Abstract
Blastic plasmacytoid dendritic cell (pDC) neoplasm (BPDCN) is a relatively rare hematological malignancy with significantly complex clinicopathological features that are still unclear. This study aimed to analyze the clinicopathological data of BPDCN and evaluate immunohistochemical detection of minimal bone marrow (BM) involvement. In this study, we examined skin and BM lesions from 6 patients with BPDCN. Neoplastic cells tested positive for CD303 (polyclonal, 100%; monoclonal, 40%) in the skin lesions and for CD303 (polyclonal, 100%; monoclonal, 67%) in the BM clots. Although immunostaining of CD4, CD56, CD123, CD303, and TCLl detected minimal BM involvement in 3 patients, morphological identification was challenging in the BM clots stained with hematoxylin–eosin. In conclusion, our results demonstrate the significance of observing BM smears to detect neoplastic cells and that immunohistochemical examination, including CD303 antibodies, is useful to detect minimal BM involvement. This study is the first to report the expression of thymic stromal lymphopoietin (TSLP) and its receptor in BPDCN cells. Therefore, the TSLP/TSLP receptor axis may be associated with the proliferation of BPDCN, and consequently, the survival of patients.
Keywords: blastic plasmacytoid dendritic cell neoplasm, minimal bone marrow involvement, CD123, CD303, TSLP, TSLPR
INTRODUCTION
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a relatively rare hematological malignancy,1 accounting for 0.44% of all hematological malignancies and 0.7% of all cutaneous lymphomas.2 BPDCN is a clinically aggressive tumor derived from the precursors of plasmacytoid dendritic cells (pDC).1 It usually occurs at the age of 50–70 and is rare in pediatric patients; the ratio of male to female patients is 2.5–2.7:1, and the median survival is 12–14 months.1-4
The clinical features and evolution of BPDCN are rather homogenous and categorized into dermatopathic (>90% of cases) and leukemic patterns.5 The dermatopathic pattern is characterized by a deceptive indolent onset dominated by skin lesions, which is the prominent and only detectable clinical feature in nearly 50% of patients, followed by tumor dissemination. Conversely, the leukemic variant is characterized by an elevated white blood cell count, circulating neoplastic cells, and massive bone marrow (BM) infiltration. Pure leukemic presentation is very rare (7% of 756 cases) and is mostly associated with multiple skin lesions. Other manifestations are related with tumor infiltration into the lymph nodes (localized and generalized lymphadenopathy), spleen, and liver.
Notably, BPDCN can be precisely diagnosed by the immunohistochemical scoring system using CD4, CD56, and pDC-related markers, including CD123, CD303 (BDCA2/CLEC4), and T-cell leukemia/lymphoma 1 (TCL1). Furthermore, additional immunostaining of cutaneous lymphocyte-associated antigen (CLA), CD43, and terminal deoxynucleotidyl transferase (TdT) often facilitates BPDCN diagnosis.4,6-8
Previously, some prognostic factors for BPDCN, including age, tumor involvement at the time of diagnosis, immunophenotype, and genetic abnormality, have also been reported.3,5,7,9 Of note, early diagnosis is essential to determine the state of disease and devise an appropriate treatment plan. In particular, the detection of minimal BM involvement of BPDCN at the time of diagnosis may be crucial for treatment selection because high marrow blastosis is a poor prognostic factor for BPDCN.5
Recently, the association of thymic stromal lymphopoietin (TSLP) with tumor survival and progression of several kinds of tumors, such as breast, lung, gastric, pancreatic, and cervical cancers, and mycosis fungoides have been reported.10-12 The TSLP receptor (TSLPR) complex has also been found in pediatric B-cell acute lymphocytic leukemia.10 Although TSLPR expression is observed in multiple myeloma cell lines, TSLP does not affect the proliferation or drug resistance of multiple myeloma cells.13 In addition, non-neoplastic pDCs express TSLPR and interleukin-7 receptor.14
This study examined the clinicopathological features of 6 patients with BPDCN, and evaluated the immunohistochemical detection of minimal BM involvement,15 focusing on CD303 immunostaining. This is the first report of the expression of TSLP and TSLPR in BPDCN cells.
MATERIALS AND METHODS
Patients
In this study, we examined 6 patients who were diagnosed with BPDCN at Yamagata University Hospital and Yamagata City Hospital Saiseikan between 2008 and 2017 (Tables 1 and 2, respectively). BPDCN was pathologically diagnosed by skin biopsy and BM aspiration biopsy in 5 patients (cases 1, 3, 4, 5, and 6), and by BM aspiration biopsy alone in 1 patient (case 2). The diagnosis was further confirmed using the following two immunohistochemical criteria: (a) the scoring system of immunophenotype for CD123, CD303, CD304, CD4, CD56, MPO, CD79a, and CD3, excluding CD11c6; and (b) the expression of four markers among CD4, CD56, CD123, TCL1, and CD303.5
Table 1. Clinical features of the patients of BPDCN.
