Abstract
Background:
There are numerous recommendations from expert sources that help guide primary care providers in cancer screening, infectious disease screening, metabolic screening, monitoring of drug levels, and chronic disease management. Little is known about the potential effort needed for a healthcare system to address these recommendations, or the patient effort needed to complete the recommendations.
Methods:
For 73 recommended population healthcare items, we examined each of 28,742 patients in a primary care internal medicine practice to determine whether they were up-to-date on recommended screening, immunizations, counseling, and chronic disease management goals. We used a rule-based software tool that queries the medical record for diagnoses, dates, laboratory values, pathology reports, and other information used in creating the individualized recommendations. We counted the number of uncompleted recommendations by age groups and examined the healthcare staff needed to address the recommendations and the potential patient effort needed to complete the recommendations.
Results:
For the 28,742 patients, there were 127,273 uncompleted recommendations identified for population health management (mean recommendations per patient 4.36, standard deviation of 2.65, range of 0–17 recommendations per patient). The age group with the most incomplete recommendations was age of 50–65 years with 5.5 recommendations per patient. The 18–35 years age group had the fewest incomplete recommendations with 2.6 per patient. Across all age groups, initiation of these recommendations required high-level input (physician, nurse practitioner, or physician’s assistant) in 28%. To completely adhere to recommended services, a 1000-patient cross-section cohort would require a total of 464 procedures and 1956 lab tests.
Conclusion:
Providers and patients face a daunting number of tasks necessary to meet guideline-generated recommendations. We will need new approaches to address the burgeoning numbers of uncompleted recommendations.
Keywords: Population health, primary care, guidelines, preventive services, primary care workload, provider work
Introduction
Comprehensive population healthcare management is a major challenge in primary care. Although treating acute illness is a large part of the day-to-day work of a primary care provider, comprehensive population health management requires attention to chronic disease management, disease screening, and prevention.
Several expert panels have published guidelines and recommendations for disease screening and prevention. These expert panels have issued evidence-based guidelines and recommendations for cancer screening, immunizations, infectious disease surveillance, and counseling for health maintenance and disease prevention. From the US Centers for Disease Control and Prevention (CDC),1 there are recommendations for infectious disease screening, including screening for human immunodeficiency virus (HIV), hepatitis C virus (HCV), chlamydia, and gonorrhea.2–4 The Advisory Committee on Immunization Practices (ACIP) of the CDC has 13 recommended vaccines listed on the recommended adult immunization schedule.5 The United States Preventive Services Task Force (USPSTF)6 has 98 published recommendations for primary care which include screening, counseling, and preventive medications.
Chronic diseases such as diabetes, hypertension, and hyperlipidemia are common in a primary care practice and are also a large component of comprehensive population healthcare management. The Institute for Clinical Systems Improvement (ICSI)7 has endorsed guidelines for treating lipids, chronic obstructive pulmonary disease (COPD), and asthma, among others. In addition, specialty societies have published guidelines on chronic disease management including diabetes management from the American Diabetes Association (ADA)8 and management of hypertension and congestive heart failure from the American College of Cardiology (ACC) and the American Heart Association (AHA).9,10
New medical informatics tools can give primary care practices a better sense of the numbers of recommendations for individual patients. By conducting a software query of the medical record for immunizations, diagnoses, procedures, laboratory tests, and dates completed, we can determine that a specific patient should have a PCV13 immunization, screening for colon cancer, screening for diabetes, consideration for a statin, screening for HCV, and counseling for tobacco cessation.
We used this technology to examine the volume and scope of recommendations across an entire adult primary care population. In this article, we examine the volume of recommendations, the healthcare staff categories needed to fulfill them, and the patient effort required to complete the recommendations. Using this information, practice administrators may be better able to prioritize efforts to address specific tasks involved in population health management.
Methods
Setting
This study took place in the Division of Primary Care Internal Medicine at Mayo Clinic Rochester. The Division of Primary Care Internal Medicine is a general internal medicine practice that cares for adult patients living in Rochester, Minnesota. The medical practice has an academic mission that includes education of internal medicine residents. However, this study included only patients who were assigned to staff physicians, or advanced practice providers (APPs) with certifications of nurse practitioners (NPs) or physician’s assistants (PAs). The study patients had longer term continuity of care with their providers and would be more typical of a medical practice that did not incorporate an education role.
Data capture and generic disease management system
We used the Mayo Clinic’s Generic Disease Management System (GDMS) to obtain the data for this study. GDMS captures information from multiple data sources within the patient electronic medical record to give a succinct summary of a patient’s most current medical information including diagnosis, immunizations, and lab and procedure reports.11–15 Utilizing this information, GDMS applies rules and calculators to expand that information into usable knowledge for enhanced patient care. For example, GDMS takes the current age, sex, cholesterol, and blood pressure information to automatically calculate the American Heart Association/American College of Cardiology 10 year cardiovascular risk.14,16 GDMS examines previous immunization histories to determine what immunizations are due based on whether the immunizations were given and the time interval since last given. GDMS also identifies cancer screening tests that may be indicated such as mammography and colon cancer screening using guidelines to identify the age of screening initiation, and intervals between screening using the date of the most recent previous screening.
Mayo Clinic has a group of generalist, specialist, and subspecialist physicians who author Ask Mayo Expert, an online knowledge delivery tool that contains more than 1500 clinical topics and more than 225 care process models that deliver information to the clinician at the point of care. The recommendations in GDMS are based on national and international guidelines and reviewed by the Ask Mayo Expert physicians, who take into consideration competing guidelines and the evidence base for the guidelines. Ask Mayo Expert physicians cover a broad scope of medical practice and review guidelines coming from multiple sources, including resources such as USPSTF and CDC, specialty societies such as the American College of Cardiology, and other groups authoring guidelines such as the Institute for Clinical Systems Improvement, American Cancer Society, and the American Heart Association.
