Abstract
Objectives
To examine the associations between serum magnesium (Mg) concentration with the prevalence of metabolic syndrome (MetS), diabetes mellitus (DM), hypertension (HP) and hyperuricaemia (HU) in patients with radiographic knee osteoarthritis (OA).
Methods
The present study was conducted at the Health Management Center of Xiangya Hospital. Radiographic OA was evaluated for patients aged over 40 years with basic characteristics and blood biochemical assessment. Serum Mg concentration was measured using the chemiluminescence method. MetS, DM, HP and HU were diagnosed based on standard protocols. The associations between serum Mg concentration with MetS, DM, HP and HU were evaluated by conducting multivariable adjusted logistic regression.
Results
A total of 962 patients with radiographic knee OA were included. Compared with the lowest quintile, the multivariable adjusted ORs and related 95% CIs of DM were 0.40 (95% CI 0.23 to 0.70, p=0.001), 0.33 (95% CI 0.18 to 0.60, p<0.001), 0.27 (95% CI 0.14 to 0.52, p<0.001) and 0.22 (95% CI 0.11 to 0.44, p<0.001) in the second, third, fourth and highest quintiles of serum Mg, respectively (p for trend <0.001); the multivariable adjusted ORs of HU were 0.33 (95% CI 0.19 to 0.59, p<0.001), 0.52 (95% CI 0.30 to 0.91, p=0.022) and 0.39 (95% CI 0.22 to 0.70, p=0.001) in the third, fourth and highest quintiles of serum Mg, respectively (p for trend <0.001); and the multivariable adjusted ORs of MetS were 0.59 (95% CI 0.36 to 0.94, p=0.027) in the second and 0.56 (95% CI 0.34 to 0.93, p=0.024) in the highest quintiles of serum Mg. However, the inverse association between serum Mg and the prevalence of MetS was non-linear (p for trend=0.067). There was no significant association between serum Mg and HP in patients with OA.
Conclusions
The serum Mg concentration was inversely associated with the prevalence of MetS, DM and HU in patients with radiographic knee OA.
Level of evidence
Level III, cross-sectional study.
Keywords: osteoarthritis, magnesium, metabolic syndrome, diabetes, hypertension, hyperuricemia
Strengths and limitations of this study.
This is the first study examining the associations between serum magnesium (Mg) and the prevalence of metabolic syndrome, diabetes mellitus, hypertension and hyperuricaemia in patients with radiographic knee osteoarthritis.
The multivariable logistical regression models in this study were adjusted for a considerable number of potential confounding factors, which greatly improved the reliability of the results.
The kidney is the key organ in maintaining Mg homoeostasis. This study conducted a sensitivity analysis by adding estimated glomerular filtration rate into the multivariable logistic regression models, and the reverse associations remained significant.
This study adopted cross-sectional design, which precluded causal correlations.
Serum Mg concentration was adopted as the indicator of body Mg content in this study, which may not be the best indicator of body status.
Introduction
The association between osteoarthritis (OA) and metabolic diseases, especially metabolic syndrome (MetS)1 2 and diabetes mellitus (DM),3–5 has drawn increasing attention in the past few years. OA includes three specific phenotypes: metabolic OA, age-related OA and injury-related OA.6 A large number of studies have indicated that the prevalence of MetS,7–9 DM10–18 and hypertension (HP)7 9–13 19 20 is either higher in patients with OA or associated with OA. In addition, some other studies reported that MetS,21 22 DM23 24 and HP21 22 are risk factors of OA progression. Thus, it appears necessary to pay more attention and adopt appropriate measures to reduce the high prevalence of metabolic diseases in patients with OA, which also seems to be beneficial in delaying OA progression.
Serum magnesium (Mg), one of the most important micronutrients for human health, has been reported to be negatively associated with MetS,25–29 DM30–38 and HP30 39–41 by lots of studies. Meanwhile, our previous study showed an inverse association between serum Mg and hyperuricaemia (HU).42 However, to the best knowledge of the authors, there is not yet a study examining the association between the serum Mg concentration and the aforementioned metabolic diseases (MetS, DM, HP and HU) in patients with OA. On the other hand, we have previously shown that the serum Mg concentration may be inversely associated with radiographic knee OA.43 Therefore, we speculate that the prevalence of MetS, DM, HP and HU in patients with OA may be reduced by elevating the level of serum Mg, which can in turn delay OA progression. Thus, the objective of the present study was to examine the associations between the serum Mg concentration with the prevalence of MetS, DM, HP and HU in patients with radiographic knee OA. It was hypothesised that serum Mg concentration was inversely associated with these diseases.
Methods
Study population
The present study was conducted at the Health Management Center of Xiangya Hospital between October 2013 and November 2014. The study design has been published previously.42–46 Registered nurses were engaged to interview all participants during the examination using a standard questionnaire, with the purpose to collect information on demographic characteristics and health-related habits. Participants were selected based on the following inclusion criteria: (1) 40 years old or above; (2) undergoing weight-bearing bilateral anteroposterior radiography of the knee, and diagnosed with knee OA according to the Kellgren-Lawrence (K-L) radiographic atlas (knee joint was graded K-L 2 or above); (3) availability of all basic characteristics, including age, gender, Body Mass Index (BMI) and blood pressure; (4) availability of biochemical test results, including serum Mg concentration; (5) availability of information related to the living habits, including education background, activity level, smoking, drinking and medication status. Initially, the present cross-sectional study retrieved 1820 patients with radiographic knee OA aged over 40 years who exhibited sound basic characteristics and required blood biochemical assessment (including serum Mg concentration). Among them, 962 patients offered demographic characteristics and health-related habits and were finally included in this study.
Blood biochemistry
All blood samples were drawn after a 12-hour overnight fast and were kept at 4°C until analysis. Blood tests were undertaken using the Beckman Coulter AU 5800 (Beckman Coulter, Brea, California, USA). The inter-assay and intra-assay coefficients of variation were tested at both low concentrations (2.5 mmol/L for glucose, 118 μmol/L for uric acid and 0.60 mmol/L for serum Mg) and high concentrations (6.7 mmol/L for glucose, 472 μmol/L for uric acid and 1.00 mmol/L for serum Mg) of standard human samples. The intra-assay coefficients of variation were 0.98% (2.5 mmol/L) and 1.72% (6.7 mmol/L) for glucose, 1.39% (118 μmol/L) and 0.41% (472 μmol/L) for uric acid, and 1.86% (0.60 mmol/L) and 1.65% (1.00 mmol/L) for serum Mg, respectively. The inter-assay coefficients of variation were 2.45% (2.5 mmol/L) and 1.46% (6.7 mmol/L) for glucose, 1.40% (118 μmol/L) and 1.23% (472 μmol/L) for uric acid, and 1.87% (0.60 mmol/L) and 1.70% (1.00 mmol/L) for serum Mg, respectively.
