Abstract
Objective
Immunosuppressed individuals are at a high risk of latent tuberculosis infection (LTBI) and clinical practice guidelines for the screening and management of LTBI in at-risk patients have been developed. We assessed the scope, quality and consistency of clinical practice guidelines on screening for LTBI and the prevention of tuberculosis infection (TB) in high-risk patient populations.
Design
We conducted a systematic review of clinical practice guidelines. Methodological quality of these guidelines was assessed using the Appraisal of Guidelines for Research and Education (AGREE) II instrument. Textual synthesis was used to summarise and compare the recommendations.
Data sources
Electronic databases (MEDLINE, EMBASE, PsycINFO) and guideline registries were searched from inception to December 2017.
Results
Thirty-eight guidelines were included. Nineteen focused on patients receiving medical immunosuppression, seven on transplantation, three on patients with HIV and nine were generalised across all at risk populations. Most guidelines (n=32, 84%) used a systematic approach to identify and appraise the evidence. The methodological quality of the guidelines varied with the overall mean AGREE II scores ranging from 35% to 80%. Guidelines performed poorly in terms of editorial independence (average score 35%, range 0%–92%); however, most were robust in defining their scope and purpose (average score 80%, range 56%–100%). Guidelines recommended either or both the tuberculin skin test and the interferon gamma release assay for screening. Treatment of LTBI with isoniazid was consistently recommended.
Conclusion
Clinical practice guidelines on LTBI vary in quality and scope. The recommendations for screening varied across guidelines, while recommendations for treatment were largely consistent. Improving the consistency and quality of guidelines may help to optimise the screening and management of LTBI for improved patient outcomes.
Keywords: latent tuberculosis, immunosuppression, screening
Strengths and limitations of this study.
This study systematically reviewed published clinical practice guidelines for screening and management of latent tuberculosis infection in immunosuppressed patients.
We used the Appraisal of Guidelines for Research and Evaluation II instrument, an internationally validated tool, to assess the quality of the guidelines.
We included 38 guidelines and 11 non-English guidelines were excluded, with only few guidelines published in low-resource settings.
Introduction
Immunosuppression increases the risk of reactivation of prior infection with Mycobacterium tuberculosis leading to tuberculosis (TB) disease. In high-income countries, the baseline risk of reactivation of latent TB infection (LTBI) varies between 6 and 20 per 100 000 persons per year.1 2 The magnitude of the risk of TB reactivation among those who are immunosuppressed varies depending on the types of immunosuppression. The excess risk is highest among solid organ transplant recipients, particularly in lung (15-fold higher compared with the general population)3 and stem cell transplant recipients (6–10-fold higher),4 followed by recipients of tumour necrosis factor (TNF) antagonists (5–7-fold higher).5–8 The risk of TB reactivation in patients with HIV infection is 3–20 times higher than the general population9 10 and causes up to 25% of deaths in these patients.9
Early detection of LTBI through screening of patients at increased risk for TB may provide a window of opportunity for interventions such as treatment to prevent the development of active TB. Screening often involves the use of the commercially available tuberculin skin test (TST) and an interferon gamma release assay (IGRA). IGRAs include the QuantiFERON-TB Gold Plus (Cellestis, Australia) and the T-SPOT test (Oxford Immunotec, UK). However, there are potential drawbacks associated with screening. False negative results (2.8% in one setting11) with attendant false assurance may lead to late or missed diagnoses and delayed treatment. Conversely, false positive results may lead to unnecessary and inappropriate investigations which may be harmful.12 There is also a lack of a valid and accurate reference standard for diagnosing LTBI in immunosuppressed populations, rendering the true test performance characteristics of IGRA difficult to ascertain.
To advise health practitioners, clinical practice guidelines have provided evidence-based recommendations that inform practitioner and patient decisions about appropriate healthcare for specific clinical circumstances.13 As such, guidelines on screening for LTBI and treatment in at-risk populations have been developed in various healthcare settings. However, it is unclear if these recommendations may be generalisable to others or if there is variability. Therefore, this review aims to assess and compare the rationale, scope, quality and consistency of clinical practice guidelines and consensus statements for the screening of LTBI, as well as for treatment against LTBI in immunosuppressed individuals.
Methods
Selection criteria
Evidence-based clinical practice guidelines and consensus statements on screening for LTBI and treatment for LTBI in immunosuppressed individuals published in English were eligible for inclusion. Patients who were medically immunocompromised (including chemotherapy, disease modifying agents and biological therapy), had received a solid organ or stem cell transplant or HIV positive were included. Draft or unpublished guidelines, conference or discussion papers, opinions and guidelines and consensus statements replaced by updated and/or revised recommendations were excluded.
Literature search
We searched MEDLINE, Embase and PsycINFO from database inception to December 2017. Medical Subject Heading (MeSH) terms and text words for ‘tuberculosis’, ‘immunosuppressed’ and ‘immunocompromised’ were combined with terms relating to clinical practice guidelines and consensus statements (online supplementary appendix 1). Clinical guideline registries and reference lists were searched for additional clinical practice guidelines. Titles and abstracts were reviewed by two authors (TH and EA), and those which did not meet the inclusion criteria were excluded. Full text versions of potentially relevant guidelines or consensus statements were examined for eligibility.
bmjopen-2018-022445supp001.pdf (83.9KB, pdf)
Appraisal of guidelines and consensus statements
The methodological quality was assessed independently by TH and EA, using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument.14 AGREE II is an internationally validated, rigorously developed 23-item tool used to evaluate independent domains of guideline development including: scope and purpose, stakeholder involvement, rigour of development, clarity and presentation, applicability and editorial independence. Each item was rated on a seven-point scale ranging from strongly disagree (score 1) to strongly agree (score 7). The domain score was obtained by summing all scores of the individual items per domain and then standardising the total as a percentage of the maximum possible score for that domain:
The minimum possible domain score would be the number of questions multiplied by the number of appraisers, multiplied by 1 (strongly disagree). The maximum possible domain score is the number of questions multiplied by the number of appraisers, multiplied by 7 (strongly agree). The AGREE scores were rated independently for each guideline by TH/EA and a quadratic weighted kappa (κ) score for each guideline and across all guidelines were calculated as a measure of inter-rater agreement. An overall weighted kappa was also calculated across all guidelines.
Textual synthesis
All text from each guideline were entered into the HyperRESEARCH software (ResearchWare 2011, V.3.0.3, Randolph, Massachusetts, USA) for storing, coding and searching textual data. Data were categorised by subheadings based on immunosuppression modality and by screening and treatment methods. Subsequently, we conducted a textual descriptive synthesis to analyse the content, consistency and evidence base of the recommendations.
Patient and public involvement
There was no patient or public involvement in this study.
Results
Characteristics of clinical practice guidelines
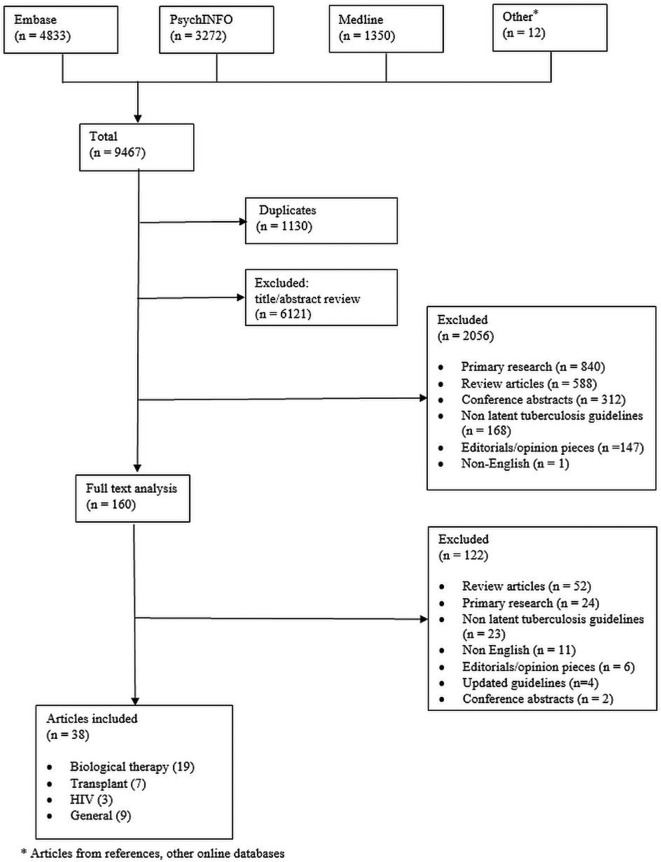
We included 38 guidelines (figure 1) published from 2002 to 2017. These guidelines focused on medical immunosuppression (19 guidelines),1 15–32 solid organ and stem cell transplantation (seven guidelines)3 33–38 and in HIV settings (three guidelines).9 39 40 Nine were general guidelines which were not specific to a particular patient group and covered the detection of LTBI and its management.10 41–46 These guidelines were published across 16 different countries from regions including North America, Western Europe, Asia, Australia and South Africa. A summary of the guideline characteristics is provided in table 1.
