Abstract
Pregnancy is a normal physiologic process with the potential for pathologic states. Pregnancy has several unique characteristics including an utero-placental interface, a physiologic stress that can cause pathologic states to develop, and a maternal–foetal interface that can affect two lives simultaneously or in isolation. Critical illness in pregnant women may result from deteriorating preexisting conditions, diseases that are co-incidental to pregnancy, or pregnancy-specific conditions. Successful maternal and neonatal outcomes for parturients admitted to a maternal critical care facility are largely dependent on a multidisciplinary input to medical or surgical condition from critical care physicians, obstetric anaesthesiologists, obstetricians, obstetric physicians, foetal medicine specialists, neonatologists, and concerned specialists. Pregnant women requiring maternal critical care unit admission are relatively low in developed nations and range from 0.9% to 1%; but in our country, the admission rates of critically ill parturients range from 3% to 8%. Two-thirds of pregnant women requiring critical care are often unanticipated at the time of conception. In this review, we will look at critical illnesses in pregnant women with a specific focus on pregnancy-induced illnesses.
Key words: Critical care, hypertension, pregnancy, resuscitation
INTRODUCTION
Maternal mortality rates remain relatively high in India although there is a declining trend in recent years.[1] The National Family Health Survey 4 from India (2015–2016) reported that institutional births increased from 38.7% to 78.9%, and child births by caesarean sections increased to 17.2% from 8.5%.[1] The maternal mortality rates (per 100,000 live births) have also shown a decline from 254 in 2004–2006 to 167 in 2011–2013.[2] Approximately 800 maternal deaths occur daily worldwide.[3] A study from the Netherlands reported a case fatality rate of 1:53 among pregnant women with severe maternal morbidity.[4] Knowledge deficit, communication lapses, and poor resuscitation skills are identified as major contributors to poor outcomes in persons requiring resuscitation.[5,6,7,8]
PHYSIOLOGIC CHANGES IN PREGNANCY
A normal pregnant parturient is an anatomically challenging and physiologically compromised patient. These changes are exaggerated when there is underlying pathology such as eclampsia, pulmonary oedema, and trauma in pregnancy.
There are several important physiologic changes in pregnancy that may influence resuscitation. There is an increase of 30%–50% in cardiac output during pregnancy due to increased stroke volume and increased maternal heartbeat.[9,10] The mean arterial pressure is reduced due to decreased systemic vascular resistance that may be mediated by an increase in progesterone, oestrogen, and nitric oxide, which are endogenous vasodilators.[11] The enlarging uterus may compress the aorta to produce increased afterload and compress the inferior vena cava leading to decreased cardiac return.[12] This can lead to hypotension in the supine position, which is the most favourable position for resuscitation.[12,13] Left uterine displacement (LUD) is a very important manoeuvre during resuscitation to improve cardiac output as it enhances the preload by more than 25% in a term gravida and also reduces afterload. LUD can be done by either manually, by lifting the uterus with two hands cephalad and displacing towards left side, or by putting a double pillow or wedge under the right hip.
Functional residual capacity (FRC) decreases by 10% to 25% in pregnancy as the uterus enlarges and elevates the diaphragm.[14] Pregnancy results in increased tidal volume and minute ventilation mediated by elevated serum progesterone levels that may result in mild alkalosis and compensatory renal excretion of bicarbonate.[14,15] Reduced FRC reserves and increased oxygen consumption lead to rapid development of hypoxia in response to apnoea in pregnant women.[16] The oxyhaemoglobin dissociation curve shifts to the right in the woman (a higher partial pressure of oxygen is required to achieve maternal oxygen saturation) while it shifts to the left in the foetus. Pregnancy also leads to altered renal tubular functions, a narrowing of the oncotic pressure–wedge pressure gradient that increases risk for pulmonary oedema, progesterone-mediated gastroesophageal sphincter relaxation, and prolonged intestinal transit times, and altered drug metabolism.[17,18,19] Upper airway oedema occurring as a result of hormonal changes may reduce visualisation during laryngoscopy and increase risk of bleeding. Creatinine clearance is increased in pregnancy to 120–160 mL/min and serum creatinine level decreases to 0.4–0.7 mg/dL.
The interpretation of arterial blood gas analyses in parturients is little different, because of the above physiological changes. The pH is 7.44, little alkalotic, and PaCO2 is in the range of 28–32 mmHg. Base deficit up to −5 is considered normal and bicarbonates are in the range of 20–22 mmol/L. PaCO2 >35 mmHg is considered as imminent respiratory failure and >40 mmHg is respiratory failure. Pregnancy is a hyperoxic state, and PaO2 is in the range of 95–105 mmHg. Hence, during management of parturients with respiratory failure, it is aimed to maintain PaO2 >70 mmHg. These factors and the gestational age of the foetus have to be considered during resuscitation.
RECOGNISING A CRITICALLY ILL PARTURIENT
The Confidential Enquiry into Maternal and Child Health in the United Kingdom highlighted the importance of early recognition and management of severely ill pregnant women and routine use of Maternal Early Warning Scoring Systems to be used for obstetric patients.[20] Early recognition of critical illness is essential for a favorable outcome for mother and baby. Prognostic criteria such as Acute Physiological and Chronic Health Evaluation (APACHE) scoring and Sequential Organ Functional Assessment (SOFA) score may not predict mortality as accurately in pregnancy as they do outside of pregnancy. One of the reasons for this difference is the physiologic change in pregnancy such as an increase in heart rate, change in white cell count, or even a decrease in normal values for creatinine that can affect the score. In many cases, delivery results in a drastic improvement in the disease course and a lower mortality, even when initial indicators suggest a high mortality.