| No | Age/ Sex |
Neoplastic cell involvement at the time of diagnosis |
G-band | Treatment | Outcome & neoplastic cell involvement at the end stage |
|---|---|---|---|---|---|
| 1 | 73/ M |
Skin, BM (Minimal involvement) | Skin
sample: 96,XXYY,i(1)(q10),+8,+8,+8,+8,inc(4) |
Hyper CVAD/MA | Dead, 38 months Skin, BM, LN, PB, cerebrum, cerebellum |
| 2 | 83/ M |
Skin, BM (Leukemic), LN, PB, spleen | BM
sample: 45,X,-Y,add(6)(q21),add(9)(q22?), add(17)(q11.2)[12]/46,XY[4] |
Supportive therapy | Dead, 5 months Skin, BM, LN, PB |
| 3 | 73/ M |
Skin, BM (Leukemic), LN, PB | BM
sample: 46,XY,add(11)(q13),?der(20)t(11;20)(q13;q11.2)[9]/45-46,XY,add(11)(q13), ?der(20)t(11;20)(q13;q11.2)[cp4]/46,XY |
Hyper CVAD/MA Radiation therapy to the head lesion of skin |
Dead, 15 months Autopsy; Skin, BM, LN, PB, heart, lung, liver, spleen, kidney, pancreas, adrenal gland, thyroid gland, testis, esophagus, urinary bladder, peritoneum |
| 4 | 37/ M |
Skin, BM (Minimal involvement), PB | BM sample: Normal karyotype |
Hyper CVAD/MA Allo-HSCT |
Alive, 34 months (on follow-up) |
| 5 | 35/ M |
Skin, BM (Minimal involvement) | BM sample: Normal karyotype |
Hyper CVAD/MA Auto-PBSCT |
Alive, 75 months (The follow-up was ended at this time due to the patient’s opinion) |
| 6 | 74/ M |
Skin, BM (Leukemic), PB | ND | CHOP | Alive, 4 months (on follow up) |
M, male; BM, bone marrow; LN, lymph node; PB, peripheral blood; CVAD, cyclophosphamide, doxorubicin, vincristine and dexamethasone; MA, methotrexate and cytarabine; Allo-HSCT, allogeneic hematopoietic stem cell transplantation; Auto-PBSCT, autologous peripheral blood stem cell transplantation; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisolone, ND; no data.
Table 2. Immunohistochemical results of skin and bone marrow (BM) lesions at the times of diagnosis.
| Antigen | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin | BM (M) |
BM (L) |
Skin | BM (L) |
Skin | BM (M) |
Skin | BM (M) |
Skin | BM (L) |
||||||
| CD4 | + | + | + | + | + | + | + | - | UC | + | + | |||||
| CD56 | + | - | - | + | + | + | + | + | - | + | + | |||||
| CD123 | + | + | + | + | + | + | + | + | + | + | + | |||||
| CD303 (polyclonal) | + | + | + | + | +† | + | + | + | + | + | + | |||||
| CD303 (124B3.13) | - | - | + | + | + | + | + | - | + | - | - | |||||
| CD304 | + | UC | + | + | ND | + | UC | + | UC | - | - | |||||
| TCL1 | - | UC | + | + | +† | - | UC | + | + | + | + | |||||
| CLA | +* | +* | + | + | ND | +* | UC | - | UC | +* | +* | |||||
| CD43 | - | - | - | - | ND | + | - | - | - | + | + | |||||
| TdT | - | - | - | - | - | f+ | + | + | + | - | - | |||||
| CD83 | - | - | - | - | ND | - | - | - | - | - | - | |||||
| TSLP | + | UC | + | + | ND | + | UC | + | UC | + | + | |||||
| TSLP receptor | + | UC | + | + | ND | + | UC | + | UC | + | + | |||||
| Cyclin A index | 35.8±0.6 | UC | 12.4±6.9 | 1.1±0.2 | ND | 37.5±3.8 | UC | 30.6±8.3 | UC | 15.2±2.7 | 6.6±1.8 | |||||
| Cyclin B index | 8.2±0.9 | ND | 4.8±2.7 | 0.5±0.3 | ND | 23.7±4.4 | ND | 11.8±2.4 | ND | ND | ND | |||||
| MIB 1 index | 71.1±4.6 | UC | 66.6±6.9 | 21.0±2.4 | ND | 79.7±2.0 | UC | 71.8±6.0 | UC | 23.6±4.0 | 21.4±4.6 | |||||
M, minimal involvement; L, leukemic; TSLP, thymic stromal lymphopoietin; -, negative; f+, focal positive; +, diffuse positive (less than 30% of neoplastic cells); ND, no data; UC, uncountable due to other hematopoietic cells staining; *, a small-dot paranuclear pattern of positive immunostaining; †, only positive for BM smear.
Tissue and cytological specimens
We obtained skin (n = 7) and BM (n = 8) tissue specimens from patients with BPDCN at the time of diagnosis (skin, n = 5; BM, n = 6; Table 2) and latest biopsy (skin, n = 2; BM, n = 2; case 1 and 3). Systemic organs, pleural effusion, and ascites were collected and examined from an autopsy case. In case 2, the skin lesion was not biopsied. Resected tissue specimens, including non-neoplastic tonsils and thymuses, were obtained from patients with chronic tonsillitis and neonatal autopsy cases, respectively. Then, tissues were fixed in 10% neutral buffered formalin for 6–12 hours at room temperature, embedded in paraffin, and used for hematoxylin–eosin (H&E) staining and immunohistochemistry as described previously.16 In addition, we examined touch imprint specimens (case 1) and BM smears (all cases) by Papanicolaou and Giemsa staining. BM smear specimens were dried and stored in a deep freezer. Before use in immunocytochemistry, they were fixed in 10% neutral buffered formalin for 1 hour at room temperature.