The GDMS recommendations were based on algorithms handled by a computer system separate from the electronic health record (EHR), but generated from patient data files shared with the EHR. So, patient information such as diagnoses, completed immunizations, and completed screening tests were fed into GDMS on a real-time basis with recommendations then transmitted into the EHR in real time. There was no paper-based data system. When patients arrived for visits, a paper list of recommendations from GDMS was printed for the provider and rooming staff. This paper list was to help prompt providers at the point of care to address the recommendations. The paper copy was also designed for patients to take home and contained other information such as recent lab test results. Providers also had access to recommendations within their panel of patients so that they could manage the recommendations on a population level as well as during face to face visits.
Using GDMS, patients can be individually identified for targeted therapy. For example, GDMS uses a combination of diagnosis, lab, and medication information to determine whether an individual with diabetes should be considered for an angiotensin-converting enzyme (ACE) inhibitor. In addition to general cancer screening recommendations, GDMS can also use diagnosis, laboratory, and procedure information to identify very specific screening recommendations such as for hepatocellular carcinoma in high-risk individuals such as those with cirrhosis and chronic hepatitis B. In all, GDMS captures 108 diagnoses that are used in its rules engine. GDMS uses a combination of diagnoses, medications, and previous lab test dates and values to calculate when certain lab tests are due such as hemoglobin A1c, microalbumin, or thyroid stimulating hormone (TSH). GDMS reviews if monitoring questionnaires (such as for asthma or depression) are indicated, based on diagnosis lists and medications. GDMS also uses age and EHR documents to determine whether advance care planning is needed. Patient-provided information such as tobacco use in the social history is used by GDMS to identify patients for tobacco cessation advice.
Although our study included just those aged 18 years and over, GDMS did query the medical record for information that may have occurred at an earlier age. For example, those aged 18 years and over would have had their records electronically queried for previous human papillomavirus (HPV) vaccinations, so as not to make an incorrect recommendation if that recommended vaccination had already been completed.
In summary, GDMS uses a variety of data sources, rules, and calculators to create recommendations for providers to use. When this study took place in 2017, GDMS had an inventory of 73 recommendations. Patients could each have multiple recommendations. However, recommendations with the same outcome were not duplicated. For example, a patient recommendation for a glucose screen because of use of a high-risk medication (e.g. olanzapine) would not also be prompted for a duplicate glucose for an age-driven diabetes screen. Likewise, a recommendation for colonoscopy because of a previous polyp would not have a duplicate recommendation for colonoscopy because of age.
Processes for managing recommendations
There are several different avenues for managing recommendations at Mayo Clinic in primary care. During a clinic face-to-face visit, the standardized rooming process includes printing a paper GDMS summary sheet at all face-to-face primary care visits (i.e. not just at preventive health or chronic disease visits). This printed summary, which can be given to the patient, contains the recommendations for the patient along with current lab values and calculations of the ACC-AHA 10- and 30-year cardiac risks if applicable. There are also additional recommendations contained in the electronic GDMS summary, available to the clinician at the time of the appointment, that often require more shared decision-making and thus are not displayed on the paper form. Examples of additional recommendations included on the electronic version are prostate cancer screening (which can require more shared decision-making), and recommendations concerning potentially sensitive topics such as screening for sexually transmissible infections.
In the face-to-face visit, the provider has the option of giving the patient the GDMS summary sheet and additionally discussing any recommendations not visible on the paper form. Licensed Practical Nurse (LPN) roomers are enabled to discuss and give vaccinations that GDMS has determined are due.
Processes of care outside the office visit also use information from GDMS. If patients have access to the patient portal (patient online services), they are sent twice yearly notifications to have selected screening tests and chronic disease monitoring tests that are due according to GDMS. Those without patient portal access receive the same notifications by mail. For example, a woman with diabetes could receive twice yearly notifications having to do with mammography (if not done in the previous 12 months), hemoglobin A1c, microalbumin, and an eye exam. An otherwise healthy 40-year-old male might get a notification for a glucose and lipid panel.
An additional non-visit avenue of care involves panel managers who may also send notifications about chronic disease monitoring. This might take the form of notifying a patient with asthma that it is time for an asthma control questionnaire or notifying a patient with depression that a mood questionnaire is due.
Assigning categories to recommendations
A three-physician panel (F.N., J.M., and J.P.) jointly developed a classification terminology to collapse the 73 different recommendations into 11 broader categories of healthcare. The 11 categories were mutually exclusive and serve to divide the recommendations into discrete categories of healthcare activity. The classification of the individual 11 groups and their component recommendations are shown in Table 1.
Table 1.
Classification of recommendations into healthcare categories.
| Category | Recommendation content |
|---|---|
| Cancer screen | Screen for colon cancer, breast cancer, cervical cancer, prostate cancer, hepatocellular carcinoma |
| Disease monitoring | Monitoring of anti-seizure medications (phenytoin and carbamazepine), heart failure medication (digoxin), thyroid medication (TSH), diabetes monitoring (hemoglobin A1c, microalbumin), anti-hypertensive monitoring (sodium and potassium), vitamin B12 monitoring in high-risk individuals (bariatric surgery and intestinal disorders) |
| Disease screen | Diabetes and lipid screening (includes patients on high-risk medications such as olanzapine) |
| Documents | Documents requiring patient input (advance directive, asthma control questionnaire, and mood status questionnaire) |
| Immunizations | Hepatitis B vaccine, human papilloma virus, herpes zoster, pneumonia PPSV 23, pneumonia PCV 13, tetanus-diphtheria, tetanus-diphtheria-pertussis |
| Infectious disease screen | Screen for chlamydia, hepatitis C, human immunodeficiency virus |
| New medication | Recommendation for initiation of aspirin, angiotensin-converting enzyme inhibitor, beta blocker |
| Optimize management | Management of blood pressure, diabetes, prediabetes, hypercholesterolemia |
| Preventive counseling | Identification of alcohol issues, tobacco use, and obesity |
| Procedure screen, not cancer | Abdominal ultrasound screen for aneurysm, eye exam due for retinopathy screening, osteoporosis screening |
| Specialty referral | Referral alert for kidney disease (low estimated glomerular filtration rate), cardiovascular referral for defibrillator shared decision-making (heart failure with low ejection fraction and no implanted defibrillator) |
TSH: thyroid stimulating hormone.
