Abstract
During pregnancy, the body goes through various anatomical and physiological changes to provide suitable environment for foetal development, to cater to the increased metabolic demands and to prepare for the childbirth. These changes have notable anaesthetic implications in determining the optimal anaesthetic technique, while also keeping in mind the gestational age, type of procedure and any coexisting medical condition. It is important to note that these changes revert to baseline (pre-pregnancy) levels at different time intervals during the postpartum period which is important while managing postpartum patients. None of the anaesthetic agents are known teratogens; however, there is concern regarding the effects of some agents on the developing brain.
Key words: Anaesthetic implications, physiologic changes, pregnancy, transplacental drugs transfer
INTRODUCTION
During pregnancy, anatomical and physiological changes occur to meet the increased metabolic needs, to permit appropriate development of foetus and to prepare the body for childbirth. The changes begin to occur early in the first trimester, peaking at the term or labour and revert to pre-pregnancy levels by a few weeks into the postpartum. These changes are well tolerated in healthy females but may aggravate or unmask a pre-existing disease or a pregnancy-related pathophysiology.
A thorough understanding of the physiological changes is the key to successful anaesthetic management of both obstetric and non-obstetric procedures during pregnancy. This conceptual knowledge will also help the anaesthesiologists to tailor the anaesthetic technique to accommodate coexisting diseases and to manage critically ill pregnant patients.
This narrative review will discuss the anatomical and physiological changes during pregnancy and their implications to the practice of anaesthesia.
CARDIOVASCULAR SYSTEM
Due to the effects of increased levels of oestrogen and progesterone, peripheral vasodilatation and resultant decrease in systemic vascular resistance (SVR) begin to occur by 8th week of gestation.[1] Since there is no autoregulation in utero placental circulation, cardiac output (CO) has to increase in order to maintain blood pressure (CO × SVR). In early pregnancy, this increase in CO is achieved by an increase in heart rate (HR) by 15–25% followed by an increase in stroke volume (SV) by 20–30%.[2] Most of the increase in CO goes to the uterus, kidneys and skin to provide nutrients to the foetus, excrete maternal and foetal waste products and assist in maternal temperature control, respectively.[3,4,5]
Blood volume increases, beginning from 6 to 8 weeks of gestation to reach a maximum increase of about 20% by mid-third trimester.[6] A wide pulse pressure and reduced mean arterial pressure leads to sodium and water retention by activating renin–angiotensin system. This results in an increase in plasma volume by 40–50%. The left ventricular end-diastolic volume is increased while end-systolic volume is unchanged leading to an increase in ejection fraction. Central venous and pulmonary capillary wedge pressures remain unchanged.[7]
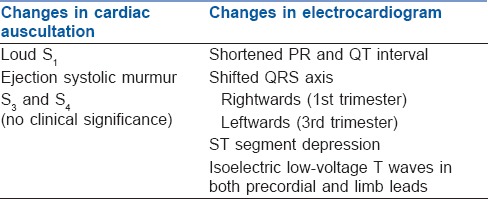
Anatomical changes due to a gravid uterus cause the heart to be displaced cephalad and laterally. Table 1 lists the changes in cardiac auscultation and electrocardiogram.[8] By 20 weeks of gestation, the gravid uterus begins to cause mechanical compression of inferior vena cava (IVC) and descending aorta in supine position. This leads to a decrease in venous return and CO resulting in maternal hypotension and foetal compromise (acidaemia). To compensate for aortocaval compression, sympathetic tone and HR increase and blood from lower limb is shunted to the right side of heart through vertebral plexus and azygos veins. In many parturient, these compensatory mechanisms may be inadequate to maintain blood pressure in supine position and result in supine hypotensive syndrome (or aortocaval compression syndrome).[9,10] It is characterised by pallor, transient tachycardia followed by bradycardia, sweating, nausea, hypotension and dizziness in supine position which get relieved by turning lateral. In its severe form, it can lead to unconsciousness or sudden maternal death.