Immunostaining
Immunostaining was performed using antibodies against CD4 (4B12; mouse IgG1κ; Nichirei, Tokyo, Japan), CD56 (123C3; mouse IgG1κ; DAKO, Glostrup, Denmark), CD123 (BR4MS; mouse IgG2b; Novocastra, Leica Biosystems, Nussloch, Germany), CD303 (BDCA-2/CLEC4C, Human Protein Atlas Number HPA029432, rabbit IgG, polyclonal; Sigma-Aldrich, St. Louis, MO), CD303 (124B3.13; mouse IgG1; Dendritics SAS, Lyon, France), CD304 (BDCA-4/Neuropilin 1, EPR3113; rabbit IgG; Abcam, Cambridge, UK), TCL1 (27D6/20; mouse IgG1; Medical & Biological Laboratories, Nagoya, Japan), CLA (CD162, HECA-452; rat IgMκ; BD, Franklin Lakes, NJ), CD43 (DF-T1; mouse IgG1κ; DAKO), TdT (SEN28; mouse IgG2a; Nichirei), CD83 (1H4b; mouse IgG1κ; Novocastra, Leica Biosystems), TSLP (rabbit IgG, polyclonal; Abcam, Cambridge, UK), TSLPR (goat IgG, polyclonal; R&D Systems, Minneapolis, MN), CD3 (PS1; mouse IgG2a; Nichirei), CD79a (JCB117; mouse IgG1κ; Nichirei), myeloperoxidase (MPO) (59A5; mouse IgG2bκ; Novocastra, Leica Biosystems), cyclin A (6E6; mouse IgG1κ; Novocastra, Leica Biosystems), cyclin B1 (7A9; mouse IgG1κ; Novocastra, Leica Biosystems), and Ki-67 (MIB-1; mouse IgG1κ; DAKO). Heat-induced antigen retrieval was performed with chemicals such as citric acid (pH 6; LSI Medience, Tokyo, Japan), EDTA (Epitope Retrieval Solution pH8; Leica Biosystems), EDTA (Antigen Retrieval Solution pH9; Nichirei), and 0.05% citraconic anhydride solution (pH7.4; Immunosaver; Nissin EM, Tokyo, Japan). In addition, enzyme-induced antigen retrieval treatment was performed with proteases such as trypsin (Difco Trypsin 250; BD) and proteinase K (Proteinase K; DAKO). Tissue sections were incubated with primary antibodies at 4°C overnight. Histofine SAB-PO (MULTI) kit (Nichirei), Dako EnVision+ System-HRP Anti-Mouse, and Anti-Rabbit (DAKO) were used as secondary antibodies. Positive reactions were detected as brown coloration with 3, 3'-diaminobenzidine tetrahydrochloride. Tonsils were used as a positive control for CD303 (polyclonal)/(124B3.13), whereas thymuses were used as a positive control for TSLP and TSLPR. In contrast, FLEX Universal Negative Control Mouse and Rabbit (DAKO) were used as the negative controls for primary antibodies.
Reactivity was defined as follows; -, negative; f+, focal positive; and +, diffusely positive (<30% of neoplastic cells). Positive neoplastic cells for cyclin A, cyclin B, and Ki-67 were counted in a total of ≥9,000 neoplastic cells, and each labeling index (LI) was estimated as a percentile.
Minimal BM involvement
Although BPDCN cells are often lymphoblast-like, they are occasionally myeloid-like.8,17 Cytologically, neoplastic cells had round to oval nuclei, finely dispersed chromatin, prominent nucleoli, scant pale-blue agranular cytoplasm, occasional cytoplasmic microvacuoles, and pseudopodia-shaped cytoplasmic extensions.4 In this study, minimal BM involvement was identified as small clusters of neoplastic cells expressing CD123 and/or CD303, which was not easily recognized in BM clots stained by H&E (case 1, 4 & 5).
Statistical analysis
The spearman’s rank correlation was used to compare the LIs of cyclin A, cyclin B, and Ki-67. The spearman’s coefficient was denoted by rs. P < 0.05 was considered significant for each marker.
Research ethics
This study was approved by the Research Ethics Committee (H27-59) of Yamagata University Faculty of Medicine (Yamagata, Japan).
RESULTS
Clinical findings
Table 1 summarizes the clinical results of this study. All 6 patients enrolled in this study were males aged 35–83 (mean age: 62.5) years. Of the 6 patients, 4 exhibited nodular skin lesions of approximately 5 cm (cases 1, 2, 3, and 6), and the other 2 exhibited small lesions, such as erythema, up to several centimeters (cases 4 and 5). Three patients (cases 2, 3, and 6) were at the leukemic stage at the time of diagnosis. In G-banding, excluding case 6, 3 patients (cases 1, 2, and 3) had abnormal karyotypes, whereas the other 2 patients had normal karyotypes. Four patients (cases 1, 3, 4, and 5) were treated with hyper-CVAD (cyclophosphamide, doxorubicin, vincristine, and dexamethasone)/MA (methotrexate and cytarabine) and CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone), whereas 1 patient (case 2) was treated with only supportive therapy as he was at the end stage of the disease. After chemotherapy, cases 4 and 5 underwent allogenic hematopoietic stem cell transplantation (ASCT) and autologous peripheral blood SCT, respectively. Both cases 4 and 5 have been alive without relapse for 34 and 75 months, respectively. Only case 6 developed hepatocellular carcinoma.
Histological and cytological findings
Seven skin samples (cases 1, 3, 4, 5, and 6) demonstrated diffuse dermal and subcutaneous infiltration with a grenz zone of medium-sized cells having a high nucleus/cytoplasm ratio with relatively scant cytoplasm and finely dispersed chromatin. Morphologically, neoplastic cells were round, oval, and spindle shaped (Fig. 1a). In the autopsy of case 3, blastic involvement was observed in the skin, BM, peripheral blood, and other systemic organs (Table 1).
