Abstract
Human laryngeal squamous cell carcinoma (LSCC) is a malignant cancer type. Epithelial-mesenchymal transition marker Snail family transcriptional repressor 1 (SNAI1) is associated with the occurrence, development, invasion and metastasis of numerous tumor types, such as lung, liver and ovarian cancer. Previous studies have indicated that microRNA-153 (miR-153) may serve as a novel tumor suppressor, which is involved in tumor metastasis; however, the role and clinical significance of miR-153 in LSCC are not fully understood. The aim of the present study was to determine the role of miR-153 in the growth and aggressiveness of LSCC cells. Bioinformatics prediction method, western blot analysis, Matrigel invasion assay and immunofluorescence were used to analyze whether SNAI1 can be regulated and controlled by miR-153 in LSCC cells. An inverse association between miR-153 and SNAI1 was observed in LSCC tissues. It was demonstrated that SNAI1 is a direct target of miR-153 in LSCC. In addition, the results indicated that miR-153 knockdown inhibited PCI-13 cell migration and invasion by targeting SNAI1, which may be a potential marker that can reflect the degree of malignancy in patients with LSCC. Furthermore, miR-153 knockdown decreased Twist family BHLH transcription factor 1 and metastasis-associated 1 family member 3 expression in LSCC cells. In conclusion, these data indicated that miR-153 regulates LSCC migration via the targeting of SNAI1 gene, which may be a potential predictor for patients with LSCC.
Keywords: laryngeal squamous cell carcinoma, microRNA-153, Snail family transcriptional repressor 1, migration, invasion
Introduction
Human laryngeal squamous cell carcinoma (LSCC) originates from the laryngeal epithelium (1,2). Advanced cervical lymph node metastasis frequently occurs in patients with LSCC, which may result in a poor prognosis (3). The 5-year survival rate (72.5%) is low due to tumor metastasis being responsible for >90% of cancer-associated mortalities in the Southeast Asia region, as found between 2000 and 2006 (4). Currently the primary treatment of LSCC is surgery supplemented with radiotherapy, chemotherapy and biological therapy (5,6); however, to the best of our knowledge, no significant progress in the molecular biomarkers of the most common cervical lymph node metastasis of LSCC and targeted therapies has been proposed for patients with advanced LSCC (7–9). Therefore, investigating molecular biological markers, in order to analyze the invasion and migration of LSCC, is vital for improving the treatment of local invasion and cervical lymph node metastasis in patients with LSCC (10,11). Notably, microRNA (miRNA), such as miRNA-1 and miRNA-205, has been reported to be associated with the invasion and migration of LSCC, which contributed to the understanding of the molecular mechanism underlying the invasion and migration of LSCC (12–14).
Numerous studies have demonstrated that miRNA serves a critical role in the regulation of LSCC metastasis (15–17). A report has indicated that miR-153 inhibited the proliferation and invasion of human LSCC by targeting Kruppel-like factor 5 (18). The microRNA-153 (miR-153) in glioblastoma is significantly lower, compared with non-neoplastic brain tissues (19). Previous reports indicated that miR-153 suppresses breast cancer cell proliferation and induces apoptosis by targeting HECT domain E3 ubiquitin protein ligase 3 or myeloid cell leukemia 1 (20,21). In addition, miR-153 serves as a tumor suppressor and the association between miR-153 and metadherin is a promising therapeutic target for breast cancer (22). Furthermore, the downregulation of miR-153 accelerates epithelial-mesenchymal transition (EMT) via the regulation of the Snail family transcriptional repressor 1 (SNAI1) expression level (23). Clinical analysis indicated that the low expression level of miR-153 in patients with gastric cancer was associated with poor prognosis and tumor metastasis (24). In addition, a previous report indicated that miR-153 expression was decreased in lung cancer tissues compared with that in adjacent noncancerous tissues (24). Furthermore, miR-153 can decrease the migration and invasion of non-small-cell lung cancer cells by targeting ADAM metallopeptidase domain 19 (25,26); however, the association between miR-153 and SNAI1 in human LSCC has not been investigated previously.
EMT is associated with the occurrence, development, invasion and metastasis of numerous malignancy types, including lung and pancreatic cancer (27,28). In the present study, the expression level of EMT inducible factor SNAI1 was detected and the expression pattern of SNAI1 was determined in LSCC tissues and cells. The association between SNAI1 and tumor metastasis was also investigated in LSCC cells.
Materials and methods
Tissue samples and cell lines
LSCC and adjacent non-tumorous tissues (5 samples) were collected from the Department of Otorhinolaryngology and Maxillofacial Oncology, Tianjin Medical University Cancer Institute and Hospital (Tianjin, China). All cases were pathologically ascertained. All tissue samples were stored at −80°C until gene expression analysis. Written informed consent for research purposes was obtained from each patient. The characteristics of the patients were summarized (Table I). The study protocol was approved by the Committee of Tianjin Medical University Cancer Institute and Hospital. SNAI1 expression levels were analyzed in LSCC and normal tissues from 148 patients from The Cancer Genome Atlas (TCGA) database (https://cancergenome.nih.gov/). Associations among Twist family BHLH transcription factor 1 (TWST1), metastasis-associated 1 family member 3 (MTA3), glycogen synthase kinase 3β (GSK3B), lysine demethylase 1A (KDM1A), histone deacetylase 1 (HDAC1), ubiquitin C (UBC) and cadherin 1 (CDH1), SMAD3, SMAD4 and body resistance tumor cluster were analyzed based on TCGA data on 148 patients with LSCC. The analysis of the patients' survival curve was conducted on the oncolytic website (https://www.cancer.org/), according to the statistical data of the 148 patients with LSCC. Human laryngeal cancer cell line PCI-13 was purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) with 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 µg/ml penicillin and streptomycin (Sigma-Aldrich; Merck KGaA) and 10% glutamine.
Table I.
