Abstract
The United States is in the midst of an opioid public health emergency, one that is also influenced by a convergence of Internet-based technology, health policy, and the need for stakeholder collaboration and action around the need to combat the illicit online sales of opioids by illegal online pharmacies and digital drug dealers. This risk is not new, however, with calls to actively reduce online opioid availability as online pharmacies use a growing array of digital channels, including search engines, social media platforms, and the dark Web. In response, the US Food and Drug Administration convened a special June 2018 summit bringing together technology companies, government agencies, researchers, and advocacy groups with the goal of collaboratively developing and implementing solutions to tackle the problem. Yet after this meeting, stakeholders remain fragmented in approaches despite the availability of technology that can detect, classify, and report illicit sellers who are in direct violation of Federal law. Despite ongoing challenges, advances in data science and the resources and expertise technology companies can contribute will be a key factor in ensuring that the Internet helps end and not fuel the public health emergency of opioid abuse.
Keywords: Opioids, illicit online pharmacies, controlled substances, social media, machine learning, public health, health policy
On June 27, 2018, the US Food and Drug Administration (FDA) held a one-of-a-kind meeting that brought together stakeholders from Internet/e-commerce technology companies (including Google, Facebook, Twitter, Alibaba, Pinterest, and others), government agencies (FDA, the US Department of Health and Human Services, the US Drug Enforcement Agency [DEA], and the US Department of Justice [DOJ]), advocacy groups (the Alliance for Safe Online Pharmacies [ASOP] and Center for Safe Internet Pharmacies and others), and members from the academic community to address a critical public health issue: combating the illicit online sale of controlled substances.1 The FDA’s “Online Opioid Summit” was a first of its kind. However, the warning signs that the Internet has enabled diversion and abuse of opioids have been blaring for more than a decade. This risk continues to grow as part of the larger public health crisis of the opioid epidemic that now claims the lives of an average of 115 people who daily overdose due to their addiction (which includes prescription opioids, heroin, and synthetics such as fentanyl).2,3
The Internet, Opioids, and Illicit Online Pharmacies
As early as 2001, the patient safety ramifications associated with the convergence of Internet technology and opioid abuse have been tragically apparent.3,4 Specifically, the 2008 Ryan Haight Online Pharmacy Consumer Protection Act (RHA) was enacted following the death of an 18-year-old adolescent who purchased Vicodin from an online seller and died from overdose.3 The RHA responded by requiring mandatory DEA registration and Web site disclosures for any online pharmacy selling controlled substances in an effort to avert future deaths and reign in this alternative and easily accessible channel of opioid access.5
However, the RHA was not able to anticipate how the Internet would evolve from static Internet Web sites (Web 1.0) to interactive and socially enabled content (Web 2.0), nor how ubiquitous e-commerce would become due to greater connectivity and widespread adoption of mobile-connected devices. For example, approximately 80% of US adult Internet users look for health information online, an estimated 35% go online to find information to diagnose a medical condition, and a survey conducted by the FDA found 23% of its respondents purchased a prescription medicine from the Internet.4,6,7 From a platform adoption side, in 2018, 88% of 18- to 29-year olds (a crucial age for substance use initiation) reported using any form of social media, and 77% of Americans owned a smartphone that enables them convenient access to online services and sellers without the need for broadband connectivity.8,9
Accompanying rapid adoption of Web-based technology, Internet, or “cyber” pharmacies have also proliferated, with an estimated 30 000 currently in existence, although some 96% operate illegally (eg, out of compliance with state and Federal laws or safety and practice standards) according to the National Association of Boards of Pharmacy.10 A smaller percentage (estimated at 9%-13%) of these online pharmacies engage in clearly dangerous and illegal activities, namely, the sale of opioids and other controlled substances via the Internet, constituting a direct violation of the RHA.4,10
