Abstract
Premature birth is associated with increased risk of autism spectrum disorder. Antenatal maternal magnesium administration is known to reduce subsequent risk of cerebral palsy including among premature infants, suggesting a potentially broader neuroprotective role for magnesium. Our objective was to determine whether magnesium could be protective against autism spectrum disorders in premature infants. A cohort of 4855 preterm children was identified, magnesium levels from 24 to 48 hours of life recorded, and subsequent autism spectrum disorder status determined. Adjusted relative risk of autism spectrum disorder with each 1 mg/dL increase in neonatal magnesium level was 1.15 (95% confidence interval: 0.86-1.53). Analysis of variance indicated that magnesium levels varied by gestational age and maternal antenatal magnesium supplementation, but not autism spectrum disorder status (F 1,4824 = 1.43, P = .23). We found that neonatal magnesium levels were not associated with decreased autism spectrum disorder risk. Future research into autism spectrum disorder risks and treatments in premature infants is needed.
Keywords: autism, magnesium, prematurity, preterm
Preterm birth is defined as delivery occurring before 37 weeks’ gestation and is a major cause of morbidity and mortality.1 Neurodevelopmental disabilities associated with prematurity include cerebral palsy,2 intellectual disability,3 and autism spectrum disorder.4 Although each represents distinct areas of disability, all 3 disorders may cooccur. Intellectual impairment affects cognitive functioning; cerebral palsy presents with delayed motor milestones and abnormal muscle tone2; and autism spectrum disorder is characterized by social communication impairment and repetitive, nonfunctional behaviors and routines.5 Cerebral palsy in children born prematurely is most commonly associated with periventricular white matter injury,6 but autism spectrum disorder’s pathophysiology among children born preterm is more elusive and complex. Further, while not associated with periventricular injury, children with cerebral palsy are at higher risk for developing autism spectrum disorder, with a prevalence of autism spectrum disorder among children with cerebral palsy estimated at 7%.7
Interventions in the peri- and neonatal period can reduce some complications of preterm birth. Maternal antenatal steroids and infant surfactant administration attenuate respiratory distress syndrome and hyaline membrane disease8 (although postnatal steroid administration may worsen neurodevelopmental outcome [Doyle et al, 2014]),9 antibiotics treat infection, and temperature, fluid, and electrolyte management prevent hypothermia and dehydration.10 With the exception of erythropoietin,11 there are no treatments in premature infants for the long-term neurological sequelae of prematurity.
However, antenatal magnesium administration to women in preterm labor has demonstrated a modest protective effect against cerebral palsy when provided immediately before birth.12,13 The American College of Obstetricians and Gynecologists recommends the administration of antenatal magnesium to mothers facing imminent delivery between 24 and 31 6/7 weeks’ gestation.14 Serum magnesium levels in newborns track closely with maternal serum magnesium levels at the end of pregnancy for neonates both exposed and not exposed to magnesium supplementation.15 In neonates born from mothers not supplemented with antenatal magnesium, infant magnesium levels have been found to decrease with increasing gestational age.16–19 Less is known about how magnesium levels vary with gestational age among neonates exposed to maternal antenatal magnesium supplementation or how serum magnesium level variation among neonates corresponds with the magnitude of cerebral palsy risk reduction. Evidence is sparse and results are mixed in regard to the protective effect conferred by neonates’ innate magnesium level against cerebral palsy.20,21 Magnesium may provide neuroprotection by several possible mechanisms, including reducing brain injury from hypoxic ischemic events by blocking N-methyl-D-aspartic acid receptors and reducing calcium influx into neuronal cells,22 attenuating cell damage from free radical activity and inflammatory cytokines,22 and restoring normal axon connection development disrupted by hypoxia.23
To our knowledge, no prior studies have investigated the potential influence of neonatal magnesium levels on long-term autism spectrum disorder risk among children born preterm. The objective of the current study was to investigate the association between innate neonatal magnesium levels immediately following birth and autism spectrum disorder risk among a large cohort of children born before 37 weeks’ gestation.
