Abstract
Objective
Primary intraventricular hemorrhage (pIVH) uncommonly presents with seizures. There are no prior data regarding the frequency of seizures, periodic and rhythmic patterns on continuous electroencephalography (EEG), (cEEG) in these patients.
Methods
We retrospectively assessed frequency of seizures, periodic discharges, and rhythmic patterns in pIVH patients undergoing cEEG monitoring. We reviewed indications for cEEG, demographics, GCS at presentation and during cEEG, modified Graeb score (mGS), presence of hydrocephalus, cEEG duration, findings and use of antiseizure medications (ASM). cEEG patterns were classified according to location and morphology. All patterns were considered “hyperexcitable” except GRDA. The ictal‐interictal continuum (IIC) was defined as LRDA, PDs, and/or SW >1 Hz but <2.5 Hz, not meeting criteria for definite electrographic seizures.
Results
Eleven patients had pIVH with median age of 81 (46–87) years and median mGS of 15 (9–23). Hydrocephalus was present in 7 (63.6%) and all underwent external ventricular drain (EVD) placement. Median cEEG recording was 19 (12–156) hours. Periodic or rhythmic EEG patterns were seen in 7 of 11 (64%), 5 of which were “hyperexcitable”. For the 5 patients with pIVH, EVDs, and hyperexcitable patterns, 4 (80%) were lateralized contralateral to the EVD and 1 (20%) was generalized to the EVD. The only significant difference between the hyperexcitable and non‐hyperexcitable group was duration of cEEG monitoring (P = 0.007).
Interpretation
Hyperexcitable patterns were common in our cases. Further research is warranted to assess prevalence of hyperexcitable patterns, their risk factors, underlying pathophysiology, and association with neuronal injury in pIVH.
Introduction
Primary intraventricular hemorrhage (pIVH) is restricted to the ventricular system and originates from the ventricles or in close proximity without visible parenchymal involvement. Clinical seizures are uncommon,1 but have been reported in pIVH.
While hyperexcitable patterns on cEEG have been reviewed in neurocritically ill patients with intracranial hemorrhage,2, 3, 4 specifically with parenchymal,5 subdural,6 and subarachnoid hemorrhages,7, 8 studies analyzing patients with pIVH are lacking. Given that intraventricular blood by itself can cause significant variability in the level of consciousness,9 excluding nonconvulsive seizures is paramount, especially in the setting of a fluctuating or limited neurologic exam. Therefore, we sought to establish the frequency of periodic discharges, rhythmic patterns, and seizures in patients with pIVH at a large academic medical center.
Methods
Using a retrospective cohort of patients with spontaneous intracerebral hemorrhage (sICH), we analyzed the prevalence of periodic discharges and rhythmic patterns as well as seizures in patients with pIVH who underwent cEEG as part of routine clinical care between January 2013 and December 2016 at Yale‐New Haven Hospital. PIVH was defined as intraventricular blood on head CT, in the absence of intraparenchymal, subdural, or subarachnoid blood. Furthermore, every patient received either a CT angiogram or conventional angiogram of the head as part of their diagnostic evaluation. Finally, if an MRI of the brain was obtained, this was also reviewed.
All patients of the original sICH cohort as well as the selected pIVH cases who underwent cEEG monitoring were identified through a query of the electronic medical record in Epic® as part of a quality improvement project.
We recorded demographic data (age, gender), Glasgow Coma Scale (GCS) at the time of admission and at the beginning of cEEG recording, reason for cEEG, extent of IVH as measured with the modified Graeb score (mGS),10 presence of radiographic hydrocephalus, placement and location of EVD, duration of and findings on cEEG, and use of antiseizure medications (ASM) prior to or during the study period.
cEEG findings were divided into electrographic or clinical seizures and rhythmic or periodic patterns. Patterns were classified by location (generalized ‐ G, lateralized ‐ L, bilateral independent‐ BI, or multifocal ‐ Mf) and morphology (periodic discharges – PDs, spike‐wave‐ SW or rhythmic delta activity‐RDA) according to the American Clinical Neurophysiology Society's Standardized Critical Care EEG terminology (2012 version) (Hirsch 2013). All patterns but GRDA were considered “hyperexcitable” given their known association with an increased seizure risk.2, 3, 11 The pIVH cohort was divided into a hyperexcitable and nonhyperexcitable group based on presence or absence of these patterns.
