Abstract
The prognostic value of squamous differentiation (SD) in urothelial carcinoma (UC) of the bladder is unclear. The aim of this study was to identify the clinical significance of SD in UC in terms of oncological outcomes in patients undergoing radical cystectomy (RC). We evaluated consecutive patients with muscle-invasive bladder cancer (MIBC; clinical T2-4aN0M0) treated with RC at our institution from March 2003 to March 2017. We enrolled 20 and 81 patients with UC with SD (UCSD) and pure UC, respectively. Postoperative survival outcomes were compared between the patients with UCSD and pure UC using the Kaplan-Meier method. Pre- and postcystectomy factors that influenced the overall survival (OS) and recurrence-free survival (RFS) were investigated in these patients. Multivariate Cox regression models were used to identify the predictors of OS and RFS. With a median follow-up time of 31 months, the 5-year OS rate of the UCSD and pure UC groups was 41.1% and 69.7% (P = .002) and the 5-year RFS rate was 51.8% and 59.5% (P = .027), respectively. The shape of the Kaplan-Meier curves for UCSD suggested a more rapid course of the disease within the first 2 years than observed in pure UC. Multivariate analyses suggested that SD in UC was significantly associated with OS (hazard ratio [HR]: 4.22; 95% confidence interval [CI]: 1.20-14.8; P = .024) and close to significance for a lower RFS (HR: 2.13, 95% CI: 0.74-6.15, P = .064). Our results indicate that SD may be an independent predictor of OS and RFS in UC of MIBC in patients undergoing RC.
Keywords: squamous differentiation, urothelial carcinoma, variant histology, radical cystectomy, prognosis
Introduction
Urothelial carcinoma with squamous differentiation (UCSD) of the bladder typically presents in cases that are more invasive and advanced than in cases observed in pure urothelial carcinoma (UC),1,2 with 60% to 70% of the cases being muscle-invasive bladder cancer (MIBC).3,4 Although squamous differentiation (SD) is the most common variant in bladder cancer, occurring in up to 20% of UC in bladder cancer cases,1,5 the biological characteristics and clinical significance of SD are poorly understood.
Several studies have suggested that UCSD has more aggressive behavior and worse survival outcomes when compared to pure UC in locally invasive bladder cancer.6-8 We also previously reported a poor response of UCSD to cisplatin-based chemotherapy.9 In contrast, recent studies reported that patients with UCSD and those with pure UC had similar survival rates.2,10 Most previous studies included SD and other variants such as glandular differentiation in a single-variant histology group.11, 12 These inclusions could have contributed to the heterogeneity of the outcomes in these previous studies; thus, the significance of SD may be unclear. The aim of this study was to assess the clinical significance of SD in UC in patients with MIBC undergoing radical cystectomy (RC).
Patients and Methods
Patient Population
We conducted a retrospective review of 139 consecutive patients with MIBC (clinical T2-4aN0M0) who had undergone RC at the University of Occupational and Environmental Health Hospital (Kitakyushu, Japan) between March 2003 and March 2017. Only patients who had histologically confirmed UCSD or pure UC were included. Patients with other histological variants and those without complete clinical data were excluded, resulting in a total of 101 patients in the analysis.
Patient Management
Patients underwent a routine precystectomy assessment, including physical examination, laboratory tests, transurethral resection of the bladder tumor (TURBT), chest-abdominal-pelvic computed tomography (CT) scan, and bone scintigraphy. The histological features and muscular invasion of tumors were confirmed in TURBT specimens by a single genitourinary pathologist who was blinded to the clinical outcome. Urothelial carcinoma with squamous differentiation was defined as the presence of UC and SD in the same tumor. In addition, SD required the presence of intercellular bridges or keratinization for diagnosis.5 The clinical tumor-node-metastasis stage was determined according to the World Health Organization criteria.13 Precystectomy factors were retrospectively collected, including age, sex, performance status, age-adjusted Charlson comorbidity index score,14 clinical stage, presence of hydronephrosis, presence of carcinoma in situ, multifocality, neutrophil to lymphocyte ratio (NLR) level, C-reactive protein (CRP) level, hemoglobin level, and history of neoadjuvant chemotherapy (NAC). Patients with clinical stage ≥T3 were eligible for NAC. The laboratory values were collected at the closest available time point to the treatment and no longer than 1 month before RC. The NLR values were derived as the ratio of the absolute neutrophil to absolute lymphocyte count in the peripheral blood. The preoperative NLR and CRP values were not assessed for patients with active infection or those receiving steroids or growth factors.