Characteristics of the patients with LSCC (n=22).
| Characteristics | No. of patients |
|---|---|
| Sex | |
| Male | 15 |
| Female | 7 |
| Age (years) | |
| Mean | 56 |
| Range | 42–84 |
| Tumor location | |
| Supraglottic | 7 |
| Glottic | 6 |
| Subglottic | 9 |
| Histological differentiation (47) | |
| Well differentiation LSCC | 8 |
| Moderately differentiation LSCC | 6 |
| Poorly differentiation LSCC | 3 |
| Undifferentiation. | 3 |
| Verrucous differentiation. | 2 |
LSCC, laryngeal squamous cell carcinoma.
Metabolic Gene Rapid Visualizer (MERAV)
The MERAV website (http://merav.wi.mit.edu/) provided additional and more advanced tools for analyzing gene expression, and was capable of analyzing multiple genes in parallel. SNAI1 expression in normal tissues, primary tumors and cancer cell lines were analyzed, and the analysis was performed using the MERAV website, in order to compare the multiple SNAI1 expression levels in LSCC and normal tissues.
RNA isolation and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from tissues and PCI-13 cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to manufacturer's protocols. Total RNA was reverse transcribed using TransScript First-Strand cDNA Synthesis Super Mix kit (Beijing Transgen Biotech Co., Ltd., Beijing, China) and TaqMan Human MiRNA Assay kit (both from Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to analyze miRNA expression according to the manufacturer's protocols. The Homo sapiens genes primers used were: SNAI1; frizzled class receptor 2 (FZD2); KDM1A; HDAC1; TWST1; CDH1; MTA3; GSK3B; and β-actin, which were synthetized from Genewiz, Inc. (South Plainfield, NJ, USA). The primer sequences are detailed in Table II. The PCR amplification for the quantification of the gene mRNAs was performed using an ABI 7500 Sequence Detection system (Applied Biosystems; Thermo Fisher Scientific, Inc.) and a SYBR® Premix rx Taq™ II (Perfect Real-Time) kit (Takara Bio, Inc., Otsu, Japan), as previously described (29). Relative genes expression levels were calculated using the comparative Cq method (30). The PCR steps were: 2 min at 95°C; followed by 40 cycles of 10 sec at 95°C, annealing for 20 sec at 55°C and a primer extension for 30 sec at 72°C; and then 10 min at 72°C. Results were expressed as a fold-change relative to β-actin mRNA expression.
Table II.
Sequences of the primers used in the present study.
| Sequence | ||
|---|---|---|
| Gene name | Reverse | Forward |
| SNAI1 | 5′-GCGGGATCCATGCCGCGCTC-3′ | 5′-CGCCTCGAGTCATTTGTCATCATCG-3′ |
| KDM1A | 5′-CTGTGGCCATCTGCCTAGT −3′ | 5′-GGGACGCAGCAACTGACATT −3′ |
| HDAC1 | 5′-CGCTTCCACTCCGAGGACTA-3′ | 5′-CTGGGCAGTCATCGCCTAC-3′ |
| TWST1 | 5′-AAGGCATCACTATGGACTTTCTCT-3′ | 5′-GCCAGTTTGATCCCAGTATTTT-3′ |
| CDH1 | 5′-TGATCCCAGGTCTTAGTGAG-3′ | 5′-AGTCTGAACTGACTTCCGCA-3′ |
| MTA3 | 5′-AAGGCGAGAGATTCTTTCCCTG′-3′ | 5′-ACTGGGGACAATTCACTAGAGC-3′ |
| GSK3B | 5′-AAAAGTTTAAAAAGGTCTGGAAAGC-3′ | 5′-TAGTGTTGCTTTTAAGACAGGGTTT-3′ |
| β-actin | 5′-CGGAGTCAACGGATTTGGTC-3′ | 5′-AGCCTTCTCCATGGTCGTGA-3′ |
SNAI1, Snail family transcriptional repressor 1; KDM1A, lysine demethylase 1A; HDAC1, histone deacetylase 1; TWST1, Twist family BHLH transcription factor 1; CDH1, cadherin 1; MTA3, metastasis-associated 1 family member 3; GSK3B, glycogen synthase kinase 3β.
miR-153, miR-199, small interfering-SNAI1 (si-SNAI1) and plasmids
miR-153, miR-199 negative control miRNA and si-SNAI1 were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China). The human SNAI1 3′-untranslated region (3′-UTR) was amplified by PCR, which was aforementioned, and inserted downstream of the firefly luciferase gene into the pMIR-REPORT vector (Promega Corporation, Madison, WI, USA), as described previously (31). The mutated 3′-UTR of SNAI1 in PCI-13 cells were created using overlap PCR, as described previously (32) and the mutated SNAI13′-UTR was also inserted into the pMIR-REPORT vector. The constructs of the pMIR-REPORT-UTR vectors were confirmed via sequencing. Renilla luciferase-expressing plasmid pRL-TK (used as an internal control; Promega Corporation) was purchased from Promega Corporation. A total of three independent experiments were performed for each assay.
Cell viability assay
PCI-13 cells or miR-153-transfected PCI-13 cells were cultured in 96-well plates (4,000 cells/well) in RPMI-1640 medium with 10% FBS for 24 h at 37°C. Cell viability was measured using the Cell-Counting kit-8 (CCK-8) assay (Sigma-Aldrich; Merck KGaA), according to the manufacturer's protocols. The result was quantitated spectrophotometrically by measuring the optical density at 450 nm, with a reference wavelength of 650 nm.
Matrigel invasion assay
Matrigel invasion assays were carried out in 24-well plates. PCI-13 cells (1×106) in HuMEC Basal serum-free medium (cat. no. 12753018; Thermo Fisher Scientific, Inc.) containing Matrigel insert filters at a 1:6 dilution. The lower chamber contained Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.) with 10% FBS. After incubation for 48 h at 37°C, cells were fixed with 5% paraformaldehyde for 10 min at room temperature and stained with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA) for 30 min at room temperature. The cells that invaded through the Matrigel membrane were quantified under a light microscope at ×40 magnification.
