consisting of vincristine, cyclophosphamide, prednisone, and doxorubicin; MTX, methotrexate; DCF, Deoxycoformycin; CHOP, consisting of cyclophosphamide, doxorubicin, vincristine, and prednisolone; VCAP-AMP-VECP, sequential combination chemotherapy consisting of VCAP (vincristine, cyclophosphamide, doxorubicin, and prednisolone), AMP (doxorubicin, ranimustine, and prednisolone), and VECP (vindesine, etoposide, carboplatin, and prednisolone); JCOG, Japan Clinical Oncology Group
The Japan Clinical Oncology Group-Lymphoma Study Group (JCOG-LSG) conducted four clinical trials on advanced aggressive non-Hodgkin’s lymphoma between 1978 and 1993 in order to evaluate the efficacy of VEPA, consisting of vincristine, cyclophosphamide, prednisone, and doxorubicin. The number of patients with ATL included in these trials were 18, 54, 42, and 60, and MSTs were 5.0, 7.5, 8.0, and 7.4 months in JCOG7801,16 JCOG8101,17 JCOG 8701,18 and JCOG9109 (combined with deoxycoformycin),19 respectively (Table 3). However, multi-agent chemotherapies based on VEPA did not improve prognoses. Hence, a phase 2 study using dose-intensified multi-agent chemotherapy, VCAP-AMP-VECP (JCOG9303),11 was conducted between 1994 and 1996. This study showed promising findings with complete response and partial response rates of 36% and 45%, and MST and OS at 2 years of 13.0 months and 31.3%, respectively. A subsequent phase 3 trial (JCOG9801)12 compared OSs between VCAP-AMP-VECP and bi-weekly CHOP, consisting of cyclophosphamide, doxorubicin, vincristine, and prednisone, in patients younger than 70 years of age in order to test the superiority of dose-intensified multi-agent chemotherapy. MSTs were 12.7 and 10.9 months and OSs at 3 years were 24% and 13.0% in VCAP-AMP-VECP and bi-weekly CHOP, respectively (P = .085). Regarding adverse events, the rates of grade 4 neutropenia were 98% and 83%, grade 4 thrombocytopenia 74% and 17%, and grade 3 or 4 infection 32% and 15% in VCAP-AMP-VECP and bi-weekly CHOP, respectively. According to a subgroup analysis, populations with a good performance status or younger than 56 years of age were more likely to benefit from VCAP-AMP-VECP.
Mogamulizumab
CCR4 is selectively expressed on Th2 cells and regulatory T cells, and tumor cells in more than 90% of patients with ATL.20,21 Mogamulizumab is a humanized anti-CCR4 immunoglobulin G1 monoclonal antibody with a defucosylated Fc region. A single agent phase 2 trial in patients with relapsed aggressive ATL showed a response rate of 50%, progression-free survival of 5.3 months, and MST of 13.7 months.13 These findings indicated the potential for a survival advantage with mogamulizumab in patients with relapsed aggressive ATL. In the USA, European Union, and Latin America, a prospective study comparing mogamulizumab with the investigator’s choice in the treatment of patients with relapsed or refractory ATL was conducted.22 The investigator’s choice regimens included the combination of gemcitabine and oxaliplatin, pralatrexate, or DHAP consisting of dexamethasone, high-dose cytarabine, and cisplatin. The overall response rate for mogamulizumab was 14.9%, while there were no confirmed responses in the investigator’s choice group. MSTs were 4.9 and 6.9 months, and OSs at 1 year were 38.9% and 37.5% in the mogamulizumab and investigator’s choice groups, respectively. No survival advantage was observed in the mogamulizumab group, while the response rate supported the therapeutic potential of mogamulizumab in this setting. Regarding first-line treatments, a randomized phase 2 study comparing VCAP-AMP-VECP with or without mogamulizumab in patients with aggressive ATL also showed an improved response with the addition of mogamulizumab; complete response and overall response rates of 52% and 86%, respectively.14 The benefit for progression-free survival and OS by adding mogamulizumab was not observed. It might be due to the small number of patients and the short-term observations for these secondary endpoint. In a retrospective multicenter study, mogamulizumab for the treatment of ATL slightly improved MST; MSTs of 382 and 240 days in patients with and without mogamulizumab, respectively (P=.167, Log-rank test).23
In terms of the adverse events of mogamulizumab, according to a phase 2 trial, skin disorders, cytomegalovirus infection, pyrexia, hyperglycemia, and interstitial lung disease were only observed in the combination of mogamulizumab arm.14 Mogamulizumab may delete regulatory T-cells, resulting in the potential to cause immune-related adverse events such as Stevens-Johnson syndrome and toxic epidermal necrolysis. The use of mogamulizumab before allo-HSCT correlated with an increased risk of graft-versus-host disease (GVHD)-related mortality.24 Further studies are needed in order to clarify appropriate settings and patient populations that would benefit from treatments with mogamulizumab.