All patients underwent RC, pelvic lymphadenectomy, and urinary diversion. The final histopathologic diagnosis and extent of SD in the lesions were rereviewed in the cystectomy specimens. Postcystectomy factors were retrospectively collected, including the pathologic tumor stage (pT), the pathologic node stage (pN), lymphovascular invasion (LVI), number of lymph nodes removed, and history of adjuvant chemotherapy (AC). Patients with pT3-4 or pN+ were eligible for AC. Postoperative follow-up examinations comprised physical examinations, laboratory tests, and CT scans, which were conducted every 6 months until the fifth year and annually thereafter. When symptoms appeared, the appropriate additional examinations were conducted.
Assessment
The primary outcomes of interest were overall survival (OS) and recurrence-free survival (RFS). The OS duration was calculated from the date the RC was performed to the date of death due to any causes or to the date of the last follow-up, if the patient was alive. The RFS duration was calculated from the date the RC was performed to the date of the first clinical recurrence or the last follow-up, if the patient had no known recurrence. Thus, the aim of this study was to identify the impact of SD in UC on OS and RFS. The study was approved by the institutional review board of our institution.
In addition, the follow-up information was obtained for all patients through the medical records of our institute or local hospitals. For some patients without follow-up, information was obtained through contacting by telephone.
Statistical Analysis
All statistical analyses were performed using EZR (Easy R, Vienna, Austria), which is a graphical user interface for R (The R Foundation for Statistical Computing). The Fisher exact test examined the associations between categorical variables. Continuous variables were divided into 2 groups according to their median values. The Mann-Whitney U test compared the continuous variables. Overall survival and RFS were estimated using the Kaplan-Meier method and the log-rank test. The statistical significance of the relationship between survival outcomes and each pre-/postcystectomy factor was analyzed using the Cox proportional hazards model. A value of P < .05 was considered statistically significant.
Results
Patient Characteristics
The patient characteristics and outcomes are shown in Table 1. Of the 101 patients, 20 (19.8%) had UCSD and 81 (80.2%) had pure UC. The median follow-up time was 31 months (range, 5-165 months), during which 37 (36.6%) patients experienced recurrence and 33 (32.7%) died. The significant differences between the groups were in terms of the clinical inflammatory markers NLR and CRP. The median NLR value was 3.08 (interquartile range [IQR], 2.05-4.09) and 2.27 (IQR, 1.80-2.88) in patients with UCSD and pure UC, respectively (P = .038). The median CRP values was 0.4 mg/dL (IQR, 0.14-0.65 mg/dL) and 0.12 mg/dL (IQR, 0.06-0.47 mg/dL) in patients with UCSD and pure UC, respectively (P = .012). Neoadjuvant chemotherapy was provided to 9 (45%) and 29 (35.8%) patients with UCSD and pure UC, respectively (P = .1 96). Twenty patients (UCSD: 2; pure UC: 18) received a combination of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) and 18 (UCSD: 7; pure UC: 11) received a combination of gemcitabine and cisplatin (P = .058). Patients with UCSD had a significantly higher rate of pathologic stage ≥T3 than those with pure UC (60.0% vs. 30.9%, respectively; P = .020), although this imbalance did not reach significance regarding the clinical stage ≥T3 (60.0% vs. 35.8%, respectively; P = .074). Additionally, there were statistically significant differences in the rate of pN+ (UCSD: 45% vs pure UC: 21%; P = .044) and LVI (UCSD: 70.0% vs pure UC: 44.4%; P = .048).
Table 1.
Patient Characteristics.
