Abstract
The efficiency of telestroke programs in improving the rates of recombinant tissue plasminogen activator (rtPA) in stroke patients has been reported. Previous studies have reported favorable treatment outcomes with the use of telestroke programs to improve the use of rtPA, but functional outcomes are not fully understood. This study investigated the effect of telestroke technology in the administration of rtPA and related functional outcomes associated with baseline clinical variables. Retrospective data of a telestroke registry were analyzed. Univariate analysis was used to compare demographic and clinical variables in the rtPA group and the no rtPA group and between the improved functional ambulation group and the no improvement group. A stepwise binary logistic regression identified factors associated with improved functional outcome in the total telestroke population and in the subset of the telestroke population who received rtPA. In adjusted analysis and elimination of any multicollinearity for patients who received rtPA in the telestroke setting, obesity (odds ratio [OR] = 2.138, 95% confidence interval [CI], 1.164-3.928, P < .05), higher systolic blood pressure at the time of presentation (OR = 1.015, 95% CI, 1.003-1.027, P < .05), and baseline high-density lipoprotein at the time of admission (OR = 1.032, 95% CI, 1.005-1.059, P < .05) were associated with improved functional outcomes. Increasing age (OR = 0.940, 95% CI, 0.916-0.965, P < .0001) and higher calculated National Institutes of Health Stroke Scale (OR = 0.903, 95% CI, 0.869-0.937) were associated with a poorer outcome in rtPA-treated patients. Telestroke technology improves functional outcomes at spoke stations where neurological expertise is unavailable. Further studies are necessary to determine how telestroke technology can be optimized, especially to improve contraindications and increase eligibility for thrombolysis therapy.
Keywords: Acute ischemic stroke, recombinant tissue plasminogen activator (rtPA), telestroke
Introduction
Telestroke programs are a means to reach out to patients who are eligible for intravenous thrombolytic therapy but may not otherwise treated because of lack of immediate access to neurology expertise at their respective community hospitals. The accuracy of telestroke evaluated rtPA-treated patients compared with bedside evaluations has been investigated extensively.1–3 Findings of no significant difference between the 2 approaches justify that telestroke evaluations are indeed effective in accurately diagnosing acute ischemic strokes. Telestroke technology supports the safe use of intravenous rtPA with emphasis on effective treatment strategies. Such strategies have been optimized at both the hub and spoke sites to promote the accuracy of stroke diagnosis in patients receiving rtPA via telemedicine.4,5 The diagnosis of acute ischemic stroke in telestroke-guided rtPA-treated patients is based on the accuracy of video and teleradiological evaluations of brain scans.6 This ensures that physicians make appropriate treatment decisions with good treatment outcomes.7 The accuracy in treatment decision-making strengthens the ability of telestroke programs to improve the rates of rtPA administration in stroke patients.8
Treatment outcomes in telestroke programs have been favorable and consistent with good expectations in several studies.9,10 However, specific functional outcomes in patients receiving rtPA via telestroke technology are not fully understood. Functional outcome data are necessary to further ensure that telestroke medicine can reliably and effectively improve the rate of rtPA in the treatment of acute ischemic stroke patients.11 A functional outcome study is necessary to identify baseline clinical variables that are associated with rtPA treatment in a telestroke setting. For instance, if a specific clinical or demographic factor is associated with improvement or no improvement in thrombolytic therapy, that particular risk factor associated with thrombolysis efficacy may not be present in the same proportions among rtPA-treated and rtPA-excluded stroke populations. Our first objective was to identify various risk factors within rtPA-treated stroke populations and determine whether these risk factors differed between rtPA-treated and rtPA-excluded stroke populations. Because rtPA-treated patients do not present the same clinical variables in a telestroke stroke population, our second objective was to determine whether the interaction of different baseline clinical variables with rtPA treatment is associated with improved functional outcome using retrospective data of acute ischemic stroke patients from a telestroke registry.
Materials and Methods
The goal of this study was to investigate functional outcomes associated with rtPA treatment of stroke patients and identify clinical variables that are associated with functional outcomes in telestroke-facilitated rtPA treatment of stroke patients. This study was approved by the Institutional Review Board of Greenville Health System (GHS) institutional committee for ethics. Retrospective data collected from the GHS telestroke system (Neuro-Direct) were used. Charts were reviewed manually to extract variables from the registry. Patients treated at the telestroke spokes between October 14, 2014, and June 25, 2016, included 559 telestroke patients. Out of this, 454 had a final diagnosis of acute ischemic stroke, 343 presented within the 4.5-hour time protocol for rtPA administration. Of these patients, 36 were excluded because of contraindications and 307 were treated with rtPA. Reasons for exclusion include mild stroke, clinical improvement in symptoms, too mild or rapidly improving symptoms, failure to meet 4.5-hour time protocol, emergency department referral delay, and significant comorbidity such as hemorrhage on computed tomography (CT).
Data were collected from patients with signs and symptoms of acute ischemic stroke and those who used telestroke technology within 4.5 hours of symptom onset. Chart review identified patients who were diagnosed with CT for radiological interpretation of intracranial hemorrhage. Baseline data including demographic characteristics such as age, sex, race, and ethnicity were collected. Clinical variables including National Institutes of Health Stroke Scale (NIHSS) score, history of hypertension, diabetes mellitus, atrial fibrillation, smoking, substance abuse, and alcohol use were collected. Ambulatory outcomes associated with acute ischemic stroke after intravenous rtPA administration were also documented. Ambulatory ability prior to the current event, at admission, and at discharge was determined. Patients were assigned a score (0-3) based on the following possible respective outcomes: undocumented (0), unable to ambulate (1), able to ambulate with assistance (2), and able to ambulate independently (3). Changes in ambulation were tracked from admission to discharge to evaluate potential risks and benefits of rtPA therapy in stroke patients.
Data analysis
All statistical analyses were performed using SPSS Statistics Software version 15.0 (Chicago, IL, USA) and a P < .05 was used to establish statistical significance in all comparisons between groups. The data set of acute ischemic stroke patients presented via telestroke was divided into 2 groups based on administration of recombinant tissue plasminogen activator (“rtPA group” and “no rtPA group”). The data set was further divided based on functional outcome as measured by improved ambulatory status (“improved functional outcome”) at discharge compared with the time of presentation. Patients’ ambulatory status was used as a metric to measure functional outcomes in rtPA-treated patients. The reliability and validity of ambulation as an assessment tool for functional recovery in the mobility of stroke patients is well-documented in the literature.12,13 The study assesses recovery progress in each patient and determines efficacy of rtPA therapy on restoration of functional mobility. Independent ambulation was evaluated and used to develop a model for functional outcome. This is important considering that recovery of upper or lower limb motor impairment after ischemic stroke may be proportional to the degree of recovery in affected corticospinal tract and general motoric functions after stroke. To compute functional ambulatory improvement, a new variable was defined from the existing data. If there was improvement from the time of admission to the time of discharge, a value of 1 was given, if there was no improvement, a value of 0 was given. This was used to build a model for improved functional outcomes for stroke patients that received rtPA.
