Abstract
Umbilical cord blood (UCB) has advantages over other tissues because it can be obtained without an invasive procedure and complex processing. We explored the availability of cryopreserved UCB cells as a source of mesenchymal stromal/stem cells (MSCs). MSCs were successfully isolated from six of 30 UCB units (median volume, 34.0 mL; median nucleated cell number, 4.4×108) that were processed and cryopreserved using CP-1/human serum albumin. This isolation rate was lower than that (57%) from non-cryopreserved UCB cells. The number of nucleated cells before and after hydroxyethyl starch separation, UCB unit volume, and cell viability after thawing did not significantly differ between UCB units from which MSCs were successfully isolated and those from which they were not. When CryoSure-DEX40 was used as a cryoprotectant, MSCs were isolated from two of ten UCB units. Logistic regression analysis demonstrated that the cryopreservation method was not significantly associated with the success of MSC isolation. The isolated MSCs had a similar morphology and surface marker expression profile as bone marrow-derived MSCs and were able to differentiate into osteogenic, adipogenic, and chondrogenic cells. In summary, MSCs can be isolated from cryopreserved UCB cells. However, the cryopreservation process reduces the isolation rate; therefore, freshly donated UCB cells are preferable for the isolation of MSCs.
Keywords: human mesenchymal stromal/stem cells, umbilical cord blood, cryopreservation
INTRODUCTION
Cell therapy using human mesenchymal stromal/stem cells (MSCs) has been extensively explored for the treatment of a variety of diseases based on their multiple biological and therapeutic effects.1 An off-the-shelf allogeneic bone marrow (BM)-derived MSC product is currently available in the clinic for the treatment of the intractable acute graft-versus-host disease after hematopoietic stem cell transplantation (HSCT).2,3 BM is the most commonly used source of MSCs.4 However, invasive procedures are required to obtain BM cells from donors. Although other sources of MSCs that can be obtained without invasive treatments have been sought, including expulsed placenta and exfoliated teeth, complex procedures with enzymatic digestion are necessary to prepare cells from these tissues.5,6 The demand for allogeneic MSCs is expected to increase in the future; therefore, appropriate sources of MSCs that can be alternatives to BM need to be explored for the further development of MSC-based therapy.
In a previous study, we demonstrated that MSCs can be isolated from freshly donated umbilical cord blood (UCB) units that do not qualify for the Japanese banking system as a hematopoietic stem cell (HSC) source because of their small volume.7 UCB has advantages over other tissues because it can be obtained from donors without invasive procedures, and a simple adhesion method without complex procedures can be used to isolate MSCs. In addition, infrastructure for processing and cryopreserving UCB cells is established in the banking system for HSCT in Japan. More importantly, about 90% of donated UCB units are discarded because they do not meet the requirements for an HSC source in terms of volume and/or cell number.8 We have further extended our investigation of the availability of such UCB units as a source of MSCs. In this study, we examined the efficacy of MSC isolation from cryopreserved UCB cells.
MATERIAL AND METHODS
UCB collection
UCB was collected in bags with an anticoagulant (acid citrate dextrose) from umbilical cords after delivery of placentas at Japanese Red Cross Kyoto Daini Hospital. UCB units that did not qualify for the banking system for HSCT were delivered to Kyoto University Hospital. Maternal informed consent for the donation of UCB to be used in this study was obtained prior to delivery. This study was approved by the Internal Review Board of Kyoto University Hospital and Japanese Red Cross Kyoto Daini Hospital.
Cell processing of UCB
UCB was subjected to cell processing when: 1) blood clots were not found in the bag of UCB and 2) cell processing could be started within 8 hr after delivery. Hydroxyethyl starch 40 (HES; NIPRO, Osaka, Japan) was used to deplete red blood cells from UCB. A one-fifth volume of HES was added to UCB, which was then centrifuged at 50 g for 5 min at room temperature. The supernatant was collected 30 min later (first supernatant). The remaining fractions were resuspended with Dulbecco’s phosphate-buffered saline (DPBS; Wako Pure Chemical Industries, Osaka, Japan) and separated using HES as outlined above, and the supernatant was collected (second supernatant). The first and second supernatants were mixed and centrifuged at 500 g for 10 min at room temperature to concentrate nucleated cells. The nucleated cells were washed with DPBS twice (UCB cells) and used for MSC isolation.