This illegal activity has also evolved along with expansion of the Internet, with online opioid sales first appearing on Web site forums, then moving to search engine results, and now populating popular social media sites such as Facebook, Twitter, Instagram, and LinkedIn while also appearing on the dark Web (Web sites not indexed or visible to the public without additional software).11-18 Hence, the risk of illegal online pharmacies is no longer confined to one part of the Internet ecosystem, instead infiltrating multiple digital services, platforms, and communities. These different parts of the Internet can also interact with each other to enable multiple marketing and selling portals for illegal opioid access. For example, dark Web direct-to-consumer and business-to-business sales can be advertised on social media channels, in addition to social media channels and search engines that advertise sale for “clear” or “open” online pharmacy Web sites (Web sites viewable to the public that are indexed or otherwise searchable).19,20
The reality of these risks are now becoming abundantly clear, with reports from the US Government Accountability Office and a more recent investigation by the US Senate Permanent Subcommittee on Investigations (part of the Senate Homeland Security and Government Affairs Committee) evidencing just how easy it is to buy opioids and even extremely dangerous fentanyl and its analogues online.21,22 Policymakers are also responding with increased scrutiny of technology companies whose platforms enable this illegal activity, with US Congressman David McKinley (HR-West Virginia) recently questioning Facebook CEO Mark Zuckerberg in congressional testimony before the House Energy and Commerce Committee about what steps the platform is taking to remove illegal Web sites and protect its users.23 In short, Zuckerberg responded to this line of inquiry that the enormity of data makes it hard for the company to monitor its platforms but that “artificial intelligence”–based approaches to “proactively” find content were needed.23 In September 2018, representative McKinley also challenged Twitter CEO Jack Dorsey in Congressional testimony to aggressively remove opioid sales being promoted via Twitter.
FDA Online Opioid Summit: Bringing Stakeholders to the Table for Action?
In response to these growing concerns, the June 27, 2018, FDA’s Online Opioid Summit was designed to bring to the table central stakeholders that have the ability to significantly reduce illegal online opioid sales, with parts of the meeting available to the public but most of the program held behind closed doors to facilitate open conversation.1 Specifically, the Summit’s agenda included the following:
Statements from the FDA (including directly from Commissioner Scott Gottlieb) on how the Internet negatively affects the national opioid crisis;
Presentations from academia, enforcement agencies, and advocacy groups on research and consumer protection activities;
Discussion of actions taken by technology and e-commerce companies;
Roundtable discussions among all participants about solutions and next steps forward.
However, even before the meeting began, concerns about representations made by technology companies and their trade associations arose in the media, namely, efforts to divert the blame for illegal opioid access solely to the dark Web.20 This arguably myopic view of scope of online opioid sales was largely debunked by regulators and law enforcement officials who have actively prosecuted cases of open Web opioid sales, as well as research analyzing public criminal court filings evidencing numerous online pharmacy prosecutions involving controlled substances and criminal organizations.5 In addition, the original Summit agenda included a voluntary pledge that the FDA proposed as a means for technology companies to demonstrate their commitment to tangible action. However, this component was removed from the final program likely due to pressure from industry stakeholders who are still assessing their legal responsibilities and how they should appropriately respond to the issue from a public relations standpoint.20
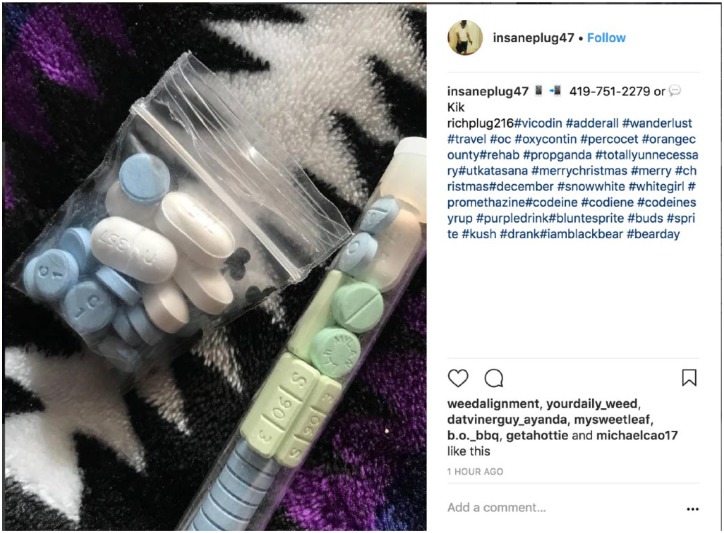