Allo-HSCT
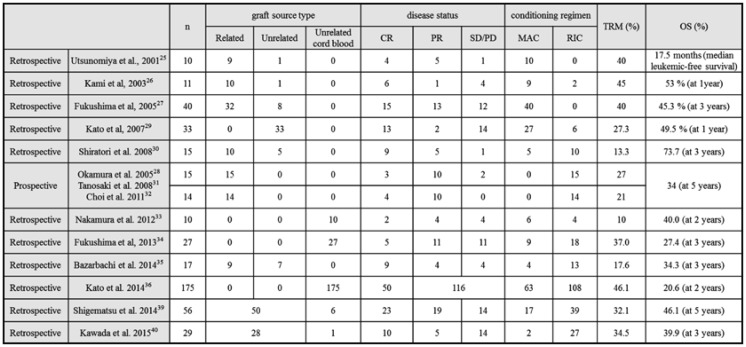
Several studies have reviewed the outcomes of allo-HSCT in patients with aggressive ATL (Table 4).25-40 Utsunomiya et al. reported that allo-HSCT using myeloablative conditioning regimens (MAC) improved the outcome of ATL in 2001.25 However, 4 out of 10 patients died of treatment-related mortality (TRM). High TRM from 10 to 45% was subsequently reported. In order to prevent TRM, reduced intensity conditioning regiments (RIC) using peripheral blood stem cells from HLA sibling donors were prospectively assessed in patients older than 50 years of age. TRM was 27%28 and OS at 5 years was 34%.32 A retrospective study that analyzed 586 patients who received allo-HSCT between 1992 and 2009 showed similar OSs between MAC and RIC, and no significant difference in the intensity of the conditioning regimen on survival.37 They also identified 5 prognostic factors associated with poor OS; an older age, male sex, not achieving complete remission, a poor performance status, and transplantation from unrelated donors. The analysis of the ATL-PI project showed MST of 22.0 months and OS at 5 years of 37.3% in patients who received allo-HSCT at first remission, with a significant difference being observed according to disease status at the time of transplantation. Kanda et al. reported the effects of acute and chronic GVHD on OS in 294 patients treated with allo-HSCT.38 The development of grade 1-2 acute GVHD correlated with a higher survival rate than its absence. These findings indicated the presence of a graft-versus-ATL effect and the potential curability of allo-HSCT, particularly in patients in remission.
Table 4. Outcomes from allogeneic hematopoietic stem cell transplantation in ATL.
Abbreviations: CR; complete response; PR, partial response; SD, stable disease; PD, progressive disease; MAC, myeloablative conditioning regimens; reduced intensity conditioning regimens, RIC; treatment-related mortality, TRM; OS, overall survival rate
Retrospective analyses of the outcomes of unrelated cord blood transplantation were performed by Nakamura et al. and Fukushima et al.: OSs of 40% at 2 years and 27.4% at 3 years, and TRM of 10 - 20% and 37%, respectively.33,34 A Japanese nationwide retrospective survey reported the outcomes of 175 patients undergoing cord blood transplantation; OS at 2 years of 20.6% and TRM of 46%, respectively.36 A prospective study to confirm the feasibility of unrelated cord blood transplantation has been completed in Japan.
IFN/AZT
The effects of the combination of IFN/AZT on ATL patients were initially reported in 1995.41,42 According to the study by Gill et al., the overall response rate and MST were 58% and 3 months in 19 aggressive ATL including 12 patients previously treated. Several studies were subsequently reported the outcome of IFN/AZT.43,44 A meta-analysis performed by Bazarbachi et al. showed that MSTs for a first-line treatment with IFN/AZT were 9 months, 7 months, and not achieved, while OSs at 5 years were 28%, 0% and 100% in patients with acute, lymphoma, and chronic and smoldering ATL, respectively.15 Patients with leukemic subtypes significantly benefited from first-line antiviral therapy. The retrospective analysis conducted by Hodson et al. supported the use of IFN/AZT with chemotherapy as the first-line treatment for lymphoma ATL as well as acute ATL. The use of IFN/AZT at any time prolonged survival, and was the only factor associated with a reduction in the risk of death among patients with aggressive ATL. Most patients in this study were treated with a CHOP-like regimen, and none of the patients received a dose-intensified multi-agent regimen.45 Although this anti-viral therapy is considered to have beneficial effects on ATL, these finding have been brought from the small phase 2 studies and several retrospective studies. Therefore, a phase 3 study was conducted by JCOG-LSG to compare IFN/AZT with watchful waiting in patients with indolent ATL, and is currently in progress (UMIN000011805).