Wound healing assay
The wound healing assay was performed by following the protocol of a previous study (33). PCI-13 cells were cultured in a 12-well plate in RPMI-1640 medium with 10% FBS and treated with siRNA-SNAI1 (si-SNAI1) or siRNA-mimic (si-NC; Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h at 37°C. After three-time washing with DMEM medium to remove cell debris, the cells were allowed to migrate for 48 h at 37°C, followed by observation under a light microscope at ×40 magnification.
Luciferase reporter assay
The 3′-UTR sequence of SNAI1 was used to predict to interact with miR-153 or a mutated sequence within the predicted target sites, was transfected into the pGL3 control vector (Promega Corporation). These constructs were designated as wild-type (wt) SNAI1-3′-UTR or mutant SNAI1-3′-UTR. For the reporter assay, PCI-13 cells (1×104/well) were seeded in 24-well plates and transfected with the aforementioned constructs, miR-153 expression vector or negative control using Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.). After 48 h, the cells were collected and Renilla luciferase activity was measured using the Dual-Luciferase Reporter Assay system (Promega Corporation), according to the manufacturer's protocols. Results were obtained from three independent experiments performed in duplicate.
Western blotting
PCI-13 cells were lysed using radioimmunoprecipitation assay lysis buffer (Thermo Fisher Scientific, Inc.). Western blotting was performed according to a previously described method (34). Protein concentration was determined using a BCA Protein Assay Reagent kit (Pierce; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocols. Proteins (20 µg) were separated using SDS-PAGE and then transferred to a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). Following blocking with 5% bull serum albumin (Sigma-Aldrich; Merck KGaA) for 1 h at 37°C, the primary antibodies used in the immunoblotting assays were: SNAI1 (1:1,000; cat. no. ab117866; Abcam, Cambridge, UK) and β-actin (1:1,000; cat. no. G8144; United States Biological, Salem, MA, USA), for 12 h at 4°C. Horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no. 1662408; Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used at a 1:5,000 dilution for 2 h at 37°C and detected using a Western Blotting Luminol reagent (EMD Millipore). Protein was quantified and normalized to β-actin using ImageJ software (version 1.38; National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence
PCI-13 cells were fixed with formaldehyde-sucrose for 4 h at 37°C. Following dehydration in graded ethanol (70, 80, 90, 95 and 100%) and xylene. Cells stained with 1% DAPI at 37°C for 30 min. Cells were washed twice with PBS and then stained with Ki67 for 2 h at 37°C. Immunostained cells were visualized using an inverted Olympus fluorescence microscope at ×100 magnification (LSM 700; Carl Zeiss AG, Oberkochen, Germany).
Statistical analysis
Results were calculated as the mean ± standard error of the mean. Significance was established using the SPSS statistical 18.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). All data were analyzed using Student's t-test or one-way analysis of variance with a Tukey's Honest Significant Difference test. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression level of SNAI1 in LSCC is higher, compared with normal tissue
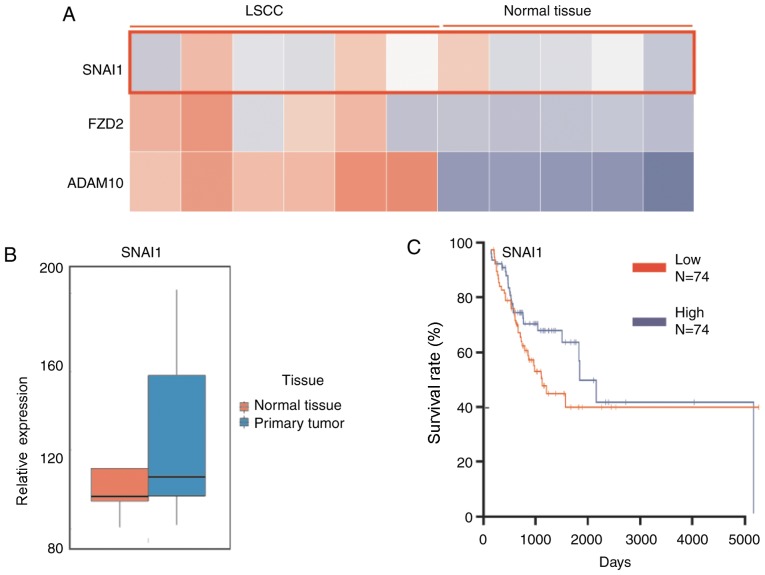
The SNAI1 expression level in LSCC and normal tissues was analyzed using MERAV image Heat-map database, with preliminary differential expression analysis described previously (35). The results demonstrated that the level of SNAI1 in LSCC tissues was generally higher, compared with normal tissues (Fig. 1A). Additionally, two cancer cell invasion-associated genes SNAI1 and FZD2 were determined to be markedly upregulated in LSCC, which may explain the high invasiveness of LSCC. Furthermore, the database was also used for the analysis of mRNA expression levels. The results demonstrated that LSCC tumor tissues (n=5) exhibited higher miR-153 level, compared with normal tissues (n=5; Fig. 1B). The analysis of the patients' survival curve from the oncolytic website demonstrated that a higher expression level of SNAI1 was negatively associated with a lower survival rate of patients with LSCC in 148 patients (Fig. 1C). These results indicated that expression level of SNAI1 is higher in LSCC tissue, compared with normal tissue, and lower SNAI1 expression presents longer survival, compared with higher SNAI1 expression.
Figure 1.
