Abstract
Development of an α-synuclein (α-Syn) positron emission tomography agent for the diagnosis and evaluation of Parkinson disease therapy is a key goal of neurodegenerative disease research. BF-227 has been described as an α-Syn binder and hence was employed as a lead to generate a library of α-Syn-binding compounds. [3H]BF-227 bound to α-Syn and amyloid β peptide (Aβ) fibrils with affinities (KD) of 46.0 nM and 15.7 nM, respectively. Affinities of BF-227-like compounds (expressed as Ki) for α-Syn and Aβ fibrils were determined, along with 5 reference compounds (flutafuranol, flutemetamol, florbetapir, BF-227, and PiB). Selectivity for α-Syn binding, defined as the Ki(Aβ)/Ki(α-Syn) ratio, was 0.23 for BF-227. A similar or lower ratio was measured for analogues decorated with alkyl or oxyethylene chains attached to the oxygen at the 6 position of BF-227, suggesting a lack of involvement of the side chain in fibril binding. BF-227-like iodobenzoxazoles had lower affinities and poor α-Syn selectivity. However, BF-227-like fluorobenzoxazoles had improved α-Syn selectively having Ki(Aβ)/Ki(α-Syn) ranging from 2.2 to 5.1 with appreciable fibril affinity, although not sufficient to warrant further investigation. Compounds based on fluorobenzoxazoles might offer an approach to obtaining an α-Syn imaging agent with an appropriate affinity and selectivity.
Keywords: α-synuclein, amyloid β peptide, binding affinity, fibrils, BF-227, PET
Introduction
Development of an α-synuclein (α-Syn) selective, positron emission tomography (PET) imaging agent for early diagnosis and evaluation of therapies in Parkinson disease is one of the most sought after goals in neurodegenerative disease research.1-3 Fluorine-18-labeled BF-227 (2-(2-[2-dimethylaminothiazol-5-yl]ethenyl)-6-(2-[fluoro]ethoxy)benzoxazole) was first used as a PET tracer for amyloid β peptide (Aβ) plaques, with a reported KD for synthetic Aβ fibrils of 4.1 nM.4 Later, it was determined that BF-227 was not selective and bound to both synthetic Aβ fibrils (KD = 1.31 nM) and synthetic α-Syn fibrils (KD = 9.3 nM).5 Histochemical analysis with a high concentration (100 μM) of BF-227 led to Lewy body fluorescence.5 However, subsequent studies indicated [18F]BF-227 failed to bind to α-Syn-positive, Aβ-negative