Figure 1.
Database search strategy The medical databases EMBASE, PsychINFO and Medline were searched for articles relevant to tuberculosis in an immunosuppressed setting, using the search strategy described in online supplementary appendix 1. A total of 9467 articles were found and compiled into the EndNote software (Clarivate Analytics 2017, V.X7), of which 1130 articles were duplicate articles. From the remaining articles, 6121 articles were excluded by abstract review, primarily because they were irrelevant. A further 2056 articles were removed during a second review of titles and abstracts. A total of 160 articles were reviewed in full of which 122 were excluded as they did not fulfil guideline or relevance criteria. A total of 38 articles were included in our final review.
Table 1.
Characteristics of the studies
| Guidelines | Funding body | Country | Population | Target users | Writers | Evidence base | Evidence level | Grading | Guideline review | Update |
| ARA 2010 1 | Professional society | Australia | Biological therapy | Rheumatologists | Rheumatologists | Guidelines | NS | NS | NS | NS |
| Aguado et al 20093 | Industry, Professional society | Spain | Organ transplant | Transplant physicians |
Transplant infectious disease specialists | Literature, consensus, experts | I-III* | A-E † | NS | NS |
| CDC 20169 | Office of AIDS Research, | USA | HIV | Clinicians | Multidisciplinary | Literature, experts | I-III‡ | A-C§ | Expert review, public consultation | 6 months |
| WHO 201510 | Ministry of health Italy, WHO, | WHO | All | Tuberculosis physicians |
Multidisciplinary | Literature, experts | GRADE¶ | Strong/conditional** | Expert review, peer review | 2020 |
| Beglinger et al 200715 | NS | Switzerland | Anti TNF-alpha therapy | Clinicians | Multidisciplinary | Literature, experts | NS | NS | NS | NS |
| Cantini et al 201516 | NS | Italy | Biological therapy | Clinicians | Multidisciplinary | Literature, experts | NS | NS | NS | NS |
| Doherty et al 200817 | Professional body | USA | Patients with psoriasis | NS | Dermatologists | Literature, experts | I-IV (Shekelle et al)†† |
NS | Medical Board | NS |
| Duarte et al 201218 | NS | Portugal | Biological therapy | Clinicians | Multidisciplinary | Guidelines, experts | A-D‡‡ | NS | NS | NS |
| Fonseca et al 200819 | NS | Portugal | Biological therapy | Rheumatologists | Multidisciplinary | Literature, guidelines | NS | NS | Expert, public consultation | NS |
| Hodkinson et al 201320 | Professional body | South Africa | Patients with rheumatoid arthritis |
Clinicians | Rheumatologists | Literature, guideline, expert, stakeholder |
NS | NS | Public/stakeholder consultation |
2 years |
| Kavanagh et al 200821 | Professional body | Ireland | Anti TNF-alpha therapy | Clinicians | Multidisciplinary | Literature, guidelines, experts | NS | NS | NS | NS |
| Keith et al 201422 | Nil | USA | Immunosuppression | Dermatologists | Multidisciplinary | Literature, guidelines | NS | NS | NS | NS |
| Koike et al 200723 | Professional body, Government |
Japan | Anti-TNF alpha therapy | Rheumatologists | NS | Experts | NS | NS | NS | NS |
| Lichauco et al 200624 | NS | Philippines | Biological therapy | Physicians | Multidisciplinary | Literature, guidelines | Level 1-4§§ | PHEX guidelines¶¶ | Expert peer review, public consultation |
NS |
| Salmon 200225 | Not specified | France | Rheumatoid arthritis | Rheumatologists | Multidisciplinary | NS | NS | NS | NS | NS |
| Mir Viladrich et al 201626 | NS | Spain | Biological therapy | Clinicians | Multidisciplinary | Guidelines, experts | NS | A-C, I-III*** | NS | NS |
| Mok et al 201127 | NS | Hong Kong | Rheumatoid arthritis | Rheumatologists | Rheumatologists | Guidelines | A-D††† | NS | NS | As required |
| Nordgaard-Lassen et al 201228 | NS | Denmark | Biological therapy | Clinicians | Gastroenterologists | Literature | I-IV‡‡‡ | A-C§§§ | NS | NS |
| BTS 200529 | NS | UK | Anti TNF-alpha therapy | Physician | Multidisciplinary | Literature, experts | SIGN¶¶¶ | SIGN**** | Professional membership consultation, peer review |
2008 |
| Smith et al 201730 | British Association of Dermatologists |
UK | Psoriasis | Dermatologists | Multidisciplinary | Literature | GRADE¶ | GRADE: Strong/weak/no†††† | Professional membership consultation, peer review |
As required |
| Solovic et al 201031 | NS | Europe | Biological therapy | Clinicians | Multidisciplinary | Literature | NS | A-D‡‡‡‡ | NS | NS |
| Carrascosa et al 201632 | Gebro Pharma | Spain | Methotrexate therapy | Dermatologists | Dermatologists | Literature, guidelines | SIGN¶¶¶ | SIGN**** | NS | NS |
| Bumbacea et al 201233 | Professional society | Europe | All transplant | Transplant physicians | Transplant infectious disease specialists | Literature, guidelines | NS | A-D‡‡‡‡ | NS | NS |
| KDIGO 200934 | KDIGO, multiple sponsors | International | Kidney transplant recipients | Clinicians | Multidisciplinary | Literature, experts | A-D§§§§ | Level 1–2, not graded¶¶¶¶ | NS | NS |
| Meije et al 201435 | NS | Spain | Solid organ transplant | Transplant physicians | Multidisciplinary | Literature | Level A-D, I-IV‡‡ | NS | NS | NS |
| EBPG 200236 | NS | Europe | Renal transplant | Transplant physicians | NS | NS | A-D***** | NS | NS | NS |
| Subramanian and Morris 201337 | American Society of Transplantation | USA | Solid organ transplant recipients | Transplant physicians | Transplant infectious disease physicians | Literature, experts | I-III‡‡ | NS | NS | NS |
| Tomblyn et al 200938 | Member societies | International/ USA/Canada |
Stem cell transplant recipients |
Clinicians | Multidisciplinary | Literature, experts | I-III††††† | A-E§§§§§ | NS | NS |
| Pozniak et al 201139 | Nil | UK | HIV | Physicians | HIV physicians | Literature, guidelines | I-III‡‡‡‡‡ | A-E¶¶¶¶¶ | NS | NS |
| SA 201040 | NS | South Africa | HIV | HIV treatment providers | NS | NS | NS | NS | NS | NS |
| Santin et al 201641 | SEPAR, SEIMC | Spain | All | Clinicians | Multidisciplinary | Literature | GRADE¶ | GRADE: weak/strong | NS | 5 years |
| Al Jahdali et al 201042 | Professional society | Saudi Arabia | All susceptible patients | Clinicians | Multidisciplinary | Experts | NS | NS | NS | NS |
| ECDC 201143 | ECDC | Europe | Immunocompromised | National bodies | Multidisciplinary | Literature, experts | NS | NS | NS | NS |
| Mazurek et al 201044 | CDC | USA | All | Public health officials, physicians, others |
Multidisciplinary | Literature, experts | NS | NS | NS | NS |
| Taylor et al 200545 | Professional bodies | USA | All | Healthcare workers | Multidisciplinary | Literature, experts | I-III****** | A-C†††††† | NS | NS |
| CTC 200846 | Public Health Agency | Canada | Immunocompromised patients | NS | Multidisciplinary | Literature, experts | NS | NS | NS | Periodic |
| Japanese Society for Tuberculosis 201447 | NS | Japan | All susceptible populations | Clinicians | NS | NS | NS | NS | NS | NS |
| NICE 201648 | NCCCC | UK | All susceptible populations | All healthcare workers and public |
Multidisciplinary | Literature review | GRADE¶ | Offer/do not offer/consider‡‡‡‡‡‡ |
Stakeholders, peer review |
As required |
| *I: Evidence from at least 1 well-designed and performed trial, II: Evidence from at least one well designed non-RCT, cohort or case control or non-controlled experimental study with non-conclusive results, III: Expert opinion based on clinical experience, descriptive studies, report from expert panel. †A: Solid evidence of clinical benefit, B: Solid or moderately solid evidence for efficacy, but clinical benefit is limited, C: Insufficient evidence for efficacy, D: Moderately solid evidence for lack of efficacy, E: Strong evidence for lack of efficacy. ‡I: One or more RCT with clinical outcomes and/or validated laboratory endpoints, II: One or more well-designed, non-randomised trials or observational cohort studies with long-term clinical outcomes III: Expert opinion. §A: Strong recommendation for the statement, B: Moderate recommendation for the statement, C: Optional recommendation for the statement. ¶GRADE—High: Further research is very unlikely to change our confidence in the estimate of effect. Moderate; Further research is likely to have an important impact on our confidence in the effect. Low: Further research is very likely to have an impact on the estimate of effect and is likely to change the estimate. Very low: Any estimate of effect is very uncertain. **(1) A strong recommendation is one for which the Panel was confident that the desirable effects of adherence to the recommendation outweigh the undesirable effects. This could be either in favour of or against an intervention. (2) A conditional recommendation is one for which the Panel concluded that the desirable effects of adherence to the recommendation probably outweigh the undesirable effects, but the Panel was not confident about these trade-offs. Reasons for not being confident included: absence of high-quality evidence (data to support the recommendation are scant); presence of imprecise estimates of benefits or harms (new evidence may result in changing the balance of risk to benefit); uncertainty or variation regarding how different individuals value the outcomes (only applicable to a specific group, population or setting); small benefits and benefits that may not be worth the costs (including the costs of implementing the recommendation). | ||||||||||
††IA: Evidence includes evidence from meta-analysis of randomised controlled trials; IB: Evidence includes evidence from at least one randomised controlled trial; IIA: Evidence includes evidence from at least one controlled study without randomisation; IIB: Evidence includes evidence from at least one other type of quasi-experimental study; III: Evidence includes evidence from non-experimental descriptive studies, such as comparative studies, correlation studies and case-control studies; IV: Evidence includes evidence from expert committee reports or opinions or clinical experience of respected authorities or both.