We recorded if patterns had a “plus” modifier12: superimposed fast activity (+F) or associated sharp waves or sharp morphology (+S) for RDA, or associated fast activity (+F) or rhythmicity (+R) if PDs. Patterns were assessed further for being on the (IIC)4, 11: PDs, SW, or LRDA at a frequency greater than 1 Hz but less than 2.5 Hz and not meeting criteria for definite electrographic seizures.
mGSs were obtained by a board‐certified neurologist (CS) following a standardized approach10: the mGS is calculated as a sum of blood within each ventricle, higher scores (range 0–32) denote larger volume or expansion of ventricle size: it includes the fourth, third, right and left lateral ventricles, right and left occipital horns, and the right and left temporal horns.10 An additional point is given to each compartment if expanded beyond the normal anatomic limits due to clot.10
CEEGs were reviewed by clinical neurophysiology board eligible (ZS, CM) and board‐certified (LJH, EJG) epileptologists. Categorical variables were reported in absolute number and percentage; continuous variables were reported with median and range. Differences between the hyperexcitable and nonhyperexcitable groups were assessed with the Fisher's exact test for categorical variables and the Mann–Whitney U test for continuous variables with P < 0.05 being significant.
Results
We identified 194 adult patients with sICH who underwent cEEG monitoring as part of the aforementioned study. Of these cases, 11 (6% of all sICH) patients, 7 of whom were female, had pIVH. Patient's median age was 81 (range 46–87) years (Table 1). GCS scores for the entire group at the time of admission and at the beginning of cEEG recording are displayed in Table 1. There was no significant difference in GCS between the hyperexcitable and nonhyperexcitable group at either time point (P = 0.55 and P = 0.23, respectively). Results of diagnostic imaging for pIVH (CTA or conventional angiography and MRI) revealed no underlying vascular malformation, aneurysm, or brain tumor in any patient. All patients underwent CTA. For the patients who underwent a conventional angiogram or MRI of the brain, there was no significant difference in acquisition of these studies between groups (Table 1).
Table 1.
Demographic, clinical, radiologic, and EEG data characteristic
| Entire group (N = 11) | Hyperexcitable group (N = 5) | Nonhyperexcitable group (N = 6) | P value | |
|---|---|---|---|---|
| Age (median, range) | 81 (46–87) | 80 (59–84) | 82 (46–86) | 0.26 |
| Female, N (%) | 7 (64) | 2 (40) | 5 (83) | 0.13 |
| GCS at diagnosis (median, range) | 11 (3–15) | 10 (3–15) | 14.5 (6–15) | 0.55 |
| GCS at time of cEEG (median, range) | 10.4 (6–14) | 9 (7–11) | 12.5 (6–15) | 0.23 |
| Imaging, N (%) CTA | 11 (100) | 5 (100) | 6 (100) | 1 |
| MRI brain | 8 (72) | 4 (80) | 4 (66) | 1 |
| Diagnostic angiogram | 4 (36) | 2 (40) | 2 (33) | 1 |
| Hydrocephalus, N (%) | 7 (64) | 5 (100) | 2 (33) | 0.06 |
| EVD, N (%) | 7 (64) | 5 (100) | 2 (33%) | 0.06 |
| mGS (median, range) | 15 (9–23) | 17 (12–22) | 12 (9–23) | 0.28 |
| Rhythmic and/or periodic patterns, N (%) | 7 (64) | 5 (100) | 2 (33) | 0.06 |
| Electrographic seizures, N (%) | 1 (9) | 1 (9) | 0 (0) | 1 |
| Monitoring duration in hours (median, range) | 19 (12–156) | 98 (36–156) | 18 (12–38) | 0.007 |
| No. monitoring Sessions (median, range) | 1 (1–7) | 5 (1–7) | 1 (1–2) | 0.08 |
mGS, modified Graeb score; EVD, external ventricular drain.
Hydrocephalus was present in 7 (63.6%) patients. The median mGS score was 15 (range 9–23). The most common indication for cEEG was to evaluate for nonconvulsive seizures in comatose patients (N = 9, 81.8%), followed by evaluation of paroxysmal events concerning for seizures (N = 2, 18.2%): One patient had an episode of unresponsiveness with loss of sphincter control and the other one had fluctuating mental status with intermittent posturing.
