Abstract
Intravascular large B-cell lymphoma (IVLBCL) is a rare and clinically distinctive entity characterized by the almost exclusive growth of large cells within the lumen of blood vessels in particular capillaries. Reports of this peculiar disease, do not commonly address the PD-L1 expression on IVLBCL tumor cells. Here, we describe a 51-year-old Japanese woman who presented with rapidly progressive cognitive decline and higher brain dysfunction. CT scan and MRI revealed multiple ischemic foci in the cerebral hemispheres, ground-glass opacity in the lungs, and splenomegaly. Random skin biopsy for IVLBCL diagnosis yielded negative results. The patient experienced a rapidly deteriorating clinical course with no treatment, and died from the disease after 3 months of hospitalization. Post-mortem examination revealed systemic intravascular plugging of lymphoma cells, without mass lesions in the central nervous system or in visceral organs such as the lungs, liver, pituitary gland, ovaries, and uterus. The tumor cells were positive for CD10, CD20, BCL2, BCL6, and MUM1, but not other lineage-specific markers. Notably, the tumor cells showed strong PD-L1 expression. Our case was diagnosed as IVLBCL with neoplastic PD-L1 expression. These findings suggest that PD-L1 is associated with immune evasion of IVLBCL and may play a role in the pathogenesis and peculiar biological behavior of this unique disease. Additionally, PD-L1 may represent a possible therapeutic target for immune check-point inhibitors.
Keywords: intravascular large B-cell lymphoma, neoplastic PD-L1 expression
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous disease comprising multiple morphologic variants, immunophenotypic and molecular subgroups, and distinct disease entities.1 One particularly aggressive DLBCL subtype is intravascular large B-cell lymphoma (IVLBCL), which is characterized by selective growth of large tumor cells within the lumen of blood vessels of varying size.1 Since IVLBCL can potentially occur in any organ, it exhibits highly heterogeneous clinical features and is challenging to diagnose, although patients with IVLBCL may predominantly exhibit fever, neurological symptoms, and cutaneous symptoms.2,3 During its clinical course, this disease often affects the immunological sanctuary, including the central nervous system (CNS). However, the reasons for this peculiar localization of tumor cells remain unclear.
The introduction of programmed death 1 (PD1)/PD1 ligand (PD-L1) pathway inhibitors has revolutionized cancer treatment, producing impressive responses in a broad variety of tumor types.4,5 Based on the established breakthrough effect of PD1 blockade, researchers are now investigating PD1 and PD-L1 targeted antibodies for treatment of various lymphomas, especially in relapsed or refractory disease. In parallel, studies have focused on analyzing of the PD1/PD-L1 axis for prognostic relevance and prediction of treatment response.4,6-10 With regards to lymphoid malignancies, tumor cell PD-L1 expression is frequently reported in classic Hodgkin lymphoma (CHL), primary mediastinal large B-cell lymphoma, and Epstein-Barr virus (EBV)-associated lymphomas, including EBV-positive diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS) type, and extranodal NK/T-cell lymphoma, nasal type11 according to the WHO 2017 classification.1 Moreover, a recent report describes a higher incidence of neoplastic PD-L1 overexpression in primary DLBCL of the CNS (PCNS-DLBCL) compared to systemic ordinal cases.12 This suggests that PD-L1 expression in tumor cells may play an important role in PCNS-DLBCL pathogenesis.
Here, we present the case of IVLBCL in an immunocompetent patient which initially presented with neurological symptoms and unfortunately had a lethal clinical course of 3 months with no treatment. The autopsy findings revealed strong PD-L1 expression on the tumor cells as well as typical histopathologic features of IVLBCL, supporting the possibility that such PD-L1 expression may be related to the pathogenesis and/or peculiar clinical behavior of this unique lymphoma.
CASE PRESENTATION
A 51-year-old woman was admitted to our affiliated hospital after experiencing cognitive deficits, disorientation with poor short-term memory, and truncal ataxia for the past 2 months. She had no remarkable family history, and exhibited no fever, weight loss, or night sweating. Physical examination findings were largely normal, with no observed skin lesions or rashes. Cerebral magnetic resonance imaging (MRI) revealed multifocal ischemic lesions with occlusion of the bilateral middle cerebral arteries. Upon computed tomography (CT) of the chest, the periphery of both lungs exhibited a ground-glass appearance. Abdominal CT revealed mild splenomegaly.
After an extensive work-up that did not yield a definitive diagnosis, we considered the possibility of IVLBCL based on the patient’s mental status decline. Examination of a random skin biopsy (RSB) of normal-appearing skin did not reveal any pathological changes. The patient subsequently suffered a rapidly progressive decline in cognitive function and higher brain dysfunction. She died of multiple organ failure after 3 months of hospitalization. Over her entire clinical course, the patient did nod exhibit lymphadenopathy. Post-mortem examination was performed.