There are certain disease-related obstetric risks scoring systems. Shock Index (SI), defined as the ratio between heart rate and systolic blood pressure, has been proposed as a useful and reliable tool to predict hypovolaemic states and early haemodynamic compromise (e.g., major obstetric haemorrhage) in obstetric populations even when the individual vital signs are within the normal values. Score less than 0.9 indicates that risk of massive resuscitation is low and >1.4 indicates urgent intervention or stabilisation and transfer to tertiary care facility.
The miniPIERS (Pre-eclampsia Integrated Estimate of RiSk) risk prediction model provides a simple tool to identify pregnant women at increased risk of death or major complications of pre-eclampsia. This model included the following: parity (nulliparity versus multiparity), gestational age on admission, headache/visual disturbances, chest pain/dyspnoea, vaginal bleeding with abdominal pain, systolic blood pressure, and dipstick proteinuria. The full PIERS model is used to identify women with pre-eclampsia at high risk of adverse maternal outcomes in need of immediate interventions. This model requires laboratory testing of platelet count, serum creatinine, lactate dehydrogenase, and aspartate transaminase and alanine aminotransaminase levels.
Recently, the obstetrically modified quick-SOFA score (omqSOFA) requires only clinical data for assessment and thus can be performed quickly without waiting for the results of biochemical or laboratory tests [Table 1].
Table 1.
Obstetrically modified qSOFA score (obstetrically modified qSOFA)

OBSTETRIC VERSUS NON-OBSTETRIC DISORDERS
The majority of cases that get admitted to critical care unit are primarily obstetric disorders. They constitute nearly 50%–80% of admissions during pregnancy and the puerperium in all parts of the world. More than 80% of these admissions are because of pre-eclampsia and its complications, haemorrhage, and sepsis. Non-obstetric disorders in pregnancy show large geographic variations. In South-East Asian countries including India, we more often see critical illness in pregnancy complicated by tropical and other infectious diseases such as malaria, leptospirosis, dengue, viral hepatitis, influenza, tuberculosis, rheumatic valvular heart diseases, cerebral sinus venous thrombosis, and endocrine disorders (diabetic keto-acidosis). In the developed nations, pneumonia, bronchial asthma, trauma, cancers, drug abuse, complicated urinary infections, preexisting autoimmune disorders, chronic pulmonary disease, endocrine disorders, and pulmonary thromboembolism are common.
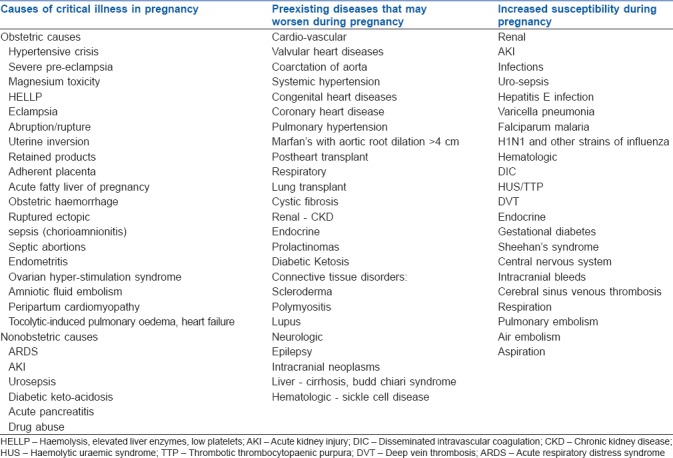
Furthermore, with the advances in medicine both in developing and developed nations, intensive care units (ICUs) are increasingly challenged with a unique subgroup of pregnant women with disorders such as surgically corrected complex congenital heart disease and organ transplant. Pregnant women with these conditions have increased morbidity and tend to require intensive medical care.[21] Common conditions associated and aggravated or render the pregnant women susceptible to critical illness.[21] are depicted in Table 2.
Table 2.
Causes of critical illness in pregnancy
PRINCIPLES OF MANAGEMENT OF A CRITICALLY ILL PARTURIENT
The initial assessment of a sick parturient should be no different from critically ill nonpregnant patient. Airway, Breathing and Circulation (ABC) are assessed and parturient is stabilised and transferred to appropriate level of care, as follows, and critical care pathway is established.
Levels of care: Level 0 – patients whose needs can be met through normal ward care, Level 1 – patients at risk of their condition deteriorating and needing a higher level of observation or those recently relocated from higher levels of care, Level 2 – patients requiring invasive monitoring/intervention that includes support for a single failing organ system (excluding advanced respiratory support), Level 3 – patients requiring advanced respiratory support (mechanical ventilation) alone or basic respiratory support along with support of at least one additional organ, and Level 4 – patients requiring highly advanced supports such as extra corporeal membrane oxygenation (ECMO) and intra-aortic balloon pump.