| Pathologic Features | UCSD | Pure UC | P Value |
|---|---|---|---|
| No. of patients, n (%) | 20 (19.8) | 81 (80.2) | |
| Age, median (IQR) | 67 (64–73) | 69 (63–75) | .511 |
| Sex, n (%) | .391 | ||
| Male | 13 (65.0) | 62 (76.5) | |
| Female | 7 (35.0) | 19 (23.5) | |
| Performance status (ECOG), n (%) | .175 | ||
| 0 | 11 (55.0) | 59 (72.8) | |
| ≥1 | 9 (45.0) | 22 (27.2) | |
| ACCI score, median (IQR) | 4 (3-5) | 3 (3-4) | .177 |
| Clinical T stage, n (%) | .074 | ||
| T2 | 8 (40.0) | 52 (64.2) | |
| ≥T3 | 12 (60.0) | 29 (35.8) | |
| Hydronephrosis, n (%) | 5 (25.0) | 18 (22.2) | .772 |
| Carcinoma in situ, n (%) | 4 (20.0) | 21 (25.9) | .774 |
| Multifocality, n (%) | 9 (45.0) | 50 (61.7) | .209 |
| NLR, median (IQR) | 3.08 (2.05–4.09) | 2.27 (1.80–2.88) | .038 |
| CRP (mg/dL), median (IQR) | 0.40 (0.14–0.65) | 0.12 (0.06–0.47) | .012 |
| Hemoglobin (g/dL), median (IQR) | 12.4 (9.6–13.1) | 12.7 (11.4–13.9) | .177 |
| Neoadjuvant chemotherapy, n (%) | .058 | ||
| MVAC | 2 (22.2) | 18 (62.1) | |
| GC | 7 (77.8) | 11 (37.9) | |
| Pathologic T stage, n (%) | .020 | ||
| ≤T2 | 8 (40.0) | 56 (69.1) | |
| ≥T3 | 12 (60.0) | 25 (30.9) | |
| Pathologic N stage, n (%) | .044 | ||
| Negative | 11 (55.0) | 64 (79.0) | |
| Positive | 9 (45.0) | 17 (21.0) | |
| Lymphovascular invasion, n (%) | 14 (70.0) | 36 (44.4) | .048 |
| No. of lymph node removed (IQR) | 9 (6–13) | 8 (6–13) | .710 |
| Adjuvant chemotherapy, n (%) | 1.00 | ||
| MVAC | 2 (50.0) | 10 (50.0) | |
| GC | 2 (50.0) | 8 (40.0) | |
| PG | 0 (0.0) | 2 (10.0) |
Abbreviations: ACCI, age-adjusted Charlson comorbidity index; CRP, C-reactive protein; ECOG, Eastern Cooperative Oncology Group; GC, gemcitabine, cisplatin; IQR, interquartile range; MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin; NLR, neutrophil to lymphocyte ratio; PG, paclitaxel, gemcitabine; UC, urothelial carcinoma; UCSD, urothelial carcinoma with squamous differentiation.
Univariate Associations With Clinical Outcome
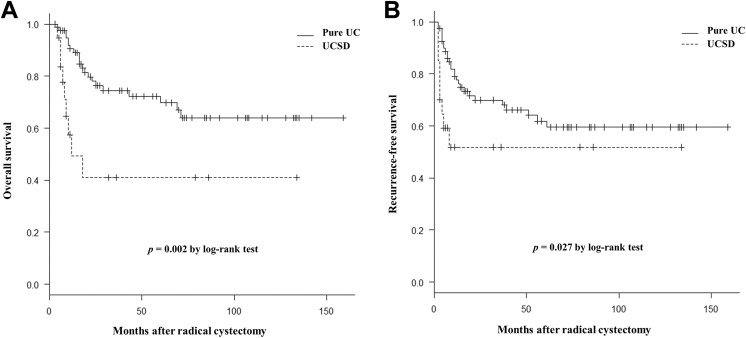
On comparison, the patients with UCSD had poorer OS and RFS values than those with pure UC (Figure 1). The 5-year OS rate of the UCSD and pure UC groups was 41.1% and 69.7% (P = .002) and the 5-year RFS rate was 51.8% and 59.5% (P = .027), respectively. The shape of the OS and RFS curves for the UCSD group presented with a steep descent within the first 2 years (Figure 1).
Figure 1.
Kaplan-Meier curves show the overall survival (A) and recurrence-free survival (B) stratified by patients with urothelial carcinoma with squamous differentiation (UCSD) and pure urothelial carcinoma (UC) in muscle-invasive bladder cancer.
As shown in Table 2, the associations of the pre- and postcystectomy factors with OS and RFS were investigated using univariate analyses. Among several factors, performance status, multifocality, NLR level, pT stage, pN stage, LVI, and presence of SD were significantly associated with OS and RFS.
Table 2.
Univariate Associations With Clinical Outcome.
