Abstract
Langerhans cell (LC) histiocytosis (LCH) and LC sarcoma (LCS) are proliferative processes consisting of cells having morphologic and phenotypic features of Langerhans cells (LCs), although the latter may have lost some of these features. Because neoplastic nature of LCH as well as LCS is more likely by recent studies, a category of LC hyperplasia can be better characterized. LCH and LCS are rarely seen in daily pathology practice, but it is important to accurately characterize these lesions. For this purpose, an outline covering proliferations of LC and related cells was constructed. The scheme of this outline is based especially on evaluating borderline lesions, neoplastic trans-differentiation, and degree of similarity with the normal counterparts. In addition, the organization and update of the current classification scheme for histiocytic and dendritic-cell proliferations is presented.
Keywords: Langerhans cell histiocytosis, Langerhans cell sarcoma, BRAF mutation, trans-differentiation, differential diagnosis
INTRODUCTION
Langerhans cell (LC) histiocytosis (LCH) and LC sarcoma (LCS) constitute proliferative disorders of LCs. The nature of LCH and LCS has been actively discussed as exemplified by the title of an article, “The Langerhans cell histiocytosis X files revealed”1 (Table 1). The complexity of the lesions was due largely to the absence of data regarding clonality and genetic abnormalities until the 1990s, when clonality of LCH was identified in female patients by examination of the pattern of X chromosome inactivation.2-4 Although this method was not applicable to male individuals who constitute the majority of LCH patients (See EPIDEMIOLOGY section.) and clonality itself does not always indicate a neoplastic process, a recurrent BRAF V600E mutation was found recently in more than half of the patients with the disease.5 The BRAF mutation is not specific for LCH,6-8 but it has been reported that LCH in both genders is a neoplastic disease that may respond to RAF pathway inhibitors.5,9 From these findings, LC hyperplasia, the non-neoplastic counterpart of the LC proliferations,10,11 has been better characterized.
Table 1. History of Langerhans cell proliferations1.
| ▪The first suggestive case (Smith T, 1865) |
| ▪ |
| Discovery of Langerhans cells (Langerhans P, 1868): non-pigmentary cutaneous dendritic cells |
| ▪case-reports (Hand A, 1893/ Schüller A, 1915/ Christian H, 1920) |
| → [Hand- Schüller-Christian disease] |
| ▪case-reports (Letterer E, 1924/ Siwe S, 1933) |
| → [Letterer-Siwe disease] |
| ▪ |
| case-reports (Fraser J, 1935/ Otani S and Ehrlich J, 1940/ Lichtenstein L, 1940) |
| → [solitary granuloma of bone/ eosinophilic granuloma of bone] |
| ▪Unifying concept of ‘histiocytosis X’ (Lichtenstein L, 1953) |
| ▪Ultrastructure of the cells (Birbeck M, et al., 1961) |
| [originally, ‘Langerhans bodies’; later, ‘Birbeck granules’] |
| ▪ |
| Ultrastructural similarities between histiocytosis X cells and Langerhans cells (Nezelof C, et al., 1973) |
| → [Langerhans cell histiocytosis (LCH)] |
| ▪Malignant histiocytosis X (Wood G, et al., 1984) |
| ▪The first workshop on histiocytosis (D’Angio G, 1985) |
| → [ |
| Foundation of Histiocytic Society with Dr. Nezelof as the first President] |
| ▪Histiocytic sarcoma in LCH (Elleder M, et al., 1986) |
| ▪Precursor LCH (Segal GH, et al., 1992) |
| ▪Clonality in 9/10 female patients (William CL, et al., 1994) |
| ▪ |
| Chromosomal instability (Betts D, et al. 1998; Scappaticci S, et al., 2000) |
| ▪ |
| The first international randomized LCH study for multisystem disease (LCH-1) (Gadner H, et al., 2001) |
In this review, hematopathologic features of LCS are described in detail according to the authors’ experience because they have not been well characterized due to marked rarity of the disease. Adult pulmonary LCH, which is clinicopathologically different from other LCH types,12,13 can be either non-clonal or clonal,4 and is described in parallel as necessary. Based on these features together with traditional classification and recent molecular findings on LCH, an outline covering proliferations of LC and related cells, such as indeterminate dendritic cells, interdigitating dendritic cells, plasmacytoid dendritic cells, non-LC-type histiocytes, and follicular dendritic cells (FDCs), was constructed for more accurate diagnosis.
DEFINITION
LCH and LCS were separately described in the classification of tumors of the hematopoietic and lymphoid systems by the World Health Organization (WHO) in 2001 (WHO-2001), where LCH was defined as a ‘neoplastic proliferation of LC, which expresses S100 protein and CD1a, and has Birbeck granules on electron microscopy’.14 LCS, on the other hand, is defined as a ‘neoplastic disorder of LC with apparent malignant cytologic features and possible inclusion of both LCS progressed from LCH and de novo LCS’.15 In the updated version (WHO-2008), both were included in a single category, i.e., tumors derived from LCs, and were defined together as ‘neoplastic proliferation of cells having immunophenotypic and electron microscopic features of LCs.16 They are separated into either LCH or LCS based on the degree of cytologic atypia and clinical aggressiveness, but there are rare examples with difficulty in distinction.16 In the latter version, CD207 (langerin) expression was added to the definition of LCH, while the description regarding the possible two forms, progressed or de novo lesion, was eliminated from the definition of LCS. In the WHO classification of skin tumors (WHO-2006), only LCH was defined as ‘a generalized clonal disorder consisting of proliferation of dendritic cells having morphologic and phenotypic features (positive Birbeck granules, CD1a, and S100 protein) of LCs’.17 Although LCH and LCS were characterized to be clonal or neoplastic by these classifications, the clonal nature of LCH in male patients had not yet been confirmed at that time. In addition, LCH is not always a generalized disorder.
The revision of WHO-2008 (WHO-2016) has been completed,18,19 but the classification of LCH, LCS, and related disorders is similar to that in WHO-2008 with the exceptions of minimal alterations to the order of each disease and the addition of Erdheim-Chester disease (a proliferative process of non-LC-type histiocytes).18
SYNONYM
LC granuloma/granulomatosis and histiocytosis X are the former terms for LCH, which consists of three well known classical forms, i.e, Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma. The fourth subtype, congenital self-healing reticulohistiocytosis (CSR), which is also referred to as Hashimoto-Pritzker disease, congenital reticulohistiocytosis, or congenital self-healing LC histiocytosis, is included in WHO-2006.17
LCS was referred to by some authors as malignant histiocytosis X (Table 1).
