Abstract
Acute lymphoblastic leukemia (ALL) is an aggressive hematologic malignancy affecting pediatric and adult populations. Although the outcomes of ALL in children have improved markedly in previous years, limited treatment strategies are available at present for adult patients with ALL. Wee1 is a crucial cell cycle checkpoint kinase of G2/M that regulates cell cycle progression and maintains chromatin integrity. MK-1775, a selective inhibitor of Wee1 has recently been identified to be able to induce apoptosis of tumor cells by abrogating G2/M checkpoint. The present study investigated the anti-leukemic activity of MK-1775 alone and in combination with doxorubicin (Adriamycin®; ADM) in various human ALL cell lines. MK-1775 treatment induced apoptosis of ALL cells, accompanied by unscheduled mitotic entry and downregulation of Notch pathway. The anti-leukemic activity of MK-1775 was in a concentration- and time-dependent manner. The data also indicated that it decreased the half-maximal inhibitory concentration (IC50) of ADM compared with the control group. The combination of MK-1775 and ADM induced an increased apoptotic rate compared with each agent alone. In addition, the human bone marrow stromal cell HS-5 cell line was detected to exhibit an increased IC50 value of MK-1775 treatment in contrast to ALL cell lines. It indicates that the hematopoietic supportive capability may remain intact during the treatment of MK-1775. Taken together, the Wee1 inhibitor MK-1775 may be an attractive agent in the treatment of patients with ALL.
Keywords: Wee1, MK-1775, acute lymphoblastic leukemia, apoptosis, Notch
Introduction
Acute lymphoblastic leukemia (ALL) is one of the most common types of leukemia, which represents 75–80% of acute leukemia in children and ~20% of all types of leukemia in adults (1–3). The cure rate and 5-year disease free survival (DFS) rate of children patients have markedly improved in previous years (4,5). However, the outcomes of adult patients remain poor (6). The 5-year overall survival (OS) rate of adults of young age (15–39 years old) was demonstrated to be between 42 and 63%. It decreased to 24.1% in those patients between 40 and 59 years old, and to 17.7% in patients between the ages of 60 and 69 (7). Resistance to chemical drugs is one of the primary factors that leads to treatment failure (8). Therefore, novel agents with high efficacy rates are required to treat this threating malignancy.
Wee1 encodes a nuclear protein measuring 96 KD, which is a tyrosine kinase belonging to the Ser/Thr family of protein kinases (9). It is markedly active during S and G2 phase of the cell cycle, and acts as a crucial regulator of the G2/M and DNA damage checkpoints (9). Cyclin-dependent kinase 1 (CDK1) and cyclin B comprise the maturation promoting factor, which initiates the process of mitosis (10,11). Wee1 phosphorylates the amino acids Tyr15 and Thr14 of CDK1, thus inactivating the CDK1-cyclinB complex, arresting cell cycle and preventing mitotic entry (10,11). MK-1775 is a selective Wee1 tyrosine kinase inhibitor that inhibits the activity of Wee1 in an adenosine 5′-triphosphate-competitive manner (12). It induces cell apoptosis by abrogating the G2/M checkpoint in a variety of solid tumors, including: Neuroblastoma (13); Ewing sarcoma (14); glioblastoma (15); pancreatic (16), prostate (17) and breast cancer (18); hepatic carcinoma (19); and acute myeloid leukemia (20,21). However, the role of Wee1 in ALL and the potential anti-ALL activity of MK-1775 have not been well elucidated. The present study evaluated the anti-ALL activity of MK-1775 as a single agent and in combination with the cytotoxic drug doxorubicin (Adriamycin®; ADM). Potential involvement of the Notch and RAC-alpha serine/threonine-protein kinase (Akt)/mechanistic target of rapamycin (mTOR) signaling pathway during the treatment was also investigated. The results provided the preliminary evidence for the clinical application of MK-1775 in the treatment of patients with ALL.
Materials and methods
Cell lines, culture mediums and reagents
Human ALL cells Nalm-6 [B-cell-ALL (B-ALL)], Molt-4 [T-cell-ALL (T-ALL)], Jurkat (T-ALL), Sup-T1 (T-ALL) and the human bone marrow stromal cell HS-5 cell line were purchased from The Cell Bank of Type Culture Collection of Chinese Academy of Medical Science (Shanghai, China) and cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin (Solarbio Life Sciences, Beijing, China) in a 37°C humidified incubator containing 5% CO2 and 95% air. The Wee1 inhibitor, MK-1775 (Selleck Chemicals, Houston, TX), was dissolved in concentrations of dimethyl sulfoxide (DMSO) ranging from 0, 50, 100, 300, 500 to 1,000 nM and stored at −80°C. ADM (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was dissolved in 0.9% saline and stored at 4°C.
Cell culture and viability assays
Cells were seeded at a density of 5,000 cells/well in 96-well plates and incubated at 37°C with MK-1775 (0, 50, 100, 300, 500 and 1,000 nM), ADM (0, 0.1, 0.2, 0.3, 0.4 and 0.5 mg/ml) or a combination of two drugs for indicated time intervals (0, 24, 48 and 72 h). Then, 10 µl MTT was added to each well. Following an additional 4 h of incubation at 37°C, the supernatant was removed following centrifugation at 300 × g and 25°C for 10 min, and 100 µl DMSO was added to each well. Then, the optical density was measured at 570 nm on a spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The half-maximal inhibitory concentration (IC50) was defined as the drug concentration required to reduce cellular viability to 50% of the control well (without treatment). The IC50 was calculated according to the following formulas: Y1-Y2/X1-X2=M; Y1-MX1=B; B-50/-M=IC50, where (X1,Y1) and (X2,Y2) are two points below and above 50% inhibition rate, and where X denotes drug concentration and Y represents the % inhibition rate.
Apoptosis assay
Cells were collected by centrifugation at 300 × g and 4°C for 5 min and washed twice with cold PBS, then suspended in 400 µl 10-fold diluted Annexin V connection solution (cat. no., BB-4101-2, Best Bio, China) and incubated with 5 µl Annexin V-fluorescein isothiocyanate (FITC) (cat. no., BB-4101-2, Best bio) solution at 4°C in dark for 15 min. A total of 10 µl propidium iodide (PI; (cat. no., BB-4101-2 Best bio) solution was added and cells were incubated at 25°C in the dark for 5 min. Following staining, cells were analyzed immediately with flow cytometry (FCM). Annexin V-positive cells were considered as apoptotic. The data was analyzed with Summit 5.2 software (Beckman Coulter, Inc.).