Methods
Study Site and Population
Eligible infants were born <37 weeks’ gestation at an Intermountain Healthcare facility in Utah in calendar years 2002 to 2010. Intermountain Healthcare is a vertically integrated, not-for-profit healthcare system in the Intermountain West encompassing 23 hospitals including the region’s only children’s hospital. Utah’s ethnic distribution is predominantly white, non-Hispanic (79%)24; Utah’s prematurity rate (9.1%) is similar to the national average (9.6%).25 Inclusion criteria required that infants link to (1) maternal medical records, and have records for (2) birthweight and (3) magnesium level(s) (mg/dL) drawn 24 to 48 hours following birth. Exclusion criteria were the presence of known/likely genetic disorders, congenital hydrocephalus/brain malformations, epileptic encephalopathies, congenital heart disease, meningitis, encephalitis, stroke, traumatic head injury, or death.
Magnesium Exposure and Covariates
The first magnesium level drawn 24 to 48 hours following birth was chosen as the exposure of interest to (1) minimize the influence of maternal magnesium administration on measured levels and (2) maximize the number of preterm neonates with available magnesium levels, as levels were inconsistently drawn after 48 hours following birth. Pre/perinatal characteristics and maternal medical comorbidities obtained from the Intermountain Healthcare Enterprise Data Warehouse were as follows: date of birth, sex, race/ethnicity, birthweight, gestational age, 5-minute Apgar score, maternal diabetes (preexisting or gestational), maternal Medicaid insurance, and antenatal maternal magnesium administration. Normalized growth curves26 were used to stratify size for gestational age with 3 levels: small (birthweight <10th percentile), average, and large (birthweight >90th percentile) for gestational age.
Autism Spectrum Disorder Case Identification
Autism spectrum disorder case status was determined through linkage of the preterm cohort to the Utah Registry of Autism and Developmental Disabilities, a population-wide autism spectrum disorder surveillance system that identifies persons with ASD primarily using an administrative record approach inclusive of education and health sources.27 Autism spectrum disorder is a reportable health condition under Utah Health Code Chapter 26 Title 7 Section 4. Utah Registry of Autism and Developmental Disabilities is designated by the Utah Department of Health to maintain a registry of individuals who have received autism spectrum disorder–specific diagnostic codes (ie, International Classification of Diseases, Ninth Revision 299*). As part of a longstanding collaboration, the Utah State Board of Education provides information on children eligible for autism special education services. Utah Registry of Autism and Developmental Disabilities identifies a child with autism spectrum disorder based on receipt of an autism spectrum disorder medical diagnosis from a qualified provider and/or a Utah State Board of Education autism special education report. Autism spectrum disorder case status was ascertained through 2014 for the 2002 to 2010 birth cohorts.
Statistical Analyses
Wilcoxon signed-rank, Student t, and χ2 tests were used to compare children’s characteristics with and without autism spectrum disorder. For evaluation of the relationship between neonatal magnesium level and autism spectrum disorder risk, a method similar to that of Ostrander et al21 was used: Discrete proportional hazards regression models were fit using generalized estimating equations with logarithmic link functions and a working independent correlation structure. Further, the age of autism spectrum disorder, diagnosis was related to neonatal magnesium level with separate binary indicator variables for a positive diagnosis defined for the following age intervals: birth to age 3, age 4 through age 6, and age 7 or older. By defining that autism spectrum disorder is present or absent at birth, and assuming that the age at which autism spectrum disorder diagnosis is made is unrelated to neonatal magnesium or the other covariates, the relative risks from the generalized estimating equations analysis can be interpreted as closely approximating the relative risks relating autism spectrum disorder to neonatal magnesium.21 Models were adjusted for potential confounders including infant sex, birth weight, gestational age, race/ethnicity, 5-minute Apgar score, maternal magnesium administration, maternal diabetes mellitus and gestational diabetes, and Medicaid insurance. To estimate subgroup-specific associations, the model was further stratified by early preterm birth (≤32 weeks’ gestation) and exposure to antenatal maternal magnesium administration.
To explore how neonatal magnesium levels varied across gestational age at delivery among mothers who did and did not receive antenatal magnesium supplementation, a 3-way analysis of variance (ANOVA) including the 3-way interaction term was used to test for mean differences in magnesium levels by gestational age, autism spectrum disorder case status, and presence or absence of antenatal magnesium administration. For the ANOVA, gestational age for all weeks other than 36 weeks was binned into 2-week groups (eg, 22-23, 24-25 weeks, etc) due to low numbers of autism spectrum disorder cases by individual gestational age weeks (eg, no children with autism spectrum disorder were 22 or 24 weeks’ gestational age at birth).
