Abstract
Cell adhesion molecule 1 (CADM1) is a cell adhesion molecule that is expressed in brain, liver, lung, testis, and some kinds of cancer cells including adult T-cell leukemia/lymphoma (ATLL). Recent studies have indicated the involvement of CADM1 in cell-cell contact between cytotoxic T-lymphocytes and virus infected cells. We previously reported that cell-cell interaction between lymphoma cells and macrophages induces lymphoma cell proliferation. In the present study, we investigated whether CADM1 is associated with cell-cell interaction between several human lymphoma cell lines and macrophages.
CADM1 expression was observed in the ATLL cell lines, ATN-1, ATL-T, and ATL-35T, and in the B cell lymphoma cell lines, TL-1, DAUDI, and SLVL, using western blotting. Significant cell-cell interaction between macrophages and ATN-1, ATL-T, ATL-35T and MT-2, DAUDI, and SLVL cells, as assessed by induction of cell proliferation, was observed. Immunohistochemical analysis of human biopsy samples indicated CADM1 expression in 10 of 14 ATLL cases; however, no case of follicular lymphoma or diffuse large B-cell lymphoma was positive for CADM1. Finally, the interaction of macrophages with cells of the CADM1-negative ED ATLL cell line and CADM1-transfected ED cells was tested. However, significant cell-cell interaction between macrophage and CADM1-transfected ED cells was not observed. We conclude that CADM1 was not associated with cell-cell interaction between lymphoma cells and macrophages, although CADM1 may be a useful marker of ATLL for diagnostic procedures.
Keywords: CADM1, TSLC1, ATLL, macrophage, TAM
INTRODUCTION
Cell adhesion molecule 1 (CADM1) is a cell adhesion molecule that is expressed on in brain, liver, lung, testis, and some kinds of cancer cells.1,2 CADM1 was first identified as a spermatogenic immunoglobulin superfamily member in a cDNA library of mouse testis. It was later found to be involved in cell-cell interaction between spermatogenic cells and Sertoli cells via binding to the poliovirus receptor.3-5 CADM1 was subsequently found to be expressed on mast cells and to be involved in cell-cell contact of mast cells with fibroblasts.6 CADM1 was also demonstrated to be expressed in lung adenocarcinoma cells and esophageal squamous cell carcinoma, where it was considered to act as a tumor suppressor.7-9 Adult T-cell leukemia/lymphoma (ATLL) is a T-cell leukemia/lymphoma that is associated with long-term infection of human T-cell leukemia virus type 1 (HTLV-1).10-12 CADM1 is expressed on ATLL cells and is involved in the attachment of ATLL cells to vascular endothelial cells.13 CADM1 has also been shown to be important for lymphoma proliferation and organ infiltration.14 The function of CADM1 in cancer cells therefore appears to differ depending on the origin of the cell in which it is expressed.
Recent studies have also indicated that CADM1 is involved in cell-cell contact between immune cells and target cells. CADM1 was found to be a ligand of class I-restricted T cell-associated molecule (CRTAM) that is expressed on activated natural killer (NK) cells and T-cells.15 It was demonstrated that NK cells and cytotoxic T-cells (CTL) recognize cancer cells and that the cytotoxicity of these immune cells is mediated by the binding of CADM1 and CRTAM.16 CADM1 is considered as a marker for high-risk HTLV-1 carriers since T-cells infected with human T cell lymphotropic virus-1 (HTLV-1) express CADM1.17 CADM1 expressed on HLTV-1-infected T cells was shown to be associated with enhanced susceptibility to CTL responses.18
Macrophages are critical components of stromal cells in the tumor microenvironment, and are considered as target cells for anti-cancer therapy.19,20 We previously reported that a high density of M2-like protumor macrophages was closely related to a worse clinical course of ATLL patients, and that direct cell-cell interaction between ATLL cells and macrophages induced lymphoma cell proliferation.21 We also observed similar interactions between macrophages and B-cell lymphoma cell lines in in vitro studies.22 However, the detailed mechanisms of direct cell-cell contact between protumor macrophages and lymphoma cells remain unclear. In the present study, we therefore investigated whether CADM1 is associated with cell-cell contact between lymphoma cells and macrophages.
