Abstract
Macrophages are closely related to various diseases and it is therefore important that the properties of macrophages are adequately evaluated in human diseases and mouse disease models. Immunohistochemistry (IHC) of formalin fixed paraffin-embedded (FFPE) samples is a very useful tool for examination of macrophages; however, an adequate IHC protocol is required for the examination of macrophage states. In this study, we assessed various antigen retrieval methods in order to devise the optimal protocols for staining of macrophages with a range of antibodies. Optimum combinations of primary antibodies and antigen retrieval protocols were determined; for example, heat treatment with ethylenediamine tetraacetic acid solution, pH 8.0, was the best procedure for IHC using mouse anti-Iba1 and human anti-CD11b, -CD163, -CD169, -CD204, and -CD206 antibodies. Moreover, we found that the immunoreactivity of sliced tissue sections decreased gradually over time in long term storage but that this immunoreactivity was preserved in storage at -80 oC in a deep freezer. The optimal IHC protocols and storage procedures that were determined in this study should be a useful tool for macrophage research.
Keywords: FFPE, immunohistochemistry, macrophage, human, mouse
INTRODUCTION
Macrophages are a type of leukocyte that are characterized by a high phagocytic activity. Macrophages are not only critically involved in host defense against microbes, but they also act as antigen presenting cells as well as dendritic cells, although their ability to present antigen is less than that of dendritic cells.1,2 It has long been known that macrophages are closely associated with the development of atherosclerosis and tissue remodeling. In addition, over the last decade macrophages have also been shown to be associated with the pathogenesis of many human diseases including malignant tumors and metabolic syndromes; however, the details of the relationships between disease progression and macrophage activation are unclear. Macrophages are classified into exudate and resident macrophages.3,4 Exudate and some of the resident macrophages are derived from circulating monocytes, and some resident macrophages such as microglia originate from fetal yolk sac macrophages.5-7 The novel concept of macrophage polarization was suggested in the late 1990s. In macrophage polarization, bacterial components and interferons differentiate macrophages to an inflammatory phenotype (classical activation, M1 macrophages) whereas Th2-type cytokines such as IL-4, IL-10, or IL-13 induce an immunosuppressive macrophage phenotype (alternative activation, M2 macrophages).8-10 However, recent studies suggest that macrophage differentiation or polarization is not that simple, and further studies are necessary to clarify the detailed mechanisms of macrophage differentiation/polarization.11,12 For this purpose, it is important to identify and adequately evaluate macrophage infiltration and activation status in human diseases and in mouse disease models. In the present study, we determined the optimal antigen retrieval methods for identification of individual macrophage-specific antigens in formalin fixed paraffin-embedded (FFPE) samples using immunohistochemistry (IHC).
MATERIALS AND METHODS
Samples
We prepared the spleen tissue samples derived from both humans and mice. Human spleens were obtained from autopsy cases with ethical approval authorized by a written informed consent and an approval by the Kumamoto University Review Board. The resected human spleens were fixed in 10% formalin neutral buffer solution for 5-7 days. Mice were purchased from Clear Japan (Shizuoka, Japan). Animal care and experiments were approved by the Animal Committee at Kumamoto University. Murine spleens were obtained from specific pathogen free Balb/c mice. The murine spleens were fixed in 10% formalin neutral buffer solution for 1 days. The spleen tissue specimens were embedded in paraffin wax after fixation.
Immunohistochemistry protocol
Sections (3 μm thick) were cut and mounted on a glass slide and were then deparaffinized in xylene and rehydrated in a graded ethanol series. Antigen retrieval steps were performed as follows. Samples were incubated with proteinase K (PK, DAKO, Glostrup, Denmark) for 5 min at room temperature (RT) and were then washed in phosphate buffered saline (PBS), or sections were immersed in citrate buffer, pH 6.0, ethylenediamine tetraacetic acid (EDTA) solution, pH 8.0, target retrieval solution (TRS) pH 9.0 (Nichirei, Tokyo, Japan), or TRS, pH 6.0 (DAKO), and samples were heated in a microwave (5 min at 100 oC) or a pressure cooker (30 sec at 125 oC). After heat treatment, the slides were cooled to RT and were washed in PBS. The sections were then treated with 0.3% H2O2 in methanol (30 min at RT) and were subsequently incubated with blocking solution (5% goat serum) for 20 min at RT, following which the sections were reacted with primary antibodies (Table 1) for 90 min at RT. The antigen retrieval methods indicated in Table 1 are those used by the respective company from which the antibody was obtained. The sections were then washed three times in PBS and were incubated with secondary anti-mouse or anti-rabbit IgG antibodies labelled with polymer and horseradish peroxidase (Histofine, Nichirei) for 30 min at RT. The immunoreactions were visualized using a diaminobenzidine substrate kit (Nichirei). All sections were counterstained with Mayer’s hematoxylin for 20 sec. After washing in flowing tap-water for 5 min, the samples were dehydrated and immersed in xylene. The sections were mounted with malinol (Muto Pure Chemicals, Tokyo, Japan). Signals were classified into 4 grades of intensity as follows: (-) negative; (+) weakly positive, (++) moderately positive, and (+++) strongly positive.