Table 1.
Changes in electrocardiogram and cardiac auscultation during pregnancy
The changes in CO during labour and postpartum period are summarised in Figure 1.[11,12]
Figure 1.
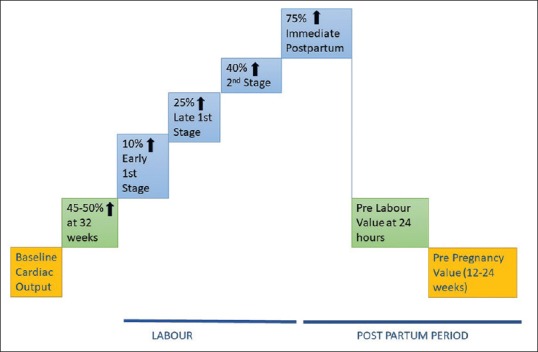
Graphical representation of changes in cardiac output during pregnancy, labour and postpartum. (↑:Increase)
Anaesthetic implications
Due to anatomical changes, the apical impulse is shifted laterally and cephalad to the fourth intercostal space. Increased blood volume provides some reserve for the normal blood loss during delivery (about 300–500 ml for vaginal delivery and 600–1000 ml for caesarean delivery) and peripartum haemorrhage. But due to this increase in blood volume, pregnant patients may not manifest the signs and symptoms of hypovolemia (tachycardia, hypotension, oliguria) till about 1500 ml of blood loss has occurred.
Engorgement of epidural venous plexus can result in increased risk of bloody tap and intravascular catheter placement during epidural anaesthesia and analgesia. Due to down-regulation of adrenergic receptors, higher doses of vasopressors like phenylephrine are required in the event of hypotension.
Reduction in SV and CO during general anaesthesia (GA) and sympathetic blockade during neuraxial anaesthesia can aggravate supine hypotensive syndrome. So, supine position should be avoided or the uterus should be displaced laterally with a wedge under hip. The adverse effects of aortocaval compression are reduced once the foetal head is engaged. For neuraxial anaesthesia, the Oxford position has been found to have better haemodynamic stability, more reproducible block height and prevents adverse effects of aortocaval compression. It is a modified lateral position with an upward slope in the thoracic region with avoidance of supine position till surgery begins.
RESPIRATORY SYSTEM
Due to the effect of oestrogen, there is capillary engorgement of nasal, oropharyngeal and laryngeal mucosa. There is an increase in anteroposterior and transverse diameters of chest wall by 2 cm each and a resultant increase in circumference by 5–7 cm.[1] Changes in lung mechanics are depicted in Table 2.
Table 2.
Changes in respiratory mechanics during pregnancy
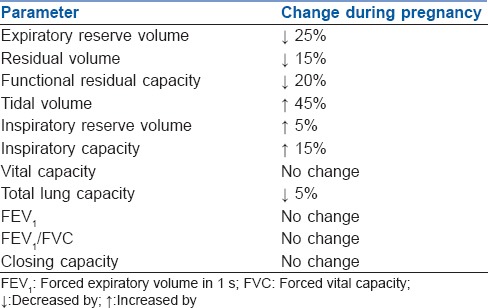
Minute ventilation (MV) increases mainly due to increase in TV with minimal rise in respiratory rate (1–2 breaths/min). There is a corresponding increase in alveolar ventilation. Progesterone is a respiratory stimulant and sensitises chemoreceptors to carbon dioxide (CO2).[13] There is an increased production of CO2 (about 300 ml/min) and due to increased MV, PaCO2 falls to 30–32 mmHg in first trimester and remains in this range throughout pregnancy. There is no gradient between end-tidal CO2 and PaCO2. Respiratory alkalosis is incompletely compensated by reduction in serum bicarbonate levels to about 20–21 mEq/L and resultant pH of 7.42–7.44. As a result of increased alveolar ventilation, there is an increased PaO2 during pregnancy but after mid-gestation, PaO2 falls in supine position as functional residual capacity (FRC) falls below closing capacity leading to closure of airways during tidal volume breathing.