With regards to ASM use, two patients, who did not have any hyperexcitable patterns or clinical events, remained on their home dose of gabapentin for pain management (300 mg three times per day). Furthermore, ASM were started for 1/11 patients (9%) due to electrographic seizures and for 2/11 (18%) due to cEEG findings on the IIC (Table 2).
Table 2.
Recorded patterns in patients with hyperexcitability on cEEG
| Pattern | ASM initiation | EVD location | Outcome (mRS)a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDs (n = 3) | RDA (n = 5; only 1 LRDA) | GSW | IIC | Seizures | ||||||||||
| LPD | LPD + R | BIPD + R | GPD + R | LRDA | LRDA + S | GRDA | I/L | C/L | ||||||
| Patient | ||||||||||||||
| 1 | X | X | X | 4 | ||||||||||
| 2 | X | X | X | 4 | ||||||||||
| 3 | X | X | LEV/LAC/PHE | X | 6 | |||||||||
| 4 | X | X | X | LEV | X | X | 5 | |||||||
| 5 | X | X | LEV/PHE | X | 6 | |||||||||
| 6 | X | 4 | ||||||||||||
| 7 | X | 0 | ||||||||||||
I/L and C/L – location of EVD ipsilateral or contralateral to EVD if patterns include a unilateral pattern.
EVD, external ventricular drain; PDs, periodic discharges; LPD (+R), lateralized periodic discharges (+ rhythmicity); BIPD +(R), bilateral independent periodic discharges (+ rhythmicity); GPD (+R), generalized periodic discharges (+rhythmicity); RDA, rhythmic delta activity; LRDA (+S), lateralized rhythmic delta activity (+sharps); GSW, generalized spike wave and/or sharp waves; IIC , ictal‐interictal continuum; LEV, levetiracetam; LAC, lacosamide; PHE, phenytoin. Note that GRDA is not a hyperexcitable pattern.
mRS is a measure of disability ranging from 0 (no symptoms) to 6 (death).
Patients were monitored for a median duration of 19 (range 12–156) hours, with significantly longer cEEG monitoring in the hyperexcitable group (Table 1). Seven patients (64%) had periodic and/or rhythmic patterns of which 5 (45%) were hyperexcitable (Table 2). Electrographic seizures were captured in 1 of 11 (9.1%) and hyperexcitable patterns without seizures recorded in 4 of 11 (36.3%) patients, respectively. Recording of cEEG began within 24 h of pIVH in seven, and after 24 h in four patients. Five patients had more than one monitoring session. Of patients with hyperexcitable patterns, PDs (L‐, BI‐, G‐) ± R were recorded in three patients, LRDA ± S was recorded in one patient and SW was recorded in one patient (Table 2). No multifocal patterns were captured and associated +F as a modifier was not seen in this case series.
For the five patients with pIVH, EVDs, and hyperexcitable patterns, 4 (80%) were lateralized contralateral to the EVD and 1 (20%) was generalized in relation to the EVD. GRDA (without modifiers) was detected in three patients (27.2%). Outcomes data (mRS at time of hospital discharge) are displayed in Table 2.
For patient #3, who had electrographic seizures, different from the two patients who had paroxysmal events concerning for seizures, seizure onset was over the right parieto‐temporal region. He was found to have a contralateral tract hemorrhage from left frontal EVD placement. Seizure control was obtained after initiation of levetiracetam 1000 mg twice a day, lacosamide 400 mg loading dose, followed by 250 mg twice a day and phenytoin loading dose at 1000 mg and subsequent 100 mg three times a day. Figure 1 shows a sample EEG tracing with LPDs (Fig. 1A) and during aforementioned electrographic seizures (Fig. 1B). Figure 2 shows a head CT (axial cut) of the intraventricular hemorrhage of patient#3.
Figure 1.