NOVEL AGENTS FOR ATL
Molecular targeted therapies have currently focused on the development of anti-cancer drugs, and are a cornerstone of precision medicine. Molecular targeted therapies that markedly improve survival have not been sufficiently developed in the field of the clinical setting of ATL. Several novel agents that may improve survival among ATL patients are described below from recent clinical trials.
Lenalidomide
Lenalidomide, which is an immunomodulatory drug with anti-proliferative and anti-tumor potential, is a key drug for multiple myeloma. E3 ubiquitin ligase cereblon was identified as a direct protein target required for the anti-myeloma and anti-lymphoma effects of lenalidomide as well as thalidomide.46,47 Two phase 2 clinical trials on lenalidomide monotherapy for patients with recurrent and refractory T-cell lymphoma showed overall response rates of 26% and 22%, and MST of 12 months in one of the trials.48,49 In relapsed and recurrent patients with aggressive ATL, the findings of a phase 2 study on lenalidomide showed an overall response rate, median progression-free survival, and MST of 42%, 3.8 months, and 20.3 months, respectively.50 In Japan, lenalidomide has been approved for relapsed/refractory ATL in March 2017 based on these findings.
Histone deacetylase inhibitors
Histone deacetylases (HDACs) and histone acetylases are enzymes that are responsible for deacetylating and acetylating, respectively. The chromatin alterations induced by these enzymes regulate transcription and many other nuclear events. HDACs also regulate many non-histone protein substrates such as hormone receptors, chaperone proteins, and cytoskeleton proteins, which control cell proliferation and cell death. HDAC inhibitors repress the expression of these genes, resulting in the induction of cell cycle arrest and apoptosis in tumor cells.51
A phase 2 study on an oral HDAC inhibitor (Panobinostat, LBH589) in patients with cutaneous T-cell lymphoma and ATL was conducted in Japan. However, the study was terminated because of severe infections associated with the formation of ulcers in patients with ATL.52 A phase 1 study on another HDAC inhibitor (HBI-8000), which inhibits cancer-associated HDAC enzymes (Class I and IIb), was subsequently reported.53 A phase 2 trial to evaluate efficacy and safety in patients with recurrent and refractory ATL in Japan is ongoing (NCT02955589).
Alemtuzumab
Alemtuzumab, a humanized monoclonal antibody, targets CD52 on the surfaces of lymphocytes and monocytes; however, monocytes and natural killer cells appear to be resistant to alemtuzumab-mediated lysis.54 Since alemtuzumab may strongly deplete the number of T-cells, it has been used to reduce the risk of GVHD in patients undergoing allo-HSCT. In ATL cells, more than 90% of tumor cells strongly express CD52. Sharma et al. reported the findings of a single institution phase 2 study on intravenous alemtuzumab administered at 30 mg three times weekly for a maximum of 12 weeks to patients with acute, lymphoma, and chronic ATL, and patients who received prior treatment accounted for 69% of subjects.55 The overall response rate was 51.7% including 12 out of 15 patients with the acute type, 2 out of 3 patients with the chronic type, and one out of 11 patients with the lymphoma type. The median progression-free survival rate and MST were 2.0 and 5.9 months, respectively. All patients developed cytomegalovirus antigenemia, three were symptomatic, and all responded to antiviral therapy. Grade 3 or 4 infections were reported in 14% of patients. They further demonstrated an increase in the duration of the anti-tumor response with the use of alemtuzumab with IL-15 through antibody-dependent cell-mediated cytotoxicity.56 A phase 1 study to establish a safe dose of rhIL-15 combined with alemtuzumab and identify adverse effects in patients with refractory or relapsed acute and chronic ATL is currently in progress (NCT02689453).
Brentuximab vedotin
CD30, which is a TNF receptor family member, is associated with cell proliferation. Brentuximab vedotin (BV) is a human CD30-directed chimeric antibody bonded to the microtubule-disrupting agent. BV represents a major breakthrough in the treatment of CD30-positive lymphomas including Hodgkin’s lymphoma and anaplastic large cell lymphoma.57,58 In T-cell lymphoma, the response rate was 41% among patients with relapsed T-cell lymphomas, including 54% of AITL patients, and there was also no correlation between CD30 expression and responses.59 Fanale et al. conducted a phase 1 study on BV administered in sequence with CHOP or in combination with CHP (CHOP without vincristine) to treatment-naive patients with CD30-expressing peripheral T-cell lymphoma, including 2 ATL patients.60 The initial therapy with BV in combination with CHP induced long-term remission with a tolerable safety profile. A phase 3 trial comparing BV in the combination of CHP with CHOP as first-line treatment for CD30-expressing peripheral T-cell lymphoma is ongoing (NCT01777152).