Assessment of other exposures
Blood pressure was measured by an electronic sphygmomanometer. The weight and height of each subject were measured respectively to calculate the BMI. Information on the average frequency of physical activity (never, one to two times per week, three to four times per week, five times and above per week) and average duration of physical activity (less than half an hour, half an hour to 1 hour, 1 to 2 hours, more than 2 hours) were collected through survey questionnaire. The smoking, alcohol drinking and medication status were collected during the face-to-face interview.
Assessment of MetS, DM, HP and HU
MetS was diagnosed based on the Chinese Diabetes Society criteria,47–49 which requires meeting at least three of the following four items: (1) BMI ≥25 kg/m2; (2) fasting plasma glucose ≥6.1 mmol/L or diagnosed DM; (3) systolic blood pressure (BP) ≥140 mm Hg or diastolic BP ≥90 mm Hg, or treatment of previously diagnosed HP; (4) triglycerides ≥1.7 mmol/L and/or high-density lipoprotein (HDL) cholesterol <0.9 mmol/L in men or <1.0 mmol/L in women, or treatment for this lipid abnormality. Subjects with fasting glucose ≥7.0 mmol/L or currently undergoing drug treatment for blood glucose control were regarded as patients with DM, and subjects with systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg or currently undertaking antihypertensive medication were regarded as patients with HP. HU was defined as uric acid ≥416 μmol/L for men and ≥360 μmol/L for women or currently undergoing drug treatment for uric acid control.
Statistical analysis
The continuous data were expressed as mean with SD, and the category data were expressed in percentage. Differences in continuous data were evaluated by one-way classification analysis of variance (normally distributed data) or Kruskal-Wallis H test (non-normally distributed data), while differences in category data were assessed by the χ2 test. The serum Mg was classified into five categories based on the quintile distribution: ≤0.85, 0.86–0.89, 0.90–0.92, 0.93–0.96 and ≥0.97 mmol/L. The prevalence of MetS, DM, HP and HU in each quintile of serum Mg in patients with OA was assessed by scatter plots.
Logistic regression was conducted to calculate the ORs with 95% CIs for the associations between serum Mg and MetS, DM, HP and HU. Specifically, model 1 was adjusted by covariates of age (continuous data) and gender (male, female). Then, model 2 was adjusted by additional covariates of BMI (continuous data), educational level (high school or above, lower than high school), smoking status (yes, no), activity level (continuous data), alcohol drinking status (yes, no), HP (yes, no), DM (yes, no) and dyslipidemia (yes, no) on the basis of model 1. Dyslipidemia was defined as triglycerides ≥1.7 mmol/L and/or HDL cholesterol <0.9 mmol/L in men or <1.0 mmol/L in women, or treatment for this lipid abnormality. Notably, the selection of covariates in model 2 varied slightly for examining different associations (between serum Mg and MetS, DM, HP or HU). For example, BMI, HP and dyslipidemia were adjusted for the association between serum Mg and DM, but not for the association between serum Mg and MetS, simply because MetS was diagnosed based on BMI, HP and dyslipidemia status. Model 3 was established based on model 2, with adjustment of an additional covariate, estimated glomerular filtration rate (eGFR). eGFR (continuous data) was calculated from the Chronic Kidney Disease Epidemiology Collaboration equation.50 All covariates in the present study were chosen referring to some of the previous similar studies.27 33 51 52 Tests for linear trends were conducted based on logistic regression using a median variable of Mg concentration in each category.
Scatter plots were plotted using R V.3.4.4.53 Other data analyses were performed using SPSS V.17.0; p value ≤0.05 was considered to be statistically significant. All tests were two tailed.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in the design or implementation of the study. There were no plans to disseminate the results of the research to study participants.
Results
A total of 962 subjects (377 women, accounting for 39.2%) were included in the present cross-sectional study. The characteristics of the study population according to quintiles of serum Mg are presented in table 1. The mean age of the subjects was 54.9±7.6 years. The overall prevalence of MetS, DM, HP and HU in patients with OA were 21.4%, 12.0%, 38.5% and 18.3%, respectively. Significant differences were observed across the quintiles of serum Mg for fasting glucose, as well as the prevalence of DM and HU.
Table 1.
Basic characteristics of included subjects according to quintiles of serum Mg (n=962)
| Quintiles of serum Mg | P values | |||||
| Q1 (lowest) | Q2 | Q3 | Q4 | Q5 (highest) | ||
| Median Mg concentration (mmol/L) | 0.82 | 0.87 | 0.91 | 0.94 | 0.99 | – |
| Participants (n) | 200 | 215 | 190 | 168 | 189 | – |
| Age (years) | 53.8 (7.3) | 54.6 (7.6) | 55.2 (7.9) | 55.3 (7.1) | 56.1 (8.0) | 0.062 |
| BMI (kg/m2) | 25.2 (3.2) | 24.9 (3.2) | 25.0 (3.7) | 25.2 (3.4) | 24.6 (3.2) | 0.464 |
| Female (%) | 37.5 | 42.3 | 36.8 | 42.3 | 37.0 | 0.627 |
| Smoking (%) | 27.5 | 27.4 | 21.6 | 24.4 | 21.7 | 0.457 |
| Alcohol drinking (%) | 34.5 | 36.3 | 40.5 | 41.1 | 38.1 | 0.645 |
| High school diploma (%) | 45.0 | 47.4 | 45.3 | 56.5 | 48.1 | 0.184 |
| Activity level (hour/week) | 2.0 (3.5) | 2.0 (3.3) | 2.3 (3.5) | 2.1 (3.1) | 2.4 (3.5) | 0.457 |
| Fasting glucose (mmol/L) | 6.6 (3.0) | 5.7 (1.7) | 5.7 (1.4) | 5.5 (0.9) | 5.5 (1.6) | 0.009 |
| Systolic pressure (mm Hg) | 129.2 (16.9) | 128.3 (17.9) | 130.4 (16.2) | 128.8 (16.3) | 129.6 (17.7) | 0.837 |
| Diastolic pressure (mm Hg) | 81.2 (11.8) | 79.8 (12.1) | 80.7 (11.0) | 80.7 (10.7) | 80.3 (10.5) | 0.654 |
| HDL cholesterol (mmol/L) | 1.5 (0.4) | 1.5 (0.4) | 1.5 (0.4) | 1.5 (0.3) | 1.5 (0.4) | 0.374 |
| Triglyceride (mmol/L) | 2.1 (1.9) | 1.8 (1.5) | 2.0 (2.1) | 1.8 (1.0) | 2.3 (2.9) | 0.620 |
| Uric acid (μmol/L) | 337.3 (101.7) | 329.0 (80.7) | 321.3 (86.3) | 331.5 (78.0) | 329.4 (81.7) | 0.590 |
| eGFR (mL/min/1.73 m2) | 80.2 (14.4) | 77.7 (10.7) | 76.0 (10.6) | 75.8 (10.7) | 74.3 (12.0) | <0.001 |
| MetS (%) | 26.5 | 17.7 | 25.8 | 19.6 | 17.5 | 0.059 |
| DM (%) | 23.5 | 10.7 | 10.0 | 8.3 | 6.3 | <0.001 |
| HP (%) | 40.0 | 33.5 | 37.4 | 42.3 | 40.2 | 0.432 |
| HU (%) | 25.5 | 19.1 | 13.2 | 18.5 | 14.8 | 0.018 |
Data are mean (SD), unless otherwise indicated. P values are for test of difference across all quintiles of serum Mg.