Study approvals were obtained from the Utah Registry of Autism and Developmental Disabilities Oversight Committee, University of Utah Institutional Review Board, Intermountain Healthcare Institutional Review Board, Utah State Board of Education Institutional Review Board, and Utah Department of Health Institutional Review Board. All analyses were conducted in SAS version 9.4 (SAS Institute Inc, Cary, North Carolina); an α of .05 was selected to assess statistical significance.
Results
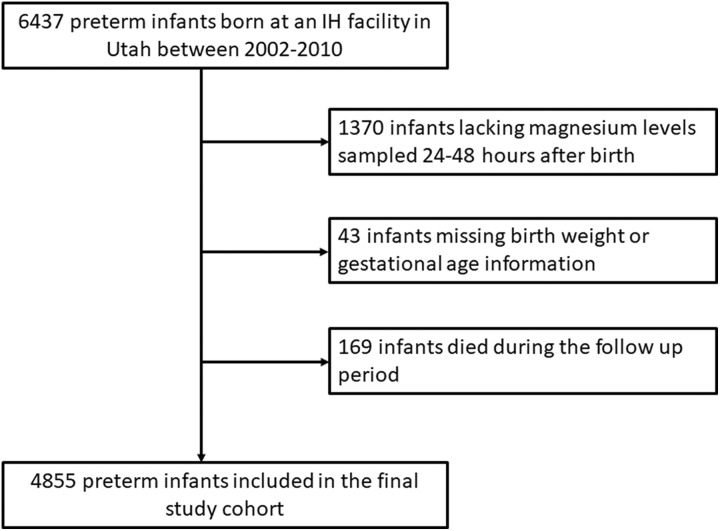
Between 2002 and 2010, there were a total of 6437 preterm births at an Intermountain Healthcare facility in Utah who met the inclusion and exclusion criteria (see “Methods”). In all, 1582 patients had no birthweight record, magnesium level in the first 48 hours of life, or had died prior to determination of autism spectrum disorder status; the final study cohort consisted of 4855 preterm infants (Figure 1). One hundred twelve children in this cohort were classified with autism spectrum disorder (2.3%) between 2004 and 2014. The following differences were observed between children with and without autism spectrum disorder: proportion of males (77% vs 55%, P < .0001), median gestational age (32 vs 33 weeks, P = .008), median birthweight (1685 vs 1990 g, P = .001), and median 5-minute Apgar score (8 vs 9, P = .009; Table 1).
Figure 1.
Flow diagram depicting identification of the final study cohort.
Table 1.
Characteristics of Children With and Without Autism Spectrum Disorder.
| Characteristic | Unaffected (N = 4743) | Autism Spectrum Disorder cases (N = 112) | P | ||
|---|---|---|---|---|---|
| n (%) or as Shown | n (%) or as Shown | ||||
| Median serum magnesium, mg/dL (IQR) | 1.80 (1.60-2.30) | 1.90 (1.70-2.50) | .29a | ||
| Male | 2676 | (56.4%) | 86 | (77%) | <.0001b |
| Median gestational age, weeks (IQR) | 33.00 (30.00-35.00) | 32.00 (27.50-35.00) | .008a | ||
| Median birthweight, g, (IQR) | 1990.00 (1503.00-2435.00) | 1685.50 (1030.50-2433.00) | .001a | ||
| Median 5 minute Apgar score, (IQR) | 9 (8-9) | 8 (7-9) | .009a | ||
| Size for gestational age | .18b | ||||
| Small for gestational age | 704 | (14.8%) | 19 | (17%) | |
| Average for gestational age | 3910 | 82.5% | 93 | 83% | |
| Large for gestational age | 129 | 2.7% | 0 | 0% | |
| Race/ethnicityc | .43b | ||||
| White | 3819 | 84.3% | 95 | 89% | |
| Hispanic | 531 | 11.7% | 9 | 9% | |
| Other | 187 | 4% | 3 | 3% | |
| Antenatal magnesium administration | 1558 | 32.9% | 39 | 35% | .66b |
| Medicaid insurance | 1533 | 32.3% | 33 | 30% | .52b |
| Diabetesd | 423 | 8.9% | 13 | 12% | .33b |
Abbreviation: IQR, interquartile range.
a Wilcoxon signed-rank test P value.
b χ2 test P value.
c Number with missing data = 211.
d Includes diabetes mellitus and gestational diabetes.