Others
Programmed cell death ligand 1 (PD-L1) is an immunomodulatory glycoprotein expressed on antigen-presenting cells and human cancers. The binding of PD-L1 to its receptor (PD-1) on the surface of T cells regulates the function of effector T-cells. Targeted therapy against PD-1 or PD-L1 has exhibited significant clinical activity in various cancers such as melanoma, renal cell carcinoma, non-small cell lung cancer, and Hodgkin’s lymphoma.61-63 Miyoshi et al. reported that PD-L1 was expressed in 7.4% of 135 patients with ATL using the immunostaining of biopsy samples, and the OS of these patients was lower than that of PD-L1 negative patients.64 A phase 2 study on nivolumab, a PD-1-blocking antibody, for relapsed and refractory ATL is ongoing (UMIN000020601).
Polycomb group proteins, which mediate the regulation of epigenetics, have been shown to play a role in the development of ATL. Enhancer of zeste homolog 2 (EZH2) expression with the trimethylation of lysine 27 on the histone H3 (H3K27me3) mark was altered in ATL cells.65 Fujikawa et al. performed integrative analyses on the epigenome and transcriptome of ATL cells in order to assess epigenetic events and their roles in the pathogenesis of ATL.66 Polycomb-repressive complex 2-mediated trimethylation at H3K27 was significantly and frequently reprogrammed in 50% of the genes in ATL cells and occurred at an early stage of the development of ATL. Furthermore, EZH2 inhibitors had the ability to reverse epigenetic disruption, and selectively eliminate ATL and HTLV-1-infected cells. A phase 1 study on DS-3201b, a histone-lysine N-methyltransferase EZH1/2 dual inhibitor, for patients with non-Hodgkin’s lymphoma including ATL is in progress (NCT02732275).
CONCLUSION
Treatment advances for ATL in the past two decades were reviewed herein. The dose-intensified multi-agent regimen (VCAP-AMP-VECP) resulted in improved responses by aggressive ATL; however, survival is still very poor. Analyses of outcomes from allo-HSCT have been reported since the early 2000s. Although high TRM was the initial crucial issue associated with this treatment, the introduction of RIC appears to have reduced the risk of TRM and improved prognoses. Allo-HSCT undoubtedly represents a cure for specific patients with aggressive ATL. While mogamulizumab also showed an improved response with the addition of cytotoxic chemotherapy, it should be elucidated the impact on survival. Several clinical trials on novel agents are currently in progress, and may contribute to improving the survival of ATL patients. Efforts to identify the subset of patients that will benefit the most from new therapies are also needed in the future.
ACKNOWLEDGMENTS
The authors thank the investigators that participated in the study of the ATL-PI project.
This work was supported by JSPS KAKEHI Grant Number 16K19580 and 16KK0206 (H. K.)
Footnotes
CONFLICT OF INTEREST: H.K. has no conflicts of interest to declare.
K.I. reports grants and personal fees from Kyowa Hakko Kirin, Chugai Pharmaceutical, Takeda Pharmaceutical, Novartis, Eisai, and Taiho Pharmaceutical; personal fees from Bristol-Myers Squibb, Celgene, Janssen Pharmaceutical, and Pfizer; and grants from Yakult Pharmaceutical, MSD, and Japan Blood Products Organization, outside the submitted work.
