Abstract
Objective
Prominent research in patients with disorders of consciousness investigated the electrophysiological correlates of auditory deviance detection as a marker of consciousness recovery. Here, we extend previous studies by investigating whether somatosensory deviance detection provides an added value for outcome prediction in postanoxic comatose patients.
Methods
Electroencephalography responses to frequent and rare stimuli were obtained from 66 patients on the first and second day after coma onset.
Results
Multivariate decoding analysis revealed an above chance‐level auditory discrimination in 25 patients on the first day and in 31 patients on the second day. Tactile discrimination was significant in 16 patients on the first day and in 23 patients on the second day. Single‐day sensory discrimination was unrelated to patients’ outcome in both modalities. However, improvement of auditory discrimination from first to the second day was predictive of good outcome with a positive predictive power (PPV) of 0.73 (CI = 0.52–0.88). Analyses considering the improvement of tactile, auditory and tactile, or either auditory or tactile discrimination showed no significant prediction of good outcome (PPVs = 0.58–0.68).
Interpretation
Our results show that in the acute phase of coma deviance detection is largely preserved for both auditory and tactile modalities. However, we found no evidence for an added value of somatosensory to auditory deviance detection function for coma‐outcome prediction.
Introduction
Coma is a severe clinical condition characterized by a pathological loss of consciousness that may result from diffuse bihemispheric lesions of the cortex or underlying white matter, or a bilateral thalamic damage.1 In particular, after cardiac arrest, the majority of resuscitated patients fall in a deep coma in which even brainstem function may be absent, and ventilation support becomes necessary. Over time, comatose patients may evolve toward a good outcome and waking up within days or may transition to the unresponsive wakefulness syndrome where the patient is awake but unaware and unresponsive to the environment. At different stages of pathological loss of consciousness, accurate evaluation of preserved brain functions can significantly improve patients’ outcome prediction, as defined by standardized clinical scales at 3 months such as the Cerebral Performance Category (CPC,2).
Within such preserved brain functions, neural detection of novel or infrequent sounds within regular auditory sequences (i.e. auditory deviance detection) is typically reported in comatose and disorders of consciousness patients who will eventually survive.3 The brain mechanisms underlying auditory deviance detection might still be preserved during early stages of coma and further improve over time in long‐term survivors, whereas deviance detection appears to degenerate over time in those patients who eventually die (see4, 5 for a discussion).
In line with these results an improvement in auditory deviance detection positively correlated with functional and cognitive performance levels at awakening in coma survivors.6 To what extent deviance detection capacity in comatose patients persists as a function of stimulus complexity and stimulus modality is currently a focus of an intense research activity.7, 8, 9
Previous studies in healthy subjects showed that deviance detection can be evoked both in the auditory (see10 for a review), somatosensory,11, 12, 13, 14 and visual modality,15, 16, 17 and even across modalities.18, 19, 20 Neuroimaging studies suggest that the underlying neural representations are encoded in distinct modality‐specific brain regions,21, 22 however, cross‐talk between sensory modalities at the functional and neuroanatomical level has been reported.21, 23 Based on these observations in healthy subjects, we hypothesized that diffuse cerebral injury in comatose patients might differently impair deviance detection for auditory and somatosensory stimuli. Thus, we hypothesized that the assessment of somatosensory deviance detection might provide complementary information to auditory deviance detection for increasing the number of predicted survivors and thereby improving coma‐outcome prediction.4, 5, 24
We tested this prediction in postanoxic comatose patients that within other etiologies, (i.e. pharmacological, traumatic etc.) represents one of the major causes of admission at the intensive care units in developed country and mostly following cardiac arrest (CA; with a frequency of 38–84/100′000 in Europe).25 We recorded twice in each patient over the first 2 days of coma, neural responses to auditory and somatosensory stimuli in order to quantify the degree of preserved deviance detection in each sensory modality in acute coma. Specifically, we implemented an auditory mismatch negativity (MMN) protocol (i.e. reported previously4, 5, 24) and a tactile MMN protocol where the stimuli were differentiated by their duration, a stimulus feature allowing a direct comparison between the two modalities.
Materials and Methods
Postanoxic comatose patients
We recorded data from 95 consecutive patients older than 18 years resuscitated from CA who were admitted to the intensive care units of the University Hospitals Lausanne (65 patients), Bern (23 patients), Sion (5 patients), and Fribourg (2 patients) between December 2016 and May 2017. Data were collected from all patients admitted during the study period who were treated with targeted temperature management (TTM;26, 27), given the availability of EEG recording system and experimenter and a high probability of the patient being still alive for the second day recording according to the treating clinician. Informed written consent for participation in this study was obtained prior to EEG recordings from a family member, legal representative, or treating clinician not involved in this study. The experimental protocol was approved by the ethical committees of the Cantons Bern, Fribourg, Valais and Vaud and all methods were carried out in accordance with relevant guidelines and regulations. Based on our previous studies showing similar prognostic performance of our method across patients receiving TTM at different target temperatures for the present study we included both patients receiving TTM at 36°C (75 patients) and patients receiving TTM at 33°C (20 patients;4, 5, 24). TTM was applied for 24 h using ice packs or intravenous ice‐cold fluids together with a feedback controlled cooling device (Arctic Sun System, Medivance, Louisville or Thermogard XP; ZOLL Medical, Zug, Switzerland) followed by removal of TTM after 24 h. Propofol (2–3 mg/kg/h), Midazolam (0.1 mg/kg/h) and Fentanyl (1.5 μg/kg/h) were given for analgesia‐sedation, and Vecuronium, Rocuronium, or Atracurium for controlling shivering.