dementia with Lewy body human brain homgenates and lacked a sufficient affinity or selectivity to serve as an α-Syn imaging agent.3 Since BF-227 has been described as having a high affinity for pathological forms of α-Syn, we were interested in using its scaffold as a lead molecule to create a focused library of BF-227-like compounds with the following objectives: (1) to further characterize the interaction of BF-227 with α-Syn and Aβ fibrils and (2) to identify compounds with improved binding affinity and selectivity for α-Syn fibrils. The affinities of the BF-227-like compounds and reference compounds were determined for reconstituted α-Syn and Aβ fibril preparations. Reference compounds were 5 compounds used clinically to image Aβ (flutafuranol, flutemetamol, florbetapir, BF-227, and PiB), Thioflavin S, benzotriazole-1 (BTA), 1,4-bis(paminostyryl)-2-methoxy benzene (BMB)-1, Clorglyine, and RO-16-6491. The rationale for reference compound selection is given subsequently.3
Materials and Methods
Compound Sources
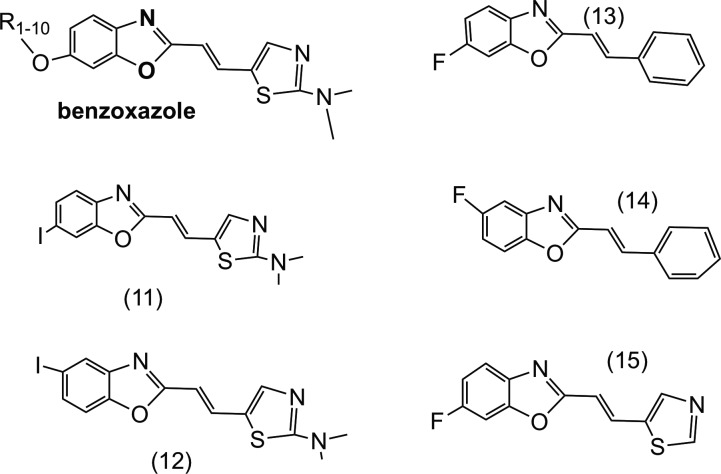
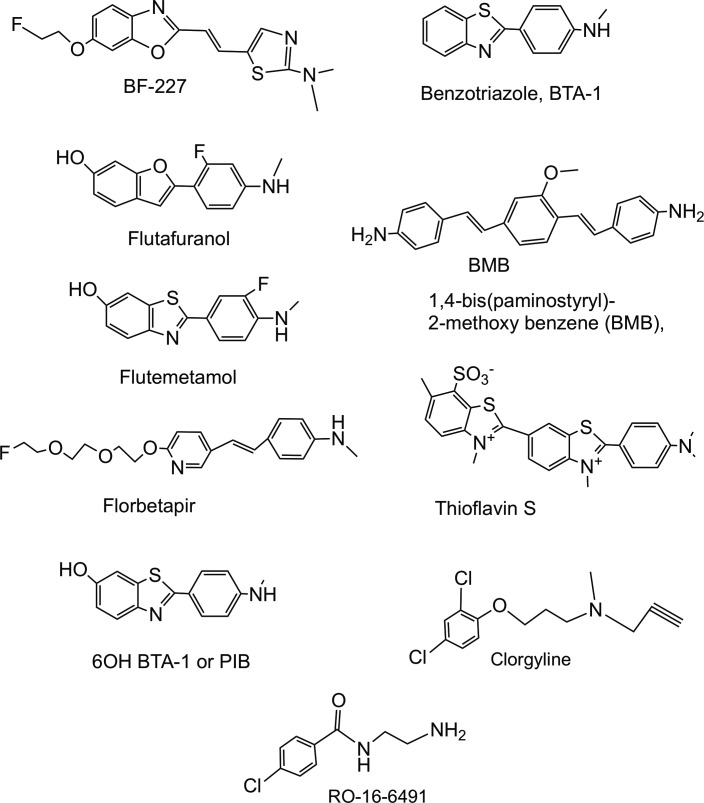
BF-227-like compounds (Figure 1) were provided by MedChem Imaging LLC (Boston, Massachusetts). Reference compounds (Figure 2) obtained from Sigma-Aldrich (St. Louis, Missouri) were BTA-1, BMB, clorgyline, and Thioflavin S; from WuXi Pharma Tech (Shanghai, China) were BF-227, florbetapir (AV-45), and flutafuranol (NAV 4694); from ABX GmbH (Radeberg, Germany) 6-OH-BTA1 (PiB); from Santa Cruz Biotechnology (Dallas, Texas) RO-16-6491; from GE Healthcare (Oslo, Norway) flutemetamol. Radiolabeled [3H]BF-227 (66 Ci/mmole; 1 mCi/mL) was from Vitrax Radiochemicals (Placenta, California). Compound structures are provided (Figures 1 and 2) with molecular properties (MW, tPSA, LogP, CLogP) determined using Chemdraw 16.01.04 (Tables 1 and 2).
Figure 1.
Structure of BF-227-like compounds. Structures 1-10 are benzoxazole derivatives with R, a fluoroethyl group, with varying length hydrocarbon chains or oxyethylene groups appended (see Table 1 for individual compound designations). The structure of compound (1) BF-227 is shown in Figure 2.
Figure 2.
Structures of reference compounds.
Table 1.
BF-227-Like Compound Affinity (Ki, nM) for α-Synuclein and Amyloid β Fibrils.