A critical component of the Summit was also the discussion of technology approaches to combat illicit online pharmacies. This included a presentation of recently published research, funded in part by ASOP through a research grant and in part by the US National Institutes of Health through a start-up award, using big data, artificial intelligence, and Web forensic approaches to detect, categorize, and report illicit online sales of opioids.4,15 Results using this digital surveillance-based research approach included evidence of continued “no-prescription” opioid marketing and sales on Twitter and Instagram detected as early as a few weeks before the FDA meeting (see Figure 1), in addition to evidence of “digital drug dealers” who use social media platforms to sell drugs using traditional means (eg, street sales facilitated through direct communication via WhatsApp, email, or mobile; see Figure 2).
Figure 1.
Screenshots of Illicit Online Pharmacies Tweeting and Selling Controlled Substances Online Detected Using Machine Learning.
Figure 2.
Screenshot from Instagram of Digital Drug Dealer Detected Using Machine Learning.
Select tech companies also explained their approaches to addressing illegal online drug sales. This included discussion about how Facebook recently instituted a program that redirects user searches for opioid keywords to educational resources (though not explicitly removing illegal online pharmacy content), Pinterest blocking opioid keywords from user searchers on its platform, and Google de-indexing and demoting certain opioid search results based on warning letters from the FDA.24,25 Companies also referenced their existing policies prohibiting online sales of controlled substances in their sponsored and Internet advertisement content.
Despite these new and existing approaches, consensus around best practices to better coordinate proactive monitoring and takedown of illicit online pharmacies with regulators and law enforcement was illusive. Part of this challenge is explained by the unique characteristics and variety of Internet platforms that enable illicit online pharmacies, differences in their data features and service delivery models, and the expectation of privacy and security of platform users. Moreover, the lack of concrete solutions and meaningful collaboration exposed underlining policy and public relations challenges about which technology firms remain acutely cautious, including issues of Internet content censorship, costs of monitoring and compliance, business and user experience impact, and broader public scrutiny tech companies face on other issues (ie, fake news, election interference, cyber bullying, etc) These concerns may predominate the conversation despite the fact that illegal online opioid marketing and sales are explicitly illegal under RHA, that no online pharmacy is currently legally registered to sell controlled substances by the DEA, and the fact that the country remains in the midst of an opioid public health crisis with opioid sales still occurring online.4,5
Policy Approaches: Technology, Legislation, or Enhanced Enforcement?
Despite the numerous challenges highlighted by the FDA Online Opioid Summit, advances in technology and the reality that online pharmacies require Internet-based services (eg, Web site hosting services, indexing, registrar services, electronic payment, mail and shipping services, and organic advertising on search and social media platforms) to operate provides unique opportunities to respond to this public health challenge leveraging this same technology as a part of the solution.10,26 For example, machine learning algorithms (both supervised and unsupervised) can accurately identify and classify illegal sales of opioids, registration of suspicious online seller accounts can be blocked, and Internet service providers can be forced to suspend or takedown illegally operating online pharmacy Web sites.4,15
Many of these technology-based solutions are available now and can be immediately deployed to help technology firms, regulators, and law enforcement officials identify and remove content in near real-time to proactively protect the public. Proactive data-based surveillance approaches, such as using big data and machine learning, could also aid in blacklisting illegal Web sites from other traditional e-commerce services, including payment processing and product shipping, 2 key components needed for online drug sales and distribution. Although individual firms may employ these techniques through their own internal data trust, safety, and integrity departments, there remains lack of formal coordination and cooperation between industry actors who operate sites/platforms, researchers who often develop innovative and cutting-edge techniques, and regulators who need this information to protect the public and enforce against illegal sellers.
