Description
A 40-year-old male patient presented at the outpatient department with ulcerative swelling with foul smelling discharge from lower half of abdomen for last 3 months. He had a history of haematuria with increased frequency of micturition for last 6 months accompanied by loss of appetite and subsequent weight loss. There was no history of nocturia, urgency or incontinence.
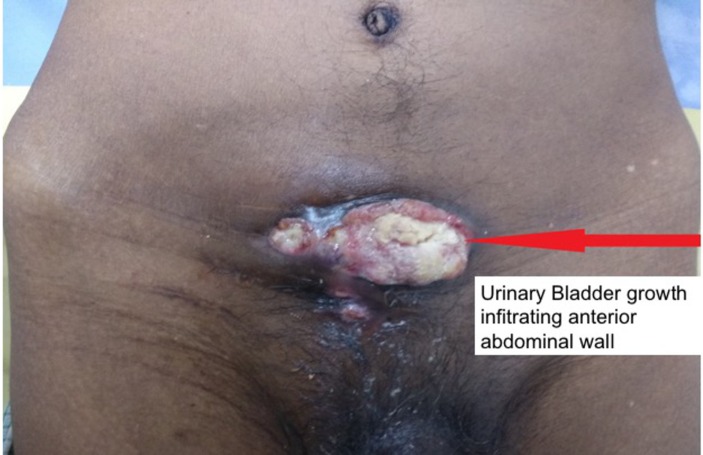
On physical examination, he was found to have an ulceroproliferative growth around 5×3 cm size in the infraumbilical region with urinary discharge near one end as shown in figure 1.
Figure 1.
Ulceroproliferative growth in the infraumbilical region arising from urinary bladder.
It was small to begin with and progressively increased in size to reach the present state.
The growth demonstrated an unhealthy ulcer with everted margins and areas of slough. It was friable and bled actively on manipulation. Rest of the abdominal examination was unremarkable. His complete blood haemogram revealed a low Hb of 8.5 gm/dL and normal renal function test. Urine culture revealed growth of E coli for which appropriate antibiotics were started.
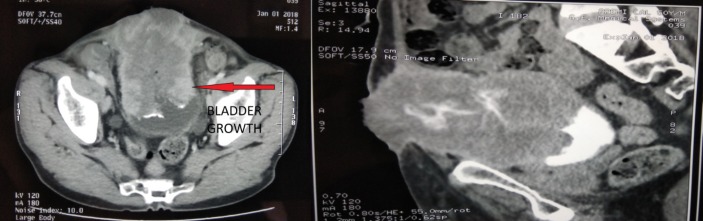
Imaging was ordered for further evaluation of the mass. On ultrasound, he was found to have a growth around 6×8 cm in size involving the anterior wall and dome of the urinary bladder reaching till abdominal wall with absence of bilateral hydronephrosis. Additional evaluation with contrast-enhanced CT confirmed a large exophytic mass lesion 8×7 cm in size arising from the anterior wall and dome of urinary bladder and infiltrating the anterior abdominal wall musculature with loss of fat planes. The ulcerative growth reached up to skin as shown in figure 2.
Figure 2.
Transverse and sagittal CT sections showing urinary bladder growth infiltrating abdominal wall.
The surrounding structures were free from invasion. A further evaluation by CT thorax and 18-fluorodeoxyglucose PET(Positron Emission Tomography) scan did not reveal any regional or distant metastasis. Colonoscopy did not show any primary cancer involving colon.
On rigid cystoscopy, there was diffuse mucosal erosion throughout the bladder wall. A solid necrotic growth was seen involving whole of dome and anterior wall of urinary bladder. Bilateral ureteric orifices showed clear efflux of urine with normal trigone. Multiple biopsies were taken.