MATERIALS AND METHODS
Macrophage culture
Peripheral blood mononuclear cells (PBMCs) were obtained from three healthy volunteer donors in accordance with protocols approved by the Kumamoto University Hospital Review Board. CD14+ monocytes were isolated by using CD14-microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). These monocytes were plated in 96-well plates (5000 cells/well) and were cultured with 2% human serum, granulocyte macrophage-colony stimulating factor (1 ng/mL, GM-CSF, WAKO, Tokyo, Japan), and macrophage-colony stimulating factor (100 ng/mL, M-CSF, WAKO) for 7 days to induce differentiation into macrophages.
Cell lines
The human ATLL cell lines (ED, ATL-T, ATL-2s, ATL-35T, MT-1, MT2) were previously established by Matsuoka M and Morikawa S.23,24 The ATLL cell line, ATN-1, and all B cell lymphoma cell lines (TL-1, DAUDI, SLVL, BALL1, NALM, and RAJI) were obtained from the RIKEN Cell Bank (Tsukuba, Japan). TL-1, DAUDI, and RAJI cells were established from patients with Burkitt lymphoma. SLVL cells were established from patient with splenic lymphoma with villous lymphocytes. BALL1 and NALM cells were from patients with patients with B-cell leukemia. All cell lines were maintained in RPMI supplemented with 10% fetal bovine serum. ED/neo (stably expressed neomycin-resistant gene) and ED/CADM1 (stably expressed CADM1 gene) cells, which stably express neomycin and CADM-1 genes, respectively, were previously established by Morishita K.2,13
Co-culture and 5-bromo-2’-deoxyuridine (BudU) incorporation assay
The following ATLL cell lines (10000 cells/well) and macrophages were directly co-cultured in 96-well plates for 2 days. BrdU incorporation was assayed using the BrdU Cell Proliferation Kit (Roche, Basel, Switzerland) according to the manufacturer’s protocol.
Western blot analysis
Cells were lysed in ice-cold lysis buffer (50 mM Tris pH 8.0, 1 mM EDTA, 150 mM NaCl, 1% NP-40) with a phosphatase inhibitor cocktail (R&D, Minneapolis, MN, USA) and a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Lysates were analyzed using SDS-PAGE and the proteins were blotted to a PVDF membrane. The PVDF membrane was reacted with antibodies against CADM1 (rabbit polyclonal, previously established by Wakayama T.) or with β-actin antibodies (Santa Cruz Biotechnology). HRP-Goat anti-mouse or anti-rabbit IgG (Invitrogen, Camarillo, CA) was used as the second antibody. Immunoreactive bands were visualized using the Pierce Western Blotting Substrate Plus Kit (Thermo Scientific, Rockford, IL) and ImageQuant LAS-4000 mini (Fuji Film, Tokyo, Japan).
Tissue samples
Paraffin-embedded tumor samples from lymph node biopsies diagnosed as ATLL (16 cases), follicular lymphoma (25 cases), and diffuse large B-cell lymphoma (56 cases) during 2005 – 2014 were examined. Written informed consent was obtained from all patients in accordance with protocols approved by the Kumamoto University Review Board. The same set of lymphoma cases of the present study were used in previous studies.25,26
Immunohistochemistry
Briefly, samples were first reacted with anti-CADM1 (x100), anti-CD3 (x1, rabbit monoclonal, Nichirei, Tokyo, Japan), and anti-Iba-1 (also known as allograft inflammatory factor 1; AIF1) (x1000, rabbit polyclonal, WAKO, Tokyo, Japan) antibodies, following which they were incubated with horseradish peroxidase (HRP)-labeled goat anti-rabbit secondary antibodies (Nichirei). Reactions were visualized using the diaminobenzidine substrate system (Nichirei). Antigen retrieval method was heat in pressure cooker with 1mM EDTA (pH8.0) buffer. Iba-1 was used as a marker for pan-macrophages.
