Abstract
Introduction:
This study aimed to evaluate the influence of central angiotensin IV (Ang IV) infusion on chronic cerebral hypoperfusion (CCH)-related neuropathological changes including amyloid-β (Aβ), hyperphosphorylated tau (p-tau) and the inflammatory response.
Materials and methods:
Rats with CCH received central infusion of Ang IV, its receptor AT4R antagonist divalinal-Ang IV or artificial cerebrospinal fluid for six weeks. During this procedure, the systolic blood pressure (SBP) was monitored, and the levels of Aβ42, p-tau and pro-inflammatory cytokines in the brain were detected.
Results:
Rats with CCH exhibited higher levels of Aβ42, p-tau and pro-inflammatory cytokines in the brain when compared with controls. Infusion of Ang IV significantly reduced the expression of pro-inflammatory cytokines in the brains of rats with CCH. Meanwhile, the reduction of pro-inflammatory cytokines levels caused by Ang IV was reversed by divalinal-Ang IV. During the treatment, the SBP in rats was not significantly altered.
Conclusion:
This study demonstrates for the first time that Ang IV dose-dependently suppresses inflammation through AT4R in the brains of rats with CCH, which is independent from SBP. These findings suggest that Ang IV/AT4R may represent a potential therapeutic target for CCH-related neurological diseases.
Keywords: Angiotensin IV, chronic cerebral hypoperfusion, inflammation, vascular dementia, Alzheimer’s disease
Introduction
Chronic cerebral hypoperfusion (CCH) usually results from vascular and metabolic diseases including hypertension, diabetes and atherosclerosis, and is considered as a critical risk factor for both vascular dementia (VaD) and Alzheimer’s disease (AD).1 Pathologically, CCH triggers the formation of amyloid-β (Aβ), tau hyperphosphorylation and the inflammatory response in the brain, and subsequently leads to white matter damage, neurodegeneration and cognitive impairments.2 To date, there is no effective treatment for these CCH-related neuropathological changes in the brain.
Previously, the renin–angiotensin system (RAS) was identified as a crucial component of the circulatory system, functioning as a regulator of water and sodium homeostasis.3 In the brain, independent local RAS has been found in several regions and structures, and is involved in the pathogenesis of several neurological diseases including ischaemic stroke, Parkinson’s disease, VaD and AD.4–7 As an important component of the brain RAS, angiotensin IV (Ang IV) is reported to bind to its receptor AT4R and thus restore cognitive function following a variety of central insults, including CCH.8,9 However, whether this hexapeptide has beneficial effects on CCH-related neuropathological changes is less well studied thus far.
In the present study, we hypothesized that exogenous Ang IV infusion might influence CCH-related neuropathological changes such as Aβ, hyperphosphorylated tau (p-tau) and the inflammatory response via a dose-dependent manner. To evaluate this hypothesis, a CCH rat model induced by bilateral common carotid artery (CCA) ligation was employed. For the first time, we showed that Ang IV dose-dependently suppressed inflammation through AT4R in the brains of rats with CCH, whereas the levels of Aβ and p-tau were unaffected. This beneficial effect of Ang IV seemed to be independent from systolic blood pressure (SBP). Taken together, these findings suggest that Ang IV/AT4R may represent a potential therapeutic target for CCH-related neurological diseases.
Materials and methods
Reagents
Ang IV and its receptor antagonist divalinal-Ang IV were synthesized by Nanjing Maoyuan Biological Technology Company. They were dissolved in an artificial cerebrospinal fluid (aCSF, composition in mM: NaCl 130, KCl 2.99, CaCl2 0.98, MgCl2·6H2O 0.8, NaHCO3 25, Na2HPO4·12H2O 0.039 and NaH2PO4·2H2O 0.46) as described.4
Animals
A total of 56 male wistar rats (10–12 weeks old, 300–350 g) were included in this study. They were purchased from the Experimental Animals Center of Nanjing Medical University, were housed in a standard animal room with a 12 h light/dark cycle, and were given free access to food and water. The Animal Care and Management Committee of Nanjing First Hospital approved the whole study protocol. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and were reported in accordance with the Animal research: Reporting of in vivo experiments (ARRIVE) guidelines.10 The number of animals used was minimized within the constraints of statistical power.