‡‡Evidence level definitions not specified.
§§Level 1: An RCT that demonstrates a statistically significant difference in at lest one major outcome or if the difference is not statistically significant, an RCT of adequate sample size to exclude 25% difference in relative risk with 80% power, given the observed results. Level 2: An RCT that does not meet the Level 1 criteria. Level 3: A non-randomised trial with concurrent controls selected by some systematic method. Level 4: Before-after study or case series (at least 10 patients) with historical controls or controls drawn from other studies. Level 5: Case series (at least 10 patients) without controls. Experts’ opinion and clinical experience are included.
¶¶Level 1: Evaluation of evidence satisfies all of the following criteria: (1) effective treatment is documented in RCTs that observe effects on clinical outcomes; (2) the condition being screened has local prevalence data; (3) the screening test is validated and (4) the cost-effectiveness of the screening test, as well as treatment for the disease have been evaluated. Level 2: Evaluation of evidence satisfies #1 but not all of #2, #3 and #4. Level 3: Evaluation of evidence satisfies #2, #3 or #4 but not #1. Level 4: Evaluation of evidence satisfies none of the criteria.
***Recommendations according to categories of strength: (A) Good evidence to support the recommendation, (B) moderate evidence to support the recommendation, (C) poor evidence that does not enable the recommendation to be either supported or rejected. Recommendations according to the scientific quality. Grade I: Recommendation based on at least one well-designed, controlled, RCT. Grade II: Recommendation based on at least one well-designed, but not RCT, cohort studies, multiple time-series studies or very evident results in uncontrolled trials. Grade III: Recommendation based on the opinion of experts, descriptive studies or clinical experience.
††† Category A: At least one RCT or meta-analyses of RCTs or reviews if these contain category A references. Category B: At least one controlled trial without randomisation or at least one other type of experimental study or extrapolated recommendations from RCTs or meta-analyses. Category C: Non-experimental descriptive studies, such as comparative studies, correlational studies and case-control studies, which are extrapolated from RCTs, non-randomised controlled studies or other experimental studies. Category D: Expert committee reports or opinions or clinical experience of respected authorities. Also includes all abstracts.
‡‡‡I: Randomised, controlled clinical trials (therapeutic or diagnostic) and meta-analyses of randomised, controlled clinical trials or systematic reviews. II: Prospective and controlled but non-randomised investigations (cohort studies); diagnostic testing evaluated by direct methods. III: Studies that are controlled but not prospective (case-control studies); diagnostic testing evaluated by indirect methods. IV: Descriptive studies, expert opinions and narrative reviews.
§§§(A) randomised, controlled clinical trials (therapeutic or diagnostic) and meta-analyses of randomised, controlled clinical trials or systematic reviews. (B) Prospective and controlled but nonrandomised investigations (cohort studies); diagnostic testing evaluated by direct methods, OR Studies that are controlled but not prospective (case-control studies); diagnostic testing evaluated by indirect methods. (C) Descriptive studies, expert opinions and narrative reviews.
¶¶¶1++: High quality meta-analyses, systematic reviews of RCTs or RCTs with a very low risk of bias. 1+: Well conducted meta-analyses, systematic reviews of RCTs or RCTs with a low risk of bias. 12: Meta-analyses, systematic reviews of RCTs or RCTs with a high risk of bias. 2++: High-quality systematic reviews of case-control or cohort studies. High-quality case-control or cohort studies with a very low risk of confounding, bias or chance and a high probability that the relationship is causal. 2+: Well-conducted case-control or cohort studies with a low risk of confounding, bias or chance and a moderate probability that the relationship is causal. 22: Case-control or cohort studies with a high risk of confounding, bias or chance and a significant risk that the relationship is not causal. 3: Non-analytical studies (eg, case reports, case series). 4: Expert opinion.
****(A) At least one meta-analysis, systematic review, or RCT rated as 1++ and directly applicable to the target population or a systematic review of RCTs or a body of evidence consisting principally of studies rated as 1+ directly applicable to the target population and demonstrating overall consistency of results. (B) A body of evidence including studies rated as 2++ directly applicable to the target population and demonstrating overall consistency of results or extrapolated evidence from studies rated as 1++ or 1+. (C) A body of evidence including studies rated as 2+ directly applicable to the target population and demonstrating overall consistency of results or extrapolated evidence from studies rated as 2+. (D) Evidence level 3 or 4 or extrapolated evidence from studies rated as 2+.
††††Strong recommendation for use of an intervention: Benefits of the intervention outweigh the risks; most patients would choose the intervention while only a small proportion would not; for clinicians, most of their patients would receive the intervention; for policy makers, it would be a useful performance indicator. Weak recommendation for the use of an intervention: Risks and benefits of the intervention are finely balanced; many patients would choose the intervention but many would not; clinicians would need to consider the pros and cons for the patient in the context of the evidence; for policy makers, it would be a poor performance indicator where variability in practice is expected. No recommendation: Insufficient evidence to support any recommendation. Strong recommendation against the use of an intervention: Risks of the intervention outweigh the benefits; most patients would not choose the intervention while only a small proportion would; for clinicians, most of their patients would not receive the interventions.
‡‡‡‡(A) Evidence is from end-points of well-designed RCTs that provide a consistent pattern of findings in the population for which the recommendation is made. Category A requires substantial numbers of studies involving substantial numbers of participants. (B) Evidence is from end-points of intervention studies that include only a limited number of patients, posthoc or subgroup analysis of RCTs or meta-analysis of RCTs. In general, category B pertains when few randomised trials exist, they are small in size, they were undertaken in a population that differs from the target population of the recommendation or the results are somewhat inconsistent. (C) Evidence is from outcomes of uncontrolled or non-randomised trials or from observational studies. (D) This category is used only in cases where the provision of some guidance was deemed valuable but the clinical literature addressing the subject was insufficient to justify placement in one of the other categories. The Panel consensus is based on clinical experience or knowledge that does not meet the criteria listed above.
§§§§(A) High, (B) moderate, (C) low, (D) very low.
¶¶¶¶Level 1: We recommend. Level 2: We suggest. No grade: Used, typically, to provide guidance based on common sense or where the topic does not allow adequate application of evidence.
*****(A) Guidelines are supported by at least one large published RCT or more. (B) Guidelines are supported by large open trials or smaller trials with consensus results. (C) Guidelines are derived from small or controversial studies or represent the opinion of the group of experts.
†††††I: Evidence from at least one well-executed randomised, controlled trial. II: Evidence from at least one well-designed clinical trial without randomisation; cohort or case-controlled analytic studies (preferably from more than one centre); multiple time-series studies or dramatic results from uncontrolled experiments. III: Evidence from opinions of respected authorities based on clinical experience, descriptive studies or reports of expert committees.
§§§§§(A) Both strong evidence for efficacy and substantial clinical benefit support recommendation for use. Should always be offered. (B) Moderate evidence for efficacy—or strong evidence for efficacy, but only limited clinical benefit—supports recommendation for use. Should generally be offered. (C) Evidence for efficacy is insufficient to support a recommendation for or against use or evidence for efficacy might not outweigh adverse consequences (eg, drug toxicity, drug interactions) or cost of the chemoprophylaxis or alternative approaches. Optional. (D) Moderate evidence for lack of efficacy or for adverse outcome supports a recommendation against use. Should generally not be offered. (E) Good evidence for lack of efficacy or for adverse outcome supports a recommendation against use. Should never be offered.
