Abstract
Introduction:
Obesity is associated with increased risk of hypercapnic respiratory failure, prolonged duration on mechanical ventilation, and extended weaning periods.
Objective:
Pilot study to determine whether morbidly obese adult tracheotomized subjects (body mass index [BMI] ⩾ 40) can be more efficiently weaned from the ventilator by optimizing their positive end-expiratory pressure (PEEP) using either an esophageal balloon or the best achieved static effective compliance.
Methods:
We randomly assigned 25 morbidly obese adult tracheotomized subjects (median [interquartile range] BMI 53.4 [26.4]; range 40.4-113.8) to 1 of 2 methods of setting PEEP; using either titration guided by esophageal balloon to overcome negative transpulmonary pressure (Ptp) (goal Ptp 0-5 cmH2O) (ESO group) or titration to maximize static effective lung compliance (Cstat group). Our outcomes of interest were number of subjects weaned by day 30 and time to wean.
Results:
At day 30, there was no significant difference in percentage of subjects weaned. 8/13 subjects (62%) in the ESO Group were weaned vs. 9/12(75%) in the Cstat Group (P = 0.67). Among the 17 subjects who weaned, median time to ventilator liberation was significantly shorter in the ESO group: 3.5 days vs Cstat group 14 days (P = .01). Optimal PEEP in the ESO and Cstat groups was similar (ESO mean ± SD = 26.5 ± 5.7 cmH2O and Cstat 24.2 ± 7 cmH2O (P = .38).
Conclusions:
Optimization of PEEP using esophageal balloon to achieve positive transpulmonary pressure did not change the proportion of patients weaned. Among patients who weaned, use of the esophageal balloon resulted in faster liberation from mechanical ventilation. There were no adverse consequences of the high PEEP (mean 25.4; range 13-37 cmH2O) used in our study. The study was approved by the Institutional Review Board at our institution (UMCIRB#10-0343) and registered with clinicaltrials.gov (NCT02323009).
Keywords: Morbidly obese, tracheotomized, esophageal balloon, transpulmonary pressure, static compliance (Cstat), positive end-expiratory pressure (PEEP), ventilator weaning, speaking valve
Introduction
Obesity is associated with increased risk of hypercapnic respiratory failure,1,2 prolonged duration on mechanical ventilation and extended weaning periods.3,4 It is also a known cause of restrictive respiratory physiology, low lung volumes,5 and overall decreased lung, chest wall, and respiratory system compliance.6,7 It is postulated that chest wall compliance in the obese subjects may be as low as 35% of that of normal subjects8 and studies have shown that this compliance is further decreased in the supine and recumbent positions.7,9,10
When airflow is zero (end-inspiration or end-expiration), the principal force maintaining lung inflation is transpulmonary pressure (Ptp),11 which is the difference between alveolar pressure and pleural pressure (Ppl).12 In mechanically ventilated subjects without active respiratory efforts or air-trapping, the airway (alveolar) pressure measured at end-expiration is equivalent to the applied positive end-expiratory pressure (PEEP). Although PEEP has traditionally been used to improve oxygenation, prevent atelectasis, and avoid atelectrauma, the optimal levels for alveolar recruitment and stabilization remain elusive.13-21 In subjects with acute respiratory distress syndrome, esophageal balloon–guided setting of PEEP to achieve end-expiratory Ptp between 0 and 10 cm of water was shown to significantly improve oxygenation and respiratory system compliance.22 There is one case report of dramatic improvements in oxygenation and ventilator weaning after PEEP was adjusted to maintain a positive Ptp.23
Pleural pressures are estimated in clinical settings by measurement of esophageal pressures using an esophageal balloon.11,24,25 This technique has been validated in healthy human subjects (obese and morbidly obese subjects inclusive) and shown to be applicable in critically ill subjects6,12,26-32 as well as in subjects being weaned from mechanical ventilation.24 A recent report summarizes the current physiological and technical knowledge on esophageal pressure measurements and its utility in patients receiving mechanical ventilation.24
We speculated that mechanically ventilated morbidly obese subjects may fail to wean because traditionally applied PEEP levels are insufficient to generate adequate airway pressures necessary to overcome the high pleural pressures and decreased chest wall compliance imposed by the excessive chest and abdominal wall mass.6,7,33-36 We acknowledged that PEEP levels may be titrated using an esophageal balloon or by adjustment to best achieved static compliance (Cstat)37,38 and posited that PEEP levels titrated using an esophageal balloon to maintain a positive Ptp 0 to 5 cmH2O (targeting as close to 0 as possible) might lead to improved outcomes with respect to weaning this subset of subjects.
Materials and Methods
Study design
The study was conducted in a university teaching hospital with a 9-bed respiratory intermediate unit (RIU). Prospectively enrolled morbidly obese subjects admitted from January 2011 through September 2013 were randomized in a simple 1:1 ratio to 1 of 2 protocols for setting PEEP using either an esophageal balloon (ESO group) or static effective compliance (Cstat group) as further explained below.
The study was approved by the Institutional Review Board at our institution (UMCIRB#10-0343) and registered with clinicaltrials.gov (NCT02323009). Written informed consent was obtained from all subjects, their next of kin or other surrogate decision maker as appropriate.