| # | R (Side Chain) | MW | n | α-Syn Ki | AβKi | Ki(Aβ)/Ki(α-Syn) | Log P | tPSA | CLogP |
|---|---|---|---|---|---|---|---|---|---|
| 1 | FCH2 (CH2)O- | 333.38 | 6 | 53 (46; 60) | 12 (11; 13) | 0.23 | 2.94 | 46.42 | 2.59 |
| 2 | FCH2 (CH2)2O- | 347.41 | 3 | 157 (126; 188) | 9.01 (3; 14) | 0.06 | 3.05 | 46.42 | 2.82 |
| 3 | FCH2 (CH2)3O- | 361.44 | 3 | 378 (306; 451) | 31 (25; 36) | 0.08 | 3.50 | 46.62 | 3.20 |
| 4 | FCH2 (CH2)4O- | 375.46 | 3 | 297 (222; 371) | 28 (24; 31) | 0.09 | 3.92 | 46.42 | 3.73 |
| 5 | FCH2 (CH2)5O- | 389.49 | 3 | 462 (439; 486) | 23 (20; 27) | 0.05 | 4.34 | 46.42 | 3.79 |
| 6 | FCH2 (CH2)6O- | 403.52 | 3 | 212 (163; 260) | 27 (25; 29) | 0.13 | 4.75 | 46.42 | 4.79 |
| 7 | FCH2[O-CH2-CH2]O- | 377.43 | 3 | 83 (79; 88) | 11 (10; 12) | 0.13 | 2.79 | 55.65 | 2.34 |
| 8 | FCH2[O-CH2-CH2]2O | 421.49 | 3 | 52 (33; 73) | 13 (12; 15) | 0.25 | 2.63 | 64.88 | 2.20 |
| 9 | FCH2[O-CH2-CH2]3O- | 465.54 | 3 | 100 (84; 117) | 26 (20; 31) | 0.26 | 2.47 | 74.11 | 2.07 |
| 10 | FCH2[O-CH2-CH2]4O- | 509.59 | 3 | 75 (59; 92) | 18 (16; 20) | 0.24 | 2.32 | 83.34 | 1.94 |
| Iodo benzoxazoles | |||||||||
| 11 | Not applicable | 397.23 | 3 | 74 (382; 1110) | 223 (188; 259) | 0.30 | 4.23 | 37.19 | 3.54 |
| 12 | Not applicable | 397.23 | 3 | 276 (127; 426) | 96 (94; 97) | 0.35 | 4.23 | 37.19 | 3.54 |
| Fluoro benzoxazoles | |||||||||
| 13 | Not applicable | 239.25 | 3 | 328 (251; 405) | 1247 (1079; 1414) | 3.8 | 4.10 | 21.59 | 4.25 |
| 14 | Not applicable | 239.25 | 3 | 238 (178; 298) | 528 (486; 570) | 2.2 | 4.10 | 21.59 | 4.25 |
| 15 | Not applicable | 246.26 | 3 | 286 (255; 316) | 1446 (1065; 1826) | 5.1 | 2.34 | 33.95 | 2.40 |
Table 2.
Reference Compound Affinity (Ki in nM) for α-Synuclein and Amyloid β Fibrils.
| Compound | MW | n | α-SynKi | Aβ Ki | Ki (Aβ)/Ki (α-Syn) | Log P | tPSA | CLogP |
|---|---|---|---|---|---|---|---|---|
| BF-227 | 333.38 | 6 | 53 (46; 60) | 12 (11; 13) | 0.23 | 2.94 | 46.42 | 2.59 |
| Flutafuranol (NAV 4694) | 258.25 | 5 | 32 (27; 38) | 42 (34; 50) | 1.34 | 2.82 | 53.85 | 2.28 |
| Flutemetamol | 274.31 | 3 | 48 (44; 53) | 65 (60; 70) | 1.35 | 3.44 | 44.62 | 2.96 |
| Florbetapir (AV-45) | 360.43 | 5 | 24 (19; 29) | 12 (9; 15) | 0.46 | 2.85 | 52.1 | 3.02 |
| PiB | 256.32 | 6 | 55 (51; 59) | 77 (68; 87) | 1.4 | 3.28 | 44.65 | 2.82 |
| BTA-1 | 240.32 | 8 | 64 (61; 67) | 146 (129; 163) | 2.3 | 3.67 | 24.39 | 3.48 |
| BMB | 342.44 | 6 | 184 (109; 258) | 76 (62; 91) | 0.35 | 4.68 | 61.27 | 4.99 |
| Thioflavin S | 510.66 | 3 | >9000 | 2150 (1865; 2435) | ≤0.2 | N.O. | N.O. | N.O. |
| Clorgyline | 272.17 | 3 | >9000 | >5000 | NO | 3.35 | 12.7 | 4.31 |
| RO-16-6491 | 198.65 | 3 | >9000 | >7000 | NO | 0.85 | 55.12 | 1.09 |
| PET agent training set, mean (SD) | 5 | 3.03 (0.28) | 53.1 (11.2) | 2.77 (0.26) |
Abbreviations: BMB, 1,4-bis(paminostyryl)-2-methoxy benzene; BTA, benzotriazole; PET, positron emission tomography; SD, standard deviation.