| Variable | Overall Survival | Recurrence-Free Survival | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, years (≥69 vs <69) | 1.72 (0.83-3.58) | .146 | 1.37 (0.71-2.64) | .352 |
| Sex (female vs male) | 1.15 (0.51-2.57) | .736 | 0.99 (0.47-2.10) | .983 |
| Performance status (≥1 vs 0) | 2.47 (1.20-5.09) | .014 | 2.32 (1.20-4.50) | .013 |
| ACCI score (≥4 vs 0-3) | 1.12 (0.92-1.35) | .232 | 1.09 (0.92-1.28) | .305 |
| Hydronephrosis (present vs absent) | 1.77 (0.81-3.87) | .152 | 1.51 (0.73-3.14) | .269 |
| Carcinoma in situ (present vs absent) | 0.55 (0.21-1.44) | .221 | 0.67 (0.29-1.53) | .343 |
| Multifocality (multiple vs single) | 2.27 (1.04-4.94) | .039 | 2.06 (1.02-4.08) | .045 |
| NLR (≥2.35 vs <2.35) | 2.88 (1.32-6.31) | .008 | 2.59 (1.29-5.19) | .007 |
| CRP, mg/dL (≥0.14 vs <0.14) | 1.58 (0.76-3.28) | .222 | 1.62 (0.83-3.18) | .158 |
| Hemoglobin, g/dL (<12.5 vs ≥12.5) | 2.58 (1.18-5.64) | .017 | 1.86 (0.95-3.63) | .071 |
| Neoadjuvant chemotherapy (yes vs no) | 1.56 (0.76-3.22) | .227 | 1.27 (0.64-2.52) | .488 |
| Pathologic T stage (≥T3 vs ≤T2) | 3.23 (1.13-9.20) | .028 | 2.67 (1.08-6.58) | .033 |
| Pathologic N stage (positive vs negative) | 5.92 (2.70-12.99) | <.001 | 4.88 (2.47-9.66) | <.001 |
| Lymphovascular invasion (positive vs negative) | 5.18 (2.53-10.60) | <.001 | 3.87 (2.02-7.40) | <.001 |
| No. of lymph node removed (≥9 vs <9) | 0.91 (0.45-1.84) | .787 | 1.02 (0.97-1.08) | .466 |
| Adjuvant chemotherapy (yes vs no) | 1.77 (0.85-3.69) | .129 | 1.66 (0.83-3.30) | .153 |
| Squamous differentiation (present vs absent) | 3.14 (1.43-6.91) | .004 | 2.29 (1.07-4.91) | .034 |
Abbreviations: ACCI, age-adjusted Charlson comorbidity index; CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; NLR, neutrophil to lymphocyte ratio.
Multivariate Associations With Clinical Outcome
Using the same variables as the univariate analysis, the results of the multivariate Cox proportional hazard regression analyses for OS and RFS are shown in Table 3. Multifocality (hazard ratio [HR]: 4.66; 95% confidence interval [CI]: 1.54-14.07; P = .006), NLR level (HR: 3.06; 95% CI: 1.00-9.40; P = .049), pT stage (HR: 3.23; 95% CI: 1.13-9.20; P = .028), pN stage (HR: 4.68; 95% CI: 1.45-15.05; P = .005), and presence of SD (HR: 4.22; 95% CI: 1.20-14.80; P = .024) were identified as significant independent predictors of OS. Multifocality (HR: 3.54; 95% CI: 1.31-9.59; P = .013), NLR level (HR: 2.78; 95% CI: 1.10-7.01; P = .031), pT stage (HR: 2.67; 95% CI: 1.08-6.58; P = .033), pN stage (HR: 2.91; 95% CI: 1.75-5.86; P = .028), and LVI (HR: 5.83; 95% CI: 1.62-20.94; P = .007) were identified as significant independent predictors of RFS. The presence of SD showed a trend toward a lower RFS (HR: 2.13; 95% CI: 0.74-6.15; P = .064).
Table 3.
Multivariate Analysis Predicting Overall Survival and Recurrence-Free Survival.