The components of care pathways for the critically ill pregnant woman include recognition, response, and Levels 2, 3, and 4 critical care. Recognition is the detection of clinical deterioration in pregnant woman that is life-threatening but potentially reversible and transferred to appropriate level. Response is the provision of a multidisciplinary care plan with obstetric interventions/specialty interventions (e.g., radiological intervention as needed. Level 2 critical care is the care provided in a delivery suite of a maternity unit or in a high dependency unit, justifying the recent terminology of mobile maternal critical care unit. Levels 3 and 4 critical care is the care provided in a critical care unit.
ORGAN DYSFUNCTION AND ORGAN SUPPORT IN A CRITICALLY ILL PARTURIENT
Common organ dysfunction or failure in a critically ill parturient varies whether it is obstetric or non-obstetric in aetiology. In several studies and with our institute's experience, the lung is the most frequently involved organ, followed by haematological, cardiovascular, renal, central nervous systems, and the multiorgan dysfunction syndrome.[22]
The trend of organ dysfunction of sick parturients admitted in our unit is presented in Figure 1. The goal of organ supports should be ‘giving best to both the lives’ (mother and the foetus). The organ supports provided in a critically ill parturients should not only provide targeted goals in mother but also take care of foetal well-being. Essentially there are subtle differences which the clinician should be aware, the bottom line being what is good for the mother is good for the foetus.
Figure 1.
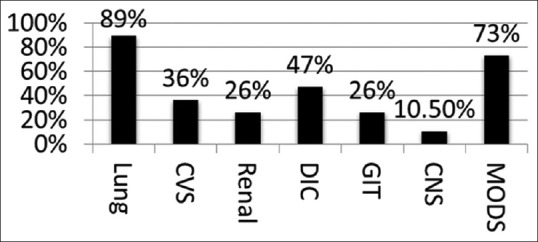
Obstetric critical care unit common organ dysfunction audit trend at Fernandez Hospital from 2012 to 2015, Department of Anaesthesia, Pain Medicine and Obstetric Critical Care
AIRWAY
Airway evaluation and management remains the top priority as pregnant patients tolerate hypoxaemia very poorly and they desaturate very rapidly. Furthermore, the risk of difficult intubation in pregnancy is increased 8–10 times and the clinician involved in the patient care has to be well versed with the difficult airway guidelines (Difficulty Airway Society, UK – DAS guidelines and All-India Difficult Airway Association guidelines).[23,24] The ICU should be equipped with all difficult airway equipments such as second-generation laryngeal mask airway (LMA) and video-laryngoscopes, and skills of doing crico-thyrotomy should also be present.
BREATHING AND VENTILATION
Adequacy of gas exchange is assessed and efforts to maintain it are ascertained. Supplemental oxygen, high concentration oxygen mask, transnasal high-flow humidified oxygen, and noninvasive ventilation (NIV) have all been reported to maintain targeted gas exchange. From our institute's protocol and NIV policy, we have data of >300 patients who received NIV to maintain safe targets, with zero incidence of aspiration and 2% incidence of conversion to invasive ventilation. However, NIV and transnasal high-flow oxygen have not been adequately studied and should be used with caution and by experienced teams only to mitigate the risk of aspiration. Tracheal intubation is performed and lung protective mechanical ventilation is initiated without any further delay, if the ventilation targets are not achieved (PaO2 >65–70 mmHg and PaCO2 <45 mmHg, PaO2 – FiO2/PF ratio >150 mmHg). Plateau pressure should be maintained less than 28 cm of H2O and PEEP should be used judiciously. Permissive hypercapnoea (upper permissible limit of PaCO2 <50 mmHg) is poorly tolerated in pregnant women, as a PaCO2 gradient of 10 mmHg is needed for foeto-maternal transfer, failing which foetal respiratory acidosis sets in and compromises foetal survival. For refractory hypoxaemia [severe acute respiratory distress syndrome (ARDS)], joint family multidisciplinary counseling has to be mandated, and pros and cons of options are to be discussed. The options may include semi-prone ventilation (personal experience of four cases) and complete prone ventilation postnatal has been reported with variable success rate; termination of pregnancy, if >22 weeks gestational age – foetal viability issues and prognostication should be properly explained and veno-venous extra-corporeal membrane oxygenation (ECMO), if available or transfer to a unit where such facility and experience to handle a pregnant patient on ECMO exists.
CIRCULATION
The crux of circulation is maintaining adequate utero-placental perfusion. Common shock states in pregnancy (hypovolaemic haemorrhagic, septic, and cardiogenic shock) should be appropriately and aggressively treated with two large bore (14G or 16G) peripheral venous access and minimally invasive monitoring (arterial line mandatory and central line if possible). Preferably, femoral route for venous or arterial access is avoided, and sub clavian route for Central Venous Pressure (CVP) line is usually avoided as coagulopathy is very common in situations of shock in pregnancy. Internal jugular route is safe and ultrasound-guided central venous access is strongly recommended. If the gestational age is greater than 20 weeks, it is ensured that LUD is maintained, using a 27° wedge.
Crystalloid resuscitation and appropriate transfusion triggers and end points should be followed. For sepsis, revised survival sepsis guidelines (2018) should be followed.
One should not hesitate to start appropriate vasopressors and inotropes for fear of foetal transfer. Thumb rule is what is good for the mother and stabilises the foetus.