Cryopreservation of UCB cells
The suspension of UCB cells was equally divided into two aliquots. One aliquot (cryopreserved cell aliquot) was mixed with an equal volume of cryopreservation cocktails containing clinically available CP-1 (12% HES and 10% dimethyl sulfoxide; Kyokuto Pharmaceutical Industrial, Tokyo, Japan) and 8% human serum albumin, frozen with a conventional protocol (-1°C/min), and then preserved in liquid nitrogen for 30 days (CP-1 method). The other aliquot (non-cryopreserved cell aliquot) was directly used for MSC isolation without cryopreservation (non-cryopreserved method). In some experiments, the suspension of UCB cells was mixed with a one-fifth volume of CryoSure-DEX40 (55% dimethyl sulfoxide and 5% dextran 40; WAK-Chemie Medical GmbH, Steinbach/Ts, Germany), frozen with a conventional protocol (-1°C/min), and then preserved in liquid nitrogen for 30 days (CryoSure-DEX40 method).
Isolation and culture of MSCs from UCB cells
Cryopreserved UCB cells were quickly thawed at 37°C in a water bath. The viability of cells after thawing was evaluated by trypan blue dye exclusion. The UCB cells were seeded onto culture dishes at a density of 5–10×106/cm2 in α-Minimal Essential Medium supplemented with 15% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin (all from GIBCO, Carlsbad, CA), and 100 μg/mL ascorbic acid 2-phosphate (Wako Pure Chemical Industries). Dexamethasone (Sigma-Aldrich, St. Louis, MO) was added to the culture at a final concentration of 10-7 M for the first week. The culture medium was changed the day after seeding cells and twice per week thereafter. After the first week of culture, suspension cells were removed and adherent cells remaining in the dish continued to be cultured. The culture medium was changed twice per week. When colonies of adherent spindle-shaped fibroblastic cells were obtained, they were dispersed by passaging using 0.05% trypsin/0.5 mM EDTA and culture-expanded in new dishes (passage 1). Cells at passage 3–5 were used in this study. Successful MSC isolation from UCB cells was defined as follows: (i) colonies of adherent spindle-shaped fibroblastic cells containing more than 50 cells were obtained; (ii) after culture expansion, these cells were positive for CD105, CD73, and CD90 and were negative for CD45, CD34, CD14, CD19, and human leukocyte antigen (HLA)-DR, as assessed by flow cytometric analysis; and (iii) after culture expansion, these cells exhibited osteogenic, adipogenic, and chondrogenic differentiation in vitro, as assessed by Alizarin Red S staining, Oil Red O staining, and Alcian blue staining, respectively.9
Flow cytometric, differentiation, and growth analyses of MSCs
Single-cell suspensions of adherent cells were stained with fluorescence-conjugated antibodies and analyzed with a FACSCanto II (Becton Dickinson, Franklin Lakes, NJ). Data were analyzed using FlowJo software (Tree Star, Ashland, OR). Multilineage differentiation assays were performed as described previously.7,10,11 For adipogenic induction, 0.5 mM isobutyl-methylxanthine, 60 μM indomethacin, 0.5 μM hydrocortisone, and 10 μg/mL insulin (all from Sigma-Aldrich) were added to the culture media. For osteogenic induction, 1.8 mM potassium dihydrogen phosphate (Sigma-Aldrich), 100 μM ascorbic acid, and 100 nM dexamethasone were added to the culture media. For chondrogenic induction, cell pellets were cultured in chondrogenic induction medium comprising Dulbecco’s Modified Eagle Medium (GIBCO) supplemented with 15% FBS, insulin/transferrin/selenous acid mixture (BD Biosciences, San Jose, CA), 100 μM ascorbic acid, 2 mM sodium pyruvate (GIBCO), 100 nM dexamethasone, 10 ng/mL transforming growth factor (Pepro-Tech Inc., Rocky Hill, NJ), 100 U/mL penicillin, and 100 mg/mL streptomycin. Three weeks later, Oil Red O staining, Alizarin Red S staining, or Alcian blue staining was performed to evaluate lipid-laden fat cells, calcium deposition, or cartilage matrix deposition, respectively. To examine growth, 5×104 cells were seeded on a 10 cm dish. On days 4, 7, and 11 after cell seeding, the number of viable cells was counted using trypan blue.