From recommendations to tasks
Recommendations are associated with tasks necessary to complete the recommendations. For example, a screening recommendation for chlamydia would include at least two separate tasks: the ordering of the test (initiation of the recommendation) and a follow-up of the test result. Although some of the 73 recommendations could require more than just those two tasks (initiation and follow-up), we used recommendation initiation and recommendation follow-up as two different tasks involved with each recommendation. For each of the 73 recommendations, the three-physician panel (F.N., J.M., and J.P.) came to a consensus on staff and training that are needed for the task initiation and task follow-up for these recommendations. We looked at these tasks separately because the initiation of a recommendation might requires a different level of training than a follow-up. For example, initiating a recommendation for colon cancer screening often falls within the scope of training of a clinician (physician, N.P. or P.A.) because there is shared decision-making around the options for screening (colonoscopy vs stool DNA). Colon cancer screening also may require clinician-level training to initiate a recommendation because previous colonoscopy results, pathology results, and family history may influence shared decision-making around the frequency and type of screening. Follow-up of colon cancer screening could involve simply a letter or portal message reporting negative results if there were no concerning findings. In this scenario, the colon cancer screening follow-up would not necessarily require a clinician unless there was a significant finding. This demonstrates how a colon cancer screening follow-up could be within a level of training different than the colon cancer screening initiation. A nurse could triage negative results to a secretary (to send out a letter or portal message reporting normal results), while sending positive results to the ordering clinician for more in-depth follow-up.
Training needed for recommendation initiation
The three-physician panel evaluated each of the 73 recommendations and came to a consensus about the training level needed to initiate the recommendation. Each recommendation associated with task initiation was assigned to one of the following staff categories: clinician (physician, N.P. or P.A.), nursing staff, or automated.
Healthcare system effort needed for follow-up of recommendations
If initiated, many of these recommendations would require follow-up. For example, follow-up would be necessary to notify patients of the results of cervical cancer screening. For each of the 73 recommendations, our three-physician panel came to a consensus about the type of healthcare staff needed for follow-up of the recommendations. Each recommendation follow-up was assigned to one of the following categories: physician or advanced practice provider (N.P. and P.A.), nursing staff/nursing staff triage, radiology follow-up, or follow-up not needed. Radiology follow-up is the legally required notification of results for mammography follow-up and is done through radiology; it does not require primary care personnel as the first step in follow-up. For the follow-up not needed category, we included recommendations such as immunizations which do not require a follow-up.
Patient effort to complete recommendations
For each of the 73 different recommendations, the three-physician panel came to a consensus about patient effort needed to initiate and complete the recommendation. For example, to adhere to a recommendation for a mammogram would require two steps, a communication (via phone or patient portal) to schedule the mammogram and then the actual visit to the imaging location. A recommendation for blood testing would also require at least two steps, a communication to set up the laboratory appointment, then the visit with a phlebotomist. A recommendation for a colonoscopy would require at least three effort components, a communication to schedule, a visit to a pharmacy to pick up the laxative prep, and a visit for the endoscopy itself.
For the patient effort, we only categorized the patient task of initiating and completing the recommendation. Although there can be patient effort associated with follow-up of the recommendation, this follow-up can be highly variable, so we did not attempt to categorize patient work or inconvenience beyond the initiation and completion of the recommendation. For example, follow-up of an unacceptable hemoglobin A1c level may not only require a visit to discuss changes in therapy, but also trips to the pharmacy and additional trips to a diabetes specialist. We assigned the patient tasks involved in initiating the recommendation into the following eight categories: communication (to schedule), documents (e.g. patient filling out a depression or asthma questionnaire), 15-min office visit, 30-min office visit, lab visit, pharmacy visit, procedure (radiology, endoscopy, etc.), and specialist visit. Patient effort most often involved more than one of these categories. As noted above, a colonoscopy would involve three patient effort categories: (1) communication (to schedule), (2) pharmacy (laxative prep), and (3) procedure (colonoscopy). Almost all the recommendations could involve a patient communication effort to schedule what was needed.
Within our categories, there may be some wide differences in patient effort, but we did not classify this effort to a more granular level. For example, within the procedures category, a procedure like a screening mammogram will take less patient effort than a colonoscopy that involves a laxative prep, taking some time off work, and arranging transportation to return home while the effects of sedation wear off.
Analysis and statistics
We used JMP 13.0 (SAS Institute, Cary, NC) for descriptive statistics used in the study.
Results
The initial dataset contained 30,895 patients from 53 providers (44 physicians and 9 APPs). After excluding 2140 patients who did not give research authorization and excluding 13 patients under age 18 years or with missing age data, the final dataset contained 28,742 unique patients. Table 2 shows the demographics of the patient population for analysis.
Table 2.
Demographics of study primary care population (n = 28,742).
| Demographic | Female, % (count); n = 16,735 | Male, % (count); n = 12,007 | Total population, % (count); n = 28,742 |
|---|---|---|---|
| Age group (years) | |||
| 18–34 | 13 (2174) | 10.5 (1255) | 11.9 (3429) |
| 35–49 | 18.2 (3053) | 18.3 (2193) | 18.3 (5246) |
| 50–64 | 28.6 (4787) | 29.8 (3577) | 29.1 (8364) |
| 65–79 | 26.4 (4420) | 28 (3366) | 27.1 (7786) |
| 80 and older | 13.7 (2301) | 13.5 (1616) | 13.6 (3917) |
| Sex | 58 (16,735) | 42 (12,007) | 100 (28,742) |
| Race | |||
| White | 88.4 (14,795) | 88.6 (10,638) | 88.5 (25,433) |
| Diagnoses | |||
| Diabetes | 9.8 (1634) | 15.2 (1824) | 12 (3458) |
| Hypertension | 37.3 (6245) | 42.8 (5138) | 39.6 (11,383) |
| Coronary artery disease | 7 (1167) | 17.7 (2121) | 11.4 (3288) |
| Atrial fibrillation | 9.8 (1634) | 15.2 (1824) | 12 (3458) |
| Congestive heart failure | 3.9 (659) | 5.9 (706) | 4.7 (1365) |
| COPD | 2.7 (460) | 4.3 (515) | 3.4 (975) |
| Risk factors | |||
| BMI of 25–30 | 28.6 (4793) | 37 (4446) | 32.1 (9239) |
| BMI ⩾ 30 | 33.8 (5650) | 36.1 (4332) | 34.7 (9982) |
| BMI > 40 | 7.1 (1188) | 4.5 (542) | 6 (1730) |
| Current tobacco use | 6.7 (1123) | 10.6 (1271) | 8.3 (2394) |
| Colon cancer FH | 3.8 (640) | 3.2 (386) | 3.6 (1026) |
| ASCVD 10 year risk ⩾ 7.5% | 18.5 (3102) | 31.8 (3816) | 24.1 (6918) |
COPD: chronic obstructive pulmonary disease; BMI: body mass index; FH: family history; ASCVD: atherosclerotic coronary vascular disease.