Study subjects
Tracheotomized morbidly obese adult subjects (body mass index [BMI] ⩾ 40; age > 18 years) with recent admission to the intensive care unit, for non-ARDS hypoxic and/or hypercapnic respiratory failure with need for intubation and mechanical ventilation, with subsequent tracheostomy creation for failure to liberate from the ventilator were enrolled if they had failed at least one post-tracheostomy weaning attempt during their index hospitalization. Subjects were excluded if there was any active lung, neuromuscular, or cardiac disease that would preclude ventilator weaning. We also excluded subjects with naso-facial, esophageal, or other gastrointestinal abnormalities that would contraindicate placement of an esophageal balloon. Subjects were required to tolerate pressure support ventilation (PSV) mode with fractional inspired oxygen (FiO2) of 0.6 or less for at least 24 hours prior to enrollment. Subjects who were hemodynamically unstable or unable to tolerate PSV were excluded from the study. A total of 25 subjects were prospectively enrolled.
Study treatments
Subjects randomized to the Cstat group had PEEP adjusted to achieve the best static effective compliance (Cstat) as automatically calculated and displayed on the graphic interphase of the Hamilton G5 or Galileo ventilator.37,38 Positive end-expiratory pressure was adjusted in increments of 3 cmH2O until there was a less than 5% observed improvement in the measured Cstat averaged over 5 breaths. The PEEP with the best achieved Cstat was chosen.
Subjects randomized to the ESO group had PEEP adjusted to overcome negative Ptp with a goal to maintaining Ptp positive (Ptp 0-5 cmH2O), maintaining as close to zero as possible. As noted above, we elected to use the esophageal balloon because we posited that PEEP levels titrated using an esophageal balloon to maintain a positive Ptp might lead to improved outcomes with respect to weaning morbidly obese subjects by generating adequate airway pressures necessary to overcome the high pleural pressures and decreased chest wall compliance imposed by the excessive chest and abdominal wall mass. Ptp was calculated as airway pressure (Paw) minus esophageal pressure (Pes). For these subjects, the measured Cstats before and after PEEP adjustment were also recorded.
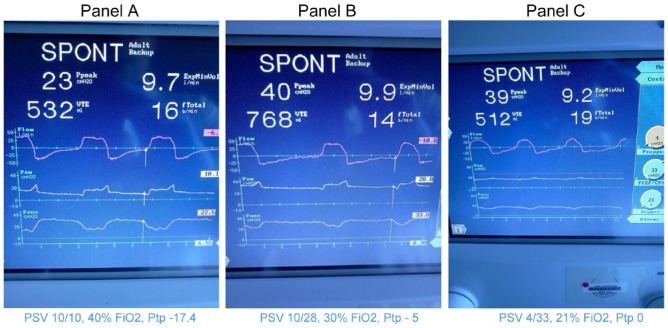
Placement of the esophageal balloon (Ackrad Labs, Adult Esophageal Balloon Catheter Set, manufactured by CooperSurgical, Trumbull, CT, USA) was performed using previously described technique.12,22,26 With the subject supine and head of the bed at 45°, the esophageal balloon was inserted trans-nasally into the stomach with its tip 60 cm from the nares. The balloon was then inflated with 1.0 mL of air and intragastric position confirmed by gentle compression of the epigastrium causing an increased pressure reading on the manometer. The catheter was then withdrawn to approximately 40 cm mark while watching for cardiac oscillations on the esophageal pressure wave form to confirm appropriate mid-esophageal placement. The waveforms of airway pressure, airflow, and Pes were then synchronized on the ventilator graphics screen, which was then frozen to allow measurements. Using the cursor, we identified the point of end-expiration and measured Paw and Pes, from which we calculated the Ptp (Paw − Pes). The PEEP was then adjusted to maintain Ptp ⩾ 0 cmH2O. Serial ventilator screenshots from an ESO subject adjustment are attached (Image 1).
Image 1.
Serial ventilator screen shots from an esophageal balloon subject adjustment.
Note. Panel A shows an airway pressure of 10.1 cmH20 (Paw); An esophageal pressure of 27.5 H20 (Paux). This corresponds to a transpulmonary pressure of -17.4 cmH20 (Paux – Paw). Measurements were made at end-expiration.
Panels B through C show increasing PEEP levels from 28 cmH20 in Panel B to 33 cm H20 in Panel C with a corresponding improvement in the transpulmonary pressure until a transpulmonary pressure of zero (0) is achieved in panel C with the final ventilator settings as shown.
Please note the significant increase in expired tidal volume from 532cc to 768cc with PEEP adjustment from Panel A to B, necessitating a decrease in applied pressure Support in Panel C.
FiO2 was also decreased from 40% to 21% to maintain oxygen saturations of 90% or greater.
Once optimal PEEP was achieved (using either Cstat or Ptp), we adjusted pressure support (PS) to maintain tidal volumes (VT) at 6 to 8 cm3/kg IBW14 and titrated FiO2 to maintain oxygen saturation of 90% or greater (SaO2 ⩾ 90%). Subjects were rested on their optimized ventilator settings overnight and spontaneous tracheostomy-collar trials (trach-collar-trials) initiated the following morning. Repeat PEEP assessments were made daily at 8 am prior to initiating trach-collar-trials to ensure that PEEP remained optimized prior to spontaneous breathing trial. If PEEP settings were found on the following morning to be suboptimal (measured Ptp now negative or measured Cstat less than previous day’s values), PEEP would be re-optimized as above. The rationale behind PEEP re-optimization prior to trach-collar trials was to minimize atelectasis and ensure subjects were fully recruited prior to initiation of spontaneous breathing trials. Repeat PEEP assessments were made daily at 8 am for the first 7 days as long as weaning trials were ongoing and/or in the case of ESO patients, the esophageal balloon was indwelling. The esophageal balloon remained in place for a maximum of 7 days but was removed earlier if a subject weaned.