Statistical analyses
Logistic regression analysis was performed to evaluate characteristics that were associated with the successful isolation of MSCs. The unpaired Student t-test was used unless specified otherwise.
RESULTS
Characteristics of UCB units
A total of 38 UCB units that did not qualify for the banking system were initially obtained. Of these, eight UCB units were excluded from further evaluation for the following reasons: 1) blood clots were found in the bag of UCB, and 2) cell processing could not be started within 8 hr after delivery. Consequently, 30 UCB units were evaluated. The characteristics of the UCB units are summarized in Table 1. All UCB units were collected following a normal pregnancy with a median gestation period of 39 weeks (range, 36–40 weeks). The median volume of the 30 UCB units was 34.0 mL (range, 9.0–100 mL) without anticoagulants. The median number of nucleated cells per UCB unit was 4.4×108 (range, 1.1–13.3×108).
Table 1. Characteristics of UCB units that were processed and cryopreserved by the CP-1 method. UCB: umbilical cord blood.
| Characteristic | Value |
|---|---|
| Baby’s gender (male/female) | 16/14 |
| Gestation period (weeks), median [range] | 39 [36–40] |
| Volume of UCB unit (mL), median [range] | 34.0 [9.0–100] |
| Nucleated cell count of UCB (×108), median [range] | 4.4 [1.1–13.3] |
Isolation of MSCs from UCB cells that were cryopreserved using CP-1
An overview of the processing of UCB for MSC isolation is shown in Figure 1. MSCs were isolated from six of 30 UCB units (20%) that were processed and cryopreserved by the CP-1 method (Table 2). For the six cryopreserved UCB units from which MSCs were successfully isolated, the median volume of the UCB units, the median nucleated cell count before and after HES separation, and the median viability of nucleated cells after thawing were 21.5 mL, 2.7×108 cells, 2.1×108 cells, and 83.6%, respectively. On the other hand, for the 24 cryopreserved UCB units from which MSCs failed to be isolated (80%), these values were 17.0 mL, 2.0×108 cells, 1.5×108 cells, and 86.3%, respectively. The statistical associations between the characteristics of UCB units and the success or failure of MSC isolation were analyzed. Although there were no significant associations, all these values, except cell viability after thawing, tended to be inferior for UCB units from which MSCs failed to be isolated compared with UCB units from which MSCs were successfully isolated.
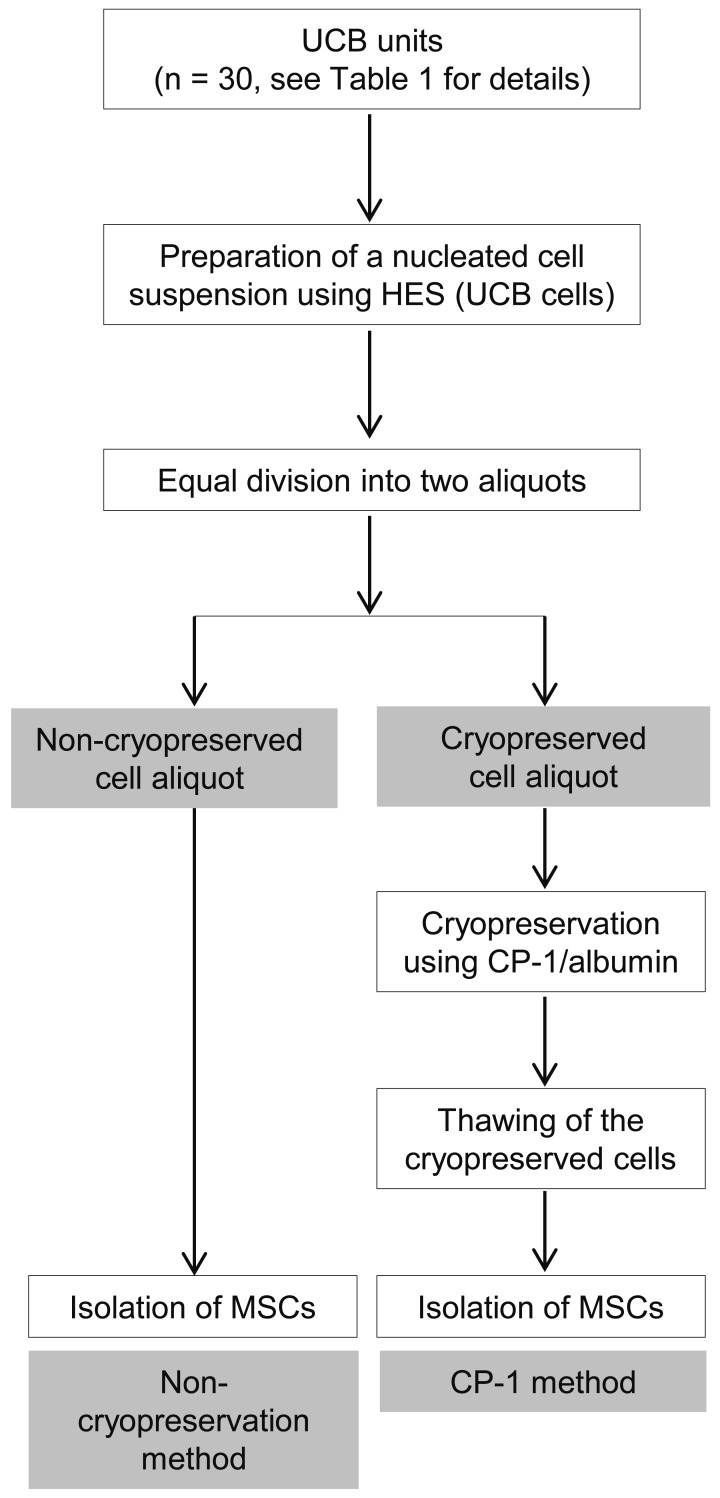
Fig. 1.