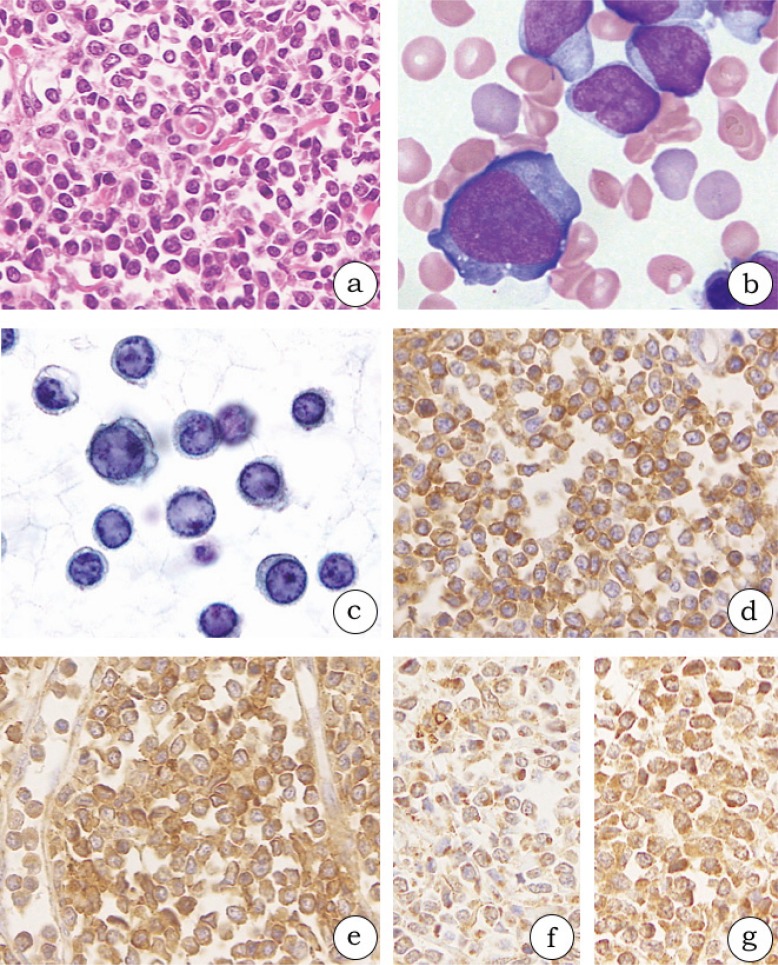
Fig. 1.
Histological and cytological findings in blastic plasmacytoid dendritic neoplasms (1a-g, case 3). (1a) The oval to spindle neoplastic cells diffusely proliferate in the dermis. (1b) The neoplastic cells have scant to modest pale-blue agranular cytoplasm and extend cytoplasmic pseudopodial projections on bone marrow smears. (1c) Neoplastic cells in the pleural effusion specimen have ground glass chromatin, a round nucleus, and some small nucleoli. (1d-g) The neoplastic cells in formalin-fixed and paraffin-embedded specimens from skin lesions are diffusely positive for CD123 (1d), CD303 (polyclonal) (1e), thymic stromal lymphopoietin (1f), and thymic stromal lymphopoietin receptor (1g).
Among eight BM samples, three BM clots demonstrated leukemic marrow at the time of diagnosis (cases 2, 3, and 6) and one BM clot did so during autopsy (case 3). The morphology of neoplastic cells in the BM clot was nearly equivalent with that of cells in the skin lesion. The BM smear features were as follows: scant to modest pale-blue agranular cytoplasm, cytoplasmic microvacuoles, and pseudopodia-like shape (Fig. 1b). No neoplastic cells were identified in four BM clots stained by H&E at the time of diagnosis (cases 1, 4, and 5) or in one BM clot from the latest biopsy (case 1; described later). Although case 1 progressed to a leukemic stage, the BM sample at the leukemic stage was not biopsied.
Neoplastic cells had a moderate amount of cytoplasm and resembled pseudopodia in shape in the touch imprint specimen (case 1), and had round nuclei with some distinct nucleoli in the pleural effusion and ascites specimens (case 3; Fig. 1c). With regard to cytology, the morphological features of neoplastic cells identified using Papanicolaou staining were consistent with those of BM smears observed using Giemsa staining.
Immunostaining
We examined antigen retrieval with heat-induced (e.g., pH6, pH8, pH9, and citraconic anhydride) and enzyme-induced (e.g., trypsin and protease K) factors for CD303 antibodies. The best results for the CD303 (polyclonal) antibody and CD303 (124B3.13) antibody were observed at pH 8 and pH 9, respectively. More than 30% positive neoplastic cells was considered as positive at the time of diagnosis (Table 2). All 6 patients satisfied the two immunohistochemical criteria of BPDCN.5,6 Neoplastic cells of BPDCN were positive for CD123 (Fig. 1d) in all. In addition, neoplastic cells were positive for CD303 (polyclonal; 100%)/(124B3.13; 40%) in the skin lesions and CD303 (polyclonal; 100%)/(124B3.13; 67%) in the BM clots (Fig. 1e). Furthermore, they were positive for CD4 (83%), CD56 (83%), CD304 (83%), TCL1 (67%), CLA (83%), CD43 (33%), TdT (33%), TSLP (100%; Fig. 1f), and TSLP-R (100%; Fig. 1g). In 4 patients (cases 1, 3, 4, and 6), CLA was positive in a paranuclear dotted pattern as reported in a previous study.18 Conversely, CD3, CD79a, CD83, and MPO were negative in all. The immunophenotype of neoplastic cells at the latest biopsy (cases 1 and 3) was similar with that at the time of diagnosis. As one BM clot at the time of diagnosis was not sufficient for examination (case 3), CD123 was expressed on cell membranes (Fig. 2a), CD303 (polyclonal) on the cell membrane and cytoplasm (Fig. 2b), and TCL1 in the nucleus and cytoplasm of neoplastic cells on the BM smear (Fig. 2c).