Trach-collar-trials were conducted by disconnecting subjects from the ventilator and placing them on equivalent amounts of tracheostomy-collar delivered supplemental oxygen to maintain SaO2 ⩾ 90%. Subjects performed incremental trach-collar-trials starting with an initial prescription of 1 to 2 hours; however, a trach-collar-trial was continued for as long as subjects remained clinically stable with respect to respiratory rate (RR), oxygen saturation, and hemodynamics. Subjects who showed signs of fatigue (sustained RR ⩾ 30 and/or verbal or nonverbal indication of inability to continue) and/or who failed a trach-collar-trial by any of the following parameters (sustained oxygen desaturation >5% for 15 minutes, sustained hypoxia <88% for 15 minutes or any hemodynamic decompensation) were promptly returned to their previous ventilator settings and repeat attempts made the following day.39,40 For subjects who showed signs of fatigue, but did not otherwise clinically decompensate, the next day’s trach-collar-trial was targeted to double the time of the previous day’s trial. For subjects who decompensated by any of the parameters listed above, a thorough search for and correction of the reason for the decompensation were performed, and barring no contraindications to further trach-collar-trials, the next day’s trial was planned to exceed the previous day’s trial by at least 2 hours. Subjects who were doing well on subsequent trials were allowed to continue as tolerated. All trach-collar-trials were conducted with patients in the sitting position.41
After rapid decompensation of our first 2 subjects following transition from high PEEP levels to a spontaneous trach-collar-trial (presumed zero-PEEP), our protocol was modified to require performance of trach-collar-trials with a Passy Muir speaking valve in place. All enrollees had to be fit with a speaking valve prior to initiating trach-collar-trials and where necessary, the tracheostomy tube was downsized to improve tolerance. Fitting with a speaking valve was performed by a speech language pathologist who, in conjunction with the respiratory therapist, performed a 30-minute bedside subject observation, monitoring voice quality, cough, and vital signs (including oxygen saturation, RR, and work of breathing) to ensure tolerance. Subjects were required to be stable without any changes in their ventilator settings overnight prior to an initial Passy Muir speaking valve assessment and fitting. Our modified protocol also mandated that all enrollees wear their speaking valves continuously once disconnected from the ventilator. The rationale for use of the speaking valve is further addressed below.
Subjects were otherwise treated the same with respect to nutrition, physical and occupational therapy, mobility, and treatment of all other comorbidities.
Study outcomes
All subjects were followed for 30-days after randomization. The study outcomes were the percentage of subjects weaned from the ventilator at day 30 and time to wean from the ventilator. Intervention effects on FiO2, RR, VT, and PS levels were noted.
A subject was considered successfully weaned if spontaneously breathing without ventilator support for at least 24 hours by day 30. Subjects who required resumption of mechanical ventilation after an initial liberation were presumed to have failed if they were still on the ventilator by day 30. The time to wean was measured from the date of randomization to the date of liberation from mechanical ventilation.
Statistical analysis
We determined that enrollment of 28 subjects per group would detect a 2-day difference in time to wean from mechanical ventilation with 80% power assuming a standard deviation of 3 days at a 2-sided α level of .05. We elected to power the study for time to wean as this was of greater importance to our RIU management and represented our primary outcome of interest. All analyses were performed in the intention-to-treat population which was defined as all subjects who had undergone randomization except those where technical difficulties precluded passing the esophageal balloon or obtaining Cstat measurements. Two subjects with very early protocol violations from whom no data were collected were also excluded from the intention-to-treat population. Comparisons were made between the 2 groups using either the Student t test or the Wilcoxon-Mann-Whitney test as appropriate. Dichotomous or nominal categorical variables were compared with Pearson χ2 or Fisher exact test as appropriate. We compared the initial and final respiratory variables within each group using the paired t test. Kaplan-Meier analysis with the log-rank test was applied to compare time to achieve ventilator independence between the groups.
All analysis was performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corporation; Armonk, NY, USA). A P value less than .05 was considered statistically significant.
Results
A total of 25 subjects were enrolled from January 1, 2011, to September 30, 2013. We did not achieve target enrollment because of significant changes in ventilator weaning practices at our institution. Essentially, more and more morbidly obese subjects were either directly weaned from the ventilator prior to tracheostomy or shortly after tracheostomy in a manner like our study protocol. Consequently, there were fewer failures and therefore much fewer eligible study subjects. After 6 months of minimal subject recruitment, the authors decided to prematurely terminate the study.
Baseline characteristics were similar in the 2 groups (Table 1). The median (interquartile range) BMI in the ESO group was 68.4 (31.3) kg/m2 (range 45-113.8) vs 73.9 (26) kg/m2 in the Cstat group (range 45.4-93.8). Initial PS mean (±SD) in both groups was 13 ± 4 cmH2O and initial PEEP in ESO group was 14 ± 5 cmH2O vs 14 ± 2 cmH2O in Cstat group.
Table 1.
Baseline patient characteristics.
| Patient characteristics | ESO group (N = 13) Mean ± SD |
Cstat group (N = 12) Mean ± SD |
P value |
|---|---|---|---|
| Age, y | 58 ± 9 | 53 ± 15 | .34 |
| Female sex, No (%) | 8 (62) | 7 (58) | >.99 |
| Height, m (median [IQR]) | 1.68 (0.23) | 1.61 (0.23) | .18 |
| BMI, kg/m2 (median [IQR]) | 68.4 (31.3) | 73.9 (26) | .47 |
| Pressure support, cmH2O | 13 ± 4 | 13 ± 4 | .77 |
| PEEP, cmH2O | 14 ± 5 | 14 ± 2 | .67 |
| Tidal volume, cm3 | 414 ± 100 | 375 ± 107 | .35 |
| Tidal volume/IBW, cm3/kg | 6.5 ± 1.2 | 6.4 ± 1.5 | .82 |
| Respiratory rate, breaths/min | 21 ± 7 | 21 ± 8 | .84 |
| FiO2 | 0.37 ± 0.07 | 0.38 ± 0.12 | .77 |
| Cstat | 40 ± 18 | 36 ± 16 | .54 |
| Primary physiologic injurya, No (%) | .58 | ||
| Pulmonary, No (%) | 7 (54) | 7 (58) | |
| Pulmonary ± other comorbidity, No (%) | 3 (23) | 4 (33) | |
| Nonpulmonary (No (%) | 3 (23) | 1 (8) | |
Abbreviations: BMI, body mass index; PEEP = positive end-expiratory pressure.