Preparation of Aβ Fibrils
Aβ1-42 peptide 5 mg, Cat #20276-5 (Anaspec, Fremont, California) was dissolved in 250 µL dimethyl sulfoxide (DMSO) for 2 hours with occasional swirling, followed by water bath sonication, (B-200 Branson [Danbury, Connecticut]) for 5 minutes. The clear peptide solution was transferred to a 15 mL conical tube. (Cat #430052, Corning [from Sigma-Aldrich]), and the original vial was washed with 625 µL of double deionized Milli-Q-H2O, which was then combined with 250 µL peptide/DMSO solution in the conical tube. Subsequently, 4 mL of Milli-Q-H2O was added to the conical tube followed by the addition of 125 µL of 1 M Tris–HCl, pH 7.5 with gentle mixing of the solution. The final volume was 5 mL, with the starting peptide concentration at 1 mg/mL. The peptide solution was divided into 5 × 1 mL aliquots with 1.5 mL Eppendorf tubes and incubated for 72 hours at 37°C with shaking at 1000 rpm in an Eppendorf Thermomixer. Fibril formation was confirmed by visual inspection for turbidity of the solution and further confirmation with Thioflavin T fluorescence spectroscopy (Sigma-Aldrich, Cat#T3516). Centrifugation at 15 000g for 15 minutes was performed to pellet fibrils, and the supernatant was assessed for protein, which was minimal by A280 or the BCA protein assay. The supernatant was discarded, and the pelleted fibrils were resuspended (1 mg/pellet) with phosphate-buffered saline (PBS; pH7.4) to obtain a stock concentration of 444 µM (expressed as a monomer equivalent). Fibril stock solutions were stored at −80°C.
Preparation of α-Syn Fibrils
Human full-length α-Syn (NM_000345) was cloned into the ampicillin-resistant Escherichia coli expression vector PET7-7 and transformed into BL21 (D3) strains for expression. The recombinant α-Syn was expressed and purified to ≥95% purity as described previously.6 The monomeric α-Syn was formulated in 10 mM Tris–HCl, pH 7.6, 50 mM NaCl at 5 mg/mL. For fibril formation, 500 µL of purified α-Syn monomer was subjected to continuous shaking at 1000 rpm in an Eppendorf Thermomixer at 37°C for 7 days. The monomer was removed from fibrils by high-speed centrifugation with a Beckman-Coulter tabletop Optima MAX-XP (Beckman-Coulter, Indianapolis, Indiana) ultra-centrifuge at 600 000g for 40 minutes, followed by 3 PBS/centrifugation wash steps. After each centrifugation step, the supernatant was removed and the concentration of α-Syn in the supernatant was calculated from absorbance at 280 nm. The pellet was resuspended in PBS, and the concentration of fibrils was estimated by subtracting the total amount of α-Syn in the supernatant from all the wash steps and confirmed by BCA assay before use.
Binding Studies With [3H]BF-227
Saturation binding of [3H]BF-227 was determined with increasing concentrations (1-400 nM) of radioligand and nonspecific binding obtained with 10 µM BTA-1, a reference agent structurally similar to imaging agent PiB. Competition studies were carried out using 5 nM [3H]BF-227. Experiments were performed in 50 mM Tris–HCl pH 7.5, 150 mM NaCl, and 0.1% bovine serum albumin in a reaction volume of 200 µL. Incubations were initiated with the addition of human α-Syn (0.5 mM/well) or Aβ1-42 (0.1 μM/well) fibrils at room temperature and terminated 2 hours later by rapid vacuum filtration over Whatman GF/C 96-well Unifilters (Brandel [Gaithersburg, Maryland]) presoaked in cold wash buffer (50 mM Tris–HCl, pH7.4), followed by four 200 µL washes with cold wash buffer. Filters containing bound ligand were mixed with 50 µL Microscint-PS (Perkin-Elmer [Waltham, Massachusetts]) and counted with a MicroBeta2 Scintillation Counter (Perkin Elmer). All data points were performed in triplicate. Values for the saturation binding dissociation constant (KD) and the maximal number of binding sites (Bmax) were determined by fitting the data to the equation Y = Bmax × X/(X + KD) using nonlinear regression analysis. IC50 values were generated from the concentration–response curves using nonlinear regression analysis and converted to Ki values with the Cheng-Prusoff equation.7 Data analysis was performed with GraphPad Prism version 7.02.