BMI, Body Mass Index; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HP, hypertension; HU, hyperuricaemia; MetS, metabolic syndrome; Mg, magnesium.
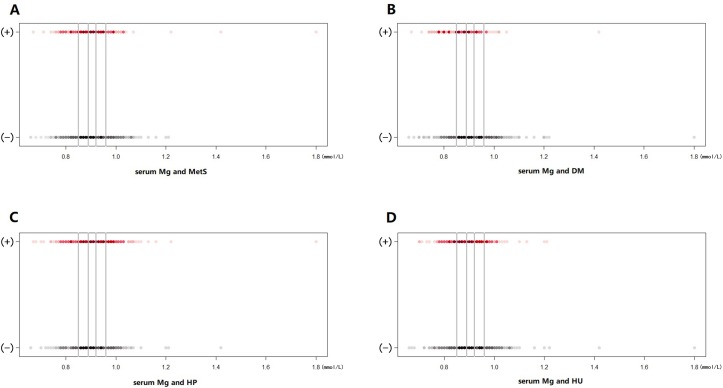
The prevalence of MetS in each quintile of serum Mg in patients with OA is shown in figure 1A. The outcomes of multivariable adjusted associations between MetS and serum Mg concentration are shown in table 2. Compared with the lowest quintile, the age–gender adjusted ORs (model 1) suggested significant decreased prevalence of MetS in the second (OR 0.61, 95% CI 0.38 to 0.97, p=0.038) and the highest (OR 0.59, 95% CI 0.36 to 0.96, p=0.035) quintiles of serum Mg; the multivariable adjusted ORs (model 2) also suggested significant decreased prevalence of MetS in the second (OR 0.60, 95% CI 0.37 to 0.96, p=0.035) and the highest (OR 0.61, 95% CI 0.37 to 0.99, p=0.047) quintiles. The sensitivity analysis, by adding eGFR into model 2, also reached similar results—significant lower prevalence of MetS in the second (OR 0.59, 95% CI 0.36 to 0.94, p=0.027) and the highest quintiles (OR 0.56, 95% CI 0.34 to 0.93, p=0.024) compared with the reference quintile of serum Mg. No clear trend was evident in the third and fourth quintiles of serum Mg. The p values for trend were 0.090 (model 1), 0.120 (model 2) and 0.067 (model 3), respectively.
Figure 1.
Prevalence of metabolic syndrome (MetS) (A), diabetes mellitus (DM) (B), hypertension (HP) (C) and hyperuricaemia (HU) (D) in each quintile of serum Mg in patients with radiographic knee OA. The figures above present the prevalence of MetS (A), DM (B), HP (C) and HU (D) among the 962 patients with OA under different quintiles of serum Mg levels. The horizontal axis denotes the serum Mg level, and the vertical axis indicates whether a subject is diagnosed with the specific disease: (+), disease; (−), no disease. The solid grey lines represent the boundaries in between the five quintiles of serum Mg levels. The red and black spots represent the prevalence of diseases and no diseases at each serum Mg level, respectively. The darker the colour of a spot, the more patients with OA there are at the corresponding concentration.
Table 2.
Multivariable adjusted relations of serum Mg and MetS in patients with OA (n=962)
| Quintiles of serum Mg | P for trend | |||||
| Q1 (lowest) | Q2 | Q3 | Q4 | Q5 (highest) | ||
| Median Mg concentration (mmol/L) | 0.82 | 0.87 | 0.91 | 0.94 | 0.99 | – |
| Participants (n) | 200 | 215 | 190 | 168 | 189 | – |
| MetS (%) | 26.5 | 17.7 | 25.8 | 19.6 | 17.5 | – |
| Model 1* | 1.00 (reference) | 0.61 (0.38 to 0.97) | 0.97 (0.61 to 1.52) | 0.69 (0.42 to 1.14) | 0.59 (0.36 to 0.96) | 0.090 |
| P values | – | 0.038 | 0.881 | 0.150 | 0.035 | – |
| Model 2* | 1.00 (reference) | 0.60 (0.37 to 0.96) | 1.00 (0.63 to 1.57) | 0.70 (0.42 to 1.15) | 0.61 (0.37 to 0.99) | 0.120 |
| P values | – | 0.035 | 0.985 | 0.160 | 0.047 | – |
| Model 3* | 1.00 (reference) | 0.59 (0.36 to 0.94) | 0.95 (0.60 to 1.51) | 0.67 (0.40 to 1.10) | 0.56 (0.34 to 0.93) | 0.067 |
| P values | – | 0.027 | 0.830 | 0.114 | 0.024 | – |
Data are adjusted OR (95% CI), unless otherwise indicated.
*Model 1 was adjusted for age (continuous data) and gender (male, female); model 2 was adjusted for age (continuous data), gender (male, female), educational level (high school or above, lower than high school), smoking status (yes, no), activity level (continuous data) and alcohol drinking status (yes, no); model 3 was adjusted based on model 2, with additional factor of estimated glomerular filtration rate (continuous data).