Of 95 patients, 21 were excluded from analysis because of missing second EEG recording (i.e. 13 awoke, 7 deceased, and 1 patient was transferred to a different hospital within 48 h following CA). For our main analysis, we excluded eight patients because a relevant comorbidity (e.g. second CA, multiorganic failure, or intracerebral bleeding) unrelated to the initial CA was diagnosed only after our recordings, and caused death within 3 months. As our approach is based on EEG recordings during the first 2 days following CA, our method cannot foresee such secondary events (for similar approach see4). Thus, the number of patients included for the analysis was 66 (15 treated with TTM at 33°C, 23%).
Patients’ outcome was defined as the best functional level reached within 3 month after CA based on regular assessment of neurological state during hospitalization and a semistructured phone interview at 3 months after CA using CPC2 CPC 1 indicates full recovery; CPC 2 conscious with moderate disability; CPC 3 conscious with severe disability; CPC 4 coma or persistent vegetative state, and CPC 5 death. Patients with CPC 1–3 at any time within 3 months after coma onset were considered patients with good outcome (in the following referred to as ‘survivors’, n = 41). All patients who died within 3 months from coma onset without ever awakening are considered within the poor outcome group (in the following ‘Nonsurvivors’; n = 25). The vast majority of Nonsurvivors died after interdisciplinary decision of withdrawal of supporting care, based on our previously published multimodal protocol (e.g.,28)
We note that this study was part of a larger study aiming to validate the auditory discrimination method in a larger cohort of comatose patients. For consistency across studies, all patients completed first the auditory protocol (i.e. as in4, 5, 24), which was followed by the administration of the tactile protocol (i.e. the total duration of both protocols was 90 min). We note that of the 66 patients analyzed for the present study, data from the auditory protocol from 49 patients receiving TTM at 36°C were previously reported.4 Here, we aimed to compare the predictive value of data from the auditory to the tactile protocol – not reported before – and therefore included here all patients who took part in both the auditory and the tactile protocol receiving either TTM at either 33 or 36°C.
Clinical assessments
Neurological examination of pupillary, oculocephalic, corneal reflexes and motor reactivity to pain stimulation was assessed by a certified neurologist after withdrawal of TTM and weaning of pharmacological sedation (at least twice between 36 and 72 h after CA, or more often if needed). Two clinical EEG recordings were performed, within 24 h (at least 6 h) after CA during TTM, and at 36–48 h after CA and withdrawal of TTM at the time of clinical examination.29 EEG background reactivity interpretation was performed by experienced electroencephalographers. Epileptiform EEG was defined as any repetitive periodic or rhythmic spikes, or sharp waves, or spike‐waves.30 Bilateral median nerve somatosensory evoked potentials (SSEP) were recorded at least 24 h after CA. Neuron‐Specific Enolase (NSE) was measured at 24 and 48 h after CA and analyzed with an automated immunofluorescent assay (Thermo Scientific Brahms NSE Kryptor Immunoassay, Hennigsdorf, Germany; and Roche Cobas Elecsys; Roche Diagnostics, Rotkreuz, Switzerland). Exclusive palliative care was decided using a multidisciplinary approach, if two or more of the following criteria were present31, 32: (1) Unreactive EEG background after TTM and off sedation, (2) Treatment‐resistant myoclonus, (3) Bilateral absence of N20 in SSEP, and (4) Incomplete return of brainstem reflexes.
Experimental protocol and EEG acquisition
Each patient was presented with an auditory MMN, previously used in24 and originally introduced by33 and a tactile MMN protocol. Details about the experimental protocols, EEG acquisition, and data preprocessing can be found in the Supplemental Data S1.