Oxygen delivery to foetus is increased by rightward shift in maternal oxygen dissociation curve and an increase in P50 value is observed at term (30 vs. 26 mmHg). Foetal haemoglobin has a higher affinity for oxygen and has a P50 of about 18 mmHg.
Despite the cephalad displacement of diaphragm, the excursion of diaphragm during breathing increases by 2 cm while there is a reduction in chest wall excursion. No change is thus observed in flow volume loops.[14] During labour, MV increases by 70–200%, PaCO2 decreases to 10–15 mmHg and oxygen consumption increases by 40–75% due to increased metabolic demands. MV, TV and oxygen consumption attain pre-pregnancy values by 6–8 weeks postpartum.
Anaesthetic implications
Mallampati classification worsens during pregnancy and more so during labour.[15] Upper airway changes, enlarged breasts and obesity can make intubation difficult during pregnancy. Laryngoscopes with short handles, smaller diameter endotracheal tubes and ramp position at the head end might be needed in difficult scenarios. Nasotracheal intubation should be avoided as there is an increased risk of nasal bleed during pregnancy.
A decreased FRC and increased oxygen consumption can lead to rapid desaturation during apnoea despite adequate pre-oxygenation.[16] An increased MV and low FRC result in faster de-nitrogenation (pre-oxygenation) and rapid uptake of inhalational agents. Hyperventilation should be avoided as it may cause respiratory alkalosis, leftward shift in oxygen dissociation curve and decreased oxygen delivery to foetus.
Uncontrolled maternal pain during labour can further increase the metabolic demands with resultant increase in maternal lactate levels indicating that oxygen requirements are increased more than supply. Despite enhanced response to hypoxic ventilatory drive, it is not possible to meet increased oxygen demand without supplemental oxygen in susceptible parturient. Epidural analgesia is beneficial by decreasing the metabolic demands during labour.[17]
HAEMATOLOGICAL AND IMMUNE SYSTEM
Discrepancy in increase in plasma volume (40–50%) and red cell mass (20%) results in physiological anaemia of pregnancy. A lower haematocrit decreases the blood viscosity and lowers the resistance to blood flow in utero placental circulation.
The leukocyte count gradually increases to around 15,000/mm.[3] Major contribution in this increase is by polymorphonuclear cells, which have impaired function. This explains the increased severity of infections but this apparently impaired immunity does not make parturient prone to infections.[18] The autoantibody production and levels of immunoglobulins A, G and M are unaltered.
There is an increased production of platelets but due to enhanced destruction and haemodilution, rise in count does not occur. In a minority, platelet count decreases (90,000–100,000) which is physiological (gestational thrombocytopaenia) and resolves in the postpartum period.[19]
The coagulation and fibrinolytic pathways are altered with an increased risk of thromboembolism during pregnancy (10 times) and postpartum (25 times). The net result of the physiologic changes is a hypercoagulable state of pregnancy.[20] The concentration of all clotting factors increases except factor II, V, XI and XIII. There is a reduction in prothrombin time and activated partial thromboplastin time by 20%. Also, elevated levels of fibrin degradation products and plasminogen are observed.[21] The hypercoagulable state is maintained up to 5–7 days postpartum with increased risk of thrombotic complications and reverts to baseline by 2 weeks postpartum.
Anaesthetic implications
In a healthy pregnant female, investigating the platelet count is not mandatory before neuraxial anaesthesia or analgesia. There is an increased risk of epidural haematoma in patients with severe preeclampsia due to exponential fall in platelets and platelet count should be obtained within 6 h before placing an epidural or catheter removal. It is however advisable to test for both platelet count and function (aggregability). In an acute condition, laboratory tests can be time-consuming and thromboelastography proves to be useful in assessment of the whole coagulation process (initial fibrin plug formation, platelet aggregation, strengthening of clot and fibrinolysis).[22]
GASTROINTESTINAL SYSTEM
The secretory and absorptive functions of the gastrointestinal (GI) system are not much affected but the motility is. There is displacement of intra-abdominal portion of oesophagus into the thorax in a majority. In addition, progesterone causes relaxation of lower oesophageal sphincter (LOS).[23] These anatomic and hormonal effects cause a decrease in the tone of LOS manifesting as gastro-oesophageal reflux disease of pregnancy.