MetS, metabolic syndrome; Mg, magnesium; n, number; OA, osteoarthritis.
Figure 1B shows the prevalence of DM in each category of serum Mg in patients with OA. Table 3 illustrates the multivariable adjusted relations between serum Mg and DM in patients with OA. Both the age–gender adjusted OR values (model 1) and the multivariable adjusted OR values (model 2) suggested a strong inverse association between serum Mg and DM. The age–gender adjusted ORs for the prevalence of DM were 0.38 (95% CI 0.22 to 0.66, p=0.001), 0.34 (95% CI 0.19 to 0.61, p<0.001), 0.29 (95% CI 0.15 to 0.55, p<0.001) and 0.20 (95% CI 0.10 to 0.40, p<0.001) in the second, third, fourth and fifth quintiles of serum Mg, respectively, and the p value for trend was <0.001. The multivariable adjusted ORs for the prevalence of DM were 0.40 (95% CI 0.23 to 0.70, p=0.001), 0.32 (95% CI 0.18 to 0.59, p<0.001), 0.26 (95% CI 0.13 to 0.50, p<0.001) and 0.21 (95% CI 0.11 to 0.42, p<0.001) in the second, third, fourth and fifth quintiles of serum Mg, respectively, and the p value for trend was <0.001. The sensitivity analysis, by adding eGFR into model 2, showed similar results—significant lower prevalence of DM in the second (OR 0.40, 95% CI 0.23 to 0.70, p=0.001), third (OR 0.33, 95% CI 0.18 to 0.60, p<0.001), fourth (OR 0.27, 95% CI 0.14 to 0.52, p<0.001) and highest quintiles (OR 0.22, 95% CI 0.11 to 0.44, p<0.001) compared with the reference quintile of serum Mg, and the p value for trend was <0.001.
Table 3.
Multivariable adjusted relations of serum Mg and DM in patients with OA (n=962)
| Quintiles of serum Mg | P for trend | |||||
| Q1 (lowest) | Q2 | Q3 | Q4 | Q5 (highest) | ||
| Median Mg concentration (mmol/L) | 0.82 | 0.87 | 0.91 | 0.94 | 0.99 | – |
| Participants (n) | 200 | 215 | 190 | 168 | 189 | – |
| DM (%) | 23.5 | 10.7 | 10.0 | 8.3 | 6.3 | – |
| Model 1* | 1.00 (reference) | 0.38 (0.22 to 0.66) | 0.34 (0.19 to 0.61) | 0.29 (0.15 to 0.55) | 0.20 (0.10 to 0.40) | <0.001 |
| P values | – | 0.001 | <0.001 | <0.001 | <0.001 | – |
| Model 2* | 1.00 (reference) | 0.40 (0.23 to 0.70) | 0.32 (0.18 to 0.59) | 0.26 (0.13 to 0.50) | 0.21 (0.11 to 0.42) | <0.001 |
| P values | – | 0.001 | <0.001 | <0.001 | <0.001 | – |
| Model 3* | 1.00 (reference) | 0.40 (0.23 to 0.70) | 0.33 (0.18 to 0.60) | 0.27 (0.14 to 0.52) | 0.22 (0.11 to 0.44) | <0.001 |
| P values | – | 0.001 | <0.001 | <0.001 | <0.001 | – |
Data are adjusted OR (95% CI), unless otherwise indicated.
*Model 1 was adjusted for age (continuous data) and gender (male, female); model 2 was adjusted for age (continuous data), Body Mass Index (continuous data), gender (male, female), educational level (high school or above, lower than high school), smoking status (yes, no), activity level (continuous data), alcohol drinking status (yes, no), hypertension (yes, no) and dyslipidemia (yes, no); model 3 was adjusted based on model 2, with additional factor of estimated glomerular filtration rate (continuous data).
DM, diabetes mellitus; Mg, magnesium; n, number; OA, osteoarthritis.
The prevalence of HP in each quintile of serum Mg in patients with OA is depicted in figure 1C. The multivariable adjusted relations between serum Mg and HP in patients with OA are illustrated in table 4. According to both the age–gender adjusted ORs (model 1) and the multivariable adjusted ORs (model 2), there was no significant association between serum Mg and HP, and the p values for trend were 0.929 and 0.377, respectively. The sensitivity analysis, by adding eGFR into model 2, reached the same results.
Table 4.
Multivariable adjusted relations of serum Mg and HP in patients with OA (n=962)
| Quintiles of serum Mg | P for trend | |||||
| Q1 (lowest) | Q2 | Q3 | Q4 | Q5 (highest) | ||
| Median Mg concentration (mmol/L) | 0.82 | 0.87 | 0.91 | 0.94 | 0.99 | – |
| Participants (n) | 200 | 215 | 190 | 168 | 189 | – |
| HP (%) | 40.0 | 33.5 | 37.4 | 42.3 | 40.2 | – |
| Model 1* | 1.00 (reference) | 0.71 (0.47 to 1.06) | 0.83 (0.54 to 1.25) | 1.00 (0.66 to 1.54) | 0.89 (0.59 to 1.35) | 0.929 |
| P values | – | 0.095 | 0.368 | 0.987 | 0.582 | – |
| Model 2* | 1.00 (reference) | 0.77 (0.50 to 1.19) | 0.89 (0.57 to 1.39) | 1.10 (0.70 to 1.74) | 1.08 (0.69 to 1.68) | 0.377 |
| P values | – | 0.245 | 0.608 | 0.686 | 0.744 | – |
| Model 3* | 1.00 (reference) | 0.77 (0.50 to 1.19) | 0.88 (0.56 to 1.38) | 1.09 (0.68 to 1.72) | 1.05 (0.67 to 1.65) | 0.434 |
| P values | – | 0.235 | 0.574 | 0.727 | 0.818 | – |
Data are adjusted OR (95% CI), unless otherwise indicated.
*Model 1 was adjusted for age (continuous data) and gender (male, female); model 2 was adjusted for age (continuous data), Body Mass Index (continuous data), gender (male, female), educational level (high school or above, lower than high school), smoking status (yes, no), activity level (continuous data), alcohol drinking status (yes, no), diabetes (yes, no) and dyslipidemia (yes, no); model 3 was adjusted based on model 2, with additional factor of estimated glomerular filtration rate (continuous data).
HP, hypertension; Mg, magnesium; n, number; OA, osteoarthritis.