‡‡‡‡‡(I) At least one properly randomised trial with clinical endpoints. (II) Clinical trials either not randomised or conducted in other populations. (III) Expert opinion.
¶¶¶¶¶(A) Preferred; should generally be offered. (B) Alternative; acceptable to offer. (C) Offer when preferred or alternative regimens cannot be given. (D) Should generally not be offered. (E) Should never be offered.
******I: Evidence from at least one RCT; II: Evidence from (1) at least one well-designed clinical trial, without randomisation, (2) cohort or case-controlled analytic studies, (3) multiple times series, (4) dramatic results from uncontrolled experiments III evidence from opinions of respected authorities on the basis of cumulative public health experience, descriptive studies or reports of expert committees.
††††††A highly recommended in all circumstances, II recommended; implementation might be dependent on resource availability, C might be considered under exceptional circumstances.
‡‡‡‡‡‡(A) Level 1++ and directly applicable to the target population or level 1+ and directly applicable to the target population AND consistency of results. Evidence from NICE technology appraisal. (B) Level 2++, directly applicable to the target population and demonstrating overall consistency of results or extrapolated evidence from 1++ or 1+. (C) Level 2+, directly applicable to the target population and demonstrating overall consistency of results or extrapolated evidence from 2++. (D) Level 3 or 4 or extrapolated from 2+ or formal consensus or extrapolated from level 2 clinical evidence supplemented with health economic modelling. D (GPP): A good practice point (GPP) is a recommendation based on the experience of the GDG.
ARA, Australian Rheumatological Association; BTS, British Thoracic Society; CDC, centre for disease control; CTC, Canadian Tuberculosis Committee; EBPG, European Best Practice Guideline Expert Group on Renal Transplantation; ECDC, European Centre for Disease Prevention and Control; GRADE, Grading of Recommendations Assessment, Development and Evaluation; KDIGO, Kidney Disease Improving Global Outcomes; NCCCC, The National Collaborating Centre for Chronic Conditions; NICE, National Institute for Health and Care Excellence; NS, not specified; PHEX, Philippine Guidelines on Periodic Health Examination; RCT, randomised control study; SA, South Africa; SEIMC, Spanish Society of Infectious Disease and Clinical Microbiology; SEPAR, Spanish Society of Respiratory Disease and Thoracic Surgery; SIGN, Scottish Intercollegiate Guidelines Network; TNF, tumour necrosis factor.
Of the guidelines that discussed medical immunosuppression, nine provided recommendations for treatment across various medical specialties including dermatology, rheumatology, gastroenterology and respiratory medicine.15 16 18 21 24 26 28 29 31 Four were specific to patients with rheumatoid arthritis,20 23 25 27 of which one focused only on patients receiving infliximab,23 while two guidelines were specific to patients with psoriasis.18 30 One guideline focused on patients with rheumatological or gastroenterological disease.15 There were specific guidelines addressing inflammatory joint disease,19 rheumatological disease1 and autoimmune bullous diseases.22 One guideline discussed patients at risk due to methotrexate therapy.32 Of the transplantation guidelines, two guidelines were for kidney transplantation,34 36 one for stem cell transplantation,38 one for both solid organ and stem cell transplantation33 and three for all forms of solid organ transplantation.3 35 37
Three guidelines addressed LTBI in patients with HIV.9 39 40 There were nine other guidelines which discussed screening in all at risk populations.10 41–48 Six of these also included discussion on patients with HIV41–45 47 and four were IGRA specific guidelines, although these guidelines also used TST as part of their screening strategies.41 43 44 46 Three guidelines were developed in countries with a high prevalence of TB (South Africa and Philippines).20 24 40
Across the guidelines, the methods for literature review were not always specified. Literature review was conducted in 32 guidelines (84%),1 3 9 10 15–22 24 26–35 37–39 41–46 48 of which 12 based their recommendations on a combination of the literature review and expert consensus.3 9 10 15–18 20 21 26 29 34 37 43–46 Two guidelines were based on expert consensus alone.23 42 Twenty guidelines graded the level of evidence.3 9 10 17 18 24 27–30 32 34–39 42 46 48 Furthermore, 17 guidelines graded the strength of their recommendations.3 9 10 24 26 28–34 38 39 41 45 48 Where evidence was graded, it was often of low quality. Only nine (24%) guidelines were peer reviewed,9 10 17 19 20 24 29 30 48 with five (13%) made available for public consultation prior to publication.9 19 20 24 48 Only one guideline included a formal cost-effectiveness analysis48 which suggested that TST was more cost effective compared with the IGRA. The incremental cost-effectiveness ratio was influenced by prevalence of disease and age of the patients.
Methodological quality
Table 2 summarises the AGREE domain scores of each guideline. The mean AGREE score (and range) for all guidelines was 55% (0%–100%). In terms of scope and purpose, on average 80% (56%–100%) of criteria were met for all guidelines. The average score for stakeholder involvement was 51% (11%–97%), for rigour of development 47% (10%–93%), clarity and presentation 74% (50%–92%), applicability 47% (0%–92%) and editorial independence 35% (0%–92%). The overall domain mean score was 55% (35%–80%).
Table 2.
Grade of recommendation
| Guideline name | Scope and purpose (%) | Stakeholder involvement (%) | Rigour of development (%) | Clarity and presentation (%) | Applicability (%) | Editorial independence (%) | Weighted Kappa Scores (Quadratic) | 95% CI |
| ARA 20101 | 75 | 31 | 10 | 67 | 25 | 0 | 0.74 | 0.56 to 0.92 |
| Aguado et al 20093 | 72 | 28 | 24 | 72 | 29 | 58 | 0.76 | 0.62 to 0.90 |
| CDC 20169 | 89 | 89 | 81 | 75 | 77 | 83 | 0.29 | −0.14 to 0.71 |
| WHO 201510 | 97 | 94 | 88 | 89 | 92 | 88 | 0.67 | 0.27 to 1.00 |
| Beglinger et al 200715 | 75 | 42 | 23 | 67 | 25 | 0 | 0.72 | 0.54 to 0.91 |
| Cantini et al 201516 | 89 | 53 | 55 | 89 | 56 | 38 | 0.80 | 0.63 to 0.97 |
| Doherty et al 200817 | 92 | 44 | 75 | 86 | 71 | 58 | 0.55 | 0.19 to 0.91 |
| Duarte et al 201218 | 86 | 44 | 31 | 83 | 52 | 0 | 0.67 | 0.46 to 0.89 |
| Fonseca et al 200819 | 92 | 72 | 73 | 86 | 60 | 4 | 0.74 | 0.53 to 0.95 |
| Hodkinson et al 201320 |
83 | 83 | 56 | 75 | 71 | 25 | 0.00 | −0.27 to 0.27 |
| Kavanagh et al 200821 |
64 | 33 | 29 | 67 | 15 | 0 | 0.61 | 0.39 to 0.82 |
| Keith et al 201422 | 83 | 42 | 45 | 50 | 19 | 42 | 0.61 | 0.27 to 0.92 |
| Koike et al 200723 | 78 | 33 | 28 | 56 | 10 | 29 | 0.41 | 0.08 to 0.75 |
| Lichauco et al 200624 | 89 | 69 | 67 | 78 | 65 | 0 | 0.64 | 0.27 to 1.00 |
| Mir Viladrich et al 201626 |
81 | 42 | 29 | 75 | 40 | 42 | 0.66 | 0.44 to 0.88 |
| Mok et al 201127 | 69 | 36 | 28 | 53 | 27 | 33 | 0.53 | 0.24 to 0.82 |
| Nordgaard-Lassen et al 201228 | 78 | 39 | 48 | 64 | 35 | 0 | 0.75 | 0.60 to 0.90 |
| Salmon 200225 | 72 | 42 | 13 | 64 | 0 | 0 | 0.76 | 0.55 to 0.97 |
| BTS 200529 | 92 | 69 | 91 | 89 | 71 | 63 | 0.32 | −0.05 to 0.70 |
| Smith et al 200930 | 94 | 61 | 80 | 83 | 65 | 75 | 0.77 | 0.51 to 1.00 |
| Solovic et al 201031 | 69 | 33 | 35 | 81 | 44 | 38 | 0.66 | 0.41 to 0.92 |
| Carrascosa et al 201632 | 67 | 42 | 46 | 61 | 21 | 83 | 0.71 | 0.56 to 0.87 |
| Bumbacea et al 201233 | 69 | 44 | 43 | 81 | 40 | 67 | 0.48 | 0.13 to 0.84 |
| KDIGO 200934 | 100 | 78 | 67 | 75 | 65 | 92 | 0.21 | −0.07 to 0.48 |
| Meiji et al 201435 | 64 | 25 | 28 | 72 | 25 | 38 | 0.67 | 0.43 to 0.89 |
| EBPG 200236 | 86 | 67 | 68 | 89 | 77 | 75 | 0.18 | −0.05 to 0.41 |
| Subramanian 201337 | 75 | 42 | 42 | 78 | 54 | 42 | 0.31 | −0.10 to 0.71 |
| Tomblyn et al 200938 | 81 | 58 | 43 | 69 | 35 | 17 | 0.44 | 0.15 to 0.74 |
| Pozniak et al 201139 | 81 | 42 | 38 | 64 | 56 | 0 | 0.73 | 0.51 to 0.95 |
| SA 201040 | 78 | 19 | 10 | 78 | 69 | 0 | 0.91 | 0.85 to 0.98 |
| Santin et al 201641 | 92 | 58 | 74 | 83 | 67 | 88 | 0.73 | 0.49 to 0.97 |
| Al Jahdali et al 201042 | 83 | 58 | 32 | 75 | 46 | 0 | 0.58 | 0.35 to 0.81 |
| ECDC 201143 | 72 | 31 | 33 | 69 | 29 | 17 | 0.41 | 0.14 to 0.67 |
| Mazurek et al 201044 | 78 | 72 | 71 | 72 | 60 | 8 | 0.57 | 0.33 to 0.81 |
| Taylor et al (CDC 2005) 45 | 75 | 44 | 28 | 58 | 38 | 0 | 0.26 | 0.09 to 0.47 |
| CTC 200846 | 83 | 50 | 52 | 69 | 40 | 46 | 0.29 | 0.01 to 0.58 |
| Japanese Society for Tuberculosis 201447 | 56 | 11 | 26 | 67 | 60 | 0 | 0.67 | 0.52 to 0.82 |
| NICE 201648 | 100 | 97 | 93 | 92 | 69 | 83 | 0.52 | 0.09 to 0.96 |
ARA, Australian Rheumatological Association; BTS, British Thoracic Society; CDC, centre for disease control; CTC, Canadian Tuberculosis Committee; EBPG, European Best Practice Guideline Expert Group on Renal Transplantation; ECDC, European Centre for Disease Prevention and Control; KDIGO, Kidney Disease Improving Global Outcomes; NICE, National Institute for Health and Care Excellence; SA, South Africa.