Expression levels of SNAI1 in LSCC tissue are higher, compared with normal tissue. (A) Expression levels of SNAI1 between LSCC and normal tissue. (B) Expression mRNA levels of SNAI1 between LSCC and normal tissue. (C) Association of patients' survival rate and SNAI1 expression. LSCC, laryngeal squamous cell carcinoma; SNAI1, Snail family transcriptional repressor 1; ADAM19, ADAM metallopeptidase domain 19; FZD2, frizzled class receptor 2.
miR-153 negatively regulates the expression of SNAI1
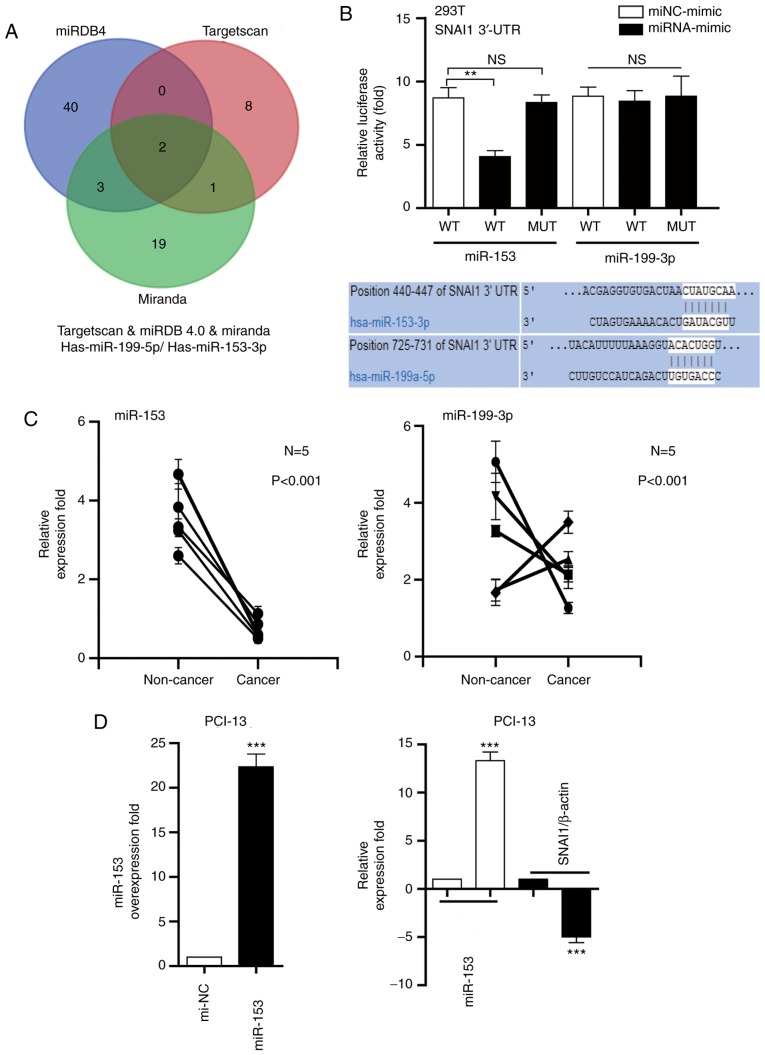
As depicted in Fig. 2A, two miRNAs (miR-153 and miR-199) were directly targeted by the 3′UTR of SNAI1 (Fig. 2A), using software programs TargetScan (http://www.targetscan.org), miRDB4.0 (http://www.mirdb.org/) and Miranda (http://www.microrna.org/microrna/home.do). The dual luciferase reporter assay demonstrated that the relative luciferase activity containing the wt SNAI1 3′-UTR was markedly decreased via miR-153 in PIC-13 cells, compared with miRNA-mimic; however, the relative activity of the mutated SNAI1 3′-UTR reporter was not altered by miR-153. Additionally, miR-199-transfected cells did not significantly change the luciferase activity (Fig. 2B). The miR-153 and miR-199 expression in LSCC tissues was measure and compared with corresponding non-cancer tissues. It was determined that the miR-53 expression level was downregulated in cancer tissues, compared with non-cancer tissue (Fig. 2C). The association between miR-153 and SNAI1 in clinical specimens was analyzed. RT-qPCR was performed to measure the expression of SNAI1 in five LSCC samples. The results demonstrated that the miR-153 expression was inversely associated with SNAI1 mRNA expression in the five LSCC samples (Fig. 2D). These results indicated that miR-153 may negatively regulate the expression of SNAI1.
Figure 2.
miR-153 negatively regulates the expression of SNAI1 in LSCC cells. (A) miR-153 or miR-199 directly targets the 3′-UTR of SNAI1. (B) Luciferase activity of miR-153 and miR-199 in HEK293T cells. (C) Analysis of miR-153 and miR-199 expression in cancer tissues, compared with corresponding non-cancer tissues. (D) Association between miR-153 and SNAI1 in clinical specimens. **P<0.01, ***P<0.001 vs. mi-NC. miR, microRNA; NS, no significance; WT, wild-type; mut, mutated; UTR, untranslated; SNAI1, Snail family transcriptional repressor 1; NC, negative control; LSCC, laryngeal squamous cell carcinoma.