Results
Figure 1 and Table 1 show the structures of BF-227 and the BF-227-like compounds that were synthesized. For the BF-227 scaffold, the R group attached to the oxygen at the 6 position of the benzoxazole group was varied by increasing hydrocarbon chain length (#2 through #6, see Table 1) and by increasing the number of oxyethylene groups (#7 through #10, see Table 1). Two iodobenzoxazoles (#11, #12) and 3 fluorobenzoxazoles (#13, #14, #15), all lacking side chains, were also synthesized (Figure 1, Table 1). The benzoxazole ring of BF-227 and BF-227-like compounds is shown in bold.
The reference compounds used are shown in Figure 2. 18F or 11C isotopologues of 5 compounds (flutafuranol, flutemetamol, florbetapir, BF-227, and PiB) have been used to image Aβ by PET. While [11C]PiB and the Food and Drug Administration–approved [18F] versions of florbetapir, florbetaben, and flutemetamol have become important methods of analyzing neurodegenerative disease, these compounds have limitations because of a lack of correlation between amyloid deposition and disease stage and an inability to image nonfibrillar Aβ-plaques.8 Our reference thioflavin S is widely used as a histological stain for amyloid, fluorescing when bound to diverse types of fibrils including those of Aβ,9 α-Syn,10 and tau.11 Benzotriazole 1 is an uncharged compound structurally related to thioflavin used in the development of Pittsburgh compound B (PiB).3 A 11C isotopologue of BMB-1, a Congo red-like compound, has been used to image mylein basic protein.12 Clorglyine and RO-16-6491 are inhibitors of monamine oxidase A and monamine oxidase B, respectively, and were included because imaging agents designed to bind tau can bind to these targets.13-15
Characterization of [3H]BF-227 Binding to α-Syn and Aβ Fibrils
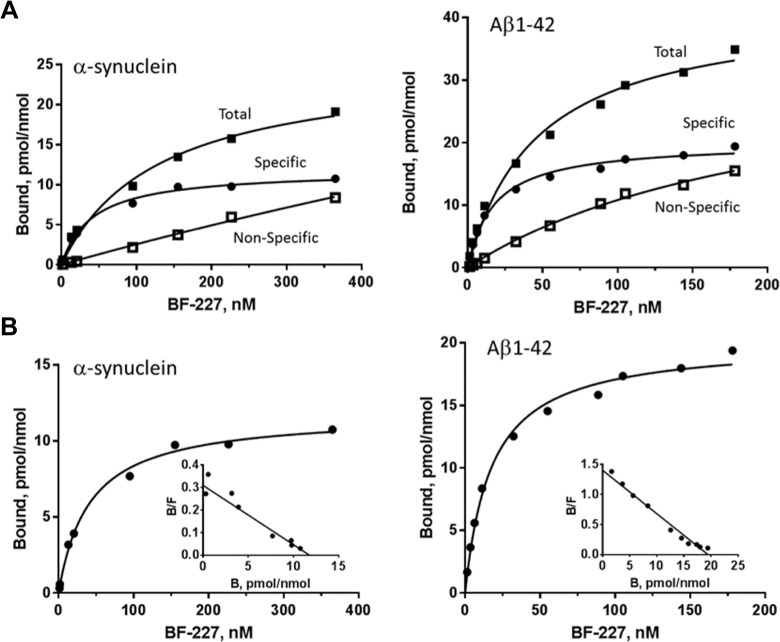
The KD values for the binding of [3H]BF-227 to α-Syn and Aβ fibrils were determined using filtration binding studies (Figure 3). The concentration dependence of specific and nonspecific binding to α-Syn and Aβ fibrils is shown in panel A. Nonspecific binding was defined by incubation with 10 μM BTA-1 and increased linearly with increasing [3H]BF-227 concentrations. Specific binding of [3H]BF-227 (specific = total − nonspecific) was fit to a single-site binding model using saturation and Scatchard plots (inset) to determine the binding affinity, KD (Figure 3B). Individual KD and Bmax values are provided in Table S1 of the supplement. For α-Syn fibrils, a KD of 46.0 (2.8) nM and a Bmax of 12.7 (1.1) pmole/nmole was obtained and is expressed as the mean and standard deviation (within the parentheses), n=2. For Aβ fibrils, a KD of 15.7 (3.1) nM and a Bmax of 19.4 (2.7) pmol/nmol was obtained (mean [SD], n = 3) .