Weighted Kappa scores (κ) to assess inter-rater agreement ranged from a score between poor to very good, with the majority being moderate (0.41–0.60) to very good (0.81–1.00). The overall weighted score was 0.65 (95% CI 0.60–0.69), with good concordance between reviewers. The AGREE scores did not improve with later guidelines and over time.
Textual synthesis
A summary of the guidelines and the recommendations are provided in table 3. Most guidelines recommended screening in all immunosuppressed patients, and treatment if there was clinical evidence of LTBI.
Table 3.
Summary of recommendations
| Guidelines | Population | Screening process | Treatment method | Treatment duration | Timing before immunosuppression | |||
| History | TST | IGRA | CXR | |||||
| ARA 20101 | Biological therapy | X | X | X | Isoniazid* | 6–9 months | 1–2 months | |
| Aguado et al 20093 | Transplant recipients | X | X | X | Isoniazid | 9 months | Before transplant | |
| CDC 20169 | Patients with HIV | X | X | Isoniazid | 9 months | NS | ||
| WHO 201510 | Low-middle income countries | X | X | Isoniazid | 6 months | NS | ||
| Beglinger et al 200715 | Biological therapy | X | X | X | Isoniazid OR rifampicin | NS | 1 month | |
| Cantini et al 201516 | Biological therapy | X | X | X | Isoniazid | 9 months | 1 month | |
| Doherty 200817 | Patients with psoriasis | X | X | X | Isoniazid | 9 months | 1–2 months or longer | |
| Duarte et al 201218 | Biological therapy | X | X | X | Isoniazid | 9 months | 1–2 months | |
| Fonseca et al 200819 | Biological therapy | X | X | X | Isoniazid | 6–9 months | 1 month | |
| Hodkinson et al 201320 | Patients with rheumatoid arthritis | X | X | X | X | Isoniazid | 9 months | 1 month |
| Kavanagh et al 200821 | Biological therapy | X | X | X | Isoniazid | 9 months | Pre-immunosuppression | |
| Keith et al 201422 | Bullous dermatosis | X | X | NS | NS | NS | ||
| Koike et al 200723 | Biological therapy | X | X | X | Isoniazid | NS | NS | |
| Lichauco et al 200624 | Biological therapy | X | X | Isoniazid | 9 months | 1 month | ||
| Salmon200225 | Biological therapy | X | X | Rifampicin and pyrazinamide | 2 months | 3 weeks | ||
| Mir Viladrich et al 201626 | Biological therapy | X | X | X | Isoniazid | 9 months | 4 weeks | |
| Mok et al 201127 | Biological therapy | X | Isoniazid | 9 months | 4 weeks | |||
| Nordgaard-Lassen et al 2012 28 | Biological therapy | X | Isoniazid | 9 months | 4 weeks | |||
| BTS 200529 | Biological therapy | X | X | X | Isoniazid | 6 months | Concurrent | |
| Smith et al 200930 | Biological therapy | X | X | Isoniazid OR Isoniazid and rifampicin | 6 months OR 3 months | 2 months | ||
| Solovic et al 201031 | Biological therapy | X | X | X | X | Isoniazid | 9 months | 4 weeks |
| Carrasoca et al 201632 | Methotrexate therapy | X | X | X | Isoniazid | NS | NS | |
| Bumbacea et al 201233 | Transplant recipients | X | X | NS | NS | Before transplant | ||
| KDIGO 200934 | Renal transplant | X | X | Isoniazid | 9 months | NS | ||
| Meije et al 201435 | Transplant recipients | X | X | Isoniazid | 9 months | NS | ||
| EBPG 200236 | Renal transplant recipients | X | X | X | Isoniazid | 9 months | NS | |
| Subramanian 201337 | Transplant recipients | X | X | X | X | Isoniazid | 9 months | Before or after transplant |
| Tomblyn et al 200938 | SCT recipients | X | X | X | Isoniazid | 9 months | NS | |
| Pozniak et al 201139 | Patients with HIV | X | X | Isoniazid | 6 months | NS | ||
| SA 201040 | Patients with HIV | X | Isoniazid | 6 months | NS | |||
| Santin et al 201741 | Patients with HIV | X | X | X | NS | NS | NS | |
| Biological therapy | X | X | X | NS | NS | NS | ||
| Transplant recipients | X | X | X | NS | NS | NS | ||
| Al Jahdali et al 201042 | Susceptible populations | X | X | Isoniazid | 9 months | NS | ||
| ECDC 201143 | Immunocompromised | X | X | NS | NS | NS | ||
| Mazurek et al 201044 | Susceptible populations | X | X | X | X | NS | NS | NS |
| Taylor et al (CDC 2005)45 | Susceptible populations | X | X | X | Isoniazid | NS | NS | |
| CTC 200846 | Immunocompromised | X | X | NS | NS | NS | ||
| Japanese Society for Tuberculosis 201447 | Susceptible populations | X | X | X | Isoniazid | 6–9 months | 3 weeks before immunosuppression NS for transplant |
|
| NICE 201648 | Susceptible populations | X | X | X | Isoniazid OR Isoniazid and rifampicin | 6 months OR 3 months | NS | |
*Where isoniazid is used, it is always provided concurrently with pyridoxine prophylaxis.
ARA, Australian Rheumatological Association; BTS, British Thoracic Society; CDC, centre for disease control; CTC, Canadian Tuberculosis Committee; CXR, chest X-ray; EBPG, European Best Practice Guideline Expert Group on Renal Transplantation; ECDC, European Centre for Disease Prevention and Control; IGRA, interferon gamma release assay; KDIGO, Kidney Disease Improving Global Outcomes; NICE, National Institute for Health and Care Excellence; NS, not specified; SA, South Africa; SCT, Stem cell transplant, TST, tuberculin skin test.