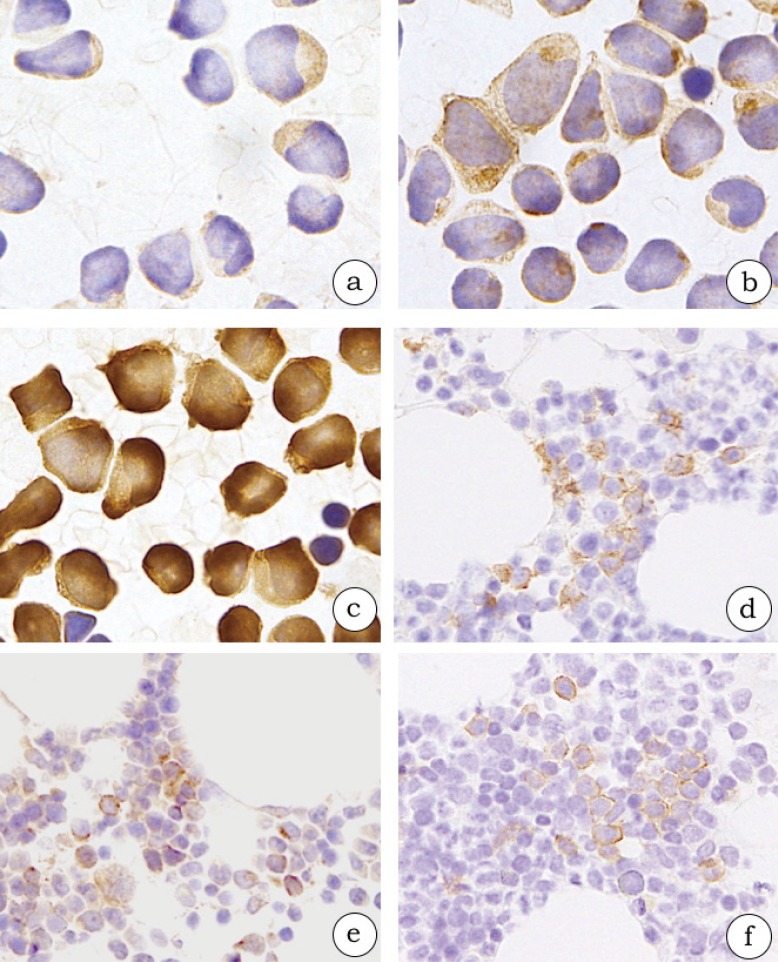
Fig. 2.
Bone marrow (BM) involvement of blastic plasmacytoid dendritic neoplastic cells (2a-c, case 3; 2d-f, case 4). The neoplastic cells in BM smears are diffusely positive for CD123 (2a), CD303 (polyclonal) (2b), and TCL1 (2c) in a leukemic case. The neoplastic cells in BM clots are positive for CD123 (2d), CD303 (polyclonal) (2e), and CD303 (124B3.13) (2f) in a minimal BM involvement case.
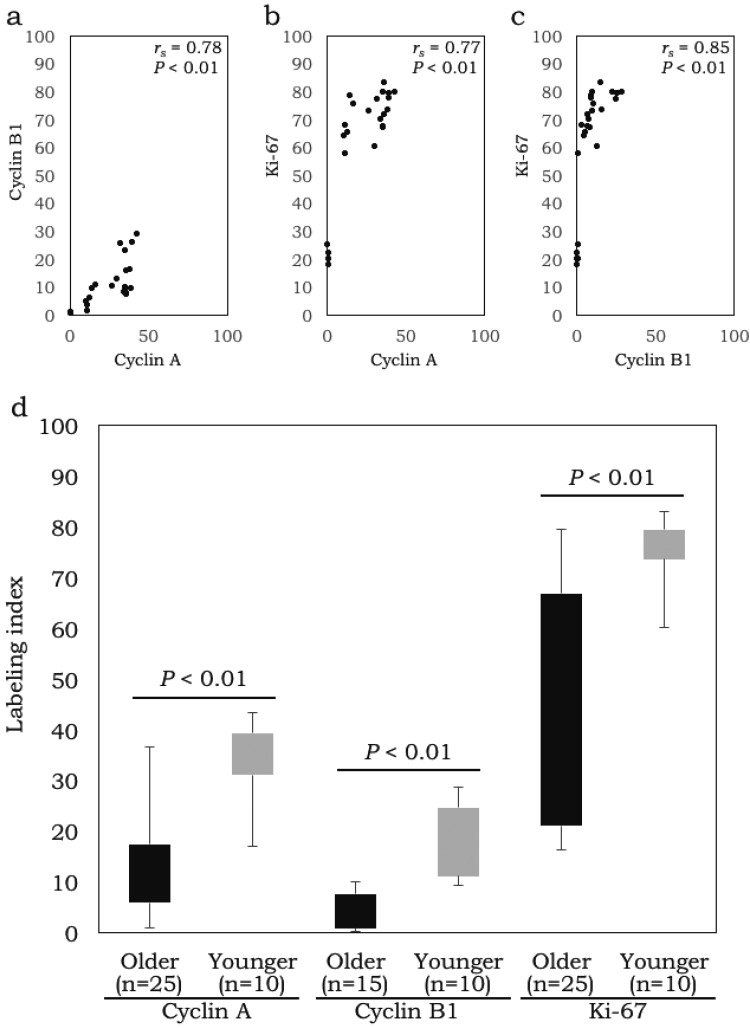
Cyclin A LIs were positively correlated with cyclin B1 LIs (rs = 0.78; P < 0.01; n = 25) and Ki-67 LIs (rs = 0.77; P < 0.01; n = 25), whereas cyclin B1 LIs were positively correlated with Ki-67 LIs (rs = 0.85; P < 0.01; n = 25) (Fig. 3a-c). Furthermore, younger patients (≤ 40 years; cases 4 and 5) had higher LIs [cyclin A LI (34.1% ± 7.7%; n = 10), cyclin B1 LI (17.7% ± 7.3%; n = 10), and Ki-67 LI (75.7% ± 6.3%; n = 10)] than older patients [cases 1, 2, 3, and 6; cyclin A LI (14.2% ± 12.2%; n = 25), cyclin B1 LI (4.5% ± 3.7%; n = 15; excluding case 6), and Ki-67 LI (40.7% ± 23.9%; n = 25; P < 0.01)] (Fig. 3d).