EPIDEMIOLOGY
The incidence of LCH is estimated at five per million per year,16 whereas that of LCS is unknown. According to the Lymphoma Study Group of Japanese Pathologists,20 the rate of histiocytic and dendritic-cell neoplasms as a whole over malignant lymphoma in Japan is calculated to be 0.31% (10 of 3,194 cases). There are contrastive differences in frequency between LCH and LCS in terms of age and gender of patients. In a large series consisting of 61 non-consecutive cases of neoplasms of histiocytes and accessory dendritic cells,21 diagnoses of 17 LCH and 9 LCS were confirmed by 26 hematopathologists. Patients with LCH were younger (median age, 33 years) than those with LCS (median age, 41 years). LCH is more often observed in males (male: female = 3.7: 1), whereas the ratio is reverse for LCS (male: female = 1: 2).21 It is possible that the difference in the male/female ratio might have resulted in deletion of ‘progressed LCS’ from the definition of LCS in WHO-2008. In other words, LCS in female patients may consist predominantly of a de novo process. Adult pulmonary LCH is a rare disease, and it occurs almost exclusively in smokers. Its precise epidemiological data are not available,12 but the crude prevalence of pulmonary LCH as a whole in Japan is estimated at 0.27 in males and 0.07 in females per 100,000 people.22 The disease predominantly affects young adults between the ages of 20 to 40 years.23
According to the experience of one of the authors of this article (H.N.), 28 LCH cases and only a single LCS case were diagnosed in daily pathology practice on consecutive, but not consultation, cases within a given period of time during which 2,407 cases of malignant lymphoma were diagnosed, resulting in a frequency of 1.2% for the above 29 cases. In our experience for LCS, all four patients were more than 40 years of age, with three being males.
CLINICAL FEATURES
Clinical features of LCH depend on the subtypes, i.e., the involved organs, which are described in the next section. Letterer-Siwe disease is seen in infants (usually within the first year of life), but occurrence in adult has been reported.24,25 These patients present with fever, weight loss, pancytopenia, lymphadenopathy, hepatosplenomegaly, cutaneous lesions, and bone lesions. Hand-Schüller-Christian disease is seen in younger children who present with osteolytic skull lesions, diabetes insipidus (due to hypopituitarism), and exophthalmos. In addition to this clinical triad, otitis media, generalized lymphadenopathy, hepatosplenomegaly, and pulmonary lesions are occasionally seen. Eosinophilic granuloma develops primarily in older children and adults who present with solitary bone lesions, skin lesions, or lymphadenopathy. CSR is seen at birth or in the neonatal period with only cutaneous lesions.17,26,27
Adult pulmonary LCH shows a diffuse pattern of involvement, but symptoms can be relatively mild or absent, with some being attributable to smoking.12
SITES OF INVOLVEMENT
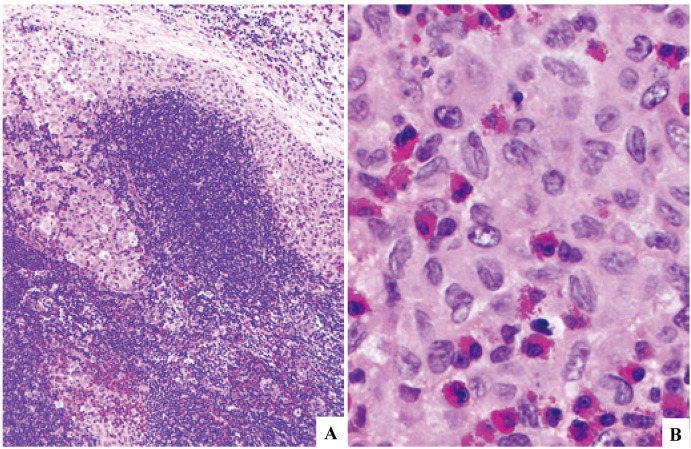
As shown in the Synonym section, LCH has traditionally been separated into Letterer-Siwe disease (multisystem involvement), Hand-Schüller-Christian disease (unisystem, multiorgan involvement), or eosinophilic granuloma (uniorgan involvement). Involvement of bone and the adjacent soft tissue is commonly seen in all types. The third type is often referred to as eosinophilic granuloma of bone, which was the term given to avoid confusion with ‘eosinophilic granuloma of soft tissue/Kimura disease’, but uniorgan involvement other than bone, such as lymph node, is occasionally seen (Fig. 1).
Fig. 1.
Langerhans cell histiocytosis involving the lymph node. Subcapsular and medullary sinuses are filled with cytoplasm-rich cells (1A). They have grooved nuclei and abundant weakly eosinophilic cytoplasm, and are associated with numerous eosinophils (1B). H&E stain. (1A) x10; (1B) x100.
In contrast to pediatric cases, adult pulmonary LCH is a single system disease in more than 80% of patients.23
PATHOLOGICAL FINDINGS
In LCH, the proliferating cells have characteristic cytologic features of LCs. They are ovoid or elliptic in shape with grooved, folded, indented, or lobulated nuclei having inconspicuous nucleoli. Cells in mitosis are variable in number depending on the case, but atypical mitosis is primarily absent. The cells have fairly abundant, weakly eosinophilic cytoplasm. Associated features in the background include increase in eosinophils, neutrophils, and monocyte-derived histiocytes. Plasma cells are virtually absent in many instances. Fibrosis develops later in the course of the disease, and a pathologic diagnosis of LCH is almost impossible when the lesion is burned out. In lymph node, involvement of the subcapsular sinus and adjacent paracortical area is rather characteristic and may recapitulate the migratory pathway of the normal counterpart (Fig. 1). In the spleen, red pulp is preferentially involved as in other myeloid neoplasms.
LCS can practically be separated into two forms, i.e., de novo lesion (Figs. 2 & 3) and that progressed from LCH (Fig. 4), although the difference of gender between LCH and LCS appears to be reverse. The de novo LCS is composed of cells having atypical/pleomorphic nuclei, at least in part, with features reminiscent of LC derivation. Both LCH and LCS foci may be seen in the same tissue in the latter lesion (Fig. 4). It is, however, occasionally difficult to characterize a given lesion to be either LCH or LCS due probably to biologic spectrum of LC neoplasms.
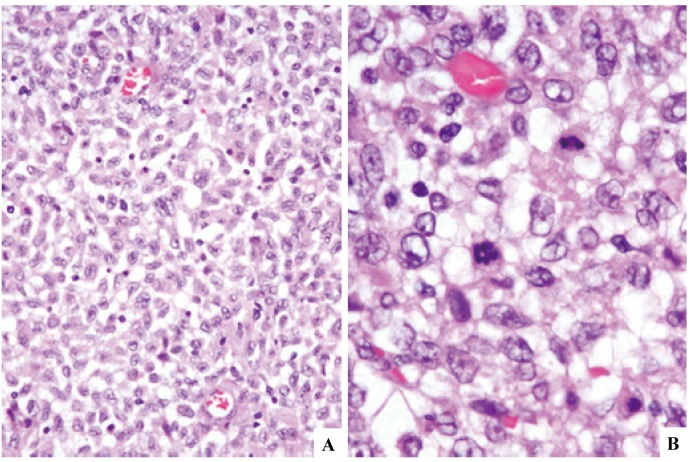
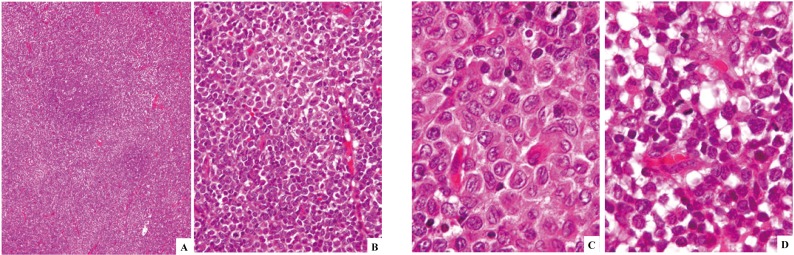
Fig. 2.