Statistics
Statistical analysis of in vitro and in vivo data was carried out using StatMate III (ATOMS, Tokyo, Japan). Student’s t-test was used for statistical analysis, and a P value of <0.05 was considered statistically significant. All data shown for the cell-culture studies are representative of at least three independent experiments.
RESULTS
Significant interaction was observed between macrophages and ATN-1, ATL-T, ATL-35T, and MT-2 cells.
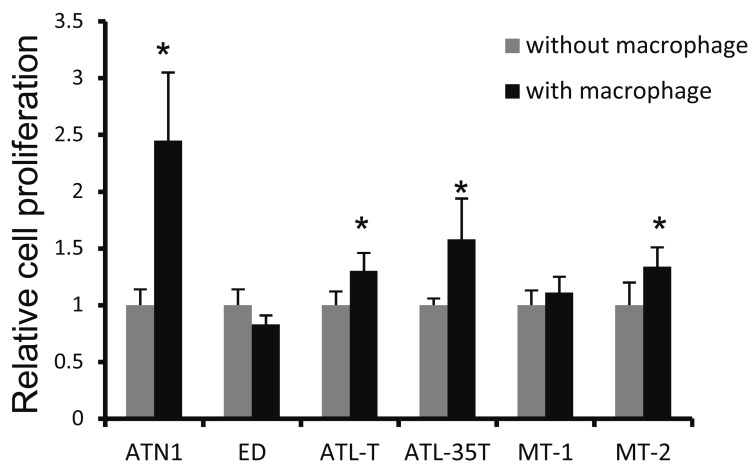
We previously demonstrated that the proliferative activity of three B-cell lymphoma cell lines (DAUDI, SLVL, RAJI) was significantly up-regulated by direct co-culture with macrophages.22 Regarding ATLL cell-macrophage interaction, we previously showed that BrdU incorporation into ATN-1 cells was significantly increased by co-culture with macrophages.21 In the present study, we tested the 6 ATLL cell lines, ATN-1, ED, ATL-T, ATL-35T, MT-1 and MT-2, to determine which of these cell line might be activated by cell-cell interaction with macrophages. The proliferation of ATN-1, ATL-T, ATL-35T, and MT-2 cells, but not of ED and MT-1 cells, as assessed using a BrdU incorporation assay, was significantly induced by co-culture with macrophages (Figure 1).
Fig. 1.
Assay of the proliferation of ATLL cell lines co-cultured with macrophages. Six ATLL cell lines were co-cultured with macrophages for 2 days and cell proliferation was then tested using a BrdU incorporation assay. Proliferation is shown relative to that of cells cultured in the absence of macrophages. *: p <0.05.
CADM1 expression was detected on several T- and B-cell lymphoma cell lines.