| Variable | Overall Survival | Recurrence-Free Survival | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, years (≥69 vs <69) | 1.06 (0.41-2.74) | .900 | 0.63 (0.26-1.48) | .286 |
| Sex (female vs male) | 0.71 (0.22-2.24) | .554 | 0.62 (0.24-1.61) | .326 |
| Performance status (≥1 vs 0) | 0.99 (0.41-2.42) | .664 | 1.17 (0.51-2.70) | .706 |
| ACCI score (≥4 vs 0-3) | 0.90 (0.70-1.16) | .431 | 1.01 (0.80-1.26) | .948 |
| Hydronephrosis (present vs absent) | 1.93 (0.70-5.35) | .204 | 1.96 (0.79-4.83) | .144 |
| Carcinoma in situ (present vs absent) | 0.45 (0.11-1.15) | .133 | 0.52 (0.19-1.39) | .193 |
| Multifocality (multiple vs single) | 4.66 (1.54-14.07) | .006 | 3.54 (1.31-9.59) | .013 |
| NLR (≥2.35 vs <2.35) | 3.06 (1.00-9.40) | .049 | 2.78 (1.10-7.01) | .031 |
| CRP, mg/dL (≥0.14 vs <0.14) | 1.33 (0.53-3.37) | .546 | 1.06 (0.46-2.46) | .889 |
| Hemoglobin, g/dL (<12.5 vs ≥12.5) | 1.87 (0.66-5.27) | .236 | 1.06 (0.44-2.53) | .902 |
| Neoadjuvant chemotherapy (yes vs no) | 0.63 (0.21-1.80) | .410 | 0.64 (0.24-1.69) | .366 |
| Pathologic T stage (≥T3 vs ≤T2) | 3.23 (1.13-9.20) | .028 | 2.67 (1.08-6.58) | .033 |
| Pathologic N stage (positive vs negative) | 4.68 (1.45-15.05) | .005 | 2.91 (1.75-5.86) | .028 |
| Lymphovascular invasion (positive vs negative) | 2.32 (0.49-10.95) | .286 | 5.83 (1.62-20.94) | .007 |
| No. of lymph node removed (≥9 vs <9) | 0.97 (0.91-1.04) | .433 | 0.96 (0.89-1.02) | .183 |
| Adjuvant chemotherapy (yes vs no) | 1.05 (0.44-2.52) | .916 | 0.34 (0.09-1.33) | .122 |
| Squamous differentiation (present vs absent) | 4.22 (1.20-14.80) | .024 | 2.13 (0.74-6.15) | .064 |
Abbreviations: ACCI, age-adjusted Charlson comorbidity index; CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; NLR, neutrophil to lymphocyte ratio.
Evaluation of the Extent of SD on Survival
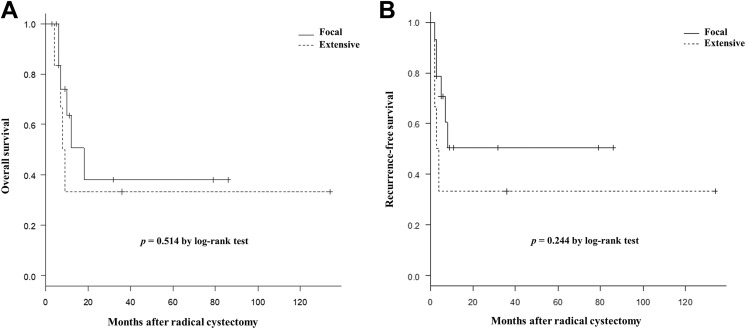
To assess the extent of SD, the UCSD group was divided into 2 groups according to the proportion of SD in the lesions (identified and measured using the cystectomy specimens). Six patients with ≥50% differentiation (extensive) and 14 patients with <50% differentiation (focal) were identified. The rates of OS and RFS were compared between the 2 groups using the Kaplan-Meier method (Figure 2). The analysis identified no significant difference in outcome between the groups. Although the percentage of SD was also analyzed as a continuous variable in relation to survival using the Cox proportional hazards model, the occupancy response relationship was not observed in OS (HR: 1.01; 95% CI: 0.98-1.20; P = .581) and RFS (HR: 1.02; 95% CI: 0.99-1.04; P = .224).
Figure 2.
The Kaplan-Meier curves show the overall survival (A) and recurrence-free survival (B) stratified by patients with urothelial carcinoma with squamous differentiation (UCSD) with extensive (≥50%) and focal (<50%) differentiation.
Discussion
In this study, SD is identified in 19.8% of patients with MIBC, which is a rate similar to those previously reported.1,5 Urothelial carcinoma with squamous differentiation presents with a significantly higher pathologic stage compared to pure UC. Liu et al1 reported that UCSD was frequently identified in patients with advanced T stage (pT3-4: 72.3%) and nodal metastasis (pN+: 46.2%). Although SD is the most common variant observed in UC, the clinical significance of SD in UC has not yet been well defined. In the present study, we demonstrate that SD in UC is significantly associated with poor oncological outcomes in patients with MIBC after undergoing an RC.
Few studies have investigated the outcomes of RC as a treatment for MIBC with UCSD.11,12 Additionally, the number of patients in these studies is small. Some reports indicated lower survival rate of patients with UCSD compared to those with pure UC, whereas others reported similar survival rates for both types of patients.2,6-8,10 This study aims to clarify the clinical significance of the survival rates in MIBC of patients with SD. In a previous study, the 5-year OS rates following RC for MIBC (clinicalT2-4a) were 50% to 60%.15 In the present study, patients with UCSD had reduced 5-year OS and RFS rates compared to those with pure UC. In past reports on UCSD, the survival rates were inconsistent, with 5-year OS and RFS rates varying from 34% to 64.7% and 36% to 62%, respectively.2-4,6-8,10,11 Monn et al16 reported that UCSD was not associated with a risk of mortality compared to risk in pure UC in multivariate analysis. In a Japanese cohort study, Nishiyama et al17 reported that histological variants were not independent predictors of mortality after RC. In contrast, our results reveal that the presence of SD is associated with increased risk of mortality. Honma et al18 indicated that concomitant SD in UC was an independent predictor of local recurrence after RC.