Langerhans cell sarcoma (case-1) involving the lymph node. The nodal architecture is obliterated, but a reactive lymph follicle remains (2A). The proliferative process consists of cells having oval- or spindle-shaped nuclei (2B). Hypercellularity, nuclear atypia, and increase in mitotic cells are noted in contrast to Langerhans cell histiocytosis cells in Fig. 1B, but the nuclear groove is still appreciable (3C). Atypical multinucleated cells or those having lobulated nuclei are occasionally present (3D). See Fig. 5 for immunophenotypic features. H&E stain. (2A) x4; (2B) x40; (2C) and (2D) x100.
Fig. 3.
Langerhans cell sarcoma (case-2) involving the lung. Hypercellular proliferation of cells with oval-shaped nuclei is seen throughout (3A). The cells show anisokaryosis and inconspicuous nuclear grooves, with increase in mitotic cells (3B). See Fig. 6 for Ki-67 staining. H&E stain. (3A) x40; (3B) x100.
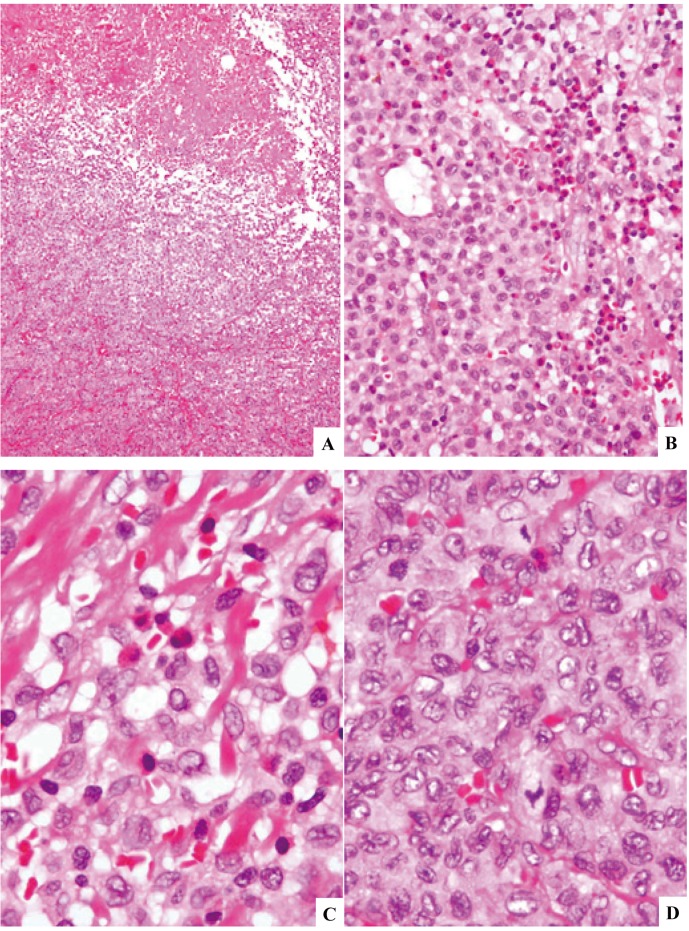
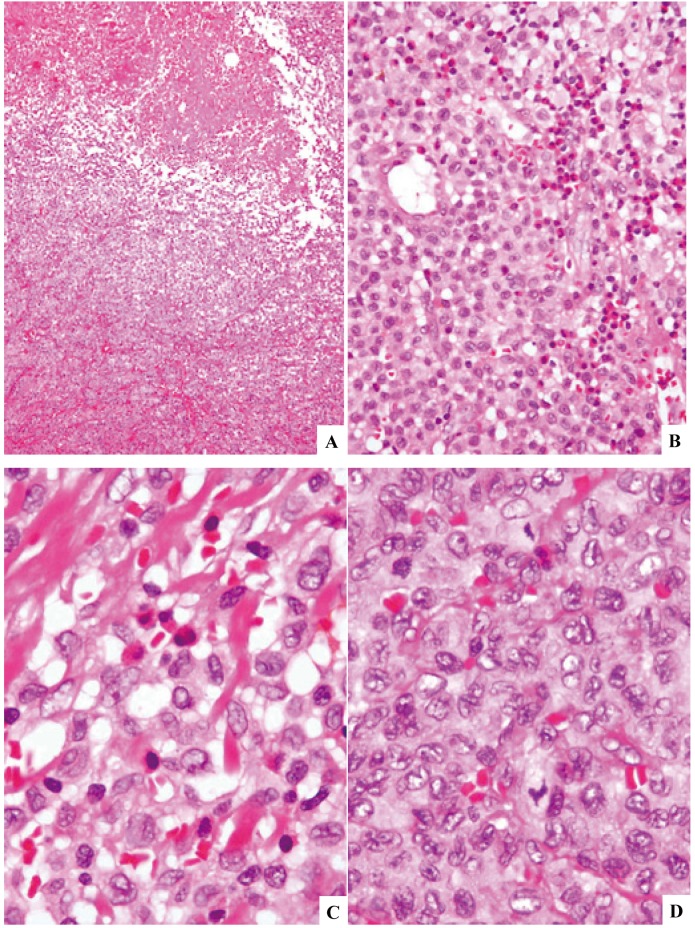
Fig. 4.
Langerhans cell sarcoma (case-3) involving the lymph node. The lesion is associated with zonal necrosis (4A). The proliferating cells are rather uniform in size having fairly abundant, weakly eosinophilic cytoplasm. There are many eosinophils in some areas (4B). Higher magnification shows relatively hypocellular areas with eosinophils (4C) and hypercellular areas rich in mitosis without eosinophils (4D). The lesion was characterized to be composed of both LCH (4C) and LCS (4D). H&E stain. (4A) x10; (4B) x40; (4C) and (4D) x100.