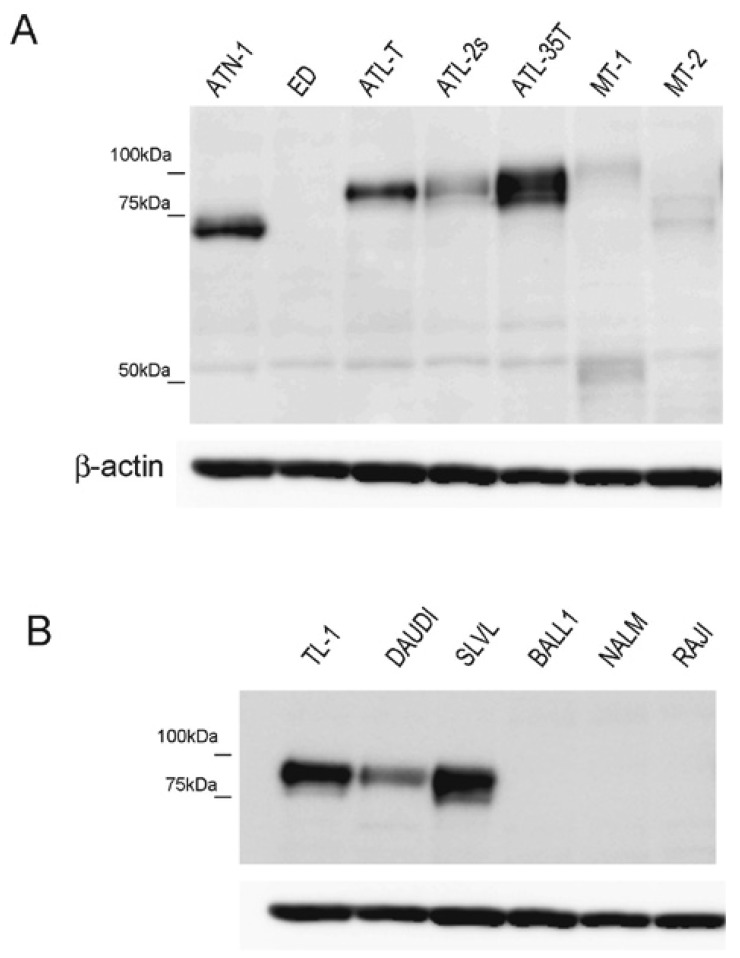
We then analyzed CADM1 expression in these 6 ATLL cell lines as well as in a number of B-cell lymphoma cell lines, by using western blotting. High expression of CADM1 was observed in ATN-1, ATL-T, ATL-35T, TL-1, DAUDI, and SLVL cells (Figure 2A, 2B). The results of figure 1 and 2 are summarized in Table 1, which indicates the cell-macrophage interaction and CADM-1 expression of each tested cell line. The macrophage-cell interaction of the B cells shown in the Table are data from a previous study.22
Fig. 2.
CADM1 expression in lymphoma cell lines. CADM1 expression in ATLL cell lines (uncropped western blot data) (A) and in B-cell lymphoma cell lines (cropped data) (B) was examined using western blot analysis. β-actin was blotted as a loading control.
Table 1. CADM-1 expression and cell-cell interaction.
| Cell-cell interaction | CADM-1 expression | |
|---|---|---|
| ATN-1 | Yes | Positive |
| ED | No | Negative |
| ATL-T | Yes | Positive |
| ATL-35T | Yes | Positive |
| MT-1 | No | Weak |
| MT-2 | Yes | Weak |
| ATL-2s | Not done | Positive |
| TL-1 | Not done | Positive |
| DAUDI | Yes | Positive |
| SLVL | Yes | Positive |
| BALL1 | Not done | Negative |
| NALM | Not done | Negative |
| RAJI | Yes | Negative |
CADM1 expression was observed in 10 of 14 ATLL cases, but not in FL and DLBCL.
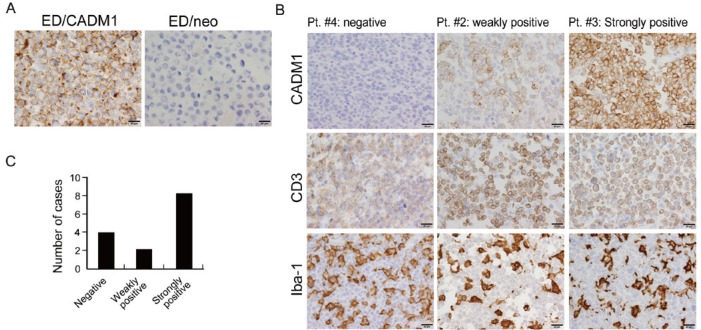
Immunostaining of CADM1 in human biopsy samples (n=14) diagnosed as ATLL was then performed to evaluate CADM1 expression in ATLL tissues. The specificity of the anti-CADM1 antibody was confirmed using immunohistochemistry of cell blocks of ED/neo and ED/CADM1 cells (Figure 3A). Representative negative, weak and strong CADM1 staining is shown in Figure 3B. Strong CADM1 expression was observed in 8 cases (57%), weak CADM1 expression was seen in 2 cases (14%), and CADM1-positive cells were not observed in 4 cases (29%) (figure 3C). The CADM1-positive signal was detected on the cell-surface membrane (Figure 3A,3B). No CADM1 expression was observed in all FL and DLBCL cases (data not shown).