Although no change has been observed in gastric emptying time (even in obese females), it is slowed during labour and in the immediate postpartum period.[24] Owing to the inhibition of GI contractile activity by progesterone, oesophageal peristalsis and intestinal transit slow down resulting in constipation. A majority (80%) of pregnant females experience nausea and vomiting. The changes in GI system return to baseline within 1–2 days postpartum.[24]
Spider nevi and palmar erythema, indicators of liver disease, can be physiologically seen during pregnancy due to raised oestrogen levels.[25] Despite an increase in CO, proportionate increase in hepatic blood flow is not observed. Due to an increase in splanchnic, portal and oesophageal venous pressure, more than 50% of the pregnant females develop oesophageal varices which rapidly resolve postpartum.[26] Dilution due to an increased plasma volume causes a decline in serum albumin concentration by up to 60%.[27] Plasma cholinesterase levels begin to fall (by 25%) in the first trimester and this level is maintained till the term.
Anaesthetic implications
No alteration is required in the fasting guidelines. Gastric emptying is delayed in pregnant and peripartum females receiving systemic or neuraxial opioids.[28] There is an increased risk of aspiration of gastric contents due to increased intra-abdominal pressure and a low LOS tone. The risk is increased during GA and intubation. Important steps in prevention include preference to neuraxial techniques and use of aspiration prophylaxis. If GA is indicated, rapid sequence induction is recommended. In uncomplicated labour, moderate amount of clear fluids is recommended.[29]
Despite a decrease in plasma cholinesterase, clinically significant prolongation of effect of a single dose of succinylcholine does not occur. This is probably due to increased volume of distribution and decreased sensitivity.
NERVOUS SYSTEM
Cerebral blood flow is increased due to a decrease in cerebrovascular resistance. Permeability of the blood–brain barrier increases. There is an increase in threshold to pain at full term and in labour probably due to increased levels of plasma endorphins and progesterone. Due to the compression of the IVC by the gravid uterus, dilatation of the epidural venous plexus occurs. There is an increase in epidural fat and decrease in epidural free space and spinal cerebrospinal fluid (CSF) volume.[30] CSF pressure remains unchanged during pregnancy but is increased during uterine contractions and bearing down. There is more dependence on sympathetic nervous system for maintenance of haemodynamics.
Anaesthetic implications
There is up to 30% decrease in minimum alveolar concentration of volatile anaesthetic agents.[31] Pregnant females are physiologically more sensitive to intravenous induction and sedative agents.[32] There is a 25–40% decrease in spinal dose of local anaesthetics (LA) since the end of first trimester implying that changes in epidural space anatomy is not the sole reason. It has been found that progesterone increases the sensitivity of neuronal membranes to LA.[33] Pregnant females are more prone to hypotension and haemodynamic instability following sympathetic blockade caused by neuraxial anaesthesia.
RENAL SYSTEM
Renal blood flow and glomerular filtration rate (GFR) are increased but no change is observed in histology or number of nephrons.[5] Due to progesterone and mechanical compression of ureters, renal pelvis and calyces are dilated. Increase in GFR causes decrease in serum creatinine (normal range: 0.4–0.8 mg/dl) and blood urea nitrogen (normal range: 8–10 mg/dl). Reduced vascular responsiveness to vasopressors (angiotensin II, norepinephrine and antidiuretic hormone) is observed due to an altered vascular receptor expression. Nitric oxide synthesis is increased during pregnancy resulting in systemic and renal vasodilation.