Plus-minus values are mean ± SD values; ESO group, patients randomized to the esophageal balloon protocol for adjusting PEEP; Cstat group, patients randomized to PEEP adjustment protocol using the best achieved static compliance.
Primary pulmonary injuries include OSA/OHS (8 patients); OSA + COPD ± pneumonia (4 patients). One patient had diffuse alveolar hemorrhage, another had influenza A pneumonia and another had sarcoidosis and renal failure.
Other associated comorbidities include predominantly congestive heart failure, myocardial infarction, leg abscess, sepsis, upper gastrointestinal bleeding, and renal failure.
Nonpulmonary etiologies include case of scrotal abscess, very large goiter after thyroidectomy, traumatic quadriplegia, and incarcerated umbilical hernia with prolonged postsurgical recovery.
Fisher exact P value was nonsignificant for the combined groups of pulmonary and pulmonary + other comorbidities vs nonpulmonary diagnoses.
Within the ESO group (Table 2, top panel [A]), there was a significant increase in PEEP (mean ± SD) from 14 ± 5 cmH2O at baseline to 27 ± 6 cmH2O post adjustment (P < .001). Ptp increased from a mean value of minus 11.2 ± 5.8 cmH2O at baseline to positive 0.7 ± 3.6 cmH2O postintervention (P < .001). The PS decreased from 13 ± 4 cmH2O to 8 ± 3 cmH2O postadjustment (P = .001) and the FiO2 decreased from 0.37 at baseline to 0.27 (P < .001) postintervention. The observed Cstat was noted to increase from 40 ± 18 at baseline to 63 ± 19 (P = .001) postadjustment. The RR, VT, and Pes were unchanged.
Table 2.
Pre- and post-intervention effects.
| Respiratory characteristic | Initial measure Mean (±SD) |
Postadjustment measure Mean (±SD) |
Delta measure (Postadjustment − initial measure) Mean (±SD) |
P value |
|---|---|---|---|---|
| A: Pre- and post-intervention effects for ESO group (N = 13) | ||||
| PS, cmH2O | 13 (4) | 8 (3) | −5 (4) | .001* |
| PEEP, cmH2O | 14 (5) | 27 (6) | 13 (7) | <.001* |
| FiO2 | 0.37 (0.07) | 0.27 (0.09) | −0.09 (0.07) | <.001* |
| Ptp, cmH2O | −11.2 (5.8) | 0.7 (3.6) | 11.8 (6.7) | <.001* |
| End Exp AWP, cmH2O | 13.2 (4.7) | 26.8 (5.5) | 13.5 (6.8) | <.001* |
| Cstat | 40 (18) | 63 (19) | 23 (19) | .001* |
| RR, breaths/min | 21 (7) | 22 (5) | 1 (5) | .751 |
| VT, cm3 | 414 (100) | 420 (70) | 6 (101) | .827 |
| VT/IBW, cm3/kg | 6.5 (1.2) | 6.8 (1.4) | 0.3 (1.6) | .568 |
| Pes, cmH2O | 24.4 (3.4) | 26.1 (6.6) | 1.7 (4.8) | .223 |
| B: Pre- and post-intervention effects for Cstat group (N = 12) | ||||
| PS, cmH2O | 13 (4) | 7 (4) | −7 (5) | <.001* |
| PEEP, cmH2O | 14 (2) | 24 (7) | 10 (8) | .001* |
| FiO2 | 0.38 (0.12) | 0.25 (0.04) | −0.12 (0.13) | .006* |
| Cstat | 36 (16) | 67 (34) | 31 (27) | .002* |
| RR, breaths/min | 21 (8) | 22 (6) | 2 (9) | .525 |
| VT, cm3 | 375 (107) | 385 (103) | 10.2 (120) | .773 |
| VT/IBW, cm3/kg | 6.4 (1.5) | 6.6 (1.3) | 0.13 (1.9) | .815 |
Abbreviations: Cstat, static effective compliance; end-exp. AWP, end-expiratory airway pressure; FiO2, fractional inspired oxygen; Pes, esophageal pressure; PEEP, positive end-expiratory pressure; PS, pressure support; Ptp, transpulmonary pressure; RR, respiratory rate; VT, tidal volume; VT per IBW, tidal volume per ideal body weight in kilograms.
Statistically significant difference.
Within the Cstat group (Table 2, bottom panel [B]), there was a significant increase in the static effective compliance from 36 ± 16 at baseline to 67 ± 34 post adjustment (P = .002), with a corresponding significant increase in PEEP from 14 ± 2 cmH2O at baseline to 24 ± 7 cmH2O post adjustment (P = .001). The PS and FiO2 decreased significantly from 13 ± 4 cmH2O to 7 ± 4 cmH2O postintervention (P < .001) and from 0.38 to 0.25 (P = .006), respectively. As in the ESO group, the RR and VT were unchanged.