For the 28,742 patients, there were 127,273 uncompleted recommendations identified for population health management. There was a mean of 4.36 recommendations per patient with median of 4, interquartile range (25%–75%) of 2–6, standard deviation (SD) of 2.65, and range of 0–17 recommendations per patient. Figure 1 shows the histogram of the total recommendations per patient. Of the 28,742 patients, there were only 711 (2.5%) who had no recommendations captured by GDMS.
Figure 1.
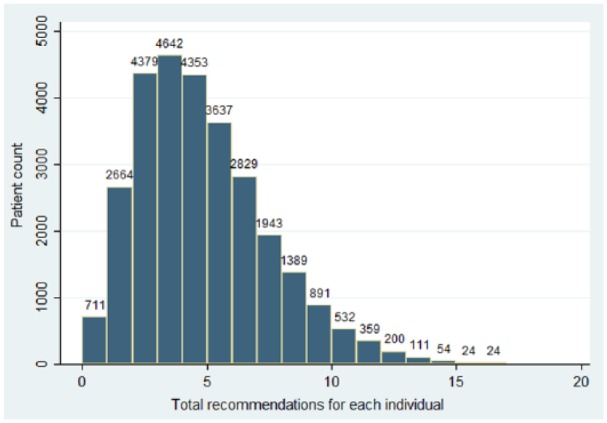
Histogram of total recommendations per patient.
There were 24,445 of the 28,742 (85%) with at least one of 108 diagnoses collected in GDMS that are used to generate recommendations. Only 4297 (15%) did not have at least one of the 108 diagnoses that were used to prompt a recommendation for a needed task.
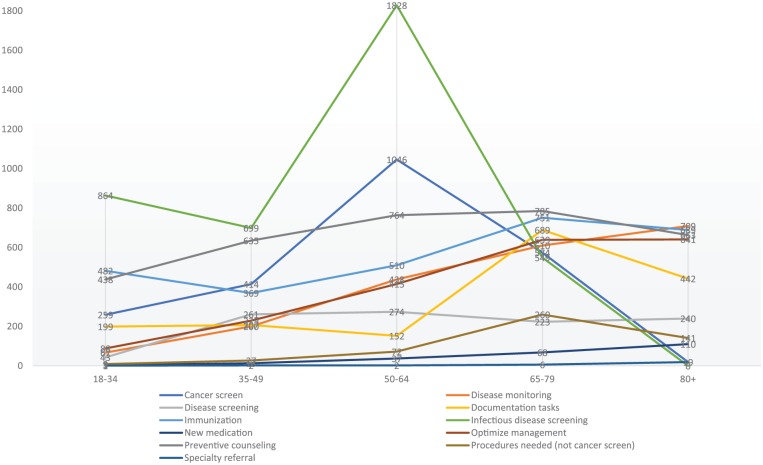
Table 3 shows the overdue recommendations per 1000 patients by recommendation category type and age group. Infectious disease screening had a large number of overdue recommendations in the 50–64 years age group because of age-related guidelines for HCV testing. Cancer screening in the 18–34 years age group was almost exclusively cervical cancer screening (see also Table 4). Immunizations are a prominent recommendation across age groups because of human papilloma virus (HPV) vaccine recommendations for younger ages and herpes zoster vaccination recommendations for older ages. Preventive counseling is primarily for obesity which involves all age groups. Documentation recommendations peak at age of 65–79 years to account for advanced directive recommendations in ages above 65 years. Infectious disease screening peaks at the 50- to 64-year-old group because of HCV and HIV screening recommendations in this age group.
Table 3.
Uncompleted healthcare recommendations per 1000 patients by age group to address all 73 recommendations.
| Recommendation category | Age groups (counts per 1000 patients), years |
All age groups | ||||
|---|---|---|---|---|---|---|
| 18–34 | 35–49 | 50–64 | 65–79 | 80+ | ||
| Cancer screening | 259 | 414 | 1046 | 574 | 19 | 569 |
| Disease monitoring | 67 | 200 | 438 | 610 | 709 | 434 |
| Disease screening | 43 | 261 | 274 | 223 | 240 | 226 |
| Documentation tasks | 199 | 207 | 152 | 689 | 442 | 353 |
| Immunization | 482 | 369 | 510 | 751 | 689 | 571 |
| Infectious disease screening | 864 | 699 | 1828 | 549 | 0 | 912 |
| New medication | 3 | 13 | 37 | 68 | 110 | 47 |
| Optimize management | 88 | 229 | 415 | 639 | 641 | 433 |
| Preventive counseling | 438 | 635 | 764 | 785 | 663 | 693 |
| Procedures needed (not cancer screen) | 9 | 27 | 72 | 260 | 141 | 117 |
| Specialty referral | 1 | 2 | 2 | 6 | 19 | 5 |
| Total | 2453 | 3056 | 5537 | 5156 | 3672 | 4359 |
Table 4.