The relative risk ratio of autism spectrum disorder associated with each 1 mg/dL increase in neonatal magnesium level was 1.15 (95% confidence interval: 0.91-1.39, P = .29; Table 2). The null relationship between neonatal magnesium level and autism spectrum disorder risk was robust to adjustment by potential confounders (relative risk =1.15; 95% confidence interval: 0.86-1.53, P = .36) and by subgroup stratification by early preterm birth or presence of maternal antenatal magnesium administration. The risk of autism spectrum disorder associated with neonate magnesium level among early and late preterm birth was 1.20 (95% confidence interval: 0.87-1.64, P = .26) and 0.91 (95% confidence interval: 0.48-1.74, P = .78), respectively. The risk of autism spectrum disorder associated with neonate magnesium level among mothers with and without antenatal magnesium administration was 1.21 (95% confidence interval: 0.87-1.68; P = .26) and 0.83 (95% confidence interval: 0.41-1.68; P = .61), respectively.
Table 2.
Adjusted Relative Risk of Autism Spectrum Disorder Associated With Neonatal Serum Magnesium Levels From a Multivariable Discrete Proportional Hazard Regression Model.
| Variable | RR (95% CI) | P |
|---|---|---|
| Serum magnesium, mg/dL | 1.15 (0.86-1.53) | .36 |
| Male | 2.81 (1.78-4.44) | <.0001 |
| 5-minute Apgar score | 0.96 (0.86-1.07) | .45 |
| Birthweight, g | 0.75 (0.51-1.09) | .13 |
| Gestational age, weeks | 0.97 (0.88-1.06) | .49 |
| Race/ethnicity | ||
| White | Reference | |
| Hispanic | 0.60 (0.28-1.31) | .20 |
| Other | 0.62 (0.20-1.99) | .43 |
| Antenatal magnesium administration | 0.79 (0.47-1.34) | .38 |
| Medicaid insurance | 0.94 (0.59-1.50) | .79 |
| Diabetesa | 1.24 (0.68-2.25) | .49 |
Abbreviations: CI, confidence interval; RR, relative risk.
a Includes diabetes mellitus and gestational diabetes.
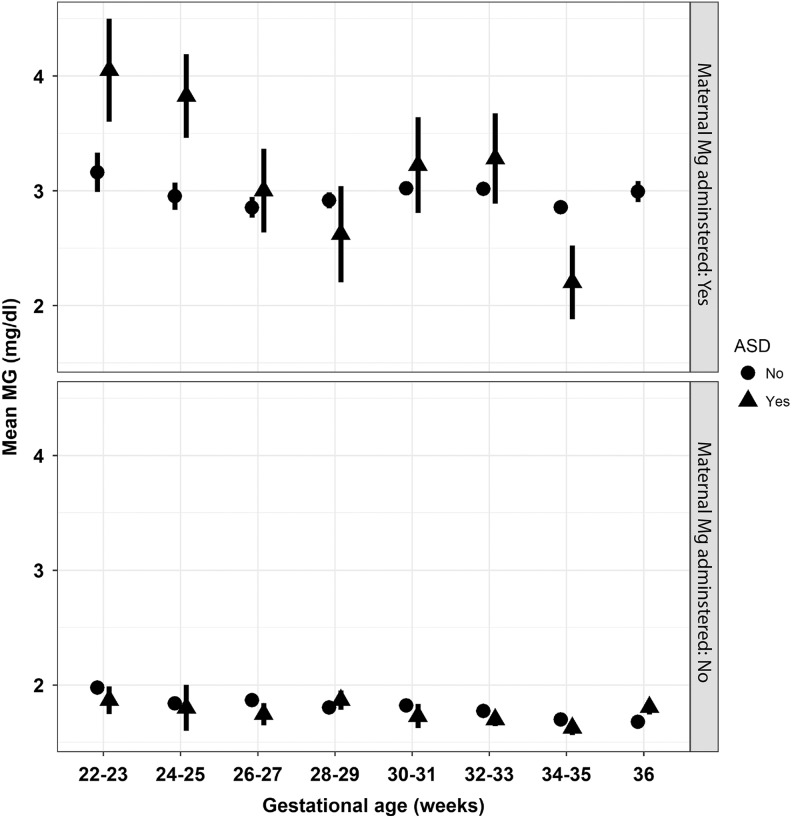
Figure 2 displays mean (±standard error) magnesium levels among preterm infants across gestational age by autism spectrum disorder status (yes vs no) and stratified by the presence or absence of antenatal maternal magnesium supplementation. The 3-way ANOVA (F 30,4824 = 148.28, P < .0001) demonstrated mean differences in magnesium levels by gestational age (F 7,4824 = 3.13, P = .0027) and maternal antenatal magnesium supplementation, but not autism spectrum disorder case status (mean [standard deviation] = 2.23 [0.95] among autism spectrum disorder cases vs mean [standard deviation] = 2.15 [0.83] among non-autism spectrum disorder–affected neonates (F 1,4824 = 1.43, P = .23)). Higher mean magnesium levels were associated with the presence versus absence of antenatal magnesium administration (mean [standard deviation] = 2.97 [0.97] vs 1.75 [0.30]; F 1,4824 = 287.78, P < .0001, respectively). While the gestational age by autism spectrum disorder case status (F 7,4824 = 1.67, P = .11) and the antenatal magnesium administration by autism spectrum disorder case status (F 1,4824 = 3.02, P = .08) interactions were not statistically significant, the interaction between gestational age and antenatal magnesium administration was statistically significant (F 7,4824 = 2.55, P = .01). Finally, the 3-way interaction between gestational age, antenatal magnesium supplementation, and autism spectrum disorder case status was not statistically significant (F 6,4824 = 1.79, P = .10).