Descriptive statistics were calculated for the demographic and clinical characteristics of patients in each group. For all continuous variables, the mean, standard deviation, and range were calculated and presented in Table 1. Comparisons were made between the group of patients with improved functional outcomes and the group of patients without improved functional outcomes using 2-tailed, independent sample Student t tests (parametric test). For all discrete variables, the number of patients and percentage of patients in that category were also calculated. Comparisons between the improved functional outcome group and the no improvement group were made using Pearson χ2 analyses (nonparametric test). A binary logistic regression was performed to further explore clinical variables that were associated with improved functional outcomes within the acute ischemic stroke population (rtPA and no rtPA). The results of this multivariate analysis are presented in Table 2. A stepwise binary logistic regression was performed with variables added to the regression model on the condition that P < .10 for that particular variable. This was used to determine the factors associated with improved functional outcome in the rtPA-treated stroke population and in the telestroke population who did not receive rtPA (Table 3). All 3 binary logistic regression models were further tested using a Hosmer-Lemeshow test, overall correct classification percentage, and the area under the receiver operating characteristic (ROC) curve for score prediction. Multicollinearity and significant interactions between independent variables were examined using variance inflation factors.
Table 1.
Comparison of demographics and clinical characteristics of acute ischemic stroke patients in the telestroke unit.
| Characteristic | No rtPA group |
rtPA group | ||||
|---|---|---|---|---|---|---|
| No improvement | Improvement | No improvement | Improvement | |||
| No. of patients | 12 | 24 | P value | 85 | 222 | P value |
| Patient age, y | ||||||
| Mean ± SD | 71.7 ± 17.7 | 62.4 ± 10.3 | .115 | 71.6 ± 11.0 | 61.2 ± 14.7 | <.001* |
| Age group, y, No. (%) | ||||||
| <50 | 1 (8.3) | 1 (4.2) | .013* | 1 (1.2) | 28 (12.6) | <.001* |
| 50-59 | 2 (16.7) | 4 (16.7) | 7 (8.2) | 46 (20.7) | ||
| 60-69 | 1 (8.3) | 9 (37.5) | 14 (16.5) | 51 (23.0) | ||
| 70-79 | 1 (8.3) | 8 (33.3) | 24 (28.2) | 56 (25.2) | ||
| ⩾80 | 7 (58.3) | 2 (8.3) | 39 (45.9) | 41 (18.5) | ||
| Gender | ||||||
| Male | 7 (58.3) | 13 (54.2) | .813 | 39 (45.9) | 115 (51.8) | .353 |
| Female | 5 (41.7) | 11 (45.8) | 46 (54.1) | 107 (48.2) | ||
| Race, No. (%) | ||||||
| White | 8 (66.7) | 20 (83.3) | .269 | 65 (76.5) | 182 (82) | .187 |
| African American | 1 (8.3) | 4 (16.7) | 17 (20) | 32 (14.4) | ||
| Other | 1 (8.3) | 0 (0.0) | 0 (0.0) | 5 (2.3) | ||
| Hispanic ethnicity, No. (%) | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 7 (3.2) | .098 |
| Body mass index | ||||||
| Mean ± SD | 27.7 ± 5.7 | 31.3 ± 9.8 | .244 | 28.5 ± 7.7 | 29.6 ± 8.6 | .334 |
| Medical history, No. (%) | ||||||
| Atrial fibrillation/flutter | 3 (25.0) | 2 (8.3) | .173 | 15 (17.6) | 16 (7.2) | .007* |
| Coronary artery disease | 3 (25.0) | 9 (37.5) | .453 | 31 (36.5) | 74 (33.3) | .604 |
| Carotid stenosis | 0 (0.0) | 1 (4.2) | .473 | 4 (4.7) | 12 (5.4) | .805 |
| Pregnant | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA |
| Depression | 0 (0.0) | 4 (16.7) | .134 | 12 (14.1) | 37 (16.7) | .585 |
| Diabetes | 4 (33.3) | 14 (58.3) | .157 | 39 (45.9) | 75 (33.8) | .050* |
| Substance abuse | 0 (0.0) | 1 (4.2) | .473 | 0 (0.0) | 12 (5.4) | .029* |
| Dyslipidemia | 5 (41.7) | 15 (62.5) | .236 | 46 (54.1) | 121 (54.5) | .951 |
| Family history of stroke | 0 (0.0) | 0 (0.0) | NA | 8 (9.4) | 33 (14.9) | .209 |
| Heart failure | 4 (33.3) | 3 (12.5) | .137 | 15 (17.6) | 16 (7.2) | .007 |
| Hormone replacement therapy | 0 (0.0) | 0 (0.0) | NA | 2 (2.4) | 7 (3.2) | .710 |
| Hypertension | 9 (75.0) | 20 (83.3) | .551 | 70 (82.4) | 168 (75.7) | .210 |
| Migraine | 0 (0.0) | 1 (4.2) | .473 | 2 (2.4) | 8 (3.6) | .581 |
| Obesity | 2 (16.7) | 14 (58.3) | .018* | 38 (44.7) | 130 (58.6) | .029* |
| Previous stroke | 0 (0.0) | 7 (29.2) | .037* | 12 (14.1) | 60 (27.0) | .017* |
| Previous TIA | 1 (8.3) | 3 (12.5) | .708 | 7 (8.2) | 28 (12.6) | .280 |
| Prosthetic heart valve | 0 (0.0) | 0 (0.0) | NA | 1 (1.2) | 0 (0.0) | .106 |