Outside of technology, and in the absence of adequate self-regulation by technology and Internet service providers, legislation updating the RHA to modernize it to the realities of a diverse and socially interactive Internet ecosystem could also be explored. This could include expanding the remit of the RHA to explicitly cover platforms and companies outside of the actual illicit online pharmacies that sell drugs. This could include explicit reference and penalties for technology companies that run Web search engines, social media platforms, messaging and mobile applications, Internet registrar and domain services (including the Internet Corporation for Assigned Names and Numbers), and payment and logistic providers.
Other legislation, including the recently proposed the Fight Illicit Networks and Detect (FIND) Trafficking Act (115th Congress, 2d Session, S.3179), propose conducting more research to better understand drug trafficking via online marketplaces. However, given existing research and recent investigations, the focus of this legislation could shift to requiring technology companies or independent third party watchdogs to conduct their own investigations and proactively remove content from their systems that directly enable both illegal opioid sales and digital drug dealing and report findings from these actions to Congress and the FDA on at least an annual basis. Additional legislative action may not be necessary as interpretation of the RHA as currently constructed arguably already requires such compliance. However, some technology companies have expressed confusion about the applicability of the RHA to their platforms and services, perhaps necessitating further legislative clarification or additional Federal guidance affirming this responsibility along with more explicit penalties for noncompliance.
Enhanced enforcement action may be necessary to sufficiently deter Internet platform facilitation of illegal online opioid activity, which continues despite numerous published studies and separate enforcement efforts by the FDA and DEA. Such enforcement is not without precedent, with Google fined US$500 million in 2011 for knowingly enabling illegal online pharmacy sales via sponsored ads, and FedEx Corp. and United Parcel Services also prosecuted by the DOJ.5 Hence, extending more robust FDA regulatory oversight (through warning and cease and desist letters), greater on the ground enforcement and investigation by the DEA, and exercising DOJ prosecutorial discretion against Internet providers who facilitate these illegal transactions might be a needed response in the event of further inaction. A more favorable outcome is the one originally envisioned by the FDA and its Summit objectives of stakeholders voluntarily “collaboratively take(ing) stronger action in combating the opioid crisis by reducing the availability of illicit opioids online.1”
Conclusions
Only time, greater public access to social media data, additional dialogue, continued public pressure, and refocused efforts on collaborative approaches (with FDA continuing to take a leadership role) can ensure success in combating the illegal online opioid marketplace. To be clear, these sales are just one part of a complex public health challenge that requires sustained efforts to enhance substance abuse treatment and recovery, community-based approaches and interventions, prevention efforts focused on substance abuse behavior, addressing overprescribing and pain management, investing in health promotion and education, and combating drug diversion.27,28 Importantly, broader actions to address opioid diversion are not in isolation from Internet sales, as making it harder for users to “doctor shop,” obtain opioids from family members, or buy from the street may have unintended consequences of redirecting users to more convenient yet equally risky channels of access: illegal Internet pharmacies.
What is also self-evident is that no sustainable solution exists without technology companies taking an active role and leveraging their expansive resources and expertise, including their understanding of ways criminals take advantage of their tools to profit from addiction. Although the current administration has generally supported an antiregulation or deregulation approach to many industries (including investment regulations, Internet privacy, climate change, etc), on the subject of combating the opioid crisis, the FDA and Congress appear to be taking a much more direct approach that may eventually involve additional regulation or enforcement. Internet technology stakeholders should recognize these potential challenges and take advantage of their unique opportunity to help prevent further escalation of this declared public health emergency. This FDA Summit participant hopes they choose to exercise this public health responsiblity.