GI SUPPORT
Pregnancy is a high calorific state. They are prone for ketosis very early if starved. Hence, prolonged starvation is avoided. The caloric requirement is increased during labour and highest in the lactating period. Accordingly, extra allowances are made. Enteral route is well tolerated and should be the primary choice. Feeds appropriate to the disease conditions are made and administered.[25]
ECMO
Successful outcomes following use of both veno-venous ECMO (VV ECMO) and veno-arterial ECMO (VA ECMO) have been reported for both antepartum and postpartum period.[26] Initiating ECMO during pregnancy requires specialised and multidisciplinary input especially about the mode of delivery and timing of delivery.
OTHER ORGAN SUPPORTS
Renal and other organ supports are the same as in nonpregnant patients. Organ protective strategies should be wisely followed and hospital-acquired infection control policies should strictly be followed and outcomes audited.
MATERNAL MONITORING IN OBSTETRIC CRITICAL CARE UNIT
All critically ill parturients requiring moderate to stiff organ supports merit invasive monitoring. There are no defined standards, but CVP (internal jugular, most preferred) and invasive arterial pressure monitoring are mandatory. CVP values are spuriously high in antepartum patients with gestational age beyond 24–26 weeks and should be wisely judged for volume replacement. Similarly, IVC ultrasound in a term gravid may not be accurate. Volume assessment is done best by trans-thoracic echocardiography. Continuous cardiac output monitoring devices have become popular in the recent past.[27] Most studies have been carried out in healthy parturients. However, our own institute's experience in using continuous cardiac output monitoring devices in sick pregnant women, from pulse volume and stroke volume variation, shows that cardiac output indices are more useful in postpartum period compared with antepartum period (because the aorto-caval component).
DECISION TO DELIVER AND MODE OF DELIVERY
Once the mother is stabilised, foetal evaluation should be done and the following principles are followed: a multidisciplinary team approach is essential with intensivist, obstetrician, foetal medicine specialist, obstetric anaesthesiologist, and concerned specialist jointly involved in patient care and counseling. Risk of preterm delivery should be explained at admission and delivery set should be kept in the ICU. Primarily, all resuscitation efforts should focussed be towards maternal stabilisation. If the foetal age is greater than 22–24 weeks gestational age, steroids should be considered for foetal lung maturity. If time permits and there is no medical contraindication, betamethasone (two intramuscular doses of 12 mg, 24 h apart) is given. The clinician has to keep in mind that the combination of steroids, tocolytics, and occult septic foci can be lethal. Role of tocolytics should best be decided jointly by the obstetrician and anaesthesiologist and or intensivist. It is better to avoid Beta 2 agonists. Foetal well-being is closely monitored in critically ill parturient. The biophysical profile has gained popularity as a test of foetal well-being. This includes foetal breathing, tone, movement, amniotic fluid volume, and the results of a nonstress test. If foetal delivery is imminent, loading dose of magnesium sulphate 4 g IV slowly followed by 1G/hour infusion is administered for 4 h for foetal brain protectionM mode (Abdominal versus vaginal) and timing of delivery should be discussed with the family and decision should be taken keeping in mind the maternal safety.
Type of anaesthesia – General versus neuraxial should be taken keeping in mind the coagulation parameters, airway needs, and haemodynamic stability. Several factors have to be considered. These include foetal oxygen delivery, the determinants of which are haemoglobin, haemoglobin saturation, utero-placental blood flow (maximally dilated). The utero-placental blood flow is dependent on the mean arterial pressure. Maternal hypotension is the single most important factor to reduce foetal blood flow. The lethal triad for foetal demise: hypotension, hypoxia and anaemia should be minimised or prevented. Maintaining maternal haemoglobin of 7g/dL and above, adequate cardiac output, and circulating blood volume are the basic fundamental requirements in managing a critically ill parturient in ICU and operating room. Coagulopathy should be completely corrected before surgery or vaginal delivery. Kleihauer–Betke analysis should be performed to detect the presence of foetal red cells in maternal circulation in all Rh-negative maternal trauma victims, massive abruption, and or amniotic fluid embolism (AFE). If positive, anti-D immunoglobulin should be administered. It is also administered if Rh-negative mother has received multiple random donor platelets. This is done to minimise the risk of Rh iso-immunisation in future pregnancies.
BREAST FEEDING IN ICU
After delivery, early maternal bonding is established. Very few drugs contraindicate breastfeeding; encourage breast feeds early even if the mother is on ventilator support. Early maternal bonding facilitates rapid weaning from mechanical ventilation, and even the incidence of lactation failure rates is lower, after a major illness (our institution audit).
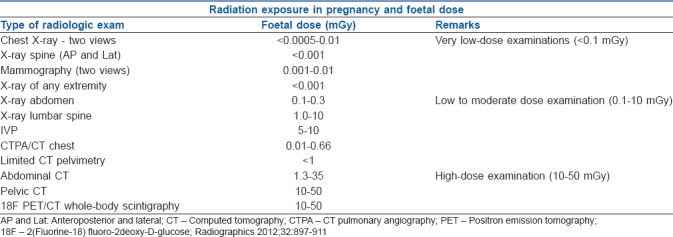
SAFE RADIATION IN PREGNANT WOMEN
Radiology and Imaging exams are part of routine exams for pregnant women. These include very low dose exams, low to moderate dose exams and very high dose exams.[28] Radiation may affect both the mother and the growing foetus. Table 3 shows the safe radiation limits in a parturient.