There is a decrease in normal plasma osmolality during pregnancy (about 270 mosmol/kg vs. 275–290 mosmol/kg pre-pregnancy) and a proportional decrease in plasma sodium concentration (4–5 meq/l below pre-pregnancy values). Thirst and release of Antidiuretic Hormone (ADH) from the pituitary, which are normal physiological responses to changes in osmolality, remain intact.
Urinary protein excretion rises (150–200 mg/day at term vs. about 100 mg/day pre-pregnancy) which is even more with multiple gestation. Urinary protein excretion >300 mg/day should be evaluated further.[34] The physiologic hypoalbuminaemia of pregnancy may result in fall in anion gap (from 10.7 to 8.5). Glucosuria and aminoaciduria may be observed in the absence of diabetes or renal disease due to impaired tubular function and decreased fractional reabsorption.[1]
Hydronephrosis and hydroureter are common occurrence during pregnancy due to hormonal effects, external compression and intrinsic changes in the ureteral wall. Urinary frequency, urinary tract infections, urinary incontinence and nocturia are also frequent. All the changes in renal system return to pre-pregnancy state by 4–6 weeks postpartum.
Anaesthetic implications
Since pregnant females have a lower normal range of serum creatinine, a small rise in values reflects a larger reduction in renal function. Low albumin levels lead to increased free levels of highly protein-bound drugs such as digoxin, midazolam, thiopentone sodium and phenytoin.[1]
ENDOCRINE SYSTEM
Thyroid gland is enlarged due to both follicular hyperplasia and increased vascularity. Due to the increase in thyroid-binding globulin caused by oestrogen, total T3 and T4 levels are increased by 50% but free T3 and T4 levels do not change. Thyroid stimulating hormone levels fall during first trimester but recover during rest of the pregnancy. Both subclinical hypo- and hyperthyroidism occur and are not associated with adverse outcomes.[35,36]
Human placental lactogen causes reduced tissue sensitivity to insulin and thus higher blood glucose levels after carbohydrate-rich meals during pregnancy when compared to pre-pregnancy state. The pregnant females rapidly develop hypoglycaemia and ketoacidosis during starvation.
Placental lactogen and dopamine cause hyperprolactinaemia during pregnancy. There is a 30% increase in oxytocin stores in the pituitary, which is released during labour and just after delivery.[37] The oxytocin response to stress is diminished during pregnancy to prevent preterm labour.
Anaesthetic implications
Pathological states arising due to iodine-deficient hypothyroidism or hyperthyroidism have much relevance to the practice of anaesthesia and should be managed keeping in mind the physiological changes of pregnancy. GA can mask the signs and symptoms of hypoglycaemia while neuraxial anaesthesia can lead to exaggerated haemodynamic instability in patients with autonomic dysfunction related to diabetes mellitus or diabetic ketoacidosis.
MUSCULOSKELETAL SYSTEM
Hormonal changes and weight gain result in a series of musculoskeletal effects. To compensate for the change in centre of gravity, the lumbar lordosis is exaggerated with anterior flexion of neck and downward movement of shoulders. Due to relaxin, progesterone and mechanical effects of pregnancy, joint laxity is increased to prepare for childbirth.[1]
Anaesthetic implications
Lordosis can decrease the distance between the spinous processes and can make lumbar flexion and neuraxial techniques difficult. Widening of the pelvis causes a head down position in lateral decubitus and may lead to cephalad spread of LA during spinal anaesthesia in lateral position.[1] A pillow placed beneath the dependent shoulder can negate this effect.
TRANSPLACENTAL TRANSFER OF DRUGS AND SAFETY
Transplacental transfer of drugs has been studied in animals (pregnant ewes, guinea pigs) and in vitro human placental models but application of these data to clinical practice is questionable. Due to inaccessibility of placenta in vivo, human studies are impractical and majority of studies provide data from single measurement of maternal and umbilical cord drug concentrations from samples obtained at delivery.[1]
Factors such as lipid solubility, protein binding, tissue binding, pKa, pH and blood flow determine the extent of drug transfer across the placenta [Table 3]. Highly lipid soluble drugs may easily enter the placenta but then get trapped within the placental tissue. Transplacental transfer of highly protein bound drugs depends on the concentration of maternal and foetal plasma proteins which may vary with gestational age and disease. Non-ionised fraction of a drug at physiological pH is determined by pKa. During foetal acidosis, maternal–foetal transfer of basic drugs like opioids and LA is enhanced and results in “ion trapping.”[38] Table 4 lists the drugs which do or do not get transferred across placenta.