Uncompleted healthcare recommendations per 1000 patients by age group and sex.
| Recommendation category | Age groups (counts per 1000 patients), years |
All age groups |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18–34 |
35–49 |
50–64 |
65–79 |
80+ |
||||||||
| Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | |
| Cancer screening | 407 | 3 | 691 | 27 | 1023 | 1076 | 627 | 506 | 15 | 24 | 639 | 471 |
| Disease monitoring | 48 | 100 | 172 | 238 | 409 | 477 | 594 | 631 | 731 | 678 | 412 | 464 |
| Disease screening | 22 | 80 | 131 | 442 | 262 | 289 | 213 | 237 | 251 | 223 | 192 | 272 |
| Documentation tasks | 205 | 190 | 236 | 167 | 177 | 119 | 690 | 687 | 428 | 462 | 361 | 340 |
| Immunization | 445 | 546 | 328 | 427 | 468 | 565 | 724 | 787 | 734 | 624 | 544 | 608 |
| Infectious disease screening | 850 | 888 | 589 | 853 | 1820 | 1840 | 552 | 545 | 0 | 0 | 884 | 950 |
| New medication | 1 | 5 | 6 | 23 | 24 | 55 | 51 | 90 | 78 | 155 | 32 | 67 |
| Optimize management | 68 | 122 | 169 | 312 | 353 | 497 | 617 | 668 | 648 | 631 | 393 | 490 |
| Preventive counseling | 385 | 530 | 563 | 736 | 682 | 872 | 716 | 876 | 595 | 759 | 619 | 798 |
| Procedures needed (not cancer screen) | 7 | 13 | 20 | 38 | 64 | 83 | 259 | 262 | 164 | 108 | 114 | 121 |
| Specialty referral | 0 | 2 | 1 | 3 | 1 | 3 | 5 | 8 | 10 | 33 | 3 | 8 |
| Total | 2438 | 2479 | 2906 | 3266 | 5283 | 5876 | 5048 | 5297 | 3654 | 3697 | 4193 | 4589 |
Figure 2 is a graph of the data in Table 3 to provide a visual representation of the change in recommendations between age categories.
Figure 2.
Graph of recommendations per 1000 in each category by age group.
Table 4 shows the uncompleted healthcare recommendations by sex. There are major differences between male and female uncompleted recommendations in cancer screening. This is mostly due to cervical cancer screening for women. Except for the recommendation categories of cancer screening and documentation tasks, males tend to have more uncompleted recommendations across age groups. Over all age groups, men have about 10% more uncompleted recommendations than women.
Table 5 categorizes the staff level for initiating the uncompleted recommendations. Physicians or advanced practice providers are needed for ordering colon screening (shared decision-making about colonoscopy vs Cologuard®) and for shared decision-making with prostate cancer screening. Mammography is often an accepted screening practice that requires little explanation so could be done with an automated process. We categorized some infectious disease screening to involve nursing input because of potentially sensitive screening discussions around sexually transmitted infections.
Table 5.
Counts of recommendation initiations needed by training level (per 1000 patients).
| Task description | Automated ordera | Clinician | Nurse | Total |
|---|---|---|---|---|
| Cancer screening | 280 | 289 | 0 | 569 |
| Disease monitoring | 431 | 0 | 3 | 434 |
| Disease screening | 226 | 0 | 0 | 226 |
| Documentation tasks | 0 | 202 | 151 | 353 |
| Immunization | 422 | 0 | 149 | 571 |
| Infectious disease screening | 424 | 0 | 488 | 912 |
| New medication | 0 | 34 | 13 | 47 |
| Optimize managementb | 210 | 0 | 223 | 433 |
| Preventive counseling | 0 | 693 | 0 | 693 |
| Procedures needed (not cancer screen) | 99 | 1 | 16 | 117 |
| Specialty referral | 0 | 5 | 0 | 5 |
| Total | 2092 | 1224 | 1042 | 4359 |
Automated process such as computer-generated order and portal message to the patient.
Optimize chronic disease management (e.g. hemoglobin A1c above goal or blood pressure above goal requiring action, but could potentially be directed by a nurse if a specific protocol available).
Table 6 shows the staff level needed for follow-up. Follow-up was not needed by the primary care team in 1513 of 4539 tasks (35%). This included tasks such as immunizations and mammograms. Mammograms were coded as not requiring follow-up by primary care as the department of radiology is required to follow-up on all mammograms. Mammograms comprised 4% of the total. Physicians/NPs/PAs were needed to do the initial follow-up about 11% of the time. The procedures requiring follow-up with this higher training level include procedure results such as liver ultrasounds for hepatocellular carcinoma screening.
Table 6.
Counts of recommendation follow-ups by staff training level (per 1000 patients).
| Task description | Follow-up not needed | Clinician | Radiology follow-up | Nurse follow-up or triage | Total |
|---|---|---|---|---|---|
| Cancer screening | 0 | 297 | 169 | 102 | 569 |
| Disease monitoring | 0 | 0 | 0 | 434 | 434 |
| Disease screening | 0 | 0 | 0 | 226 | 226 |
| Documentation tasks | 202 | 0 | 0 | 151 | 353 |
| Immunization | 571 | 0 | 0 | 0 | 571 |
| Infectious disease screening | 0 | 0 | 0 | 912 | 912 |
| New medication | 47 | 0 | 0 | 0 | 47 |
| Optimize managementa | 0 | 77 | 0 | 356 | 433 |
| Preventive counseling | 693 | 0 | 0 | 0 | 693 |
| Procedures needed (not cancer screen) | 0 | 100 | 0 | 16 | 117 |
| Specialty referral | 0 | 5 | 0 | 0 | 5 |
| Total | 1513 | 480 | 169 | 2197 | 4359 |
Optimize chronic disease management (e.g. hemoglobin A1c above goal or blood pressure above goal requiring action, but could potentially be directed by a nurse if a specific protocol available).
Over 50% of the follow-up could be at least partially done by nursing staff. Nursing staff can triage laboratory results and some imaging and procedures. If, upon triaging, the results are found to be abnormal, nursing staff would then refer the results to a physician/NP/PA. Thus, out of the 50% of follow-up listed as being performed by nursing staff, some of this follow-up would be triaged by nursing staff and ultimately passed on to a clinician.