In our study, the rates of OS and RFS for the UCSD group showed a steep decline within 2 years after cystectomy. However, the recurrence of cystectomy after 5 years is extremely rare, regardless of the histology. Izard et al2 also indicated a rapid course of the disease in patients with UCSD within the first 2 years after cystectomy. These results indicate that close follow-up should be mandatory for the 2 years after surgery.
Zahoor et al19 reported a retrospective review of patients with UCSD and pure squamous cell carcinoma (SCC). In patients who underwent RC, the 2-year OS and RFS rates were 61% and 50%, respectively. The authors showed that older age, hydronephrosis, advanced pT stage, pN stage, and LVI were associated with shorter OS and RFS. Other earlier studies showed comparable oncologic outcomes between patients with UCSD and those with SCC.2,4,19
If the presence of SD is associated with the oncological outcome, the proportion of SD area in whole-cancer lesions may be important, with a higher percentage of SD area potentially being associated with lower OS. However, in our cohort study, the proportion of SD in cancer lesions was not associated with the survival outcomes. Other reports also demonstrated that the percentage of SD in cystectomy specimens was not associated with the postoperative survival.20,21 Therefore, the extent of SD in cancer lesions may not correlate with prognosis in patients with MIBC. Further studies are warranted to determine the impact of SD on the oncological outcome in patients with MIBC.
Patients with an MIBC histological variant are typically treated in the same manner as those with pure UC, although no high-level evidence supports this approach.7,22 In general, patients with locally advanced bladder cancer have a more favorable prognosis with RC after NAC than those with only RC.23 NAC has been reported to increase the proportion of downstaging and provide a survival benefit, especially in stage III cases.24 Therefore, NAC is indicated and performed only in patients with clinical T3-4 cancer and not in those with clinical T2 cancer in this study. However, NAC is not significantly associated with OS and RFS. This selection bias of patients may influence the efficacy of NAC, and NAC fails to show the better outcomes. We have reported that UCSD of the bladder was less sensitive to cisplatin-based NAC when compared to pure UC.9 The proportion of pathological downstaging was significantly lower in patients with UCSD than in those with pure UC (11.1% vs 51.7%; P = .031).9 Scosyrev et al25 reported that survival was significantly improved in patients with histological variants who received NAC. In their cohort, variant histology consisted of SD and glandular differentiation, although the NAC regimen was only for patients with MVAC. On the other hand, patients with pure UC did not have a significant survival benefit (HR: 0.90; 95% CI: 0.67-1.21; P = .48).25 Buisan et al20 indicated that patients with UCSD with an NLR ≥5 showed poor response to NAC and had short survival rate. In this study, we show that patients with UCSD have higher levels of NLR than those with pure UC.
Recently, Li et al26 reported significant differences in terms of recurrence and survival rates in patients with UCSD between re-TURBT and RC groups in recurred pT1. The authors recommended early cystectomy for patients with SD in non-muscle-invasive bladder cancer (NMIBC) because it was an independent, negative prognostic factor. The delay in RC could be associated with lowered survival rates.
In addition to SD, independent predictors of mortality or recurrence were multifocality, NLR level, pT stage, pN stage, and LVI. Advanced T stage, lymph node metastasis, and LVI at the time of RC were well-documented poor prognostic features.27,28 Several studies also reported that an elevated preoperative NLR level (cutoff value, ≈2.5) could be a potential predictor of oncological outcomes in MIBC.29-31 Moreover, recently published research by Ornstein et al32 showed that circulating myeloid-derived suppressor cells were significantly correlated with NLR in patients with UC undergoing RC, and these cells played an important role in tumor progression as immunosuppressive cells. The number of tumors was associated with the risk of recurrence after TURBT in NMIBC.33 Multifocality in MIBC caused increases in the tumor burden. Cheng et al34 suggested that an evaluation of the tumor burden and size could provide significant prognostic information in patients with MIBC.