Cell cycle analysis
Cultured cells were collected by centrifugation 300 × g at 4°C for 10 min, washed twice with cold PBS and fixed with 75% ice-cold ethyl alcohol for 1 h. Then, cells were collected by centrifugation with 300 × g at 4°C for 10 min and re-suspended in 200 µl cold PBS. A total of 400 µl PI solution was added to the tube and incubated at 4°C in dark for 30 min. The DNA index (22) was measured using FCM. Cell cycle distribution was calculated according to the DNA index with ModFit LT 3.1 software (Verity software House, Inc., Topsham, ME, USA).
Protein extraction and western blot analysis
Cells were collected and lysed in lysis buffer (50 mmol/l Tris (pH7.5), 100 mmol/l NaCl, 1 mmol/l EDTA, 0.5% NP40, 0.5% Triton X-100, 2.5 mmol/l sodium orthovanadate, 10 µl/ml protease inhibitor cocktail, 1 mmol/l phenylmethylsulfonyl fluoride) for 20 min at 4°C. The total protein was obtained by centrifugation at 20,000 × g at 4°C for 15 min. Protein concentrations were determined with the BCA Protein Assay reagent (Pierce; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Protein (20 µg/lane) was separated by 5% SDS-PAGE, and transferred onto the nitrocellulose membrane. Membranes were blocked with blocking buffer [0.1 mol/l Tris (pH 7.5), 0.9% NaCl and 0.05% Tween-20 (TBST) containing 5% non-fat milk powder] for 1 h at 25°C, then incubated at 4°C overnight with primary antibodies p-CDK1 (Tyr15; cat. no., ab47594; Abcam, Cambridge, UK), total CDK1 (cat. no., A17; Abcam), Notch1-intracellular domain (ICN; cat. no., ab8925; Abcam), Notch3-ICN (cat. no., ab23426; Abcam), HES1 (cat. no., ab108937; Abcam), β-actin (cat. no., 4967; Cell Signaling Technology, Inc., Beverly, MA, USA), γ-H2AX (p-H2AX Ser139) (cat. no., 9718, Cell Signaling Technology, Inc.), phospho-Histone H3 (Ser10) (cat. no., 53348; Cell Signaling Technology, Inc.), Akt (cat. no., 2920; Cell Signaling Technology, Inc.), p-AKT (Ser473; cat. no., 4060, Cell Signaling Technology, Inc.), mTOR (cat. no., 2983; Cell Signaling Technology, Inc,), phosphorylated-mTOR (Ser-2448; cat. no., 2971; Cell Signaling Technology, Inc.), poly (ADP-ribose) polymerase 1 PARP1 (cat. no., SC-8007; Santa Cruz Biotechnology, Inc., Hercules, CA, USA), all at 1:1,000 dilution. Followed by incubation with anti-rabbit (cat. no., ZB-2301; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. Beijing, China) or mouse (cat. no., ZK-9600; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. Beijing, China) horseradish peroxidase (HRP)-conjugated antibodies at 1:5,000 dilution at 4°C for 1 h and reaction with chemiluminescent HRP substrate (EMD Millipore, Billerica, MA, USA), probed proteins were detected on a chemiluminescent autography machine Fluor Chem E (ProteinSimple, San Jose, CA, USA). Software ImageJ 1.44p (National Institute of Health, Bethseda, MD, USA) was used to perform densitometric analysis.
Statistical analysis
Measurement data are presented as mean ± standard deviation. Differences among ≥ three groups were determined by one-way analysis of variance, followed by Student-Newman-Keuls post-hoc test for multiple comparisons, whereas differences between two groups were evaluated by a Student's t-test. P<0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using the SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Results
MK-1775 inhibits the viability of ALL cells
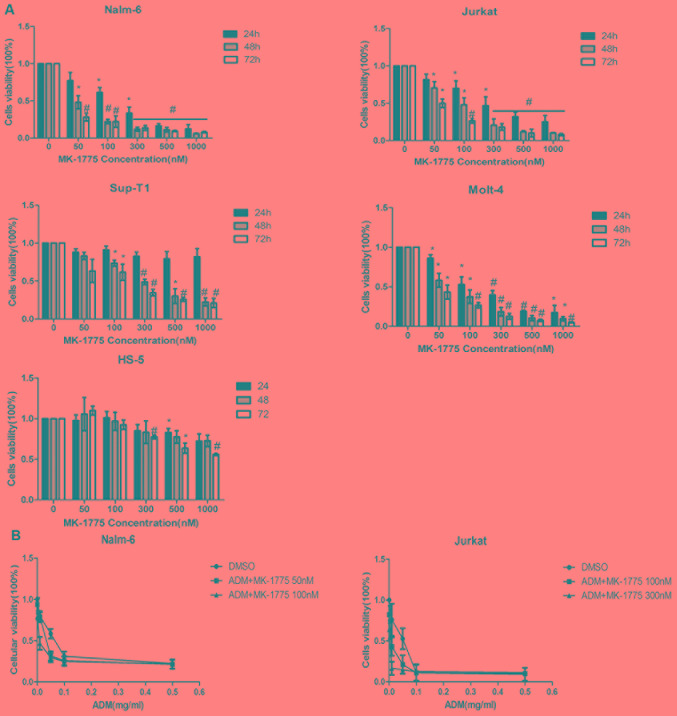
To determine the effect of MK-1775 on cell viability, human ALL Nalm-6, Molt-4, Jurkat and Sup-T1 cell lines and human bone marrow stromal cell HS-5 cell line were treated with MK-1775 in increasing concentrations for 24, 48 and 72 h, and cell viability was detected by MTT assay. As demonstrated in Fig. 1A, cell viability was suppressed in a concentration- and time-dependent manner. The IC50 values of the ALL cells were in a range of 45.9±3.9 to 347.4±95 nmol/l at 48 h, and the IC50 of the HS-50 cell line was 1472.0±304.8 nmol/l, which was increased compared with each ALL cell (P<0.05; Table I). Then, the effects of MK-1775 on the anti-leukemia effect of ADM on the ALL cells was detected. Nalm-6 (B-ALL) and Jurkat (T-ALL) cells were treated with increased doses of ADM with or without MK-1775 for 48 h. The data indicated that the IC50 of ADM was decreased with the additional application of MK-1775 compared with single ADM treatment (Fig. 1B).