miR-153 inhibits PCI-13 cell proliferation, migration and invasion in vitro
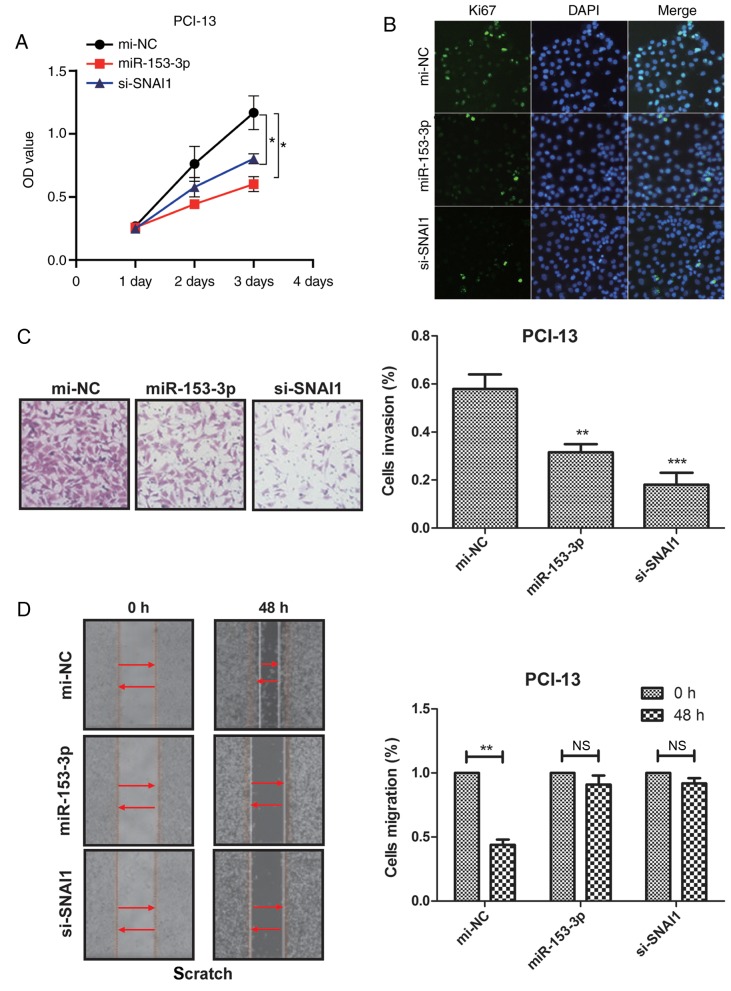
To determine the role of miR-153 in PCI-13 cells, miR-153 and si-SNAI1 were transfected into PCI-13 cells via adding the miR-153 or si-SNAI1. As measured by RT-qPCR and western blot analysis, the expression of miR-153 and si-SNAI1 were overexpressed by miR-153 and si-SNAI1 mimics in PCI-13 cells (Fig. 3A). CCK-8 assay and Ki67 staining were used to detect cell proliferation and it was determined that after 72 h, miR-153 and si-SNAI1 markedly inhibited cell proliferation, compared with si-NC (Fig. 3A and B). Cell wounding assays were performed to determine the effect of increased miR-153 and si-SNAI1 levels on tumor cell migration. It was demonstrated that the upregulation of miR-153 and si-SNAI1 resulted in a significant reduction of migration of PCI-13 cells, compared with mi-NC (Fig. 3C). Furthermore, as determined by Matrigel assays, the number of invaded PCI-13 cells decreased in cells transfected with miR-153 and si-SNAI1 mimics, compared with mi-NC (Fig. 3D). These results indicated that miR-153 could inhibit PCI-13 cell proliferation, migration and invasion in vitro.
Figure 3.
miR-153 inhibits PCI-13 cell proliferation, migration and invasion in vitro. (A) Expression of miR-153 and si-SNAI1 in miR-153 and si-SNAI1 mimics in PCI-13 cells. (B) miR-153 or si-SNAI1 inhibits PCI-13 cells proliferation. (C) miR-153 and si-SNAI1 significantly reduces the cell invasion of PCI-13 cells. (D) miR-153 and si-SNAI1 mimics decrease the migration of PCI-13 cells. *P<0.05, **P<0.01, ***P<0.001, vs. mi-NC. miR, microRNA; NC, negative control; SNAI1, Snail family transcriptional repressor 1; OD, optical density; si, small interfering; NS, no significance.
Association analysis of the SNAI1 gene
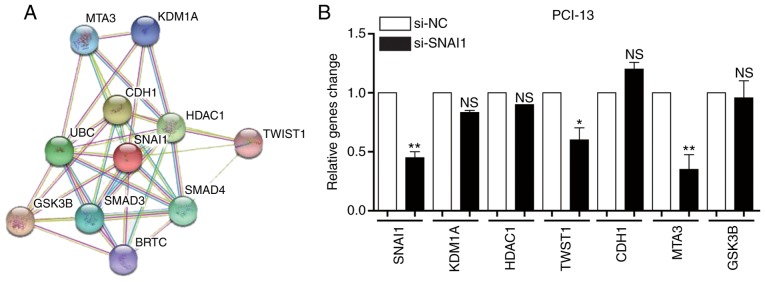
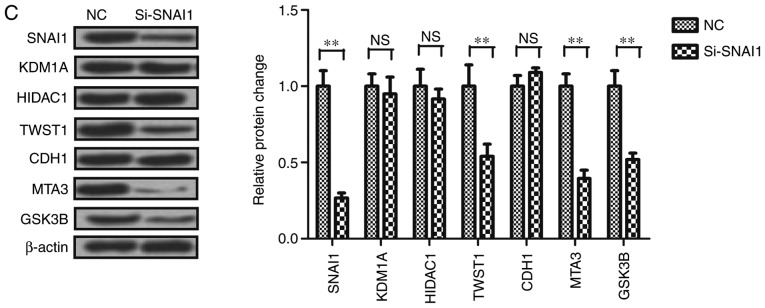
A number of genes were determined to be associated with the SNAI1 gene using STRING (http://string-db.org/) and a protein-associated online analysis software (Fig. 4A). The expression level of genes were verified and it was determined that the expression levels of KDM1A, HDAC1 and CDH1 were not significantly altered by si-SNAI1, whilst the expressions levels of TWST1, MTA3 and GSK3B were markedly downregulated by si-SNAI1 in PCI-13 cells. These genes were associated with cell invasion, indicating that SNAI affected cell invasion (Fig. 4B). The results demonstrated that changes in the expression of metastatic proteins TWST1, MTA3, GSK3B, KDM1A, HDAC1 and CDH1 were in accordance with gene expression (Fig. 4C). These results indicated that the SNAI1 gene is associated with LSCC cell invasion.
Figure 4.
Analysis of the association between the SNAI1 gene and SNAI1-associated genes in PCI-13 cells. (A) Association of SNAI1 and its associated genes in PCI-13 cells. (B) Effects of knockdown of SNAI1 on its associated genes in PCI-13 cells. (C) Effects of knockdown of SNAI1 on its associated proteins in PCI-13 cells. *P<0.05, **P<0.01 vs. NC. SNAI1, Snail family transcriptional repressor 1; si, small interfering; NC, negative control; NS, no significance; MTA3, metastasis-associated 1 family member 3; KDM1A, lysine demethylase 1A; CDH1, cadherin 1; HDAC1, histone deacetylase 1; UBC, ubiquitin C; TWST1, Twist family BHLH transcription factor 1; GSK3B, glycogen synthase kinase 3β; BRTC, body resistance tumor cluster.