Acknowledgments
TKM would like to thank ASOP for their support of prior work associated with developing a methodology to detect and characterize illicit online pharmacies using social media platforms. He also would like to thank the FDA for its invitation to present and travel support for the FDA Online Opioid Summit.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: TKM received funding for a pilot grant to research social media and illicit online pharmacies from the Alliance for Safe Online Pharmacies (ASOP), a 501(c)(4) social welfare organization engaged on the issue of illicit online pharmacies. TKM is also the co-founder of the company S-3 Research, LLC, a company that received funding through the National Institutes of Health, National Institute on Drug Abuse Start an SUD Startup Challenge Award and the company is also supported by an Accelerating Innovations to Market Award provided by the UCSD Institute for the Global Entrepreneur. S-3 Research, LLC, has not received any non-public funds related to its activity. TKM also received travel support from the FDA for an invited accredited educational activity on drug safety and surveillance, which included discussion regarding the results of this study in January 2018 and also received travel support to attend and present at the FDA Online Opioid Summit in June 2018 as referenced in this study. In the past, he has also acted as a consultant for the US Department of Justice and US Secretary of State on the issue of illicit online pharmacies and counterfeit medicines.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: TKM formulated, conducted the analysis, drafted and completed the final version of the manuscript.
References
- 1. Center for Drug Evaluation Research. FDA Commissioner Scott Gottlieb, M.D., invites internet stakeholders to opioid summit, June 27, 2018 [Internet]. fda.gov (Center for Drug Evaluation and Research), 2018. https://www.fda.gov/drugs/newsevents/ucm608633.htm. Accessed August 6, 2018.
- 2. NIDA. Opioid overdose crisis [Internet]. drugabuse.gov. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-overdose-crisis. Accessed August 6, 2018.
- 3. Mackey TK, Liang BA, Strathdee SA. Digital social media, youth, and nonmedical use of prescription drugs: the need for reform. J Med Internet Res. 2013;15:e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mackey T. Solution to detect, classify, and report illicit online marketing and sales of controlled substances via Twitter: using machine learning and web forensics to combat digital opioid access. J Med Internet Res. 2018;20:e10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guerra C, Mackey TK. USA criminal and civil prosecutions associated with illicit online pharmacies: legal analysis and global implications [published online ahead of print December 14, 2017]. Med Access @ Point of Care. doi: 10.5301/maapoc.0000020. [DOI] [Google Scholar]
- 6. Fox S, Duggan M. Health online 2013 [Internet]. pewinternet.org, 2013. http://www.pewinternet.org/2013/01/15/health-online-2013/. Accessed August 6, 2018.
- 7. Amante DJ, Hogan TP, Pagoto SL, English TM, Lapane KL. Access to care and use of the Internet to search for health information: results from the US National Health Interview Survey. J Med Internet Res. 2015;17:e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith A, Anderson M. Social media use in 2018 [Internet]. pewinternet.org, 2018. http://www.pewinternet.org/2018/03/01/social-media-use-in-2018/. Accessed March 22, 2018.
- 9. PewInternet. Mobile fact sheet [Internet]. pewinternet.org, 2018. http://www.pewinternet.org/fact-sheet/mobile/. Accessed August 6, 2018.
- 10. Mackey TK, Nayyar G. Digital danger: a review of the global public health, patient safety and cybersecurity threats posed by illicit online pharmacies. Br Med Bull. 2016;118:110–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forman RF. Availability of opioids on the Internet. JAMA. 2003;290:889. [DOI] [PubMed] [Google Scholar]
- 12. Forman RF, Woody GE, McLellan T, Lynch KG. The availability of web sites offering to sell opioid medications without prescriptions. Am J Psychiatry. 2006;163:1233–1238. [DOI] [PubMed] [Google Scholar]
- 13. Raine C, Webb DJ, Maxwell SRJ. The availability of prescription-only analgesics purchased from the internet in the UK. Br J Clin Pharmacol. 2009;67:250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DEA. Counterfeit prescription pills containing fentanyls: a global threat [Internet]. dea.gov, 2016. https://content.govdelivery.com/attachments/USDOJDEA/2016/07/22/file_attachments/590360/fentanyl%2Bpills%2Breport.pdf. Accessed February 17, 2017.