Figure 2.
Mean magnesium levels (±standard error) across gestational age among children with and without autism spectrum disorder and stratified by the presence or absence of antenatal magnesium supplementation (top and bottom panels, respectively).
Discussion
To our knowledge, this is the first study to examine for the association between neonatal magnesium levels and autism spectrum disorder risk. We hypothesized a protective relationship between higher neonatal magnesium levels and lower autism spectrum disorder risk based on the well-supported yet modest association found between antenatal magnesium administration and reduced cerebral palsy risk.12,22 However, our study did not find a statistically significant association. Further, our null finding was robust to stratification by early preterm birth and exposure to maternal antenatal magnesium supplementation. In contrast, complementary studies investigating the association between neonatal magnesium levels and cerebral palsy risk have yielded mixed results (Doll et al, 2014).21 Doll et al20 reported an inverse relationship between higher average serum magnesium levels and motor functioning measured at 20 to 36 months of age among 75 preterm infants. Conversely, Ostrander et al21 did not find a protective effect of magnesium levels drawn between 24 and 48 hours of life on risk of developing epilepsy or motor impairment in a large cohort of preterm Utah births. However, it is notable that Ostrander et al also did not find a reduction in cerebral palsy from maternal magnesium administration. This suggests that the efficacy of magnesium on outcomes may be tempered by an unrecognized effect, for example, socioeconomic or genetic background of the study population.
Mechanisms that may impart magnesium’s neuroprotective effects for cerebral palsy could also be relevant to the pathophysiological origins of autism spectrum disorder. Magnesium may have multiple effects, including preventing axon pathfinding errors23 and limiting neuronal injury from hypoxic events and inflammation by reducing glutamate excitotoxicity and neuronal cell death.28 Further, cerebral palsy and autism spectrum disorder could be caused by separate mechanisms. Even if cerebral palsy and autism spectrum disorder share common pathophysiological features, our study suggest that cerebral palsy risk is more amenable than autism spectrum disorder risk to antenatal magnesium administration. Finally, differences in timing or mechanism of neuronal injury leading to cerebral palsy or autism spectrum disorder would then affect the timing and effectiveness of magnesium administration.