| Peripheral vascular disease | 0 (0.0) | 1 (4.2) | .473 | 8 (9.4) | 17 (7.7) | .615 |
| Renal insufficiency | 0 (0.0) | 1 (4.2) | .473 | 7 (8.2) | 9 (4.1) | .140 |
| Sickle cell | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA |
| Sleep apnea | 0 (0.0) | 1 (4.2) | .473 | 4 (4.7) | 7 (3.2) | .512 |
| Smoking | 3 (25.0) | 9 (37.5) | .453 | 17 (20.0) | 70 (31.5) | .045* |
| Initial vital signs, No. (%) | ||||||
| Pulse | 81.2 ± 12.9 | 73.3 ± 14.2 | .116 | 80.2 ± 19.2 | 76.8 ± 16 | .152 |
| Systolic blood pressure | 135.9 ± 22.3 | 157.4 ± 22.1 | .010* | 145.4 ± 25.6 | 144.5 ± 26.9 | .8 |
| Diastolic blood pressure | 74.4 ± 10.5 | 79.7 ± 14.7 | .279 | 78.4 ± 19.3 | 80 ± 17.7 | .476 |
| Initial labs, No. (%) | ||||||
| Total cholesterol | 172.2 ± 59.5 | 171.3 ± 44.1 | .96 | 169.7 ± 42.7 | 166.5 ± 41.6 | .554 |
| Triglycerides | 100.2 ± 31.7 | 151.3 ± 78.8 | .011* | 144.4 ± 120.3 | 145.6 ± 92.4 | .929 |
| HDL | 37.7 ± 20.7 | 41.1 ± 9.5 | .613 | 40.5 ± 11.3 | 40.1 ± 13.1 | .854 |
| LDL | 114.5 ± 56.0 | 102.8 ± 34.2 | .458 | 104.4 ± 35.7 | 101.1 ± 33.9 | .481 |
| Lipids | 6.1 ± 1.3 | 6.9 ± 2.0 | .232 | 6.7 ± 1.9 | 6.3 ± 1.7 | .047* |
| Blood glucose | 126.2 ± 34.0 | 158.8 ± 127.3 | .25 | 151.3 ± 84.3 | 123.9 ± 65.0 | .008* |
| Creatinine | 1 ± 0.3 | 1.5 ± 2.0 | .36 | 1.1 ± 0.6 | 1.0 ± 0.5 | .007* |
| INR | 0.7 ± 0.6 | 0.8 ± 0.6 | .503 | 0.9 ± 0.4 | 0.7 ± 0.5 | .006* |
| Initial NIH Stroke Scale | ||||||
| Mean ± SD | 11.6 ± 9.6 | 5.3 ± 6.7 | .034* | 13.2 ± 9.6 | 6.1 ± 6.4 | <.001* |
| Medications prior to admission, No. (%) | ||||||
| Antiplatelet or anticoagulant | 10 (83.3) | 24 (100) | .040* | 84 (98.8) | 221 (99.5) | .479 |
| Antihypertensive | 9 (75.0) | 17 (70.8) | .792 | 61 (71.8) | 153 (68.9) | |
| Cholesterol reducer | 5 (41.7) | 17 (70.8) | .091 | 36 (42.4) | 109 (49.1) | |
| Diabetic medication | 2 (16.7) | 10 (41.7) | .134 | 31 (36.5) | 61 (27.5) | |
| Antidepressant | 0 (0.0) | 3 (12.5) | .425 | 14 (16.5) | 35 (15.8) | |
| Ambulation status prior to event, No. (%) | ||||||
| Ambulate independently | 9 (75) | 23 (95.8) | .200 | 73 (85.9) | 222 (100.0) | <.001* |
| Ambulate with assistance | 1 (8.3) | 0 (0.0) | 3 (3.5) | 0 (0.0) | ||
| Unable to ambulate | 1 (8.3) | 1 (4.2) | 5 (5.9) | 0 (0.0) | ||
| Not documented | 1 (8.3) | 0 (0.0) | 4 (4.7) | 0 (0.0) | ||
| Ambulation status on admission, No. (%) | ||||||
| Ambulate independently | 1 (8.3) | 14 (58.3) | .001* | 7 (8.2) | 63 (28.4) | <.001* |
| Ambulate with assistance | 5 (41.7) | 1 (4.2) | 25 (29.4) | 39 (17.6) | ||
| Unable to ambulate | 6 (50.0) | 5 (20.8) | 48 (56.5) | 27 (12.2) | ||
| Not documented | 0 (0.0) | 4 (16.7) | 5 (5.9) | 93 (41.9) | ||
| Ambulation status on discharge, No. (%) | ||||||
| Ambulate independently | 0 (0.0) | 19 (79.2) | <.001* | 0 (0.0) | 168 (75.7) | <.001* |
| Ambulate with assistance | 6 (50.0) | 3 (12.5) | 30 (35.3) | 54 (24.3) | ||
| Unable to ambulate | 4 (33.3) | 1 (4.2) | 36 (42.4) | 0 (0.0) | ||
| Not documented | 2 (16.7) | 1 (4.2) | 19 (22.4) | 0 (0.0) | ||
| Patient location during symptom onset, No. (%) | ||||||
| Not in a health care setting | 10 (83.3) | 22 (91.7) | .357 | 80 (94.1) | 210 (94.6) | .571 |
| Chronic health care facility | 1 (8.3) | 2 (8.3) | 3 (3.5) | 3 (1.4) | ||
| Another acute care facility | 1 (8.3) | 0 (0.0) | 1 (1.2) | 4 (1.8) | ||
| Stroke occurred after hospital arrival | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| ND or cannot be determined | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Outpatient health care setting | 0 (0.0) | 0 (0.0) | 1 (1.2) | 5 (2.3) | ||
| First care received, No. (%) | ||||||
| Emergency department | 7 (58.3) | 13 (54.2) | .813 | 26 (30.6) | 56 (25.2) | .342 |
| Direct admit | 5 (41.7) | 11 (45.8) | 59 (69.4) | 166 (74.8) | ||
| Patient care team: No. (%) | ||||||
| Neurology admit | 10 (83.3) | 20 (83.3) | 1.000 | 85 (100.0) | 220 (99.1) | .380 |
| Stroke unit | 7 (58.3) | 17 (70.8) | .453 | 53 (62.4) | 137 (61.7) | .918 |
| Stroke consult | 1 (8.3) | 1 (4.2) | .607 | 0 (0.0) | 1 (0.5) | .535 |
Abbreviations: HDL, high-density lipoprotein; INR, international normalized ratio; LDL, low-density lipoprotein; rtPA, recombinant tissue plasminogen activator.
P<0.05
Table 2.
Factors associated with improved functional outcome in acute ischemic stroke population in the telestroke unit.