Fig. 3.
Immunohistochemistry of CADM1 in lymphoma cases. (A) The specificity of the anti-CADM1 antibody was tested by using ED/CADM1 and ED/neo cell lines. (B) CADM1 expression in 3 representative cases of ATLL is shown. Lymphoma cells were positive for CD3, and infiltrating macrophages were labelled with anti-Iba-1 antibody. (C) A bar graph shows the total number of cases that were negative, weakly positive or strongly positive for CADM1 expression.
CADM1 expression was not associated with lymphoma cell-macrophage interaction.
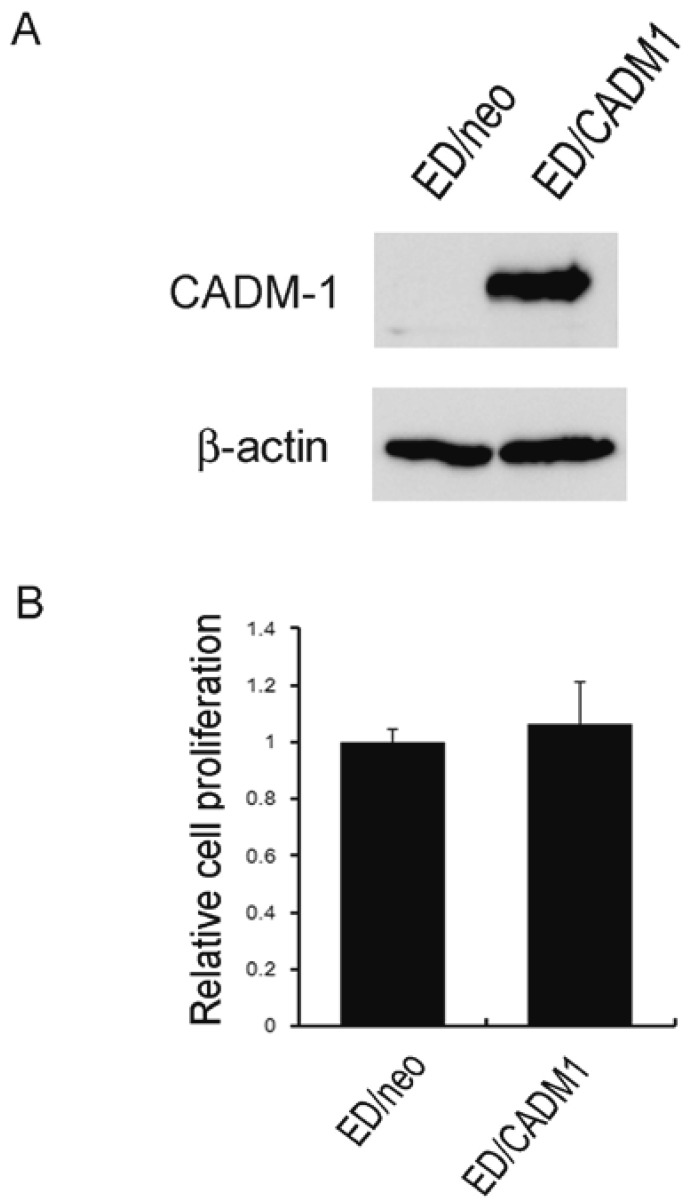
Next, ED/neo and ED/CADM1 cells were co-cultured with macrophages to evaluate if CADM1 is involved in cell-cell interaction between macrophages and lymphoma cells. CADM1 expression in the ED/CADM1 cells was confirmed by western blot analysis (Figure 4A). However, no increased BrdU incorporation was observed in ED/CADM1 cells compared to ED/neo cells following their co-culture with macrophages (Figure 4B).
