Abstract
Renal cell carcinoma (RCC) accounts for approximately 80% of all primary renal neoplasms in United States causing approximately 65 000 new cases of RCC and 14 000 deaths each year. Symptoms of RCC typically include weight loss and night sweats but may also feature paraneoplastic phenomena in advanced stages as well as flank pain, gross hematuria, scrotal varicocele, inferior vena cava pathology, and a palpable abdominal mass. In this article, we present the course of a patient with advanced RCC, from initial presentation through workup and to eventual diagnosis. The case features late-onset symptoms, extensive paraneoplastic phenomena, and significant physical examination findings. We also review the literature available on RCC and critically analyze inefficiencies of the workup retrospectively.
Keywords: advanced renal cell carcinoma, RCC, metastatic, paraneoplastic
Background
In the United States, renal cell carcinoma (RCC) accounts for approximately 80% of all primary renal neoplasms.1 Each year, there are approximately 65 000 new cases of RCC and 14 000 deaths.1 Typical symptoms of RCC include the insidious findings of most malignancies such as weight loss and night sweats. Uniquely, findings may additionally include flank pain, gross hematuria, scrotal varicocele, inferior vena cava pathology, and a palpable abdominal mass in late stages. The classic triad of RCC taught in medical school includes flank pain, hematuria, and a palpable abdominal renal mass. The triad, however classic, is relatively uncommon, but when present is strongly linked with advanced disease. Unfortunately, many patients are asymptomatic or mildly symptomatic until the disease is advanced and approximately one-quarter of patients have metastatic or locally advanced malignancy at presentation.2 The diagnostic approach of RCC is not standardized, generally multifaceted, and should initially include laboratory and imaging modalities after thorough review of systems, medical history taking, and physical examination. The diagnosis is confirmed by tissue biopsy and augmented by staging imaging studies, the results of which are imperative to guide appropriate treatment.
In this article, we present the course of a patient with advanced renal cell carcinoma, from initial presentation through workup and to eventual diagnosis. The case features late onset symptoms, sufficing two of three criteria of the RCC triad, and extensive paraneoplastic phenomena. We also offer a review of the available literature on RCC and its relation to primary care.
Clinical Report
The patient is a 52-year-old man who initially presented with a chief complaint of 3 months of bilateral flank pain when sleeping at night. He also noted significant fatigue that had been present during this time which he attributed to poor sleep. Review of systems at that visit was positive for night sweats throughout this time period that were sufficient to soak through his sheets. He denied weight loss, recent illnesses, trauma, or changes in environment. As a rare visitor to the doctor’s office, we had no prior weights or vital signs on this patient in the previous 10 years to aid in objective comparison. At initial visit, he was taking no regular medications and had no allergies. Social history was pertinent for active tobacco use and a 35-pack-year smoking history, occasional alcohol use, and no illicit drug use history. A family history was noncontributory. Objectively, his vital signs on presentation were entirely within normal limits. Physical examination was significant for right upper quadrant tenderness without peritoneal signs or costovertebral angle tenderness. He also had reproducible tenderness over the iliac crests bilaterally. He appeared tired and thin for his height. Initial labs that showed microcytic anemia (mean corpuscular volume MCV 74 fL, hemoglobin 11 g/dL), normal thyroid-stimulating hormone, erythrocyte sedimentation rate of 38 mm/h, C-reactive protein of 80 mg/L, sodium, potassium, chloride, and creatinine within normal limits and a urinalysis that was entirely normal. He received a diagnosis of polymyalgia rheumatica given presumed flank pain originating from his hips and elevated inflammatory markers. He was given an empiric short course of prednisone with a plan for telephone reassessment in 2 to 3 days to confirm the diagnosis. Unfortunately, the patient felt no benefit at recheck and so this was discontinued and proceeded with further workup of his laboratory abnormalities.
A return provider visit 1 week later did not yield new history but nontender hepatomegaly was noted on examination. Lab workup from the second visit showed stable microcytic anemia confirmed with peripheral smear, elevated lactate dehydrogenase, low iron, very elevated ferritin with decreased total iron binding capacity. Inflammatory markers were persistently elevated. Alanine transaminase, aspartate transaminase, alkaline phosphatase, albumin, international normalized ratio were all normal. Antinuclear antibodies and rheumatoid factor were negative. Prostate-specific antigen was normal. He had a newfound diagnosis of diabetes with hemoglobin A1c of 7% and was also found to have hypercalcemia of 11.5 mg/dL.