Figure 1.
Effects of MK-1775 on the viability of ALL cells and human bone marrow stromal cell. (A) ALL Nalm-6, Jurkat, Molt-4, Sup-T1 and bone marrow stromal cell HS-5 cell lines were treated with 0, 50, 100, 300, 500 and 1,000 nM of MK-1775 for 24, 48 and 72 h. MTT was used to detect cell viability. Data is presented as the percentages of the corresponding untreated control, and as mean ± SD. *P<0.05 and #P<0.01 compared with the control (0 nM). The results are representative of 3 independent experiments. (B) Nalm-6 and Jurkat were treated with ADM and different concentrations of MK-1775 for 48 h. The half-maximal inhibitory concentration of ADM was evaluated. Values are presented as mean ± SD. Results are representative of 3 independent experiments. SD, standard deviation; ADM, doxorubicin.
Table I.
IC50 of MK-1775 against ALL cell lines.
| Cell line | Cell type | p53 | IC50, nM |
|---|---|---|---|
| Nalm-6 | B-ALL | WT | 45.9±3.9a |
| Sup-T1 | T-ALL | MUT | 347.4±95.0a |
| Jurkat | T-ALL | MUT | 137.4±33.9a |
| Molt-4 | T-ALL | MUT | 67.6±31.8a |
| HS-5 | BMSC | WT | 1,472.0±304.8 |
ALL, acute lymphoblastic leukemia; B-ALL, B-cell acute lymphoblastic leukemia; T-ALL, T-cell acute lymphoblastic leukemia; WT, wild type; MUT, mutation. MUT, mutated; WT, wild type; p%£, tumor protein 53; IC50, half-maximal inhibitory concentration.
P<0.05 compared with HS-5 cell line.
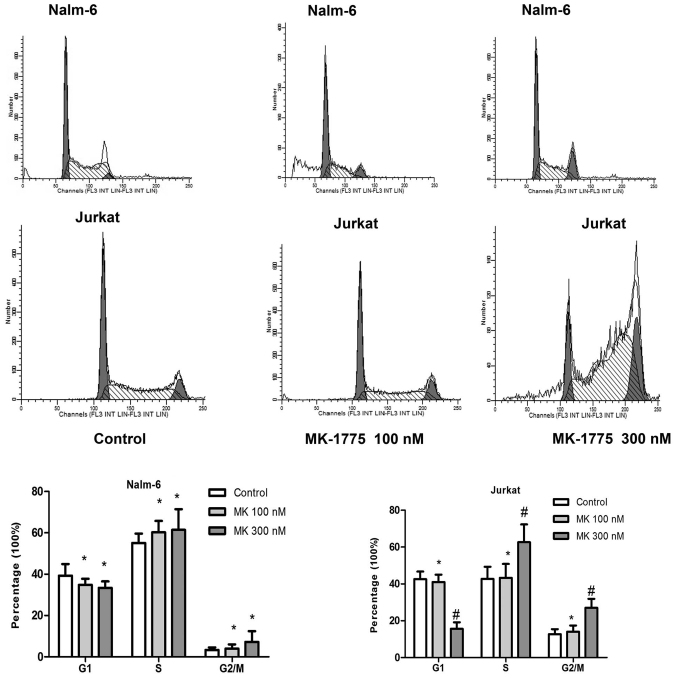
MK-1775 promotes cells into G2/M phase
To determine the effect of MK-1775 on the cell cycle progression of ALL cells in vitro, Nalm-6 and Jurkat cells were cultured in 6-well plates with 0, 100 or 300 nM MK-1775 for 24 h. Cells were collected and stained with PI. Cell cycle analysis was performed with an FCM assay. As indicated in Fig. 2, compared with the control group, treatment with 300 nM MK-1775 decreased the proportion of Jurkat cells in G1 phase, and more cells were arrested at G2/M phase (P<0.05). The proportion of each phase of Nalm-6 cells treated with 100 or 300 nM MK-1775 did not change significantly compared with the control group (P>0.05).
Figure 2.
Effect of MK-1775 on the cell cycle progression of acute lymphoblastic leukemia cells. Nalm-6 and Jurkat were treated with 0, 100 and 300 nM of MK-1775 for 24 h. Flow cytometry was used to detect the cell cycle distribution. Values are presented as mean ± standard deviation. Results are representative of 3 independent experiments. *P>0.05 and #P<0.05 compared with control.