Multivariate EEG decoding
The absence of stereotypical evoked responses in comatose patients recorded during acute coma encourages the use of data‐driven single patient analysis for the assessment of sensory discrimination. Single‐patient EEG data was analyzed with a multivariate decoding algorithm based on EEG responses across the whole 19‐channel montage.34 This method can be used to quantify the differential responses to standard versus deviant sounds at the level of each single patient and recording. It has been previously used for decoding responses in healthy subjects and comatose patients.5, 7, 24, 35, 36, 37, 38 This algorithm consists of modeling the distribution of single‐trial EEG responses across all electrodes using a mixture of Gaussian models (GMM) in an n‐dimensional space where n represents the number of electrodes.34, 39 The models are computed through an expectation‐maximization algorithm for each patient and recording (first day, second day) separately, using only one part of the available data (training data set, consisting of 90% of the artifact‐free single trials;40). Posterior probabilities are computed for each topographies recorded at each single time frames and trial and discriminative time‐periods between conditions of interest are derived.41 Each trial in the training data set is decoded as being a response to a standard or a deviant sound based on the average posterior probabilities values computed across trials at each time frame. The performance of the decoding algorithm is then assessed on the remaining 10% of the available single trials (test data set) and by assigning the test trials in one of the two experimental conditions (i.e., responses to standard vs. deviant sounds).
Decoding performance is measured as the area under the receiver operating characteristic curve (AUC;42) and it is computed for standard versus each type of deviant sound. The GMM model's parameters are optimized by repeating this whole procedure 10 times by splitting the data in training and test data sets in a way that the 10 test data sets never overlap. The AUC values reported for the auditory protocol correspond to the mean value across all three contrasts (i.e., responses to standard sounds vs. deviant sounds in pitch, duration, or location), and for the tactile protocol to duration deviant and standard sounds. To allow a full comparison between the auditory and tactile protocol, we report also the AUC values separately for each of the three auditory deviants (see Supplemental Data S1).
Sensory discrimination on single day and outcome prediction
The outcome prediction was based on the average AUC values obtained on the test datasets across the three deviants. All AUC values were considered in this analysis irrespective of their significance at the single subject level. More specifically, outcome prediction was based on the change of decoding performance from Day 1 (AUCDAY1) to Day 2 (AUCDAY2) and specifically on the percentage change in AUC values: 100× (AUCDAY2 − AUCDAY1)/AUCDAY1. Significance of outcome prediction results was assessed with 95% confidence intervals (CIs) based on a binomial distribution.
In a separate analysis we assessed the significance of the AUC values for each recording separately by evaluating the decoding methods on the validation dataset, i.e. an independent dataset from that used for feature selection and that was not used for outcome prediction at any step, and by comparing this value to chance level using a permutation test. Results based on the validation dataset and corresponding chance levels are reported in the section ‘Sensory discrimination on single day’.
We further assessed the significance at group level of the decoding values for each day separately by computing the probability of the having k success out of n tests using a binomial cumulative distribution function (in Matlab it is implemented in the function binocdf;37, 43); The number of success was based on the number of AUC that were significant based on the permutation test. The probability of significance for each AUC was assessed based on the number of times the decoding performance obtained on the validation dataset outperformed that obtained on random permutation. This test provides an estimation of the probability to observe by chance significant results in k out of n tests (here for ‘All Patients”, n = 66; Table 2).
Table 2.
Proportion of comatose patients showing an above chance‐level auditory or tactile discrimination on Day 1 during targeted temperature management (TTM) and from Day 2 after removal of temperature control
| All patients | Survivors | Nonsurvivors | ||||
|---|---|---|---|---|---|---|
| Audio | Tactile | Audio | Tactile | Audio | Tactile | |
| Day 1, pts. (%) | 25/66 (38%) | 16/66 (24%) | 16/41 (39%) | 12/41 (29%) | 9/25 (36%) | 4/25 (16%) |
| Day 2, pts. (%) | 31/66 (47%) | 23/66 (35%) | 22/41 (54%) | 13/41 (32%) | 9/25 (36%) | 10/25 (40%) |
The results are shown separately for the total sample, and Survivors and Nonsurvivors. Above chance level performance at group level across all patients is highlighted in bold.
Results
Comatose patients’ outcome
Of the 66 patients analyzed (14 women, age mean = 65 years, SD = 13 years), 41 (62%) had a good outcome, 25 (38%) had a poor outcome, and no patient was in a persistent vegetative state.
Outcome prediction based on the progression of sensory discrimination
The average decoding performance for the auditory stimulation protocol for 41 Survivors was AUCDAY1 = 0.615 ± 0.005 and AUCDAY2 = 0.616 ± 0.005, and for the 25 Nonsurvivors decoding performance was AUCDAY1 = 0.627 ± 0.006 and AUCDAY2 = 0.614 ± 0.006 (Fig. 1A). An improvement was observed in 19 of 41 Survivors (46%), whereas the majority of Nonsurvivors (18 of 25, 72%) showed a decrease in decoding performance (Fig. 2A). Overall, across all 66 patients, an improvement in AUC from Day 1 to Day 2 was observed in 26 patients, among whom 19 awoke from coma, resulting in 73% predictive value of good outcome (95% CI = 0.52–0.88; Table 1). The sensitivity (i.e., ratio of Survivors showing an increase) was 46% (95% CI = 0.31–0.63) and the specificity (i.e., ratio of Nonsurvivors showing a decrease) was 72% (95% CI = 0.51–0.88). The predictive value of poor outcome (i.e. ratio patients showing decrease with a poor outcome) was 45% (95% CI = 0.29–0.62), and the overall accuracy was 56% (95% CI = 0.35–0.57; Table 1). For comparison with previous studies4, 5 we present in the Supplemental Data S1 complementary outcome prediction analyses for a subgroup of patients without epileptiform features (Table S1) and separate analysis based on each type of deviant (duration, location, pitch; Table S2).