REFERENCES
- 1.de The G : Bomford R: An HTLV-I vaccine: why, how, for whom? AIDS Res Hum Retroviruses 9: 381-386, 1993. 10.1089/aid.1993.9.381 [DOI] [PubMed] [Google Scholar]
- 2.Gessain A, Cassar O: Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front Microbiol 3: 388, 2012. 10.3389/fmicb.2012.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL: Global epidemiology of HTLV-I infection and associated diseases. Oncogene 24: 6058-6068, 2005. 10.1038/sj.onc.1208968 [DOI] [PubMed] [Google Scholar]
- 4.Iwanaga M, Watanabe T, Yamaguchi K: Adult T-cell leukemia: a review of epidemiological evidence. Front Microbiol 3: 322, 2012. 10.3389/fmicb.2012.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimoyama M: Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87). Br J Haematol 79: 428-437, 1991. 10.1111/j.1365-2141.1991.tb08051.x [DOI] [PubMed] [Google Scholar]
- 6.Shimoyama M: Chemotherapy of ATL, in Takatsuki K (ed): Adult T-Cell Leukemia. Oxford, United Kingdom, Oxford University Press, 1994, pp 221-237 [Google Scholar]
- 7.Katsuya H, Ishitsuka K, Utsunomiya A, Hanada S, Eto T, et al. : Treatment and survival among 1594 patients with ATL. Blood 126: 2570-2577, 2015. 10.1182/blood-2015-03-632489 [DOI] [PubMed] [Google Scholar]
- 8.Takasaki Y, Iwanaga M, Imaizumi Y, Tawara M, Joh T, et al. : Long-term study of indolent adult T-cell leukemia-lymphoma. Blood 115: 4337-4343, 2010. 10.1182/blood-2009-09-242347 [DOI] [PubMed] [Google Scholar]
- 9.Katsuya H, Yamanaka T, Ishitsuka K, Utsunomiya A, Sasaki H, et al. : Prognostic index for acute- and lymphoma-type adult T-cell leukemia/lymphoma. J Clin Oncol 30: 1635-1640, 2012. 10.1200/JCO.2011.38.2101 [DOI] [PubMed] [Google Scholar]
- 10.Katsuya H, Shimokawa M, Ishitsuka K, Kawai K, Amano M, et al. : Prognostic index for chronic and smoldering types adult T-cell leukemia/lymphoma. J Clin Oncol 33: 8522-8522, 2015. [abstract] [Google Scholar]
- 11.Yamada Y, Tomonaga M, Fukuda H, Hanada S, Utsunomiya A, et al. : A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br J Haematol 113: 375-382, 2001. 10.1046/j.1365-2141.2001.02737.x [DOI] [PubMed] [Google Scholar]
- 12.Tsukasaki K, Utsunomiya A, Fukuda H, Shibata T, Fukushima T, et al. : VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol 25: 5458-5464, 2007. 10.1200/JCO.2007.11.9958 [DOI] [PubMed] [Google Scholar]
- 13.Ishida T, Joh T, Uike N, Yamamoto K, Utsunomiya A, et al. : Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol 30: 837-842, 2012. 10.1200/JCO.2011.37.3472 [DOI] [PubMed] [Google Scholar]
- 14.Ishida T, Jo T, Takemoto S, Suzushima H, Uozumi K, et al. : Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: a randomized phase II study. Br J Haematol 169: 672-682, 2015. 10.1111/bjh.13338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bazarbachi A, Plumelle Y, Carlos Ramos J, Tortevoye P, Otrock Z, et al. : Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol 28: 4177-4183, 2010. 10.1200/JCO.2010.28.0669 [DOI] [PubMed] [Google Scholar]
- 16.Shimoyama MIM, Yunoki K, Oota K, Ogawa M: Final results of cooperative study of VEPA [vincristine, cyclophosphamide (endoxan), prednisolone and adriamycin] therapy in advanced adultnon-Hodgkin’s lymphoma: relation between T- or B-cell phenotype and response. Jpn J Clin Oncol 12, 1982 [Google Scholar]
- 17.Shimoyama M, Ota K, Kikuchi M, Yunoki K, Konda S, et al. : Chemotherapeutic results and prognostic factors of patients with advanced non-Hodgkin’s lymphoma treated with VEPA or VEPA-M. J Clin Oncol 6: 128-141, 1988. 10.1200/JCO.1988.6.1.128 [DOI] [PubMed] [Google Scholar]
- 18.Tobinai KSM, Minato K, Shirakawa S, Kobayashi T, Hotta T, et al. : Japan Clinical Oncology Group phase II trial of second-generation ‛LSG4 protocol’ in aggressive T- and B-lymphoma: a new predictive model FOR T-and B-lymphoma. Proc Am Soc Clin Oncol, 1994. [abstract] [Google Scholar]
- 19.Tsukasaki K, Tobinai K, Shimoyama M, Kozuru M, Uike N, et al. : Deoxycoformycin-containing combination chemotherapy for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study (JCOG9109). Int J Hematol 77: 164-170, 2003. 10.1007/BF02983215 [DOI] [PubMed] [Google Scholar]