Surgical procedure
The rat model of CCH was established by bilateral CCA ligation surgery, as previously described.6 Rats were anaesthetized with 10% chloral hydrate and the bilateral CCAs were isolated through a ventral midline incision as described.6 Afterward, the bilateral CCAs were ligated with a 4-0 surgical silk suture in the CCH group, whereas they were not ligated in controls. Throughout the procedure, body temperature was monitored with a rectal probe and maintained in the range of 37.0 ± 0.5°C with a heating pad.
Treatment
Eight weeks after bilateral CCA ligation surgery, rats were anaesthetized with 10% chloral hydrate and placed in a stereotactic frame. The scalp was reflected under sterile surgical conditions. A brain-infusion cannula (Brain Infusion Kit 2; ALZET Inc.) coupled via vinyl tubing to an osmotic pump (Model 2006; ALZET Inc.) was implanted into the third cerebral ventricle by surgeons who were blinded to the experimental groups. Osmotic pumps were placed subcutaneously between the scapulae and used to infuse two doses of Ang IV (20 nM, 0.15 µl/h and 100 nM, 0.15 µl/h), divalinal-Ang IV (500 nM, 0.15 µl/h) or aCSF (0.15 µl/h) into the third cerebral ventricle, lasting for six weeks. Following this surgery, the wounds were carefully closed with sutures. The dose and route of administration for Ang IV were chosen based on a previous study by Paris et al.11
Blood pressure measurement
In this study, SBP was measured by a tail cuff method using a non-invasive blood pressure analyser (BP-2000, Visitech Systems, Inc.), as previously described.12 Measurements were performed on week 0 (before surgery), week 8 (eight weeks after bilateral CCAs ligation surgery) and week 14 (six weeks after treatment) in rats between 08:00 a.m. and 12:00 a.m. Each measurement was performed three times to obtain a mean SBP. It is worth noting that the SBP was not significantly affected by bilateral CCA ligation surgery, osmotic pump implantation, or infusion of Ang IV (20 nM or 100 nM) or divalinal-Ang IV (500 nM).
Brain tissue preparation
Rats were killed under deep anaesthesia following six weeks of treatment. They (n = 8 per group) were perfused transcardially with 0.9% saline (pH 7.4), after which brains were removed and stored in liquid nitrogen until use.
Enzyme-linked immunosorbent assay
For the measurement of pro-inflammatory cytokines, brains were lysed as described.13 The protein levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-12 were measured by specific enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems Inc.). For the measurement of Aβ42, the cerebral cortex and hippocampus were separately homogenized in 10 volumes of tris-buffered saline containing 5 mM ethylene diamine tetraacetic acid (EDTA), phosphatase inhibitor, EDTA-free protease inhibitor cocktail and 2 mM 1,10-phenanthroline at 4°C. The homogenate was centrifuged for 1 h at 4°C. Supernatants were collected and the levels of Aβ42 were detected by a specific ELISA kit. For the measurement of p-tau (Thr181) levels, the cerebral cortex and hippocampus were separately homogenized and lysed as previously described.14 The levels of p-tau (Thr181) were assessed by a specific ELISA kit.
Statistical analysis
Data were analysed using GraphPad Prism 6 (GraphPad Software, Inc.). One-way analysis of variance (ANOVA) followed by Tukey’s post hoc test was employed to analyse differences among groups. Statistical power was estimated using STPLAN version 4.3 software. Data are expressed as mean ± SD. P < 0.05 was considered significant.
Results
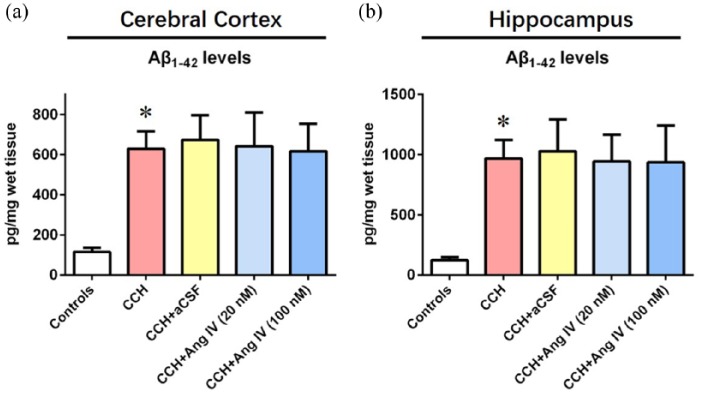
Ang IV does not influence Aβ levels in the brains of rats with CCH
As demonstrated by Figure 1(a) and (b), rats with CCH exhibited higher levels of Aβ42 in the cerebral cortex and hippocampus when compared with controls (for cerebral cortex: F (4, 35) = 31.41, 629.3 ± 86.4 vs 115.7 ± 21.2 pg/mg wet tissue, P < 0.05; for hippocampus: F (4, 35) = 24.49, 966.8 ± 155.4 vs 123.1 ± 25.9 pg/mg wet tissue, P < 0.05). Ang IV infusion (20 nM or 100 nM) did not alter the levels of Aβ42 in the cerebral cortex of rats with CCH (Figure 1(a)). Meanwhile, the levels of Aβ42 in the hippocampus of controls were not significantly affected by Ang IV infusion (20 nM or 100 nM, Figure 1(b)).