Figure 1.
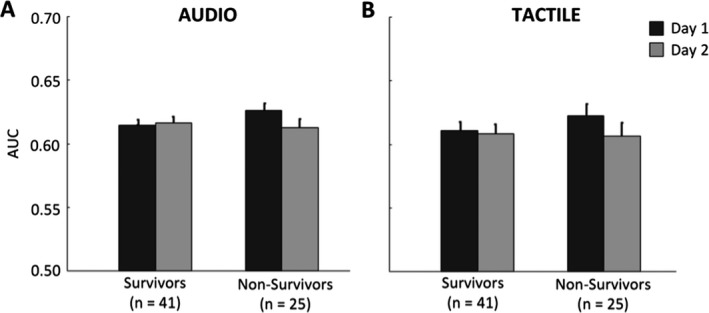
Average decoding performance of comatose patients for the Auditory (A) and Tactile (B) stimulation protocols, split according to patients’ outcome (Survivors, Nonsurvivors). Black bars refer to the area under the curve (AUC) values obtained for the first day recording (Day 1) under targeted temperature management and grey bars refer to AUC values of the second day recording (Day 2) after removal of temperature control. Decoding performance corresponds to average AUC values for decoding EEG responses to standard versus deviant stimuli evaluated for each patient/recording separately.
Figure 2.
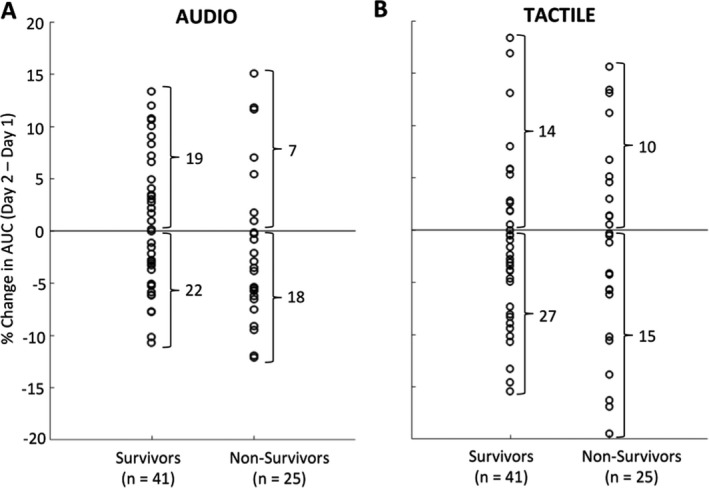
Outcome prediction results based on the progression of auditory (A) and tactile (B) discrimination in comatose patients, split according to patients’ outcome (Survivors, Nonsurvivors). Circles refer to the percentage change in decoding performance for individual patients from Day 1 under targeted temperature management to Day 2 after withdrawal of temperature control. AUC, area under the curve.
Table 1.
Prognostic values for good outcome for comatose patients based on the progression of auditory, tactile, or combinations of auditory and tactile discrimination results
| Audio | Tactile | Audio and Tactile | Audio or Tactile | |
|---|---|---|---|---|
| Positive predictive value (95% CI) | 0.73 (0.52–0.88) | 0.58 (0.37–0.78) | 0.63 (0.24–0.91) | 0.68 (0.49–0.83) |
| Sensitivity (95% CI) | 0.46 (0.31–0.63) | 0.34 (0.20–0.51) | 0.12 (0.04–0.26) | 0.56 (0.40–0.72) |
| Specificity (95% CI) | 0.72 (0.51–0.88) | 0.60 (0.39–0.79) | 0.88 (0.69–0.97) | 0.56 (0.35–0.76) |
| Negative predictive value (95% CI) | 0.45 (0.29–0.62) | 0.36 (0.22–0.52) | 0.38 (0.26–0.52) | 0.44 (0.26–0.62) |
| Accuracy (95% CI) | 0.56 (0.35–0.57) | 0.44 (0.25–0.46) | 0.41 (0.19–0.37) | 0.56 (0.39–0.63) |
Values above chance level are highlighted in bold.