Fig. 3.
Correlation of cyclin A, cyclin B1, and Ki-67 labeling indices (LIs) (3a-c), and relationship of LIs in younger patients and in older patients (3d). Cyclin A LIs were positively correlated with cyclin B1 LIs and Ki-67 LIs, whereas cyclin B1 LIs were positively correlated with Ki-67 LIs (3a-c). Younger patients (≤ 40 years; cases 4 and 5) had higher LIs than older patients (3d).
Minimal BM involvement
Minimal BM involvement was observed in 3 patients (cases 1, 4, and 5), excluding the 3 leukemic patients (cases 2, 3, and 6; Tables 1 and 2). Three BM clots at the time of diagnosis exhibited normocellular marrow in which neoplastic cells was not easily detected using H&E staining. However, neoplastic cells with morphological characteristics of BPDCN were scattered on BM smears as mentioned earlier. In an immunohistochemical re-evaluation of the BM clots, neoplastic cells were positive for CD123 (Fig. 2d; Table 2). Regarding the other BPDCN markers, CD303 (polyclonal) was expressed in 3 patients (cases 1, 4, and 5; Fig. 2e), CD303 (124B3.13) in 2 (cases 4 and 5; Fig. 2f), CD4 in 2 (cases 1 and 4), CD56 in 1 (case 4), and TCL1 in 1 (case 5). Of these five markers, CD4 and TCL1 were also detected in hematopoietic cells other than BPDCN cells. In addition, CD123 was expressed on the cell membrane (Fig. 2d), CD303 (polyclonal) on the cell membrane and cytoplasm (Fig. 2e), and CD303 (124B3.13) on the inner part of the cell membrane of neoplastic cells (Fig. 2f).
DISCUSSION
Although the immunophenotype of immature pDC is CD303++CD304++CD83-CD86++CD11c- and that of mature pDC is CD303+/-CD304+++CD83+CD86+++CD11c+/-.19 Facchetti F, et al. suggested that BPDCN could be precisely diagnosed when four antigens among CD4, CD56, CD123, CD303, and TCLl were expressed by neoplastic cells.5 Of these markers, CD123 and CD303 were included in the scoring system for BPDCN due to their relative specificity.6 However, occasional CD123, CD303, and TCL1 expression was noted in myeloid and myelomonocytic leukemia with skin involvement.4 Hwang K, et al. demonstrated the effectiveness of immunohistochemistry for CD4, CD56, and CD123 in detecting minimal BM involvement by BPDCN.15 Their method of confirming minimal BM involvement was to detect positivity for CD4, CD56, and CD123 on clustered neoplastic cells in morphologically normal BM confirmed by BM smear, biopsy, and BM clot. In this study, CD303 (polyclonal) and CD303 (124B3.13) immunostaining was examined. Our results demonstrated different positive rates between CD303 (polyclonal) and CD303 (124B3.13) antibodies. CD303 is a type II transmembrane glycoprotein that belongs to the C-type lectin superfamily and is the most specific marker for human pDC.20 CD303 consists of a single extracellular carbohydrate recognition domain, a transmembrane region, and a short cytoplasmic domain.20 In addition, the CD303 (polyclonal) antibody recognizes the part of the carbohydrate recognition domain that is conserved between the molecules of the same family, such as CD209 (DC-SIGN) and CD367 (DCIR/CLEC4A), whereas the CD303 (124B3.13) antibody recognizes the intracytoplasmic part of the molecule confirmed by each manufacturer and the designed sequence.21 In this study, the staining pattern of the CD303 (polyclonal) antibody was the cell membrane and cytoplasm (Figs. 1e and 2b, and e), and that of the CD303 (124B3.13) antibody was the inner part of the cell membrane (Fig. 2f). Fixation agents, such as buffered formalin, mask antigens. It is possible that the CD303 (polyclonal) antibody recognizes more antigen epitopes. Conversely, the CD303 (124B3.13) antibody recognizes the limited region 10–20 amino acid sequence.21 In this study, the best results for the CD303 (polyclonal) antibody and CD303 (124B3.13) antibody were achieved with antigen retrieval agents at pH 8 and pH 9, respectively. As the difference in regions recognized by the two antibodies may affect the positive rates and staining patterns, our results strongly recommend the use of CD303 markers together. Notably, immunostaining of CD4, CD56, CD123, and TCL1 should be considered because these markers also detect other hematopoietic cells. To detect the minimal BM involvement in the clot or for screening of neoplastic cells on BM smears, it is essential to consider the morphology of BPDCN such as scant pale-blue agranular cytoplasm, cytoplasmic microvacuoles, and pseudopodia-like shape.4
Of the cell markers, cyclin A and B1 LIs were positively correlated with Ki-67 LI in this study. Cyclin A (S-G2 phase) and cyclin B (M phase)22 may be alternatives to Ki-67 (non-cycle-specific), and reliably evaluate the proliferation potential of BPDCN because these markers are less susceptible to genomic abnormalities frequently associated with genomic loss involving tumor-suppressor genes or genes related with the Gl/S transition that have been reported in BPDCN.5 Cyclin A and cyclin B1 LIs were lower than the Ki-67 LI with a 67% cut off,7 and more countable than Ki-67 LI.