| Variable | B value | Adjusted odds ratio | Wald | P value |
|---|---|---|---|---|
| NIH Stroke Scale | −0.095 | 0.910 (0.871-0.950) | 17.962 | <.001* |
| Direct admission | 0.324 | 1.382 (0.640-2.985) | 0.678 | .410 |
| Neurology admission | −0.399 | 0.671 (0.036-12.399) | 0.072 | .789 |
| Stroke consult | −1.942 | 0.143 (0.000-582.685) | 0.210 | .647 |
| Stroke unit | 0.247 | 1.280 (0.650-2.519) | 0.509 | .476 |
| Demographics | ||||
| Increasing age | −0.096 | 0.909 (0.870-0.949) | 18.732 | <.001* |
| Age more than 80 years old | 0.864 | 2.373 (0.741-7.606) | 2.116 | .146 |
| Female gender | −0.387 | 0.679 (0.314-1.470) | 0.965 | .326 |
| Body mass index | −0.026 | 0.975 (0.923-1.030) | 0.828 | .363 |
| African American race | −0.780 | 0.458 (0.170-1.238) | 2.370 | .124 |
| Past medical history | ||||
| Atrial fibrillation/flutter | 0.006 | 1.006 (0.349-2.903) | 0.000 | .991 |
| Coronary artery disease | 0.347 | 1.415 (0.662-3.025) | 0.801 | .371 |
| Depression | −0.492 | 0.611 (0.229-1.631) | 0.966 | .326 |
| Diabetes mellitus | −0.175 | 0.839 (0.266-2.645) | 0.089 | .765 |
| Dyslipidemia | 0.176 | 1.193 (0.524-2.716) | 0.177 | .674 |
| Family history of stroke | 0.027 | 1.027 (0.351-3.002) | 0.002 | .961 |
| Heart failure | −0.952 | 0.386 (0.127-1.173) | 2.818 | .093 |
| Hypertension | −0.345 | 0.708 (0.220-2.279) | 0.335 | .563 |
| Obesity | 1.205 | 3.338 (1.471-7.572) | 8.318 | .004* |
| Previous stroke | 1.065 | 2.901 (1.222-6.888) | 5.825 | .016* |
| Previous transient ischemic attack | 0.204 | 1.226 (0.386-3.891) | 0.119 | .730 |
| Peripheral vascular disease | 0.561 | 1.752 (0.492-6.238) | 0.750 | .387 |
| Smoking | 0.003 | 1.003 (0.424-2.374) | 0.000 | .994 |
| Current medications | ||||
| Antihypertensive | 0.732 | 2.080 (0.721-5.998) | 1.837 | .175 |
| Cholesterol reducer | −0.004 | 0.996 (0.414-2.395) | 0.000 | .992 |
| Diabetes medication | −0.213 | 0.808 (0.246-2.659) | 0.123 | .726 |
| Initial labs | ||||
| Total cholesterol | 0.010 | 1.010 (0.968-1.055) | 0.221 | .638 |
| Triglycerides | −0.002 | 0.998 (0.991-1.005) | 0.246 | .620 |
| HDL | 0.025 | 1.025 (0.973-1.080) | 0.873 | .350 |
| LDL | −0.017 | 0.983 (0.940-1.028) | 0.565 | .452 |
| Lipids | −0.037 | 0.964 (0.740-1.255) | 0.074 | .785 |
| Blood glucose | −0.002 | 0.998 (0.992-1.003) | 0.700 | .403 |
| Creatinine | −0.299 | 0.741 (0.341-1.613) | 0.570 | .450 |
| INR | −0.145 | 0.865 (0.410-1.824) | 0.145 | .703 |
| Initial vital signs | ||||
| Pulse | −0.011 | 0.989 (0.968-1.011) | 1.021 | .312 |
| Systolic blood pressure | 0.019 | 1.020 (1.004-1.035) | 6.021 | .014 |
| Diastolic blood pressure | −0.004 | 0.996 (0.973-1.018) | 0.144 | .704 |
Abbreviations: HDL, high-density lipoprotein; INR, international normalized ratio; LDL, low-density lipoprotein.
P<0.05
Table 3.
Stepwise regression model for functional outcome in the rtPA-treated acute ischemic stroke patients and patients who did not receive rtPA in the telestroke unit.
| rtPA-treated patients in the telestroke | rtPA-excluded patients in the telestroke | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | B value | Adjusted odds ratio | Wald | P value | B value | Adjusted odds ratio | Wald | P value |
| NIH Stroke Scale | −0.102 | 0.903 (0.869-0.937) | 28.091 | <.001* | −0.100 | 0.905 (0.871-0.940) | 26.091 | <.001* |
| Increasing age | −0.061 | 0.940 (0.916-0.965) | 21.239 | <.001* | −0.049 | 0.952 (0.929-0.976) | 15.207 | <.001* |
| Previous stroke | 1.074 | 2.927 (1.300-6.591) | 6.728 | .009* | 0.662 | 1.938 (0.892-4.213) | 2.791 | .095 |
| Blood glucose level | −0.005 | 0.995 (0.991-0.999) | 5.611 | .018* | −0.005 | 0.995 (0.991-0.999) | 5.288 | .021* |
| Systolic blood pressure | 0.015 | 1.015 (1.003-1.027) | 5.981 | .014* | 0.012 | 1.013 (1.000-1.025) | 4.107 | .043* |
| African American race | −0.846 | 0.429 (0.181-1.015) | 3.707 | .054 | ||||
| Obesity | 0.760 | 2.138 (1.164-3.928) | 5.997 | .014* | ||||
| HDL | 0.031 | 1.032 (1.005-1.059) | 5.380 | .020* | ||||
Abbreviations: HDL, high-density lipoprotein; rtPA, recombinant tissue plasminogen activator.
P<0.05
Results
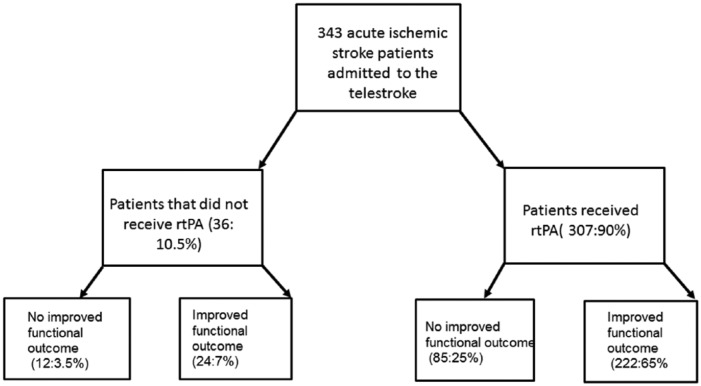
Demographic and clinical characteristics of rtPA-treated patients with improved functional outcome were compared with the no improvement group (Table 1 and Figure 1). Of the 36 patients who did not receive rtPA, 66.7% experienced improved functional outcome. Of the 307 patients who received rtPA, 72.3% experienced improved functional ambulation. In the subset of the population who did not receive rtPA, univariate statistical analysis reveals statistically significant differences between the no improvement group and the improvement group in 9 clinical characteristics. Patients who experienced improved functional ambulation were more likely to be obese (58.3% vs 16.7%), more likely to have had a previous stroke (29.2% vs 0.0%), have a higher systolic blood pressure (BP) at presentation (157.4 ± 22.1 vs 135.9 ± 22.3), have higher triglycerides at presentation (151.3 ± 78.8 vs 100.2 ± 31.7), have a lower calculated NIHSS (5.3 ± 6.7 vs 11.6 ± 9.6), and were more likely to be taking an antiplatelet or anticoagulant (100.0% vs 83.3%). Patients who experienced improved functional outcome in the no rtPA group tended to have a better ambulation status at the time of admission. In the no rtPA population, the improvement group was less likely to be more than 80 years of age (8.3% vs 58.3%). However, this apparent difference between groups could not be demonstrated when age was treated as a continuous variable.
Figure 1.
Total numbers and percentages of patients admitted to the telestroke who received and did not receive rtPA and those with and without an improvement in functional outcome.
