Abstract
Recent progress in anti-tumor therapy has revealed the significance of anti-tumor immune responses in tumor progression and clinical course in several kinds of malignant tumors. The draining lymph node is an important immune system component that contains a number of antigen-presenting cells, which induce rapid immune responses to foreign antigens. Current studies have shown that higher expression of CD169 on lymph node sinus macrophages is associated with the induction of anti-tumor immunity. In the present study, we searched for natural compounds that regulate the CD169-positive phenotype in macrophages to identify potential new anti-cancer agents targeting macrophage activation. Among 50 natural compounds, aculeatiside A, naringin, and onionin A significantly induced the CD169-positive phenotype in human monocyte-derived macrophages. These compounds also induced CD169 overexpression and secretion of inflammatory cytokines, including interleukin (IL)-1β and IL-12, in murine macrophages. Subcutaneous injection of aculeatiside A and naringin enhanced mRNA expression of IL-1β, IL12, and CD169 in regional lymph nodes in mice. These findings suggest aculeatiside A and naringin may enhance anti-tumor immune responses by inducing CD169-positive macrophages in lymph nodes.
Key words: : CD169, natural compounds, sinus macrophage, lymph node, anti-cancer immunity
INTRODUCTION
In the spleen and lymph nodes (LNs), immune responses are induced by the activation of lymphocytes and natural killer cells, which in turn are dependent on the activity of antigen-presenting cells (APCs), such as dendritic cells and macrophages. Draining LNs are located near the tumor nodule in many kinds of malignant tumors, and the LN sinus is filled with lymphatic fluid in which a large number of macrophages are found.1,2 Under normal conditions, LN sinus macrophages capture pathogens, however, they also capture abnormal antigens and debris derived from tumor tissues that flow into the lymphatic fluid in patients with malignant tumors. As such, the LN sinus is proactively associated with the induction of antigen-specific immune responses.3,4 It is well established that, together with dendritic cells, many macrophages are distributed in lymphoreticular organs such as the spleen and LNs, and those detected in the subcapsular sinus and the medullary sinus of LNs express CD169.5,6 CD169 is a type I lectin that specifically recognizes sialic acid-containing sugar chains and is involved in exosome capture and the immune response to exosomal antigens.7
In some malignant diseases, the LNs are thought to be important for anti-tumor immune responses because of the induction of cytotoxic T-lymphocytes (CTLs) into tumor tissue by APCs.8,9 Sinus macrophages in the LN engulf antigens via several receptors, including scavenger receptors, and present antigen-derived peptides to T- and B-lymphocytes.10,11 Asano et al. demonstrated that CD169-positive subcapsular sinus macrophages in LNs are preferentially involved in antigen presentation and the induction of CTLs, and these CD169-positive macrophages are more significant for anti-tumor immune responses than the CD169 antigen itself.9 Benhard et al. additionally found that macrophages generate CTLs that react to a broader range of epitopes than dendritic cells.12 CD169 has consequently been considered to be useful as a potential target for antigen delivery of vaccines.13-15 In contrast, Pucci et al. demonstrated that subcapsular sinus macrophages engulf melanoma-derived extracellular vesicles and subsequently suppress the induction of pro-tumor B-cells.16 They suggested that subcapsular sinus macrophages may act as a physical barrier to B-cell activation under specific circumstances. LN macrophage-mediated B-lymphocyte activation might thus be a novel target for anti-tumor immunotherapy.
Therefore, the induction of macrophage activation into the CD169-positive phenotype is a new strategy for anti-tumor therapy. In the present study, we searched for natural compounds that regulate the CD169-positive phenotype in macrophages in order to identify potential new anti-cancer agents targeting macrophage activation.
MATERIALS AND METHODS
Preparation of natural compounds
We selected 50 purified natural compounds known to have bioactive structures, such as flavonoid compounds, triterpenoid compounds, and steroid compounds, from our natural compound library.17 The purified natural compounds were dissolved in dimethyl sulfoxide (DMSO) to create 100-mM stock solutions.
Cells and cell culture conditions
Peripheral blood mononuclear cells were obtained from healthy volunteer donors. Informed written consent was obtained from all donors and experimental procedures were approved by Kumamoto University Review Board (#1169). CD14-positive monocytes were purified from peripheral blood mononuclear cells by positive selection via magnetic-activated cell sorting technology (Miltenyi Biotec, Bergisch Gladbach, Germany). The monocytes were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and GM-CSF (10 ng/ml, WAKO, Tokyo, Japan) for 7 days in order to differentiate them into macrophages.18 Resident peritoneal macrophages from mice (8–10 weeks of age) were obtained by peritoneal lavage using 6 ml of PBS. Cells were incubated in DMEM medium with 10% FBS.