Figure 1.
Angiotensin IV does not influence amyloid-β levels in brains of rats with chronic cerebral hypoperfusion. (a) The levels of amyloid-β42 in the cerebral cortex were detected by enzyme-linked immunosorbent assay. (b) The levels of amyloid-β42 in the hippocampus were detected by enzyme-linked immunosorbent assay. Data were analysed by one-way analysis of variance followed by Tukey’s post hoc test. Columns represent mean ± SD. n = 8 per group.
*P < 0.05 versus controls.
Aβ: amyloid-β; aCSF: artificial cerebrospinal fluid; Ang IV: angiotensin IV; CCH: chronic cerebral hypoperfusion.
Ang IV does not affect p-tau levels in the brains of rats with CCH
As illustrated by Figure 2(a) and (b), the levels of p-tau in the cerebral cortex and hippocampus of rats with CCH were markedly higher than those of controls (for cerebral cortex: F (4, 35) = 30.42, 22.3 ± 3.6 vs 1.9 ± 0.3 ng/mg wet tissue, P < 0.05; for hippocampus: F (4, 35) = 28.24, 40.9 ± 8.4 vs 3.2 ± 0.8 ng/mg wet tissue, P < 0.05). Infusion of Ang IV (20 nM or 100 nM) did not affect the levels of p-tau in the cerebral cortex of rats with CCH (Figure 2(a)). Meanwhile, p-tau levels in the hippocampus of rats with CCH were not significantly affected by infusion of Ang IV (20 nM or 100 nM, Figure 2(b)).
Figure 2.
Angiotensin IV does not affect p-tau levels in brains of rats with chronic cerebral hypoperfusion. (a) The levels of p-tau (Thr181) in the cerebral cortex were detected by enzyme-linked immunosorbent assay. (b) The levels of p-tau (Thr181) in the hippocampus were detected by enzyme-linked immunosorbent assay. Data were analysed by one-way analysis of variance followed by Tukey’s post hoc test. Columns represent mean ± SD. n = 8 per group. *P < 0.05 vs controls. aCSF: artificial cerebrospinal fluid; Ang IV: angiotensin IV; CCH: chronic cerebral hypoperfusion.
Ang IV suppresses inflammation in the brains of rats with CCH
As demonstrated by Figure 3(a)–(d), rats with CCH displayed higher protein levels of pro-inflammatory cytokines including TNF-α (F (6, 49) = 35.64, 386.2 ± 42.1 vs 76.9 ± 11.7 pg/mg wet tissue, P < 0.05), IL-1β (F (6, 49) = 27.43, 783.4 ± 128.3 vs 215.7 ± 38.5 pg/mg wet tissue, P < 0.05), IL-6 (F (6, 49) = 38.88, 1124.5 ± 158.2 vs 94.6 ± 18.5 pg/mg wet tissue, P < 0.05) and IL-12 (F (6, 49) = 39.24, 152.7 ± 32.6 vs 34.8 ± 7.3 pg/mg wet tissue, P < 0.05) in the brain when compared with controls. Infusion of Ang IV (20 nM or 100 nM) significantly reduced the protein levels of TNF-α, IL-1β, IL-6 and IL-12 in the brains of rats with CCH (Figure 3(a)–(d), all P < 0.05). Meanwhile, the reduction of TNF-α, IL-1β, IL-6 and IL-12 protein levels caused by Ang IV (100 nM) in the brains of rats with CCH was completely reversed by infusion of divalinal-Ang IV (500 nM, Figure 3(a)–(d), all P < 0.05). Of note, neither osmotic pump implantation nor divalinal-Ang IV (500 nM) infusion significantly influenced the protein levels of TNF-α, IL-1β, IL-6 or IL-12 in the brains of rats with CCH (Figure 3(a)–(d)).