Screening for latent TB infection
Populations of interest
Most clinical practice guidelines recommended screening for LTBI in patients starting immunosuppression or were highly likely to start immunosuppression, and patients immunosuppressed due to concurrent illness, including patients with HIV and/or undergoing solid organ and bone-marrow transplantation.3 15–20 22 24 26 33 35 37 39 47 48 Although medical immunosuppression was mostly biological therapy, two guidelines specified recommendations for patients who have received medical immunosuppression such as methotrexate,17 32 cyclosporine and T cell blocking agents for the management of autoimmune disease.17 A third guideline which considered all immunosuppressed patients also specified the use of non-biological therapies.47
Screening modalities and frequencies
A combination of TST and/or IGRA testing, chest X-ray, detailed background history (including previous exposure to other individuals with TB) and risk factor assessment (travel or migration from endemic areas) was the most frequent recommendation for LTBI screening in immunosuppressed individuals.1 17 18 21 23 24 26 29–32 47 The recommended choice of screening modalities and their frequency were reliant on test availability and costs. The TST is widely available and economical.10
In guidelines pertaining to medical immunosuppression, the recommendations for screening varied considerably between the use of TST and IGRA. Concurrent testing with both TST and IGRA was supported in six guidelines,16 18 20 22 26 32 however, three recommended the use of IGRA alone.15 28 30 Seven guidelines supported TST screening alone, but these recommendations were published prior to 2011.17 19 21 23 24 27 29 Two other guidelines recommended the use of either the TST or IGRA.1 22 In addition, two other guidelines recommended IGRA for BCG vaccinated individuals.16 17
In patients who require long-term maintenance medical immunosuppression, repeat testing at yearly intervals using IGRA was recommended by three guidelines,17 28 31 but two advocated against this, as the benefits of frequent IGRA screening was questionable.16 27 IGRA was recommended by one guideline in the presence of (any) skin disease due to difficulties in inoculating the TST in many of these cases.18
For transplant recipients, those with HIV and other immunosuppressed individuals, most guidelines acknowledged the added value of including TST and IGRA in the screening algorithm.9 10 33 35 37–39 41–46 48 Two guidelines specified the preference for IGRA over TST as the standard triage screening tool for LTBI, because of the high false positive rates associated with TST,34 particularly among those who had been vaccinated with BCG.47 However, across all guidelines, among BCG vaccinated individuals, two guidelines recommended a two-step strategy for screening LTBI.31 42 TST was often considered as the triage test. If negative, IGRA was recommended as the second test to confirm the diagnosis. This has also been recommended to increase case detection in five other guidelines.17 20 30 35 46
Costs were also considered as a key factor in determining the frequency and modality of screening in immunosuppressed individuals. The WHO have suggested IGRA and/or TST may be used in high-income and upper-middle-income countries.10 Given the anticipated costs of IGRA, and the general acceptance of TST by clinicians and patients, TST was preferred in low income countries, despite the lower test accuracies of TST.10 In the high prevalence settings of South Africa and the Philippines, there was no reliable testing method: a combined TST and IGRA approach was recommended in one guideline,20 treatment of all patients with HIV without screening was recommended in another40 and TST alone in one guideline.24
Defining screen positive and negative results
Criteria for TST positivity varied across guidelines. Some recommended a TST-induced reaction of at least 5 mm diameter in all populations, to allow for the treatment of patients in high-risk settings.17 19–21 26 35–37 40 48 Other recommendations for the threshold diameter ranged from 6 mm to 20 mm.18–21 23 24 26 27 31 33 Where the TST result was initially negative, two guidelines recommended repeat testing.23 45 In all guidelines, an individual was deemed to be at risk for LTBI if either the TST or IGRA was positive.
Are these recommendations valid?
There is a body of evidence assessing the test performance characteristics of TST and IGRA in the general population. However, these recommendations were sourced largely from observational studies performed in middle to high-income countries and did not include immunosuppressed patients from low-resource settings and with low certainty of the evidence. Given the low test sensitivity of TST in immunosuppressed patients, some guidelines suggested a two-stage screening, first using TST and then IGRA to increase the detection rates of LTBI.17 20 30 35 46 Among those who are immunosuppressed and had previously been vaccinated with BCG, IGRA generally performs better than TST. IGRA test sensitivity and specificity varies between 67%–75% and 93%–99%, respectively.33 43 However, given the concerns of spectrum bias, most guidelines suggested caution in the interpretation of test results among immunosuppressed hosts.
Treatment for latent TB infection
Population of interests
Either a positive TST or IGRA was considered sufficient evidence to warrant further evaluation. Prior to LTBI treatment, exclusion of active TB was recommended.1 9 15 17 18 25 26 29 30 32 35 42–44 47 48 Once active TB was excluded, LTBI treatment was recommended. Treatment for LTBI was also indicated for those who were BCG vaccinated, because BCG status may indicate time spent in an area with a high prevalence of LTBI.34 Furthermore, in South Africa, where there is a high prevalence of TB, treatment for LTBI was recommended in all patients after exclusion of active TB in the setting of HIV.40 Also, most clinical practice guidelines recommended LTBI treatment where clinical suspicion was high, regardless of the IGRA and TST test findings.1 3 15 19 20 24 26 28 29 33 35–38
Intervention and duration
Recommendations for the treatment of LTBI were largely similar across guidelines, regardless of the mode of immunosuppression. In most guidelines, isoniazid 300 mg daily with pyridoxine was recommended for a duration of 9 months.3 9 16–21 24–27 29 31 33–39 42 Six months of isoniazid therapy was considered less efficacious,18 but was recommended in one guideline.48 Three guidelines suggested a flexible treatment regimen of 6–9 months of the combined therapies.19 30 47 Four guidelines did not specify duration.15 23 32 45
Rifamycin-based therapy (10 mg/kg/day) either alone or for three10 or four1 3 9 10 15–18 24 26 27 31 33 35–39 42 months was the second most frequently reported treatment strategy among patients who tested positive for LTBI. This was thought to be useful when isoniazid was contraindicated or not tolerated,27 with one guideline describing the option as cheaper, but with more drug-drug interactions.18 Rifampicin plus isoniazid for three1 10 15–19 25 26 29–31 39 or 4 months10 24 was also an option. Rifampicin plus isoniazid for 3 months was stipulated as a primary alternative therapy to isoniazid in two guidelines.30 48 Other options included rifabutin for 4 months9 42 or 3 months of weekly rifapentine and isoniazid.9 10 Finally, rifampicin and pyrazinamide for a shorter 2-month regimen was considered as an option in eight guidelines,3 25 29 35–39 with most being in the transplantation setting. The shorter duration of treatment was considered advantageous for those maintained on the transplant waiting list.3 35–38 However, a biological therapy-based guideline advised against this option due to the increased risk of hepatotoxicity.24
In the transplantation and HIV settings, some guidelines specified avoidance of rifamycins, given the potential drug-drug interactions with calcineurin inhibitors and protease inhibitors.3 35 37 However, therapeutic drug monitoring may mitigate against the potential for such interactions.34 Several other non-rifamycin based alternatives were recommended and included ethambutol with levofloxacin or moxifloxacin for 6 months,3 3712 weeks of rifapentine and isoniazid and 6 months of isoniazid with ethambutol.24
Close monitoring with monthly liver function tests and for peripheral neuropathy was recommended while on treatment for all patients.3 9 10 17 18 26 31 35 37 40 47 Coadministration of vitamin B6 (pyridoxine) was suggested universally, to reduce the risk of peripheral neuropathy associated with isoniazid. If there were treatment interruptions for more than 2 months, one guideline recommended clinical and radiological reassessment for TB.42
Timing of preventive therapy
In patients who are medically immunosuppressed, most guidelines recommended delaying medical therapy for 1 month after commencement of LTBI treatment where possible, to reduce the risk of TB reactivation.15–18 20 24–28 Alternative waiting periods varied between 3 weeks25 47 to 2 months.30 One guideline preferred a prolonged delay, but did not provide a time frame.21 However, if the underlying disease was severe, earlier institution of immunosuppressive agents was accepted17 29 once active TB was excluded.28
In transplant setting, patients with LTBI are recommended to commence treatment on the waiting list where possible, with treatment ideally completed prior to transplantation.3 33 35 37 38 However, treatment interruption peritransplantation, with recommencement and completion of the treatment course once patients were clinically stable, may also be considered.33 35 37 LTBI treatment should not delay transplantation.38 In the setting of liver transplantation, the use of anti-TB medications has been associated with increased risk of hepatotoxicity. Thus, it was generally recommended that LTBI therapy be commenced after transplantation, to avoid drug-related fulminant hepatitis while waiting for a donor organ.3 35 37
In patients with HIV, the timing of commencement of antiretroviral therapy in relation to LTBI treatment was not specified by clinical practice guidelines. Unlike treatment for active TB, immune reconstitution related to LTBI treatment has not been documented.9 Generally, it was recommended to initiate or continue antiretroviral treatment concurrently with treatment for LTBI.39 40
Are these recommendations valid?
Overall, clinical practice guidelines recommended the use of isoniazid-based or rifamycin-based regimes for the treatment of LTBI. The evidence for recommendations was largely sourced from observational studies in high-income countries, thus limiting the ability to generalise recommendations to low-income countries. There was very little evidence about the exact time frame of delay before initiating treatment. In addition, side effects associated with the treatment of LTBI, such as hepatotoxicity, neuropathy, gastrointestinal toxicity and rash, were discussed in only 50% of the guidelines.1 3 9 10 18 19 21 24 29 31 33 35–37 39 40 42 47 48
Discussion
Clinical practice guidelines for screening and treatment of LTBI vary in scope and their recommendations for screening modalities, frequency of screening and the target populations of interest. The two-stage screening approach of TST and IGRA was most frequently recommended because of improved test performance characteristics in high risk, immunosuppressed populations. Guidelines did not specify how to interpret a mismatch in results between TST and IGRA, but recommended treatment where either test was positive. For treatment, most recommendations suggested the use of isoniazid-based therapies for LTBI, but there were discrepancies in the duration and timing of commencing treatment. Nine months of isoniazid-based therapy appeared to be the preferred therapy for LTBI, and most agreed that treatment of LTBI should be initiated prior to commencement of immunosuppressive therapies.