Fig. 4.
The involvement of CADM1 in cell-cell interaction. (A) Western blot analysis of CADM1 expression in ED/neo and ED/CADM1 cells. β-actin was blotted as a loading control. (B) The ED/neo and ED/CADM1 cells were co-cultured with macrophages for 2 days, following which cell proliferation was assayed using a BrdU incorporation assay.
DISCUSSION
In the present study, our initial data suggested that adhesion molecules might be involved in cell-cell interaction between macrophages and lymphoma cells because direct cell-cell contact of these cells. We showed in figure 1 that activation of lymphoma cell proliferation by direct cell-cell contact with macrophages was observed for 4 of 6 ATLL cell lines that strongly expressed CADM1. Regarding B-cell lymphoma, 2 out of 3 cell lines that were shown to interact with macrophages in a previous study were positive for CADM1 expression. We therefore considered that CADM1 might potentially be involved in cell-cell contact and cell-cell interaction between macrophages and lymphoma cells. However, the data shown in figure 4, which indicated that CADM1- transfected ED cells did not show enhanced proliferation versus control cells following co-culture with macrophages, suggested that CADM1 might not be involved in cell-cell interaction between lymphoma cells and macrophages.
In the present study, we showed that 3 of 6 B-cell lymphoma cell lines tested express CADM1 although no clinical case of FL and DLBCL was positive for CADM1. It was previously shown in ATLL cell lines that DNA methylation influences the expression of CADM1.13 Epigenetic modification might therefore be involved in the regulation of CADM1 expression in cell lines; however, the detailed mechanisms of the regulation of CADM1 expression were not investigated in the present study. We previously demonstrated that the BALL1, NALM, and RAJI B-cell lymphoma cell lines interact with macrophages22; however, all of these cell lines were negative for CADM1 in the present study. This observation also suggested that CADM1 was not involved in cell-cell interaction between lymphoma cells and macrophages.
It is known that CADM1 directly interacts with a spectrin-actin-binding protein DAL1 and a membrane-associated guanylate kinase PALS2,27 however, we have no data of expression of these molecules on macrophages. Regarding ATLL-macrophage interaction, CD163 was suggested to be significantly associated with direct cell-cell interaction between lymphoma cells and macrophages.21 CD163 is a scavenger receptor that acts in the clearance of hemoglobin/haptoglobin complexes.25 The detailed mechanisms of CD163 activity have not been clarified; however, our observations using a murine sarcoma model indicated that CD163 is involved in protumor activation of macrophages (unpublished data). However, we have no significant data of interaction between CADM1 and CD163 in the present study (unpublished data). Identification of unknown CD163-ligands expressed on lymphoma cells will be of interest to elucidate the detailed mechanisms of lymphoma-macrophage interaction.
In conclusion, we newly demonstrated that the proliferative activity of some ATLL cell lines was elevated by direct cell-cell interaction with macrophages and we tested the involvement of the cell adhesion molecule CADM1 in the cell-cell interaction between ATLL cells and macrophages. However, CADM1 was not associated with cell-cell interaction between the lymphoma cells and macrophages. Further studies are necessary to elucidate the detailed mechanisms of direct cell-cell interaction between lymphoma cells and macrophages.
ACKNOWLEDGMENTS
We thank Ms. Emi Kiyota, Ms. Kanako Hashimoto, Ms. Yumeka Hamada, Ms. Yui Hayashida, and Mr. Takenobu Nakagawa for their technical assistance. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No.25460497, No.25293089).
Footnotes
CONFLICT OF INTEREST: The authors have no financial competing interests to declare.