When the post intervention effects were compared between the two groups (Table 3), no statistically significant difference in the final post adjustment values was observed. The mean post adjustment PEEP levels in both groups were similar at 27 ± 6 cmH2O in the ESO group and 24 ± 7.0 cmH2O in the Cstat group (P = .38).
Table 3.
Post-intervention parameters: ESO group vs Cstat group.
| Patient characteristics | ESO group (N = 13) Mean (±SD) |
Cstat group (N = 12) Mean (±SD) |
P value |
|---|---|---|---|
| PS, cmH2O | 8 (3) | 7 (4) | .23 |
| PEEP, cmH2O | 27 (6) | 24 (7.0) | .38 |
| VT/IBW, cm3/kg | 6.8 (1.4) | 6.6 (1.3) | .66 |
| RR, breaths/min | 22 (5) | 22 (6) | .73 |
| FiO2 | 0.27 (0.09) | 0.25 (0.04) | .49 |
| Cstat | 63 (19) | 67 (34) | .76 |
Abbreviations: Cstat, static effective compliance; FiO2, fractional inspired oxygen; PEEP, positive end-expiratory pressure; PS, pressure support; RR, respiratory rate; TV/IBW, tidal volume per ideal body weight.
Outcomes
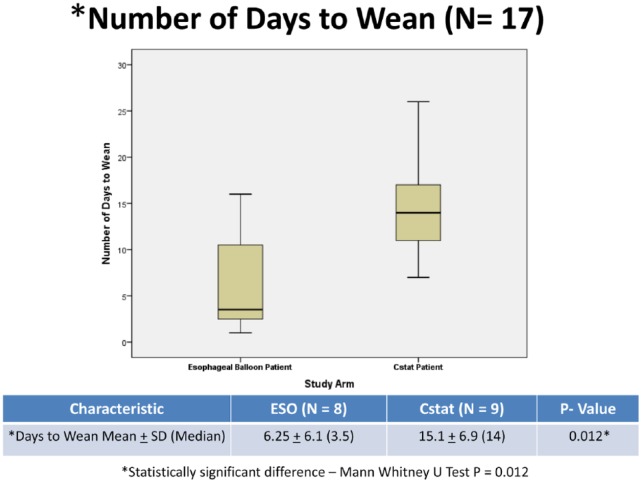
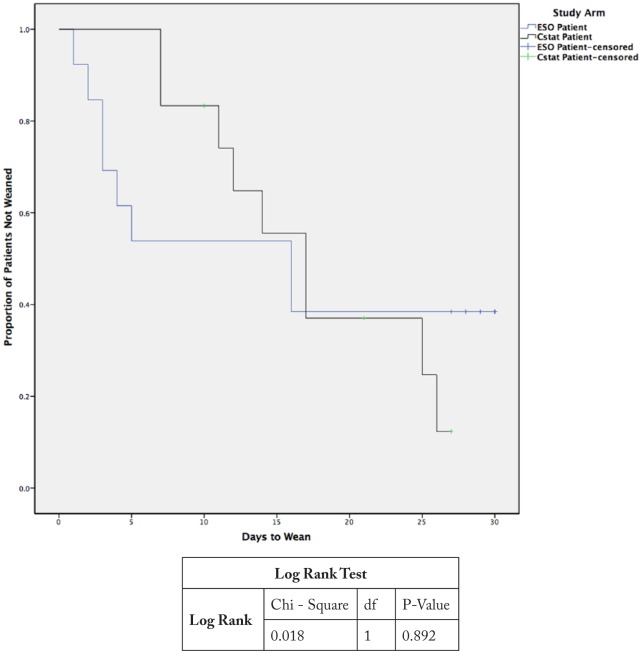
With respect to our study group, there was no significant between group difference in the percentage of subjects weaned by day 30. Eight subjects (62%) were weaned in the ESO group vs 9 (75%) in the Cstat group (P = .67). Overall, 17 subjects (68% of intervention cohort) were weaned by day 30. Among the 17 subjects who successfully weaned, subjects in the ESO group weaned significantly quicker than those in the Cstat group. Subjects in the ESO group weaned in a median of 3.5 days vs 14 days for subjects in the Cstat group (P = .012; Figure 1). When the time to wean data were analyzed in the entire cohort (N = 25) using survival analysis, we found that the greatest impact to weaning using esophageal balloon was seen in the first 15 days. If subjects were not weaned by day 15, the outcome was the same as in the Cstat group (Figure 2).
Figure 1.
Box plot comparing number of days to wean in the successfully weaned ESO vs. Cstat subjects.
Figure 2.
Kaplan-Meier curves showing the time to wean in the entire cohort (N=25).
Discussion
With respect to ventilator-dependent adult morbidly obese subjects with non-fenestrated tracheostomy tubes fit with a speaking valve, there was no significant difference in the percentage of subjects successfully weaned by day 30 whether PEEP was adjusted using an esophageal balloon to overcome negative Ptp or adjusted to maximize Cstat.
Among the 17 subjects who successfully weaned, however, subjects who had their PEEP titrated to overcome a negative Ptp using an esophageal balloon weaned faster than those who had their PEEP titrated to maximize Cstat. Subjects in the ESO group weaned in a median of 3.5 days (range 1-16 days) vs a median of 14 days (range 7-26 days) for subjects in the Cstat group (P = .012).
The mean PEEP level considered optimal was the same whether esophageal balloon or Cstat was used for adjustment (26.5 ± 5.7 cmH2O vs 24.2 ± 7.0 cmH2O; P = .38).