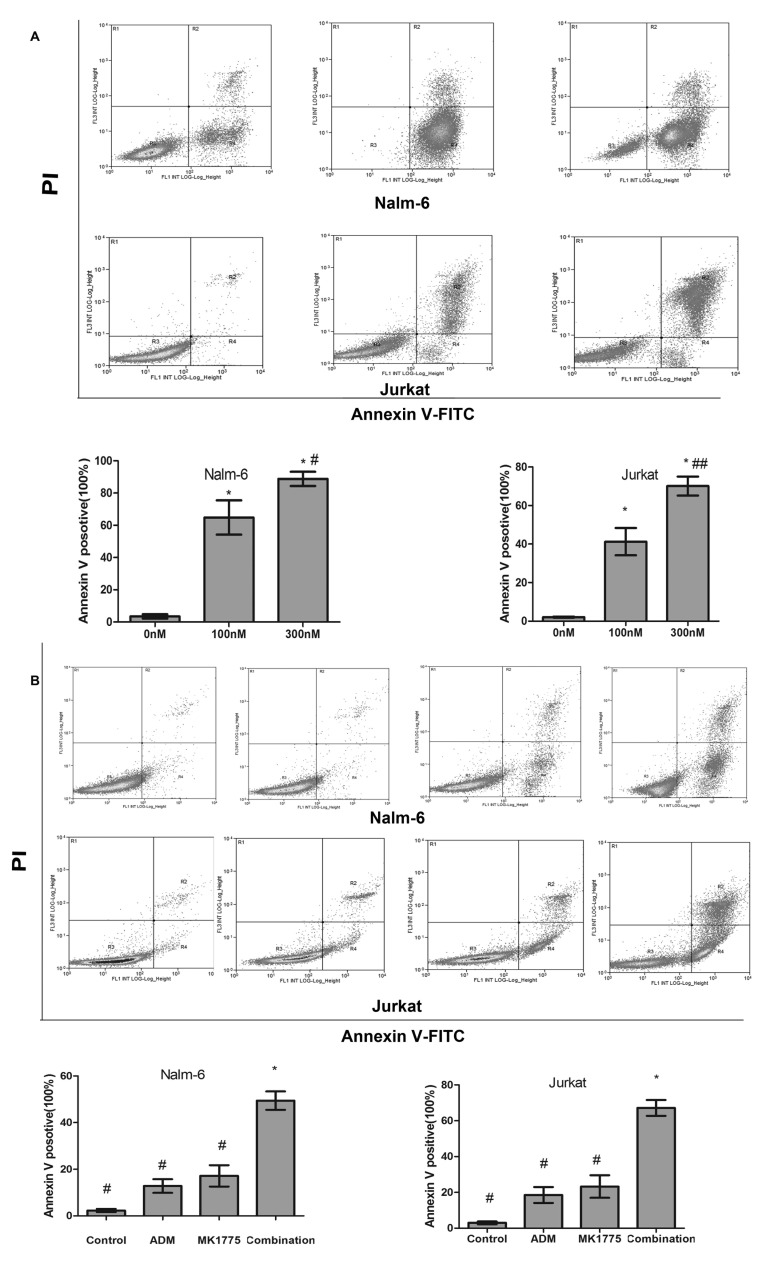
MK-1775 induces apoptosis of ALL cells
To determine the effect of MK-1775 induced apoptosis in ALL cells, Nalm-6 and Jurkat were cultured with MK-1775 (0, 100 or 300 nM) for 48 h, then Annexin V-FITC/PI dual staining followed by flow cytometric analysis was performed. The proportion of apoptotic Nalm-6 cells was 3.46±2.25, 64.82±18.44 and 88.83±7.65% respectively, and the proportion of apoptotic Jurkat cells was 2.12±0.47, 41.26±12.30 and 70.08±8.52%, respectively (Fig. 3).
Figure 3.
Effect of MK-1775 on the apoptosis of acute lymphoblastic leukemia cells. (A) Nalm-6 and Jurkat were treated with 0, 100 and 300 nM MK-1775 for 48 h. Flow cytometry was used to detect the apoptotic cells. Annexin V-FITC-positive cells were considered as apoptotic. Values are presented as mean ± standard deviation. Results are representative of 3 independent experiments. *P<0.05 compared with 0 nM, #P>0.05 compared with 100 nM group, ##P<0.05 compared with 100 nM group. (B) Nalm-6 and Jurkat cells were treated with control, 100 nM MK-1775, 0.05 mg/ml ADM or the combination for 24 h. Flow cytometry was used to detect apoptotic cells. Values are presented as mean ± standard deviation. Results are representative of 3 independent experiments. MK-1775 combined with doxorubicin induced higher apoptotic rates compared with each single agent and the control in the Nalm-6 and Jurkat cell lines (*P<0.05 compared with#). FITC, fluorescein isothiocyanate; PI propidium iodide; ADM, doxorubicin.
Then, the synergistic effect of MK-1775 and ADM was detected. Nalm-6 and Jurkat cells were treated with control, 0.05 mg/ml ADM, MK-1775 100 nM, or the combination for 24 h, and apoptosis analysis was performed with flow cytometry. The apoptotic rate of the Nalm-6 cells in the control, ADM, MK-1775 and combination groups was 2.30±1.13, 12.8±5.07, 17.15±7.89 and 49.42±6.82% respectively; the apoptotic rate of the Jurkat cells was 3.01±1.31, 18.50±7.67, 23.25±10.87 and 67.16±7.67%, respectively. MK-1775 combined with ADM induced higher apoptotic rates compared with each single agent and the control in the Nalm-6 and Jurkat cell lines (P<0.05; Fig. 3).
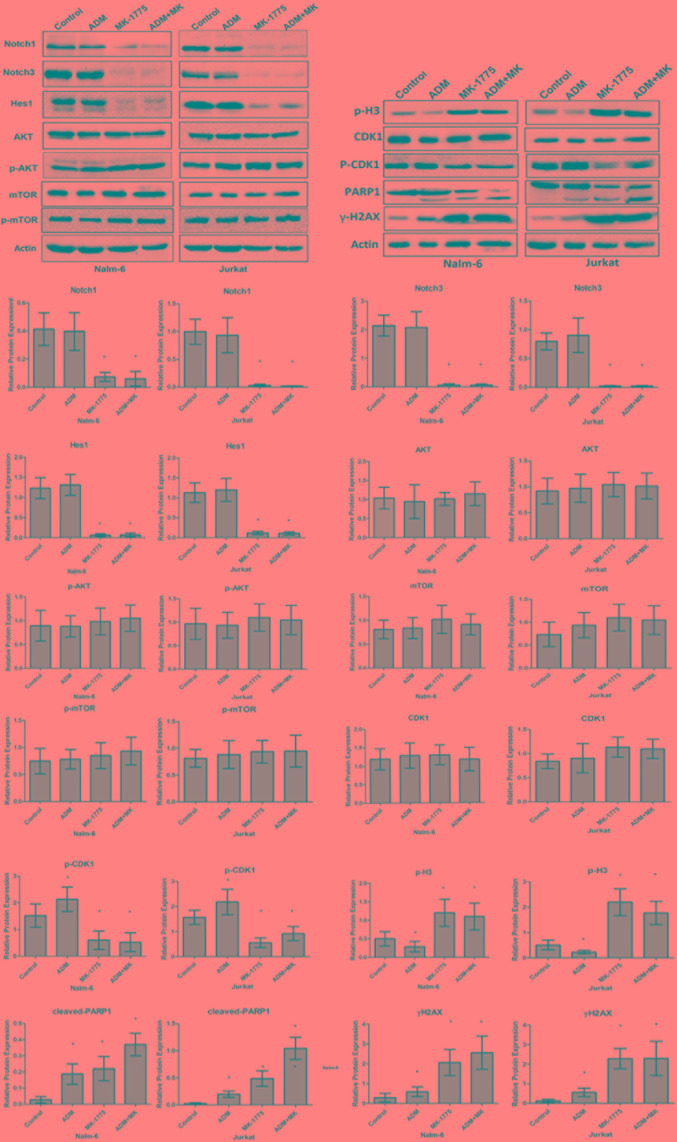
MK-1775 induces apoptosis due to unscheduled mitotic entry and downregulation of Notch pathway
To determine the underlying molecular mechanism of the apoptosis induced by MK-1775, Nalm-6 and Jurkat cells were treated with 0.05 mg/ml ADM, 300 nM MK-1775, or the combination for 24 h, then western blotting was performed to detect the expression of the associated proteins. As demonstrated in Fig. 4, MK-1775 and ADM increased the expression of H2A histone family, member X (γ-H2AX) and promoted the cleavage of PARP1, which indicated breakage of DNA strand and apoptosis of ALL cells. Additional analysis revealed that MK-1775 treatment suppressed the phosphorylation of CDK1 at Tyr15 and increased the expression of p-H3 (Ser10), which is an index of mitotic entry (23). It indicated that apoptosis of ALL cells induced by MK-1775 was due to abrogation of G2/M checkpoint and unscheduled mitosis entry. Different from MK-1775, ADM treatment increased the phosphorylation of CDK1 and decreased the expression of p-H3, which indicated that mitotic entry in the cells was inhibited. In addition, the data indicated that the combination of ADM and MK-1775 induced increased γ-H2AX levels and more cleaved PARP1 compared with treatment with ADM alone (P<0.05), which suggests that MK-1775 may enhance the efficacy of ADM in the treatment of ALL. Next, the potential signaling pathway involved in the apoptosis induced by MK-1775 treatment was detected. The results indicated that Notch1 ICN and Notch3 ICN levels were downregulated by MK-1775 (P<0.05), but that the mTOR pathway was not changed (P>0.05; Fig. 4). However, ADM treatment did not affect the Notch or mTOR pathways as significantly (P>0.05). The relative protein expression was calculated as the density ratio of the protein to be detected to actin.
Figure 4.