Table 3.
Safe radiation exposure in a parturient (borrowed from ACOG guidelines, 2017)
OBSTETRIC SPECIFIC DISORDERS
Hypertensive disorders in pregnancy
Pre-eclampsia is defined as hypertension and proteinuria occurring in pregnant women after 20 weeks of gestation and resolving within 6–12 weeks after delivery. Pre-eclampsia may be classified as mild or severe. Severe pre-eclampsia is defined as a systolic blood pressure >160 mm of Hg and/or a diastolic blood pressure >110 mm Hg, proteinuria >5 g per 24 h, oliguria <400 mL per 24 h, cerebral irritability, epigastric or right upper quadrant pain, and/or pulmonary oedema.
Eclampsia is a severe complication of pre-eclampsia defined by the presence of seizures in the absence of other neurologic disorders. Seizures may result from severe intracranial vasospasm, intracranial hypertension, ischemia, and vasogenic and cytotoxic oedema leading to endothelial dysfunction. Eclampsia may progress to hepatic failure, haemorrhage, or infarctions.
Haemolysis, elevated liver enzymes, low platelets (HELLP) syndrome includes microangiopathic haemolytic anaemia, elevated liver function tests, and thrombocytopaenia. HELLP syndrome usually occurs before 37 weeks of gestation and overlaps with pre-eclampsia. HELLP syndrome may result from generalised endothelial and microvascular injury from complement and coagulation cascade activation, increased vascular tone, and platelet aggregation. This results in liver haemorrhage and necrosis and can lead to large hepatic haematomas, capsular tears, and intraperitoneal bleeds.
Acute fatty liver of pregnancy (AFLP) is also considered as an extension of HELLP syndrome and many clinicians consider these disorders as HELLP–AFLP complex. They are often multiorgan disorders and treatment is termination of pregnancy. The mimickers of these disorders are haemolytic uraemic syndrome (HUS), thrombotic thrombocytopaenic purpura (TTP), postpartum thrombotic micro-angiopathy (PTMS), and sepsis, and they should be appropriately worked up. ADAMTS-13 assay helps in differentiating them. Very few laboratories perform this test in the world. In management of TTP and HUS, plasmapheresis plays a very important role and conventional haemodialysis may not help in HUS, TTP, and PTMS.
Amniotic fluid embolism
Amniotic fluid embolism syndrome presents with acute severe hypoxic respiratory failure, associated with shock, disseminated intravascular coagulopathy, and seizures.[29,30] AFE usually presents within 24 h of delivery and has a very high maternal mortality rate and long-term neurologic sequelae in survivors.[29,30] The cause for AFE is unclear but may result from a hypersensitivity reaction or anaphylaxis to amniotic fluid.[29,30] AFE can lead to acute lung injury, hypoxaemia, hypoxic pulmonary vasoconstriction, and acute right heart failure. Acute diastolic dysfunction followed by acute systolic failure of the left ventricle may occur. Multiorgan failure and cardiac arrest may occur. Aggressive multiorgan support, correction of coagulopathy, massive transfusion protocol, emergency delivery of foetus, Caesarean section with possible ligation of uterine/internal iliac arteries, and even hysterectomy have been performed as life-saving measures. Successful use of V-A ECMO has also been reported. With current good ICU support and care, survival rates up to 80% and above can be expected.
Sepsis
Four specific infectious complications are of interest in pregnancy – chorioamnionitis, pyelonephritis, endometritis, and pneumonia. Chorioamnionitis results from change in pH of the vagina and increased glycogen content. There is a loss of barrier for bacterial entry and it may also result from chorionic villi sampling, amniocentesis, and abortions. Pneumonia maybe caused by aspiration of gastric contents due to loss of lower oesophageal tone and elevation of the diaphragm. Pregnancy may also cause a level of immunosuppression and lead to fungal or viral pneumonia especially in those already at risk. Pyelonephritis may result from colonisation of the renal system with Gram-negative bacteria as a result of lower or loss of ureteral sphincter tone that is associated with progesterone. Mortality from sepsis is significant although progression to sepsis in pregnancy is low. Infections may involve the endometrium, spread through the uterine wall, peritonitis, or lead to thrombophlebitis of the pelvic veins.
Clinically, sepsis includes signs of systemic inflammation followed by coagulopathy, vascular anomalies, and progressive multiorgan failure. Conventional survival sepsis guidelines are followed with judicious fluid therapy.[31]
Ovarian hyperstimulation syndrome
Ovarian hyperstimulation syndrome (OHSS) is caused by controlled ovarian stimulation with gonadotropins during infertility treatment.[32] There is a sudden enlargement of the ovaries with multiple cysts that lead to vascular permeability and acute transfer of fluid into external spaces leading to pleural effusion and ascites. Increased haemoconcentration and blood viscosity may result and lead to thromboembolic events with cardiovascular and neurological complications. Pregnancy increases the risk for OHSS possibly related to an increase in endogenous human chorionic gonadotrophin (hCG) and mediated through vascular endothelial growth factor.