IMMUNOHISTOCHEMICAL FINDINGS
Before the establishment of immunohistochemical identification of CD207, ultrastructural demonstration of Birbeck granules was necessary to characterize a cell to be LC, irrespective of its reactive or neoplastic nature. CD207 is a type II transmembrane C-type lectin associated with the formation of Birbeck granules. Other markers often employed for the identification of LCs include CD1a (a molecule required for LCs to present antigen to NKT cells), S100 protein, CD68 (KP-1), CD68R (PG-M1), lysozyme, HLA-DR,21 CD4, fascin, Factor XIIIa, and cyclin D1. Regarding two types of cells closely related to LCs, interdigitating dendritic cells are S100 protein+, CD1a-, and CD207-, whereas indeterminate dendritic cells are S100 protein+, CD1a+, CD207-, in general. Markers for FDC, such as CD21, CD23, and CD35, are negative in most instances. There appears to be no clear difference in phenotypic profiles between LCH and LCS, although there may be a tendency for lower expression in LCS in terms of molecular density in a given cell, rate of positive cells in a given lesion, and rate of positive cases in a given cohort. A recent report has indicated that LCS cells are positive for cancer-associated B7 molecules including CD273 (B7-DC), CD274 (B7-H1), CD276 (B7-H3), and CD277 (B7-H4).28
In our LCS cases, all four were positive for CD207, CD1a, S100 protein, CD68, CD68R, cyclin D1, Factor XIIIa, and fascin, and three for CD4 (Fig. 5). In case when it is difficult to characterize a given lesion to be either LCH or LCS, the rate of Ki-67 expression can be helpful as in other fields of tumor pathology (Fig. 6).
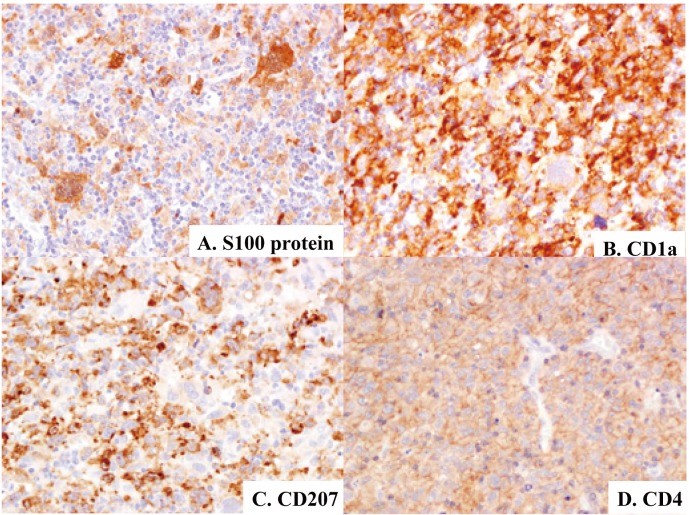
Fig. 5.
Immunophenotypic features of Langerhans cell sarcoma (case-1). Proliferating cells, including pleomorphic/atypical multinucleated giant cells, are positive for S100 protein (5A), CD1a (5B), CD207 (5C), and CD4 (5D). Immunoperoxidase stain with hematoxylin counterstain. (5A)-(5D) x40.
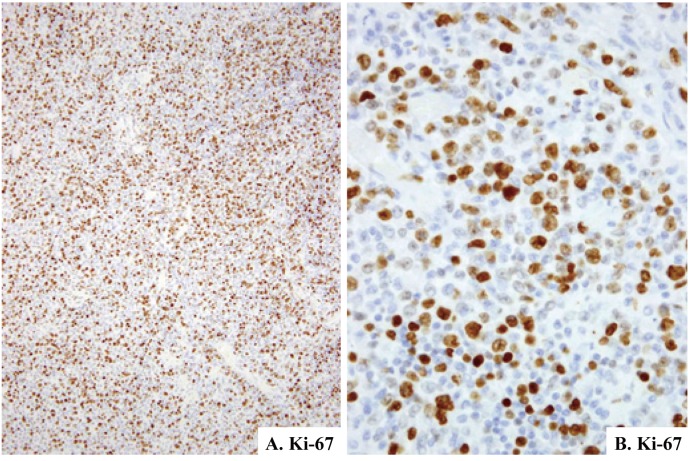
Fig. 6.
Ki-67 expression in Langerhans cell sarcoma (case-2). Positive rate is estimated at 30% (6A & 6B). Immunoperoxidase stain with hematoxylin counterstain. (6A) x10; (6B) x40.
CHROMOSOMAL AND GENETIC FINDINGS
Currently, no recurrent chromosomal abnormalities have been reported in LC proliferations. Clonality was initially shown in some or the majority of female patients with LCH by examinations targeting several genes locating on X chromosomes, one of which had been inactivated during early fetal period. These include genes coding for phosphoglycerate kinase, hypoxanthine phosphoribosyl transferase, and human androgen receptor (HUMARA), and the latter became a major target because of its high frequency of polymorphism. This analysis was initially performed by Southern blot hybridization,2,3 but later by methylation-specific polymerase-chain reaction amplification.4 Interestingly, 71% of pulmonary LCH was shown to be non-clonal by the latter method.4 Although this finding was important at that time, it might not have a large impact on the pathogenesis of LCH due to male preponderance of the disease. However, possibility of the neoplastic nature of LCH is further supported by the identification of the BRAF mutation in more than half of the patients with the disease.5 By these clonality analyses, the non-neoplastic counterpart of LC proliferation, i.e., LC hyperplasia, has been better characterized. Although the majority of pulmonary LCH has been shown to be non-clonal by HUMARA amplification,4 the possibility of oligoclonal proliferations, which would be characterized as non-clonal by this method, remains to be clarified.
Another important finding is the development of LCH or LCS in patients with non-LC lymphoid and myeloid neoplasms. The antedating or co-existing neoplasms include B-acute lymphoblastic leukemia,29 chronic lymphocytic leukemia/small lymphocytic lymphoma,30 or hairy-cell leukemia.31,32 In these patients, common clonal origin of both LC proliferation and leukemia/lymphoma was confirmed by the rearrangement pattern of the immunoglobulin heavy chain gene. Similarly, common clonal origin of LCH and T-acute lymphoblastic leukemia33 or LCH and myeloid sarcoma34 was confirmed by the rearrangement pattern of the T-cell receptor-γ gene or presence of trisomy 8 in both neoplasms, respectively. After these earlier reports, articles describing such cases are increasing and the associated neoplasms are expanding beyond those listed above. Erdheim-Chester disease, a non-LC proliferative process, is one such example, and this process and LCH in a single individual had the BRAF V600E mutation.35 This phenomenon, which can be explained by trans-differentiation, has also been reported in patients with histiocytic sarcoma.29,36,37 Hematopathologic features of such a case are shown in Figs. 7 & 8, although clonal relation of the two lesions could not be examined. Recently, Murakami and associates have shown that Merkel-cell polyoma virus (MCPyV)-related molecules are present in more than half of LCH cases as well as in some dermatopathic lymphadenopathy cases.38 Furthermore, they found that three of our seven LCS cases were positive for the viral DNA sequences.39 Based on these findings together with the requirement of interleukin-1 autocrine loop in Raf-induced transformation of NIH 3T3 cells,40 they have proposed that LCH is an inflammatory disorder prolonged by the BRAF mutation and that MCPyV is involved in the pathogenesis of LCH by triggering the IL-1 loop.41 It is, therefore, expected that these findings may be another ‘piece of the LCH puzzle’9 in terms of both oncogenesis and differential diagnosis of LC proliferations (See next section.).