A third return visit was then ordered the following week. At this visit, there were no new findings on history or physical examination, again palpating hepatomegaly and reproducible right flank pain were observed. Additional studies showed normal urine and serum protein electrophoresis, further elevated calcium to 12.4 mg/dL, normal phosphorus of 3.8 mg/dL, elevated 1,25-dihydroxyvitamin D, undetectable parathyroid hormone, and elevated parathyroid hormone–related peptide. Bone alkaline phosphatase was undetectable, urine calcium was normal, and urine phosphorus was elevated. Colonoscopy and upper endoscopy were subsequently performed 1 week later to assess a gastrointestinal source of anemia and they returned values largely within normal limits. Several days later, a computed tomography (CT) scan of the lungs, abdomen, and pelvis was obtained to assess for malignancy with these presumed paraneoplastic phenomena.
The CT scan showed a large 15 × 14 × 14 cm malignant-appearing tumor arising from the right kidney abutting the liver, chest wall, diaphragm, and right psoas muscle (Figure 1). There was evidence for metastases in both adrenal glands and bilateral pulmonary nodules were seen that were worrisome for metastasis. Referrals to Medical Oncology and Urology were ordered. The renal mass was then biopsied showing RCC clear cell subtype, grade 3/4. A multidisciplinary tumor board was organized and ultimately decided against cytoreductive nephrectomy in favor of systemic chemotherapy due to the multiple metastases. Weeks later during the staging workup and prior to starting chemotherapy, pulmonary emboli were incidentally found and he was started on systemic anticoagulation.
Figure 1.
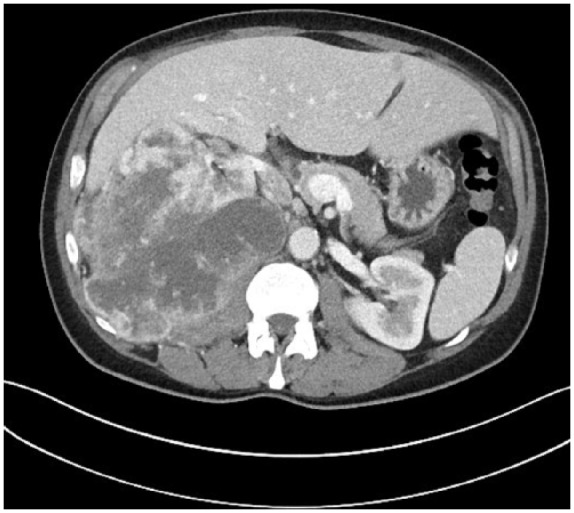
Computed tomography scan of the renal malignancy.
At the time of writing this manuscript, the patient is actively participating in multidrug systemic chemotherapy with regular monitoring for tumor response. The time between initial presentation at clinic to the time of diagnostic imaging and diagnosis was approximately one month. Thus approximately four months elapsed between initial symptoms of flank pain and night sweats and diagnosis of metastatic renal cell carcinoma.
Literature Review
Epidemiology
Each year, there are approximately 65 000 new cases of RCC and almost 14 000 deaths.1 It is approximately 50% more common in men3 with a median age at diagnosis of 64 years.4 Risk factors for the development of RCC include smoking, hypertension, obesity, polycystic kidney disease, occupational exposure, analgesics (eg, acetaminophen and non-aspirin nonsteroidal anti-inflammatory drugs have shown correlation to increased risk of RCC),5 genetics, hepatitis C infection, and nephrolithiasis.6-8 In recent years, survival has nearly doubled due in part to earlier detection of smaller sized masses as well as improved definitive surgical intervention with the most recent calculations of 5-year survival rate to be approximately 73%.4 Interestingly, the incidence of RCC has risen 3-fold higher than the mortality rate, further supporting improved detection, survival and case fatality rates.9
Pathology
Subtypes of RCC include clear cell (75%-85% of tumors), papillary (10%-15%), chromophobic (5%-10%), oncocytic (3%-7%), and collecting duct (rare).10 On initial diagnosis, approximately 65% of patients have localized disease (confined to the kidney). Furthermore, approximately 16% have regional disease with lymph node involvement, 16% have metastatic disease, and 3% are unstageable.11 Prognostication is achieved primarily through stratifying histologic subtype with the extent of metastasis. Of the various histologic subtypes, the majority of research has been in the clear cell subtype and so several genetic alterations have been noted. Common chromosomal aberrations found include a loss of 3p in 94% (genes affected including VHL, BRCA1 associated protein-1), gain of 5q (69%), and monosomy or partial loss of 14q (42%).12
Diagnosis
Ultrasonography and CT scans are generally used first line for imaging and diagnosis. Ultrasonography has the added benefit of a dynamic study and better visualization of cystic structures that may have structural complexity. Magnetic resonance imaging (MRI) is additionally more available as a diagnostic imaging choice due to cheaper cost and is particularly helpful when ultrasound and CT are nondiagnostic. CT urograms are less used but may be helpful for further delineation of genitourinary anatomy. After appropriate imaging modalities are completed, biopsy for tissue studies is necessary for histological confirmation and stratifying prognosis and risks. Staging is then begun and can be completed by reviewing the initial CT scan for metastases which has a good sensitivity/specificity. Positron emission tomography (PET), PET/CT, and MRI are generally not needed for staging in the case of RCC with the availability of high-quality CT scanning.13 Staging is generally demarcated in the TNM staging system.