An overview of the processing of UCB units. Nucleated cells were separated from UCB using HES. These UCB cells were divided equally into two aliquots and subjected to two different methods, namely, direct isolation of MSCs without cryopreservation (non-cryopreservation method) and isolation of MSCs after cryopreservation and thawing (CP-1 method).
Table 2. Characteristics of UCB units according to whether MSC isolation was a success or failure after cryopreservation using the CP-1 method (n = 30). UCB: umbilical cord blood, HES: hydroxyethyl starch, MSC: mesenchymal stromal/stem cell.
| Characteristic | Success | Failure | P value |
|---|---|---|---|
| Number of cryopreserved UCB units | 6 | 24 | - |
| Volume of UCB unit (mL), median [range] | 21.5 [15.0–40] | 17.0 [4.5–50] | 0.35 |
| Nucleated cell count of UCB before HES separation (×108), median [range] | 2.7 [1.1–4.9] | 2.0 [0.6–6.7] | 0.53 |
| Nucleated cell count of UCB after HES separation (×108), median [range] | 2.1 [0.9–3.7] | 1.5 [0.3–5.8] | 0.43 |
| Cell viability after thawing (%), median [range] | 83.6 [80.3–99] | 86.3 [75.9–99] | 0.78 |
MSCs were isolated from 17 of 30 (57%) non-cryopreserved aliquots of UCB cells (Table 3). In all cases of MSC isolation failure from a non-cryopreserved cell aliquot (n = 13), MSCs were not isolated from the corresponding cryopreserved cell aliquot. In all cases of successful MSC isolation from a cryopreserved cell aliquot (n = 6), MSCs were also isolated from the corresponding non-cryopreserved cell aliquot. We compared the characteristics of these six UCB units and the 11 UCB units for which MSCs were successfully isolated from non-cryopreserved cell aliquots, but not from cryopreserved cell aliquots (Table 4). The median volume of the UCB units, the median nucleated cell count of UCB before and after HES separation, and the median cell viability after thawing did not significantly differ between these two groups. These results suggested that freezing by the CP-1 method and thawing reduced the efficacy of MSC isolation from UCB cells.
Table 3. The number of UCB units according to the success/failure of MSC isolation from cryopreserved and non-cryopreserved cell aliquots. MSC, mesenchymal stromal/stem cell, UCB: umbilical cord blood.
| MSC isolation from non-cryopreserved cell aliquot | ||||||
|---|---|---|---|---|---|---|
| Success | Failure | Subtotal | ||||
| MSC isolation from cryopreserved cell aliquot (CP-1 method) |
Success | 6 | 0 | 6 | ||
| Failure | 11 | 13 | 24 | |||
| Subtotal | 17 | 13 | 30 | |||
Table 4. Characteristics of UCB units according to whether MSC isolation was successful from only the non-cryopreserved cell aliquot or from both the cryopreserved and non-cryopreserved cell aliquots. UCB: umbilical cord blood, HES: hydroxyethyl starch, MSC: mesenchymal stromal/stem cell.