MK-1775 induced apoptosis due to unscheduled mitotic entry and downregulation of Notch pathway. MK-1775 inhibited the phosphorylation of CDK1, induced apoptosis characterized by cleavage of PARP-1 and increase of γ-H2AX expression. Notch1 ICN and Notch3 ICN were downregulated, but mTOR levels were not altered by MK-1775 treatment. Results are representative of 3 independent experiments. β-actin protein served as a protein loading control. *P<0.05 vs. control. ADM, doxorubicin; CDK1, cyclin-dependent kinase 1; mTOR, mechanistic target of rapamycin; ICN, intracellular domain; p, phosphorylated; Hes1, hairy and enhancer of split-1; Akt, RAC-alpha serine/threonine protein kinase; p-H3, phosphorylated histone H3; PARP1, poly (ADP-ribose) polymerase 1; rH2AX, H2A histone family, member X.
Discussion
The DNA repairing pathway serves a critical role in the survival of cancer cells (24). The majority of traditional anti-leukemia chemicals are DNA damaging agents, including antimetabolites, alkylating agents and topoisomerase inhibitors (25). Tumor protein 53 (p53) serves as a key regulator of G1/S checkpoint (26). It blocks the initiation of S-phase and activates DNA repairing proteins when DNA has sustained damage (27). However, p53 was mutated in a variety of types of cancer; consequently, there is dysfunction in the G1/S checkpoint and cells with DNA lesions can enter S phase without DNA repair. In these cancer cells, DNA repair relies on the G2/M rather than the G1/S checkpoint (28).
Wee1 is a crucial cell cycle checkpoint kinase of G2/M that regulates cell cycle progression and maintains chromatin integrity (29). In addition, Wee1 serves a critical role in regulating histone synthesis and spindle formation (30). It phosphorylates the amino acids Tyr37 of H2B to terminate the synthesis of histone to maintain the proper histone/DNA stoichiometry before mitotic entry. Wee1 inhibition may force cells in S phase directly into mitosis without completing DNA synthesis, resulting in highly abnormal mitoses characterized by dispersed chromosomes and disorganized bipolar spindles, ultimately resulting in mitotic exit with gross micronuclei formation and apoptosis (31–33). MK-1775 is a selective Wee1 kinase inhibitor that has demonstrated a high rate of anti-tumor activity in a variety of types of cancer.
The data of the present study indicated that the B-ALL and T-ALL cell lines were sensitive to the treatment of Wee1 inhibitor MK-1775 in vitro. It inhibited cell viability and induced apoptosis in a concentration- and time-dependent manner. MK-1775 treatment suppressed the phosphorylation of CDK1 and increased the expression levels of p-H3, which is an indicator of mitotic entry (23). Increased expression levels of γ-H2AX suggested that the unscheduled mitotic entry induced by MK-1775 led to more extensive DNA lesions. PARP is a crucial enzyme responsible for the repair of single-strand DNA breaks (34). It is cleaved by caspase during the process of apoptosis, and consequently becomes inactive (35). The data of the present study indicated that MK-1775 treatment led to DNA repair suppression and apoptosis of ALL cells, characterized by cleavage of PARP1. ADM is a common agent applied in the treatment of ALL that destroys DNA structure and inhibits the replication of DNA (36). The results of the present study indicated that the combination of ADM and MK-1775 led to increased levels of DNA damage compared with each single agent, and as a result induced and increased level of apoptosis. MK-1775 treatment decreased the IC50 of ADM in the Jurkat and Nalm-6 cells, indicating that it may increase the sensitivity of ALL cells to ADM treatment. In addition, the present study demonstrated that the IC50 of MK-1775 in the HS-5 cell line was increased compared with all ALL cell types. It indicates that the hematopoietic supportive capacity of bone marrow stromal cells may be reserved during the treatment of MK-1775. It also agrees with the conclusion that MK-1775 is a well-tolerated agent with high safety (37). In addition, it was identified that MK-1775 exhibited an anti-ALL effect not only in cells with p53 mutations (Jurkat, Sup-T1 and Molt-4), but also in cells with wild type p53 (Nalm-6). A similar result was also identified in sarcomas (38), which indicates that the status of p53 is not the only predicator of response to the Wee1 inhibitor MK-1775. Other studies revealed that the level of Wee1 expression and CDK1 phosphorylation are also responsible for the efficiency of Wee1 inhibitor in untreated glioblastoma and osteosarcoma (39,40).