The prevalence of HU in each category of serum Mg in patients with OA is shown in figure 1D. The multivariable adjusted relations between serum Mg and HU in patients with OA are illustrated in table 5. Both the age–gender adjusted OR values (model 1) and the multivariable adjusted OR values (model 2) suggested significant decreased prevalence of HU in the third quintile (age–gender adjusted OR 0.44, 95% CI 0.26 to 0.75, p=0.002; multivariable adjusted OR 0.38, 95% CI 0.22 to 0.67, p=0.001) and fifth quintile (age–gender adjusted OR 0.51, 95% CI 0.30 to 0.85, p=0.010; multivariable adjusted OR 0.50, 95% CI 0.29 to 0.87, p=0.013) compared with the lowest quintile of serum Mg, and the p values for trend were 0.008 and 0.006, respectively. The sensitivity analysis, by adding eGFR into model 2, showed similar outcomes—significant lower prevalence of HU in the third (OR 0.33, 95% CI 0.19 to 0.59, p<0.001), fourth (OR 0.52, 95% CI 0.30 to 0.91, p=0.022) and highest quintiles (OR 0.39, 95% CI 0.22 to 0.70, p=0.001) compared with the reference quintile of serum Mg, and the p value for trend was <0.001.
Table 5.
Multivariable adjusted relations of serum Mg and HU in patients with OA (n=962)
| Quintiles of serum Mg | P for trend | |||||
| Q1 (lowest) | Q2 | Q3 | Q4 | Q5 (highest) | ||
| Median Mg concentration (mmol/L) | 0.82 | 0.87 | 0.91 | 0.94 | 0.99 | – |
| Participants (n) | 200 | 215 | 190 | 168 | 189 | – |
| HU (%) | 25.5 | 19.1 | 13.2 | 18.5 | 14.8 | – |
| Model 1* | 1.00 (reference) | 0.71 (0.44 to 1.14) | 0.44 (0.26 to 0.75) | 0.68 (0.41 to 1.14) | 0.51 (0.30 to 0.85) | 0.008 |
| P values | – | 0.157 | 0.002 | 0.144 | 0.010 | – |
| Model 2* | 1.00 (reference) | 0.73 (0.45 to 1.20) | 0.38 (0.22 to 0.67) | 0.59 (0.35 to 1.02) | 0.50 (0.29 to 0.87) | 0.006 |
| P values | – | 0.210 | 0.001 | 0.058 | 0.013 | – |
| Model 3* | 1.00 (reference) | 0.68 (0.41 to 1.14) | 0.33 (0.19 to 0.59) | 0.52 (0.30 to 0.91) | 0.39 (0.22 to 0.70) | <0.001 |
| P values | – | 0.142 | <0.001 | 0.022 | 0.001 | – |
Data are adjusted OR (95% CI), unless otherwise indicated.
*Model 1 was adjusted for age (continuous data) and gender (male, female); model 2 was adjusted for age (continuous data), Body Mass Index (continuous data), gender (male, female), educational level (high school or above, lower than high school), smoking status (yes, no), activity level (continuous data), alcohol drinking status (yes, no), hypertension (yes, no), diabetes (yes, no) and dyslipidemia (yes, no); model 3 was adjusted based on model 2, with additional factor of estimated glomerular filtration rate (continuous data).
HU, hyperuricaemia; Mg, magnesium; n, number; OA, osteoarthritis.
Discussion
The results of this study suggested that the serum Mg concentration was negatively associated with the prevalence of MetS, DM and HU in subjects with radiographic knee OA. To control potential confounders, several covariates including characteristics, living habits and underlying diseases were selected, and even the eGFR was added into the multivariable logistic regression models to eliminate the influence of renal function on Mg excretion. The reverse associations mentioned above remained significant after adjustments of these confounders. However, the association between serum Mg and the prevalence of MetS was non-linear, with no clear trend in the third and fourth quintiles of serum Mg. Moreover, the negative association between serum Mg and the prevalence of HP was not observed in patients with radiographic knee OA.
Mg, the fourth most abundant cation in the human body and the second most profuse intracellular cation, is a metallic cofactor for over 300 enzymatic reactions. It appears to play an important role in glucose metabolism and insulin homoeostasis, which are both highly correlated with metabolic diseases, especially MetS and DM. The mechanisms involved in Mg deficiency in patients with MetS, DM and HU are probably multifactorial. The most important factor may be insulin resistance, as Mg is essential for insulin action and is a critical cofactor for several enzymes in carbohydrate metabolism, which is important for the phosphorylation reactions of tyrosine kinase in the insulin receptor.31 54–58 Of course, it is necessary to highlight the fact that insulin can also induce Mg excretion59 and produce a significant decline of plasma Mg through ion exchange.60 Thus, there seems to be a vicious circle between Mg deficiency and insulin resistance.
Other potential mechanisms include glucose transportation,57 oxidative stress57 and inflammatory cytokines,61–63 and cellular calcium homoeostasis.55 Mg is an essential cofactor of the high-energy phosphate-bound enzymatic pathways involved in the modulation of glucose transport across cell membranes.57 It also plays a role in the mechanisms of cellular antioxidant defence.64 The oxidative stress, defined as a persistent imbalance between the excessive production of reactive oxygen species and/or defects in antioxidant defence, has been implicated in the pathogenesis of diabetic complications.57 Moreover, low serum Mg levels are strongly related to elevated serum concentrations of both tumour necrosis factor alpha and C reactive protein (CRP),65 suggesting that Mg deficiency may contribute to the development of low-grade chronic inflammation syndrome and the development of glucose metabolic disorders through the former pathway. In addition, lower Mg concentration can enhance calcium-mediated vasoconstriction, blunt cardiac and smooth muscle relaxation, and thus contribute to BP elevation.55 However, the decreased serum calcium concentration in patients with radiographic knee OA may weaken the association between Mg and HP.66
MetS21 22 and DM4 23 24 were reported to be the risk factors of OA progression. Moreover, serum Mg level has been proven to be significantly associated with CRP concentration,27 67–69 and higher CRP might serve as a prediction factor for OA progression.70 71 Thus, OA progression may be delayed by elevating the serum Mg level through reducing the prevalence of MetS and DM and decreasing the level of CRP. Above all, the present study indicated that the elevation of serum Mg level has the potential to reduce the prevalence of MetS, DM and HU in patients with knee OA and may delay the progression of knee OA. However, the specific mechanism needs to be further explored.