Treatment Modalities
Medical and surgical routes are both viable options for treatment, depending on severity and subtype. For localized RCC, definitive and first-line treatment is surgical excision which is generally curative. There is no clear role in adjuvant chemotherapy after complete surgical resection of a localized RCC.14 In advanced RCC, surgery has a smaller role to play than systemic chemotherapy, immunotherapy, hormonal therapy, and molecular targeted therapy. Cytoreductive nephrectomy is still often performed/offered prior to attempting systemic therapy.15 Radiotherapy is not used as RCC is characterized as a radioresistant tumor.
Surveillance and Screening
Surveillance for patients in remission of their RCC is usually done by regular examinations and imaging modalities depending on initial staging criteria. For stage I [T1] (<7 cm and localized to the kidney), an office visit at 6, 12, 24, and 36 months is recommended along with kidney function labs and urinary analysis. In stage I, renal imaging is offered at the same interval as office visits are offered for patients with partial nephrectomy, but imaging is only needed once at 6 months for patients treated with total nephrectomy. Chest imaging is recommended annually for 3 years postoperatively in patients with low-risk tumors and every 6 months in patients with T2 staging or greater for 3 years postoperatively. For more advanced malignancies, all the above is recommended to be performed every 6 months for the first 5 years of remission as outlined by American Urological Association guidelines.16 As evidenced by this case, providers should be vigilant for late-stage and large masses on abdominal examination and ensure accurate differentiation between organomegaly.
Screening of asymptomatic individuals is generally not recommended because of the low prevalence of RCC in the general population. However, individuals at high risk for the development of RCC, such as related genetic syndromes/mutations (eg, von Hippel–Landau syndrome or tuberous sclerosis), those with a strong family history of RCC, prior kidney irradiation or a young patient with end-stage renal disease do warrant screening. Periodic monitoring with abdominal ultrasonography, CT, or MRI are reasonable in such cases to help detect early disease; however, this is expert opinion and no specific frequency and modality has been delineated as a standard.17
Case Discussion
Several aspects of paraneoplastic phenomena are displayed in this case and are pertinent to highlight. Notably, this case included anemia, hypercalcemia, and cachexia. Interestingly, the literature does report several syndromic type findings in patients with RCC that display similarities with polymyalgia rheumatica,18 which was the initial presumptive diagnosis in our patient. In contrast to polymyalgia rheumatica, the symptoms in such a case do not respond to prednisone but are often corrected entirely by nephrectomy. Anemia, which can precede the diagnosis of RCC by several months, has been previously reported in patients with advanced disease.19 The anemia is often disproportionately severe, can be either normocytic or microcytic, and is frequently associated with iron studies typical of those observed with the anemia of chronic disease as was seen in our patient (microcytosis and elevated ferritin) 1 month prior to initial diagnosis. Hypercalcemia occurs in up to 15% of patients with advanced RCC, and can result from primarily 2 different mechanisms: lytic bone metastases and overproduction of parathyroid hormone–related protein, the latter being the primary driver of hypercalcemia in our specific patient.19 As with other tumors, patients with RCC may suffer from significant cachexia which is thought to be due to the elevated tumor necrosis factor–α that is inherent to malignancy and was likely the driving factor for our patient’s appearance. Thrombocytosis is rare in patients with RCC, but its presence is associated with a poor prognosis and advanced disease.19
RCC can also less commonly feature hepatic dysfunction, fever, erythrocytosis, and amyloidosis as part of additional paraneoplastic phenomena. Hepatic dysfunction is an uncommon occurrence in patients with RCC and is termed Stauffer syndrome when it occurs in the absence of liver metastases.20 Liver function was evaluated in our patient and found to be within normal limits and without evidence of metastasis. Fever, which occurs in up to 20% of patients,19 is usually intermittent and is frequently accompanied by night sweats, anorexia, weight loss, and fatigue-all of which our patient experienced. Its etiology is unclear but is an insidious finding of malignancy that is not unique to RCC. Erythrocytosis occurs in 1% to 5% of patients with advanced RCC and appears to be due to constitutive production of erythropoietin.19 Finally, Secondary amyloidosis is found in up to 5% of patients. This finding reflects a chronic inflammatory response as the amyloid fibrils are composed of fragments of the acute phase reactant serum amyloid A protein.21
Reviewing the case retrospectively, the patient carried a single but strong risk factor for RCC with his extensive smoking history and active tobacco use at presentation. The entirely normal urinalysis, while reassuring, did admittedly stray the differential diagnosis. After reviewing the literature there is no clear estimate on the sensitivity of finding hematuria on urinalysis for RCC. This leads to the apparent teaching point that a negative urine analysis does not rule out RCC definitively. We note the patient’s abdominal malignancy was likely palpated during several examinations and was assumed to be hepatomegaly. An ultrasound or CT scan could have reasonably been ordered earlier once hepatomegaly was found on examination given the above assumptions were avoided, although it is unlikely to have changed the clinical course of this advanced malignancy. The primary question that arises from our case is if such an advanced malignancy could have been found earlier. Unfortunately, there are no data on the benefit of preventive examinations with a primary care provider in order to find renal malignancies earlier, but we anticipate this would make for an interesting question to study for future researchers.