- 20.Yoshie O, Fujisawa R, Nakayama T, Harasawa H, Tago H, et al. : Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood 99: 1505-1511, 2002. 10.1182/blood.V99.5.1505 [DOI] [PubMed] [Google Scholar]
- 21.Ishida T, Utsunomiya A, Iida S, Inagaki H, Takatsuka Y, et al. : Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res 9: 3625-3634, 2003 [PubMed] [Google Scholar]
- 22.Phillips A, Fields P, Hermine O, Ramos JC, Beltran BE, et al. : A Prospective, Multicenter, Randomized Study of Anti-CCR4 Monoclonal Antibody Mogamulizumab Versus Investigator’s Choice in the Treatment of Patients with Relapsed/Refractory Adult T-Cell Leukemia-Lymphoma: Overall Response Rate, Progression-Free Survival, and Overall Survival. Blood 128: 4159-4159, 2016. [abstract] [Google Scholar]
- 23.Iyama S, Sato T, Ohnishi H, Kanisawa Y, Ohta S, et al. : A Multicenter Retrospective Study of Mogamulizumab Efficacy in Adult T-Cell Leukemia/Lymphoma. Clin Lymphoma Myeloma Leuk 17: 23-30.e2, 2017. 10.1016/j.clml.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 24.Fuji S, Inoue Y, Utsunomiya A, Moriuchi Y, Uchimaru K, et al. : Pretransplantation Anti-CCR4 Antibody Mogamulizumab Against Adult T-Cell Leukemia/Lymphoma Is Associated With Significantly Increased Risks of Severe and Corticosteroid-Refractory Graft-Versus-Host Disease, Nonrelapse Mortality, and Overall Mortality. J Clin Oncol 34: 3426-3433, 2016. 10.1200/JCO.2016.67.8250 [DOI] [PubMed] [Google Scholar]
- 25.Utsunomiya A, Miyazaki Y, Takatsuka Y, Hanada S, Uozumi K, et al. : Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 27: 15-20, 2001. 10.1038/sj.bmt.1702731 [DOI] [PubMed] [Google Scholar]
- 26.Kami M, Hamaki T, Miyakoshi S, Murashige N, Kanda Y, et al. : Allogeneic haematopoietic stem cell transplantation for the treatment of adult T-cell leukaemia/lymphoma. Br J Haematol 120: 304-309, 2003. 10.1046/j.1365-2141.2003.04054.x [DOI] [PubMed] [Google Scholar]
- 27.Fukushima T, Miyazaki Y, Honda S, Kawano F, Moriuchi Y, et al. : Allogeneic hematopoietic stem cell transplantation provides sustained long-term survival for patients with adult T-cell leukemia/lymphoma. Leukemia 19: 829-834, 2005. 10.1038/sj.leu.2403682 [DOI] [PubMed] [Google Scholar]
- 28.Okamura J, Utsunomiya A, Tanosaki R, Uike N, Sonoda S, et al. : Allogeneic stem-cell transplantation with reduced conditioning intensity as a novel immunotherapy and antiviral therapy for adult T-cell leukemia/lymphoma. Blood 105: 4143-4145, 2005. 10.1182/blood-2004-11-4193 [DOI] [PubMed] [Google Scholar]
- 29.Kato K, Kanda Y, Eto T, Muta T, Gondo H, et al. : Allogeneic bone marrow transplantation from unrelated human T-cell leukemia virus-I-negative donors for adult T-cell leukemia/lymphoma: retrospective analysis of data from the Japan Marrow Donor Program. Biol Blood Marrow Transplant 13: 90-99, 2007. 10.1016/j.bbmt.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 30.Shiratori S, Yasumoto A, Tanaka J, Shigematsu A, Yamamoto S, et al. : A retrospective analysis of allogeneic hematopoietic stem cell transplantation for adult T cell leukemia/lymphoma (ATL): clinical impact of graft-versus-leukemia/lymphoma effect. Biol Blood Marrow Transplant 14: 817-823, 2008. 10.1016/j.bbmt.2008.04.014 [DOI] [PubMed] [Google Scholar]
- 31.Tanosaki R, Uike N, Utsunomiya A, Saburi Y, Masuda M, et al. : Allogeneic hematopoietic stem cell transplantation using reduced-intensity conditioning for adult T cell leukemia/lymphoma: impact of antithymocyte globulin on clinical outcome. Biol Blood Marrow Transplant 14: 702-708, 2008. 10.1016/j.bbmt.2008.03.010 [DOI] [PubMed] [Google Scholar]
- 32.Choi I, Tanosaki R, Uike N, Utsunomiya A, Tomonaga M, et al. : Long-term outcomes after hematopoietic SCT for adult T-cell leukemia/lymphoma: results of prospective trials. Bone Marrow Transplant 46: 116-118, 2011. 10.1038/bmt.2010.92 [DOI] [PubMed] [Google Scholar]
- 33.Nakamura T, Oku E, Nomura K, Morishige S, Takata Y, et al. : Unrelated cord blood transplantation for patients with adult T-cell leukemia/lymphoma: experience at a single institute. Int J Hematol 96: 657-663, 2012. 10.1007/s12185-012-1177-8 [DOI] [PubMed] [Google Scholar]