While most guidelines conducted a comprehensive literature review, the evidence base supporting the recommendations was limited to observational studies without trial-based evidence to support routine screening and treatment for LTBI in immunosuppressed patients. The rigour of guideline development lacks robustness. Less than half of the guidelines provided grading of the evidence and recommendations. Details regarding the methods used for formulating the recommendations were not adequately described, lacking transparency in the methodology and did not consistently link the recommendations to the corresponding level of evidence, both for screening and treatment of LTBI and the benefit-harm-cost relationship.
In this review, we found that public and stakeholder consultation was rarely reported in the development of the guidelines. Only 22% underwent a peer review process and 11% underwent public consultation. Engaging experts may improve guidelines by allowing criticism and suggestions.19 Expert clinicians were consulted in guideline development and included clinicians such as rheumatologists, gastroenterologists, dermatologists, thoracic physicians, infectious diseases physicians and clinicians involved in treating patients with HIV. Public consultations and patient participation can also improve guideline applicability.49 Although four guidelines used public consultation, none elaborated on how they have contributed to guideline development. Guideline applicability may be improved by active consumer involvement and engagement in the development, design and implementation process.
Inconsistencies exist in the recommendations for screening modalities and frequencies for LTBI. The TST evokes delayed hypersensitivity after intradermal application of a purified protein derivative.33 TST generally performs poorly in immunosuppressed patients, with reported estimates of 89% and 71% for test sensitivity and specificity, respectively.43 The lower test specificity may be due to the cross-reactivity with prior BCG vaccination15 34 and infections with non-TB mycobacteria. Testing with IGRA identifies adaptive immune response to TB-specific antigens which are not present in BCG strains, enabling greater specificity.42 43 Test sensitivity of TST and IGRA is uncertain or may be reduced among immunosuppressed hosts because of anergy.33 Determining the diagnostic accuracy of the IGRA and TST is complicated because of the absence of an accurate and valid reference standard. For example, underestimation of the true test sensitivity and specificity of the new test may occur if the imperfect reference incorrectly classifies those with disease as no disease (false negative) and those without disease as disease (false positive).
Multiple diagnostic algorithms for LTBI have been proposed to overcome the shortcomings of IGRA and TST, including the use of predefined multiple imperfect diagnostic tests and clinical data to inform the prevalence estimates of LTBI in different settings. Despite this, prevalence of LTBI varies substantially, even in high-risk patients.50 Statistical methods such as latent class and Bayesian mixture analyses may overcome this limitation.51 52
Most guidelines recommended treatment for LTBI, including those who were screened negative but of high clinical risk. While this is of relevance and importance to at-risk immunosuppressed patients, interventions such as isoniazid and alternatives including rifampicin are not without adverse complications. No guidelines specified contraindications to treatment, except in the case of liver transplantation, where treatment was recommended to be delayed until after transplantation due to the increased risk of hepatotoxicity.3 35 37 Treatment of LTBI also has other potential drug toxicities, including neuropathy and drug-drug interactions, particularly for rifampicin-based regimens. Although many guidelines acknowledged these toxicities, the impact of overtreatment and the potential risk of adverse drug reactions were not quantified. Only two guidelines specified the growing concern of increasing rates of multidrug-resistant TB secondary to excess exposure to drug therapy.23 47 Furthermore, barriers to screening and treatment are only considered in one guideline, which stated that there were no barriers in a public hospital.41 This therefore would not apply in under-resourced settings or where public healthcare is not available.
In our systematic review, we used a reliable and validated method using the AGREE II to assess guidelines for the screening for and treatment of LTBI. There was good agreement between the two reviewers. We have summarised the variability in the literature pertaining to LTBI, allowing for a consolidated approach to recommendations for screening and management of LTBI. However, limitations of our review are that we have only included guidelines written in the English language. Therefore, applicability of our findings to other settings, particularly those in low-income countries are uncertain. Future guidelines should consider the specific health issues that are applicable to the population of interest, such as in low-income settings, and consider cost implications and barriers to screening and treatment. Very few guidelines discussed non-TNF based immunosuppression. This included two well-established medications—methotrexate and cyclophosphamide—for the management of autoimmune disease as well as newer biological treatments.17 Only one guideline included newer monoclonal agents30 and one for patients on regular methotrexate therapy.32 One of the key challenges for guideline developers is the translation and dissemination of these recommendations in clinical practice, which may transform care and improve health of the target population. Currently, there are limited training initiatives in the implementation of these guidelines in different cultural and resource settings. Future research, through direct engagement with local stakeholders, clinicians and patients should therefore assess the features and processes that underpin success in research translation and adapt these strategies in practice.
Overall, the current clinical guidelines reaffirm the importance of LTBI screening and treatment. Although there are some discrepancies in terms of screening modalities, recommendation for the treatment of LTBI was consistent across all guidelines. Quality of evidence and rigour of guideline development varied. Therefore, there is a need to undertake better-quality studies, with international, multidisciplinary and stakeholder involvement to consolidate current evidence. This is critical to support evidence-based guidelines development and patient-centred practice to improve patient outcomes.
Supplementary Material
Footnotes
Contributors: TH: database search, selection of guidelines; grading of guidelines, assessing quality, interpretation; preparation of manuscript and editing. EA: selection of guidelines; grading of guidelines, assessing quality, interpretation; preparation of manuscript and editing. SC, AT, GW: preparation of manuscript and editing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: SC reports grants from MSD Australia, outside the submitted work.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available on request.
References
- 1. Australian Rheumatology Association. Screening for latent tuberculosis infection (LTBI) prior to use of biological agents in Australia. 2010. https://rheumatology.org.au/downloads/April2010-FINAL-SCREENINGFORLATENTTUBERCULOSISINFECTION.pdf (accessed 25 Oct 2016).
- 2. Gómez-Reino JJ, Carmona L, Valverde VR, et al. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum 2003;48:2122–7. 10.1002/art.11137 [DOI] [PubMed] [Google Scholar]
- 3. Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis 2009;48:1276–84. 10.1086/597590 [DOI] [PubMed] [Google Scholar]
- 4. Budak-Alpdogan T, Tangün Y, Kalayoglu-Besisik S, et al. The frequency of tuberculosis in adult allogeneic stem cell transplant recipients in Turkey. Biol Blood Marrow Transplant 2000;6:370–4. 10.1016/S1083-8791(00)70013-9 [DOI] [PubMed] [Google Scholar]
- 5. Askling J, Fored CM, Brandt L, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum 2005;52:1986–92. 10.1002/art.21137 [DOI] [PubMed] [Google Scholar]
- 6. Carmona L, Gómez-Reino JJ, Rodríguez-Valverde V, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum 2005;52:1766–72. 10.1002/art.21043 [DOI] [PubMed] [Google Scholar]
- 7. Tubach F, Salmon D, Ravaud P, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: the three-year prospective French research axed on tolerance of biotherapies registry. Arthritis Rheum 2009;60:1884–94. 10.1002/art.24632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolfe F, Michaud K, Anderson J, et al. Tuberculosis infection in patients with rheumatoid arthritis and the effect of infliximab therapy. Arthritis Rheum 2004;50:372–9. 10.1002/art.20009 [DOI] [PubMed] [Google Scholar]
- 9. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. 2016. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf (accessed 27 Oct 2016).