Table 3.
Factors increasing transplacental transfer of drugs

Table 4.
Transplacental transfer of drugs related to anaesthesia
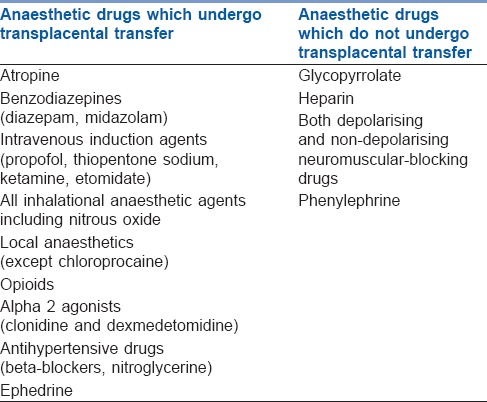
None of the anaesthetic drugs and other commonly used drugs during anaesthesia are teratogenic. However, United States Food and Drug Administration has issued a warning that repeated or prolonged use (>3 h) of general anaesthetic and sedative drugs during procedures in children younger than 3 years or in full-term pregnant women may affect the developing brain. Thus, the benefits of a certain anaesthetic techniques in this population should be balanced against potential risks.[39,40]
SUMMARY
Knowledge of various physiological changes which occur during pregnancy is crucial in the anaesthetic management of both healthy females and those with coexisting diseases. Proper preparation of equipment, drugs, availability of qualified anaesthesiologists and adaptation of anaesthetic technique to suit these changes are must for successful conduct of procedures during pregnancy, thereby contributing to reduction in maternal–foetal morbidity and mortality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gaiser R. Physiologic changes of pregnancy. In: Chestnut DH, Wng CA, Tsen LC, Ngan Kee WD, Beilin Y, Mhyre JM, et al., editors. Chestnut’s Obstetric Anesthesia: Principles and Practice. 5th ed. Philadelphia: Elsevier Saunders; 2014. pp. 15–38. [Google Scholar]
- 2.Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256:H1060–5. doi: 10.1152/ajpheart.1989.256.4.H1060. [DOI] [PubMed] [Google Scholar]
- 3.Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG, et al. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol. 1992;80:1000–6. [PubMed] [Google Scholar]
- 4.Katz M, Sokal MM. Skin perfusion in pregnancy. Am J Obstet Gynecol. 1980;137:30–3. doi: 10.1016/0002-9378(80)90381-6. [DOI] [PubMed] [Google Scholar]
- 5.Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis. 2013;20:209–14. doi: 10.1053/j.ackd.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueland K. Maternal cardiovascular dynamics. VII. Intrapartum blood volume changes. Am J Obstet Gynecol. 1976;126:671–7. [PubMed] [Google Scholar]
- 7.Clark SL, Cotton DB, Lee W, Bishop C, Hill T, Southwick J, et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989;161:1439–42. doi: 10.1016/0002-9378(89)90900-9. [DOI] [PubMed] [Google Scholar]
- 8.Sunitha M, Chandrasekharappa S, Brid SV. Electrocradiographic QRS axis, Q wave and T-wave changes in 2nd and 3rd trimester of normal pregnancy. J Clin Diagn Res. 2014;8:BC17–21. doi: 10.7860/JCDR/2014/10037.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinsella SM, Lohmann G. Supine hypotensive syndrome. Obstet Gynecol. 1994;83:774–88. [PubMed] [Google Scholar]
- 10.Lanni SM, Tillinghast J, Silver HM. Hemodynamic changes and baroreflex gain in the supine hypotensive syndrome. Am J Obstet Gynecol. 2002;187:1636–41. doi: 10.1067/mob.2002.127304. [DOI] [PubMed] [Google Scholar]