In the subset of the population who did receive rtPA, univariate statistical analysis identified all the variables that were statistically significant between the clinical characteristics of the no improvement group and the improvement group. The improvement group was younger in age (61.2 ± 14.7 vs 71.6 ± 11.0) years, more likely to have a history of substance abuse (5.4% vs 0.0%), more likely to be obese (58.6% vs 44.7%), more likely to have had a previous stroke (27.0% vs 14.1%), more likely to be a smoker (31.5% vs 20.0%), and less likely to have atrial fibrillation or atrial flutter (7.2% vs 17.6%). The improvement group’s laboratory values at the time of presentation tended to show lower total lipids (6.3 ± 1.7 vs 6.7 ± 1.9), lower blood glucose levels (123.9 ± 65.0 vs 151.3 ± 84.3), lower creatinine (1.0 ± 0.5 vs 1.1 ± 0.6), and a lower international normalized ratio (INR) (0.7 ± 0.5 vs 0.9 ± 0.4). The improvement group tended to have a lower calculated NIHSS (6.1 ± 6.4 vs 13.2 ± 9.6), a better ambulation status prior to admission, at the time of presentation and improved ambulation at discharge.
Multivariate analysis revealed that a past medical history of obesity (odds ratio [OR] = 3.338, 95% confidence interval [CI], 1.471-7.572, P < .05), previous stroke (OR = 1.065, 95% CI, 1.222-6.888, P < .05), and a higher systolic BP at the time of presentation (OR = 1.020, 95% CI, 1.004-1.036, P < .05) was associated with improved functional outcome in the population of telestroke patients. However, a higher calculated NIHSS (OR = 0.911, 95% CI, 0.871-0.953, P < .001) and age (OR = 0.902, 95% CI, 0.862-0.944, P < .001) were not associated with an improved functional outcome in acute ischemic stroke patients (rtPA and no rtPA) who were treated in the telestroke unit (Table 2). The ROC curve associated with prediction of functional outcome for acute ischemic stroke population in the telestroke is presented in Figure 2. In adjusted analysis and elimination of any multicollinearity for patients who received rtPA, obesity (OR = 2.138, 95% CI, 1.164-3.928, P < .05), higher systolic BP at the time of presentation (OR = 1.015, 95% CI, 1.003-1.027, P < .05), and baseline high-density lipoprotein (HDL) (OR = 1.032, 95% CI, 1.005-1.059, P < .05) were associated with improved functional outcomes. By contrast, increasing age (OR = 0.940, 95% CI, 0.916-0.965, P < .0001) and higher calculated NIHSS (OR = 0.903, 95% CI, 0.869-0.937) were associated with a poorer outcome in rtPA-treated patients (Table 3). The ROC curve associated with prediction of functional outcome for rtPA-treated acute ischemic stroke in the telestroke is presented in Figure 3. It is also important to point out that the effect of previous history of stroke was not significant (P > .05). Systolic BP (OR = 1.013, 95% CI, 1.000-1.025, P < .05) was slightly associated with improved functional outcome after adjustment and elimination of any multicollinearity for the stroke patient population who did not receive rtPA (Table 3). The ROC curve associated with prediction of an improved functional outcome for patients who were excluded from rtPA is presented in Figure 4.
Figure 2.
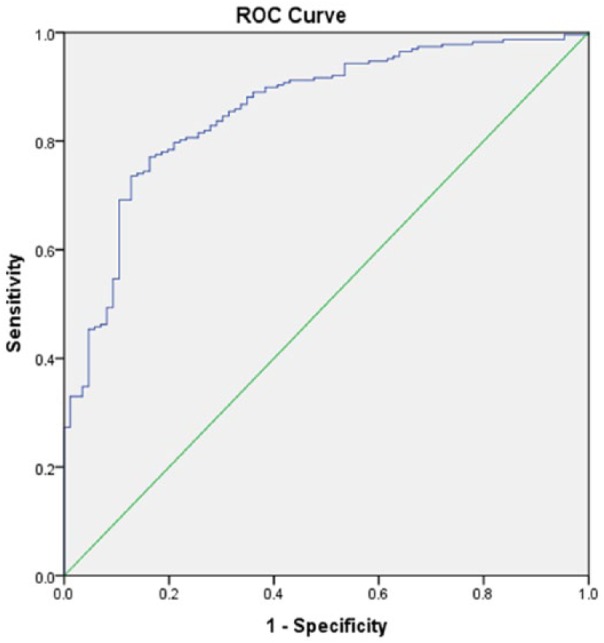
Receiver operating characteristic (ROC) curve associated with prediction of functional outcome for acute ischemic stroke population in the telestroke. Higher area under the curve (AUC) values in ROC analysis indicate better discrimination of the score for the measured outcome. Classification table (overall correctly classified percentage = 79.9%) and area under the ROC curve (AUC = 0.858, 0.813-0.903) were applied to check the model fitness.
Figure 3.
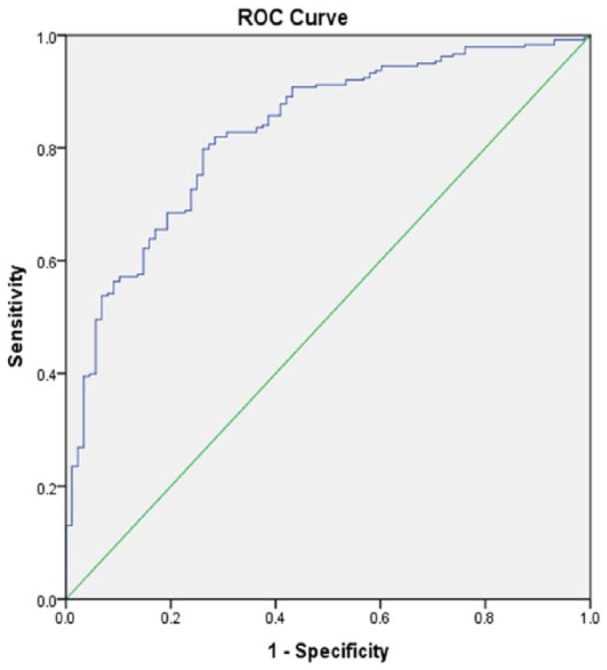
Receiver operating characteristic (ROC) curve associated with prediction of functional outcome for rtPA-treated acute ischemic stroke in the telestroke. Higher area under the curve (AUC) values in ROC analysis indicate better discrimination of the score for the measured outcome. Classification table (overall correctly classified percentage = 79.9%) and area under the ROC curve (AUC = 0.829, 0.780-0.877) were applied to check the model fitness.
Figure 4.