OHSS can be classified as mild (incidence is 20%–33% of in vitro fertilization (IVF) cycles), moderate (3%–6%), and severe and critical (0.1%–2% incidence) categories. The following risk factors[33] have been identified: poly cystic ovarian syndrome, previous history of OHSS. Anti-Muellerian hormone (AMH) concentration [AMH >0.47 pmol/L (3.36 ng/mL)] is a useful predictor of developing OHSS (sensitivity 90.5%, specificity 81.3%). Antral follicle count (AFC) ≥24 is correlated with an increased risk of moderate to severe OHSS in comparison to an AFC <24 (8.6% versus 2.2%). Combination of ≥18 follicles on ultrasound (diameter ≥11 mm) and estradiol E2 ≥5000 ng/L on the day of hCG trigger is more useful (sensitivity 83%, specificity 84%) than E2 concentrations alone in the prediction of severe OHSS.
OHSS is classified into mild, moderate, severe, and critical. Severe and critical types need ICU-based symptomatic and supportive care.
Mild OHSS is associated with abdominal bloating and mild abdominal pain; ovarian size is usually <8 cm2. Moderate OHSS in addition to above symptoms has nausea ± vomiting; there is ultrasound evidence of ascites, and ovarian size is usually 8–12 cm2. Severe OHSS presents with clinical ascites (±hydrothorax), oliguria (<300 mL/day or <30 mL/hour), haematocrit >0.45, hyponatraemia (sodium <135 mmol/L), hypo-osmolality (osmolality <282 mOsm/kg), hyperkalaemia (potassium >5 mmol/L), hypoproteinaemia (serum albumin <35 g/L), and ovarian size is usually >12 cm2. Critical OHSS is associated with tense ascites, large hydrothorax, oliguria/anuria, thromboembolism, and acute respiratory distress syndrome.
Treatment is mainly supportive and consists of judicious hydration, correction of dyselectrolytaemia, antiemetics, pain management (avoid nonsteroidal anti-inflammatory drugs), anti-deep vein thrombosis prophylaxis, maintenance of colloid oncotic pressure, and relief of abdominal compartment syndrome by tapping. Pleurocentesis is done for hydrothorax. Watch for torsion ovaries and thrombosis. Continue heparin for up to 1 week after the symptoms resolve and if the patient conceives, up to first trimester.
Cardiac Arrest in pregnancy
The principles of resuscitating a pregnant woman are nearly the same as resuscitating any nonpregnant person; however, one has to consider that there are two lives in a pregnant woman – the woman and the foetus. Resuscitation maybe necessary in a maternal near miss that is defined as a woman who nearly died but survived a complication that occurred during pregnancy, childbirth, or within 42 h of birth.
In the event of cardiac arrest, published guidelines should be followed.[34,35,36,37] Common causes of cardiac arrest in pregnancy can be remembered by the acronym – ABCDE UPS. A – Anaphylaxis, Anaesthetic; B – Bleeding, Bleeds in Brain, Bleeds in Liver; C – Cardiac; D – Drugs (Magnesium toxicity), E – Eclampsia, Embolism (Amniotic, Air and Thrombo-embolism); U – Uterine (Abruptio, Rupture, couvelaire, Inversion); P (Pulmonary – ARDS, Pulmonary oedema, Placental – Adherent placenta, Retained placenta), and S – Sepsis. ABC and CAB should proceed simultaneously as they are prone to severe hypoxaemia. Supine position with LUD is recommended during chest compressions. Venous access in upper limb or neck is recommended. Standard 200J shock is given using biphasic defibrillator if the rhythm is shockable. If return of spontaneous circulation (ROSC) cannot be established in 4 min, resuscitative hysterotomy [peri mortem caesarean section (PMCS)] should be done at the site of cardiac arrest. Do not delay PMCS beyond 4 min, if gestational age is beyond 22–24 weeks. Association of Obstetric Anaesthesiologists (AOA), India guidelines recommend vascular compression of aorta or clamping of aorta/common iliacs (needs practice or availability of vascular surgeon) if PMCS is being done in a lady with placenta percreta. AOA guidelines further recommend prophylactic compressive sutures on uterus if it is flabby post delivery. Use of oxytocin in atonic uterus can lead to recurrent cardiac arrest. Judicious balance of various uterotonic agents is recommended. V-A ECMO can used if ROSC cannot be established with the above methods. Standard postresuscitation care in ICU is given, and usual organ supportive and protective strategies are followed.
Ethical dilemmas in obstetric critical care
Critically ill pregnant women sometime can pose ethical dilemmas in critical care.[38] Brain dead parturient with foetus near viability age, dilemma to prolong the supports to maximise foetal viability, and deliver a healthy baby? Post delivery, she would be the ideal candidate for organ donation. Decision to deliver in critically ill mothers – what should be the ideal time? If the unstable sick mother is term pregnant woman with foetal distress, would termination of pregnancy be ideal option to save the baby and risk mothers' life? Co-ICU admissions – sick mother often delivers a sicker baby and both mother and neonate may require intense organ supports. Channelisation of resources should be wisely done so that both the lives can be saved. Saving mothers' life should be prime priority. In the above situation, there is a possibility of double whammy, losing both mother and foetus, if delivery is delayed (e.g., term parturient with dengue shock syndrome). The above situations warrant experience, wisdom, evidence, and multidisciplinary, ethical committee, and family input.