There were no observed adverse consequences of the high PEEP levels (range 13-37 cmH2O) used in our study. This is consistent with prior findings indicating that higher PEEP levels targeted to Ptp are safe and well tolerated.22,23,35,42
After rapid decompensation of the first 2 subjects following transition to a trach-collar-trial from high PEEP levels, all subsequent subjects were required to perform trach-collar-trials with a speaking valve in place. The speaking valve is a 1-way valve which when placed on the hub of the tracheostomy tube redirects airflow during expiration through the vocal folds and upper airways allowing audible speech.43 Creation of a tracheostomy results in disruption of glottic closure and leads to loss of subglottic back pressure which has been shown to be necessary for efficient swallowing, clearing of secretions, and maintenance of a tracheal airway column.43-46 We postulated that the open tracheostomy with larger sized tracheostomy tubes disrupts the normal anatomy of a closed respiratory system and impairs the normal ability of obese subjects to maintain a normal positive subglottic back pressure47 and intrinsic PEEP.48 We reasoned that this positive subglottic back pressure is transmitted to the lower airways and thus possibly plays a role in maintaining some intrinsic PEEP48,49 which limits end-expiratory airway closure and atelectasis in the stable obese subjects. The speaking valve is suited to this function because it maintains a closed position during expiration, thereby restoring a relatively closed respiratory system which allows subjects to generate and maintain some positive subglottic airway pressure with larger tracheostomy tubes in place. To the best of our knowledge, there have not been any previous studies looking at the role of speaking valves in weaning ventilator-dependent tracheotomized subjects.
When we examined only the subjects that weaned, we noted that subjects who had their PEEP adjusted to overcome negative Ptp weaned significantly faster than subjects who had their PEEP optimized to achieve the best effective static compliance. It is not entirely clear why this is so. We think some of this may be explained physiologically; however, there may also be a more fundamental institutional component.
Although speculative, it is physiologically plausible that lung recruitment in the ESO group was immediate, whereas that in the Cstat group was more gradual. This seems possible if one considers that the use of the esophageal balloon to overcome negative Ptp is more expeditious and prompt than the more gradual process, whereby PEEP is adjusted stepwise in increments of 3 cmH2O until the best observed Cstat is achieved. Indeed, although initial PEEP adjustments in both groups on study day 1 took approximately 1 hour from setup to final determination of optimal PEEP, we noted that subjects in the Cstat group required more PEEP adjustments over ensuing days than did subjects in the ESO group. There were 0.7 PEEP adjustments/ESO subjects on day 2 vs 1.9 PEEP adjustments/CStat subjects on days 2 and 3. There were no indicated PEEP adjustments in either group after day 3. This greater need to adjust PEEP in the Cstat group on subsequent days may suggest an overall more gradual process of lung recruitment in that group. It is to be noted that while all subsequent PEEP adjustments were completed by day 2 in the ESO group, subjects in the Cstat group still required additional PEEP adjustments on day 3. Perhaps immediate lung recruitment resulting from restoration of positive Ptp in the ESO group produced quicker resolution of atelectasis and a less inflamed state,50-53 that fostered more rapid weaning. We did not measure any inflammatory biomarkers in our subjects and are unable to substantiate this claim. This unfortunately represents a significant flaw in our study design that raises questions that warrant further study.
On the more practical institutional component, we found that because ESO subjects required minimal PEEP adjustments after study day 1, they were able to get fitted with their speaking valves by the following morning and could proceed immediately to trach-collar-trials. Cstat subjects, however, were still undergoing PEEP titrations by day 2 and sometimes by day 3, thus potentially delaying their speaking valve fittings and subsequent initiation of trach-collar-trials. Although this institutional component may be regarded as a potential bias against Cstat patients, it does in its own way also speak to the more expeditious nature of the esophageal balloon as a way of setting PEEP. Our post study institutional experience has been that use of the esophageal balloon for adjusting PEEP facilitates planning and interdisciplinary coordination between the respiratory therapists and speech language pathologists with respect to scheduling bedside patient visits for speaking valve assessments and initiation of trach-collar-trials. The speech language pathologists can now plan on a speaking valve fitting the day following a balloon-guided PEEP optimization with subsequent initiation of a trach-collar trial. Much of the guesswork about which patients are “ready” for fitting or “when best” to get to the bedside have been eliminated. It is to be noted, however, that the speech language pathologists were not part of our study and had no prior knowledge of which patients were enrolled in the ESO or Cstat arm. Although attractive as a theory, we do not think, however, that the institutional component alone is sufficient to explain the difference in time to wean between the 2 study groups.
Whatever the reason, the more rapid wean observed in the esophageal balloon group is a significant clinical end point. If the average length of stay on a mechanical ventilator in a weaning unit can be decreased by a week or more, we can potentially achieve significant cost savings while improving outcome. This cost savings could potentially be greater if indeed fewer ventilator adjustments and by extension less intense bedside respiratory therapist presence are required for weaning tracheotomized ventilator-dependent subjects.
Our study has several limitations. First, we had a small sample size of 25 subjects and did not achieve sufficient enrollment as per our a priori sample size and power calculations. It is therefore very possible that our small sample size may have magnified our findings. Second, we did not measure any inflammatory biomarkers and thus do not know if indeed the more gradual lung recruitment with the Cstat-guided PEEP titration truly reflects a more inflamed state as compared with the more immediate recruitment achieved with esophageal balloon-guided PEEP adjustment. Third, due to the nature of our intervention, our study could not be blinded to either the subjects or the therapists so it is possible that ESO patients may have been encouraged to remain on trach-collar-trials longer than Cstat patients. We do not think, however, that this was the case, as there were very clear rules for stopping a trial and placing patients back on the ventilator if they failed and/or fatigued. Furthermore, patients pushed to continue suboptimal spontaneous breathing trials often do poorly and have larger setbacks than if the trial was aborted and patient rested. Finally, this is a single-center study in an institution with experience in managing morbidly obese subjects and our findings may not be generalizable to centers with less experience in the care of the morbidly obese.