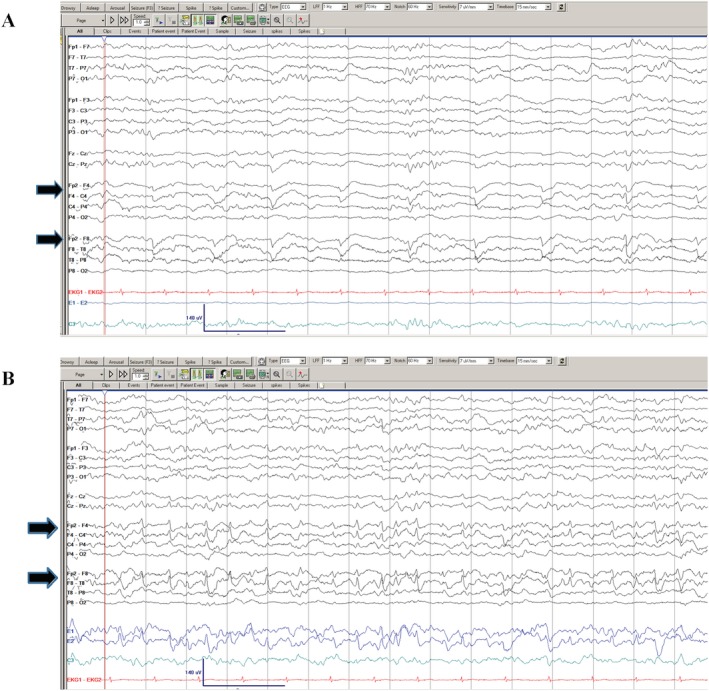
EEG of patient #3 showing right fronto‐temporal LPDs (A, shown in solid black arrows) and evolution to an electrographic seizure in the same patient (B, shown in solid black arrows).
Figure 2.
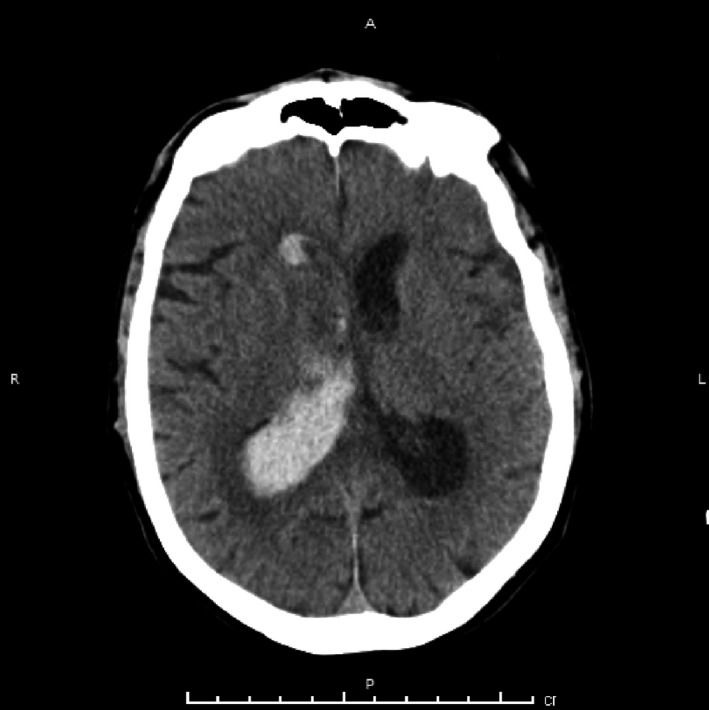
Head CT (axial cuts) of the intraventricular hemorrhage of patient #3.
Two patients’ patterns met criteria for the IIC (patients #4 and 5 in Table 2): in patient #4, the prevalence of SW was initially more than 90% at a frequency of 1.5–2.5 Hz. Following administration of levetiracetam of 1000 mg IV and maintenance dosing of 750 mg PO twice a day, prevalence of SW decreased to <10% and frequency of SW to 1–1.5 Hz. These electrophysiologic changes coincided with an improved clinical exam.
In patient #5, BIPD +R were initially prevalent for 85% of the recording at a frequency of 1–2.5 Hz, which improved to a prevalence of 20% following administration of levetiracetam (loading dose 2 g, followed by 1 gram twice a day) and the addition of phenytoin (maintenance dose 150 mg twice a day). Given no clinical improvement, ASM were discontinued. Subsequently, BIPD + R then occupied 80% of the record and levetiracetam was restarted at 1000 mg twice a day without bolus. While BIPD + R were no longer seen, GPD +R emerged at 1–2 Hz, on 10–49% of the record, which resolved with continuation of levetiracetam.
Discussion
Frequency of IVH in ICH cases
To date, this represents the first report characterizing cEEG patterns of hyperexcitability and seizures in a selected population of patients with isolated pIVH. With 6% of sICH patients having pIVH and having undergone cEEG monitoring, this represents an overall small group.