SUMMARY
Critical illness may complicate any pregnancy. Early warning scores can predict clinical deterioration. Blood gas analysis is often a very good tool to risk categorise a sick parturient. Obstetricians must be familiar with the issues pertaining to care of pregnant women with multiple organ failures. Many obstetric disorders may mimic medical disorders. Constitute a multidisciplinary team and lynchpin the correct diagnosis even while resuscitation measures are on. The team must decide whether delivery will alter the natural history of the disease process and improve maternal survival. If the maternal condition is expected to improve after delivery, then the consensus decision to deliver vaginally or by caesarean section must be made. Foetal viability should obviously be taken into consideration. Hypovolaemia, hypotension, coagulopathy, and respiratory failure are treated while preparations are made to deliver the foetus. Timely delivery improves not only maternal outcome but also foetal outcome. No efforts should be spared in the management of critically ill obstetric patients because their outcomes are often dramatically better than expected from the initial severity of illness.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.National Family Health Survey 4, India. [Last accessed on 2018 Mar 18]. Available from: http://www.rchiips.org/NFHS/pdf/NFHS4/India.pdf .
- 2.NITI Aayog. National Institution for Transforming India. [Last accessed on 2018 Mar 18]. Available from: http://www.niti.gov.in/content/maternal-mortality-ratio-mmr-100000-live-births .
- 3.Estimates by WHO, UNICEF, UNFPA, The World Bank and the United Nations Population Division. Geneva, Switzerland: World Health Organization; 2014. [Last accessed on 2018 Jun 01]. World Health Organization, UNICEF, UNFPA, The World Bank, United Nations Population Division. Trends in Maternal Mortality: 1990 to 2013. Available from: http://www.apps.who.int/iris/bitstream/10665/112682/2/9789241507226_eng.pdf?ua=1 . [Google Scholar]
- 4.Zwart JJ, Richters JM, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J, et al. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: A nationwide population-based study of 371,000 pregnancies. BJOG. 2008;115:842–50. doi: 10.1111/j.1471-0528.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 5.Cohen SE, Andes LC, Carvalho B. Assessment of knowledge regarding cardiopulmonary resuscitation of pregnant women. Int J Obstet Anesth. 2008;17:20–5. doi: 10.1016/j.ijoa.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Einav S, Matot I, Berkenstadt H, Bromiker R, Weiniger CF. A survey of labour ward clinicians' knowledge of maternal cardiac arrest and resuscitation. Int J Obstet Anesth. 2008;17:238–42. doi: 10.1016/j.ijoa.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Lewis G, editor. The Confidential Enquiry into Maternal and Child Health (CEMACH): Saving Mothers' Lives: Reviewing Maternal D Detahs to Make Motherhood Safer 2003-2005: The Seventh Confidential Enquiry into Maternal Deaths in the United Kingdom. London, UK: CEMACH; 2007. [Google Scholar]
- 8.Bajwa SJ, Bajwa SK. Critical care in obstetrics: Essentiality, initiatives, and obstacles in Indian scenario. J Anaesthesiol Clin Pharmacol. 2014;30:459–61. doi: 10.4103/0970-9185.142798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan EK, Tan EL. Alterations in physiology and anatomy during pregnancy. Best Pract Res Clin Obstet Gynaecol. 2013;27:791–802. doi: 10.1016/j.bpobgyn.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 10.San-Frutos L, Engels V, Zapardiel I, Perez-Medina T, Almagro-Martinez J, Fernandez R, et al. Hemodynamic changes during pregnancy and postpartum: A prospective study using thoracic electrical bioimpedance. J Matern Fetal Neonatal Med. 2011;24:1333–40. doi: 10.3109/14767058.2011.556203. [DOI] [PubMed] [Google Scholar]
- 11.Carbillon L, Uzan M, Uzan S. Pregnancy, vascular tone, and maternal hemodynamics: A crucial adaptation. Obstet Gynecol Surv. 2000;55:574–81. doi: 10.1097/00006254-200009000-00023. [DOI] [PubMed] [Google Scholar]
- 12.McLennan C, Minn M. Antecubital and femoral venous pressure in normal and toxemic pregnancy. Am J Obstet Gynecol. 1943;45:568–91. [Google Scholar]
- 13.Ueland K, Novy MJ, Peterson EN, Metcalfe J. Maternal cardiovascular dynamics. IV. The influence of gestational age on the maternal cardiovascular response to posture and exercise. Am J Obstet Gynecol. 1969;104:856–64. [PubMed] [Google Scholar]
- 14.Contreras G, Gutiérrez M, Beroíza T, Fantín A, Oddó H, Villarroel L, et al. Ventilatory drive and respiratory muscle function in pregnancy. Am Rev Respir Dis. 1991;144:837–41. doi: 10.1164/ajrccm/144.4.837. [DOI] [PubMed] [Google Scholar]
- 15.Lucius H, Gahlenbeck H, Kleine HO, Fabel H, Bartels H. Respiratory functions, buffer system, and electrolyte concentrations of blood during human pregnancy. Respir Physiol. 1970;9:311–7. doi: 10.1016/0034-5687(70)90088-5. [DOI] [PubMed] [Google Scholar]