- 34.Fukushima T, Itonaga H, Moriuchi Y, Yoshida S, Taguchi J, et al. : Feasibility of cord blood transplantation in chemosensitive adult T-cell leukemia/lymphoma: a retrospective analysis of the Nagasaki Transplantation Network. Int J Hematol 97: 485-490, 2013. 10.1007/s12185-013-1307-y [DOI] [PubMed] [Google Scholar]
- 35.Bazarbachi A, Cwynarski K, Boumendil A, Finel H, Fields P, et al. : Outcome of patients with HTLV-1-associated adult T-cell leukemia/lymphoma after SCT: a retrospective study by the EBMT LWP. Bone Marrow Transplant 49: 1266-1268, 2014. 10.1038/bmt.2014.143 [DOI] [PubMed] [Google Scholar]
- 36.Kato K, Choi I, Wake A, Uike N, Taniguchi S, et al. : Treatment of patients with adult T cell leukemia/lymphoma with cord blood transplantation: a Japanese nationwide retrospective survey. Biol Blood Marrow Transplant 20: 1968-1974, 2014. 10.1016/j.bbmt.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 37.Ishida T, Hishizawa M, Kato K, Tanosaki R, Fukuda T, et al. : Allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia-lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood 120: 1734-1741, 2012. 10.1182/blood-2012-03-414490 [DOI] [PubMed] [Google Scholar]
- 38.Kanda J, Hishizawa M, Utsunomiya A, Taniguchi S, Eto T, et al. : Impact of graft-versus-host disease on outcomes after allogeneic hematopoietic cell transplantation for adult T-cell leukemia: a retrospective cohort study. Blood 119: 2141-2148, 2012. 10.1182/blood-2011-07-368233 [DOI] [PubMed] [Google Scholar]
- 39.Shigematsu A, Kobayashi N, Yasui H, Shindo M, Kakinoki Y, et al. : High level of serum soluble interleukin-2 receptor at transplantation predicts poor outcome of allogeneic stem cell transplantation for adult T cell leukemia. Biol Blood Marrow Transplant 20: 801-805, 2014. 10.1016/j.bbmt.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 40.Kawada H, Yoshimitsu M, Nakamura D, Arai A, Hayashida M, et al. : A retrospective analysis of treatment outcomes in adult T cell leukemia/lymphoma patients with aggressive disease treated with or without allogeneic stem cell transplantation: A single-center experience. Biol Blood Marrow Transplant 21: 696-700, 2015. 10.1016/j.bbmt.2014.12.020 [DOI] [PubMed] [Google Scholar]
- 41.Gill PS, Harrington W, Jr, Kaplan MH, Ribeiro RC, Bennett JM, et al. : Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med 332: 1744-1748, 1995. 10.1056/NEJM199506293322603 [DOI] [PubMed] [Google Scholar]
- 42.Hermine O, Bouscary D, Gessain A, Turlure P, Leblond V, et al. : Brief report: treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N Engl J Med 332: 1749-1751, 1995. 10.1056/NEJM199506293322604 [DOI] [PubMed] [Google Scholar]
- 43.White JD, Wharfe G, Stewart DM, Maher VE, Eicher D, et al. : The combination of zidovudine and interferon alpha-2B in the treatment of adult T-cell leukemia/lymphoma. Leuk Lymphoma 40: 287-294, 2001. 10.3109/10428190109057927 [DOI] [PubMed] [Google Scholar]
- 44.Matutes E, Taylor GP, Cavenagh J, Pagliuca A, Bareford D, et al. : Interferon alpha and zidovudine therapy in adult T-cell leukaemia lymphoma: response and outcome in 15 patients. Br J Haematol 113: 779-784, 2001. 10.1046/j.1365-2141.2001.02794.x [DOI] [PubMed] [Google Scholar]
- 45.Hodson A, Crichton S, Montoto S, Mir N, Matutes E, et al. : Use of zidovudine and interferon alfa with chemotherapy improves survival in both acute and lymphoma subtypes of adult T-cell leukemia/lymphoma. J Clin Oncol 29: 4696-4701, 2011. 10.1200/JCO.2011.35.5578 [DOI] [PubMed] [Google Scholar]
- 46.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, et al. : Identification of a primary target of thalidomide teratogenicity. Science 327: 1345-1350, 2010. 10.1126/science.1177319 [DOI] [PubMed] [Google Scholar]
- 47.Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, et al. : Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 26: 2326-2335, 2012. 10.1038/leu.2012.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morschhauser F, Fitoussi O, Haioun C, Thieblemont C, Quach H, et al. : A phase 2, multicentre, single-arm, open-label study to evaluate the safety and efficacy of single-agent lenalidomide (Revlimid) in subjects with relapsed or refractory peripheral T-cell non-Hodgkin lymphoma: the EXPECT trial. Eur J Cancer 49: 2869-2876, 2013. 10.1016/j.ejca.2013.04.029 [DOI] [PubMed] [Google Scholar]