While the retrospective case series on pIVH by Flint et al. (2008) not only focused on those patients with cEEG, the authors found nontraumatic pIVH to account for 2.7% of intracerebral hemorrhage admissions to a tertiary referral center. Seizures (without distinction of clinical vs. electrographic) were reported to occur in 1 of 15 patients (7%).13 In our cohort, cEEG was obtained to assess for nonconvulsive seizures and to characterize events which were concerning for seizures. Hyperexcitable patterns were common (in 45%), with electrographic seizures recorded in 1 of 11 patients.
Potential explanations of hyperexcitable patterns in pIVH
Why did the above‐mentioned periodic and rhythmic patterns occur in our cases of pIVH patients? We did not identify clear causes of (worsening) hydrocephalus, toxic‐metabolic encephalopathy or increased intracranial pressure at the time of cEEG recording to account for generalized, lateralized, or bilateral independent patterns.
Lateralized patterns (LPD (+), LRDA (+s)) can occur in the setting of focal CNS lesions; while acute stroke is reported as the most common etiology for LPDs,14, 15, 16, 17 LRDA has most commonly been observed in intracerebral and subarachnoid hemorrhages.3 Interestingly, lateralized patterns occurred contralateral to the site of EVD placement and no other focal lesions (hemorrhagic or ischemic) were found ipsilateral to these patterns in our patient cases. Until validated in another cohort, this likely reflects a chance finding, the significance of which remains unclear.
Bilateral independent periodic discharges (BIPDs) can emerge with various etiologies of acute and subacute brain injury,18 but are rarely seen in ICH5 and have not specifically been reported in patients with pIVH. Given the bilateral independent foci of periodic discharges, unilateral EVD placement in itself cannot fully account for this pattern.
On the other hand, generalized periodic discharges have been reported in patients with severe encephalopathy or coma19 and are thought to be more reflective of a global injury18 such as in our patient with GPD+R. While not reported as a pattern with increased seizure risk,2 GRDA can be seen in several brain pathologies, including metabolic encephalopathies (most commonly), as well as structural lesions.20, 21
The case for EEG monitoring in ICH and pIVH
Based on our retrospective data showing patterns of hyperexcitability in nearly one in two patients with pIVH, it appears reasonable to monitor all patients with pIVH and impaired mental status with cEEG until further data is prospectively collected, especially if the clinical exam is limited. While the 2015 AHA Guidelines on the Management of ICH do not specifically comment on pIVH, obtaining cEEG in patients with decreased mental status out of proportion to the amount of brain injury is recommended (Class IIa, Level C).22 However, one could argue that even in patients without clinical‐radiographic mismatch, cEEG may be indicated. Rodriguez Ruiz et al. (2017)2 provide data allowing to stratify seizure risk based on pattern frequency and modifiers: In their review of cEEG recordings of 4772 critically ill patients, which included patients with intraventricular hemorrhage, they found that LPDs, LRDA, and GPDs were associated with seizures. While LPDs were associated with seizures even at 1 Hz, LRDA and GPDs had a statistically significant association with seizures only at or above 1.5 Hz.2
Data by Struck et al. (2017)23 may help inform the decision on duration of monitoring: they provide prospectively collected data allowing one to adjust duration of cEEG to the individual patient's risk profile on the grounds of two simple clinical risk factors (coma and prior seizures) and the cEEG findings early in the recordings.
Proposed workup and treatment for hyperexcitable patterns and the IIC
For patients with intracerebral hemorrhage, the guidelines do not support the routine use of ASM in the absence of seizures.22 Whether to treat or not treat hyperexcitable patterns in this patient population and in general remains an area of active research.24 Our institutional standard practice, often tailored to the individual patient, is based on the algorithm described by Gilmore et al. (2016)25 for patients with ICH, that is, to treat patients with high‐risk patterns—PDs, SW, and LRDA—during the acute phase of injury empirically. Regarding patterns on the IIC, our standardized approach is trifold, based on expert opinion11, 26, 27 and includes investigation of etiology, starting or increasing doses of ASM, assessing for treatment response, and monitoring for development of nonconvulsive status epilepticus (NCSE) with cEEG.11, 26
The recently developed 2HELPS2B model28 is a risk score based on EEG and evidence of the effort to inform treatment decisions based on the likelihood for seizures in critically ill patients. Once validated prospectively, this score may provide guidance as to which patients warrant treatment.