Determination of the inducing effect of natural compounds on CD169 expression
Human monocyte-derived macrophages (5 × 104 cells per well of a 96-well plate) were incubated with natural compounds (30 µM) for 24 h, followed by the determination of CD169 expression by a cell enzyme-linked immunosorbent assay (Cell-ELISA).
Cell-ELISA
CD169 expression on human monocyte-derived macrophages (HMDMs) was evaluated using a Cell-ELISA. After cells were fixed with paraformaldehyde, each well of a 96-well plate was blocked with Block Ace (DS Pharma Biomedical, Osaka, Japan) and washed three times with PBS containing 0.05% Tween 20 (washing buffer). The wells were then incubated with anti-CD169 antibody (sc-53442, 0.1 µg/ml; Santa Cruz Biotechnology, Dallas, TX, USA) for 1 h. Thereafter, wells were washed with washing buffer three times and reacted with HRP-conjugated anti-mouse IgG antibody, followed by a reaction with ULTRASENSITIVE TMB (Cosmo Bio, Tokyo, Japan). The reaction was terminated by the addition of 1 M sulfuric acid, and the absorbance at 450 nm was read by a micro-ELISA plate reader.
Animals
C57BL/6N mice were obtained from CLEA Japan (Shizuoka, Japan). Mice were housed in a temperature-controlled room with a 12-h light/dark cycle under specific-pathogen-free conditions. All animal experiments were approved by the Ethics Committee for Animal Experiments of Kumamoto University (#28-003) and performed in accordance with the Guidelines for Animal Experiments of the laboratories.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using RNAiso Plus (Takara Bio Inc., Shiga, Japan). RNA was reverse-transcribed with a PrimeScript RT Reagent Kit (Takara Bio Inc.). RT-qPCR was then performed using Taq polymerase with SYBR Green fluorescence (Takara Bio Inc.) and an ABI PRISM 7300 Sequence Detector (Applied Biosystems, Foster City, CA, USA). The following primers were used for RT-qPCR: 1) IL-1β, sense 5′-TGACGGACCCCAAAAGATG-3′ and antisense 5′- GCGAGATTTGAAGCTGGATG-3′; 2) IL-12, sense 5′-AGCACCAGCTTCTTCATCAG-3′ and antisense 5′-CTTGAGGGAGAAGTAGGAATGG-3′; 3) TNF-α, sense 5′-CCAAAGGGATGAGAAGTTCC-3′ and antisense 5′-TCCACTTGGTGGTTTGCTAC-3′; 4) CD169, sense 5′-ACCTGCCCAGCTAACTCTCA-3′ and antisense 5′-CCTTCCAGCAGAAGTTCCAG-3′; and 5) β-actin, sense 5′-TTTCCAGCCTTCCTTCTTGG-3′ and antisense 5′-TGGCATAGAGGTCTTTACGGATG-3′.
Murine subcutaneous interferon (IFN)-α and natural compound administration model
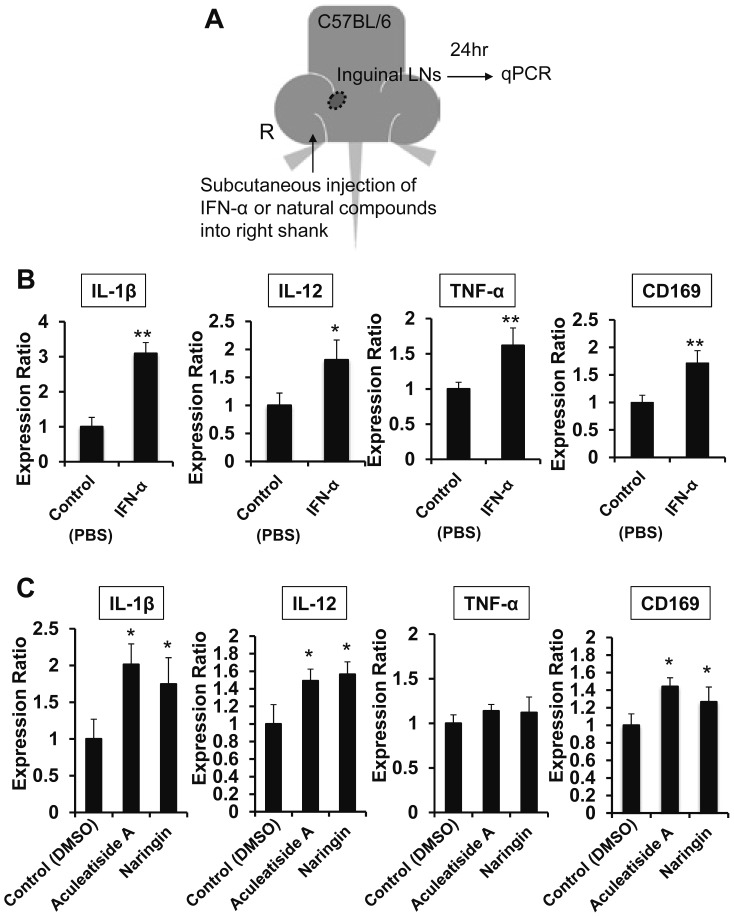
IFN-α and tested natural compounds were dissolved in 100 μl of PBS or DMSO and subcutaneously injected into the right shank of mice. The mice were sacrificed at 24 h after the injection, followed by the determination of CD169 and cytokine expression in inguinal LNs by real-time PCR analysis.