Figure 3.
Angiotensin IV suppresses inflammation in the brains of rats with chronic cerebral hypoperfusion. (a) The protein levels of tumour necrosis factor-α in the brain were detected by enzyme-linked immunosorbent assay. (b) The protein levels of interleukin-1β in the brain were detected by enzyme-linked immunosorbent assay. (c) The protein levels of interleukin-6 in the brain were detected by enzyme-linked immunosorbent assay. (d) The protein levels of interleukin-12 in the brain were detected by enzyme-linked immunosorbent assay. Data were analysed by one-way analysis of variance followed by Tukey’s post hoc test. Columns represent mean ± SD. n = 8 per group.
*P < 0.05 versus controls. #P < 0.05 versus CCH+aCSF group. &P < 0.05 versus CCH+Ang IV (100 nM) group.
aCSF: artificial cerebrospinal fluid; Ang IV: angiotensin IV; CCH: chronic cerebral hypoperfusion; IL: interleukin; TNF: tumour necrosis factor.
Discussion
Emerging evidence has indicated that CCH is associated with an increased inflammatory response in the brain.15,16 This may be a consequence of Aβ formation and tau hyperphosphorylation induced by CCH.17,18 Although the inflammatory response is crucial for the brain to remove senescent cells and extrinsic pathogenic substances, long-lasting neuroinflammation is toxic to neurons and synapses, and thus contributes to neurodegeneration, white matter damage and cognitive impairments.19–22 In this study, we confirmed previous findings by showing higher protein levels of pro-inflammatory cytokines including TNF-α, IL-1β, IL-6 and IL-12 in the brains of rats with CCH. More importantly, we revealed for the first time that Ang IV suppressed this inflammatory response via a dose-dependent manner, which was independent from SBP. The anti-inflammatory property of Ang IV has been previously validated under various conditions. In a rat model of myocardial ischaemia-reperfusion, Park and colleagues found that Ang IV suppressed the inflammatory response in cardiomyocytes and thus exerted cardioprotective effects.23 In a previous study by Kong et al., treatment with a medium dose of Ang IV markedly reduced the expression of pro-inflammatory cytokines in a mouse model of abdominal aortic aneurysm induced by angiotensin II.24 More recently, in a mouse model of AD, Royea and colleagues revealed that the anti-inflammatory effect of losartan was actually achieved by Ang IV.25 Meanwhile, in this study, we also revealed that the anti-inflammatory property of Ang IV was mediated by AT4R, and this finding was consistent with previous observations from other groups.23–25 Nevertheless, Kong and colleagues showed that Ang IV may potentially affect the expression of AT2R.24 Meanwhile, recent evidence suggests that Ang IV may exert its function via hepatocyte growth factor (HGF)/c-Met signalling.26 Interestingly, activation of AT2R or HGF/c-Met signalling was revealed to suppress the inflammatory response in different tissues.27–30 Therefore, whether AT2R or HGF/c-Met signalling was involved in the anti-inflammatory properties of Ang IV under the context of CCH deserves further investigation in the future.
Lastly, some minor issues should be mentioned. First, in the present study, we did not elucidate the precise cellular mechanisms by which Ang IV suppressed inflammation in the brain with CCH. Since AT4R has been identified in rat astrocytes,31 we speculated that Ang IV may exert its anti-inflammatory function by inhibition of the astrocyte-mediated inflammatory response. This speculation needs to be verified in the future. Second, in this study, although Ang IV infusion significantly suppressed inflammation in the brains of rats with CCH, it did not affect AD-like neuropathology including Aβ42 and p-tau. These findings were in accordance with a previous study from Royea and colleagues showing that Ang IV did not influence AD-like neuropathology in a mouse model of AD,25 suggesting that this hexapeptide may not be involved in the formation or degradation of these neuropathological changes. However, it is also possible that the six-week central infusion of Ang IV is not enough to cause obvious alterations in Aβ42 and p-tau levels. Unfortunately, the limited working duration of osmotic pumps suitable for rats (maximum six weeks, Model: 2006D) prevented us from observing longer effects of Ang IV. Third, we delivered Ang IV to the brain via an intracerebroventricular infusion strategy because this peptide cannot readily penetrate the blood brain barrier (BBB) and is rapidly degraded by several proteases in the peripheral tissues.32 This property largely restricts the application of Ang IV in animal and clinical research. In view of this fact, artificial synthetic Ang IV analogues with oral efficacy, extended half-life and increased BBB penetrability such as Nle1-AngIV and Dihexa will be employed in our future studies.33,34
In conclusion, this study provided the first evidence that Ang IV dose-dependently suppresses inflammation through AT4R in the brains of rats with CCH, whereas the levels of Aβ42 and p-tau were unaffected. Meanwhile, the anti-inflammatory effect of Ang IV seemed to be independent from SBP. These findings suggest that Ang IV/AT4R may represent a potential therapeutic target for CCH-related neurological diseases.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81771140), Natural Science Foundation of Jiangsu Province (BK20151084), Key Research and Development Project of Jiangsu Province (BL2014014), “Six Talent Summit” Foundation of Jiangsu Province (2016-WSN-180), Youth Medical Talent Program of Jiangsu Province (QNRC2016068), Medical Innovation Team of Jiangsu Province (CXTDA2017030), and Nanjing Medical Science and Technology Development Foundation for Distinguished Young Scholars (JQX17008).