For the tactile stimulation protocol, the average decoding performance for 41 Survivors was AUCDAY1 = 0.611 ± 0.007 and AUCDAY2 = 0.608 ± 0.008, and for the 25 Nonsurvivors decoding performance was AUCDAY1 = 0.618 ± 0.010 and AUCDAY2 = 0.605 ± 0.010 (Fig. 1B). The change in decoding performance from Day 1 to Day 2 showed an improvement in 14 of 41 Survivors (34%), and a decrease in decoding performance was found in 15 of 25 Nonsurvivors (60%; Fig. 2B). Overall, across all 66 patients, an improvement in AUC from Day 1 to Day 2 was observed in 24 patients, among whom 14 awoke from coma, resulting in 58% predictive value of good outcome (95% CI = 0.37–0.78; Table 1). The sensitivity (i.e., ratio of Survivors showing an increase) was 34% (95% CI = 0.20–0.51) and the specificity (i.e., ratio of Nonsurvivors showing a decrease) was 60% (95% CI = 0.39–0.79). The predictive value of poor outcome (i.e. ratio patients showing decrease with a poor outcome) was 36% (95% CI = 0.22–0.52), and the overall accuracy was 44% (95% CI = 0.25–0.46; Table 1).
By combining the decoding results from the auditory and tactile tasks, we found no improvement of outcome prediction as compared to analysis based solely on result from the auditory task (see Table 1 for an overview of results). Of the 66 patients analyzed, 8 (12%) showed an improvement of both auditory and tactile discrimination, of which only five survived (i.e. PPV = 0.63, 95% CI = 0.24–0.91; see Table 1: Audio and Tactile). By considering those patients with improvement in either auditory or tactile discrimination (i.e. 34 of 66 patients, 52%), decoding performance showed no significant prediction of good outcome (i.e. PPV = 0.68; 95% CI = 0.49–0.83; Table 1: Audio or Tactile).
Sensory discrimination on single day
To assess whether the absence of predictive value for the progression of tactile discrimination was related to a poor sensory discrimination performance, we evaluated the statistical significance of the neural discrimination for each patient on a single day, separately for the tactile and the auditory protocol (i.e. duration deviant only) using a permutation test. Across the total of 66 patients tested, an above‐chance level auditory discrimination was found in 25 patients (38%) on the first day and in 31 patients (47%) on the second day. Tactile discrimination was above chance level in 16 patients (24%) on the first day and 23 patients (35%) on the second day. The notable increase of the proportion of participants showing sensory discrimination from the first (24–38%) to the second day (35–47%) is in line with the idea of an improvement of sensory discrimination over time in comatose patients. In addition, across all recordings in 66 patients, an above‐chance level discrimination was found in 42 patients (64%) for the auditory protocol and in 31 patients (47%) for the tactile protocol, indicating that sensory discrimination function was preserved for substantial proportion of comatose patients for both the auditory and somatosensory modalities.
Importantly, these results for single days by itself were not informative about patient outcome (Table 2; see also Supplemental Material in our previous paper for similar considerations4). At group level, single‐day discrimination results were significant (P < 0.05) for auditory discrimination on both days and for tactile discrimination for Day 1.
Discussion
We tested and compared auditory and tactile discrimination as measured by EEG for predicting postanoxic comatose patients’ outcome.
Following previous works, we focused on the improvement of sensory discrimination over 2 days4, 5, 24, 44 and we replicated that the improvement in auditory discrimination between standard and deviant stimuli was informative of the patients’ chances of awakening (73% PPV;4, 5, 24). The progression of tactile discrimination assessed in the same patients was not predictive of the patients’ outcome (58% PPV). Tactile discrimination was nevertheless significant in 31 of 66 tested patients and significant at group level on the first day, thus suggesting that data quality did not prevent the detection of the predictive value of the tactile discrimination for outcome prediction. Our study shows that auditory discrimination results outperformed tactile discrimination for the prediction of coma outcome and that tactile discrimination provided no additional predictive value.
We note that the experimental protocols for auditory and tactile discrimination assessment slightly differed in terms of deviance feature (i.e. audio: duration, pitch, and location deviants; tactile: duration deviant only), proportion of deviant to the total number of stimuli (i.e. audio: 30%; tactile: 20%), and a fixed order of presentation (i.e. auditory task before tactile task), which may have contributed to the present results. Accordingly, a direct comparison between auditory and tactile results should be considered preliminary. Complementary analyses were carried out for computing the prediction performance separately for each of the auditory deviants (Supplemental Data S1). This analysis confirmed that the progression of auditory discrimination showed significant positive predictive power for the duration and the location deviant, comparable to data based on the average of three deviants. Thus, even single auditory deviants provided a superior predictive power to the tactile duration deviant. These results indicate that the prediction of patients’ outcome is not based on feature detection across modalities (e.g. duration) but rather on the sensory modality of the stimuli across different features types.