REFERENCES
- 1.Wakayama T, Iseki S: Role of the spermatogenic-Sertoli cell interaction through cell adhesion molecule-1 (CADM1) in spermatogenesis. Anat Sci Int 84: 112-121, 2009. 10.1007/s12565-009-0034-1 [DOI] [PubMed] [Google Scholar]
- 2.Nakahata S, Morishita K: CADM1/TSLC1 is a novel cell surface marker for adult T-cell leukemia/lymphoma. J Clin Exp Hematop 52: 17-22, 2012. 10.3960/jslrt.52.17 [DOI] [PubMed] [Google Scholar]
- 3.Wakayama T, Ohashi K, Mizuno K, Iseki S: Cloning and characterization of a novel mouse immunoglobulin superfamily gene expressed in early spermatogenic cells. Mol Reprod Dev 60: 158-164, 2001. 10.1002/mrd.1072 [DOI] [PubMed] [Google Scholar]
- 4.Wakayama T, Sai Y, Ito A, Kato Y, Kurobo M, et al. : Heterophilic binding of the adhesion molecules poliovirus receptor and immunoglobulin superfamily 4A in the interaction between mouse spermatogenic and Sertoli cells. Biol Reprod 76: 1081-1090, 2007. 10.1095/biolreprod.106.058974 [DOI] [PubMed] [Google Scholar]
- 5.Wakayama T, Koami H, Ariga H, Kobayashi D, Sai Y, et al. : Expression and functional characterization of the adhesion molecule spermatogenic immunoglobulin superfamily in the mouse testis. Biol Reprod 68: 1755-1763, 2003. 10.1095/biolreprod.102.012344 [DOI] [PubMed] [Google Scholar]
- 6.Ito A, Jippo T, Wakayama T, Morii E, Koma Y, et al. : SgIGSF: a new mast-cell adhesion molecule used for attachment to fibroblasts and transcriptionally regulated by MITF. Blood 101: 2601-2608, 2003. 10.1182/blood-2002-07-2265 [DOI] [PubMed] [Google Scholar]
- 7.Ito A, Okada M, Uchino K, Wakayama T, Koma Y, et al. : Expression of the TSLC1 adhesion molecule in pulmonary epithelium and its down-regulation in pulmonary adenocarcinoma other than bronchioloalveolar carcinoma. Lab Invest 83: 1175-1183, 2003. 10.1097/01.LAB.0000081391.28136.80 [DOI] [PubMed] [Google Scholar]
- 8.Uchino K, Ito A, Wakayama T, Koma Y, Okada T, et al. : Clinical implication and prognostic significance of the tumor suppressor TSLC1 gene detected in adenocarcinoma of the lung. Cancer 98: 1002-1007, 2003. 10.1002/cncr.11599 [DOI] [PubMed] [Google Scholar]
- 9.Ito T, Shimada Y, Hashimoto Y, Kaganoi J, Kan T, et al. : Involvement of TSLC1 in progression of esophageal squamous cell carcinoma. Cancer Res 63: 6320-6326, 2003 [PubMed] [Google Scholar]
- 10.Satou Y, Matsuoka M: HTLV-1 and the host immune system: how the virus disrupts immune regulation, leading to HTLV-1 associated diseases. J Clin Exp Hematop 50: 1-8, 2010. 10.3960/jslrt.50.1 [DOI] [PubMed] [Google Scholar]
- 11.Yoshida N, Imaizumi Y, Utsunomiya A, Miyoshi H, Arakawa F, et al. : Mutation Analysis for TP53 in Chronic-Type Adult T-Cell Leukemia/Lymphoma. J Clin Exp Hematop 55: 13-16, 2015. 10.3960/jslrt.55.13 [DOI] [PubMed] [Google Scholar]
- 12.Miyoshi H, Kiyasu J, Kato T, Yoshida N, Shimono J, et al. : PD-L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T-cell leukemia/lymphoma. Blood 128: 1374-1381, 2016. 10.1182/blood-2016-02-698936 [DOI] [PubMed] [Google Scholar]
- 13.Sasaki H, Nishikata I, Shiraga T, Akamatsu E, Fukami T, et al. : Overexpression of a cell adhesion molecule, TSLC1, as a possible molecular marker for acute-type adult T-cell leukemia. Blood 105: 1204-1213, 2005. 10.1182/blood-2004-03-1222 [DOI] [PubMed] [Google Scholar]