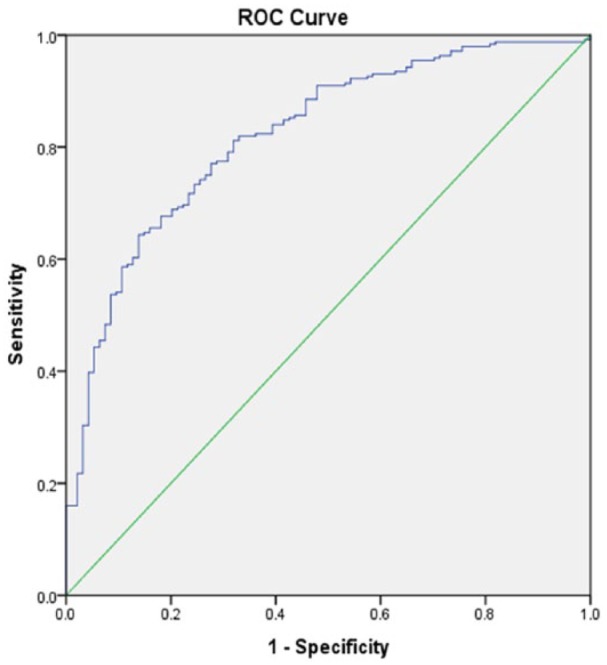
Receiver operating characteristic (ROC) curve associated with prediction of an improved functional outcome for patients excluded from in the telestroke. Higher area under the curve (AUC) values in ROC analysis indicate better discrimination of the score for the measured outcome. Classification table (overall correctly classified percentage = 79.9%) and area under the ROC curve (AUC = 0.829, 0.780-0.877) were applied to check the model fitness.
Discussion
This study characterizes functional ambulatory outcome in rtPA-treated patients evaluated with telestroke technology. There is a general trend to report stroke outcomes using a global functional outcome measure such as the Functional Independence Measure (FIM),14 the Barthel Index, or Modified Rankin Scale (mRS).15–17 The practice of using one global outcome measure is beneficial, especially in providing means of comparison. Physical abilities and disabilities experienced following discharge after treatment with rtPA are of major significance to clinicians,18 the stroke patient, and family members. Moreover, ability to walk indoors without assistance is an important component of independent living at home, with or without the assistance of family members. It has several advantages in revealing a series of clinically useful outcome measures,19 standardizing stroke outcome measures by revealing significant improvement in stroke patients’ performance,12 and documenting recovery following stroke treatments. More specifically, it reveals considerable residual mobility problems after treatment13 with rtPA.
In the rtPA-treated group, patients in the improved functional outcome group were younger in age, more likely to have a history of substance abuse, obesity, previous stroke, and were less likely to have atrial fibrillation. Lower values for NIHSS scores, total lipids, blood glucose levels, creatinine, and INR were also found among rtPA-treated patients. The initial NIHSS score prior to treatment with rtPA is a strong predictor of treatment outcome and is used as an evaluation tool to assess the efficiency of rtPA treatment.20 In this study, most of the rtPA-treated patients who presented with improved functional outcomes were younger, had history of smoking and a previous stroke (more than 3 months), were obese with history of substance abuse, and had lower values of other risk factors. This result indicates that the severity of stroke by itself does not provide an explanation for the observed functional outcome in treated patients, but several clinical factors are significant as well.21 Moreover, the lower values of NIH scores observed in this study have been reported for other telestroke programs.22
The adjusted analysis controlled for age, sex, and clinical stroke severity; our result indicates that patients with a past medical history of obesity, previous stroke, and a higher systolic BP were associated with an improved functional outcome in rtPA-treated patients. In general, the effect of obesity and prognosis on cerebrovascular disease is contentious. Many studies suggest an “obesity paradox,” a better prognosis in obese patients after stroke.23 Although obesity is an established risk factor for stroke, its influence on clinical outcomes in thrombolytic therapy for acute ischemic stroke is still under debate. One major argument against the obesity paradox is that studies which support the obesity paradox did not adjust for stroke severity,24,25 resulting in an inverse association between body mass index (BMI) and stroke severity. The initial stroke severity was presumed to be an intermediary between levels of BMI and poststroke mortality, contradicting the existence of obesity paradox.23 Our finding that rtPA-treated obese stroke patients were more likely to be associated with an improved functional outcome is consistent with studies that did not use telestroke technology.26,27 It is possible that clinicians treat obese patients more aggressively than lean patients, due to assumed increase of vascular risk,28 resulting in improved outcomes after thrombolysis.
After adjustment for multiple baselines, patients with a previous history of stroke of more than 3 months who received thrombolytic therapy were associated with an improved functional outcome. Although thrombolytics are contraindicated in patients with recent stroke or any previous hemorrhagic stroke,29,30 our finding is supported by another study31 that found patients with no recent history of stroke experienced better outcomes when treated with a thrombolytic therapy and were not at increased risk of major bleeding complications. However, previous stroke is also a predictor of cerebrovascular complications32 due to the increased risk of poor outcome. Therefore, patients with a history of previous stroke (more than 3 months) in this study benefited from application of evidence-based interventions including aspirin and statins. These are unlikely to have a poor risk-to-reward ratio.33,34 This most likely resulted in more patients with a previous history of stroke who received rtPA in this study with an improved functional outcome. Therefore, the result of this study may support further discussion to reconsider exclusion of acute ischemic stroke patients with a previous history of stroke from receiving rtPA, as previous studies indicate that patients with a history of stroke would have worse outcomes, more bleeding, and be less likely to receive thrombolysis.35
Apart from obesity, and a previous history of stroke, BP was also associated with an improved functional outcome. The ideal targets for the control of BP in patients with acute ischemic stroke are not clear, as many observational studies revealed a correlation with poor outcomes.36,37 These studies reveal poor outcomes with either very high or very low admission BP. Our finding that a higher systolic BP was associated with an improved functional outcome in rtPA-treated telestroke patients is supported by a recent finding that systolic BPs after thrombolytic therapy are associated with improved neurological outcomes.38,39 A recent study found systolic BP to be the most influential BP variable that significantly improved the prediction of functional outcome in neurological care.39 Several studies have been helpful in describing the natural history of BP changes following acute ischemic stroke.40,41 Findings from these studies support our result that a predictor for an improved functional outcome in rtPA-treated patients during the acute phase of BP control is the systolic BP.42 The association between stroke and elevated BP on admission as well as during hospitalization has prompted several studies to determine whether BP control in an acute setting would improve outcomes in a stroke population.
The effect of HDLs was not significant in the univariate analysis; however, after adjustment, stroke patients with high HDL were more likely to be associated with improved functional outcome following treatment with rtPA. High-density lipoproteins are used as a neuro- and vasculoprotective treatment in acute ischemic stroke patients.43 Apart from the known action of HDL in reverse cholesterol transport, HDLs have anti-inflammatory, antioxidant, and endothelial protective effects.44,45 Furthermore, the beneficial actions of HDL do not interfere with the fibrinolytic effect of rtPA.43,46 In our study, a higher baseline HDL at admission for rtPA-treated patients was associated with an improved functional outcome in our telestroke patients. This finding supports the emerging role of HDL as a potential target in the care of stroke patients. In the adjusted analysis for the rtPA-treated patients, higher NIH scores and old age were associated with poor functional outcome, whereas a past medical history of obesity, previous stroke, and a higher systolic BP at the time of presentation was associated with improved functional outcome.