Limitations
Our cases are limited by the nature of a single‐center study, a small sample size and the retrospective design. Furthermore, with a median age of 81 years, our cases were significantly older when compared to a median age of 55 years in published case series,13 which may limit generalizability to a larger population with pIVH. The exclusion of associated intraparenchymal, subarachnoid, or subdural blood from the initial group allowed us to potentially eliminate other etiologies of hyperexcitable patterns. As noted above, EVDs were contralateral to the side of origin of lateralized hyperexcitable patterns and seizure origin.
Our data may overestimate the true frequency of hyperexcitable patterns and seizures due to selection bias. On the other hand, while median cEEG recording was 19 h, only 45.5% of patients received ≥24 h of monitoring. It remains unclear based on currently available data whether longer monitoring would have yielded a higher frequency of hyperexcitable patterns.
The fact that patients who were found to have hyperexcitable patterns underwent significantly longer cEEG recording is likely the result of a practice strategy to allocate further monitoring resources to patients deemed at higher risk for clinical and/or electrographic seizures.
Furthermore, our small sample size and low frequency of seizures did not allow for testing of associations between hyperexcitable patterns and seizures or a correlation with hydrocephalus, EVD placement, metabolic abnormalities, or outcome, all topics for future research.
Conclusion
In patients with acute brain injury, including pIVH, our understanding of hyperexcitable patterns on the IIC, which may behave from a metabolic stress perspective with their repercussions on metabolic stress and association with seizures, is evolving.2, 4, 8, 11, 25, 27 Given the heterogeneity of this population, individualized treatment approaches based on best clinical practice are indicated.
Prospective multicenter studies are needed to more accurately assess prevalence of hyperexcitable patterns, their associations with seizures and underlying pathophysiology as well as their independent associations with functional and cognitive outcomes.
Conflict of Interest
None declared.
Acknowledgments
The authors would like to thank all the neurology, epilepsy, and neurocritical care faculty, residents and technicians from the Comprehensive Epilepsy Center and the Division of Neurocritical Care and Emergency Neurology at Yale‐New Haven Hospital who contributed to data collection and technical support. There are no sources of funding to report. Disclosures of financial support (unrelated to this work) are as follows: Drs. Stretz, Sheikh and Maciel have nothing to disclose. Dr. Hirsch has received research support from Yale University for investigator‐initiated studies from Eisai and Upsher‐Smith and consultation fees for advising from Ceribell, Eisai, Monteris, Sun Pharma, and Engage Therapeutics. Furthermore, he has received royalties for authoring chapters for UpToDate‐Neurology, chapters for Medlink— Neurology, and from Wiley for coauthoring the book “Atlas of EEG in Critical Care”, by Hirsch and Brenner, as well as honoraria for speaking from Neuropace. Dr. Gilmore has received funding from Yale's Center for Clinical Investigation, CTSA Grant (ULTR000142), Yale's Claude D. Pepper Older Americans Independence Center (P30AG021342 NIH/NIA), the American Brain Foundation, the National Institutes of Health Loan Repayment Program and consulting fees from Sage Therapeutics and Bard Medical.