PATHOLOGICAL FINDINGS
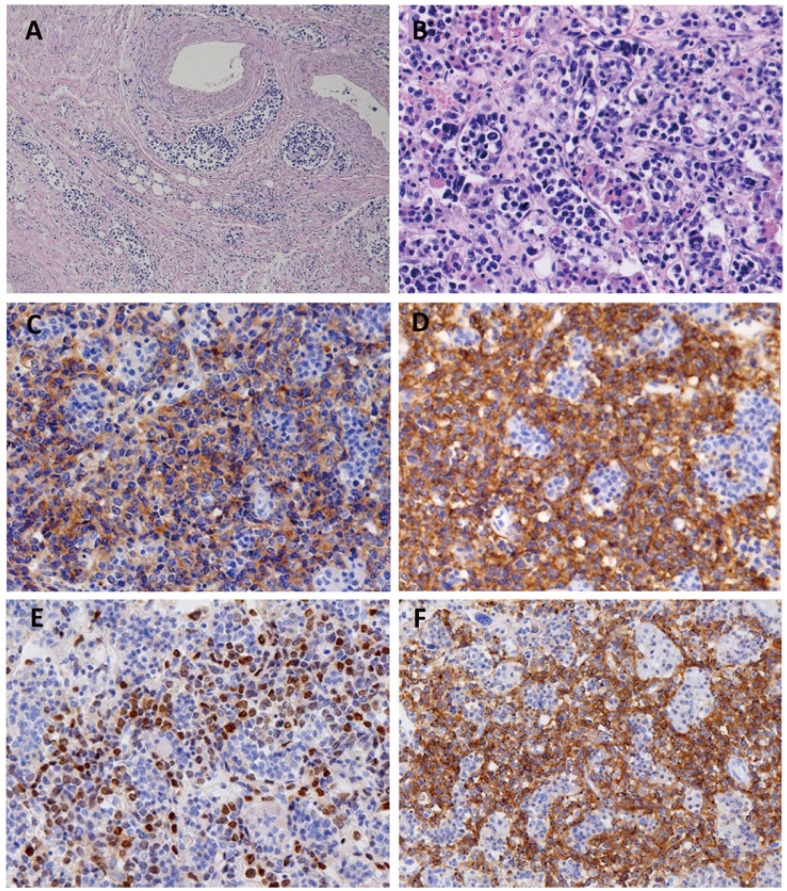
Histological examination revealed large tumor cells infiltrating into small and middle-sized blood vessels of multiple organs, including the lungs, liver, spleen, pituitary gland, ovaries, uterus, and bone marrow (Fig. 1A and 1B). We also observed extravascular spread in the spleen and liver. These morphologic features conformed to those documented in IVLBCL. The tumor cells were immunohistochemically positive for CD10 (Fig. 1C), CD20 (Fig. 1D), BCL2, BCL6, and MUM1 (Fig. 1E), but negative for CD3 and CD5. Analysis with EBV-encoded small nuclear early region in situ hybridization (EBER-ISH) showed that the tumor cells were not associated with EBV. About 50% of the tumor cells showed strong expression of PD-L1 (clone SP142, Spring Bioscience, Pleasanton, CA) (Fig. 1F).
Fig. 1.
Intravascular large B-cell lymphoma with neoplastic PD-L1 expression. A, Large lymphoma cells fill the veins and capillaries around the ovary. B-F, Large lymphoma cells fill the vascular channels in the pituitary gland (B), and are positive for CD10 (C), CD20 (D), MUM1 (E), and PD-L1 (F).
DISCUSSION
Here we describe a case of IVLBCL with both neurologic involvement and systemic symptoms. Notably, postmortem examination revealed strong neoplastic PD-L1 expression. PD-L1 represents an attractive therapeutic target in this era of immuno-oncology, and may be associated with the peculiar observation that IVLBCL preferentially affects the immunological sanctuaries, as exemplified by CNS involvement13 and neurolymphomatosis.14
IVLBCL has been described in an increasing number of reports mostly short series and single case reports. Ponzoni et al.2 reviewed our current knowledge of this rare aggressive lymphoma. By the time of diagnosis, IVLBCL is disseminated and warrants systemic chemotherapy using an anthracycline-based chemotherapy regimen, such as cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) with the addition of monoclonal antibodies rituximab (R-CHOP).
Shimada et al.3 reported that IVLBCL patients who exhibit CNS involvement at diagnosis tend to show a short interval from diagnosis to CNS recurrence. Among patients without CNS involvement at diagnosis, they found a 12% survival rate at 2 years after CNS recurrence. Furthermore, they demonstrated that the risk of CNS recurrence did not significantly differ between patients receiving chemotherapy regimens with or without rituximab. CNS recurrence is a serious complication in IVLBCL patients, and the development of optimal strategies for the treatment and prevention of CNS involvement will likely improve to clinical outcomes. Similarly, patients with PCNS-DLBCL show a remarkably worse prognosis than patients with systemic DLBCL (S-DLBCL)1 with about 25% of PCNS-DLBCL patients not responding to first-line treatment, and over 50% of these non-responders relapsing during their clinical course.