Statistical analyses
All data are representative of two or three independent experiments. Data are expressed as the mean ± standard deviation (SD). Differences between groups were examined to determine statistical significance using the Mann-Whitney U-test and a non-repeated measures analysis of variance. A P-value < 0.05 denoted the presence of a statistically significant difference.
RESULTS
Effects of natural compounds on CD169 expression in HMDMs
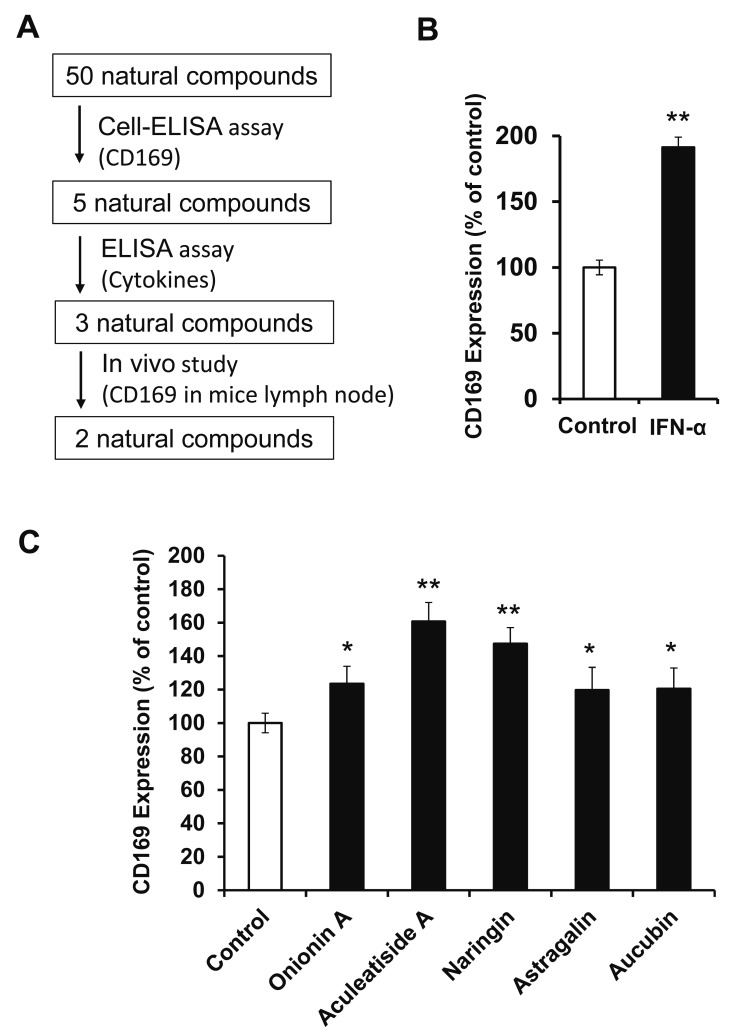
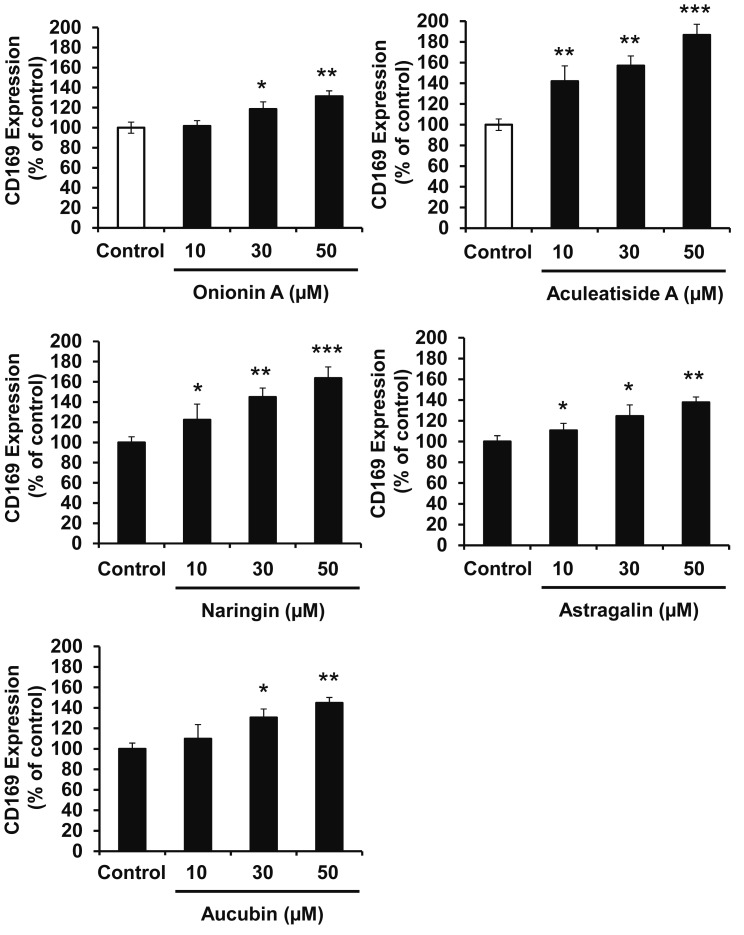
The aim of this study is to identify natural compounds that induce CD169-positive macrophages using in vitro and in vivo assays, as shown in Figure 1A. Type I interferon, such as IFN-α, is known to induce CD169 expression in macrophages,19 and we initially confirmed that IFN-α enhanced CD169 expression in HMDMs (Fig. 1B). Under assay conditions, we first measured the effect of 50 natural compounds on CD169 expression in HMDMs and found that 5 enhanced the expression (Table 1). As shown in Figure 1C, onionin A, aculeatiside A, naringin, astragalin, and aucubin (chemical structures shown in Fig. 2) significantly enhanced CD169 expression. Furthermore, these compounds induced CD169 expression in a dose-dependent manner (Fig. 3), and aculeatiside A and naringin induced CD169 expression particularly strongly (Fig. 3), suggesting that aculeatiside A and naringin are promising candidate compounds for inducing the CD169-positive phenotype in macrophages.
Fig. 1.
Effect of Natural Compounds on CD169 Expression in Human Macrophages. Experimental study design (A). HMDMs were incubated with IFN-α (5 mIU/ml) for 24 h, followed by the determination of CD169 expression by Cell-ELISA, as described in the Materials and Methods (B). HMDMs were incubated with natural compounds (30 µM) for 24 h, followed by the determination of CD169 expression by Cell-ELISA, as described in the Materials and Methods (C). Data are presented as the means ± SD. *, P < 0.01, **, P < 0.001 vs. control.