Conclusions
In conclusion, use of an esophageal balloon to adjust applied PEEP in morbidly obese subjects, to overcome negative Ptp (Ptp ⩾ 0 cmH2O) resulted in more expeditious weaning from mechanical ventilation but did not change the proportion of patients weaned. There were no adverse consequences of the high PEEP levels (mean 25.4 cmH2O, range 13-37 cmH2O) used in our study.
Acknowledgments
The authors wish to acknowledge Kevin F O’Brien, PhD (Biostatistics), for performing the initial sample size calculations and wish to thank Jason Brinkley, PhD (Biostatistics), for reviewing the initial statistical analysis.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: ONO takes responsibility for the content of the manuscript, including the data and analysis; had full access to all the data in the study; and takes responsibility for the integrity of the data and the accuracy of the data analysis. MM, CB, and RS contributed substantially to the study design, data review, data interpretation, and multiple revisions of the manuscript. CB, WTII, KS, and PLR contributed substantially to the study design, study conduct, data collection, and subject follow-up. ZK contributed substantially to the study design, subject recruitment and conduct of the study. KS contributed substantially to the review of data for accuracy. RS was the principal investigator and ONO prepared the final manuscript with critical reviews by MM, CB, and RS.
References
- 1. Powers MA. The obesity hypoventilation syndrome. Respir Care. 2008;53:1723–1730. [PubMed] [Google Scholar]
- 2. BaHammam A. Acute ventilatory failure complicating obesity hypoventilation: update on a “critical care syndrome.” Curr Opin Pulm Med. 2010;16:543–551. [DOI] [PubMed] [Google Scholar]
- 3. Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36:151–158. [DOI] [PubMed] [Google Scholar]
- 4. El-Solh A, Sikka P, Bozkanat E, Jaafar W, Davies J. Morbid obesity in the medical ICU. Chest. 2001;120:1989–1997. [DOI] [PubMed] [Google Scholar]
- 5. Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–833. [DOI] [PubMed] [Google Scholar]
- 6. Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol (1985). 2010;108:212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pelosi MP, Croci M, Ravagnan I, Vicardi P, Gattinoni L. Total respiratory system, lung and chest wall mechanics in sedated paralyzed postoperative morbidly obese patients. Chest. 1996;109:144–151. [DOI] [PubMed] [Google Scholar]
- 8. Sharp JT, Henry JP, Sweany SK, Meadows WR, Pietras RJ. The total work of breathing in normal and obese men. J Clin Invest. 1964;43:728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yap JC, Watson RA, Gilbey S, Pride NB. Effects of posture on respiratory mechanics in obesity. J Appl Physiol. 1995;79:1199–1205. [DOI] [PubMed] [Google Scholar]
- 10. Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol (1985). 1960;15:377–382. [DOI] [PubMed] [Google Scholar]
- 11. Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. [DOI] [PubMed] [Google Scholar]
- 12. Talmor D, Sarge T, O’Donnell CR, et al. Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med. 2006;34:1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slutsky AS, Hudson LD. PEEP or no PEEP—lung recruitment may be the solution. N Engl J Med. 2006;354:1839–1841. [DOI] [PubMed] [Google Scholar]
- 14. Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 15. Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637–645. [DOI] [PubMed] [Google Scholar]
- 16. Brower RG, Lanken PN, MacIntyre N, et al. ; The National Heart Lung Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. [DOI] [PubMed] [Google Scholar]
- 17. Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–655. [DOI] [PubMed] [Google Scholar]
- 18. Grasso S, Fanelli V, Cafarelli A, et al. Effects of high versus low positive end-expiratory pressures in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:1002–1008. [DOI] [PubMed] [Google Scholar]
- 19. Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–873. [DOI] [PubMed] [Google Scholar]
- 20. Fuller BM, Cinel I, Dellinger RP. General principles of mechanical ventilation. In: Parrillo JE, Dellinger R, eds. Critical Care Medicine: Principles of Diagnosis and Management in the Adult. 4th ed. Philadelphia, PA: Elsevier Saunders; 2013:138–152. [Google Scholar]
- 21. Slutsky AS. Lung injury caused by mechanical ventilation. Chest. 1999;116:9S–15S. [DOI] [PubMed] [Google Scholar]
- 22. Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piraino T, Cook DJ. Optimal PEEP guided by esophageal balloon manometry. Respir Care. 2011;56:510–513. [DOI] [PubMed] [Google Scholar]
- 24. Akoumianaki E, Maggiore SM, Valenza F, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med. 2014;189:520–531. [DOI] [PubMed] [Google Scholar]
- 25. Milic-Emili WAZ. Esophageal pressure measurement. In: Hamid Q, Shannon J, Martin J, eds. Physiologic Basis of Respiratory Disease. Montreal, QC, Canada: PMPH; 2005:639–647. [Google Scholar]
- 26. Talmor DS, Fessler HE. Are esophageal pressure measurements important in clinical decision-making in mechanically ventilated patients? Respir Care. 2010;55:162–172, discussion 172–174. [PubMed] [Google Scholar]