Table 1. Antibodies for immunohistochemistry on human and mice paraffin sections.
| Antigen | Clone | Details* | Antigen retrieval** | Company | |
|---|---|---|---|---|---|
| Mice | EMR1 | F4/80 | rat, mono | PK | Bio-Lad/DAKO, Hercules, CA, USA |
| CD68 | FA11 | rat, mono | PK, TRS(H) | Bio-Lad/DAKO, Hercules, CA, USA | |
| CD163 | - | rabbit, poly | TRS (H) | CosmoBio, Tokyo, Japan | |
| CD204 | 2F8 | rat, mono | EDTA (H) | Bio-Lad/DAKO, Hercules, CA, USA | |
| CD206 | MR5D3 | rat, mono | PK | Bio-Lad/DAKO, Hercules, CA, USA | |
| Iba1 | - | rabbit, poly | EDTA (H) | WAKO, Tokyo, Japan | |
| Human | CD11b | EP1345Y | rabbit, mono | EDTA (H) | Abcam, Cambridge, UK |
| CD68 | PG-M1 | mouse, mono | EDTA (H) | Agilent Technol., Santa Vlara, CA, USA | |
| CD163 | 10D6 | mouse, mono | EDTA (H) | Leica Biosystems, Nussloch, Germany | |
| CD169 | Hsn7D2 | mouse, mono | EDTA (H) | Santa Cruz Biotech., Santa Cruz, CA, USA | |
| CD204 | SRA-E5 | mouse, mono | EDTA (H) | CosmoBio, Tokyo, Japan | |
| CD206 | 5C11 | mouse, mono | EDTA (H) | Abnova, Taipei, Taiwan | |
| Iba1 | - | rabbit, poly | EDTA (H) | WAKO, Tokyo, Japan |
*: mono; monoclonal antibody, poly; polyclonal antibody.
**: EDTA; 1mM EDTA (pH8.0), PK; proteinase K (5 min at RT), H; heat treatment, TRS; target retreival solution (DAKO).
EMR1; EGF-like module-containing mucin-like hormone receptor-like 1, Iba1;induction of brown adipocytes 1.
RESULTS
The result of IHC of mouse spleen
To identify the most suitable method of antigen retrieval for individual antibodies, the sections were subjected to various antigen retrieval methods and then IHC was performed as described in materials and methods. The results are summarized in table 1 and 2. Figure 1 shows mouse spleen slides stained with the indicated antibodies using an optimal antigen retrieval method. For IHC of EMR1 and CD206, positive staining was observed in sections treated with PK and in those heated in TRS, pH 6.0, but the strongest staining was detected in the section treated with PK. For IHC of Iba1, although positive staining was seen in all sections, the strongest positive signal was seen in the section that was treated by heating in EDTA (1 mM, pH 8.0) buffer. The staining of Iba1 was clearer in sections that were heated in the pressure cooker as compared with sections that were heated in the microwave. For IHC of CD163, a positive signal was detected in sections treated with PK and TRS, pH 6.0, but the strongest positive signal was detected in the section that was heated in the microwave or the pressure cooker in TRS, pH 6.0. Although positive signals were detected for CD68 and CD204 in sections that were treated with PK or were heated in the pressure cooker in EDTA buffer, respectively, their positive staining was weaker than that of other antigens.