Table 7 categorizes the patient effort of completing the recommendations. Patients often must do more than one task to complete a recommendation, so the total counts per 1000 do not add up to 4359 as in the task initiation and follow-up (Tables 3–5). Patients almost always require a telephone call or some other communication to schedule appointments in addition to the appointment itself. The scheduling communication occurs whether the recommendation is for lab visits, procedures, or face-to-face visits. Of the 9379 tasks per 1000 patients, 4147 of them were communication tasks to schedule appointments. With communication removed from these counts, the patient effort would be comprised as follows: 37% lab visits, 9% procedure visits, 31% as 30-min visits, 11% as 15-min visits, 7% filling out documents, and 5% pharmacy visits.
Table 7.
Patient effort and responsibilities required per 1000 patients to address initiation of all recommendations.
| Task category | Communication | Documents | 15-min visits | 30-min visits | Lab tests | Pharmacy visit | Procedures | Specialist visits | Total |
|---|---|---|---|---|---|---|---|---|---|
| Cancer screening | 569 | 0 | 0 | 221 | 120 | 169 | 347 | 0 | 1427 |
| Disease monitoring | 434 | 0 | 0 | 0 | 434 | 0 | 0 | 0 | 868 |
| Disease screening | 226 | 0 | 0 | 0 | 226 | 0 | 0 | 0 | 451 |
| Documentation tasks | 151 | 353 | 0 | 202 | 0 | 0 | 0 | 0 | 705 |
| Immunization | 561 | 0 | 571 | 0 | 0 | 0 | 0 | 0 | 1132 |
| Infectious disease screening | 912 | 0 | 0 | 0 | 912 | 0 | 0 | 0 | 1823 |
| New medication | 47 | 0 | 0 | 34 | 15 | 47 | 0 | 0 | 142 |
| Optimize managementa | 433 | 0 | 0 | 433 | 250 | 40 | 0 | 0 | 1156 |
| Preventive counseling | 693 | 0 | 0 | 693 | 0 | 0 | 0 | 0 | 1387 |
| Procedures needed (not cancer screen) | 117 | 0 | 0 | 38 | 0 | 0 | 117 | 0 | 272 |
| Specialty referral | 5 | 0 | 0 | 5 | 0 | 0 | 0 | 5 | 16 |
| Total | 4147 | 353 | 571 | 1628 | 1956 | 256 | 464 | 5 | 9379 |
Optimize chronic disease management (e.g. hemoglobin A1c above goal or blood pressure above goal requiring action, but could potentially be directed by a nurse if a specific protocol available).
Thirty-minute visits for cancer screening would be for Pap tests. The documentation 30-min visits would be for discussion and preparation of advance directive documentation. Optimize management 30-min visits would be for managing uncontrolled diabetes, hypertension, or hyperlipidemia.
Discussion
Providing comprehensive preventive services and chronic disease management in a primary care practice can create a large volume of tasks. Even with multiple different systems, care processes, and dedicated panel health managers working to address these recommendations, our practice had over 4300 incomplete recommendations per 1000 patients. This study shows that addressing these recommendations will likely require a new approach from the healthcare system, providers, and patients.
Recommendations were not specifically clustered in one recommendation category or age group. For different age groups, there was only a little over a twofold difference between the number of uncompleted recommendations per 1000 in the age group with the fewest uncompleted recommendations (2450 per 1000 patients aged 18–34 years) to the highest recommendation group (5540 per 1000 patients aged 50–64 years). For the youngest age group, HPV immunization, chlamydia, and gonorrhea screening along with Pap tests accounted for a large number of the recommendations. Cancer screening and infectious disease screening reached a peak in the 50–64 years age group.
Incomplete recommendations for immunizations were relatively stable across all age groups. Lack of HPV vaccine accounted for many incomplete vaccinations in the young group, and the older group had recommendations for herpes zoster vaccination, pneumococcal polysaccharide vaccine (PPSV23), and pneumococcal conjugate vaccine (PCV13). Preventive counseling to mitigate risk factors also was relatively stable across all age groups. This reflected some of the stability in obesity across age groups and continued recommendations for counseling about tobacco cessation and alcohol use across age groups.
Recommendations for disease monitoring and optimizing chronic disease management increased with age. This is consistent with the increasing prevalence of diabetes, hyperlipidemia, and hypertension with age. Recommendations for new medications and specialty referrals also increased with age, although these accounted for relatively few uncompleted recommendations overall.
For task initiation, which often involved ordering lab tests, procedures, or images, we found that 48% (2092 of the 4359 recommendations per 1000) could be handled using an automated process. However, there was no easily automated way to counsel for options concerning tobacco cessation, or for obesity treatment options, such as dietary approaches, weight loss medications, bariatric endoscopic procedures, or bariatric surgery.
Many incomplete recommended tasks could be completed by nursing staff without additional communication or follow-up. Immunizations, for example, can be ordered and given during a visit for another reason. Immunizations can also be scheduled and done with a nurse-only visit, independent of a clinician office visit.
Guidelines change over time and we see some of the results of this in our study. Associated with advances in the treatment of HIV and HCV, there has been a push for earlier identification of these treatable infectious diseases.17,18 Thus, screening for HIV and HCV involves a wide segment of the population. Screening recommendations includes screening for HCV in all individuals born from 1945 to 1965 and screening for HIV is recommended for all individuals from age 13 to 64 years.1,19 Other newer guidelines may have a similar impact of a surge in recommendations. For example, we have not yet incorporated lung cancer screening into GDMS recommendations.
Not only newer guidelines are increasing the volume of tasks recommended but also the complexity of the tasks involved with older recommendations is changing. For example, colon cancer and breast cancer screening options are now more complex with the addition of the stool DNA test (Cologuard™) and molecular breast imaging.20,21 With newer options available, the scope of practice required to engage in shared decision-making with patients may change as well. With the help of medical informatics, we are also able to identify smaller groups of patients needing specific attention. For example, GDMS can identify individuals with congestive heart failure with low ejection fraction and without a defibrillator implant, so these patients can be identified for discussion of a defibrillator. We are also able to identify subgroups of patients with specific nutritional or supplemental vitamin needs such as those with bacterial overgrowth, Crohn’s disease, and those who have had bariatric surgery.