Fig. 7.
Association of T-lymphoblastic lymphoma and Langerhans cell histiocytosis in a single lymph node. There are light and dark zones around a reactive lymph follicle (7A). The light zone is composed of cells with features of Langerhans cells (7B, upper; 7C), whereas the dark zone with features of lymphoblastoid cells (7B, lower; 7D). H&E stain. (7A) x10; (7B) x40; (7C) and (7D), x100.
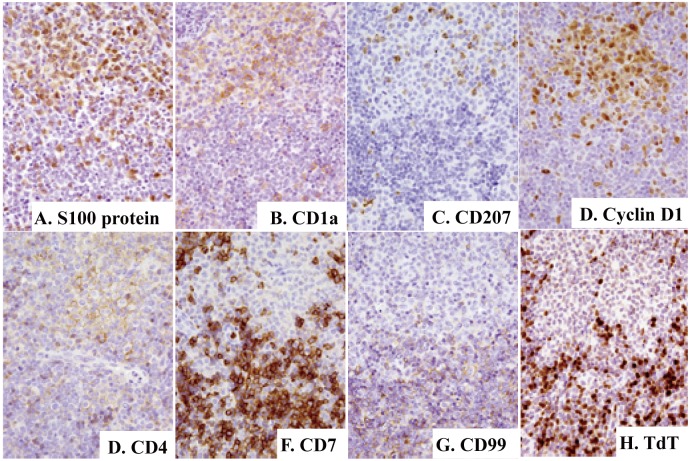
Fig. 8.
Immunophenotypic features of T-lymphoblastic lymphoma/Langerhans cell histiocytosis with Fig. 7B being a corresponding area. Majority or some of the cells in the light zone (upper) are positive for S100 protein (8A), CD1a (8B), CD207 (8C), cyclin D1 (8D), and CD4 (8E), whereas cells in the dark zone (lower) are positive for CD1a (8B), CD4 (8E), CD7 (8F), CD99 (8G), and terminal deoxynucleotidyl transferase (TdT)(8H). Immunoperoxidase stain with hematoxylin counterstain. (8A)-(8H) x40.
DIFFERENTIAL DIAGNOSIS
Although LC neoplasms and FDC neoplasms are included in a single category, i.e., dendritic-cell neoplasm, based on the functional similarity with their normal counterparts in the WHO-200842 and WHO-2016,18 they are quite different each other in their differentiation pathways (myeloid stem-cell origin for the former and mesenchymal stem-cell origin for the latter).42 Similarly, plasmacytoid dendritic-cell neoplasm, a blastic form of which is included in a category of ‘acute myeloid leukemia and related precursor neoplasms’ in the WHO-2008,43 is better fit to the category of dendritic-cell neoplasm (Table 2).
Table 2. Normal and neoplastic counterparts of histiocytic and dendritic cell neoplasms and their current classification.
| Origin | Normal counterpart | Neoplastic counterpart | Category in WHO-200842 |
|---|---|---|---|
| Myeloid | Langerhans cell | Langerhans cell neoplasm | Dendritic cell neoplasm |
| Myeloid | Indeterminate dendritic cell | Indeterminate dendritic cell tumor | Dendritic cell neoplasm |
| Myeloid | Interdigitating dendritic cell | Interdigitating dendritic cell sarcoma | Dendritic cell neoplasm |
| Myeloid | Plasmacytoid dendritic cell | Blastic plasmacytoid dendritic cell neoplasm | AML and related precursor neoplasm |
| Myeloid | Histiocyte | Histiocytic sarcoma | Histiocytic neoplasm |
| Mesenchymal | Follicular dendritic cell | Follicular dendritic cell sarcoma | Dendritic cell neoplasm |
| Mesenchymal | Fibroblastic reticular cell | Fibroblastic reticular cell tumor | Dendritic cell neoplasm |
AML, acute myeloid leukemia
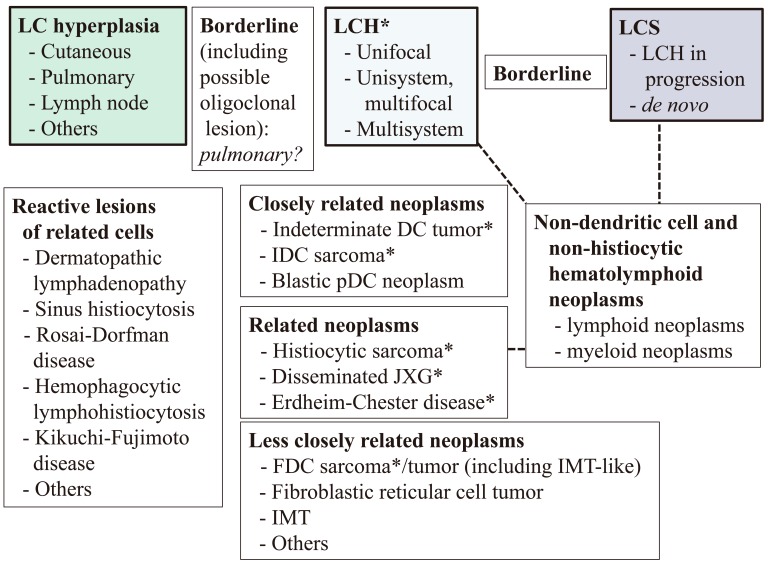
We summarized reactive and neoplastic proliferations of LC and related cells for differential diagnosis (Fig. 9). The outline of the scheme consists of three principles. 1) As there may be a spectrum among LC hyperplasia, LCH, and LCS, borderline lesions had better be inserted between the respective two lesions. The distinction of LC hyperplasia10,11 from LCH can be made by clonality/mutation analyses, but the distinction of LCH from LCS may occasionally be difficult as exemplified by the abstractive, but not specific, definition of LCS by the WHO-2008.15 In this regard, our finding for MCPyV, i.e., the viral load is higher in LCS than in LCH,39 may be helpful for differential diagnosis. 2) LCH and LCS can develop as a result of trans-differentiation of non-LC hematolymphoid neoplasms as mentioned earlier. 3) The differential diagnosis of LC proliferations from other neoplasms may be stratified based on the degree of similarity with normal counterparts of the latter to LC, i.e., closely related (indeterminate and interdigitating dendritic cells), related (non-LC-type histiocytes and plasmacytoid dendritic cells), or less closely related (FDCs and fibroblastic reticular cells). This stratification may be helpful to determine the target molecules in immunophenotypic examinations. Although the BRAF V600E mutation has been reported in indeterminate-cell tumor, interdigitating dendritic-cell sarcoma,44 Erdheim-Chester disease,35 histiocytic sarcoma, and FDC sarcoma45 as well as LC proliferations, the mutation has also been seen in non-hematolymphoid neoplasms, such as carcinomas, astrocytic neoplasms, and melanoma, showing that it is not lineage nor disease specific.