Many limitations must be considered before interpreting the findings of this study. Our data are retrospective and did not indicate time of stroke or differentiate between stroke subtypes, including hemorrhagic and ischemic strokes. Moreover, our analysis is restricted to patients without an identified contraindication to a specific procedure or medication. Furthermore, thrombolytics may have been knowingly withheld from patients with known, but unrecorded contraindications. Our study is classified as a single institution study, and it is possible that a selection bias could have affected the selection of patients. Not obtaining ambulatory data at 90 days after treatment is also a limitation. Moreover, patients with a history of stroke were considered a contraindication for rtPA, whereas ischemic strokes of more than 3 months were not. The strengths of this study stem from the fact that the registry used is a large telestroke center designed to improve stroke care in rural settings. For this reason, this study is equipped to determine functional outcome in rtPA-treated patients. Major contributions of this study to existing literature is (1) the identification of clinical and demographic variables that are associated with improvement and no improvement in the treatment of acute ischemic stroke in the telestroke registry and (2) the ability to use functional ambulation as a tool to measure functional outcome in a population of stroke-treated patients within the telestroke setting. Although a recent study47 reported no difference between no telestroke and telestroke in favorable clinical outcome at discharge and 90 days later, there was a significant decrease in the onset-to-treatment duration in the telestroke group. This finding supports our current study that telestroke is more likely to be associated with an improved functional outcome in rtPA-treated patients.
Acknowledgments
The authors thank GHS stroke unit for helping collect the data. This work was supported by grants from the Fullerton Foundation.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Statistical analysis was performed by JG. Critical interpretation of the results was performed by all authors. The manuscript was drafted by TN and revised by LB, BB, EL, AK, and LM.
ORCID iD: Thomas Nathaniel  https://orcid.org/0000-0003-0954-5050
https://orcid.org/0000-0003-0954-5050
References
- 1. Akbik F, Hirsch JA, Chandra RV, et al. Telestroke: the promise and the challenge. Part one: growth and current practice. J Neurointerv Surg. 2017;9:357–360. [DOI] [PubMed] [Google Scholar]
- 2. Demaerschalk BM. Telestrokologists: treating stroke patients here, there, and everywhere with telemedicine. Semin Neurol. 2010;30:477–491. [DOI] [PubMed] [Google Scholar]
- 3. Wechsler LR, Demaerschalk BM, Schwamm LH, et al. Telemedicine quality and outcomes in stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:E3–E25. [DOI] [PubMed] [Google Scholar]
- 4. Al-Hussain FA. Principles and practice of thrombolysis via telestroke. Neurosciences. 2014;19:178–182. [PMC free article] [PubMed] [Google Scholar]
- 5. Hubert G, Handschu R, Barlinn J, Berrouschot J, Audebert HJ. Telemedicine applications in acute stroke care. Akt Neurol. 2016;43:615–623. [Google Scholar]
- 6. Demaerschalk BM, Vegunta S, Vargas BB, Wu Q, Channer DD, Hentz JG. Reliability of real-time video smartphone for assessing National Institutes of Health Stroke Scale scores in acute stroke patients. Stroke. 2012;43:3271–3277. [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez MA, Hanna N, Rodrigo ME, Satler LF, Waksman R. Reliability of prehospital real-time cellular video phone in assessing the simplified National Institutes of Health Stroke Scale in patients with acute stroke: a novel telemedicine technology. Stroke. 2011;42:1522–1527. [DOI] [PubMed] [Google Scholar]
- 8. Demaerschalk BM, Raman R, Ernstrom K, Meyer BC. Efficacy of telemedicine for stroke: pooled analysis of the Stroke Team Remote Evaluation Using a Digital Observation Camera (STRokE DOC) and STRokE DOC Arizona telestroke trials. Telemed J E Health. 2012;18:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al Kasab S, Adams RJ, Debenham E, Jones DJ, Holmstedt CA. Medical University of South Carolina Telestroke: a telemedicine facilitated network for stroke treatment in South Carolina—a progress report. Telemed J E Health. 2017;23:674–677. [DOI] [PubMed] [Google Scholar]
- 10. Bagot KL, Bladin CF, Vu M, et al. Exploring the benefits of a stroke telemedicine programme: an organisational and societal perspective. J Telemed Telecare. 2016;22:489–494. [DOI] [PubMed] [Google Scholar]
- 11. Lazaridis C, DeSantis SM, Jauch EC, Adams RJ. Telestroke in South Carolina. J Stroke Cerebrovasc Dis. 2013;22:946–950. [DOI] [PubMed] [Google Scholar]
- 12. Kollen B, van de Port I, Lindeman E, Twisk J, Kwakkel G. Predicting improvement in gait after stroke—a longitudinal prospective study. Stroke. 2005;36:2676–2680. [DOI] [PubMed] [Google Scholar]
- 13. Mahendran N, Kuys SS, Brauer SG. Accelerometer and global positioning system measurement of recovery of community ambulation across the first 6 months after stroke: an exploratory prospective study. Arc Phys Med Rehabil. 2016;97:1465–1472. [DOI] [PubMed] [Google Scholar]
- 14. Hamilton B, Granger C. Disability outcomes following inpatient rehabilitation for stroke. Phys Ther. 1994;74:494–503. [DOI] [PubMed] [Google Scholar]
- 15. Shih MM, Rogers JC, Skidmore ER, Irrgang JJ, Holm MB. Measuring stroke survivors’ functional status independence: five perspectives. Am J Occup Ther. 2009;63:600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5:603–612. [DOI] [PubMed] [Google Scholar]
- 17. Lees KR, Bath PMW, Schellinger PD, et al. Contemporary outcome measures in acute stroke research choice of primary outcome measure. Stroke. 2012;43:1163–1170. [DOI] [PubMed] [Google Scholar]
- 18. Unsworth CA, Thomas SA, Greenwood KM. Decision polarization among rehabilitation team recommendations concerning discharge housing for stroke patients. Int J Rehabil Res. 1997;20:51–69. [DOI] [PubMed] [Google Scholar]
- 19. Buurke JH, Nene AV, Kwakkel G, Erren-Wolters V, Ijzerman MJ, Hermens HJ. Recovery of gait after stroke: what changes? Neurorehabil Neural Repair. 2008;22:676–683. [DOI] [PubMed] [Google Scholar]
- 20. Kenmuir CL, Hammer M, Jovin T, Reddy V, Wechsler L, Jadhav A. Predictors of outcome in patients presenting with acute ischemic stroke and mild stroke scale scores. J Stroke Cerebrovasc Dis. 2015;24:1685–1689. [DOI] [PubMed] [Google Scholar]