Table 2. Immunoreactivity of antibodies and antigen retreival methods.
| Antigen | Pretreatment (antigen retrieval methods) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Protainase K | CB (M) | EDTA (M) | TRSN(M) | TRSD(M) | CB (P) | EDTA (P) | TRSN(P) | TRSD(P) | |||||||||||||
| Mice | EMR1 | + | +++ | + | - | - | ++ | - | - | - | ++ | |||||||||||
| CD68 | + | ++ | - | - | - | + | - | - | - | + | ||||||||||||
| CD163 | + | ++ | - | + | - | +++ | - | + | - | +++ | ||||||||||||
| CD204 | - | + | - | - | - | - | - | ++ | + | - | ||||||||||||
| CD206 | - | +++ | - | - | - | + | - | - | - | ++ | ||||||||||||
| Iba1 | ++ | + | ++ | +++ | ++ | ++ | ++ | +++ | ++ | ++ | ||||||||||||
| Human | CD11b | + | + | ++ | +++ | +++ | + | ++ | +++ | +++ | ++ | |||||||||||
| CD68 | + | +++ | ++ | ++ | ++ | ++ | ++ | +++ | ++ | ++ | ||||||||||||
| CD163 | - | - | ++ | ++ | ++ | ++ | ++ | +++ | +++ | ++ | ||||||||||||
| CD169 | + | ++ | - | +++ | + | + | - | + | - | - | ||||||||||||
| CD204 | - | - | ++ | ++ | ++ | ++ | ++ | +++ | +++ | ++ | ||||||||||||
| CD206 | ++ | - | ++ | ++ | ++ | ++ | ++ | +++ | +++ | ++ | ||||||||||||
| Iba1 | ++ | + | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ||||||||||||
-;negative, +; weakly positive, ++; moderately positive, +++; strongly positive.
(M); treatment with microwave, 5 min at 100oC
(P); treatment with pressure cooker, 30 sec at 125oC
CB; 10mM citrate buffer (pH6.0), EDTA; 1mM EDTA buffer (pH8.0), TRSN; target retrieval solution from NICHIREI (pH9.0), TRSD; target retrieval solution from DAKO (pH6.0).
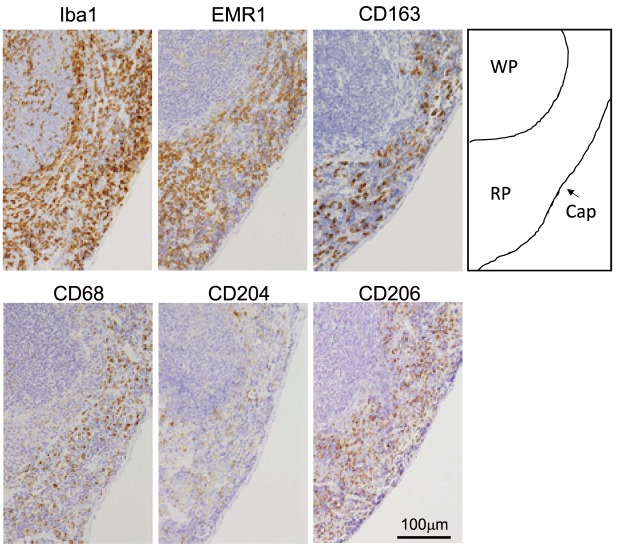
Fig. 1.
Immunohistochemistry of macrophages in mouse spleen. Iba1, EMR1, CD163, CD68, CD204, and CD206 expressions were tested using formalin fixed paraffin-embedded mouse spleen as described in materials and methods. WP; white pulp, RP; red pulp, Cap; capsule.
The results of IHC of human spleen
We next performed similar experiments using human spleen and anti-human antibodies. The results are summarized in table 1, 2, and figure 2. In IHC for CD68, positive staining was detected in all sections, and the strongest staining was detected in the sections that were treated with PK or were heated in a pressure cooker in EDTA buffer. In IHC for CD11b, positive staining was detected in all sections, and the strongest signals were detected in the sections that were treated by heating in EDTA buffer or in TRS. In IHC for Iba1, although positive staining was detected in all sections, the strongest positive signal was detected in the section that was treated by heating in EDTA buffer. In IHC for CD163, a strong positive signal was detected in sections that were heated in the pressure cooker in EDTA buffer or in TRS, pH 9.0, and those positive signals were of equal intensity. In IHC for CD204, positive staining was detected in sections that were heated in all of the buffers tested but the strongest signal was detected in sections that were heated in the pressure cooker in EDTA buffer or in TRS, pH 9.0. In IHC for CD206, positive staining was detected in both the section that was not treated for antigen retrieval and in sections that were heated in all buffers tested, but the strongest signal was detected in sections that were heated in the pressure cooker in EDTA buffer or in TRS, pH 9.0.