Although frequency of PD-L1 expression in DLBCL have varied among several studies,6-9 no study focusing on PD-L1 expression in IVLBCL has been reported. Four et al.12 recently reported a higher rate of PD-L1 expression (clone SP142) on tumor cells in PCNS-DLBCL than in S-DLBCL (58% and 37%, respectively). Moreover, Hishikawa et al.15 reviewed 12 unique cases with coexisting PCNS-DLBCL and IVLBCL, and suggested that the former may be regarded as the extensive extravascular involvement of the latter in the CNS. Indeed, one study among Japanese patient reported that PCNS-DLBCL and IVLBCL had overlapping survival curves and rarely differed in any clinicopathologic parameters, except that the international prognostic index (IPI) score was low or low-intermediate in 86% of PCNS-DLBCL cases and was high or high-intermediate in 98% of IVLBCL cases.16 These findings raise the question of what mechanism or microenvironment might explain the unique aggressive behavior shared by these diseases, which exclusively affects extranodal sites but not nodal sites. Here we detected PD-L1 overexpression in our case of IVLBCL, which may contribute to the immune escape of tumor cells, and which is absent in ordinal DLBCL, NOS.7
Our case elucidates the possibility that neoplastic PD-L1 expression in IVLBCL may represent the bridge between IVLBCL and CNS-DLBCL with regards to pathogenesis, hypothetically indicating a new category of immune escape-related aggressive extranodal large B-cell lymphoma.15,16 Moreover, the apparent importance of PD-L1 indicates that it may be a possible therapeutic target for immune check-point inhibitors.17 Of course, it is not possible to draw any definitive conclusion from a single case report, and future large series studies are warranted to investigate these possibilities.
Footnotes
CONFLICT OF INTEREST: The authors have no significant relationships with or financial interests in any commercial companies pertaining to this article.
REFERENCES
- 1.Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th edition edn, International Agency for Research on Cancer. 2017; pp. 317-318. [Google Scholar]
- 2.Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007; 25: 3168-3173. 10.1200/JCO.2006.08.2313 [DOI] [PubMed] [Google Scholar]
- 3.Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009; 10: 895-902. 10.1016/S1470-2045(09)70140-8 [DOI] [PubMed] [Google Scholar]
- 4.Ilcus C, Bagacean C, Tempescul A, et al. Immune checkpoint blockade: the role of PD-1-PD-L axis in lymphoid malignancies. Onco Targets Ther. 2017; 10: 2349-2363. 10.2147/OTT.S133385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008; 26: 677-704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013; 19: 3462-3473. 10.1158/1078-0432.CCR-13-0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015; 126: 2193-2201. 10.1182/blood-2015-02-629600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon D, Kim S, Kim PJ, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology. 2016; 68: 1079-1089. 10.1111/his.12882 [DOI] [PubMed] [Google Scholar]
- 9.Menter T, Bodmer-Haecki A, Dirnhofer S, et al. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum Pathol. 2016; 54: 17-24. 10.1016/j.humpath.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 10.Vranic S, Ghosh N, Kimbrough J, et al. PD-L1 Status in Refractory Lymphomas. PLoS One. 2016; 11: e0166266. 10.1371/journal.pone.0166266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwong YL, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017; 129: 2437-2442. 10.1182/blood-2016-12-756841 [DOI] [PubMed] [Google Scholar]
- 12.Four M, Cacheux V, Tempier A, et al. PD1 and PDL1 expression in primary central nervous system diffuse large B-cell lymphoma are frequent and expression of PD1 predicts poor survival. Hematol Oncol. 2017; 35: 487-496. 10.1002/hon.2375 [DOI] [PubMed] [Google Scholar]
- 13.Shimada K, Murase T, Matsue K, et al. Central nervous system involvement in intravascular large B-cell lymphoma: a retrospective analysis of 109 patients. Cancer Sci. 2010; 101: 1480-1486. 10.1111/j.1349-7006.2010.01555.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsue K, Hayama BY, Iwama K, et al. High frequency of neurolymphomatosis as a relapse disease of intravascular large B-cell lymphoma. Cancer. 2011; 117: 4512-4521. 10.1002/cncr.26090 [DOI] [PubMed] [Google Scholar]
- 15.Hishikawa N, Niwa H, Hara T, et al. An autopsy case of lymphomatosis cerebri showing pathological changes of intravascular large B-cell lymphoma in visceral organs. Neuropathology. 2011; 31: 612-619. 10.1111/j.1440-1789.2011.01203.x [DOI] [PubMed] [Google Scholar]
- 16.Imai H, Shimada K, Shimada S, et al. Comparative clinicopathological study of primary CNS diffuse large B-cell lymphoma and intravascular large B-cell lymphoma. Pathol Int. 2009; 59: 431-437. 10.1111/j.1440-1827.2009.02390.x [DOI] [PubMed] [Google Scholar]
- 17.Miyoshi H, Kiyasu J, Kato T, et al. PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood. 2016; 128: 1374-1381. 10.1182/blood-2016-02-698936 [DOI] [PubMed] [Google Scholar]