- 10. World Health Organization. Guidelines on the management of latent tuberculosis infection. Geneva: World Health Organization, 2015. [PubMed] [Google Scholar]
- 11. Kong FS, Tomford JW, Teixeira L, et al. Challenges of interferon- γ release assay conversions in serial testing of health-care workers in a TB control program. Chest 2011;142:55–62. [DOI] [PubMed] [Google Scholar]
- 12. Slater M, Parsonnet J, Banaei N. Investigation of false-positive results given by the QuantiFERON-TB Gold In-Tube assay. J Clin Microbiol 2012;50:3105–7. 10.1128/JCM.00730-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Field MJ, Lohr KN. Committee to Advise the Public Health Service on Clinical Practice Guidelines, Institute of Medicine. Clinical practice guidelines: directions of a new program. Washington, DC: National Academy Press, 1990. [Google Scholar]
- 14. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010;182:E839–42. 10.1503/cmaj.090449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beglinger C, Dudler J, Mottet C, et al. Screening for tuberculosis infection before the initiation of an anti-TNF-alpha therapy. Swiss Med Wkly 2007;137:620–2. doi:2007/43/smw-11939 [DOI] [PubMed] [Google Scholar]
- 16. Cantini F, Nannini C, Niccoli L, et al. Guidance for the management of patients with latent tuberculosis infection requiring biologic therapy in rheumatology and dermatology clinical practice. Autoimmun Rev 2015;14:503–9. 10.1016/j.autrev.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 17. Doherty SD, Van Voorhees A, Lebwohl MG, et al. National Psoriasis Foundation consensus statement on screening for latent tuberculosis infection in patients with psoriasis treated with systemic and biologic agents. J Am Acad Dermatol 2008;59:209–17. 10.1016/j.jaad.2008.03.023 [DOI] [PubMed] [Google Scholar]
- 18. Duarte R, Campainha S, Cotter J, et al. Position paper on tuberculosis screening in patients with immune mediated inflammatory diseases candidates for biological therapy. Acta Reumatol Port 2012;37:290–9. 10.1016/j.jpg.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 19. Fonseca JE, Lucas H, Canhão H, et al. Recommendations for the diagnosis and treatment of latent and active tuberculosis in inflammatory joint diseases candidates for therapy with tumor necrosis factor alpha inhibitors - March 2008 update. Rev Port Pneumol 2008;14:271–83. 10.1016/S0873-2159(15)30235-X [DOI] [PubMed] [Google Scholar]
- 20. Hodkinson B, Van Duuren E, Pettipher C, et al. South African recommendations for the management of rheumatoid arthritis: an algorithm for the standard of care in 2013. S Afr Med J 2013;103(8 Pt 2):576–85. 10.7196/SAMJ.7047 [DOI] [PubMed] [Google Scholar]
- 21. Kavanagh PM, Gilmartin JJ, O’Donnell J, et al. Tumour necrosis factor-alpha and tuberculosis: guidance from the National TB Advisory Committee. Ir Med J 2008;101:6–7. [PubMed] [Google Scholar]
- 22. Keith PJ, Wetter DA, Wilson JW, et al. Evidence-based guidelines for laboratory screening for infectious diseases before initiation of systemic immunosuppressive agents in patients with autoimmune bullous dermatoses. Br J Dermatol 2014;171:1307–17. 10.1111/bjd.13355 [DOI] [PubMed] [Google Scholar]
- 23. Koike R, Takeuchi T, Eguchi K, et al. Update on the Japanese guidelines for the use of infliximab and etanercept in rheumatoid arthritis. Mod Rheumatol 2007;17:451–8. 10.3109/s10165-007-0626-3 [DOI] [PubMed] [Google Scholar]
- 24. Lichauco JJT, Tankeh-Torres SA, Navarra SV, et al. Philippine guidelines on the screening for tuberculosis prior to the use of biologic agents. APLAR Journal of Rheumatology 2006;9:184–92. 10.1111/j.1479-8077.2006.00182.x [DOI] [Google Scholar]
- 25. Salmon D. Recommendations about the prevention and management of tuberculosis in patients taking infliximab. Joint, Bone . Spine 2002;69:170–2. [DOI] [PubMed] [Google Scholar]
- 26. Mir Viladrich I, Daudén Tello E, Solano-López G, et al. Consensus document on prevention and treatment of tuberculosis in patients for biological treatment. Arch Bronconeumol 2016;52:36–45. 10.1016/j.arbr.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 27. Mok CC, Tam LS, Chan TH, et al. Management of rheumatoid arthritis: consensus recommendations from the Hong Kong Society of Rheumatology. Clin Rheumatol 2011;30:303–12. 10.1007/s10067-010-1596-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nordgaard-Lassen I, Dahlerup JF, Belard E, et al. Guidelines for screening, prophylaxis and critical information prior to initiating anti-TNF-alpha treatment. Dan Med J 2012;59:C4480. [PubMed] [Google Scholar]
- 29. British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 2005;60:800–5. 10.1136/thx.2005.046797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith CH, Anstey AV, Barker JN, et al. British Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009. Br J Dermatol 2009;161:987–1019. 10.1111/j.1365-2133.2009.09505.x [DOI] [PubMed] [Google Scholar]
- 31. Solovic I, Sester M, Gomez-Reino JJ, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J 2010;36:1185–206. 10.1183/09031936.00028510 [DOI] [PubMed] [Google Scholar]
- 32. Carrascosa JM, de la Cueva P, Ara M, et al. Methotrexate in moderate to severe psoriasis: review of the literature and expert recommendations. Actas Dermosifiliogr 2016;107:194–206. 10.1016/j.ad.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 33. Bumbacea D, Arend SM, Eyuboglu F, et al. The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur Respir J 2012;40:990–1013. 10.1183/09031936.00000712 [DOI] [PubMed] [Google Scholar]
- 34. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009;9(Suppl 3):S1–155. 10.1111/j.1600-6143.2009.02834.x [DOI] [PubMed] [Google Scholar]
- 35. Meije Y, Piersimoni C, Torre-Cisneros J, et al. Mycobacterial infections in solid organ transplant recipients. Clin Microbiol Infect 2014;20(Suppl 7):89–101. 10.1111/1469-0691.12641 [DOI] [PubMed] [Google Scholar]
- 36. EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: Long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant recipients. Nephrol Dial Transplant 2002;17(Suppl 4):50–5. [PubMed] [Google Scholar]
- 37. Subramanian AK, Morris MI. AST Infectious Diseases Community of Practice. Mycobacterium tuberculosis infections in solid organ transplantation. Am J Transplant 2013;13(Suppl 4):68–76. 10.1111/ajt.12100 [DOI] [PubMed] [Google Scholar]
- 38. Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009;15:1143–238. 10.1016/j.bbmt.2009.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pozniak AL, Coyne KM, Miller RF, et al. British HIV Association guidelines for the treatment of TB/HIV coinfection 2011. HIV Med 2011;12:517–24. 10.1111/j.1468-1293.2011.00954.x [DOI] [PubMed] [Google Scholar]
- 40. Republic of South Africa Department of Health. Guidelines for tuberculosis preventive therapy among HIV infected individuals in South Africa. 2010. www.who.int/hiv/pub/guidelines/south_africa_hiv_tb.pdf (accessed 1 Nov 2016).
- 41. Santin M, García-García J-M, Domínguez J. Guidelines for the use of interferon-γ release assays in the diagnosis of tuberculosis infection. Enfermedades Infecciosas y Microbiología Clínica 2016;34:303.e1–303.e13. 10.1016/j.eimc.2015.11.022 [DOI] [PubMed] [Google Scholar]
- 42. Al Jahdali HH, Baharoon S, Abba AA, et al. Saudi guidelines for testing and treatment of latent tuberculosis infection. Ann Saudi Med 2010;30:38–49. 10.4103/0256-4947.59373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. European Centre for Disease Prevention and Control. Use of interferon-gamma release assays in support of TB diagnosis. Stockholm: European Centre for Disease Prevention and Control (ECDC), 2011. [Google Scholar]
- 44. Mazurek GH, Jereb J, Vernon A, et al. Updated guidelines for using interferon gamma release assays to detect mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep 2010;59(RR-5):1–25. [PubMed] [Google Scholar]
- 45. Taylor Z, Nolan CM, Blumberg HM. American Thoracic Society Centers for Disease Control and Prevention Infectious Diseases Society of America. Controlling tuberculosis in the United States. Recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. MMWR Recomm Rep 2005;54(RR-12):1–81. [PubMed] [Google Scholar]
- 46. Canadian Tuberculosis Committee. Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2008;34(Acs-6):1–13. [PubMed] [Google Scholar]
- 47. Prevention Committee of the Japanese Society for Tuberculosis Treatment Committee of the Japanese Society for Tuberculosis. Treatment guidelines for latent tuberculosis infection. Kekkaku 2014;89:21–37. [PubMed] [Google Scholar]
- 48. National Collaborating Centre for Chronic Conditions (UK): Centre for Clinical Practice at NICE (UK). Tuberculosis: prevention, diagnosis, management and service organisation. London: National Institure for Health and Clinical Excellence (UK), 2016. [PubMed] [Google Scholar]
- 49. Krahn M, Naglie G. The next step in guideline development: incorporating patient preferences. JAMA 2008;300:436 10.1001/jama.300.4.436 [DOI] [PubMed] [Google Scholar]
- 50. Pai M, Gokhale K, Joshi R, et al. Mycobacterium tuberculosis infection in health care workers in rural India: Comparison of a whole blood interferon-γ assay with tuberculin skin testing. JAMA 2005;22:293–302. [DOI] [PubMed] [Google Scholar]
- 51. Pai M, Dendukuri N, Wang L, et al. Improving the estimation of tuberculosis infection prevalence using T-cell-based assay and mixture models. Int J Tuberc Lung Dis 2008;12:895–902. [PMC free article] [PubMed] [Google Scholar]
- 52. Doan TN, Eisen DP, Rose MT, et al. Interferon-gamma release assay for the diagnosis of latent tuberculosis infection: A latent-class analysis. PLoS One 2017;12:e0188631 10.1371/journal.pone.0188631 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-022445supp001.pdf (83.9KB, pdf)