The degeneration of auditory discrimination performance in Nonsurvivors can result from the anoxia‐induced deterioration of brain tissue properties over time in brain regions encoding auditory discrimination function.45, 46 Diffusion tensor imaging data showed brain damaged in the thalamus, basal ganglia, cerebellum, hippocampus, frontal, and parietal cortices.47, 48, 49 In particular the hippocampus and fronto‐parietal regions are considered as part of the network underlying detection of unexpected events at different stages of sensory processing and in particular in the auditory modality.47, 48, 49, 50, 51
Somatosensory cortical processing in humans involves thalamocortical projections to primary somatosensory cortex and further relay to secondary somatosensory, parietal, and frontal cortical regions involved in different aspects of somatosensory perception52 for a review). Based on only a few available neuroimaging studies, somatosensory deviance detection appears to mainly involve processing in the secondary somatosensory cortex and intraparietal regions, partially overlapping with parietal centers involved in auditory deviance detection (e.g.22). Somatosensory processing is already exploited in clinics for predicting comatose patients’ outcome. In particular the absence of cortical SSEP responses is highly informative of poor outcome.31, 32 Other studies have proposed SSEP amplitude for predicting good outcome either at early or middle latencies in the response window.53, 54 The optimal use of the SSEP for predicting good outcome in patients especially in modern clinical application of TTM targeting 33 or 36°C is currently under exploration. Notably, the early components of the SSEP reflects merely the presence of a local cortical response in primary and secondary somatosensory cortical regions and not the ability of discriminating rare from frequent stimuli over time which is most likely based on a distributed network of cortical and subcortical regions of a deviance detection network.55 To the best of our knowledge this is the first study testing tactile discrimination in postanoxic comatose patients.
The present study aimed at comparing deviance detection for auditory and somatosensory stimulations using the same multivariate decoding analysis as used previously (single‐trial topographic analysis,4, 5, 24). We adopted this method based on previous studies investigating its sensitivity for predicting patients’ outcome in comparison to other analyses. A previous study compared the detection of the auditory MMN in postanoxic comatose patients using the proposed decoding analysis with classical waveform‐based analysis.24 Results showed that whereas decoding analysis provided a high positive predictive value, classical waveform analysis was not informative about patients’ outcome. We interpreted these results in light of the highly heterogeneous and not‐stereotypical deviance detection response in comatose patients, with different features in the evoked responses from that of healthy participants (e.g. see illustrations of exemplar waveforms in5 and56). In a different study the decoding method was compared to logistic regression analysis of the same data from comatose patients38 and in a cohort of healthy participants. We found that the prediction results based on single‐trial topographic analysis was more accurate (100% PPV) then when based on logistic regression (73% PPV) and that the proposed method could detect significant standard versus deviant discrimination in the majority of healthy participants (7 out of 11).
We note that in this study the self‐fulfilling prophecy bias was avoided by acquiring the data in a blinded fashion to the clinicians responsible for end‐of‐life decisions. Nevertheless, we cannot exclude that end‐of‐life decisions may have affected the overall results of this study. Our findings contribute to the ongoing research on coma outcome prediction by showing that the large proportion of Nonsurvivors still present a certain degree of preserved tactile discrimination function and especially during the first day where results were significant at group level. The timing of the assessment and single measurements at variable latency from coma onset even within the span of a few days provides distinct evidence about the degree of preserved functions in the same patients and encourages the use of repetitive measurements over time for a reliable estimation of the patient's overall functional state. In addition, the specificity of sensory discrimination improvement in survivors and for the auditory modality indicate a specific role of auditory stimuli for probing neural circuits involved in consciousness recovery from coma.
Supporting information
Data S1. Additional methods and supplemental analyses.
Acknowledgment
Concept and study design: C.P., M.H., R.K., F.Z., E.A., D.V., M.R., M.O., A.O.R., and M.D.L.; data acquisition and analysis: C.P., N.A.N.N., M.C., P.B., M.H., R.K., E.A., D.V., and M.R.; drafting the manuscript and figures: C.P., A.O.R., and M.D.L. This research was funded by EUREKA‐Eurostars, Grant Number E! 9361 Com‐Alert to Marzia De Lucia. The authors declare no competing financial interests. We thank all EEG technicians from the involved hospitals for invaluable help with EEG recordings. We thank Christine Staehli, R.N., and Daria Solari, M.D., from the CHUV and Sébastien Doll, M.D., from Fribourg Hospital for help with data acquisition. We are indebted to Rupert Ortner and Christoph Guger for technical support.
Funding Statement
This work was funded by EUREKA‐Eurostars grant 9361.