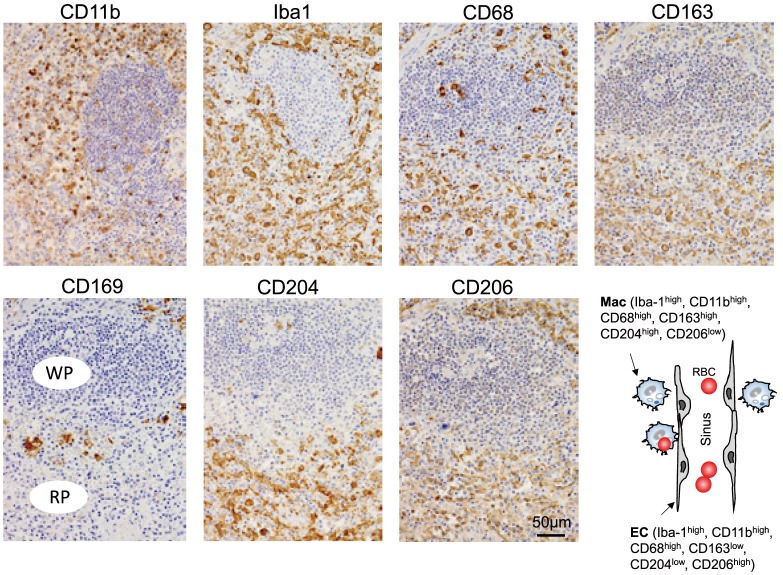
Fig. 2.
Immunohistochemistry of macrophages in human spleen. Iba1, CD68, CD163, CD169, CD204, and CD206 expressions were tested using formalin fixed paraffin-embedded human spleen as described in materials and methods. The staining pattern of macrophage-related antigens was summarized in the scheme. WP; white pulp, RP; red pulp, Mac; macrophage, EC; endothelial cell, RBC; red blood cell. Immunohistochemistry of macrophages in human spleen. Iba1, CD68, CD163, CD169, CD204, and CD206 expressions were tested using formalin fixed paraffin-embedded human spleen as described in materials and methods. The staining pattern of macrophage-related antigens was summarized in the scheme. WP; white pulp, RP; red pulp, Mac; macrophage, EC; endothelial cell, RBC; red blood cell.
The expression of macrophage-related antigens in human and mouse spleens
Regarding the IHC findings using mouse spleens and optimal antigen retrieval methods, all macrophages (red pulp macrophages, marginal zone macrophages, and marginal metallophilic macrophages) stained positive for Iba-1 and EMR1 (Figure 1). However, there were clearly more Iba-1 positive cells than EMR1-positive cells. CD68 and CD204 expression was detected in all macrophages; however, the positive signals of red pulp macrophages were stronger than those of other macrophages. Only red pulp macrophages were positive for CD163 and CD206.
With regards to the IHC staining of human spleen (Figure 2), all macrophages and venous sinus (VS) endothelial cells stained strongly positive for Iba-1, CD11b, and CD68. Staining of CD163 and CD204 was strongly positive for red pulp macrophages and marginal zone macrophages, and weakly positive for venous sinus (VS) endothelial cells. Conversely, CD206 stained strongly positive for VS endothelial cells and weakly positive for macrophages. CD169 staining was only detected on marginal metallophilic macrophages, and was negative for other macrophages and VS endothelial cells.