There are certain limitations to this study such as its retrospective, nonrandomized single-institutional design, and small sample size. The follow-up periods were relatively short, and the follow-up evaluation time points were not standardized. The introduction of confounding bias from unmeasured factors was inevitable. The treatment was not uniform, as some patients received RC alone, while others received RC with chemotherapy. Additionally, the chemotherapy regimens were not standardized. The clinical significance of SD is only assessed in patients without distant metastasis. Therefore, future studies should include prospective validation with uniform treatment and a larger sample size, including patients with metastases. As in our study, it is important to evaluate the difference in the survival outcomes between subdivided histological variants and pure UC groups in MIBC. Pathologists should report the presence of SD in UC, which may predict poor oncological outcome, regardless of the proportion of such feature in the lesions. We believe that our study will assist in better defining the disease characteristics in patients with UCSD.
Conclusion
Our study suggests that SD in UC of the bladder may be associated with poor oncological outcome after RC and predict lowered rates of OS and RFS.
Footnotes
Authors’ Note: This study (H28-047) was approved by the ethics committee of the University of Occupational and Environmental Health Hospital (Kitakyushu, Japan).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Liu Y, Bui MM, Xu B. Urothelial carcinoma with squamous differentiation is associated with high tumor stage and pelvic lymph-node metastasis. Cancer Control. 2017;24(1):78–82. [DOI] [PubMed] [Google Scholar]
- 2. Izard JP, Siemens DR, Mackillop WJ, et al. Outcomes of squamous histology in bladder cancer: a population-based study. Urol Oncol. 2015;33(10):425, e7–e13. [DOI] [PubMed] [Google Scholar]
- 3. Shah RB, Montgomery JS, Montie JE, Kunju LP. Variant (divergent) histologic differentiation in urothelial carcinoma is under-recognized in community practice: impact of mandatory central pathology review at a large referral hospital. Urol Oncol. 2013;31(8):1650–1655. [DOI] [PubMed] [Google Scholar]
- 4. Ehdaie B, Maschino A, Shariat SF, et al. Comparative outcomes of pure squamous cell carcinoma and urothelial carcinoma with squamous differentiation in patients treated with radical cystectomy. J Urol. 2012;187(1):74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gellert LL, Warrick J, Al-Ahmadie HA. Urothelial carcinoma with squamous differentiation—the pathologists perspective. Urol Oncol. 2015;33(10):437–443. [DOI] [PubMed] [Google Scholar]
- 6. Erdemir F, Tunc M, Ozcan F, et al. The effect of squamous and/or glandular differentiation on recurrence, progression and survival in urothelial carcinoma of bladder. Int Urol Nephrol. 2007;39(3):803–807. [DOI] [PubMed] [Google Scholar]
- 7. Black PC, Brown GA, Dinney CP. The impact of variant histology on the outcome of bladder cancer treated with curative intent. Urol Oncol. 2009;27(1):3–7. [DOI] [PubMed] [Google Scholar]
- 8. Antunes AA, Nesrallah LJ, Dall’Oglio MF, et al. The role of squamous differentiation in patients with transitional cell carcinoma of the bladder treated with radical cystectomy. Int Braz J Urol. 2007;33(3):339–345. [DOI] [PubMed] [Google Scholar]
- 9. Minato A, Fujimoto N, Kubo T. Squamous differentiation predicts poor response to cisplatin-based chemotherapy and unfavorable prognosis in urothelial carcinoma of the urinary bladder. Clin Genitourin Cancer. 2017;15(6):e1063–e1067. [DOI] [PubMed] [Google Scholar]
- 10. Mitra AP, Bartsch CC, Bartsch G, Jr, Miranda G, Skinner EC, Daneshmand S. Does presence of squamous and glandular differentiation in urothelial carcinoma of the bladder at cystectomy portend poor prognosis? An intensive case-control analysis. Urol Oncol. 2014;32(2):117–127. [DOI] [PubMed] [Google Scholar]
- 11. Raman JD, Jafri SM. Surgical management of bladder urothelial carcinoma with squamous differentiation. Urol Oncol. 2015;33(10):429–433. [DOI] [PubMed] [Google Scholar]
- 12. Chen Q, Li L, Wang G, Hu J, Sun T, Fu B. Do histological variants in urothelial carcinoma of the bladder portend poor prognosis? A systematic review and meta-analysis. Oncotarget. 2017;8(29):48263–48271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Comperat EM, Burger M, Gontero P, et al. Grading of urothelial carcinoma and the new “World Health Organisation classification of tumours of the urinary system and male genital organs 2016. Eur Urol Focus. 2018. doi:10.1016/j.euf.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 14. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. [DOI] [PubMed] [Google Scholar]