Fig. 9.
Outline of dendritic- and related-cell proliferations based on the morphologic features of Langerhans cell sarcoma, molecular findings on Langerhans cell proliferation, and degree of similarity with their normal counterparts. See text for details.
* BRAF mutation is present in some cases.
DC, dendritic cell; FDC, follicular dendritic cell; IDC, interdigitating dendritic cell; IMT, inflammatory myofibroblastic tumor; JXG, juvenile xanthogranuloma; LC, Langerhans cell; LCH, Langerhans cell histiocytosis; LCS, Langerhans cell sarcoma; pDC, plasmacytoid dendritic cell
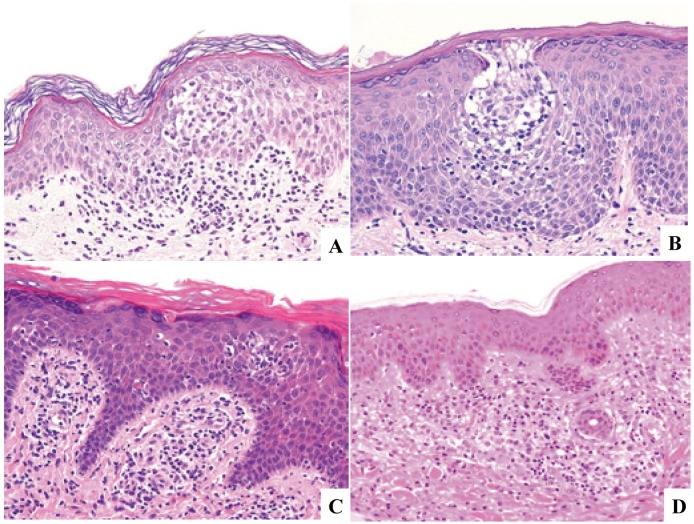
LC hyperplasia has already been well characterized in the skin and is categorized into either epidermal or dermal lesions. Epidermal LC hyperplasia, referred to as pseudo-Pautrier abscess or LC micro-granuloma by some authors,10,11 can be seen in dermatitis associated with spongiosis, psoriasiform tissue reaction, and lichenoid dermatitis, and requires differentiation from cutaneous T-cell lymphoma as well as LCH (Fig. 10). Dermal LC hyperplasia can be practically separated into either that associated with scabies46 or not,47 and the latter includes drug eruption (Fig. 10). LC hyperplasia can also be seen in other organs, such as lymph nodes, in which some or the majority of dermatopathic lymphadenopathy cases are probably one of LC hyperplasia because a considerable number of CD207+ histiocytes is present (Fig. 11). It is also of interest to characterize pulmonary LC proliferations in this respect.
Fig. 10.
Cutaneous Langerhans cell hyperplasia. Intraepidermal Langerhans cell hyperplasia (10A & 10B) may be referred to as pseudo-Pautrier abscess and a vase-like shape in some instances. Dermal Langerhans cell hyperplasia seen in drug eruption is associated with (10C) or without (10D) a lichenoid change. H&E stain. (10A)-(10D) x20.
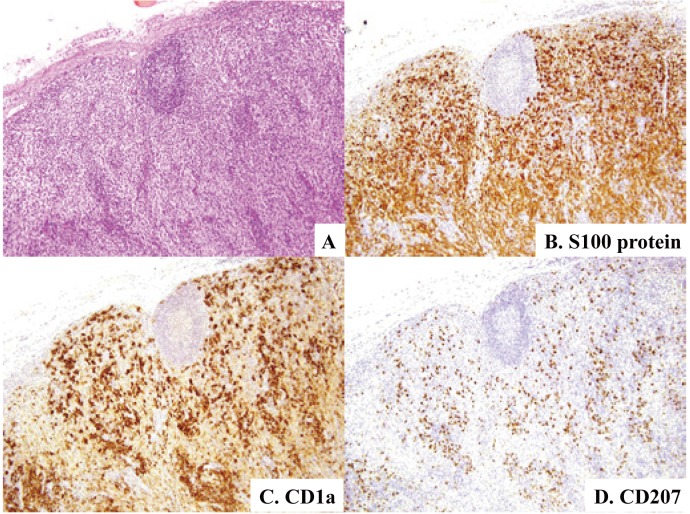
Fig. 11.
Dermatopathic lymphadenopathy. Expansion of paracortical areas with proliferation of pale-appearing cells together with decrease in lymphocytes is evident (11A). In addition to the great many cells positive for S100 protein (11B) and many cells positive for CD1a (11C), there is a considerable number of cells positive for CD207 (11D). H&E stain (11A) and immunoperoxidase stain with hematoxylin counterstain (11B-11D). (11A)-(11D) x10.
PROGNOSIS
The clinical course of LCH depends on the degree of systems or organs involved, with bone marrow, liver, or pulmonary involvement being listed as poor prognostic factors.16 However, isolated pulmonary involvement in pediatric LCH is no longer considered as a significant risk of death.48 More than 99% of patients with LCH having a single site involvement are alive,16 while the mortality rate of those with multisystem involvement is 50-66%.17 The fourth category, congenital self-healing reticulohistiocytosis, whose prognosis is generally excellent, may recur or progress to systemic disease.49 In the series described above,19 four of 13 patients (31%) with LCH and four of eight patients (50%) with LCS died of the disease. The survival of patients with adult pulmonary LCH is shorter than that of the general population.23
CONCLUSION
LCH as well as LCS is probably a neoplastic disorder by the identification of clonality and the BRAF mutation, and this has resulted in better characterization of LC hyperplasia with a possible spectrum of LC proliferations consisting of three categories. However, further studies are necessary to correctly identify LCH and LCS, the differential diagnosis of which still depends on the classical criteria in daily pathology practice. A recent discovery of trans-differentiation of non-LC hematolymphoid proliferations to LCH or LCS and the association of MCPyV in LC proliferations may give novel approaches to the better understanding of LC proliferations.
Note added in proof. After submission of our manuscript for this article, a review article entitled, “Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages”, has been published (Emile JF, Abla O, Fraitag S, Horne A, Haroche J, et al. Blood 127: 2672-2681, 2016).
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest.