The present study has several strengths. First, this is the first study examining the associations between serum Mg and the prevalence of MetS, DM, HP and HU in patients with radiographic knee OA. The results of this study will provide a new insight into the treatment of knee OA. Second, the multivariable logistical regression models were adjusted for a considerable number of potential confounding factors, which greatly improved the reliability of the results. Third, the kidney is the key organ in maintaining Mg homoeostasis. This study conducted a sensitivity analysis by adding eGFR into multivariable logistic regression models, which showed that the reverse associations remained significant.
Limitations of the present study should also be admitted. The cross-sectional design precludes causal correlations, so further prospective studies and intervention trials should be undertaken to establish a causal association between serum Mg with the prevalence of MetS, DM, HP and HU in patients with radiographic knee OA. Since no previous research investigated such associations in patients with knee OA, the value of this study should not be blotted out by the cross-sectional nature. Another limitation of this study lies in the relatively small sample size, and thus, extensive high-quality researches based on a larger sample are needed. Moreover, the dietary intake of Mg in relation to the prevalence of MetS, DM, HP and HU was not assessed in the present study. Last but not the least, it is important to highlight that Mg is an intracellular ion; therefore, the serum Mg concentration must be considered as a poor indicator of body Mg content72 even though it has been used in many studies. However, blood Mg level is the second best indicator of body status.73
Conclusions
The present study concluded that the serum Mg concentration was inversely associated with the prevalence of MetS, DM and HU in patients with radiographic knee OA.
Supplementary Material
Footnotes
Contributors: All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. GL, YW and JW conceived the study. GL, YW and JW were responsible for conception and design of the study and drafted the manuscript. CZ, TY, HL, YC and DX contributed to data collection. JW contributed to preparation and data analysis. BX, ZL, JL and SJ contributed to study retrieval. GL and YW contributed to revision of the manuscript. All the authors contributed to the interpretation of the data and critically reviewed the manuscript for publication.
Funding: This work was supported by the Innovation Foundation of the Central South University for Postgraduate (2018zzts045), the Postdoctoral Science Foundation of Central South University (182130), the National Natural Science Foundation of China (nos. 81201420, 81272034, 81472130, 81501923), the Provincial Science Foundation of Hunan (no. 14JJ3032), the Scientific Research Project of the Development and Reform Commission of Hunan Province ((2013)1199), the Scientific Research Project of Science and Technology Office of Hunan Province (2013SK2018) and the Doctoral Scientific Fund Project of the Ministry of Education of China (20120162110036).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The protocol has been reviewed and approved by the Ethics Committee of Xiangya Hospital, Central South University (reference no. 201312459), and the methods were developed in accordance with the approved guidelines.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data sets during the current study are available from the corresponding author on reasonable request.
References
- 1. Zhuo Q, Yang W, Chen J, et al. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol 2012;8:729–37. 10.1038/nrrheum.2012.135 [DOI] [PubMed] [Google Scholar]
- 2. Katz JD, Agrawal S, Velasquez M. Getting to the heart of the matter: osteoarthritis takes its place as part of the metabolic syndrome. Curr Opin Rheumatol 2010;22:512–9. 10.1097/BOR.0b013e32833bfb4b [DOI] [PubMed] [Google Scholar]
- 3. Berenbaum F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Ann Rheum Dis 2011;70:1354–6. 10.1136/ard.2010.146399 [DOI] [PubMed] [Google Scholar]
- 4. King KB, Rosenthal AK. The adverse effects of diabetes on osteoarthritis: update on clinical evidence and molecular mechanisms. Osteoarthritis Cartilage 2015;23:841–50. 10.1016/j.joca.2015.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirkman MS. Osteoarthritis progression: is diabetes a culprit? Osteoarthritis Cartilage 2015;23:839–40. 10.1016/j.joca.2015.03.030 [DOI] [PubMed] [Google Scholar]
- 6. Sellam J, Berenbaum F. Is osteoarthritis a metabolic disease? Joint Bone Spine 2013;80:568–73. 10.1016/j.jbspin.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 7. Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med 2009;121:9–20. 10.3810/pgm.2009.11.2073 [DOI] [PubMed] [Google Scholar]
- 8. Shin D. Association between metabolic syndrome, radiographic knee osteoarthritis, and intensity of knee pain: results of a national survey. J Clin Endocrinol Metab 2014;99:3177–83. 10.1210/jc.2014-1043 [DOI] [PubMed] [Google Scholar]
- 9. Calvet J, Orellana C, Larrosa M, et al. High prevalence of cardiovascular co-morbidities in patients with symptomatic knee or hand osteoarthritis. Scand J Rheumatol 2015:1–4. [DOI] [PubMed] [Google Scholar]
- 10. Rahman MM, Kopec JA, Cibere J, et al. The relationship between osteoarthritis and cardiovascular disease in a population health survey: a cross-sectional study. BMJ Open 2013;3:e2624 10.1136/bmjopen-2013-002624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inoue R, Ishibashi Y, Tsuda E, et al. Medical problems and risk factors of metabolic syndrome among radiographic knee osteoarthritis patients in the Japanese general population. J Orthop Sci 2011;16:704–9. 10.1007/s00776-011-0157-9 [DOI] [PubMed] [Google Scholar]
- 12. Hart DJ, Doyle DV, Spector TD. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol 1995;22:1118–23. [PubMed] [Google Scholar]