Discussion
Local invasion and cervical lymph node metastasis of LSCC are the primary risk factors for the recurrence and prognosis of patients with LSCC (36). The molecular mechanisms underlying tumor development have become a popular research topic, and research regarding the molecular mechanisms underlying the invasion and metastasis of LSCC also focused on the regulatory role of miRNA (12,13,37). Although the tumor suppressor effects of miR-153 have been studied in human breast cancer, colorectal cancer and epithelial cancer cells, the miR-153-mediated SNAI1 signal pathway has not been reported in LSCC cells (22,38,39). In the present study, miR-153 expression was demonstrated to be inversely associated with SNAI1 mRNA expression in the five LSCC samples. Notably, it was determined that miR-153 could inhibit the proliferation, migration and invasion of PCI-13 cells via inhibition of SNAI1 expression.
Currently, an increasing number of reports have demonstrated the association between miRNA and tumor metastasis, which is regulated by miRNA mediating a number of signal pathways (40,41). Liu et al (42) indicated that miR-153 could enhance the therapeutic effects of gemcitabine, by targeting SNAI1 in pancreatic cancer and gastric carcinoma. In the present study, the expression differences of SNAI1 in cancer and normal tissues were examined. SNAI1 serves a key role in understanding the intercellular matrix signal pathway through regulation of E-cadherin and Slug, which induces the EMT processes in LSCC. A previous study demonstrated that oxidase-like 2 can be used as a poor prognosis indicator for human squamous cell carcinomas, which could promote malignant transformation by SNAI1-dependent and SNAI1-independent pathways (43). The present study indicated that the SNAI1 gene may be the target of miR-153. The association between the SNAI1 gene and miR-153 was verified by a dual-luciferase experiment. This method can be extended to the interaction of other associated genes. Previous studies indicated that EMT markers are associated with human tumor metastasis (44–46). In the present study, only the association between miR-153 and SNAI1 in LSCC cells was analyzed. Future studies should analyze the association between miR-153 and other EMT markers in LSCC cells.
To conclude, it was determined that miR-153 may regulate the expression of SNAI1 in the LSCC cells, which may be considered as a tumor suppressor agent (34). Data indicated that miR-153 is downregulated in LSCC, whilst overexpression of miR-153 can significantly inhibit the proliferation and migration of PCI-13 cells. Data also provided a direction for investigating the molecular mechanism underlying LSCC invasion; however, further LSCC samples are require to be analyzed, to determine the association between miR-153 and SNAI1, in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
BZ and TF performed the experiments and analyzed experimental data. LZ designed this experiments.
Ethics approval and consent to participate
This study was approved by Ethics Committee of Tianjin Medical University Cancer Institute and Hospital. Written informed consent for research purposes was obtained from each patient.
Patient consent for publication
All patients have provided consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rees L, Birchall M, Bailey M, Thomas S. A systematic review of case-control studies of human papillomavirus infection in laryngeal squamous cell carcinoma. Clin Otolaryngol Allied Sci. 2004;29:301–306. doi: 10.1111/j.1365-2273.2004.00841.x. [DOI] [PubMed] [Google Scholar]
- 2.Miura K, Kum Y, Han G, Tsutsui Y. Radiation-induced laryngeal angiosarcoma after cervical tuberculosis and squamous cell carcinoma: Case report and review of the literature. Pathol Int. 2003;53:710–715. doi: 10.1046/j.1440-1827.2003.01540.x. [DOI] [PubMed] [Google Scholar]
- 3.Joos B, Joos N, Bumpous J, Burns C, French CA, Farghaly H. Laryngeal squamous cell carcinoma in a 13 year-old child associated with human papillomaviruses 16 and 18: A case report and review of the literature. Head Neck Pathol. 2009;3:37–41. doi: 10.1007/s12105-008-0093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larbcharoensub N, Cheewaruangroj W, Nitiyanant P. Laryngeal sarcocystosis accompanying laryngeal squamous cell carcinoma: Case report and literature review. Southeast Asian J Trop Med Public Health. 2011;42:1072–1076. [PubMed] [Google Scholar]
- 5.Si-Mohamed A, Badoual C, Hans S, Péré H, Tartour E, Brasnu D. An unusual human papillomavirus type 82 detection in laryngeal squamous cell carcinoma: Case report and review of literature. J Clin Virol. 2012;54:190–193. doi: 10.1016/j.jcv.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Sipaul F, Birchall M, Corfield A. What role do mucins have in the development of laryngeal squamous cell carcinoma? A systematic review. Eur Arch Otorhinolaryngol. 2011;268:1109–1117. doi: 10.1007/s00405-011-1617-8. [DOI] [PubMed] [Google Scholar]
- 7.Furusaka T, Matsuda A, Tanaka A, Matsuda H, Ikeda M. Superselective intra-arterial chemoradiation therapy for functional laryngeal preservation in advanced squamous cell carcinoma of the glottic larynx. Acta Otolaryngol. 2013;133:633–640. doi: 10.3109/00016489.2012.744144. [DOI] [PubMed] [Google Scholar]