Table 1. Effect of Natural Compounds on the CD169 Expression.
| Compound Name | CD169 expression (% of control) |
SD | Compound Name | CD169 expression (% of control) |
SD |
|---|---|---|---|---|---|
| Acteoside | 105 | 7 | Tomatine | 96 | 8 |
| Alisol A | 94 | 6 | Desgalactotigonin | 101 | 15 |
| Cilistol A | 96 | 5 | Isosteviol | 108 | 12 |
| Astragaloside I | 110 | 9 | Astragaloside II | 98 | 4 |
| Astragaloside III | 106 | 8 | Astragaloside IV | 99 | 6 |
| Astragaloside V | 103 | 11 | Astragaloside VI | 96 | 7 |
| Astragaloside VII | 100 | 7 | Isoastragaloside I | 111 | 9 |
| Onionin A | 124* | 10 | Tubocaposigenin | 93 | 12 |
| Tubocaposide B | 93 | 8 | Isotubocaposide A | 108 | 6 |
| Isotubocaposide B | 91 | 8 | Isotubocaposide C | 96 | 10 |
| Aculeatiside | 161** | 11 | Kikkanol F | 102 | 15 |
| Prunasin | 108 | 6 | Luteolin | 120 | 15 |
| Sakuranin | 123 | 21 | Naringin | 148** | 9 |
| Corydaline | 119 | 15 | Astragalin | 120* | 13 |
| Dioscin | 91 | 7 | Arbutin | 121 | 18 |
| Aucubin | 121* | 12 | Onjisaponin B | 118 | 16 |
| Baicalin | 92 | 7 | Kaempferol | 95 | 5 |
| Capsianoside II | 103 | 5 | Capsianoside III | 106 | 11 |
| Capsianoside VIII | 96 | 10 | Capsianoside IX | 116 | 17 |
| Quercetin | 105 | 8 | Abrisapogenol F | 118 | 16 |
| Solasodin | 105 | 17 | Solamargine | 97 | 12 |
| Tetrahydropalmatine | 99 | 9 | β-Sitosterol | 101 | 5 |
| Timosaponin AIII | 111 | 9 | Kaikasaponin III | 101 | 9 |
| Capsianoside AS | 95 | 11 | Capsianoside BS | 97 | 5 |
| Asperuloside | 103 | 12 | Subproside V | 92 | 7 |
HMDMs were incubated with natural compounds (30 µM) for 24 h, followed by the determination of the CD169 expression by Cell-ELISA, as described in the materials and methods. Data are presented as the means ± SD. *, P < 0.01, **, P < 0.001 vs. control.