References
- 1. Marti‐Fabregas J, Piles S, Guardia E, Marti‐Vilalta JL. Spontaneous primary intraventricular hemorrhage: clinical data, etiology and outcome. J Neurol 1999;246:287–291. [DOI] [PubMed] [Google Scholar]
- 2. Rodriguez Ruiz A, Vlachy J, Lee JW, et al. For the Critical Care EEG monitoring research consortium. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically Ill patients. JAMA Neurol. 2017;74: 181–188 [DOI] [PubMed] [Google Scholar]
- 3. Gaspard N, Manganas L, Rampal N, et al. Similarity of lateralized rhythmic delta activity to periodic lateralized epileptiform discharges in critically ill patients. JAMA Neurol 2013;70:1288–1295. [DOI] [PubMed] [Google Scholar]
- 4. Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol 2005;22:79–91. [DOI] [PubMed] [Google Scholar]
- 5. Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology 2007;69:1356–1365. [DOI] [PubMed] [Google Scholar]
- 6. Rudzinski LA, Rabinstein AA, Chung SY, et al. Electroencephalographic findings in acute subdural hematoma. J Clin Neurophysiol 2011;28:633–641. [DOI] [PubMed] [Google Scholar]
- 7. Maciel CB, Gilmore EJ. Seizures and epileptiform patterns in SAH and their relation to outcomes. J Clin Neurophysiol 2016;33:183–195. [DOI] [PubMed] [Google Scholar]
- 8. Witsch J, Frey HP, Schmidt JM, et al. Electroencephalographic periodic discharges and frequency‐dependent brain tissue hypoxia in acute brain injury. JAMA Neurol 2017;74:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hinson HE, Hanley DE, Ziai WC. Management of intraventricular hemorrhage. Curr Neurol Neurosci Rep 2010;10:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgan TC, Dawson J, Spengler D, et al. The modified Graeb score: an enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke 2013;44:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sivaraju A, Gilmore EJ. Understanding and managing the ictal‐interictal continuum in neurocritical care. Curr Treat Options Neurol 2016;18:8. [DOI] [PubMed] [Google Scholar]
- 12. Hirsch LJ, LaRoche SM, Gaspard N, et al. American clinical neurophysiology Society's Standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 13. Flint AC, Roebken A, Singh V. Primary intraventricular hemorrhage: yield of diagnostic angiography and clinical outcome. Neurocrit Care 2008;8:330–336. [DOI] [PubMed] [Google Scholar]
- 14. Claassen J, Mayer SA, Kowalski RG, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004;62:1743–1748. [DOI] [PubMed] [Google Scholar]
- 15. San Juan OD, Chiappa KH, Costello DJ, Cole AJ. Periodic epileptiform discharges in hypoxic encephalopathy: BiPLEDS and GPEDs as a poor prognosis for survival. Seizure 2009; 18:365–368. [DOI] [PubMed] [Google Scholar]
- 16. Walsh JM, Brenner RP. Periodic lateralized epileptiform discharges – longterm outcome in adults. Epilepsia 1987;28:533–536. [DOI] [PubMed] [Google Scholar]
- 17. Li HT, Wu T, Lin WR, et al. Clinical correlation and prognostic implication of periodic EEG patterns: a cohort study. Epilepsy Res 2017;131:44–50. [DOI] [PubMed] [Google Scholar]
- 18. Cormier J, Maciel CB, Gilmore EJ. Ictal‐interictal continuum: when to worry about the continuous electroencephalography pattern. Semin Respir Crit Care Med 2017;38:793–806. [DOI] [PubMed] [Google Scholar]
- 19. Orta DS, Chiappa KH, Quiroz AZ, et al. Prognostic implications of periodic epileptiform discharges. Arch Neurol 2009;66:985–991. [DOI] [PubMed] [Google Scholar]
- 20. Brigo F. Intermittent rhythmic delta activity patterns. Epilepsy Behav 2011;20:254–256. [DOI] [PubMed] [Google Scholar]
- 21. Accolla EA, Kaplan PW, Maeder‐Ingvar M, et al. Clinical correlates of frontal intermittent rhythmic delta (FIRDA). Clin Neurophysiol 2011;122:27–31. [DOI] [PubMed] [Google Scholar]
- 22. Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 23. Struck AF, Osman G, Rampal N, et al. Time‐dependent risk of seizures in critically ill patients on continuous electroencephalogram. Ann Neurol 2017;82:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krish S, Bazil CW. Interpreting periodic rhythmic electroencephalographic patterns in critical ill patients. JAMA Neurol 2017;74:150–151. [DOI] [PubMed] [Google Scholar]
- 25. Gilmore EJ, Maciel CB, Hirsch LJ, Sheth KN. Review of the utility of prophylactic anticonvulsant use in critically ill patients with intracerebral hemorrhage. Stroke 2016;47:2666–2672. [DOI] [PubMed] [Google Scholar]
- 26. Johnson EL, Kaplan PW. Population of the ictal‐interictal zone: the significance of periodic and rhythmic activity. Clin Neurophys Practice 2017;2:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez V, Rodden MF, LaRoche SM. Ictal‐interictal continuum: a proposed treatment algorithm. Clin Neurophysiol 2016;127:2056–2064. [DOI] [PubMed] [Google Scholar]
- 28. Struck AF, Ustun B, Ruiz AR, et al. Association of an electroencephalography‐based risk score with seizure probability in hospitalized patients. JAMA Neurol 2017;74:1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]