ORCID iD: Ying-Dong Zhang  https://orcid.org/0000-0002-4204-6563
https://orcid.org/0000-0002-4204-6563
References
- 1. Akinyemi RO, Mukaetova-Ladinska EB, Attems J, et al. Vascular risk factors and neurodegeneration in ageing related dementias: Alzheimer’s disease and vascular dementia. Curr Alzheimer Res 2013; 10: 642–653. [DOI] [PubMed] [Google Scholar]
- 2. Zhao Y, Gong CX. From chronic cerebral hypoperfusion to Alzheimer-like brain pathology and neurodegeneration. Cell Mol Neurobiol 2015; 35: 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nguyen Dinh Cat A, Touyz RM. A new look at the renin-angiotensin system—focusing on the vascular system. Peptides 2011; 32: 2141–2150. [DOI] [PubMed] [Google Scholar]
- 4. Jiang T, Yu JT, Zhu XC, et al. Angiotensin-(1–7) induces cerebral ischaemic tolerance by promoting brain angiogenesis in a Mas/eNOS-dependent pathway. Brit J Pharmacol 2014; 171: 4222–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ou Z, Jiang T, Gao Q, et al. Mitochondrial-dependent mechanisms are involved in angiotensin II-induced apoptosis in dopaminergic neurons. J Renin Angiotensin Aldosterone Syst 2016; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xie W, Zhu D, Ji L, et al. Angiotensin-(1–7) improves cognitive function in rats with chronic cerebral hypoperfusion. Brain Res 2014; 1573: 44–53. [DOI] [PubMed] [Google Scholar]
- 7. Jiang T, Zhang YD, Zhou JS, et al. Angiotensin-(1–7) is reduced and inversely correlates with Tau hyperphosphorylation in animal models of Alzheimer’s disease. Mol Neurobiol 2016; 53: 2489–2497. [DOI] [PubMed] [Google Scholar]
- 8. Wright JW, Harding JW. The brain renin-angiotensin system: A diversity of functions and implications for CNS diseases. Pflugers Arch 2013; 465: 133–151. [DOI] [PubMed] [Google Scholar]
- 9. Wright JW, Kawas LH, Harding JW. A role for the brain RAS in Alzheimer’s and Parkinson’s diseases. Front Endocrinol 2013; 4: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hooijmans CR, de Vries R, Leenaars M, et al. Improving planning, design, reporting and scientific quality of animal experiments by using the Gold Standard Publication Checklist, in addition to the ARRIVE guidelines. Br J Pharmacol 2011; 162: 1259–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paris JJ, Eans SO, Mizrachi E, et al. Central administration of angiotensin IV rapidly enhances novel object recognition among mice. Neuropharmacology 2013; 70: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang T, Gao L, Shi J, et al. Angiotensin-(1–7) modulates renin-angiotensin system associated with reducing oxidative stress and attenuating neuronal apoptosis in the brain of hypertensive rats. Pharmacol Res 2013; 67: 84–93. [DOI] [PubMed] [Google Scholar]
- 13. Jiang T, Gao L, Guo J, et al. Suppressing inflammation by inhibiting the NF-kappaB pathway contributes to the neuroprotective effect of angiotensin-(1–7) in rats with permanent cerebral ischaemia. Br J Pharmacol 2012; 167: 1520–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang T, Zhang YD, Chen Q, et al. TREM2 modifies microglial phenotype and provides neuroprotection in P301S tau transgenic mice. Neuropharmacology 2016; 105: 196–206. [DOI] [PubMed] [Google Scholar]
- 15. Cheng P, Zuo X, Ren Y, et al. Adenosine A1-receptors modulate mTOR signaling to regulate white matter inflammatory lesions induced by chronic cerebral hypoperfusion. Neurochem Res 2016; 41: 3272–3277. [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Zhang HY, Tang XC. Huperzine a improves chronic inflammation and cognitive decline in rats with cerebral hypoperfusion. J Neurosci Res 2010; 88: 807–815. [DOI] [PubMed] [Google Scholar]