Fig. 2.
Chemical Structures of the Five Candidate Natural Compounds.
Fig. 3.
Effects of Candidate Compounds on CD169 Expression in Murine Macrophages. HMDMs were incubated with the indicated amount of natural compounds for 24 h, followed by the determination of CD169 expression by Cell-ELISA, as described in the Materials and Methods. Data are presented as the means ± SD. *, P < 0.01, **, P < 0.001, ***, P < 0.0001 vs. control.
Effects of natural compounds on activation of murine macrophages
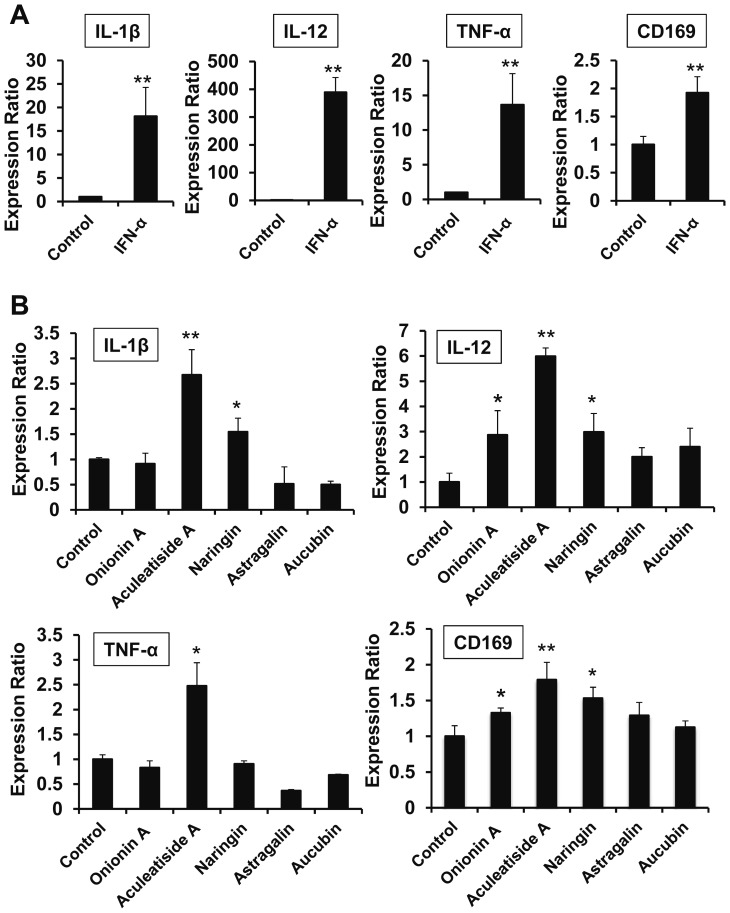
Since type I interferon, such as IFN-α and IFN-β, is known to induce an anti-tumor phenotype (inflammatory phenotype) in macrophages,20 we tested the effect of IFN-α in inducing cytokine secretion from macrophages. As shown in Figure 4A, IFN-α significantly enhanced the expression of inflammatory cytokines, such as IL-1β, IL-12, and TNF-α, in murine peritoneal macrophages. Among the five compounds examined, aculeatiside A also induced IL-1β, IL-12, and TNF-α expression, similar to IFN-α (Fig. 4B). Additionally, naringin enhanced IL-1β and IL-12 expression (Fig. 4B) and onionin A enhanced IL-12 expression (Fig. 4B). Furthermore, CD169 expression in murine peritoneal macrophages was enhanced by stimulation with both IFN-α and these natural compounds (Fig. 4A and 4B). These results suggest that aculeatiside A and naringin induce an anti-tumor phenotype in macrophages and enhance the anti-tumor immunity.
Fig. 4.
Effects of Candidate Compounds on Cytokine and CD169 Expression. Murine peritoneal macrophages were incubated with natural compounds (30 µM) for 6 h, followed by the determination of cytokine and CD169 expression by real-time PCR, as described in the Materials and Methods. Data are presented as the means ± SD. *, P < 0.05, **, P < 0.01 vs. control.
Effects of aculeatiside A and naringin on LN macrophage activation in mice
We next examined the effect of aculeatiside A and naringin on LN macrophages in mice using the methods shown in Figure 5A. IFN-α injection into the hind shank enhanced IL-1β, IL-12, and TNF-α expression in LN macrophages on the same side (Fig. 5B). Aculeatiside A and naringin injection also induced TNF-α, IL-1β, and IL-12 expression in same-side LN macrophages, similar to IFN-α (Fig. 5C). This result indicates that aculeatiside A and naringin also induce an anti-tumor phenotype in macrophages in vivo, suggesting that these compounds are promising candidates for potential new anti-cancer agents targeting macrophage activation.