Only 1% of the body’s magnesium is extracellular, plasma levels are determined by renal functioning, and homeostatic magnesium load remains constant during pregnancy.16 While the current study is consistent with previous studies that demonstrate how neonatal magnesium levels decline with advancing gestational age among neonates not exposed to antenatal maternal magnesium supplementation,16–19 findings also suggest that the same pattern exists among neonates exposed to antenatal maternal magnesium supplementation. Interestingly, although not significant, we did note that in infants of early preterm birth, compared to late preterm/near-term birth, that for infants exposed to antenatal maternal magnesium supplementation, infants who subsequently developed an autism spectrum disorder had higher serum magnesium levels. Thus, future studies may need to take into consideration the gestational age of the infant, for example, considering potential differences in magnesium stores or metabolism based on gestational age.
Our analysis was limited to neonatal magnesium levels drawn 24 to 48 hours following birth to reduce the influence of antenatal magnesium administration; however, our results clearly demonstrate that antenatal magnesium administration impacts the neonate’s plasma magnesium levels during this time. Elevated magnesium levels in neonates resulting from antenatal maternal magnesium administration have been shown to fall significantly during the first 48 hours after birth, but do remain elevated through at least the first 72 hours of life.29 It is unclear whether antenatal magnesium exposure impacts the neonate’s magnesium homeostatic load beyond elevation in the peripheral circulation.
A meta-analysis conclusively established the association between antenatal magnesium administration and reduced cerebral palsy risk, though the effect size was small.12 If a protective effect of neonatal magnesium levels on autism spectrum disorder risk was of similar magnitude, limitations inherent to this study cohort could mask detection. For example, with a mean elevation of 6100 ft,30 Utah has the third highest average elevation in the United States. Higher elevations are implicated in obstetrical complications related to prematurity (ie, low birth weight, gestational hypertension, preeclampsia)31,32 and may negate a modest protective effect against neuronal injury from inflammation or hypoxia. This is consistent with a previously published study from our group that also failed to show a protective effect of magnesium on cerebral palsy risk related to either antenatal magnesium administration or neonatal magnesium levels.21 Utah’s relative homogeneity in racial/ethnic composition also limits this cohort’s genetic variability. Homeostatic levels of magnesium are genetically determined by several gene polymorphisms,33 and subgroup analysis of neonatal outcomes associated with antenatal maternal magnesium administration supports differential effects across racial groups.34 Subsequently, further study of neonatal magnesium and autism spectrum disorder risk among more diverse groups located at sea level might yield different findings. Study limitations also include the use of autism spectrum disorder classification as a dichotomous outcome variable. Use of a continuous measure of social communication ability would better reflect the autism spectrum disorder spectrum and improve the study’s power to detect a small effect size.
Study strengths include the use of a retrospective cohort design and high autism spectrum disorder capture rate (2.3%). Utah Registry of Autism and Developmental Disabilities uses both medical and education sources to identify children with autism spectrum disorder, thereby providing thorough autism spectrum disorder ascertainment. Pre-/perinatal risk factors were also ascertained using maternal and neonatal medical records rather than birth certificate records. Medical records exceed birth certificates in accuracy and thoroughness for maternal and neonatal medical interventions and complications.35–37
Ideally, we would have available a series of serum magnesium levels at equal intervals in the premature infant, which we could examine for their relationship to the risk for autism spectrum disorder. However, because there was only the reliable presence of serum magnesium levels in the first several days of life, our study had to be constrained to the available data. Further, because the magnesium level in the first 24 hours of life corresponded precisely with whether antenatal magnesium supplementation was performed, these 2 factors (magnesium level in the first 24 hours of life and maternal magnesium supplementation) acted essentially as a single variable. For this reason, we only considered the variable of maternal magnesium supplementation.
Our findings suggest a null association between magnesium levels in preterm infants during their second day of life and autism spectrum disorder. However, animal models and human studies demonstrate magnesium’s neuroprotective effects for cerebral palsy,12,22,28 which support continued efforts to identify key biochemical and genetic targets of magnesium action in premature infants. Additional studies into the fundamental genetic and biochemical mechanisms associated with the adverse effects of prematurity could also identify other molecular targets. Future studies can enhance our understanding of magnesium’s potential influence on fetal and neonatal brain development in the context of autism spectrum disorder by including a larger, more racially/ethnically diverse sample, implementing a continuous, quantitative measure of social communication ability to assess autism spectrum disorder phenotype, and investigating this relationship in a cohort born at lower elevation.
Acknowledgments
Autism spectrum disorder surveillance efforts were supported by the Utah Department of Health through the Utah Registry of Autism and Developmental Disabilities. Thank you to our health and education data sources and to Intermountain Healthcare for their on-going collaborations.