Previously, some prognostic factors of BPDCN were reported.3,5,7,9 Age ≤ 40 years, limited infiltration into the skin, high TdT and CD303 expression, and high MIB-1 index (Ki-67 LI; ≥67%) are considered as good prognostic factors.3,7,9 Poor prognostic factors include high marrow or peripheral blood blastosis, low TdT and CD303 expression, CDKN2A/CDKN2B deletions, and mutations in DNA methylation pathway genes.5 Julia F, et al. suggested that patients with a very high proliferative index have a better response to antimitotic chemotherapy, which may prolong their survival.7 In this study, 2 younger (≤ 40 years of age) patients (cases 4 and 5) with TdT expression and high Ki-67 LI have survived longer after chemotherapy and SCT (Tables 1 and 2). Of note, ASCT after chemotherapy improves the prognosis.23 Eligible patients who achieve a complete remission after second-line therapies are recommended to undergo ASCT.2 Recently, molecular-targeted therapy for BPDCN, the immunotoxin SL-401 targeting the interleukin-3 receptor alpha (CD123), was reported to be a prospective therapy.24,25 Although no specific karyotype abnormalities were found in BPDCN, complex chromosomal aberrations of 5q (72%), 12p (64%), 13q (64%), 6q (50%), 15q (43%), and 9 (28%) were reported.5 Three dead patients (cases 1, 2, and 3; Table 1) had chromosomal aberrations of 1q (case 1), 6q (case 2), 9q (case 2), 11q (case 3), 17q (case 2), and 20q (case 3), and these should be investigated as prognostic factors.
We firstly demonstrated the expression of TSLP in BPDCN. BPDCN may proliferate via TSLP–TSLPR and IL-7R autocrine pathways because unstimulated pDCs expressed TSLPR and IL-7R, a receptor for TSLP.14 The TSLP–TSLPR axis activates multiple signaling transduction pathways, including the JAK–STAT pathway and the PI-3 kinase pathway.26 Furthermore, TSLP in BPDCN cells may also regulate Th2 polarization escaping from tumor-associated macrophages because TSLP induces Th2 response via myeloid dendritic cells.10
In conclusion, the following three steps are necessary to detect minimal BM involvement of BPDCN at the time of diagnosis: (a) precise diagnosis of skin lesions for BPDCN by immunostaining; (b) morphological examination of BM smears, paying particular attention to scant to modest pale-blue agranular cytoplasm, cytoplasmic microvacuoles, and pseudopodia-like shape; and (c) immunostaining of the BM clot using CD4, CD56, CD123, TCL1, and CD303 (including a polyclonal antibody) together.
ACKNOWLEDGEMENTS
The author is grateful to Hiromi Murata and Junko Takeda (Department of Pathological Diagnostics, Yamagata University Faculty of Medicine), and Makiko Sato (Division of Clinical Laboratory, Yamagata University Hospital) for their valuable assistance during this study.
Footnotes
CONFLICT OF INTEREST: All authors have read the journal’s policy on conflicts of interest and have none to declare.
REFERENCES
- 1.Facchetti F, Jones DM, Petrella T. Blastic plasmacytoid dendritic cell neoplasm. In: Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed, Lyon, International Agency for Research on Cancer. 2008; pp. 145-147. [Google Scholar]
- 2.Pagano L, Valentini CG, Grammatico S, et al. Blastic plasmacytoid dendritic cell neoplasm: diagnostic criteria and therapeutical approaches. Br J Haematol. 2016; 174: 188-202. 10.1111/bjh.14146 [DOI] [PubMed] [Google Scholar]
- 3.Yu G, Wang W, Han Y, et al. Blastic plasmacytoid dendritic cell neoplasm presenting with a cutaneous tumor alone as the first symptom of onset: A case report and review of literature. Oncol Lett. 2015; 9: 819-821. 10.3892/ol.2014.2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Z, Zhou J, Bentley G. Blastic plasmacytoid dendritic cell neoplasm: report of a case presenting with lung and central nervous system involvement and review of the literature. J La State Med Soc. 2014; 166: 2-9. [PubMed] [Google Scholar]
- 5.Facchetti F, Cigognetti M, Fisogni S, et al. Neoplasms derived from plasmacytoid dendritic cells. Mod Pathol. 2016; 29: 98-111. 10.1038/modpathol.2015.145 [DOI] [PubMed] [Google Scholar]
- 6.Garnache-Ottou F, Feuillard J, Ferrand C, et al. Extended diagnostic criteria for plasmacytoid dendritic cell leukaemia. Br J Haematol. 2009; 145: 624-636. 10.1111/j.1365-2141.2009.07679.x [DOI] [PubMed] [Google Scholar]
- 7.Julia F, Dalle S, Duru G, et al. Blastic plasmacytoid dendritic cell neoplasms: clinico-immunohistochemical correlations in a series of 91 patients. Am J Surg Pathol. 2014; 38: 673-680. 10.1097/PAS.0000000000000156 [DOI] [PubMed] [Google Scholar]