Fig. 5.
Effects of Candidate Compounds on Cytokine and CD169 Expression in Mice. Experimental schematic illustration of the in vivo study (A). Subcutaneous injection of 100 µl IFN-α (50 mIU) and PBS into the right shank of mice (n=6), followed by the determination of cytokine and CD169 expression in the right inguinal lymph node by real-time PCR, as described in the Materials and Methods (B). Subcutaneous injection of 100 µl natural compounds (100 µM) and DMSO into the right shank of mice (n=6), followed by the determination of cytokine and CD169 expression in the right inguinal lymph node by real-time PCR, as described in the Materials and Methods (C). Data are presented as the means ± SD. *, P < 0.05, **, P < 0.01 vs. control.
DISCUSSION
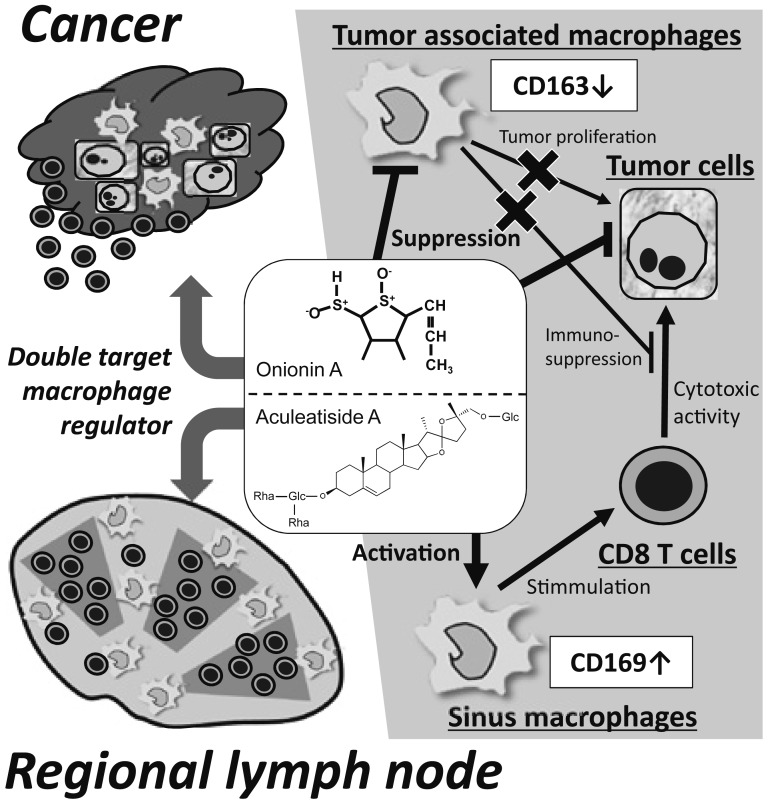
In the present study, we identified natural compounds that induce CD169 expression in macrophages. Among these compounds, aculeatiside A, naringin, and onionin A had an enhancing effect on CD169 expression in both HMDMs and murine macrophages (Figs. 1, 3, and 4), and aculeatiside A and naringin induced an anti-tumor phenotype (M1-like phenotype) in murine macrophages (Figs. 4 and 5). Several macrophage-targeting agents that inhibit macrophage recruitment into tumor tissues and/or inhibit macrophage differentiation into the M2 pro-tumor phenotype are currently being investigated.32-35 We previously reported that onionin A suppresses tumor progression by inhibiting M2 polarization of macrophages, which correlated with both tumor proliferation and immunosuppression36,37 (Fig. 6). In contrast, aculeatiside A and naringin exert a potential inhibitory effect on tumor progression by inducing CD169-positive and M1-like macrophages, potentially correlating with cytotoxic T-cell activation (Fig. 6). Therefore, our present findings suggest that aculeatiside A and naringin, as well as onionin A, may be potential new candidates that target macrophage activation for anti-cancer therapy.
Fig. 6.
Mechanisms of Compounds That Regulate Two Different Macrophage Types in the Tumor Environment. Onionin A suppresses M2 polarization of macrophages and tumor cell proliferation in tumor site. In contrast, aculeatiside A or naringin induce CD169-positive and M1-like macrophages in LN. These compounds are suggested to synergistically stimulate anti-tumor immune responses in patients with malignant tumors.