The present study additionally investigated the potential effect of MK-1775 on the Notch pathway, which serves a critical role in the survival of ALL cells (41). Notch is a conserved transmembrane pathway that consists of 5 ligands [Jagged1, Jagged2, Delta-like ligand (DLL)-1, DLL3, and DLL4] and four receptors (Notch1-4) in mammals (42). When bound with its corresponding ligands, the Notch ICN is released from the membrane, then translocated into the nucleus and associates with transcription factor Recombination signal binding protein for immunoglobulin Kappa J region, leading to the expression of Notch target genes, for example the HES1/Hes related family BHLH transcription factor with YRPW motif 1 family. A previous study indicated that >50% of T-ALL cases contain activating mutations in the Notch1 gene (43). Aberrant activation of Notch1 maintains T-ALL cells survival and promotes extra-medullary infiltration (44,45). and downregulation of Notch-1 may increase the sensitivity to chemotherapy of co-cultured human T-ALL Jurkat cells (46). In B-ALL, Notch-3 and Notch-4 signaling may rescue leukemia cells in contact with human bone marrow-derived mesenchymal stromal cells from apoptosis (47). In addition, Notch has been demonstrated to interact with other signaling pathways in the survival of tumor cells (48,49). It may positively regulate the activity of mTOR pathway in T-ALL (50). Downregulation of Notch-1 may inhibit prostate cancer cell growth and induce apoptosis via inactivation of Akt/mTOR and NF-κB signaling pathways (51). In the present study, Notch1 and Notch3 were detected to be downregulated by MK-1775 treatment, while the expression and phosphorylation level of mTOR were not altered. This indicates that the anti-ALL activity of MK-1775 was partly mediated by the downregulation of Notch pathway, rather than mTOR. However, additional study is required to identify how the Notch signaling pathway was regulated by Wee1 inhibitor MK-1775.
In conclusion, the results of the present study demonstrated that the Wee1 inhibitor, MK-1775, exhibited significant anti-ALL effects by inducing mitotic catastrophe and downregulation of the Notch pathway. The combination of MK-1775 and cytotoxic agents may be a promising anti-ALL regimen with high efficiency and good tolerance. Additional studies are warranted to examine the safety of this drug in vivo.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Nature Science Foundation of China (grant no. 81500130), Shandong Provincial Natural Science Foundation (grant no., ZR2015HQ026), Project of Shandong Province Higher Educational Science and Technology Programme (grant no., J14LL03) and Taian Science and Technology Programme (grant no., 2015NS2088).
Availability of data and materials
All datasets generated and analyzed in the present study are included in this published article.
Authors' contributions
YD and XD conducted experiments and wrote the manuscript. JN performed statistical analysis data, PL and FL analyzed data and participated in manuscript writing, and DM and CJ designed experiments.
Ethics and consent to participate
The research protocol was approved by the Human Ethics Committee of Qilu Hospital, Shandong University. Written informed consent was gained from all participants.
Patient consent for publication
All the participants provided consent for the data to be published.
Competing interests
The authors declare that they have no conflict of interest.
References
- 1.Yeoh AE, Tan D, Li CK, Hori H, Tse E, Pui CH, Asian Oncology Summit Management of adult and paediatric acute lymphoblastic leukaemia in Asia: Resource-stratified guidelines from the Asian Oncology Summit 2013. Lancet Oncol. 2013;2013;14:e508–e523. doi: 10.1016/S1470-2045(13)70452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarnas JC, Brown PA, Aoun P, Ballen KK, Bellam N, Blum W, Boyer MW, Carraway HE, Coccia PF, Coutre SE, et al. Acute lymphoblastic leukemia. J Natl Compr Canc Netw. 2012;10:858–914. doi: 10.6004/jnccn.2012.0089. [DOI] [PubMed] [Google Scholar]
- 3.Esparza SD, Sakamoto KM. Topics in pediatric leukemia-acute lymphoblastic leukemia. MedGenMed. 2005;7:23. [PMC free article] [PubMed] [Google Scholar]
- 4.Ma H, Sun H, Sun X. Survival improvement by decade of patients aged 0–14 years with acute lymphoblastic leukemia: A SEER analysis. Sci Rep. 2014;4:4227. doi: 10.1038/srep04227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenderian SS, Al-Kali A, Gangat N, Letendre L, Hogan WJ, Litzow MR, Patnaik MM. Monosomal karyotype in Philadelphia chromosome-negative acute lymphoblastic leukemia. Blood Cancer J. 2013;3:e122. doi: 10.1038/bcj.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koharazawa H, Kanamori H, Sakai R, Hashimoto C, Takemura S, Hattori M, Taguchi J, Fujimaki K, Tomita N, Fujita H, et al. Long-term outcome of L86 and L97 protocols for adult acute lymphoblastic leukemia. Leuk Lymphoma. 2008;49:2133–2140. doi: 10.1080/10428190802464711. [DOI] [PubMed] [Google Scholar]
- 7.Pulte D, Jansen L, Gondos A, Katalinic A, Barnes B, Ressing M, Holleczek B, Eberle A, Brenner H. Survival of adults with acute lymphoblastic leukemia in Germany and the United States. PLoS One. 2014;9:e85554. doi: 10.1371/journal.pone.0085554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inukai T. Mechanisms of drug resistance in acute lymphoblastic leukemia. Rinsho Ketsueki. 2016;57:2022–2028. doi: 10.11406/rinketsu.57.2022. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 9.Nurse P. Wee beasties. Nature. 2004;432:557. doi: 10.1038/432557a. [DOI] [PubMed] [Google Scholar]