- 14.Dewan MZ, Takamatsu N, Hidaka T, Hatakeyama K, Nakahata S, et al. : Critical role for TSLC1 expression in the growth and organ infiltration of adult T-cell leukemia cells in vivo. J Virol 82: 11958-11963, 2008. 10.1128/JVI.01149-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arase N, Takeuchi A, Unno M, Hirano S, Yokosuka T, et al. : Heterotypic interaction of CRTAM with Necl2 induces cell adhesion on activated NK cells and CD8+ T cells. Int Immunol 17: 1227-1237, 2005. 10.1093/intimm/dxh299 [DOI] [PubMed] [Google Scholar]
- 16.Piccio L, Vermi W, Boles KS, Fuchs A, Strader CA, et al. : Adhesion of human T cells to antigen-presenting cells through SIRPbeta2-CD47 interaction costimulates T-cell proliferation. Blood 105: 2421-2427, 2005. 10.1182/blood-2004-07-2823 [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi S, Watanabe E, Ishigaki T, Ohno N, Yuji K, et al. : Advanced human T-cell leukemia virus type 1 carriers and early-stage indolent adult T-cell leukemia-lymphoma are indistinguishable based on CADM1 positivity in flow cytometry. Cancer Sci 106: 598-603, 2015. 10.1111/cas.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manivannan K, Rowan AG, Tanaka Y, Taylor GP, Bangham CR: CADM1/TSLC1 Identifies HTLV-1-Infected Cells and Determines Their Susceptibility to CTL-Mediated Lysis. PLoS Pathog 12: e1005560, 2016. 10.1371/journal.ppat.1005560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komohara Y, Jinushi M, Takeya M: Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci 105: 1-8, 2014. 10.1111/cas.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamura T, Qian BZ, Pollard JW: Immune cell promotion of metastasis. Nat Rev Immunol 15: 73-86, 2015. 10.1038/nri3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komohara Y, Niino D, Saito Y, Ohnishi K, Horlad H, et al. : Clinical significance of CD163+ tumor-associated macrophages in patients with adult T-cell leukemia/lymphoma. Cancer Sci 104: 945-951, 2013. 10.1111/cas.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai B, Horlad H, Saito Y, Ohnishi K, Fujiwara Y, et al. : Role of Stat3 activation in cell-cell interaction between B-cell lymphoma and macrophages: the in vitro study. J Clin Exp Hematop 53: 127-133, 2013. 10.3960/jslrt.53.127 [DOI] [PubMed] [Google Scholar]
- 23.Satou Y, Matsuoka M: Implication of the HTLV-I bZIP factor gene in the leukemogenesis of adult T-cell leukemia. Int J Hematol 86: 107-112, 2007. 10.1532/IJH97.07103 [DOI] [PubMed] [Google Scholar]
- 24.Katoh T, Harada T, Morikawa S, Wakutani T: IL-2- and IL-2-R- independent proliferation of T-cell lines from adult T-cell leukemia/lymphoma patients. Int J Cancer 38: 265-274, 1986. 10.1002/ijc.2910380218 [DOI] [PubMed] [Google Scholar]
- 25.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, et al. : Identification of the haemoglobin scavenger receptor. Nature 409: 198-201, 2001. 10.1038/35051594 [DOI] [PubMed] [Google Scholar]
- 26.Horlad H, Ohnishi K, Ma C, Fujiwara Y, Niino D, et al. : TIM-3 expression in lymphoma cells predicts chemoresistance in patients with adult T-cell leukemia/lymphoma. Oncol Lett 12: 1519-1524, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoneshige A, Hagiyama M, Fujita M, Ito A: Pathogenic Actions of Cell Adhesion Molecule 1 in Pulmonary Emphysema and Atopic Dermatitis. Front Cell Dev Biol 3: 75, 2015. 10.3389/fcell.2015.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]