- 11.Robson SC, Dunlop W, Boys RJ, Hunter S. Cardiac output during labour. Br Med J (Clin Res Ed) 1987;295:1169–72. doi: 10.1136/bmj.295.6607.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robson SC, Dunlop W, Hunter S. Haemodynamic changes during the early puerperium. Br Med J (Clin Res Ed) 1987;294:1065. doi: 10.1136/bmj.294.6579.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LoMauro A, Aliverti A. Respiratory physiology of pregnancy: Physiology masterclass. Breathe (Sheff) 2015;11:297–301. doi: 10.1183/20734735.008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grindheim G, Toska K, Estensen ME, Rosseland LA. Changes in pulmonary function during pregnancy: A longitudinal cohort study. BJOG. 2012;119:94–101. doi: 10.1111/j.1471-0528.2011.03158.x. [DOI] [PubMed] [Google Scholar]
- 15.Kodali BS, Chandrasekhar S, Bulich LN, Topulos GP, Datta S. Airway changes during labor and delivery. Anesthesiology. 2008;108:357–62. doi: 10.1097/ALN.0b013e31816452d3. [DOI] [PubMed] [Google Scholar]
- 16.McClelland SH, Bogod DG, Hardman JG. Apnoea in pregnancy: An investigation using physiological modelling. Anaesthesia. 2008;63:264–9. doi: 10.1111/j.1365-2044.2007.05347.x. [DOI] [PubMed] [Google Scholar]
- 17.Hägerdal M, Morgan CW, Sumner AE, Gutsche BB. Minute ventilation and oxygen consumption during labor with epidural analgesia. Anesthesiology. 1983;59:425–7. doi: 10.1097/00000542-198311000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Stirrat GM. Pregnancy and immunity. BMJ. 1994;308:1385–6. doi: 10.1136/bmj.308.6941.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burrows RF, Kelton JG. Incidentally detected thrombocytopenia in healthy mothers and their infants. N Engl J Med. 1988;319:142–5. doi: 10.1056/NEJM198807213190304. [DOI] [PubMed] [Google Scholar]
- 20.Gerbasi FR, Bottoms S, Farag A, Mammen E. Increased intravascular coagulation associated with pregnancy. Obstet Gynecol. 1990;75:385–9. [PubMed] [Google Scholar]
- 21.Liu J, Yuan E, Lee L. Gestational age-specific reference intervals for routine haemostatic assays during normal pregnancy. Clin Chim Acta. 2012;413:258–61. doi: 10.1016/j.cca.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson O, Sporrong T, Hillarp A, Jeppsson A, Hellgren M. Prospective longitudinal study of thromboelastography and standard hemostatic laboratory tests in healthy women during normal pregnancy. Anesth Analg. 2012;115:890–8. doi: 10.1213/ANE.0b013e3182652a33. [DOI] [PubMed] [Google Scholar]
- 23.Shah S, Nathan L, Singh R, Fu YS, Chaudhuri G. E2 and not P4 increases NO release from NANC nerves of the gastrointestinal tract: Implications in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1546–54. doi: 10.1152/ajpregu.2001.280.5.R1546. [DOI] [PubMed] [Google Scholar]
- 24.Whitehead EM, Smith M, Dean Y, O'Sullivan G. An evaluation of gastric emptying times in pregnancy and the puerperium. Anaesthesia. 1993;48:53–7. doi: 10.1111/j.1365-2044.1993.tb06793.x. [DOI] [PubMed] [Google Scholar]
- 25.Angel García AL. Effect of pregnancy on pre-existing liver disease physiological changes during pregnancy. Ann Hepatol. 2006;5:184–6. [PubMed] [Google Scholar]
- 26.Paech MJ, Scott K. Liver and renal disease. In: Gambling DR, Douglas MJ, McKay RS, editors. Obstetric Anesthesia and Uncommon Disorders. 2nd ed. Cambridge: Cambridge University Press; 2008. pp. 249–57. [Google Scholar]