References
- 1. Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol 2014;10:99–114. [DOI] [PubMed] [Google Scholar]
- 2. Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA 2004;291:870–879. [DOI] [PubMed] [Google Scholar]
- 3. Morlet D, Fischer C. MMN and novelty P3 in coma and other altered states of consciousness: a review. Brain Topogr 2014;27:467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pfeiffer C, Nguissi NA, Chytiris M, et al. Auditory discrimination improvement predicts awakening of postanoxic comatose patients treated with targeted temperature management at 36°C. Resuscitation 2017;118:89–95. [DOI] [PubMed] [Google Scholar]
- 5. Tzovara A, Rossetti AO, Juan E, et al. Prediction of awakening from hypothermic post anoxic coma based on auditory discrimination. Ann Neurol 2016;79:748–757. [DOI] [PubMed] [Google Scholar]
- 6. Juan E, De Lucia M, Tzovara A, et al. Prediction of cognitive outcome based on the progression of auditory discrimination during coma. Resuscitation 2016;106:89–95. [DOI] [PubMed] [Google Scholar]
- 7. Tzovara A, Simonin A, Oddo M, et al. Neural detection of complex sound sequences in the absence of consciousness. Brain 2015;138(Pt 5):1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naccache L, King JR, Sitt J, et al. Neural detection of complex sound sequences or of statistical regularities in the absence of consciousness? Brain 2015;138(Pt 12):e395. [DOI] [PubMed] [Google Scholar]
- 9. Piarulli A, Charland‐Verville V, Laureys S. Cognitive auditory evoked potentials in coma: can you hear me? Brain 2015;138(Pt 5):1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol 2007;118:2544–2590. [DOI] [PubMed] [Google Scholar]
- 11. Näätänen R. Somatosensory mismatch negativity: a new clinical tool for developmental neurological research? Dev Med Child Neurol 2009;51:930–931. [DOI] [PubMed] [Google Scholar]
- 12. Allen M, Fardo F, Dietz MJ, et al. Anterior insula coordinates hierarchical processing of tactile mismatch responses. NeuroImage 2016. Feb;15(127):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naeije G, Vaulet T, Wens V, et al. Multilevel cortical processing of somatosensory novelty: a magnetoencephalography study. Front Hum Neurosci 2016;10:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shinozaki N, Yabe H, Sutoh T, et al. Somatosensory automatic responses to deviant stimuli. Brain Res Cogn Brain Res 1998;7:165–171. [DOI] [PubMed] [Google Scholar]
- 15. Stefanics G, Kremlacek J, Czigler I. Visual mismatch negativity: a predictive coding view. Front Hum Neurosci 2014;8:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang W, Miao D, Zhao L. Visual MMN elicited by orientation changes of faces. J Integr Neurosci 2014;13:485–495. [DOI] [PubMed] [Google Scholar]
- 17. Winkler I, Czigler I. Evidence from auditory and visual event‐related potential (ERP) studies of deviance detection (MMN and vMMN) linking predictive coding theories and perceptual object representations. Int J Psychophysiol 2012;83:132–143. [DOI] [PubMed] [Google Scholar]
- 18. Zhao C, Valentini E, Hu L. Functional features of crossmodal mismatch responses. Exp Brain Res 2015;233:617–629. [DOI] [PubMed] [Google Scholar]
- 19. Besle J, Fort A, Giard MH. Is the auditory sensory memory sensitive to visual information? Exp Brain Res 2005;166:337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stekelenburg JJ, Vroomen J, de Gelder B. Illusory sound shifts induced by the ventriloquist illusion evoke the mismatch negativity. Neurosci Lett 2004;357:163–166. [DOI] [PubMed] [Google Scholar]
- 21. Smiley JF, Falchier A. Multisensory connections of monkey auditory cerebral cortex. Hear Res 2009;258:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Butler JS, Molholm S, Fiebelkorn IC, et al. Common or redundant neural circuits for duration processing across audition and touch. J Neurosci 2011;31:3400–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bolognini N, Papagno C, Moroni D, Maravita A. Tactile temporal processing in the auditory cortex. J Cogn Neurosci 2010;22:1201–1211. [DOI] [PubMed] [Google Scholar]
- 24. Tzovara A, Rossetti AO, Spierer L, et al. Progression of auditory discrimination based on neural decoding predicts awakening from coma. Brain 2013;136(Pt 1):81–89. [DOI] [PubMed] [Google Scholar]
- 25. Beuchat I, Solari D, Novy J, et al. Standardized EEG interpretation in patients after cardiac arrest: correlation with other prognostic predictors. Resuscitation 2018;126:143–146. [DOI] [PubMed] [Google Scholar]
- 26. Scirica BM. Therapeutic hypothermia after cardiac arrest. Circulation 2013;127:244–250. [DOI] [PubMed] [Google Scholar]
- 27. Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med 2013;369:2197–2206. [DOI] [PubMed] [Google Scholar]
- 28. Oddo M, Rossetti AO. Early multimodal outcome prediction after cardiac arrest in patients treated with hypothermia. Crit Care Med 2014;42:1340–1347. [DOI] [PubMed] [Google Scholar]
- 29. Rossetti AO, Tovar Quiroga DF, Juan E, et al. Electroencephalography predicts poor and good outcomes after cardiac arrest: a two‐center study. Crit Care Med 2017;45(7):e674–e682. [DOI] [PubMed] [Google Scholar]