Footnotes
Author’s Contributors: AVB conceptualized and designed the study, coordinated the data from the Utah Registry of Autism and Developmental Disabilities, conducted the analysis, and reviewed and revised the manuscript. DAB conceptualized and designed the study and reviewed and revised the manuscript. EKK coordinated and collected the data and critically reviewed the manuscript. JLB conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript.
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Although unrelated to autism spectrum disorder, Dr. Bilder serves as a consultant and advisory board member to Audentes Therapeutics and BioMarin Pharmaceutical.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Project funding was provided in part by the National Institutes of Health grants DP2 MH100008 and R01 MH094400.
Ethical Approval: Study approvals were obtained from the URADD Oversight Committee, University of Utah Institutional Review Board (IRB), IH IRB, USBE IRB, and UDOH IRB.
References
- 1. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi:10.1016/s0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. Feb 2007;109:8–14. [PubMed] [Google Scholar]
- 3. Bilder D, Pinborough-Zimmerman J, Bakian A, et al. Prenatal, perinatal, and neonatal factors associated with intellectual disability. Am J Intellect Dev Disabil. 2013;118(2):156–176. [DOI] [PubMed] [Google Scholar]
- 4. Joseph RM, O’Shea TM, Allred EN, et al. Prevalence and associated features of autism spectrum disorder in extremely low gestational age newborns at age 10 years. Autism Res. 2017;10(2):224–232. doi:10.1002/aur.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010;167(11):1357–1363. doi:10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- 6. Hoon AH, Faria AV. Pathogenesis, neuroimaging and management in children with cerebral palsy born preterm. Dev Disabil Res Rev. 2010;16(4):302–312. doi:10.1002/ddrr.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christensen D, Van Naarden Braun K, Doernberg NS, et al. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning—Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol. 2013;56(1):59–65. doi:10.1111/dmcn.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454 doi: 10.1002/14651858.CD004454.pub3. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doyle LW, Ehrenkranz RA, Halliday HL. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2014;(5):CD001146. [DOI] [PubMed] [Google Scholar]
- 10. Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fischer HS, Reibel NJ, Bührer C, Dame C. Prophylactic early erythropoietin for neuroprotection in preterm infants: a meta-analysis. Pediatrics. 2017;139(5). pii: e20164317. [DOI] [PubMed] [Google Scholar]
- 12. Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009;(1):CD004661 doi:10.1002/14651858.cd004661.pub2. [DOI] [PubMed] [Google Scholar]
- 13. Rouse DJ, Hirtz DG, Thom E, et al. Eunice kennedy shriver NICHD maternal-fetal medicine units network: a randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359(9):895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American College of Obstetricians and Gynecologists. Magnesium sulfate before anticipated preterm birth for neuroprotection. Patient safety checklist no. 7. Obstet Gynecol. 2012;120:432–433. [DOI] [PubMed] [Google Scholar]
- 15. Rigo J, Pieltain C, Christmann V, et al. Serum magnesium levels in preterm infants are higher than adult levels: a systematic literature review and meta-analysis. Nutrients. 2017;9(10):E1125 doi:10.3390/nu9101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ariceta G, Rodriguez-Soriano J, Vallo A. Magnesium homeostasis in premature and full-term neonates. Pediatr Nephro. 1995;9(4):423–427. doi:10.1007/bf00866716. [DOI] [PubMed] [Google Scholar]
- 17. Fenton TR, Lyon AW, Rose MS. Cord blood calcium, phosphate, magnesium, and alkaline phosphatase gestational age-specific reference intervals for preterm infants. BMC Pediatr. 2011;11:76 doi:10.1186/1471-2431-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Standley CA, Whitty JE, Mason BA, Cotton DB. Serum ionized magnesium levels in normal and preeclamptic gestation. Obstet Gynecol. 1997;89(1):24–27. doi:10.1016/s0029-7844(96)00380-8. [DOI] [PubMed] [Google Scholar]
- 19. Stigson L, Kjellmer I. Serum levels of magnesium at birth related to complications of immaturity. Acta Paediatr. 1997;86(9):991–994. doi:10.1111/j.1651-2227.1997.tb15185.x. [DOI] [PubMed] [Google Scholar]
- 20. Doll E, Wilkes J, Cook LJ, et al. Neonatal magnesium levels correlate with motor outcomes in premature infants: a long-term retrospective cohort study. Front Pediatr. 2014;2:120 doi:10.3389/fped.2014.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ostrander B, Bardsley T, Korgenski EK, Greene T, Bonkowsky JL. Neonatal magnesium levels between 24 and 48 hours of life and outcomes for epilepsy and motor impairment in premature infants. Pediatr Neuro. 2016;59:41–46. doi:10.1016/j.pediatrneurol.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rouse D, Hirtz DG, Thom E, et al. A randomized controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359(9):895–905. doi:10.1016/j.ajog.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stevenson TJ, Trinh T, Kogelschatz C, et al. Hypoxia disruption of vertebrate CNS pathfinding through ephrinB2 is rescued by magnesium. PLoS Genet. 2012;8(4):e1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US Census Bureau. 2011-2015 American Community Survey 5-Year Estimates. American FactFinder—Results. 2010. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?%20src=CF. Accessed August 11, 2017.