A high density of CD8-positive CTLs infiltrating the tumor site or circulating in the peripheral blood is associated with a favorable clinical prognosis in several malignant tumors.21-24 However, it has not yet been ascertained how CTLs are generated in tumor patients. We previously reported that an elevated number of CD169-positive sinus macrophages in the regional LN and higher percentages of CD169-positive cells among CD68-positive sinus macrophages were significantly correlated with lower T- and N-stages and, notably, high CD8-positive T-cell infiltration into primary colorectal carcinoma.19 Patients with a higher density of CD169-positive macrophages showed a significantly better overall survival than those with a lower density.19 These observations suggested that elevated CD169 expression in sinus macrophages was closely associated with the induction of anti-tumor immune responses and exerted a beneficial effect on the clinical course.19 Similar observations were subsequently observed in patients with melanoma, endometrial cancer, and breast cancer.25-28
A number of methods for targeting antigens to LN macrophages have been investigated as part of the ongoing development of lymphatic-targeted vaccines. It was reported that cholesteryl pullulan (CHP) nanogels induce tumor regression in some patients with malignant tumors,29,30 and CD169-positive macrophages in LN sinus captured CHP nanogels and worked as APCs.31 Thus, lymphatic-targeting materials might be a promising approach to improving vaccine efficacy, and CD169-positive macrophages are now of interest as APCs to which antigens might be efficiently delivered.
In conclusion, we herein presented a new approach to screen for candidate drugs that influence the anti-tumor immune system. Aculeatiside A and naringin, as well as onionin A, may increase CD169 expression in cultured macrophages. Our findings suggest that enhancing anti-tumor immune responses via increasing the function of antigen presentation in regional LNs may be an effective therapeutic strategy.
ACKNOWLEDGEMENTS
We thank Mr. Takenobu Nakagawa and Ms. Ikuko Miyagawa, for their technical assistance. This work was supported by JSPS KAKENHI Grant Number JP25293089, JP25460497, JP293344 and JP860501.
Footnotes
CONFLICT OF INTEREST: All authors have no conflicts of interest in association with this manuscript.
REFERENCES
- 1.Martinez-Pomares L, Gordon S. CD169+ macrophages at the crossroads of antigen presentation. Trends Immunol. 2012; 33: 66-70. . 10.1016/j.it.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 2.Gasteiger G, Ataide M, Kastenmüller W. Lymph node - an organ for T-cell activation and pathogen defense. Immunol Rev. 2016; 271: 200-220. . 10.1111/imr.12399 [DOI] [PubMed] [Google Scholar]
- 3.Marmey B, Boix C, Barbaroux JB, et al. CD14 and CD169 expression in human lymph nodes and spleen: specific expansion of CD14+CD169− monocyte-derived cells in diffuse large B-cell lymphomas. Hum Pathol. 2006; 37: 68-77. . 10.1016/j.humpath.2005.09.016 [DOI] [PubMed] [Google Scholar]
- 4.Junt T, Moseman EA, Iannacone M, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007; 450: 110-114. . 10.1038/nature06287 [DOI] [PubMed] [Google Scholar]
- 5.O’Neill ASG, van den Berg TK, Mullen GED. Sialoadhesin - a macrophage-restricted marker of immunoregulation and inflammation. Immunology. 2013; 138: 198-207. . 10.1111/imm.12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartnell A, Steel J, Turley H, et al. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood. 2001; 97: 288-296. . 10.1182/blood.V97.1.288 [DOI] [PubMed] [Google Scholar]
- 7.Saunderson SC, Dunn AC, Crocker PR, et al. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014; 123: 208-216. . 10.1182/blood-2013-03-489732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lores B, García-Estevez JM, Arias C. Lymph nodes and human tumors (review). [review]. Int J Mol Med. 1998; 1: 729-733. [DOI] [PubMed] [Google Scholar]
- 9.Asano K, Nabeyama A, Miyake Y, et al. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011; 34: 85-95. . 10.1016/j.immuni.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 10.Martens J-H, Kzhyshkowska J, Falkowski-Hansen M, et al. Differential expression of a gene signature for scavenger/lectin receptors by endothelial cells and macrophages in human lymph node sinuses, the primary sites of regional metastasis. J Pathol. 2006; 208: 574-589. . 10.1002/path.1921 [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Asano K, Qiu CH. Immune regulation by apoptotic cell clearance. Ann N Y Acad Sci. 2010; 1209: 37-42. . 10.1111/j.1749-6632.2010.05746.x [DOI] [PubMed] [Google Scholar]
- 12.Bernhard CA, Ried C, Kochanek S, et al. CD169 + macrophages are sufficient for priming of CTLs with specificities left out by cross-priming dendritic cells. Proc Natl Acad Sci USA. 2015; 112: 5461-5466. . 10.1073/pnas.1423356112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen WC, Kawasaki N, Nycholat CM, et al. Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169. PLoS One. 2012; 7: e39039. . 10.1371/journal.pone.0039039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poderoso T, Martínez P, Álvarez B, et al. Delivery of antigen to sialoadhesin or CD163 improves the specific immune response in pigs. Vaccine. 2011; 29: 4813-4820. . 10.1016/j.vaccine.2011.04.076 [DOI] [PubMed] [Google Scholar]