- 13. Jungmann PM, Kraus MS, Alizai H, et al. Association of metabolic risk factors with cartilage degradation assessed by T2 relaxation time at the knee: data from the osteoarthritis initiative. Arthritis Care Res 2013;65:1942–50. 10.1002/acr.22093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anagnostopoulos I, Zinzaras E, Alexiou I, et al. The prevalence of rheumatic diseases in central Greece: a population survey. BMC Musculoskelet Disord 2010;11:98 10.1186/1471-2474-11-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Massengale M, Reichmann WM, Losina E, et al. The relationship between hand osteoarthritis and serum leptin concentration in participants of The Third National Health and Nutrition Examination Survey. Arthritis Res Ther 2012;14:R132 10.1186/ar3864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nieves-Plaza M, Castro-Santana LE, Font YM, et al. Association of hand or knee osteoarthritis with diabetes mellitus in a population of Hispanics from Puerto Rico. J Clin Rheumatol 2013;19:1–6. 10.1097/RHU.0b013e31827cd578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greiver M, Williamson T, Barber D, et al. Prevalence and epidemiology of diabetes in Canadian primary care practices: a report from the Canadian Primary Care Sentinel Surveillance Network. Can J Diabetes 2014;38:179–85. 10.1016/j.jcjd.2014.02.030 [DOI] [PubMed] [Google Scholar]
- 18. Rahman MM, Cibere J, Anis AH, et al. Risk of Type 2 Diabetes among osteoarthritis patients in a prospective longitudinal study. Int J Rheumatol 2014;2014:1–7. 10.1155/2014/620920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reid JL, Morton DJ, Wingard DL, et al. Obesity and other cardiovascular disease risk factors and their association with osteoarthritis in Southern California American Indians, 2002–2006. Ethn Dis 2010;20:416–22. [PubMed] [Google Scholar]
- 20. Birtwhistle R, Morkem R, Peat G, et al. Prevalence and management of osteoarthritis in primary care: an epidemiologic cohort study from the Canadian Primary Care Sentinel Surveillance Network. CMAJ Open 2015;3:E270–5. 10.9778/cmajo.20150018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoshimura N, Muraki S, Oka H, et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage 2012;20:1217–26. 10.1016/j.joca.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 22. Monira Hussain S, Wang Y, Cicuttini FM, et al. Incidence of total knee and hip replacement for osteoarthritis in relation to the metabolic syndrome and its components: a prospective cohort study. Semin Arthritis Rheum 2014;43:429–36. 10.1016/j.semarthrit.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 23. Schett G, Kleyer A, Perricone C, et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care 2013;36:403–9. 10.2337/dc12-0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eymard F, Parsons C, Edwards MH, et al. Diabetes is a risk factor for knee osteoarthritis progression. Osteoarthritis Cartilage 2015;23:851–9. 10.1016/j.joca.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 25. Guerrero-Romero F, Rodríguez-Morán M. Low serum magnesium levels and metabolic syndrome. Acta Diabetol 2002;39:209–13. 10.1007/s005920200036 [DOI] [PubMed] [Google Scholar]
- 26. Guerrero-Romero F, Rodríguez-Morán M. Hypomagnesemia, oxidative stress, inflammation, and metabolic syndrome. Diabetes Metab Res Rev 2006;22:471–6. 10.1002/dmrr.644 [DOI] [PubMed] [Google Scholar]
- 27. Evangelopoulos AA, Vallianou NG, Panagiotakos DB, et al. An inverse relationship between cumulating components of the metabolic syndrome and serum magnesium levels. Nutr Res 2008;28:659–63. 10.1016/j.nutres.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 28. Hjelmesaeth J, Hofsø D, Aasheim ET, et al. Parathyroid hormone, but not vitamin D, is associated with the metabolic syndrome in morbidly obese women and men: a cross-sectional study. Cardiovasc Diabetol 2009;8:7 10.1186/1475-2840-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lima ML, Cruz T, Rodrigues LE, et al. Serum and intracellular magnesium deficiency in patients with metabolic syndrome—evidences for its relation to insulin resistance. Diabetes Res Clin Pract 2009;83:257–62. 10.1016/j.diabres.2008.11.019 [DOI] [PubMed] [Google Scholar]
- 30. Ma J, Folsom AR, Melnick SL, et al. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis Risk in Communities Study. J Clin Epidemiol 1995;48:927–40. 10.1016/0895-4356(94)00200-A [DOI] [PubMed] [Google Scholar]
- 31. Kao WH, Folsom AR, Nieto FJ, et al. Serum and dietary magnesium and the risk for type 2 diabetes mellitus: the Atherosclerosis Risk in Communities Study. Arch Intern Med 1999;159:2151–9. [DOI] [PubMed] [Google Scholar]
- 32. Wang JL, Shaw NS, Yeh HY, et al. Magnesium status and association with diabetes in the Taiwanese elderly. Asia Pac J Clin Nutr 2005;14:263–9. [PubMed] [Google Scholar]
- 33. Chambers EC, Heshka S, Gallagher D, et al. Serum magnesium and type-2 diabetes in African Americans and Hispanics: a New York cohort. J Am Coll Nutr 2006;25:509–13. 10.1080/07315724.2006.10719566 [DOI] [PubMed] [Google Scholar]
- 34. Simmons D, Joshi S, Shaw J. Hypomagnesaemia is associated with diabetes: not pre-diabetes, obesity or the metabolic syndrome. Diabetes Res Clin Pract 2010;87:261–6. 10.1016/j.diabres.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 35. Sales CH, Pedrosa LF, Lima JG, et al. Influence of magnesium status and magnesium intake on the blood glucose control in patients with type 2 diabetes. Clin Nutr 2011;30:359–64. 10.1016/j.clnu.2010.12.011 [DOI] [PubMed] [Google Scholar]
- 36. Lecube A, Baena-Fustegueras JA, Fort JM, et al. Diabetes is the main factor accounting for hypomagnesemia in obese subjects. PLoS One 2012;7:e30599 10.1371/journal.pone.0030599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu J, Xu W, Yao H, et al. Associations of serum and urinary magnesium with the pre-diabetes, diabetes and diabetic complications in the Chinese Northeast population. PLoS One 2013;8:e56750 10.1371/journal.pone.0056750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang SJ, Hwang SY, Baik SH, et al. Serum magnesium level is associated with type 2 diabetes in women with a history of gestational diabetes mellitus: the Korea National Diabetes Program study. J Korean Med Sci 2014;29:84–9. 10.3346/jkms.2014.29.1.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh RB, Rastogi V, Niaz MA, et al. Epidemiological study of magnesium status and risk of hypertension in a rural population of north India. Magnes Res 1996;9:173–81. [PubMed] [Google Scholar]
- 40. Peacock JM, Folsom AR, Arnett DK, et al. Relationship of serum and dietary magnesium to incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol 1999;9:159–65. [DOI] [PubMed] [Google Scholar]
- 41. Guerrero-Romero F, Rodríguez-Morán M, Hernández-Ronquillo G, et al. Low serum magnesium levels and its association with high blood pressure in children. J Pediatr 2016;168:93–8. 10.1016/j.jpeds.2015.09.050 [DOI] [PubMed] [Google Scholar]
- 42. Zeng C, Wang YL, Wei J, et al. Association between low serum magnesium concentration and hyperuricemia. Magnes Res 2015;28:56–63. 10.1684/mrh.2015.0384 [DOI] [PubMed] [Google Scholar]