Author Biographies
Allen L. Pimienta is a resident in Family Medicine at Mayo Clinic.
Thomas A. Billings is a core preceptor at the Mayo Clinic Family Medicine Residency Program.
Robert G. Fish is a core preceptor at the Mayo Clinic Family Medicine Residency Program.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [DOI] [PubMed] [Google Scholar]
- 2. Garnick MB. Primary neoplasms of the kidney. In: Brady HR, Wilcox CS, eds. Therapy in Nephrology and Hypertension: A Companion to Brenner and Rector’s—The Kidney. Philadelphia, PA: WB Saunders; 1998:337-340. [Google Scholar]
- 3. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-236. [DOI] [PubMed] [Google Scholar]
- 4. Thompson RH, Ordonez MA, Iasonos A, et al. Renal cell carcinoma in young and old—is there a difference? J Urol. 2008;180:1262-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu J, Mao Y, White K. Renal cell carcinoma and occupational exposure to chemicals in Canada. Occup Med (Lond). 2002;52:157-164. [DOI] [PubMed] [Google Scholar]
- 6. Tsivian M, Moreira DM, Caso JR, Mouraviev V, Polascik TJ. Cigarette smoking is associated with advanced renal cell carcinoma. J Clin Oncol. 2011;29:2027-2031. [DOI] [PubMed] [Google Scholar]
- 7. Hidayat K, Du X, Zou SY, Shi BM. Blood pressure and kidney cancer risk: meta-analysis of prospective studies. J Hypertens. 2017;35:1333-1344. [DOI] [PubMed] [Google Scholar]
- 8. Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615-621. [DOI] [PubMed] [Google Scholar]
- 9. Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166:1611-1623. [PubMed] [Google Scholar]
- 10. Ries LA, Eisner MP, Kosary CL, et al. SEER Cancer Statistics Review, 1973-1997. Bethesda, MD: National Cancer Institute; 2000. [Google Scholar]
- 11. Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763-2771. [DOI] [PubMed] [Google Scholar]
- 12. Beroukhim R, Brunet JP, Di Napoli A, et al. Patterns of gene expression and copy-number alterations in von Hippel–Lindau disease–associated and sporadic clear cell carcinoma of the kidney. Cancer Res. 2009;69:4674-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson CD, Dunnick NR, Cohan RH, Illescas FF. Renal adenocarcinoma: CT staging of 100 tumors. AJR Am J Roentgenol. 1987;148:59-63. [DOI] [PubMed] [Google Scholar]
- 14. Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543-552. [DOI] [PubMed] [Google Scholar]
- 15. Lamb GW, Bromwich EJ, Vasey P, Aitchison M. Management of renal masses in patients medically unsuitable for nephrectomy—natural history, complications, and outcome. Urology. 2004;64:909-913. [DOI] [PubMed] [Google Scholar]
- 16. Donat SM, Diaz M, Bishoff JT, et al. Follow-up for clinically localized renal neoplasms: AUA guideline. J Urol. 2013;190:407-416. [DOI] [PubMed] [Google Scholar]
- 17. Vogelzang NJ, Stadler WM. Kidney cancer. Lancet. 1998;352:1691-1696. [DOI] [PubMed] [Google Scholar]
- 18. Sidhom OA, Basalaev M, Sigal LH. Renal cell carcinoma presenting as polymyalgia rheumatica. Resolution after nephrectomy. Arch Intern Med. 1993;153:2043-2045. [PubMed] [Google Scholar]
- 19. Gold PJ, Fefer A, Thompson JA. Paraneoplastic manifestations of renal cell carcinoma. Semin Urol Oncol. 1996;14:216-222. [PubMed] [Google Scholar]
- 20. Utz DC, Warren MM, Gregg JA, Ludwig J, Kelalis PP. Reversible hepatic dysfunction associated with hypernephroma. Mayo Clin Proc. 1970;45:161-169. [PubMed] [Google Scholar]
- 21. Pras M, Franklin EC, Shibolet S, Frangione B. Amyloidosis associated with renal cell carcinoma of the AA type. Am J Med. 1982;73:426-428. [DOI] [PubMed] [Google Scholar]