- 8.Xian JM, Zhou GY, Liang CY, Liu SX. Study on interference therapy induced by epidermal growth factor receptor-antisense cDNA in signal transduction of laryngeal squamous cell carcinoma. Hua Xi Kou Qiang Yi Xue Za Zhi. 2007;25:540–543. 547. (In Chinese) [PubMed] [Google Scholar]
- 9.Louw L, Claassen J. Rationale for adjuvant fatty acid therapy to prevent radiotherapy failure and tumor recurrence during early laryngeal squamous cell carcinoma. Prostaglandins Leukot Essent Fatty Acids. 2008;78:21–26. doi: 10.1016/j.plefa.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Butler A, Rigby MH, Scott J, Trites J, Hart R, Taylor SM. A retrospective review in the management of T3 laryngeal squamous cell carcinoma: An expanding indication for transoral laser microsurgery. J Otolaryngol Head Neck Surg. 2016;45:34. doi: 10.1186/s40463-016-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross BC, Olsen SM, Lewis JE, Kasperbauer JL, Moore EJ, Olsen KD, Price DL. Level IIB lymph node metastasis in laryngeal and hypopharyngeal squamous cell carcinoma: Single-institution case series and review of the literature. Laryngoscope. 2013;123:3032–3036. doi: 10.1002/lary.24129. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Chen Y, Yu J, Liu G, Huang Z. Integrated transcriptome analysis reveals miRNA-mRNA crosstalk in laryngeal squamous cell carcinoma. Genomics. 2014;104:249–256. doi: 10.1016/j.ygeno.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Wang F, Song G, Liu M, Li X, Tang H. miRNA-1 targets fibronectin1 and suppresses the migration and invasion of the HEp2 laryngeal squamous carcinoma cell line. FEBS Lett. 2011;585:3263–3269. doi: 10.1016/j.febslet.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 14.Wang B, Lv K, Chen W, Zhao J, Luo J, Wu J, Li Z, Qin H, Wong TS, Yang W, et al. miR-375 and miR-205 Regulate the Invasion and Migration of Laryngeal Squamous Cell Carcinoma Synergistically via AKT-Mediated EMT. Biomed Res Int. 2016;2016:9652789. doi: 10.1155/2016/9652789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo J, Wu J, Li Z, Qin H, Wang B, Wong TS, Yang W, Fu QL, Lei W. miR-375 suppresses IGF1R expression and contributes to inhibition of cell progression in laryngeal squamous cell carcinoma. Biomed Res Int. 2014;2014:374598. doi: 10.1155/2014/374598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li JZ, Gao W, Lei WB, Zhao J, Chan JY, Wei WI, Ho WK, Wong TS. MicroRNA 744-3p promotes MMP-9-mediated metastasis by simultaneously suppressing PDCD4 and PTEN in laryngeal squamous cell carcinoma. Oncotarget. 2016;7:58218–58233. doi: 10.18632/oncotarget.11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janiszewska J, Szaumkessel M, Kostrzewska-Poczekaj M, Bednarek K, Paczkowska J, Jackowska J, Grenman R, Szyfter K, Wierzbicka M, Giefing M, Jarmuz-Szymczak M. Global miRNA expression profiling identifies miR-1290 as novel potential oncomiR in laryngeal carcinoma. PloS One. 2015;10:e0144924. doi: 10.1371/journal.pone.0144924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu JY, Lu JB, Xu Y. MicroRNA-153 inhibits the proliferation and invasion of human laryngeal squamous cell carcinoma by targeting KLF5. Exp Ther Med. 2016;11:2503–2508. doi: 10.3892/etm.2016.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghasemi A, Fallah S, Ansari M. MiR-153 as a tumor suppressor in glioblastoma multiforme is downregulated by DNA methylation. Clin Lab. 2016;62:573–580. doi: 10.7754/Clin.Lab.2015.150738. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Li L, Li Y, Liu Z. MiR-153 promotes breast cancer cell apoptosis by targeting HECTD3. Am J Cancer Res. 2016;6:1563–1571. [PMC free article] [PubMed] [Google Scholar]
- 21.Zou Y, Liu W, Zhang J, Xiang D. miR-153 regulates apoptosis and autophagy of cardiomyocytes by targeting Mcl-1. Mol Med Rep. 2016;14:1033–1039. doi: 10.3892/mmr.2016.5309. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Zhai L, Zhao C, Lv S. MiR-153 inhibits epithelial-mesenchymal transition by targeting metadherin in human breast cancer. Breast Cancer Res Treat. 2015;150:501–509. doi: 10.1007/s10549-015-3346-y. [DOI] [PubMed] [Google Scholar]
- 23.Xia W, Ma X, Li X, Dong H, Yi J, Zeng W, Yang Z. miR-153 inhibits epithelial-to-mesenchymal transition in hepatocellular carcinoma by targeting Snail. Oncol Rep. 2015;34:655–662. doi: 10.3892/or.2015.4008. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Liu C. MiR-153 regulates metastases of gastric cancer through Snail. Tumour Biol. 2015 Aug 2; doi: 10.1007/s13277-015-3846-8. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 25.Yuan Y, Du W, Wang Y, Xu C, Wang J, Zhang Y, Wang H, Ju J, Zhao L, Wang Z, et al. Suppression of AKT expression by miR-153 produced anti-tumor activity in lung cancer. Int J Cancer. 2015;136:1333–1340. doi: 10.1002/ijc.29103. [DOI] [PubMed] [Google Scholar]
- 26.Shan N, Shen L, Wang J, He D, Duan C. MiR-153 inhibits migration and invasion of human non-small-cell lung cancer by targeting ADAM19. Biochem Biophys Res Commun. 2015;456:385–391. doi: 10.1016/j.bbrc.2014.11.093. [DOI] [PubMed] [Google Scholar]
- 27.Baek SH, Ko JH, Lee JH, Kim C, Lee H, Nam D, Lee J, Lee SG, Yang WM, Um JY, et al. Ginkgolic acid inhibits invasion and migration and TGF-β-induced EMT of lung cancer cells through PI3K/Akt/mTOR inactivation. J Cell Physiol. 2017;232:346–354. doi: 10.1002/jcp.25426. [DOI] [PubMed] [Google Scholar]