- 27. Loring SH, O’Donnell CR, Behazin N, et al. Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol (1985). 2010;108:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hedenstierna G. Esophageal pressure: benefit and limitations. Minerva Anestesiol. 2012;78:959–966. [PubMed] [Google Scholar]
- 29. Owens RL, Campana LM, Hess L, Eckert DJ, Loring SH, Malhotra A. Sitting and supine esophageal pressures in overweight and obese subjects. Obesity (Silver Spring). 2012;20:2354–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mead J, Gaensler EA. Esophageal and pleural pressures in man, upright and supine. J Appl Physiol (1985). 1959;14:81–83. [DOI] [PubMed] [Google Scholar]
- 31. Washko GR, O’Donnell CR, Loring SH. Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J Appl Physiol (1985). 2006;100:753–758. [DOI] [PubMed] [Google Scholar]
- 32. Bernard GR. PEEP guided by esophageal pressure—any added value? N Engl J Med. 2008;359:2166–2168. [DOI] [PubMed] [Google Scholar]
- 33. Pelosi P, Luecke T, Rocco PR. Chest wall mechanics and abdominal pressure during general anaesthesia in normal and obese individuals and in acute lung injury. Curr Opin Crit Care. 2011;17:72–79. [DOI] [PubMed] [Google Scholar]
- 34. Zerah F, Harf A, Perlemuter L, Lorino H, Lorino AM, Atlan G. Effects of obesity on respiratory resistance. Chest. 1993;103:1470–1476. [DOI] [PubMed] [Google Scholar]
- 35. Malhotra A, Hillman D. Obesity and the lung: 3. Obesity, respiration and intensive care. Thorax. 2008;63:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Jong A, Chanques G, Jaber S. Mechanical ventilation in obese ICU patients: from intubation to extubation. Crit Care. 2017;21:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iotti GA, Braschi A, Brunner JX, et al. Respiratory mechanics by least squares fitting in mechanically ventilated patients: applications during paralysis and during pressure support ventilation. Intensive Care Med. 1995;21:406–413. [DOI] [PubMed] [Google Scholar]
- 38. https://www.hamilton-medical.com/en_US/Services/Knowledge-Base/Knowledge-Base-Detail~2018-02-11~Static-compliance-(Cstat)-vs–dynamic-compliance-(Cdyn)~23980f36-edb7-41ea-b04f-fb9fe4d990b5~.html#DataTables_Table_0=od3. Accessed September 9, 2018
- 39. Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332:345–350. [DOI] [PubMed] [Google Scholar]
- 40. MacIntyre NR, Cook DJ, Ely EW, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120:375S–395S. [DOI] [PubMed] [Google Scholar]
- 41. Lemyze M, Mallat J, Duhamel A, et al. Effects of sitting position and applied positive end-expiratory pressure on respiratory mechanics of critically ill obese patients receiving mechanical ventilation. Crit Care Med. 2013;41:2592–2599. [DOI] [PubMed] [Google Scholar]
- 42. Bohm SH, Thamm OC, von Sandersleben A, et al. Alveolar recruitment strategy and high positive end-expiratory pressure levels do not affect hemodynamics in morbidly obese intravascular volume-loaded patients. Anesth Analg. 2009;109:160–163. [DOI] [PubMed] [Google Scholar]
- 43. Passy V. Passy-Muir tracheostomy speaking valve. Otolaryngol Head Neck Surg. 1986;95:247–248. [DOI] [PubMed] [Google Scholar]
- 44. Eibling DE, Gross RD. Subglottic air pressure: a key component of swallowing efficiency. Ann Otol Rhinol Laryngol. 1996;105:253–258. [DOI] [PubMed] [Google Scholar]
- 45. Christopher KL. Tracheostomy decannulation. Respir Care. 2005;50:538–541. [PubMed] [Google Scholar]
- 46. Savard P, Cole P, Miljeteig H, Haight JS. Laryngeal resistance to respiratory airflow in humans. Laryngoscope. 1993;103:785–792. [DOI] [PubMed] [Google Scholar]
- 47. Hussey JD, Bishop MJ. Pressures required to move gas through the native airway in the presence of a fenestrated vs a nonfenestrated tracheostomy tube. Chest. 1996;110:494–497. [DOI] [PubMed] [Google Scholar]
- 48. Pankow W, Podszus T, Gutheil T, Penzel T, Peter J, Von Wichert P. Expiratory flow limitation and intrinsic positive end-expiratory pressure in obesity. J Appl Physiol (1985). 1998;85:1236–1243. [DOI] [PubMed] [Google Scholar]
- 49. Ferretti A, Giampiccolo P, Cavalli A, Milic-Emili J, Tantucci C. Expiratory flow limitation and orthopnea in massively obese subjects. Chest. 2001;119:1401–1408. [DOI] [PubMed] [Google Scholar]
- 50. Retamal J, Bergamini BC, Carvalho AR, et al. Non-lobar atelectasis generates inflammation and structural alveolar injury in the surrounding healthy tissue during mechanical ventilation. Crit Care. 2014;18:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Loring SH, Pecchiari M, Della Valle P, Monaco A, Gentile G, D’Angelo E. Maintaining end-expiratory transpulmonary pressure prevents worsening of ventilator-induced lung injury caused by chest wall constriction in surfactant-depleted rats. Crit Care Med. 2010;38:2358–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wakabayashi K, Wilson MR, Tatham KC, O’Dea KP, Takata M. Volutrauma, but not atelectrauma, induces systemic cytokine production by lung-marginated monocytes. Crit Care Med. 2014;42:e49–e57. [DOI] [PubMed] [Google Scholar]
- 53. Borges JB, Costa EL, Suarez-Sipmann F, et al. Early inflammation mainly affects normally and poorly aerated lung in experimental ventilator-induced lung injury. Crit Care Med. 2014;42:e279–e287. [DOI] [PubMed] [Google Scholar]