Storage of tissue sections
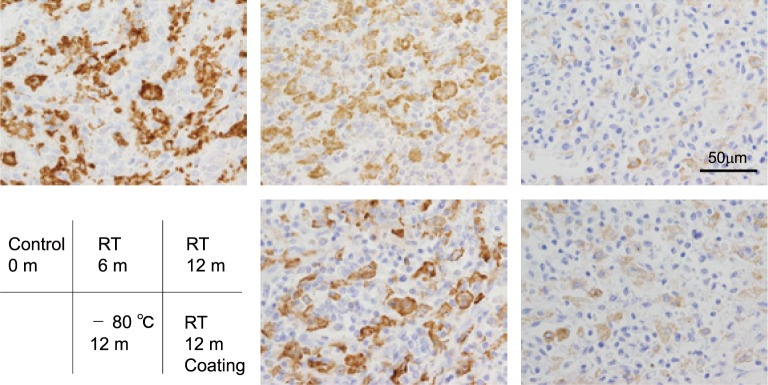
We tested if the immunoreactivity of antigens changed over long term storage using staining of CD204 as a test case. Tissue sections were stored at RT or at -80 oC for 6 or 12 months, and IHC for CD204 was performed at each time point using the same protocol for each staining. As shown in figure 3, the staining intensity on the slide that was stored at RT for 6 or 12 months was significantly decreased compared to that of the control slide (0 month), whereas staining intensity was preserved when the slides were stored at -80 oC. Paraffin coating of the sections did not significantly affect the decrease in the positive signal of CD204 following storage at RT.
Fig. 3.
IHC of CD204 using stored slides. The data of IHC in slides stored for 0 day, 6 months at RT, 12 months at RT, 12 month at -80oC, and 12 months at RT with paraffin coating were shown. Slides were preserved in sealed containers.
DISCUSSION
In the present study, we established the most adequate methods to detect macrophages in FFPE samples. FFPE samples are the most common methods of tissue preparation for pathological diagnosis at the hospitals and so many cases and kinds of disease samples are stored at hospital, and these samples must be critical resources for biomedical research. In addition, the significant involvement of macrophages to various diseases have been found by many researchers, and the importance of the pathological studies related to macrophages are more interested in biomedical fields. In the present study, we showed the most suitable methods to identify and evaluate macrophage distribution in FFPE sections of human and mice. Iba-1, CD11b, CD68, and EMR1 are well known molecules specifically expressed on pan-macrophages. Iba-1 is the easiest marker for macrophages in the present study, since anti-Iba-1 antibody strongly reacted to both human and mice and seemed to be suitable for all antigen retrieval methods. We often use anti-Iba-1 antibody for double IHC with other antigens.13 CD163, CD204, and CD206 have been suggested as markers for M2-like phenotype, however, recent findings indicated that CD204 is not specific to M2-like phenotype.14 It is also notable that the anti-mouse CD204 antibody (clone 2F8) does not react with the CD204 antigen in C57B/6 mice due to mutation of the CD204 gene.15 CD169 is known to be up-regulated by interferon signals in human macrophages, and considered to be a marker for M1-like phenotype.16 Further studies using antibodies react to these molecules and FFPE samples must give new insights and findings to the pathogenesis of various diseases.
In addition to the methods of IHC, we also checked the methods of the storage of tissue sections. As shown in figure 3, immunoreactivity of CD204 was significantly decreased on tissue section stored for more than 6 months at RT, although we did not check the immunoreactivity of other antigens. This antigen loss phenomenon was well known, and similar results in IHC of p53 were observed 20 years ago.17 Antigen loss of Ki-67, estrogen receptor, and cytokeratin in breast cancer tissue microarray sections were tested by DiVito KA et al., and they described that storage for 3 months at RT induced marked degradation of all antigens.18 However, they also demonstrated that nitrogen storage or paraffin coating with anti-oxidants significantly suppressed the antigen degradation, and these observations indicated that oxidation by air exposure was involved in antigen loss by long term storage. It is now thought that an antigenic activity is gradually attenuated by oxidation reaction. A refrigerated storage might delay the oxidation reaction. Grillo F et al. demonstrated that antigen loss or decay was seen in IHC of CD3, CD 31, CD117, estrogen and progesterone receptors, Ki67, p53, TTF-1, vimentin, but not in IHC of smooth muscle actin, keratins 7, 20, AE1/AE3, 34βE12, and they also showed cold storage is suitable methods for long term storage of tissue sections.19 The importance of cold storage (at minus degrees rather than at 4 oC) was reported by other researchers,20 and these observations were consistent with our observation of figure 3.