- 25. Murphy SL, Mathews TJ, Martin JA, Minkovitz CS, Strobino DM. Annual summary of vital statistics: 2013–2014. Pediatrics. 2017;139(6):e20163239 doi:10.1542/peds.2016-3239. [DOI] [PubMed] [Google Scholar]
- 26. Alexander G, Himes J, Kaufman R, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;7(2):163–168. [DOI] [PubMed] [Google Scholar]
- 27. Bakian AV, Bilder DA, Coon H, McMahon WM. Spatial relative risk patterns of autism spectrum disorders in Utah. J Autism Dev Disord. 2015;45(4):988–1000. doi:10.1007/s10803-014-2253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chang E. Preterm birth and the role of neuroprotection. BMJ. 2015;350:g6661 doi:10.1136/bmj.g6661. [DOI] [PubMed] [Google Scholar]
- 29. Donovan EF, Tsang RC, Steichen JJ, Strub RJ, Chen I-W, Chen M. Neonatal hypermagnesemia: effect on parathyroid hormone and calcium homeostasis. J Pediatr. 1980;96(2):305–310. doi:10.1016/s0022-3476(80)80835-3. [DOI] [PubMed] [Google Scholar]
- 30. Sawe BE. US States With The Highest Average Elevations. World Atlas; 2016. http://www.worldatlas.com/articles/us-states-with-the-highest-average-elevations.html. Accessed October 23, 2017. [Google Scholar]
- 31. Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Public Health. 1997;87(6):1003–1007. doi:10.2105/ajph.87.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zamudio S. High-altitude hypoxia and preeclampsia. Front Biosci. 2007;12:2967–2977. doi:10.2741/2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang X, Glessner J, Tin A, et al. Genome-wide association study reveals two loci for serum magnesium concentrations in European-American children. Sci Rep. 2015;5:18792 doi:10.1038/srep18792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vilchez G, Dai J, Kumar K, Mundy D, Kontopoulos E, Sokol RJ. Racial/ethnic disparities in magnesium sulfate neuroprotection: a subgroup analysis of a multicenter randomized controlled trial. J Matern Fetal Neonatal Med. 2017;31(17):2304–2311. doi:10.1080/14767058.2017.1342795. [DOI] [PubMed] [Google Scholar]
- 35. Buescher PA, Taylor KP, Davis MH, Bowling JM. The quality of the new birth certificate data: a validation study in North Carolina. Am J Public Health. 1993;83(8):1163–1165. doi:10.2105/ajph.83.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dobie SA, Baldwin LM, Rosenblatt RA, Fordyce MA, Andrilla CH, Hart LG. How well do birth certificates describe the pregnancies they report? The Washington State experience with low-risk pregnancies. Matern Child Health J. 1998;2(3):145–154. [DOI] [PubMed] [Google Scholar]
- 37. Green DC, Moore JM, Adams MM, Berg CJ, Wilcox LS, McCarthy BJ. Are we understanding rates of vaginal birth after previous cesarean birth? The validity of delivery methods from birth certificates. Am J Epidemiol. 1998;147(6):581–586. doi:10.1093/oxfordjournals.aje.a009490. [DOI] [PubMed] [Google Scholar]