- 27.Carter J. Liver function in normal pregnancy. Aust N Z J Obstet Gynaecol. 1990;30:296–302. doi: 10.1111/j.1479-828x.1990.tb02014.x. [DOI] [PubMed] [Google Scholar]
- 28.Porter JS, Bonello E, Reynolds F. The influence of epidural administration of fentanyl infusion on gastric emptying in labour. Anaesthesia. 1997;52:1151–6. doi: 10.1111/j.1365-2044.1997.238-az0373.x. [DOI] [PubMed] [Google Scholar]
- 29.Practice guidelines for obstetric anesthesia: An updated report by the American Society of Anesthesiologists Task Force on obstetric anesthesia and the society for obstetric anesthesia and perinatology. Anesthesiology. 2016;124:270–300. doi: 10.1097/ALN.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 30.Igarashi T, Hirabayashi Y, Shimizu R, Saitoh K, Fukuda H, Suzuki H, et al. The fiberscopic findings of the epidural space in pregnant women. Anesthesiology. 2000;92:1631–6. doi: 10.1097/00000542-200006000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Chan MT, Mainland P, Gin T. Minimum alveolar concentration of halothane and enflurane are decreased in early pregnancy. Anesthesiology. 1996;85:782–6. doi: 10.1097/00000542-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Christensen JH, Andreasen F, Jansen JA. Pharmacokinetics of thiopental in caesarian section. Acta Anaesthesiol Scand. 1981;25:174–9. doi: 10.1111/j.1399-6576.1981.tb01632.x. [DOI] [PubMed] [Google Scholar]
- 33.Flanagan HL, Datta S, Lambert DH, Gissen AJ, Covino BG. Effect of pregnancy on bupivacaine-induced conduction blockade in the isolated rabbit vagus nerve. Anesth Analg. 1987;66:123–6. [PubMed] [Google Scholar]
- 34.Airoldi J, Weinstein L. Clinical significance of proteinuria in pregnancy. Obstet Gynecol Surv. 2007;62:117–24. doi: 10.1097/01.ogx.0000253301.55009.ac. [DOI] [PubMed] [Google Scholar]
- 35.Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239–45. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- 36.Casey BM, Dashe JS, Wells CE, McIntire DD, Leveno KJ, Cunningham FG, et al. Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol. 2006;107:337–41. doi: 10.1097/01.AOG.0000197991.64246.9a. [DOI] [PubMed] [Google Scholar]
- 37.Scheithauer BW, Sano T, Kovacs KT, Young WF, Jr, Ryan N, Randall RV, et al. The pituitary gland in pregnancy: A clinicopathologic and immunohistochemical study of 69 cases. Mayo Clin Proc. 1990;65:461–74. doi: 10.1016/s0025-6196(12)60946-x. [DOI] [PubMed] [Google Scholar]
- 38.Zakowski MI, Ham AA, Grant GJ. Transfer and uptake of alfentanil in the human placenta during in vitro perfusion. Anesth Analg. 1994;79:1089–93. doi: 10.1213/00000539-199412000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Olutoye OA, Baker BW, Belfort MA, Olutoye OO. Food and drug administration warning on anesthesia and brain development: Implications for obstetric and fetal surgery. Am J Obstet Gynecol. 2018;218:98–102. doi: 10.1016/j.ajog.2017.08.107. [DOI] [PubMed] [Google Scholar]
- 40.FDA Drug Safety Communication: FDA Review Results in New Warnings about Using General Anesthetics and Sedation Drugs in Young Children and Pregnant Women. 2017. Apr 27, [Last accessed on 2018 Jun 30]. Available from: https://www.fda.gov/Drugs/DrugSafety/ucm532356.htm .