- 15.Detienne S, Welsby I, Collignon C, et al. Central Role of CD169+ Lymph Node Resident Macrophages in the Adjuvanticity of the QS-21 Component of AS01. Sci Rep. 2016; 6: 39475. . 10.1038/srep39475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pucci F, Garris C, Lai CP, et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science. 2016; 352: 242-246. . 10.1126/science.aaf1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujiwara Y, Komohara Y, Ikeda T, et al. Corosolic acid Inhibits Glioblastoma Cell Proliferation by Suppressing the Activation of STAT3 and NF-κB in Tumor Cells and Tumor-associated Macrophages. Cancer Sci. 2011; 102: 206-211. . 10.1111/j.1349-7006.2010.01772.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komohara Y, Ma C, Yano H, et al. Cell adhesion molecule-1 (CADM1) expressed on adult T-cell leukemia/lymphoma cells is not involved in the interaction with macrophages. J Clin Exp Hematop. 2017; 57: 15-20. . 10.3960/jslrt.17003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohnishi K, Komohara Y, Saito Y, et al. CD169-positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci. 2013; 104: 1237-1244. . 10.1111/cas.12212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014; 14: 36-49. . 10.1038/nri3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998; 58: 3491-3494. [PubMed] [Google Scholar]
- 22.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006; 313: 1960-1964. . 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 23.Gooden MJM, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011; 105: 93-103. . 10.1038/bjc.2011.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasada T, Suekane S. Variation of tumor-infiltrating lymphocytes in human cancers: controversy on clinical significance. Immunotherapy. 2011; 3: 1235-1251. . 10.2217/imt.11.106 [DOI] [PubMed] [Google Scholar]
- 25.Saito Y, Ohnishi K, Miyashita A, et al. Prognostic Significance of CD169+ Lymph Node Sinus Macrophages in Patients with Malignant Melanoma. Cancer Immunol Res. 2015; 3: 1356-1363. . 10.1158/2326-6066.CIR-14-0180 [DOI] [PubMed] [Google Scholar]
- 26.Ohnishi K, Yamaguchi M, Erdenebaatar C, et al. Prognostic significance of CD169-positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Sci. 2016; 107: 846-852. . 10.1111/cas.12929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiota T, Miyasato Y, Ohnishi K, et al. The Clinical Significance of CD169-Positive Lymph Node Macrophage in Patients with Breast Cancer. PLoS One. 2016; 11: e0166680. . 10.1371/journal.pone.0166680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komohara Y, Ohnishi K, Takeya M. Possible functions of CD169-positive sinus macrophages in lymph nodes in anti-tumor immune responses. Cancer Sci. 2017; 108: 290-295. . 10.1111/cas.13137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitano S, Kageyama S, Nagata Y, et al. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin Cancer Res. 2006; 12: 7397-7405. . 10.1158/1078-0432.CCR-06-1546 [DOI] [PubMed] [Google Scholar]
- 30.Kawabata R, Wada H, Isobe M, et al. Antibody response against NY-ESO-1 in CHP-NY-ESO-1 vaccinated patients. Int J Cancer. 2007; 120: 2178-2184. . 10.1002/ijc.22583 [DOI] [PubMed] [Google Scholar]
- 31.Muraoka D, Harada N, Hayashi T, et al. Nanogel-based immunologically stealth vaccine targets macrophages in the medulla of lymph node and induces potent antitumor immunity. ACS Nano. 2014; 8: 9209-9218. . 10.1021/nn502975r [DOI] [PubMed] [Google Scholar]
- 32.Mills CD, Lenz LL, Harris RA. A Breakthrough: Macrophage-Directed Cancer Immunotherapy. Cancer Res. 2016; 76: 513-516. . 10.1158/0008-5472.CAN-15-1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017; 14: 399-416. . 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komohara Y, Fujiwara Y, Ohnishi K, et al. Tumor-associated macrophages: potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev. 2016; 99: 180-185. . 10.1016/j.addr.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 35.Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci. 2014; 105: 1-8. . 10.1111/cas.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuboki J, Fujiwara Y, Horlad H, et al. Onionin A inhibits ovarian cancer progression by suppressing cancer cell proliferation and the protumour function of macrophages. Sci Rep. 2016; 6: 29588. . 10.1038/srep29588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujiwara Y, Horlad H, Shiraishi D, et al. Onionin A, a sulfur-containing compound isolated from onions, impairs tumor development and lung metastasis by inhibiting the protumoral and immunosuppressive functions of myeloid cells. Mol Nutr Food Res. 2016; 60: 2467-2480. . 10.1002/mnfr.201500995 [DOI] [PubMed] [Google Scholar]