- 10.Siripattarapravat K, Busta A, Steibel JP, Cibelli J. Characterization and in vitro control of MPF activity in zebrafish eggs. Zebrafish. 2009;6:97–105. doi: 10.1089/zeb.2008.0527. [DOI] [PubMed] [Google Scholar]
- 11.Adhikari D, Liu K. The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol Cell Endocrinol. 2014;382:480–487. doi: 10.1016/j.mce.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Hirai H, Iwasawa Y, Okada M, Arai T, Nishibata T, Kobayashi M, Kimura T, Kaneko N, Ohtani J, Yamanaka K, et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther. 2009;8:2992–3000. doi: 10.1158/1535-7163.MCT-09-0463. [DOI] [PubMed] [Google Scholar]
- 13.Creevey L, Ryan J, Harvey H, Bray IM, Meehan M, Khan AR, Stallings RL. MicroRNA-497 increases apoptosis in MYCN amplified neuroblastoma cells by targeting the key cell cycle regulator WEE1. Mol Cancer. 2013;12:23. doi: 10.1186/1476-4598-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackintosh C, García-Domínguez DJ, Ordóñez JL, Ginel-Picardo A, Smith PG, Sacristán MP, de Álava E. WEE1 accumulation and deregulation of S-phase proteins mediate MLN4924 potent inhibitory effect on Ewing sarcoma cells. Oncogene. 2013;32:1441–1451. doi: 10.1038/onc.2012.153. [DOI] [PubMed] [Google Scholar]
- 15.Sarcar B, Kahali S, Prabhu AH, Shumway SD, Xu Y, Demuth T, Chinnaiyan P. Targeting radiation-induced G(2) checkpoint activation with the Wee-1 inhibitor MK-1775 in glioblastoma cell lines. Mol Cancer Ther. 2011;10:2405–2414. doi: 10.1158/1535-7163.MCT-11-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang G, Niu X, Zhang W, Caldwell JT, Edwards H, Chen W, Taub JW, Zhao L, Ge Y. Synergistic antitumor interactions between MK-1775 and panobinostat in preclinical models of pancreatic cancer. Cancer Lett. 2015;356:656–668. doi: 10.1016/j.canlet.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrassa L, Chilà R, Lupi M, Ricci F, Celenza C, Mazzoletti M, Broggini M, Damia G. Combined inhibition of Chk1 and Wee1: In vitro synergistic effect translates to tumor growth inhibition in vivo. Cell Cycle. 2012;11:2507–2517. doi: 10.4161/cc.20899. [DOI] [PubMed] [Google Scholar]
- 18.Ghiasi N, Habibagahi M, Rosli R, Ghaderi A, Yusoff K, Hosseini A, Abdullah S, Jaberipour M. Tumour suppressive effects of WEE1 gene silencing in breast cancer cells. Asian Pac J Cancer Prev. 2014;14:6605–6611. doi: 10.7314/APJCP.2013.14.11.6605. [DOI] [PubMed] [Google Scholar]
- 19.Kogiso T, Nagahara H, Hashimoto E, Ariizumi S, Yamamoto M, Shiratori K. Efficient induction of apoptosis by wee1 kinase inhibition in hepatocellular carcinoma cells. PLoS One. 2014;9:e100495. doi: 10.1371/journal.pone.0100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter CC, Kim J, Fosmire S, Gearheart CM, van Linden A, Baturin D, Zaberezhnyy V, Patel PR, Gao D, Tan AC, et al. Integrated genomic analyses identify WEE1 as a critical mediator of cell fate and a novel therapeutic target in acute myeloid leukemia. Leukemia. 2012;26:1266–1276. doi: 10.1038/leu.2011.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi W, Xie C, Li C, Caldwell JT, Edwards H, Taub JW, Wang Y, Lin H, Ge Y. CHK1 plays a critical role in the anti-leukemic activity of the wee1 inhibitor MK-1775 in acute myeloid leukemia cells. J Hematol Oncol. 2014;7:53. doi: 10.1186/s13045-014-0053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tizzani A, Casetta G, Gontero P, Giammò A, Ghabin H, Demurtas S, Pacchioni D. DNA flow cytometry and 67Ki proliferating index as prognostic factors of early recurrence and progression in G1-G2/Ta-T1 and G3/Ta-T1 transitional cell carcinoma of the bladder. Minerva Urol Nefrol. 1997;49:141–143. [PubMed] [Google Scholar]
- 23.Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD. Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc Natl Acad Sci USA. 1998;95:7480–7484. doi: 10.1073/pnas.95.13.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mateo J, Boysen G, Barbieri CE, Bryant HE, Castro E, Nelson PS, Olmos D, Pritchard CC, Rubin MA, de Bono JS. DNA repair in prostate cancer: Biology and clinical implications. Eur Urol. 2017;71:417–425. doi: 10.1016/j.eururo.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 25.Shang X, Shiono Y, Fujita Y, Oka S, Yamazaki Y. Synergistic enhancement of apoptosis by DNA- and cytoskeleton-damaging agents: A basis for combination chemotherapy of cancer. Anticancer Res. 2001;21:2585–2589. [PubMed] [Google Scholar]
- 26.Carr AM, Green MH, Lehmann AR. Checkpoint policing by p53. Nature. 1992;359:486–487. doi: 10.1038/359486a0. [DOI] [PubMed] [Google Scholar]
- 27.Xie G, Habbersett RC, Jia Y, Peterson SR, Lehnert BE, Bradbury EM, D'Anna JA. Requirements for p53 and the ATM gene product in the regulation of G1/S and S phase checkpoints. Oncogene. 1998;16:721–736. doi: 10.1038/sj.onc.1201793. [DOI] [PubMed] [Google Scholar]
- 28.Hamzehloie T, Mojarrad M, Hasanzadeh Nazarabadi M, Shekouhi S. The role of tumor protein 53 mutations in common human cancers and targeting the murine double minute 2-p53 interaction for cancer therapy. Iran J Med Sci. 2012;37:3–8. [PMC free article] [PubMed] [Google Scholar]
- 29.McGowan CH, Russell P. Cell cycle regulation of human WEE1. EMBO J. 1995;14:2166–2175. doi: 10.1002/j.1460-2075.1995.tb07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia K, Stumpff J, Duncan T, Su TT. Tyrosines in the kinesin-5 head domain are necessary for phosphorylation by Wee1 and for mitotic spindle integrity. Curr Biol. 2009;19:1670–1676. doi: 10.1016/j.cub.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornago M, Garcia-Alberich C, Blasco-Angulo N, Vall-Llaura N, Nager M, Herreros J, Comella JX, Sanchis D, Llovera M. Histone deacetylase inhibitors promote glioma cell death by G2 checkpoint abrogation leading to mitotic catastrophe. Cell Death Dis. 2014;5:e1435. doi: 10.1038/cddis.2014.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Witt Hamer PC, Mir SE, Noske D, Van Noorden CJ, Würdinger T. WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res. 2011;17:4200–4207. doi: 10.1158/1078-0432.CCR-10-2537. [DOI] [PubMed] [Google Scholar]