| Characteristic | Successful MSC isolation only from non- cryopreserved cell aliquot |
Successful MSC isolation both from
non- cryopreserved and cryopreserved cell aliquots |
P value |
|---|---|---|---|
| Number of cryopreserved UCB units | 11 | 6 | - |
| Volume of UCB unit (mL), median [range] | 20.5 [13.5–50.0] | 21.5 [15.0–40.0] | 0.90 |
| Nucleated cell count of UCB before
HES separation (×108), median [range] |
2.8 [1.4–6.7] | 2.7 [1.1–4.9] | 0.82 |
| Nucleated cell count of UCB after
HES separation (×108), median [range] |
1.9 [0.6–5.8] | 2.1 [0.9–3.7] | 0.84 |
| Cell viability after thawing (%), median [range] | 87.1 [80.6–99] | 83.6 [80.3–99] | 0.33 |
Isolation of MSCs from UCB cells that were cryopreserved using CryoSure-DEX40
Dextran was recently used to cryopreserve UCB cells as a source of HSCs.12,13 We further examined the isolation of MSCs from UCB cells that were cryopreserved using CryoSure-DEX40 (CD-40 method). UCB cells were separated from ten UCB units using HES and cryopreserved using CryoSure-DEX40. MSCs were isolated from two of the ten UCB units (20%) (Table 5). There were no significant associations between the characteristics of UCB units and the success or failure of MSC isolation (Table 5). The isolation rate of MSCs was lower than that from non-cryopreserved UCB units. Logistic regression analysis demonstrated that the two different cryoprotectants (CP-1 method and CD-40 method) were not associated with the successful isolation of MSCs.
Table 5. Characteristics of UCB units according to whether MSC isolation was successful after cryopreservation using the CD-40 method (n = 10). UCB: umbilical cord blood, HES: hydroxyethyl starch, MSC: mesenchymal stromal/stem cell.
| Characteristic | Success | Failure | P value |
|---|---|---|---|
| Number of cryopreserved UCB units | 2 | 8 | - |
| Volume of UCB unit (mL), median [range] | 68.5 [51.0–86.0] | 35.0 [21.0–58.0] | 0.32 |
| Nucleated cell count of UCB before HES separation (×108), median [range] | 6.8 [6.4–7.1] | 6.1 [3.0–8.1] | 0.21 |
| Nucleated cell count of UCB after HES separation (×108), median [range] | 5.0 [4.5–5.4] | 3.9 [1.9–6.2] | 0.28 |
| Cell viability after thawing (%), median [range] | 69.1 [67.5–70.8] | 77.7 [62.0–84.0] | 0.09 |
Characteristics of MSCs isolated from cryopreserved UCB cells
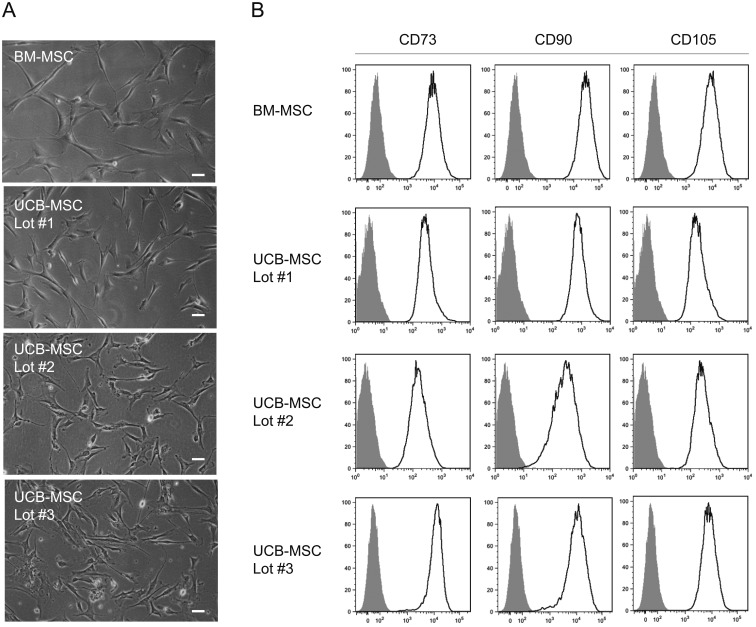
Adherent spindle-shaped fibroblastic cells appeared in the culture of cryopreserved UCB cells and had a similar morphology to BM-MSCs (Fig. 2A). Flow cytometric analysis showed that these cells (UCB-MSCs) were positive for mesenchymal stem cell-related antigens, including CD73, CD90, and CD105 (Fig. 2B), and negative for hematopoietic cell- and endothelial cell-related antigens, including CD45, CD34, CD14, CD19, and HLA-DR. The surface marker expression patterns of these UCB-MSCs were similar to those of human BM-MSCs.