This study should help other healthcare systems examine how to remove barriers to completing recommendations. For example, we found that many recommendations involved immunization and screening for infectious disease. At Mayo Clinic, patients must request online or call in to make appointments for vaccinations and lab testing. If recommendations were more easily available online and patients could make their own appointments for vaccinations and tests, this would eliminate the process of having telephone contact with appointment schedulers.
Many of these recommendations are also reportable quality measures. For example, proportions of patients who have had appropriate breast cancer, colon cancer, or cervical cancer screening are all quality measures that form a basis for comparison of different practices in Minnesota and nationally.22,23 The framework we have developed here could be used to prioritize each of the recommendations in the context of overall population health management and specific quality measures. Examining these in terms of the expertise needed for initiation and follow-up of recommendations could be a starting point for looking for more cost-effective processes to use to complete these tasks.
This study also shows some potential utility of using this information in scheduling face-to-face appointments. When patients call in for appointments, it would be useful to have the unfinished task information available to the appointment schedulers. For example, a patient calling in for a sore knee might also be asked if they wanted to update their vaccinations, and, if so, dedicated nursing time could be scheduled for that.
There were many primary care recommendations about behavioral health issues such as tobacco use, alcohol use, and eating behaviors. This study supports having strong behavioral health integration as a way to manage the behavioral health recommendations in a primary care population.
Our study demonstrates how these unfinished tasks affect more than just the healthcare system and its providers. Patients are very much affected as well. The results show that our population would have to make thousands of phone calls and thousands of visits to complete the incomplete medical recommendations. This represents potentially a large burden of time and money for these individuals, even if they have comprehensive healthcare insurance. Although we did not perform this in our study, our framework for examining these recommendations would allow a more robust cost estimate for the disease prevention and screening component of population health management.
This study is highly relevant to the emerging field of clinical informatics. To successfully manage recommendations there needs to be a technological way of capturing the different steps involved in task completion. For example, it may be that a provider has had discussions with a patient about colon cancer screening on multiple occasions, and each time the patient declined cancer screening. Information about patients declining recommendations needs to be easily searchable so that effort and resources can be allocated more effectively. Perhaps this information could be put in the form of a recommendation flowsheet where the process of recommendations, and their subsequent disposition could be tracked along a timeline. This flowsheet could note when the recommendation was initiated and the outcome in some categorical fashion. For certain recommendations, there could be a step care process involved. For example, a recommendation for mammography could start with a portal message. If there is no response, then there could be a telephone call from an appointment coordinator. If that is declined, then decision-making is shared with a nurse or clinician.
The study has several limitations. We did not take into consideration competing guidelines. For example, in our mammography recommendation, we use the age of 40 years as the starting point for initiating discussion about screening mammography and then repeating yearly. While this is consistent with some guidelines such as the American Cancer Society,24 the USPSTF25 recommends starting at age of 50 years and then repeating every 2 years. Thus, healthcare systems using different guidelines could have different results. Other healthcare systems may have greater or lesser abilities to develop this recommendation list based on the availability of diagnoses, demographics, social history, and so on. A comprehensive recommendations list may have hundreds of rules that depend on information throughout the medical record. Mayo’s GDMS currently identifies 108 separate diagnoses to use in its algorithms and considers social history (tobacco and alcohol), lab information, previous procedures accomplished, and various individual parameters (height, weight, and blood pressure). A complex rule-based system may not be not widely available to primary care practices.
Our analysis of the recommendation follow-ups (Table 5) has some limitations. For example, the nurses can triage results from various procedures, images, and lab tests. We found that this could be done in about 50% of the follow-ups. However, some of that follow-up would invariably go to the physician when the test results, procedures, or images were abnormal and needed further interpretation and patient education. It should be noted that in our practice, as well as others, patients have direct access to their lab test, image, and pathology results. Even with processes in place of having nurses triage and potentially explain these results, patients can still freely message their provider about their results through the portal, leading to another avenue for follow-up to be routed to the clinician. Thus, Table 5 is more a best case and likely underestimates the effort that eventually ends in the clinicians’ task list.
Our practice also has a relatively stable population with individuals having continuous care at Mayo Clinic for decades. As such, our software can capture the appropriate data required to generate the recommendations. For most patients in our primary care practice, we have EHR access to years of data including diagnoses, previous colonoscopy dates, pathology reports, cervical cancer screening tests, lab tests, and immunizations. Practices without computer searchable medical records over a period of years would have a difficult time replicating our ability to capture recommendations for an entire population.
We did not examine each recommendation in terms of the incomplete recommendations compared to the total recommendations. GDMS was designed to focus only on incomplete recommendations. However, looking at each recommendation by percent completed may give insights into processes and systems that could be used to achieve higher completion rates.
There are varying levels of evidence associated with recommendations. For example, the USPSTF recommendations are associated with 5 levels of evidence: A, B, C, D, and I. The Mayo GDMS algorithms that generated recommendations were based on national and international guidelines and approved by panels of Mayo Clinic physicians. GDMS did not deliver explanatory information for providers or for patients that indicated the evidence basis for those recommendations. Thus, we were unable to determine whether an evidence basis for recommendations was used by providers or patients to help prioritize certain recommendations over others.
From a broad population perspective there are many incomplete recommendations. However, each individual recommendation is in the context of a specific patient. An individual patient within the population may have a terminal illness, a mental illness, multiple comorbidities, or economic and social challenges that could dwarf healthcare recommendations. Consequently, a recommendation designed for a whole population sometimes just does not apply to a specific patient.