REFERENCES
- 1.Coppes-Zanting A, Egeler RM: The Langerhans cell histiocytosis X files revealed. Br J Haematol 116: 3-9, 2002. 10.1046/j.1365-2141.2002.03232.x [DOI] [PubMed] [Google Scholar]
- 2.Willman CL, Busque L, Griffith BB, Favara BE, McClain KL, et al. : Langerhans’-cell histiocytosis (histiocytosis X): A clonal proliferative disease. N Engl J Med 331: 154-160, 1994. 10.1056/NEJM199407213310303 [DOI] [PubMed] [Google Scholar]
- 3.Yu RC, Chu C, Buluwela L, Chu AC: Clonal proliferation of Langerhans cells in Langerhans cell histiocytosis. Lancet 343: 767-768, 1994. 10.1016/S0140-6736(94)91842-2 [DOI] [PubMed] [Google Scholar]
- 4.Yousem SA, Colby TV, Chen YY, Chen WG, Weiss LM: Pulmonary Langerhans’ cell histiocytosis: molecular analysis of clonality. Am J Surg Pathol 25: 630-636, 2001. 10.1097/00000478-200105000-00010 [DOI] [PubMed] [Google Scholar]
- 5.Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, et al. : Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood 116: 1919-1923, 2010. 10.1182/blood-2010-04-279083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, et al. : Mutations of the BRAF gene in human cancer. Nature 417: 949-954, 2002. 10.1038/nature00766 [DOI] [PubMed] [Google Scholar]
- 7.Tiacci E, Trifonov V, Schiavoni G, Holmes A, Kern W, et al. : BRAF mutations in hairy-cell leukemia. N Engl J Med 364: 2305-2315, 2011. 10.1056/NEJMoa1014209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arcaini L, Zibellini S, Boveri E, Riboni R, Rattotti S, et al. : The BRAF V600E mutation in hairy cell leukemia and other mature B-cell neoplasms. Blood 119: 188-191, 2012. 10.1182/blood-2011-08-368209 [DOI] [PubMed] [Google Scholar]
- 9.Nichols KE, Arceci RJ: BRAF, a piece of the LCH puzzle. Blood 116: 1825-1827, 2010. 10.1182/blood-2010-06-289934 [DOI] [PubMed] [Google Scholar]
- 10.Candiago E, Marocolo D, Manganoni MA, Leali C, Facchetti F: Nonlymphoid intraepidermal mononuclear cell collections (pseudo-Pautrier abscesses): A morphologic and immunophenotypic characterization. Am J Dermatopathol 22: 1-6, 2000. 10.1097/00000372-200002000-00001 [DOI] [PubMed] [Google Scholar]
- 11.LeBoit PE, Epstein BA: A vase-like shape characterizes the epidermal-mononuclear cell collections seen in spongiotic dermatitis. Am J Dermatopathol 12: 612-616, 1990. 10.1097/00000372-199012000-00014 [DOI] [PubMed] [Google Scholar]
- 12.Tazi A: Adult pulmonary Langerhans’ cell histiocytosis. Eur Respir J 27: 1272-1285, 2006. 10.1183/09031936.06.00024004 [DOI] [PubMed] [Google Scholar]
- 13.Suri HS, Yi ES, Nowakowski GS, Vassallo R: Pulmonary Langerhans cell histiocytosis. Orphanet J Rare Dis 7: 16, 2012. 10.1186/1750-1172-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss LM, Grogan TM, Müller-Hermelink HK, Stein H, Dura T, et al.: Langerhans cell histiocytosis. In: Jaffe ES, Harris NL, Stein H, Vardiman JW (eds): World Health Organization Classification of Tumours. Pathology and Genetics. Tumours of Haematopoietic and Lymphoid tissues. Lyon, IARC Press, pp.280-282, 2001 [Google Scholar]
- 15.Weiss LM, Grogan TM, Pileri SA, Favara B, Dura T, et al.: Langerhans cell sarcoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW (eds): World Health Organization Classification of Tumours. Pathology and Genetics. Tumours of Haematopoietic and Lymphoid tissues. Lyon, IARC Press, p.283, 2001 [Google Scholar]
- 16.Jaffe R, Weiss LM, Facchetti F: Tumours derived from Langerhans cells. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, et al. (eds): WHO Classification of Tumours of Haematopoietic and Lymphoid tissues. 4th ed, Lyon, IARC Press, pp.358-360, 2008 [Google Scholar]
- 17.Zelger B, Rapini RP, Burg G: Langerhans cell histiocytosis. In: LeBoit PE, Burg G, Weedon D, Sarasin S (eds): World Health Organization Classification of Tumours. Pathology and Genetics. Skin Tumours. Lyon, IARC Press, pp.217-219, 2006 [Google Scholar]
- 18.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, et al. : The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127: 2375-2390, 2016. 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, et al. : The 2016 revision of the World Health Organization classification of myeloid neoplasms and acute leukemias. Blood 127: 2391-2405, 2016. 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 20.Lymphoma Study Group of Japanese Pathologist : The World Health Organization classification of malignant lymphomas in Japan: incidence of recently recognized entities. Pathol Int 50: 696-702, 2000. 10.1046/j.1440-1827.2000.01108.x [DOI] [PubMed] [Google Scholar]
- 21.Pileri S, Grogan TM, Harris NL, Banks P, Campo E, et al. : Tumors of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology 41: 1-29, 2002. 10.1046/j.1365-2559.2002.01418.x [DOI] [PubMed] [Google Scholar]
- 22.Watanabe R, Tatsumi K, Hashimoto S, Tamakoshi A, Kuriyama T: Respiratory Failure Research Group of Japan. Clinico-epidemiological features of pulmonary histiocytosis X. Intern Med 40: 998-1003, 2001. 10.2169/internalmedicine.40.998 [DOI] [PubMed] [Google Scholar]
- 23.Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH: Clinical outcomes of pulmonary Langerhans’-cell histiocytosis in adults. N Engl J Med 346: 484-490, 2002. 10.1056/NEJMoa012087 [DOI] [PubMed] [Google Scholar]
- 24.Novice FM, Collison DW, Kleinsmith DM, Osband ME, Burdakin JH, et al. : Letterer-Siwe disease in adults. Cancer 63: 166-174, 1989. [DOI] [PubMed] [Google Scholar]
- 25.Yuasa M, Fujiwara S, Oh I, Yamaguchi T, Fukushima N, et al. : Rapidly progressing fatal adult multi-organ Langerhans cell histiocytosis complicated with fatty liver disease. J Clin Exp Hematop 52: 121-126, 2012. 10.3960/jslrt.52.121 [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto K, Pritzker MS: Electron microscopic study of reticulohistiocytoma: an unusual case of congenital, self-healing reticulohistiocytosis. Arch Dermatol 107: 263-270, 1973. 10.1001/archderm.1973.01620170071020 [DOI] [PubMed] [Google Scholar]
- 27.Schaumburg-Lever G, Rechowicz E, Fehrenbacher B, Möller H, Nau P: Congenital self-healing reticulohistiocytosis: A benign Langerhans cell disease. J Cutan Pathol 21: 59-66, 1994. 10.1111/j.1600-0560.1994.tb00692.x [DOI] [PubMed] [Google Scholar]