On Histopathological Examination examination of the biopsy, the patient was found to have low-grade adenocarcinoma with desmoplastic reaction and atypical cells. The overlying urothelium was found to have only reactive changes, and underlying bladder muscle was invaded with cancerous cells. On further evaluation with immunohistochemistry (IHC), it was found positive for Cytokeratin20(CK 20) and Cytokeratin7(CK 7) and negative for β-catenin. Prostate-specific antigen levels were 0.36 ng/mL, and carcinoembryonic antigen levels were 1.30 ng/mL (normal value). The patient was diagnosed to have non-metastatic urachal adenocarcinoma (UC).
After proper counselling and consent, the patient was operated on by radical cystectomy with ileal conduit (non-refluxing type) and standard bilateral pelvic lymphadenectomy.
At 6 months follow-up, the patient was doing well with no evidence of recurrence of cancer.
UC is a rare cancer and accounts for 0.01% of all cancers in adults. First described in 1963 by Hue and Jacquin, this condition has a male predilection and a mean age of presentation of 50–60 years.1
The urachus originates from the involution of the allantois and cloaca, as an embryonic remnant, extending from the umbilicus to the bladder dome. Although it involutes with age, its patency may persist in a minor proportion of adults. Haematuria is the most common presentation.1
The criteria for pathological diagnosis of UC according to WHO 2016 include the following: (1) tumour localised in the dome/anterior wall; (2) carcinoma epicentre in the bladder wall; (3) absence of cystitis/glandularis beyond the dome/anterior wall; (4) absence of urothelial neoplasia in the bladder (5) and absence of a known primary tumour elsewhere.2
IHC plays a pivotal role in differentiating primary UC from secondary ones. β-catenin, CK7 and CK20 are the commonly used markers. In our case, diffuse nuclear β-catenin was negative, and both CK7 and CK20 were positive. These help to differentiate UC of enteric subtype from colonic adenocarcinoma which are β-catenin+ and CK7−.
The effective role of adjuvant or neoadjuvant chemotherapy for UC, largely based on 5- fluorouracil and cisplatin, is not yet established. It is limited to patients with positive margin or metastatic disease. The choice of protocols, both chemotherapy and radiotherapy, is based on isolated case reports at present.3
Currently, surgery is the treatment of choice for UC. The gold standard surgical approach includes excision of the urachus, umbilicus and partial/radical cystectomy, combined with bilateral pelvic lymphadenectomy. We did a wide excision of the anterior abdominal wall (using component separation) with removal of umbilicus and urachus along with radical cystectomy with ileal conduit and standard bilateral pelvic lymphadenopathy.
The most significant predictor of prognosis is surgical margin status. The surgical margin in our case was negative.
Learning points.
Primary urachal adenocarcinoma is a rare entity. It has to be differentiated from adenocarcinomas arising from other organs in vicinity like colon.
The disease can present with local or distant metastasis. A thorough metastatic evaluation is necessary. The prognosis is poor with metastasis.
Surgical excision with negative margin is the gold standard of treatment. Chemoradiation is reserved for patients with positive margin or metastasis.
Footnotes
Contributors: AA: concept, design, processing, writing manuscript and critical analysis. SA: concept, writing manuscript and critical analysis. SP: processing, writing manuscript and critical analysis. SS: concept, supervision, writing manuscript and critical analysis.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bao B, Hatem M, Wong JK. Urachal adenocarcinoma: a rare case report. Radiol Case Rep 2017;12:65–9. 10.1016/j.radcr.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gopalan A, Sharp DS, Fine SW, et al. Urachal carcinoma: a clinicopathologic analysis of 24 cases with outcome correlation. Am J Surg Pathol 2009;33:659–68. 10.1097/PAS.0b013e31819aa4ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paner GP, Lopez-Beltran A, Sirohi D, et al. Updates in the pathologic diagnosis and classification of epithelial neoplasms of urachal origin. Adv Anat Pathol 2016;23:71–83. 10.1097/PAP.0000000000000110 [DOI] [PubMed] [Google Scholar]