- 16.Archer GW, Jr, Marx GF. Arterial oxygen tension during apnoea in parturient women. Br J Anaesth. 1974;46:358–60. doi: 10.1093/bja/46.5.358. [DOI] [PubMed] [Google Scholar]
- 17.Odutayo A, Hladunewich M. Obstetric nephrology: Renal hemodynamic and metabolic physiology in normal pregnancy. Clin J Am Soc Nephrol. 2012;7:2073–80. doi: 10.2215/CJN.00470112. [DOI] [PubMed] [Google Scholar]
- 18.Lawson M, Kern F, Jr, Everson GT. Gastrointestinal transit time in human pregnancy: Prolongation in the second and third trimesters followed by postpartum normalization. Gastroenterology. 1985;89:996–9. doi: 10.1016/0016-5085(85)90199-4. [DOI] [PubMed] [Google Scholar]
- 19.Chiloiro M, Darconza G, Piccioli E, De Carne M, Clemente C, Riezzo G, et al. Gastric emptying and orocecal transit time in pregnancy. J Gastroenterol. 2001;36:538–43. doi: 10.1007/s005350170056. [DOI] [PubMed] [Google Scholar]
- 20.Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving mothers' lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 21.Munnur U, Bandi V, Guntupalli KK. Management principles of the critically ill obstetric patient. Clin Chest Med. 2011;32:53–60. doi: 10.1016/j.ccm.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Karnad DR, Guntupalli KK. Critical illness and pregnancy: Review of a global problem. Crit Care Clin. 2004;20:555–76, vii. doi: 10.1016/j.ccc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Ramkumar V, Dinesh E, Shetty SR, Shah A, Kundra P, Das S, et al. All India difficult airway association 2016 guidelines for the management of unanticipated difficult tracheal intubation in obstetrics. Indian J Anaesth. 2016;60:899–905. doi: 10.4103/0019-5049.195482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obstetric Anaesthetists' Association/Difficult Airway Society Guidelines. 2015. [Last accessed 2018 May 10]. Available from: https://www.das.uk.com/guidelines/obstetric_airway_guidelines_2015 .
- 25.Pandya ST. Chapter in principles in critical care nutrition. In: Dixit Subhal, Zirpe Kapil, Khatib Khalid., editors. Nutrition in Critically Ill Parturient. 1st ed. New Delhi: Jaypee Brothers Medical Publishers; 2018. [Google Scholar]
- 26.Sharma NS, Wille KM, Bellot SC, Diaz-Guzman E. Modern use of extracorporeal life support in pregnancy and postpartum. ASAIO J. 2015;61:110–4. doi: 10.1097/MAT.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 27.Trikha A, Singh P. The critically ill obstetric patient – Recent concepts. Indian J Anaesth. 2010;54:421–7. doi: 10.4103/0019-5049.71041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guidelines for Diagnostic Imaging during Pregnancy and Lactation. ACOG COMMITTEE OPINION Number 723. 2017 Oct [Google Scholar]
- 29.Clark SL. New concepts of amniotic fluid embolism: A review. Obstet Gynecol Surv. 1990;45:360–8. doi: 10.1097/00006254-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Aurangzeb I, George L, Raoof S. Amniotic fluid embolism. Crit Care Clin. 2004;20:643–50. doi: 10.1016/j.ccc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med. 2018;44:925–8. doi: 10.1007/s00134-018-5085-0. [DOI] [PubMed] [Google Scholar]
- 32.Sansone P, Aurilio C, Pace MC, Esposito R, Passavanti MB, Pota V, et al. Intensive care treatment of ovarian hyperstimulation syndrome (OHSS) Ann N Y Acad Sci. 2011;1221:109–18. doi: 10.1111/j.1749-6632.2011.05983.x. [DOI] [PubMed] [Google Scholar]
- 33.Nastri CO, Teixeira DM, Moroni RM, Leitão VM, Martins WP. Ovarian hyperstimulation syndrome: pathophysiology, staging, prediction and prevention. Ultrasound Obstet Gynecol. 2015;45:377–93. doi: 10.1002/uog.14684. [DOI] [PubMed] [Google Scholar]
- 34.Jeejeebhoy FM, Zelop CM, Lipman S, Carvalho B, Joglar J, Mhyre JM, et al. Cardiac Arrest in Pregnancy: A Scientific Statement From the American Heart Association. Circulation. 2015;132:1747–73. doi: 10.1161/CIR.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed SM, Garg R, Divatia JV, Rao SC, Mishra BB, Kalandoor MV, et al. Compression-only life support (COLS) for cardiopulmonary resuscitation by layperson outside the hospital. Indian J Anaesth. 2017;61:867–73. doi: 10.4103/ija.IJA_636_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg R, Ahmed SM, Kapoor MC, Mishra BB, Rao SC, Kalandoor MV, et al. Basic cardiopulmonary life support (BCLS) for cardiopulmonary resuscitation by trained paramedics and medics outside the hospital. Indian J Anaesth. 2017;61:874–82. doi: 10.4103/ija.IJA_637_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garg R, Ahmed SM, Kapoor MC, Rao SC, Mishra BB, Kalandoor MV, et al. Comprehensive cardiopulmonary life support (CCLS) for cardiopulmonary resuscitation by trained paramedics and medics inside the hospital. Indian J Anaesth. 2017;61:883–94. doi: 10.4103/ija.IJA_664_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esmaeilzadeh M, Dictus C, Kayvanpour E, Sedaghat-Hamedani F, Eichbaum M, Hofer S, et al. One life ends, another begins: Management of a brain-dead pregnant mother – A systematic review. BMC Med. 2010;8:74. doi: 10.1186/1741-7015-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]