- 28.Rumman M, Jung KH, Fang Z, Yan HH, Son MK, Kim SJ, Kim J, Park JH, Lim JH, Hong S, Hong SS. HS-173, a novel PI3K inhibitor suppresses EMT and metastasis in pancreatic cancer. Oncotarget. 2016;7:78029–78047. doi: 10.18632/oncotarget.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HH, Lee YJ, Chan P, Lin CY. Use of PCR-based amplification analysis as a substitute for the southern blot method for CYP21 deletion detection in congenital adrenal hyperplasia. Clin Chem. 2004;50:1074–1076. doi: 10.1373/clinchem.2003.028597. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Stanewsky R. Analysis of rhythmic gene expression in adult Drosophila using the firefly luciferase reporter gene. Methods Mol Biol. 2007;362:131–142. doi: 10.1007/978-1-59745-257-1_9. [DOI] [PubMed] [Google Scholar]
- 32.Xiao YH, Yin MH, Hou L, Luo M, Pei Y. Asymmetric overlap extension PCR method bypassing intermediate purification and the amplification of wild-type template in site-directed mutagenesis. Biotechnol Lett. 2007;29:925–930. doi: 10.1007/s10529-007-9327-4. [DOI] [PubMed] [Google Scholar]
- 33.Sticker D, Lechner S, Jungreuthmayer C, Zanghellini J, Ertl P. Microfluidic migration and wound healing assay based on mechanically induced injuries of defined and highly reproducible areas. Anal Chem. 2017;89:2326–2333. doi: 10.1021/acs.analchem.6b03886. [DOI] [PubMed] [Google Scholar]
- 34.Huang G. Erratum: Differential expression of miR-21 and miR-75 in esophageal carcinoma patients and its clinical implication. Am J Transl Res. 2017;9:1961. [PMC free article] [PubMed] [Google Scholar]
- 35.Shaul YD, Yuan B, Thiru P, Nutter-Upham A, McCallum S, Lanzkron C, Bell GW, Sabatini DM. MERAV: A tool for comparing gene expression across human tissues and cell types. Nucleic Acids Res. 2016;44:D560–D566. doi: 10.1093/nar/gkv1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGarey PO, Jr, O'Rourke AK, Owen SR, Shonka DC, Jr, Reibel JF, Levine PA, Jameson MJ. Rigid esophagoscopy for head and neck cancer staging and the incidence of synchronous esophageal malignant neoplasms. JAMA Otolaryngol Head Neck Surg. 2016;142:40–45. doi: 10.1001/jamaoto.2015.2815. [DOI] [PubMed] [Google Scholar]
- 37.Cybula M, Wieteska L, Józefowicz-Korczyńska M, Karbownik MS, Grzelczyk WL, Szemraj J. New miRNA expression abnormalities in laryngeal squamous cell carcinoma. Cancer Biomark. 2016;16:559–568. doi: 10.3233/CBM-160598. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Pickard K, Jenei V, Bullock MD, Bruce A, Mitter R, Kelly G, Paraskeva C, Strefford J, Primrose J, et al. miR-153 supports colorectal cancer progression via pleiotropic effects that enhance invasion and chemotherapeutic resistance. Cancer Res. 2013;73:6435–6447. doi: 10.1158/0008-5472.CAN-12-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Q, Sun Q, Zhang J, Yu J, Chen W, Zhang Z. Downregulation of miR-153 contributes to epithelial-mesenchymal transition and tumor metastasis in human epithelial cancer. Carcinogenesis. 2013;34:539–549. doi: 10.1093/carcin/bgs374. [DOI] [PubMed] [Google Scholar]
- 40.Mühlberg L, Kühnemuth B, Costello E, Shaw V, Sipos B, Huber M, Griesmann H, Krug S, Schober M, Gress TM, Michl P. miRNA dynamics in tumor-infiltrating myeloid cells modulating tumor progression in pancreatic cancer. Oncoimmunology. 2016;5:e1160181. doi: 10.1080/2162402X.2016.1160181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakraborty C, Chin KY, Das S. miRNA-regulated cancer stem cells: Understanding the property and the role of miRNA in carcinogenesis. Tumour Biol. 2016;37:13039–13048. doi: 10.1007/s13277-016-5156-1. [DOI] [PubMed] [Google Scholar]
- 42.Liu F, Liu B, Qian J, Wu G, Li J, Ma Z. miR-153 enhances the therapeutic effect of gemcitabine by targeting Snail in pancreatic cancer. Acta Biochim Biophys Sin (Shanghai) 2017;49:520–529. doi: 10.1093/abbs/gmx039. [DOI] [PubMed] [Google Scholar]
- 43.Peinado H, Moreno-Bueno G, Hardisson D, Pérez-Gómez E, Santos V, Mendiola M, de Diego JI, Nistal M, Quintanilla M, Portillo F, Cano A. Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res. 2008;68:4541–4550. doi: 10.1158/0008-5472.CAN-07-6345. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Han H, Hu Y, Yang W, Lv Y, Wang L, Zhang L, Ji J. MicroRNA-130a-3p suppresses cell migration and invasion by inhibition of TBL1XR1-mediated EMT in human gastric carcinoma. Mol Carcinog. 2018;57:383–392. doi: 10.1002/mc.22762. [DOI] [PubMed] [Google Scholar]
- 45.Yang L, Wang T, Zhang J, Wang X. BTBD7 silencing inhibited epithelial- mesenchymal transition (EMT) via regulating Slug expression in human salivary adenoid cystic carcinoma. Cancer Biomark. 2017;20:461–468. doi: 10.3233/CBM-170262. [DOI] [PubMed] [Google Scholar]
- 46.Murakami K, Wu Y, Imaizumi T, Aoki Y, Liu Q, Yan X, Seino H, Yoshizawa T, Morohashi S, Kato Y, Kijima H. DEC1 promotes hypoxia-induced epithelial-mesenchymal transition (EMT) in human hepatocellular carcinoma cells. Biomed Res. 2017;38:221–227. doi: 10.2220/biomedres.38.221. [DOI] [PubMed] [Google Scholar]
- 47.Sun C, Han C, Wang P, Jin Y, Sun Y, Qu L. HOXB9 expression correlates with histological grade and prognosis in LSCC. Biomed Res Int. 2017;2017:3680305. doi: 10.1155/2017/3680305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.