- 17. McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: Implications for therapy. Acta Neuropathol 2013; 126: 479–497. [DOI] [PubMed] [Google Scholar]
- 18. Laurent C, Buee L, Blum D. Tau and neuroinflammation: What impact for Alzheimer’s Disease and tauopathies? Biomed J 2018; 41: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J Neurosci Res 2017; 95: 943–972. [DOI] [PubMed] [Google Scholar]
- 20. Zhou Y, Zhang J, Wang L, et al. Interleukin-1beta impedes oligodendrocyte progenitor cell recruitment and white matter repair following chronic cerebral hypoperfusion. Brain Behav Immun 2017; 60: 93–105. [DOI] [PubMed] [Google Scholar]
- 21. Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science 2016; 353: 777–783. [DOI] [PubMed] [Google Scholar]
- 22. Jiang T, Xue LJ, Yang Y, et al. AVE0991, a nonpeptide analogue of Ang-(1–7), attenuates aging-related neuroinflammation. Aging 2018; 10: 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park BM, Cha SA, Lee SH, et al. Angiotensin IV protects cardiac reperfusion injury by inhibiting apoptosis and inflammation via AT4R in rats. Peptides 2016; 79: 66–74. [DOI] [PubMed] [Google Scholar]
- 24. Kong J, Zhang K, Meng X, et al. Dose-dependent bidirectional effect of angiotensin IV on abdominal aortic aneurysm via variable angiotensin receptor stimulation. Hypertension 2015; 66: 617–626. [DOI] [PubMed] [Google Scholar]
- 25. Royea J, Zhang L, Tong XK, et al. Angiotensin IV receptors mediate the cognitive and cerebrovascular benefits of losartan in a mouse model of Alzheimer’s disease. J Neurosci 2017; 37: 5562–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawas LH, McCoy AT, Yamamoto BJ, et al. Development of angiotensin IV analogs as hepatocyte growth factor/Met modifiers. J Pharmacol Exp Ther 2012; 340: 539–548. [DOI] [PubMed] [Google Scholar]
- 27. Terenzi R, Manetti M, Rosa I, et al. Angiotensin II type 2 receptor (AT2R) as a novel modulator of inflammation in rheumatoid arthritis synovium. Sci Rep 2017; 7: 13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matavelli LC, Zatz R, Siragy HM. A nonpeptide angiotensin II type 2 receptor agonist prevents renal inflammation in early diabetes. J Cardiovasc Pharmacol 2015; 65: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dhande I, Ma W, Hussain T. Angiotensin AT2 receptor stimulation is anti-inflammatory in lipopolysaccharide-activated THP-1 macrophages via increased interleukin-10 production. Hypertens Res 2015; 38: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Molnarfi N, Benkhoucha M, Funakoshi H, et al. Hepatocyte growth factor: A regulator of inflammation and autoimmunity. Autoimmun Rev 2015; 14: 293–303. [DOI] [PubMed] [Google Scholar]
- 31. Greenland K, Wyse B, Sernia C. Identification and characterization of angiotensinIV binding sites in rat neurone and astrocyte cell cultures. J Neuroendocrinol 1996; 8: 687–693. [PubMed] [Google Scholar]
- 32. Wright JW, Kawas LH, Harding JW. The development of small molecule angiotensin IV analogs to treat Alzheimer’s and Parkinson’s diseases. Prog Neurobiol 2015; 125: 26–46. [DOI] [PubMed] [Google Scholar]
- 33. Benoist CC, Wright JW, Zhu M, et al. Facilitation of hippocampal synaptogenesis and spatial memory by C-terminal truncated Nle1-angiotensin IV analogs. J Pharmacol Exp Ther. 2011; 339: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCoy AT, Benoist CC, Wright JW, et al. Evaluation of metabolically stabilized angiotensin IV analogs as procognitive/antidementia agents. J Pharmacol Exp Ther 2013; 344: 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]