- 8.Tsunoda K, Satoh T, Akasaka K, et al. Blastic plasmacytoid dendritic cell neoplasm: report of two cases. J Clin Exp Hematop. 2012; 52: 23-29. 10.3960/jslrt.52.23 [DOI] [PubMed] [Google Scholar]
- 9.Bekkenk MW, Jansen PM, Meijer CJ, et al. CD56+ hematological neoplasms presenting in the skin: a retrospective analysis of 23 new cases and 130 cases from the literature. Ann Oncol. 2004; 15: 1097-1108. 10.1093/annonc/mdh268 [DOI] [PubMed] [Google Scholar]
- 10.Lo Kuan E, Ziegler SF. Thymic stromal lymphopoietin and cancer. J Immunol. 2014; 193: 4283-4288. 10.4049/jimmunol.1400864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barooei R, Mahmoudian RA, Abbaszadegan MR, et al. Evaluation of thymic stromal lymphopoietin (TSLP) and its correlation with lymphatic metastasis in human gastric cancer. Med Oncol. 2015; 32: 217. 10.1007/s12032-015-0653-4 [DOI] [PubMed] [Google Scholar]
- 12.Tuzova M, Richmond J, Wolpowitz D, et al. CCR4+T cell recruitment to the skin in mycosis fungoides: potential contributions by thymic stromal lymphopoietin and interleukin-16. Leuk Lymphoma. 2015; 56: 440-449. 10.3109/10428194.2014.919634 [DOI] [PubMed] [Google Scholar]
- 13.Nakajima S, Fujiwara T, Ohguchi H, et al. Induction of thymic stromal lymphopoietin in mesenchymal stem cells by interaction with myeloma cells. Leuk Lymphoma. 2014; 55: 2605-2613. 10.3109/10428194.2014.881478 [DOI] [PubMed] [Google Scholar]
- 14.Kopecka J, Rozkova D, Sediva A. Plasmacytoid DCs, exposed to TSLP in synergy with TLR ligands, acquire significant potential towards Th2 polarization. Med Sci Monit Basic Res. 2013; 19: 291-299. 10.12659/MSMBR.889791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang K, Park CJ, Jang S, et al. Immunohistochemical analysis of CD123, CD56 and CD4 for the diagnosis of minimal bone marrow involvement by blastic plasmacytoid dendritic cell neoplasm. Histopathology. 2013; 62: 764-770. 10.1111/his.12079 [DOI] [PubMed] [Google Scholar]
- 16.Meng HX, Li HN, Geng JS, et al. Decreased expression of follicular dendritic cell-secreted protein correlates with increased immunoglobulin A production in the tonsils of individuals with immunoglobulin A nephropathy. Transl Res. 2015; 166: 281-291. 10.1016/j.trsl.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 17.Reichard KK. Blastic plasmacytoid dendritic cell neoplasm. In Foucar K, Reichard KK, Wilson CS, et al. Diagnostic Pathology: Blood and Bone Marrow. Philadelphia, Lippincott Williams & Wilkins. 2011; chapter 9 pp. 208-213. [Google Scholar]
- 18.Petrella T, Meijer CJ, Dalac S, et al. TCL1 and CLA expression in agranular CD4/CD56 hematodermic neoplasms (blastic NK-cell lymphomas) and leukemia cutis. Am J Clin Pathol. 2004; 122: 307-313. 10.1309/0QPPAVTUPCV9UCLV [DOI] [PubMed] [Google Scholar]
- 19.Demoulin S, Herfs M, Delvenne P, et al. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol. 2013; 93: 343-352. 10.1189/jlb.0812397 [DOI] [PubMed] [Google Scholar]
- 20.Riboldi E, Daniele R, Parola C, et al. Human C-type lectin domain family 4, member C (CLEC4C/BDCA-2/CD303) is a receptor for asialo-galactosyl-oligosaccharides. J Biol Chem. 2011; 286: 35329-35333. 10.1074/jbc.C111.290494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jegouzo SA, Feinberg H, Dungarwalla T, et al. A Novel Mechanism for Binding of Galactoseterminated Glycans by the C-type CarbohydrateRecognition Domain in Blood Dendritic Cell Antigen 2. J Biol Chem. 2015; 290: 16759-16771. 10.1074/jbc.M115.660613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aleem E, Arceci RJ. Targeting cell cycle regulators in hematologic malignancies. Front Cell Dev Biol. 2015; 3: 16. 10.3389/fcell.2015.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roos-Weil D, Dietrich S, Boumendil A, et al. Stem cell transplantation can provide durable disease control in blastic plasmacytoid dendritic cell neoplasm: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2013; 121: 440-446. 10.1182/blood-2012-08-448613 [DOI] [PubMed] [Google Scholar]
- 24.Frankel AE, Woo JH, Ahn C, et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood. 2014; 124: 385-392. 10.1182/blood-2014-04-566737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angelot-Delettre F, Roggy A, Frankel AE, et al. In vivo and in vitro sensitivity of blastic plasmacytoid dendritic cell neoplasm to SL-401, an interleukin-3 receptor targeted biologic agent. Haematologica. 2015; 100: 223-230. 10.3324/haematol.2014.111740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong J, Sharma J, Raju R, et al. TSLP signaling pathway map: a platform for analysis of TSLPmediated signaling. Database (Oxford). 2014; bau007. 10.1093/database/bau007 [DOI] [PMC free article] [PubMed] [Google Scholar]