- 33.Aarts M, Sharpe R, Garcia-Murillas I, Gevensleben H, Hurd MS, Shumway SD, Toniatti C, Ashworth A, Turner NC. Forced mitotic entry of S-phase cells as a therapeutic strategy induced by inhibition of WEE1. Cancer Discov. 2012;2:524–539. doi: 10.1158/2159-8290.CD-11-0320. [DOI] [PubMed] [Google Scholar]
- 34.Heale JT, Ball AR, Jr, Schmiesing JA, Kim JS, Kong X, Zhou S, Hudson DF, Earnshaw WC, Yokomori K. Condensin I interacts with the PARP-1-XRCC1 complex and functions in DNA single-strand break repair. Mol Cell. 2006;21:837–848. doi: 10.1016/j.molcel.2006.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bharti AC, Takada Y, Aggarwal BB. PARP cleavage and caspase activity to assess chemosensitivity. Methods Mol Med. 2005;111:69–78. doi: 10.1385/1-59259-889-7:069. [DOI] [PubMed] [Google Scholar]
- 36.Cruet-Hennequart S, Prendergast ÁM, Shaw G, Barry FP, Carty MP. Doxorubicin induces the DNA damage response in cultured human mesenchymal stem cells. Int J Hematol. 2012;96:649–656. doi: 10.1007/s12185-012-1196-5. [DOI] [PubMed] [Google Scholar]
- 37.Kreahling JM, Foroutan P, Reed D, Martinez G, Razabdouski T, Bui MM, Raghavan M, Letson D, Gillies RJ, Altiok S. Wee1 inhibition by MK-1775 leads to tumor inhibition and enhances efficacy of gemcitabine in human sarcomas. PLoS One. 2013;8:e57523. doi: 10.1371/journal.pone.0057523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreahling JM, Gemmer JY, Reed D, Letson D, Bui M, Altiok S. MK1775, a selective Wee1 inhibitor, shows single-agent antitumor activity against sarcoma cells. Mol Cancer Ther. 2012;11:174–182. doi: 10.1158/1535-7163.MCT-11-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mir SE, De Witt Hamer PC, Krawczyk PM, Balaj L, Claes A, Niers JM, Van Tilborg AA, Zwinderman AH, Geerts D, Kaspers GJ, et al. In silico analysis of kinase expression identifies WEE1 as a gatekeeper against mitotic catastrophe in glioblastoma. Cancer Cell. 2010;18:244–257. doi: 10.1016/j.ccr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Posthuma DeBoer J, Würdinger T, Graat HC, van Beusechem VW, Helder MN, van Royen BJ, Kaspers GJ. WEE1 inhibition sensitizes osteosarcoma to radiotherapy. BMC Cancer. 2011;11:156. doi: 10.1186/1471-2407-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nwabo Kamdje AH, Krampera M. Notch signaling in acute lymphoblastic leukemia: Any role for stromal microenvironment? Blood. 2011;118:6506–6514. doi: 10.1182/blood-2011-08-376061. [DOI] [PubMed] [Google Scholar]
- 42.Penton AL, Leonard LD, Spinner NB. Notch signaling in human development and disease. Semin Cell Dev Biol. 2012;23:450–457. doi: 10.1016/j.semcdb.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weng AP, Ferrando AA, Lee W, Morris JP, IV, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 44.Yin C, Ye J, Zou J, Lu T, Du Y, Liu Z, Fan R, Lu F, Li P, Ma D, et al. Role of stromal cells-mediated Notch-1 in the invasion of T-ALL cells. Exp Cell Res. 2015;332:39–46. doi: 10.1016/j.yexcr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Herranz D, Ambesi-Impiombato A, Palomero T, Schnell SA, Belver L, Wendorff AA, Xu L, Castillo-Martin M, Llobet-Navás D, Cordon-Cardo C, et al. A NOTCH1-driven MYC enhancer promotes T cell development, transformation and acute lymphoblastic leukemia. Nat Med. 2014;20:1130–1137. doi: 10.1038/nm.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou J, Li P, Lu F, Liu N, Dai J, Ye J, Qu X, Sun X, Ma D, Park J, et al. Notch1 is required for hypoxia-induced proliferation, invasion and chemoresistance of T-cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2013;6:3. doi: 10.1186/1756-8722-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nwabo Kamdje AH, Mosna F, Bifari F, Lisi V, Bassi G, Malpeli G, Ricciardi M, Perbellini O, Scupoli MT, Pizzolo G, et al. Notch-3 and Notch-4 signaling rescue from apoptosis human B-ALL cells in contact with human bone marrow-derived mesenchymal stromal cells. Blood. 2011;118:380–389. doi: 10.1182/blood-2010-12-326694. [DOI] [PubMed] [Google Scholar]
- 48.Itoh M, Fu L, Tohda S. NF-kappaB activation induced by Notch ligand stimulation in acute myeloid leukemia cells. Oncol Rep. 2009;22:631–634. doi: 10.3892/or_00000482. [DOI] [PubMed] [Google Scholar]
- 49.Baldoni S, Sportoletti P, Del Papa B, Aureli P, Dorillo E, Rosati E, Ciurnelli R, Marconi P, Falzetti F, Di Ianni M. NOTCH and NF-κB interplay in chronic lymphocytic leukemia is independent of genetic lesion. Int J Hematol. 2013;98:153–157. doi: 10.1007/s12185-013-1368-y. [DOI] [PubMed] [Google Scholar]
- 50.Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ. Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood. 2007;110:278–286. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Z, Li Y, Banerjee S, Kong D, Ahmad A, Nogueira V, Hay N, Sarkar FH. Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion and induces apoptosis via inactivation of Akt, mTOR and NF-kappaB signaling pathways. J Cell Biochem. 2010;109:726–736. doi: 10.1002/jcb.22451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated and analyzed in the present study are included in this published article.