- 30. Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 31. Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Ann Neurol 2010;67:301–307. [DOI] [PubMed] [Google Scholar]
- 32. Tsetsou S, Novy J, Pfeiffer C, et al. Multimodal outcome prognostication after cardiac arrest and targeted temperature management: analysis at 36 degrees C. Neurocrit Care 2017;28:104–109. [DOI] [PubMed] [Google Scholar]
- 33. Todd J, Michie PT, Schall U, et al. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biol Psychiat 2008;63:58–64. [DOI] [PubMed] [Google Scholar]
- 34. Tzovara A, Murray MM, Plomp G, et al. Decoding stimulus‐related information from single‐trial EEG responses based on voltage topographies. Pattern Recogn 2012;45:2109–2122. [Google Scholar]
- 35. Bernasconi F, De Lucia M, Tzovara A, et al. Noise in brain activity engenders perception and influences discrimination sensitivity. J Neurosci 2011;31:17971–17981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Lucia M, Tzovara A, Bernasconi F, et al. Auditory perceptual decision‐making based on semantic categorization of environmental sounds. NeuroImage 2012;60:1704–1715. [DOI] [PubMed] [Google Scholar]
- 37. Cossy N, Tzovara A, Simonin A, et al. Robust discrimination between EEG responses to categories of environmental sounds in early coma. Front Psychol 2014;5:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Lucia M, Tzovara A. Decoding auditory EEG responses in healthy and clinical populations: a comparative study. J Neurosci Methods 2015. Jul;30(250):106–113. [DOI] [PubMed] [Google Scholar]
- 39. Tzovara A, Murray MM, Michel CM, De Lucia M. A tutorial review of electrical neuroimaging from group‐average to single‐trial event‐related potentials. Dev Neuropsychol 2012;37:518–544. [DOI] [PubMed] [Google Scholar]
- 40. Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc Ser B 1977;39:1–38. [Google Scholar]
- 41. Bishop CM. Neural networks for pattern recognition. Oxford, UK: Oxford University Press, 1995. [Google Scholar]
- 42. Green D, Swets J. Signal detection theory and psychophysics. New York, NY: John Wiley and Sons, 1966. [Google Scholar]
- 43. Hausfeld L, De Martino F, Bonte M, Formisano E. Pattern analysis of EEG responses to speech and voice: influence of feature grouping. NeuroImage 2012;59:3641–3651. [DOI] [PubMed] [Google Scholar]
- 44. Rossetti AO, Tzovara A, Murray MM, et al. Automated auditory mismatch negativity paradigm improves coma prognostic accuracy after cardiac arrest and therapeutic hypothermia. J Clin Neurophysiol 2014;31:356–361. [DOI] [PubMed] [Google Scholar]
- 45. Hirsch KG, Mlynash M, Jansen S, et al. Prognostic value of a qualitative brain MRI scoring system after cardiac arrest. J Neuroimaging 2015;25:430–437. [DOI] [PubMed] [Google Scholar]
- 46. Wijman CA, Mlynash M, Caulfield AF, et al. Prognostic value of brain diffusion‐weighted imaging after cardiac arrest. Ann Neurol 2009;65:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arbelaez A, Castillo M, Mukherji SK. Diffusion‐weighted MR imaging of global cerebral anoxia. AJNR Am J Neuroradiol 1999;20:999–1007. [PMC free article] [PubMed] [Google Scholar]
- 48. Wijdicks EF, Campeau NG, Miller GM. MR imaging in comatose survivors of cardiac resuscitation. AJNR Am J Neuroradiol 2001;22:1561–1565. [PMC free article] [PubMed] [Google Scholar]
- 49. Greer DM, Scripko PD, Wu O, et al. Hippocampal magnetic resonance imaging abnormalities in cardiac arrest are associated with poor outcome. J Stroke Cerebrovasc Dis 2013;22:899–905. [DOI] [PubMed] [Google Scholar]
- 50. Garrido MI, Kilner JM, Kiebel SJ, Friston KJ. Dynamic causal modeling of the response to frequency deviants. J Neurophysiol 2009;101:2620–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chennu S, Noreika V, Gueorguiev D, et al. Silent expectations: dynamic causal modeling of cortical prediction and attention to sounds that weren't. J Neurosci 2016;36:8305–8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kaas JH. Chapter 30 ‐ somatosensory system A2 ‐ Mai, Jürgen K In: Paxinos G, ed. The human nervous system, 3rd ed San Diego: Academic Press, 2012:1074–1109. [Google Scholar]
- 53. Cruse D, Norton L, Gofton T, et al. Positive prognostication from median‐nerve somatosensory evoked cortical potentials. Neurocrit Care 2014;21:238–244. [DOI] [PubMed] [Google Scholar]
- 54. Zanatta P, Linassi F, Mazzarolo AP, et al. Pain‐related Somato Sensory Evoked Potentials: a potential new tool to improve the prognostic prediction of coma after cardiac arrest. Crit Care 2015. Nov;17(19):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Spierer L, De Lucia M, Bernasconi F, et al. Learning‐induced plasticity in human audition: objects, time, and space. Hear Res 2011;271:88–102. [DOI] [PubMed] [Google Scholar]
- 56. De Lucia M, Tzovara A. Prognostic use of cognitive event‐related potentials in acute consciousness impairment In: Rossetti AO, Laureys S, eds. Clinical neurophysiology in disorders of consciousness. Vienna: Springer, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Additional methods and supplemental analyses.