This study gives a snapshot view of the tasks and staff needed to complete the healthcare recommendations for a primary care population. While we are making progress on the informatics challenges, each patient represents an individual challenge. Individuals have different priorities about their healthcare, and no matter what computer algorithms recommend, it comes down to individual decisions from patients and shared decisions with their physician or provider in the context of their healthcare goals and beliefs. Our study just examines the volume of recommendations involved in a primary care practice and attempts to examine the potential provider and patient efforts required to complete the recommendations. Further research will be needed to examine how to prioritize the tasks involved in the recommendations and how to develop processes for most cost-effective ways of addressing this burgeoning number of guideline-driven tasks.
Conclusion
Recommendations generated by guidelines will require a large effort from healthcare systems. Patients will also need a considerable effort to complete the recommendations. New healthcare system approaches are needed to address these large numbers of uncompleted recommendations.
Acknowledgments
FN contributed to study concept; FN, JCM, JP, and RC contributed to study design; FN, AMF, and SSJ contributed to data analysis and statistics; FN contributed to draft manuscript; FN, JP, SMT-S, JCM, RC, AMF, and SSJ contributed to final manuscript and approval.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from the Mayo Clinic Institutional Review Board (IRB 17-008632).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Mayo Clinic.
Informed consent: Informed consent waived by the Mayo Clinic Institutional Review Board (IRB 17-008632).
ORCID iDs: Frederick North  https://orcid.org/0000-0002-3696-4595
https://orcid.org/0000-0002-3696-4595
John C Matulis  https://orcid.org/0000-0001-9319-5457
https://orcid.org/0000-0001-9319-5457
Jennifer L Pecina  https://orcid.org/0000-0003-1970-1344
https://orcid.org/0000-0003-1970-1344
References
- 1. US Centers for Disease Control and Prevention (CDC). STD & HIV screening recommendations, https://www.cdc.gov/std/prevention/screeningreccs.htm (2017, accessed 9 April 2018).
- 2. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55: 1–17; quiz CE1–4. [PubMed] [Google Scholar]
- 3. Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep 2012; 61: 1–32. [PubMed] [Google Scholar]
- 4. Smith BD, Morgan RL, Beckett GA, et al. Hepatitis C virus testing of persons born during 1945–1965: recommendations from the centers for disease control and prevention. Ann Intern Med 2012; 157: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vaccine recommendations and guidelines of the ACIP, https://www.cdc.gov/vaccines/hcp/acip-recs/index.html (2018, accessed 25 March 2018).
- 6. US Preventive Services Task Force (USPSTF). Published recommendations, https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations (2017, accessed 25 March 2018).
- 7. Institute for Clinical Systems Improvement (ICSI). Endorsed guidelines, https://www.icsi.org/guidelines__more/endorsed_guidelines/ (2017, accessed 25 March 2018).
- 8. American Diabetes Association (ADA). Standards of medical care in diabetes—2018 abridged for primary care providers. Clin Diab 2017; 36: 14–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension 2018; 71: 1269–1324. [DOI] [PubMed] [Google Scholar]
- 10. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 11. Chaudhry R, Schietel SM, North F, et al. Improving rates of herpes zoster vaccination with a clinical decision support system in a primary care practice. J Eval Clin Pract 2013; 19: 263–266. [DOI] [PubMed] [Google Scholar]
- 12. Chaudhry R, Tulledge-Scheitel SM, Parks DA, et al. Use of a web-based clinical decision support system to improve abdominal aortic aneurysm screening in a primary care practice. J Eval Clin Pract 2012; 18: 666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kesman RL, Rahman AS, Lin EY, et al. Population informatics-based system to improve osteoporosis screening in women in a primary care practice. J Am Med Inform Assoc 2010; 17: 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scheitel MR, Kessler ME, Shellum JL, et al. Effect of a novel clinical decision support tool on the efficiency and accuracy of treatment recommendations for cholesterol management. Appl Clin Inform 2017; 8: 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marcelin JR, Tan EM, Marcelin A, et al. Assessment and improvement of HIV screening rates in a Midwest primary care practice using an electronic clinical decision support system: a quality improvement study. BMC Med Inform Decis 2016; 16: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. North F, Fox S, Chaudhry R. Clinician time used for decision making: a best case workflow study using cardiovascular risk assessments and Ask Mayo Expert algorithmic care process models. BMC Med Inform Decis 2016; 16: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chou R, Selph S, Dana T, et al. Screening for HIV: systematic review to update the 2005 US Preventive Services Task Force recommendation. Ann Intern Med 2012; 157: 706–718. [DOI] [PubMed] [Google Scholar]
- 18. Shiffman ML. Universal screening for chronic hepatitis C virus. Liver Int 2016; 36: 62–66. [DOI] [PubMed] [Google Scholar]
- 19. US Centers for Disease Control and Prevention (CDC). Testing recommendations for hepatitis C virus infection, https://www.cdc.gov/hepatitis/hcv/guidelinesc.htm (2015, accessed 9 April 2018).
- 20. El Zoghbi M, Cummings LC. New era of colorectal cancer screening. World J Gastrointest Endosc 2016; 8: 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shermis RB, Redfern RE, Burns J, et al. Molecular breast imaging in breast cancer screening and problem solving. Radiographics 2017; 37: 1309–1606. [DOI] [PubMed] [Google Scholar]
- 22. Minnesota HealthScores. http://www.mnhealthscores.org/all-measure-topics (2018, accessed 25 March 2018).
- 23. National Committee for Quality Assurance (NCQA). HEDIS 2018. measures, http://www.ncqa.org/hedis-quality-measurement/hedis-measures/hedis-2018; http://www.ncqa.org/Portals/0/HEDISQM/HEDIS2018/Summary%20of%20Changes%20for%20Physician%20Measurement%202018.pdf?ver=2017-12-15-070645-503 (accessed 25 March 2018).
- 24. American Cancer Society breast cancer screening guideline, https://www.cancer.org/latest-news/special-coverage/american-cancer-society-breast-cancer-screening-guidelines.html (2017, accessed 25 March 2018).
- 25. US Preventive Services Task Force (USPSTF). Recommendations—breast cancer: screening, https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1 (2016, accessed 25 March 2018).