- 15. Gakis G, Efstathiou J, Lerner SP, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63(1):45–57. [DOI] [PubMed] [Google Scholar]
- 16. Monn MF, Kaimakliotis HZ, Pedrosa JA, et al. Contemporary bladder cancer: variant histology may be a significant driver of disease. Urol Oncol. 2015;33(1):18, e15–e20. [DOI] [PubMed] [Google Scholar]
- 17. Nishiyama H, Habuchi T, Watanabe J, et al. Clinical outcome of a large-scale multi-institutional retrospective study for locally advanced bladder cancer: a survey including 1131 patients treated during 1990-2000 in Japan. Eur Urol. 2004;45(2):176–181. [DOI] [PubMed] [Google Scholar]
- 18. Honma I, Masumori N, Sato E, et al. Local recurrence after radical cystectomy for invasive bladder cancer: an analysis of predictive factors. Urology. 2004;64(4):744–748. [DOI] [PubMed] [Google Scholar]
- 19. Zahoor H, Elson P, Stephenson A, et al. Patient characteristics, treatment patterns and prognostic factors in squamous cell bladder cancer. Clin Genitourin Cancer. 2018;16(2):e437–e442. [DOI] [PubMed] [Google Scholar]
- 20. Buisan O, Orsola A, Oliveira M, et al. Role of inflammation in the perioperative management of urothelial bladder cancer with squamous-cell features: impact of neutrophil-to-lymphocyte ratio on outcomes and response to neoadjuvant chemotherapy. Clin Genitourin Cancer. 2017;15(4):e697–e706. [DOI] [PubMed] [Google Scholar]
- 21. Kim SP, Frank I, Cheville JC, et al. The impact of squamous and glandular differentiation on survival after radical cystectomy for urothelial carcinoma. J Urol. 2012;188(2):405–409. [DOI] [PubMed] [Google Scholar]
- 22. Willis D, Kamat AM. Nonurothelial bladder cancer and rare variant histologies. Hematol Oncol Clin North Am. 2015;29(2):237–252. [DOI] [PubMed] [Google Scholar]
- 23. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–866. [DOI] [PubMed] [Google Scholar]
- 24. Rosenblatt R, Sherif A, Rintala E, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61(6):1229–1238. [DOI] [PubMed] [Google Scholar]
- 25. Scosyrev E, Ely BW, Messing EM, et al. Do mixed histological features affect survival benefit from neoadjuvant platinum-based combination chemotherapy in patients with locally advanced bladder cancer? A secondary analysis of Southwest Oncology Group-Directed Intergroup Study (S8710). BJU Int. 2011;108(5):693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li G, Yu J, Song H, et al. Squamous differentiation in patients with superficial bladder urothelial carcinoma is associated with high risk of recurrence and poor survival. BMC Cancer. 2017;17(1):530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Margulis V, Lotan Y, Montorsi F, Shariat SF. Predicting survival after radical cystectomy for bladder cancer. BJU Int. 2008;102(1):15–22. [DOI] [PubMed] [Google Scholar]
- 28. Youssef RF, Lotan Y. Predictors of outcome of non-muscle-invasive and muscle-invasive bladder cancer. Scientific World Journal. 2011;11:369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim HS, Ku JH. Systemic inflammatory response based on neutrophil-to-lymphocyte ratio as a prognostic marker in bladder cancer. Dis Markers. 2016. doi:10.1155/2016/8345286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marchioni M, Primiceri G, Ingrosso M, et al. The clinical use of the neutrophil to lymphocyte ratio (NLR) in urothelial cancer: a systematic review. Clin Genitourin Cancer. 2016;14(6):473–484. [DOI] [PubMed] [Google Scholar]
- 31. Tang X, Wang S, An C, Du P, Yang Y. Preoperative high neutrophil-to-lymphocyte ratio is associated with high-grade bladder cancer. Anticancer Res. 2017;37(8):4659–4663. [DOI] [PubMed] [Google Scholar]
- 32. Ornstein MC, Diaz-Montero CM, Rayman P, et al. Myeloid-derived suppressors cells (MDSC) correlate with clinicopathologic factors and pathologic complete response (pCR) in patients with urothelial carcinoma (UC) undergoing cystectomy. Urol Oncol. 2018. doi:10.1016/j.urolonc.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 33. Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–477. [DOI] [PubMed] [Google Scholar]
- 34. Cheng L, Neumann RM, Scherer BG, et al. Tumor size predicts the survival of patients with pathologic stage T2 bladder carcinoma: a critical evaluation of the depth of muscle invasion. Cancer. 1999;85(12):2638–2647. [DOI] [PubMed] [Google Scholar]