Histiocytic sarcoma (HS) is a macrophage-derived neoplasm. Although human HS is extremely rare and aggressive neoplasm, spontaneous HS is occasionally observed in mice.21 Neoplastic cells are usually large and oval in shape, have features similar to macrophages, and are phenotypically positive for macrophage-related markers such as CD68 and CD163.22 CD204 is also reported as a marker for HS.23,24 The molecules listed in this article are suggested to be expressed on HS, and further studies are necessary to confirm the usefulness of these antibodies for diagnosis of HS. In addition, IHC of macrophage-markers is useful for the diagnosis for hemophagocytic lymphohistiocytosis or hemophanocytic syndrome (HLH/HPS) and evaluating tumor-associated macrophages (TAMs) in FFPE samples at hospital.25-27
In the present study, we showed that not only macrophages but also VS endothelial cells were positive for CD163, CD204, and CD206. Similar results were observed in previous studies.28,29 The scavenger receptors expressed by VS endothelial cells have been suggested to be involved in the clearance of blood debris and pathogens. Littoral cell angioma is a rare primary splenic tumor arising from endothelial cells, and CD163 is a useful marker for the diagnosis of littoral cell angioma.22,30
In conclusion, we summarized and listed the best methods for detecting macrophage-specific antigens in the present study. Antibodies listed in this article is also suggested to be useful for pathological diagnosis of HS and HLH/HPS, or for the assessment of TAMs. However, many antibodies against various molecules have been developed by many researchers and companies. Update for the best methods of IHC of macrophages is necessary for further researches.
ACKNOWLEDGEMENT
We thank Ms. Ikuko Miyakawa for their technical assistance. This work was supported by JSPS KAKENHI (No. 16H05162, 26460454, 16K15248).
Footnotes
CONFLICT OF INTEREST: The authors have no financial competing interests to declare.
REFERENCES
- 1.Medzhitov R, Shevach EM, Trinchieri G, Mellor AL, Munn DH, et al. : Highlights of 10 years of immunology in Nature Reviews Immunology. Nat Rev Immunol 11: 693-702, 2011. 10.1038/nri3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavaillon JM: The historical milestones in the understanding of leukocyte biology initiated by Elie Metchnikoff. J Leukoc Biol 90: 413-424, 2011. 10.1189/jlb.0211094 [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Naito M, Takeya M: Development and heterogeneity of macrophages and their related cells through their differentiation pathways. Pathol Int 46: 473-485, 1996. 10.1111/j.1440-1827.1996.tb03641.x [DOI] [PubMed] [Google Scholar]
- 4.van Furth R: Monocyte production during inflammation. Comp Immunol Microbiol Infect Dis 8: 205-211, 1985. 10.1016/0147-9571(85)90045-1 [DOI] [PubMed] [Google Scholar]
- 5.Naito M, Umeda S, Yamamoto T, Moriyama H, Umezu H, et al. : Development, differentiation, and phenotypic heterogeneity of murine tissue macrophages. J Leukoc Biol 59: 133-138, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Hoeffel G, Ginhoux F: Ontogeny of Tissue-Resident Macrophages. Front Immunol 6: 486, 2015. 10.3389/fimmu.2015.00486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perdiguero EG, Geissmann F: The development and maintenance of resident macrophages. Nat Immunol 17: 2-8, 2016. 10.1038/ni.3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mills CD: M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol 32: 463-488, 2012. 10.1615/CritRevImmunol.v32.i6.10 [DOI] [PubMed] [Google Scholar]
- 9.Gordon S: Alternative activation of macrophages. Nat Rev Immunol 3: 23-35, 2003. 10.1038/nri978 [DOI] [PubMed] [Google Scholar]
- 10.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M: Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229: 176-185, 2013. 10.1002/path.4133 [DOI] [PubMed] [Google Scholar]
- 11.Komohara Y, Fujiwara Y, Ohnishi K, Shiraishi D, Takeya M: Contribution of Macrophage Polarization to Metabolic Diseases. J Atheroscler Thromb 23: 10-17, 2016. 10.5551/jat.32359 [DOI] [PubMed] [Google Scholar]