Fig. 2.
Characteristics of MSCs isolated from cryopreserved UCB cells. (A) Morphology of UCB-MSCs. Phase contrast images of cells isolated from three different UCB units (Lots #1, #2, and #3) in comparison with that of BM-MSCs. Bars, 50 μm. (B) Expression of MSC-related antigens in UCB-MSCs. Flow cytometric analysis showing the expression of CD73, CD90, and CD105 in cells isolated from three different UCB units (Lots #1, #2, and #3) in comparison with that in BM-MSCs. Open histograms indicate expression of these molecules. Filled histograms indicate control staining.
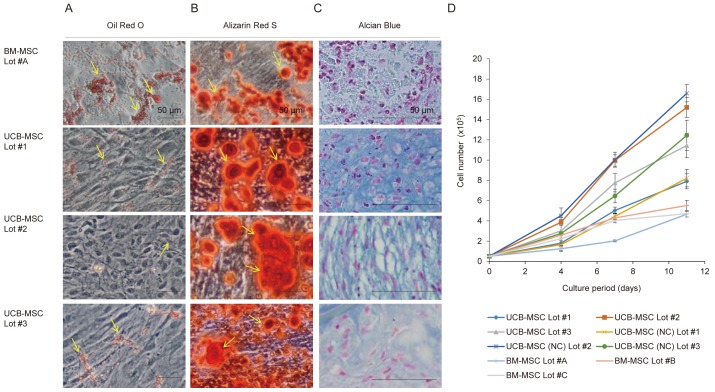
We examined the osteogenic, adipogenic, and chondrogenic differentiation of MSCs isolated from cryopreserved UCB cells in vitro. After 3 weeks of adipogenic induction, these UCB-MSCs showed a low level of fat deposition as assessed by Oil Red O staining, which was poor in comparison with that of BM-MSCs (Fig. 3A). After 3 weeks of osteogenic induction, UCB-MSCs showed mineral deposition as assessed by Alizarin Red S staining, which was comparable with that of BM-MSCs (Fig. 3B). With regard to chondrogenic induction, UCB-MSCs showed chondrogenic phenotypes as assessed by Alcian blue staining, which was comparable with that of BM-MSCs (Fig. 3C). Thus, MSCs isolated from cryopreserved UCB cells showed a multi-differentiation capability in vitro, although their adipogenic differentiation was reduced. With regard to the proliferation ability, the expansion of MSCs isolated from cryopreserved UCB cells was comparable with that of MSCs isolated from non-cryopreserved UCB cells (Fig. 3D). The expansion of these UCB-MSCs was faster than that of BM-MSCs.
Fig. 3.
Multilineage differentiation and expansion assays of UCB-MSCs isolated from cryopreserved UCB cells. (A) Oil Red O staining showing the adipogenic differentiation of UCB-MSCs in comparison with that of BM-MSCs. Yellow arrows indicate lipid-laden fat cells. (B) Alizarin Red S staining showing the osteogenic differentiation of UCB-MSCs in comparison with that of BM-MSCs. Yellow arrows indicate nodules of calcium deposits. (C) Alcian blue staining with Nuclear Fast Red counterstaining showing the chondrogenic differentiation of UCB-MSCs in comparison with that of BM-MSCs. Cartilage matrix deposits were stained blue. In (A–C), representative images were obtained from three different UCB units (Lots #1, #2, and #3). Bars, 50 μm. (D) Growth curve of MSCs isolated from three lots (Lots #1, #2, and #3) of cryopreserved UCB cells and non-cryopreserved (NC) UCB cells, and from three lots (Lots #A, #B, and #C) of BM cells. On days 4, 7, and 11 after cell seeding, the number of viable cells was counted using trypan blue. n = 3 per group. Data are mean values ± SD.