- 21. Zhang S, Liu Z, Liu YL, Wang YL, Liu T, Cui XB. Prevalence of stroke and associated risk factors among middle-aged and older farmers in western China. Environ Health Prev Med. 2017;22:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Audebert HJ, Kukla C, Vatankhah B, et al. Comparison of tissue plasminogen activator administration management between Telestroke Network Hospitals and Academic Stroke Centers—the telemedical pilot project for integrative stroke care in Bavaria/Germany. Stroke. 2006;37:1822–1827. [DOI] [PubMed] [Google Scholar]
- 23. Oesch L, Tatlisumak T, Arnold M, Sarikaya H. Obesity paradox in stroke—myth or reality? a systematic review. PLoS ONE. 2017;12:e0171334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ryu WS, Lee SH, Kim CK, Kim BJ, Yoon BW. Body mass index, initial neurological severity and long-term mortality in ischemic stroke. Cerebrovasc Dis. 2011;32:170–176. [DOI] [PubMed] [Google Scholar]
- 25. Kim Y, Kim CK, Jung S, Yoon BW, Lee SH. Obesity-stroke paradox and initial neurological severity. J Neurol Neurosurg Psychiatry. 2015;86:743–747. [DOI] [PubMed] [Google Scholar]
- 26. Burke DT, Al-Adawi S, Bell RB, Easley K, Chen S, Burke DP. Effect of body mass index on stroke rehabilitation. Arch Phys Med Rehabil. 2014;95:1055–1059. [DOI] [PubMed] [Google Scholar]
- 27. Daniel B, Nitin BJ, David TB. Overweight patients recover faster after stroke. Paper presented at: 8th Mediterranean Congress of Physical and Rehabilitation Medicine; September 29-October 2, 2010; Limassol:25-28. Bologna: Medimond. [Google Scholar]
- 28. Schenkeveld L, Magro M, Oemrawsingh RM, et al. The influence of optimal medical treatment on the “obesity paradox,” body mass index and long-term mortality in patients treated with percutaneous coronary intervention: a prospective cohort study. BMJ Open. 2012;2:e000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cotte FE, Chaize G, Kachaner I, Gaudin AF, Vainchtock A, Durand-Zaleski I. Incidence and cost of stroke and hemorrhage in patients diagnosed with atrial fibrillation in France. J Stroke Cerebrovasc Dis. 2014;23:E73–E83. [DOI] [PubMed] [Google Scholar]
- 30. Bembenek JP, Karlinski M, Kurkowska-Jastrzebska I, Czlonkowska A. Changes in pre-hospital management of vascular risk factors among patients admitted due to recurrent stroke in Poland from 1995 to 2013. Arch Med Sci. 2016;12:754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanne D, Gottlieb S, Caspi A, et al. Treatment and outcome of patients with acute myocardial infarction and prior cerebrovascular events in the thrombolytic era: the Israeli Thrombolytic National Survey. Arch Intern Med. 1998;158:601–606. [DOI] [PubMed] [Google Scholar]
- 32. Cronin L, Mehta SR, Zhao F, et al. Stroke in relation to cardiac procedures in patients with non-ST-elevation acute coronary syndrome: a study involving >18 000 patients. Circulation. 2001;104:269–274. [DOI] [PubMed] [Google Scholar]
- 33. Chin CT, Ong TK, Krittayaphong R, et al. Characteristics and outcomes of medically managed patients with non-ST-segment elevation acute coronary syndromes: insights from the multinational EPICOR Asia study. Int J Cardiol. 2017;243:15–20. [DOI] [PubMed] [Google Scholar]
- 34. Scherillo M, Cirillo P, Formigli D, et al. Antiplatelet therapy for non-ST-segment elevation myocardial infarction in complex “Real” clinical scenarios: a consensus document of the “Campania NSTEMI Study Group.” Angiology. 2017;68:598–607. [DOI] [PubMed] [Google Scholar]
- 35. Giantin V, Semplicini A, Franchin A, et al. Outcome after acute ischemic stroke (AIS) in older patients: effects of age, neurological deficit severity and blood pressure (BP) variations. Arch Gerontol Geriatr. 2011;52:E185–E191. [DOI] [PubMed] [Google Scholar]
- 36. Goodfellow JA, Dawson J, Quinn TJ. Management of blood pressure in acute stroke. Expert Review of Neurotherapeutics. 2013;13:911–923. [DOI] [PubMed] [Google Scholar]
- 37. Vemmos KN, Tsivgoulis G, Spengos K, et al. Blood pressure course in acute ischaemic stroke in relation to stroke subtype. Blood Press Monit. 2004;9:107–114. [DOI] [PubMed] [Google Scholar]
- 38. Seok J, Lee JS, Jeong KY, Lee CM. Association between systolic blood pressure after thrombolysis and early neurological improvement in ischaemic stroke patients. Hong Kong J Emer Med. 2017;24:138–144. [Google Scholar]
- 39. Kellert L, Hametner C, Ahmed N, et al. Reciprocal interaction of 24-hour blood pressure variability and systolic blood pressure on outcome in stroke thrombolysis. Stroke. 2017;48:1827–1834. [DOI] [PubMed] [Google Scholar]
- 40. Manning L, Robinson TG, Anderson CS. Control of blood pressure in hypertensive neurological emergencies. Curr Hypertens Rep. 2014;16:436. [DOI] [PubMed] [Google Scholar]
- 41. Miller J, Kinni H, Lewandowski C, Nowak R, Levy P. Management of hypertension in stroke. Ann Emer Med. 2014;64:248–255. [DOI] [PubMed] [Google Scholar]
- 42. Manning L, Hirakawa Y, Arima H, et al. Blood pressure variability and outcome after acute intracerebral haemorrhage: a post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol. 2014;13:364–373. [DOI] [PubMed] [Google Scholar]
- 43. Lapergue B, Dang BQ, Desilles JP, et al. High-density lipoprotein-based therapy reduces the hemorrhagic complications associated with tissue plasminogen activator treatment in experimental stroke. Stroke. 2013;44:699–707. [DOI] [PubMed] [Google Scholar]
- 44. Kober AC, Manavalan APC, Tam-Amersdorfer C, et al. Implications of cerebrovascular ATP-binding cassette transporter G1 (ABCG1) and apolipoprotein M in cholesterol transport at the blood-brain barrier. Biochim Biophys Acta. 2017;1862:573–588. [DOI] [PubMed] [Google Scholar]
- 45. Ruiz M, Frej C, Holmer A, Guo LJ, Tran S, Dahlback B. High-density lipoprotein-associated apolipoprotein M limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1. Arterioscler Thromb Vasc Biol. 2017;37:118–129. [DOI] [PubMed] [Google Scholar]
- 46. Puri R, Nissen SE, Shao MY, et al. The beneficial effects of raising high-density lipoprotein cholesterol depends upon achieved levels of low-density lipoprotein cholesterol during statin therapy: implications for coronary atheroma progression and cardiovascular events. Eur J Prev Cardiol. 2016;23:474–485. [DOI] [PubMed] [Google Scholar]
- 47. Alireza B, Leila R, Abdelrahman IA, Saeed S, Chung WL, Ali A. Effects of telestroke on thrombolysis times and outcomes: a meta-analysis. Prehosp Emerg Care. 2018;22:472–484. [DOI] [PubMed] [Google Scholar]