- 15. Mackey TK, Kalyanam J, Katsuki T, Lanckriet G. Machine learning to detect prescription opioid abuse promotion and access via Twitter. Am J Public Health. 2017;107:e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pergolizzi JV, LeQuang JA, Taylor R, Raffa RB. The “Darknet”: the new street for street drugs. J Clin Pharm Therap. 2017;42:790–792. [DOI] [PubMed] [Google Scholar]
- 17. Bachhuber MA, Cunningham CO. Availability of buprenorphine on the Internet for purchase without a prescription. Drug Alcohol Depend. 2012;130:238–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin J, Cunliffe J, Décary-Hétu D, Aldridge J. Effect of restricting the legal supply of prescription opioids on buying through online illicit marketplaces: interrupted time series analysis. BMJ. 2018;361:k2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lytvynenko J. Social networks are losing a deadly battle with illegal online pharmacies [Internet]. buzzfeednews.com, 2018. https://www.buzzfeednews.com/article/janelytvynenko/social-networks-are-losing-a-deadly-battle-with-illegal. Accessed August 6, 2018.
- 20. Lapowsky I. Tech companies deflect blame for opioid crisis ahead of FDA summit [Internet]. wired.com, 2018. https://www.wired.com/story/tech-companies-deflect-blame-for-opioid-crisis-fda-summit/. Accessed August 6, 2018.
- 21. US Government Accountability Office HGG. Internet pharmacies: federal agencies and states face challenges combating rogue sites, particularly those abroad [Internet]. gao.gov, 2013. http://www.gao.gov/assets/660/655751.pdf. Accessed January 11, 2016.
- 22. US Senate. Combatting the opioid crisis: exploiting vulnerabilities in international mail [Internet]. hsgac.senate.gov, 2018. https://www.hsgac.senate.gov/imo/media/doc/Combatting%20the%20Opioid%20Crisis%20-%20Exploiting%20Vulnerabilities%20in%20International%20Mail1.pdf. Accessed August 6, 2018.
- 23.What they’re saying: McKinley’s questioning of Facebook’s Zuckerberg [Internet]. mckinley.house.gov, 2018. https://mckinley.house.gov/news/documentsingle.aspx?DocumentID=1301. Accessed August 6, 2018.
- 24. LaVito A. Facebook, Google and other tech giants meet with federal regulators to tackle online opioid sales [Internet]. cnbc.com, 2018. https://www.cnbc.com/2018/06/27/facebook-google-others-to-meet-with-fda-about-online-opioid-sales.html. Accessed August 6, 2018.
- 25. Facher L. Facebook redirects users searching for opioids to federal crisis help line [Internet]. scientificamerican.com, 2018. https://www.scientificamerican.com/article/facebook-redirects-users-searching-for-opioids-to-federal-crisis-help-line/. Accessed August 6, 2018.
- 26. Orizio G, Merla A, Schulz PJ, Gelatti U. Quality of online pharmacies and websites selling prescription drugs: a systematic review. J Med Internet Res. 2011;13:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Califf RM, Woodcock J, Ostroff S. A proactive response to prescription opioid abuse. N Engl J Med. 2016;374:1480–1485. [DOI] [PubMed] [Google Scholar]
- 28. Kolodny A, Frieden TR. Ten steps the Federal Government should take now to reverse the opioid addiction epidemic. JAMA. 2017;318:1537–1538. [DOI] [PubMed] [Google Scholar]