- 12.Lewis CE, Harney AS, Pollard JW: The Multifaceted Role of Perivascular Macrophages in Tumors. Cancer Cell 30: 18-25, 2016. 10.1016/j.ccell.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komohara Y, Horlad H, Ohnishi K, Fujiwara Y, Bai B, et al. : Importance of direct macrophage-tumor cell interaction on progression of human glioma. Cancer Sci 103: 2165-2172, 2012. 10.1111/cas.12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara Y, Hizukuri Y, Yamashiro K, Makita N, Ohnishi K, et al. : Guanylate-binding protein 5 is a marker of interferon-γ-induced classically activated macrophages. Clin Transl Immunology 5: e111, 2016. 10.1038/cti.2016.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daugherty A, Whitman SC, Block AE, Rateri DL: Polymorphism of class A scavenger receptors in C57BL/6 mice. J Lipid Res 41: 1568-1577, 2000 [PubMed] [Google Scholar]
- 16.Komohara Y, Ohnishi K, Takeya M: Possible functions of CD169-positive sinus macrophages in lymph nodes in anti-tumor immune responses. Cancer Sci in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs TW, Prioleau JE, Stillman IE, Schnitt SJ: Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst 88: 1054-1059, 1996. 10.1093/jnci/88.15.1054 [DOI] [PubMed] [Google Scholar]
- 18.DiVito KA, Charette LA, Rimm DL, Camp RL: Long-term preservation of antigenicity on tissue microarrays. Lab Invest 84: 1071-1078, 2004. 10.1038/labinvest.3700131 [DOI] [PubMed] [Google Scholar]
- 19.Grillo F, Pigozzi S, Ceriolo P, Calamaro P, Fiocca R, et al. : Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem Cell Biol 144: 93-99, 2015. 10.1007/s00418-015-1316-4 [DOI] [PubMed] [Google Scholar]
- 20.Xie R, Chung JY, Ylaya K, Williams RL, Guerrero N, et al. : Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J Histochem Cytochem 59: 356-365, 2011. 10.1369/0022155411398488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackwell BN, Bucci TJ, Hart RW, Turturro A: Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol Pathol 23: 570-582, 1995. 10.1177/019262339502300503 [DOI] [PubMed] [Google Scholar]
- 22.Nguyen TT, Schwartz EJ, West RB, Warnke RA, Arber DA, et al. : Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am J Surg Pathol 29: 617-624, 2005. 10.1097/01.pas.0000157940.80538.ec [DOI] [PubMed] [Google Scholar]
- 23.Kato Y, Murakami M, Hoshino Y, Mori T, Maruo K, et al. : The class A macrophage scavenger receptor CD204 is a useful immunohistochemical marker of canine histiocytic sarcoma. J Comp Pathol 148: 188-196, 2013. 10.1016/j.jcpa.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 24.Ohnishi K, Tanaka S, Oghiso Y, Takeya M: Immunohistochemical detection of possible cellular origin of hepatic histiocytic sarcoma in mice. J Clin Exp Hematop 52: 171-177, 2012. 10.3960/jslrt.52.171 [DOI] [PubMed] [Google Scholar]
- 25.Kuriyama T, Kawano N, Yamashita K, Kikuchi I: Cord Blood Transplantation Following Reduced-Intensity Conditioning for Epstein-Barr Virus-Associated Hemophagocytic Lymphohistiocytosis during Systemic Lupus Erythematosus Treatment. J Clin Exp Hematop 56: 126-129, 2016. 10.3960/jslrt.56.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugimoto T, Watanabe T: Follicular Lymphoma: The Role of the Tumor Microenvironment in Prognosis. J Clin Exp Hematop 56: 1-19, 2016. 10.3960/jslrt.56.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komohara Y, Jinushi M, Takeya M: Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci 105: 1-8, 2014. 10.1111/cas.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linehan SA, Weber R, McKercher S, Ripley RM, Gordon S, et al. : Enhanced expression of the mannose receptor by endothelial cells of the liver and spleen microvascular beds in the macrophage-deficient PU.1 null mouse. Histochem Cell Biol 123: 365-376, 2005. 10.1007/s00418-005-0767-4 [DOI] [PubMed] [Google Scholar]
- 29.Martens JH, Kzhyshkowska J, Falkowski-Hansen M, Schledzewski K, Gratchev A, et al. : Differential expression of a gene signature for scavenger/lectin receptors by endothelial cells and macrophages in human lymph node sinuses, the primary sites of regional metastasis. J Pathol 208: 574-589, 2006. 10.1002/path.1921 [DOI] [PubMed] [Google Scholar]
- 30.Peckova K, Michal M, Hadravsky L, Suster S, Damjanov I, et al. : Littoral cell angioma of the spleen: a study of 25 cases with confirmation of frequent association with visceral malignancies. Histopathology 69: 762-774, 2016. 10.1111/his.13026 [DOI] [PubMed] [Google Scholar]