- 49.Toumishey E, Prasad A, Dueck G, Chua N, Finch D, et al. : Final report of a phase 2 clinical trial of lenalidomide monotherapy for patients with T-cell lymphoma. Cancer 121: 716-723, 2015. 10.1002/cncr.29103 [DOI] [PubMed] [Google Scholar]
- 50.Ishida T, Fujiwara H, Nosaka K, Taira N, Abe Y, et al. : Multicenter Phase II Study of Lenalidomide in Relapsed or Recurrent Adult T-Cell Leukemia/Lymphoma: ATLL-002. J Clin Oncol, 2016. 10.1200/JCO.2016.67.7732 [DOI] [PubMed] [Google Scholar]
- 51.Minucci S, Pelicci PG: Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6: 38-51, 2006. 10.1038/nrc1779 [DOI] [PubMed] [Google Scholar]
- 52.Tsukasaki K, Tobinai K: Human T-cell lymphotropic virus type I-associated adult T-cell leukemia-lymphoma: new directions in clinical research. Clin Cancer Res 20: 5217-5225, 2014. 10.1158/1078-0432.CCR-14-0572 [DOI] [PubMed] [Google Scholar]
- 53.Onizuka M, Ando K, Yoshimitsu M, Ishida T, Yoshida S, et al. : Oral HDAC Inhibitor HBI8000 in Japanese Patients with Non-Hodgkin Lymphoma (NHL): Phase I Safety and Efficacy Results. Blood 128: 1827-1827, 2016. [abstract] [Google Scholar]
- 54.Rao SP, Sancho J, Campos-Rivera J, Boutin PM, Severy PB, et al. : Human peripheral blood mononuclear cells exhibit heterogeneous CD52 expression levels and show differential sensitivity to alemtuzumab mediated cytolysis. PLoS One 7: e39416, 2012. 10.1371/journal.pone.0039416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma K, Janik JE, O’Mahony D, Stewart D, Pittaluga S, et al. : Phase II Study of Alemtuzumab (CAMPATH-1) in Patients with HTLV-1-Associated Adult T-cell Leukemia/lymphoma. Clin Cancer Res 23: 35-42, 2017. 10.1158/1078-0432.CCR-16-1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wen B, Zhang M, Dilillo D, Ravetch JV, Waldmann TA: Interleukin-15 enhances rituximab-dependent cytotoxicity ex vivo and in vivo against a mouse lymphoma expressing human CD20. Cancer Res 75: 1332-1332, 2015. [abstract] 10.1158/1538-7445.AM2015-133225634213 [DOI] [Google Scholar]
- 57.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, et al. : Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 30: 2183-2189, 2012. 10.1200/JCO.2011.38.0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, et al. : Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol 30: 2190-2196, 2012. 10.1200/JCO.2011.38.0402 [DOI] [PubMed] [Google Scholar]
- 59.Horwitz SM, Advani RH, Bartlett NL, Jacobsen ED, Sharman JP, et al. : Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood 123: 3095-3100, 2014. 10.1182/blood-2013-12-542142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fanale MA, Horwitz SM, Forero-Torres A, Bartlett NL, Advani RH, et al. : Four-Year Survival and Durability Results of Brentuximab Vedotin in Combination with CHP in the Frontline Treatment of Patients with CD30-Expressing Peripheral T-Cell Lymphomas. Blood 128: 2993-2993, 2016. [abstract] [Google Scholar]
- 61.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, et al. : Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32: 1020-1030, 2014. 10.1200/JCO.2013.53.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443-2454, 2012. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, et al. : PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372: 311-319, 2015. 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyoshi H, Kiyasu J, Kato T, Yoshida N, Shimono J, et al. : PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood 128: 1374-1381, 2016. 10.1182/blood-2016-02-698936 [DOI] [PubMed] [Google Scholar]
- 65.Sasaki D, Imaizumi Y, Hasegawa H, Osaka A, Tsukasaki K, et al. : Overexpression of Enhancer of zeste homolog 2 with trimethylation of lysine 27 on histone H3 in adult T-cell leukemia/lymphoma as a target for epigenetic therapy. Haematologica 96: 712-719, 2011. 10.3324/haematol.2010.028605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujikawa D, Nakagawa S, Hori M, Kurokawa N, Soejima A, et al. : Polycomb-dependent epigenetic landscape in adult T-cell leukemia. Blood 127: 1790-1802, 2016. 10.1182/blood-2015-08-662593 [DOI] [PubMed] [Google Scholar]