DISCUSSION
Cryopreservation enables a variety of cells to be stored for a long time until they are used. Donated UCB units that qualify for the banking system are processed and cryopreserved as a source of HSCs. Although UCB units that contain a large number of cells are in demand for HSCT, those that contain a small number of cells are less likely to be available in clinics. In Japan, cryopreserved UCB units containing fewer than 8×108 cells are unlikely to be used.14 We therefore explored whether cryopreserved UCB units containing a small number of cells (median cell number: 4.4×108 cells with the CP-1 method) can be used as an alternative source of MSCs. Our results indicated that MSCs could be isolated from cryopreserved UCB cells with a conventional adhesion method in the absence of complex procedures. However, the isolation rate was lower than that from non-cryopreserved UCB cells. A reduced isolation efficacy of MSCs is also observed with cryopreserved dental pulp tissues.15 These phenomena may result from damage caused by ice crystals during the freezing procedure. In our study, the isolation rate of MSCs was comparable between the methods using two different cryoprotectants (CP-1 method and CD-40 method). Further investigation of freezing optimization may improve the isolation efficacy of MSCs from cryopreserved tissues, such as a novel programmable freezer coupled to a magnetic field.16 Currently, most freshly donated UCB units are discarded because of their small volumes and/or cell numbers.8 Such UCB units should be processed without cryopreservation for effective MSC isolation.
In contrast with the little attention that has been paid to the isolation efficacy of MSCs from cryopreserved tissues/organs, the influence of cryopreservation on the characteristics of MSCs has been investigated.17 Allogeneic MSCs are typically culture-expanded, cryopreserved, and banked for future use. Studies to date have yielded conflicting results with regard to the impact of cryopreservation on the characteristics of MSCs. Several reports show that cryopreservation reduces the immunomodulatory capacity of MSCs.18,19 On the other hand, cryopreservation does not alter the surface marker profiles and osteogenic potential of BM-MSCs or compromise the therapeutic effects on cardiac function after direct intramyocardial injection.20,21 Difference in donors or the cultivation period after cell thawing might affect the characteristics of MSCs.18,22 In our study, MSCs that were isolated from cryopreserved UCB cells showed a similar morphology, surface marker expression profile as BM-MSCs, and had capability to differentiate into osteogenic, adipogenic, and chondrogenic cells. The expansion of these cells was faster than that of BM-MSCs. Although our study did not examine the immunomodulatory capacity of UCB-MSCs, this was demonstrated in previous reports.23,24 Therefore, it is crucial to elucidate whether MSCs isolated from cryopreserved UCB cells maintain the functions for therapeutic purposes.
In conclusion, MSCs were isolated from cryopreserved UCB units that are currently discarded as medical waste due to their inadequate volume and cell number. To reconsider such UCB units as a medical source of MSCs, processing of UCB cells without cryopreservation is favorable.
ACKNOWLEDGEMENTS
We thank Dr. Yayoi Higashi, Dr. Masaaki Fukuoka, Ms. Mieko Takumi, and the nursing staff of A7 ward in Japanese Red Cross Kyoto Daini Hospital for coordinating UCB donation. This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (#26293277 and #15K09453 to Y.M. and T.I.). This work was also supported in part by the Program of the network-type joint Usage/Research Disaster Medical Science of Hiroshima University, Nagasaki University, and Fukushima Medical University (Y.M. S.F., and T.I.). The authors declare that they have no conflict of interest.
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest in this study.