- 28.Li H, Wang C, Guo G, Gao C, Wu Y, et al. : The characteristic expression of B7-associated proteins in Langerhans cell sarcoma. Acta Histochem 114: 733-743, 2012. 10.1016/j.acthis.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 29.Ratei R, Hummel M, Anagnostopoulos I, Jähne D, Arnold R, et al. : Common clonal origin of an acute B-lymphoblastic leukemia and a Langerhans’ cell sarcoma: evidence for hematopoietic plasticity. Haematologica 95: 1461-1466, 2010. 10.3324/haematol.2009.021212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao H, Xi L, Raffeld M, Feldman AL, Ketterling RP, et al. : Clonally related histiocytic/dendritic cell sarcoma and chronic lymphocytic leukemia/small lymphocytic lymphoma: A study of seven cases. Mod Pathol 24: 1421-1432, 2011. 10.1038/modpathol.2011.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furmanczyk PS, Lisle AE, Caldwell RB, Kraemer KG, Mercer SE, et al. : Langerhans cell sarcoma in a patient with hairy cell leukemia: common clonal origin indicated by identical immunoglobulin gene rearrangements. J Cutan Pathol 39: 644-650, 2012. 10.1111/j.1600-0560.2012.01873.x [DOI] [PubMed] [Google Scholar]
- 32.Muslimani A, Chisti MM, Blenc AM, Boxwala I, Micale MA, et al. : Langerhans/dendritic cell sarcoma arising from hairy cell leukemia: A rare phenomenon. Ann Hematol 91: 1485-1487, 2012. 10.1007/s00277-011-1399-5 [DOI] [PubMed] [Google Scholar]
- 33.Feldman AL, Berthold F, Arceci RJ, Abramowsky C, Shehata BM, et al. : Clonal relationship between precursor T-lymphoblastic leukaemia/lymphoma and Langerhans-cell histiocytosis. Lancet Oncol 6: 435-437, 2005. 10.1016/S1470-2045(05)70211-4 [DOI] [PubMed] [Google Scholar]
- 34.Schmitt-Graeff AH, Duerkop H, Vollmer-Kary B, Haxelmans S, Nitschke R, et al. : Clonal relationship between Langerhans cell histiocytosis and myeloid sarcoma. Leukemia 26: 1707-1710, 2012. 10.1038/leu.2012.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hervier B, Haroche J, Arnaud L, Charlotte F, Donadieu J, et al. : Association of both Langerhans cell histiocytosis and Erdheim-Chester disease linked to BRAFV600E mutation. Blood 124: 1119-1126, 2014. 10.1182/blood-2013-12-543793 [DOI] [PubMed] [Google Scholar]
- 36.Zeng W, Meck J, Cheson BD, Ozdemirli M: Histiocytic sarcoma transdifferentiated from follicular lymphoma presenting as a cutaneous tumor. J Cutan Pathol 38: 999-1003, 2011. 10.1111/j.1600-0560.2011.01769.x [DOI] [PubMed] [Google Scholar]
- 37.Wang E, Papalas J, Hutchinson CB, Kulbacki E, Huang Q, et al. : Sequential development of histiocytic sarcoma and diffuse large B-cell lymphoma in a patient with a remote history of follicular lymphoma with genotypic evidence of a clonal relationship: A divergent (bilineal) neoplastic transformation of an indolent B-cell lymphoma in a single individual. Am J Surg Pathol 35: 457-463, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Murakami I, Matsushita M, Iwasaki T, Kuwamoto S, Kato M, et al. : Merkel cell polyomavirus DNA sequence in peripheral blood and tissue from patients with Langerhans cell histiocytosis. Hum Pathol 45: 119-126, 2014. 10.1016/j.humpath.2013.05.028 [DOI] [PubMed] [Google Scholar]
- 39.Murakami I, Matsushita M, Iwasaki T, Kuwamoto S, Kato M, et al. : High viral load of Merkel cell polyomavirus DNA sequences in Langerhans cell sarcoma tissues. Infect Agent Cancer 9: 15, 2014. 10.1186/1750-9378-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vale T, Ngo TT, White MA, Lipsky PE: Raf-induced transformation requires an interleukin 1 autocrine loop. Cancer Res 61: 602-607, 2001 [PubMed] [Google Scholar]
- 41.Murakami I, Matsushita M, Iwasaki T, Kuwamoto S, Kato M, et al. : Interleukin-1 loop model for pathogenesis of Langerhans cell histiocytosis. Cell Commun Signal 13: 13, 2015. 10.1186/s12964-015-0092-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaffe R, Pileri SA, Facchetti F, Jones DM, Jaffe ES: Histiocytic and dendritic cell neoplasms, introduction. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, et al. (eds): WHO Classification of Tumours of Haematopoietic and Lymphoid tissues. 4th ed, Lyon, IARC Press, pp.354-355, 2008 [Google Scholar]
- 43.Facchetti F, Jones DM, Petrella T: Blastic plasmacytoid dendritic cell neoplasm. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, et al. (eds): WHO Classification of Tumours of Haematopoietic and Lymphoid tissues. 4th ed, Lyon, IARC Press, pp.145-147, 2008 [Google Scholar]
- 44.O’Malley DP, Agrawal R, Grimm KE, Hummel J, Glazyrin A, et al. : Evidence of BRAF V600E mutation in indeterminate cell tumor and interdigitating dendritic cell sarcoma. Ann Diagn Pathol 19: 113-116, 2015. 10.1016/j.anndiagpath.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 45.Go H, Jeon YK, Huh J, Choi SJ, Choi YD, et al. : Frequent detection of BRAF(V600E) mutations in histiocytic and dendritic cell neoplasms. Histopathology 65: 261-272, 2014. 10.1111/his.12416 [DOI] [PubMed] [Google Scholar]
- 46.Bhattacharjee P, Glusac EJ: Langerhans cell hyperplasia in scabies: A mimic of Langerhans cell histiocytosis. J Cutan Pathol 34: 716-720, 2007. 10.1111/j.1600-0560.2006.00723.x [DOI] [PubMed] [Google Scholar]
- 47.Drut R, Peral CG, Garone A, Rositto A: Langerhans cell hyperplasia of the skin mimicking Langerhans cell histiocytosis: A report of two cases in children not associated with scabies. Fetal Pediatr Pathol 29: 231-238, 2010. 10.3109/15513811003789610 [DOI] [PubMed] [Google Scholar]
- 48.Ronceray L, Potschger U, Janka G, Gadner H, Minkov M: German Society for Pediatric H, German Society for Pediatric Hematology and Oncology, Langerhans Cell Histiocytosis Study Group. Pulmonary involvement in pediatric-onset multisystem Langerhans cell histiocytosis: effect on course and outcome. J Pediatr 161: 129-133, 2012. 10.1016/j.jpeds.2011.12.035 [DOI] [PubMed] [Google Scholar]
- 49.Longaker MA, Frieden IJ, LeBoit PE, Sherertz EF: Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol 31: 910-916, 1994. 10.1016/S0190-9622(94)70258-6 [DOI] [PubMed] [Google Scholar]