- 43. Zeng C, Wei J, Li H, et al. Relationship between serum magnesium concentration and radiographic knee osteoarthritis. J Rheumatol 2015;42:1231–6. 10.3899/jrheum.141414 [DOI] [PubMed] [Google Scholar]
- 44. Wei J, Zeng C, Gong Q-yi, et al. Associations between dietary antioxidant intake and metabolic syndrome. PLoS One 2015;10:e130876 10.1371/journal.pone.0130876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xie DX, Xiong YL, Zeng C, et al. Association between low dietary zinc and hyperuricaemia in middle-aged and older males in China: a cross-sectional study. BMJ Open 2015;5:e8637 10.1136/bmjopen-2015-008637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wei J, Zeng C, Gong QY, et al. The association between dietary selenium intake and diabetes: a cross-sectional study among middle-aged and older adults. Nutr J 2015;14:18 10.1186/s12937-015-0007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Expert panel on metabolic syndrome of Chinese Diabetes Society: recommendations on metabolic syndrome of Chinese Diabetes Society (Chinese). Chin J Diabetes 2004;14:156–61. [Google Scholar]
- 48. Pang C, Jia L, Hou X, et al. The significance of screening for microvascular diseases in Chinese community-based subjects with various metabolic abnormalities. PLoS One 2014;9:e97928 10.1371/journal.pone.0097928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou H, Guo ZR, Yu LG, et al. Evidence on the applicability of the ATPIII, IDF and CDS metabolic syndrome diagnostic criteria to identify CVD and T2DM in the Chinese population from a 6.3-year cohort study in mid-eastern China. Diabetes Res Clin Pract 2010;90:319–25. 10.1016/j.diabres.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 50. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Joosten MM, Gansevoort RT, Mukamal KJ, et al. Urinary magnesium excretion and risk of hypertension: the prevention of renal and vascular end-stage disease study. Hypertension 2013;61:1161–7. 10.1161/HYPERTENSIONAHA.113.01333 [DOI] [PubMed] [Google Scholar]
- 52. Choi MK, Bae YJ. Association of magnesium intake with high blood pressure in Korean adults: Korea National Health and Nutrition Examination survey 2007–2009. PLoS One 2015;10:e130405 10.1371/journal.pone.0130405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2016. [Google Scholar]
- 54. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–52. 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 55. Barbagallo M, Dominguez LJ, Galioto A, et al. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Aspects Med 2003;24(1-3):39–52. 10.1016/S0098-2997(02)00090-0 [DOI] [PubMed] [Google Scholar]
- 56. Song Y, Ridker PM, Manson JE, et al. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care 2005;28:1438–44. 10.2337/diacare.28.6.1438 [DOI] [PubMed] [Google Scholar]
- 57. Guerrero-Romero F, Rodríguez-Morán M. Complementary therapies for diabetes: the case for chromium, magnesium, and antioxidants. Arch Med Res 2005;36:250–7. 10.1016/j.arcmed.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 58. Huerta MG, Roemmich JN, Kington ML, et al. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care 2005;28:1175–81. 10.2337/diacare.28.5.1175 [DOI] [PubMed] [Google Scholar]
- 59. Djurhuus MS, Skøtt P, Hother-Nielson O, et al. Insulin increases renal magnesium excretion: a possible cause of magnesium depletion in hyperinsulinaemic states. Diabet Med 1995;12:664–9. 10.1111/j.1464-5491.1995.tb00566.x [DOI] [PubMed] [Google Scholar]
- 60. Paolisso G, Sgambato S, Passariello N, et al. Insulin induces opposite changes in plasma and erythrocyte magnesium concentrations in normal man. Diabetologia 1986;29:644–7. 10.1007/BF00869264 [DOI] [PubMed] [Google Scholar]
- 61. Bonora E, Targher G, Zenere MB, et al. Relationship of uric acid concentration to cardiovascular risk factors in young men. Role of obesity and central fat distribution. The Verona Young Men Atherosclerosis Risk Factors Study. Int J Obes Relat Metab Disord 1996;20:975–80. [PubMed] [Google Scholar]
- 62. Lyngdoh T, Marques-Vidal P, Paccaud F, et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS One 2011;6:e19901 10.1371/journal.pone.0019901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kirilmaz B, Asgun F, Alioglu E, et al. High inflammatory activity related to the number of metabolic syndrome components. J Clin Hypertens 2010;12:136–44. 10.1111/j.1751-7176.2009.00229.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Salmonowicz B, Krzystek-Korpacka M, Noczyńska A. Trace elements, magnesium, and the efficacy of antioxidant systems in children with type 1 diabetes mellitus and in their siblings. Adv Clin Exp Med 2014;23:259–68. doi:10.17219/acem/37074 [DOI] [PubMed] [Google Scholar]
- 65. Rodríguez-Morán M, Guerrero-Romero F. Elevated serum concentration of tumor necrosis factor-alpha is linked to low serum magnesium levels in the obesity-related inflammatory response. Magnes Res 2004;17:189–96. [PubMed] [Google Scholar]
- 66. Li H, Zeng C, Wei J, et al. Serum calcium concentration is inversely associated with radiographic knee osteoarthritis: a cross-sectional study. Medicine 2016;95:e2838 10.1097/MD.0000000000002838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chacko SA, Song Y, Nathan L, et al. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care 2010;33:304–10. 10.2337/dc09-1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bo S, Durazzo M, Guidi S, et al. Dietary magnesium and fiber intakes and inflammatory and metabolic indicators in middle-aged subjects from a population-based cohort. Am J Clin Nutr 2006;84:1062–9. 10.1093/ajcn/84.5.1062 [DOI] [PubMed] [Google Scholar]
- 69. Kim DJ, Xun P, Liu K, et al. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care 2010;33:2604–10. 10.2337/dc10-0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Spector TD, Hart DJ, Nandra D, et al. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum 1997;40:723–7. 10.1002/art.1780400419 [DOI] [PubMed] [Google Scholar]
- 71. Smith JW, Martins TB, Gopez E, et al. Significance of C-reactive protein in osteoarthritis and total knee arthroplasty outcomes. Ther Adv Musculoskelet Dis 2012;4:315–25. 10.1177/1759720X12455959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Topf JM, Murray PT. Hypomagnesemia and hypermagnesemia. Rev Endocr Metab Disord 2003;4:195–206. 10.1023/A:1022950321817 [DOI] [PubMed] [Google Scholar]
- 73. Sabatier M, Pont F, Arnaud MJ, et al. A compartmental model of magnesium metabolism in healthy men based on two stable isotope tracers. Am J Physiol Regul Integr Comp Physiol 2003;285:R656–63. 10.1152/ajpregu.00749.2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.