REFERENCES
- 1.Miura Y: Human bone marrow mesenchymal stromal/stem cells: current clinical applications and potential for hematology. Int J Hematol 103: 122-128, 2016. 10.1007/s12185-015-1920-z [DOI] [PubMed] [Google Scholar]
- 2.Muroi K, Miyamura K, Okada M, Yamashita T, Murata M, et al. : Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: a phase II/III study. Int J Hematol 103: 243-250, 2016. 10.1007/s12185-015-1915-9 [DOI] [PubMed] [Google Scholar]
- 3.Yoshioka S, Miura Y: Human mesenchymal stem cell therapy for acute graft versus host disease. Transl Med (Sunnyvale) 6: 171, 2016. 10.4172/2161-1025.1000171 [DOI] [Google Scholar]
- 4.Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR: MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell 14: 141-145, 2014. 10.1016/j.stem.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 5.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, et al. : SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 100: 5807-5812, 2003. 10.1073/pnas.0937635100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, et al. : Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22: 1338-1345, 2004. 10.1634/stemcells.2004-0058 [DOI] [PubMed] [Google Scholar]
- 7.Yoshioka S, Miura Y, Iwasa M, Fujishiro A, Yao H, et al. : Isolation of mesenchymal stromal/stem cells from umbilical cord blood units of small volumes that do not qualify for the banking system. Int J Hematol 102: 218-219, 2015. 10.1007/s12185-015-1828-7 [DOI] [PubMed] [Google Scholar]
- 8. http://www.kk.bbc.jrc.or.jp/saitai_kinki/saitai_kinkisaitai.files/160726_saisyuhozon.pdf
- 9.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. : Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317, 2006. 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 10.Miura Y, Miura M, Gronthos S, Allen MR, Cao C, et al. : Defective osteogenesis of the stromal stem cells predisposes CD18-null mice to osteoporosis. Proc Natl Acad Sci USA 102: 14022-14027, 2005. 10.1073/pnas.0409397102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaza T, Miura Y, Akiyama K, Bi Y, Sonoyama W, et al. : Mesenchymal stem cell-mediated ectopic hematopoiesis alleviates aging-related phenotype in immunocompromised mice. Blood 113: 2595-2604, 2009. 10.1182/blood-2008-10-182246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, et al. : Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA 92: 10119-10122, 1995. 10.1073/pnas.92.22.10119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. http://www.jshct.com/guideline/pdf/innai_futai_jikou.pdf
- 14. http://www.mhlw.go.jp/stf/shingi/2r9852000002ud99-att/2r9852000002uddl.pdf
- 15.Lindemann D, Werle SB, Steffens D, Garcia-Godoy F, Pranke P, et al. : Effects of cryopreservation on the characteristics of dental pulp stem cells of intact deciduous teeth. Arch Oral Biol 59: 970-976, 2014. 10.1016/j.archoralbio.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 16.Kaku M, Kamada H, Kawata T, Koseki H, Abedini S, et al. : Cryopreservation of periodontal ligament cells with magnetic field for tooth banking. Cryobiology 61: 73-78, 2010. 10.1016/j.cryobiol.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 17.Malekfar A, Valli KS, Kanafi MM, Bhone RR: Isolation and Characterization of Human Dental Pulp Stem Cells from Cryopreserved Pulp Tissues Obtained from Teeth with Irreversible Pulpitis. J Endod 42: 76-81, 2016. 10.1016/j.joen.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 18.Francois M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, et al. : Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy 14: 147-152, 2012. 10.3109/14653249.2011.623691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, et al. : Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells 32: 2430-2442, 2014. 10.1002/stem.1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotobuki N, Hirose M, Machida H, Katou Y, Muraki K, et al. : Viability and osteogenic potential of cryopreserved human bone marrow-derived mesenchymal cells. Tissue Eng 11: 663-673, 2005. 10.1089/ten.2005.11.663 [DOI] [PubMed] [Google Scholar]
- 21.Chin SP, Poey AC, Wong CY, Chang SK, Teh W, et al. : Cryopreserved mesenchymal stromal cell treatment is safe and feasible for severe dilated ischemic cardiomyopathy. Cytotherapy 12: 31-37, 2010. 10.3109/14653240903313966 [DOI] [PubMed] [Google Scholar]
- 22.Luetzkendorf J, Nerger K, Hering J, Moegel A, Hoffmann K, et al. : Cryopreservation does not alter main characteristics of Good Manufacturing Process-grade human multipotent mesenchymal stromal cells including immunomodulating potential and lack of malignant transformation. Cytotherapy 17: 186-198, 2015. 10.1016/j.jcyt.2014.10.018 [DOI] [PubMed] [Google Scholar]
- 23.Hao L, Gao L, Chen XH, Zou ZM, Zhang X, et al. : Human umbilical cord blood-derived stromal cells prevent graft-versus-host disease in mice following haplo-identical stem cell transplantation. Cytotherapy 13: 83-91, 2011. 10.3109/14653249.2010.501786 [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Hirai M, Cantero S, Ciubotariu R, Dobrila L, et al. : Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem 112: 1206-1218, 2011. 10.1002/jcb.23042 [DOI] [PubMed] [Google Scholar]