Figure 3.
Binding of [3H]BF-227 to α-synuclein and amyloid β fibrils. A, The concentration dependence of [3H]BF-227 binding is shown, where specific = total − nonspecific binding. B, Binding isotherms and Scatchard plots from (A) are shown. Data were fit to a single-site binding model. Y-axis is pmoles tritiated tracer divided nmoles fibril (expressed as monomer).
Affinities of BF-227-Like Compounds for α-Syn and Aβ1-42 Fibrils
Table 1 shows binding affinties (Ki in nM) to α-Syn and Aβ fibrils for the 15 benzoxazole compounds shown in Figure 1. Results are expressed as inhibition constants (Ki, nM) corrected for ligand occupancy.7 Each value is a geometric mean from the indicated numbers of experiments (n), with the numbers in parentheses indicating the low and high errors of the geometric mean. Also shown are the Log P, tPSA, and CLogP values for these compounds. These can be compared with means for the 5 Aβ imaging agents which can serve as an empirical training set to judge the likely in vivo behavior of new compounds.
Affinities of Reference Compounds for α-Syn and Aβ Fibrils
Table 2 gives the affinties to α-Syn and Aβ fibrils for the reference compounds (Figure 2), along with their values of MW, logP, tPSA, and cLogP. Isotopologues of BF-227, flutafuranol, flumetamol, florbetapir, and PiB have been used to image Aβ by PET. All 5 bound both α-Syn and Aβ fibrils, with 2 (BF-227 and Florbetapir) exhibitng a preference for Aβ over α-Syn, with a values of 0.23 and 0.46 for the ratio of their Ki’s (Ki[Aβ]/Ki[α-Syn]). Flutafuranol, flutemetamol, and PiB have 6-OH benzothiazoles motifs, and these had similar affinity and selectivity for Aβ fibrils and α-Syn fibrils. Florbetapir, a compound with a stilbene motif, also bound both types of fibrils.
A 11C isotopologue of BMB has been used to image myelin basic protein by PET,12 with further use as an intraoperative fluorescent agent for highlighting of nerves.16
The 5 Aβ imaging compounds have a narrow range of physical propertes (MW’s, LogP, tPSA, and cLogP) as indicated by their means, standard deviations, and coefficients of variation (SD/mean) tabulated at the bottom of Table 2.
Discussion
For the series of BF-227-like compounds (Figure 1), increasing the length of R, either as the length of hydrocarbon chain (#2 through #6) or as the number of oxyethylene groups (#7 through #10), produced minimal increases in Ki and minimal effects on selectivity (Ki α-Syn/ Ki Aβ). The minimal effect of R size on Ki is consistent with a model where the 2 rings of BF-227 (and our BF-227-like compounds) bind in a pocket of a β-sheet-based fibril, with the side chain relatively uninvolved.17-19 However, molecular docking studies have concluded that BF-227 can bind to a core binding site with an Aβ fibril.20
Our data indicate the difficulty in obtaining a highly selective α-Syn imaging agent binding to the common β-sheet structure of α-Syn and Aβ fibrils. First, all 5 of the reference PET imaging agents bound both Aβ and α-Syn fibrils, with higher affinity for Aβ fibrils. Second, BMB, which was developed as an imaging agent for mylein basic protein, had a Ki of 76 nM for Aβ fibrils which was not significantly different from the Aβ imaging agent PiB (Ki of 77 nM). In addition, a large literature with fluorescent and birefringent probes like thioflavin S, thioflavin T, and Congo red indicates they bind to the β-sheet motifs contained within many different proteins.
Removal of the 6-OH group on the benzoxazole ring and replacement with an iodine or fluorine (Table 1) reduced the affinity of BF-227 derivatives for both α-Syn and Aβ fibrils. None of the BF-227-like compounds had suffient α-Syn selectivity to be used for an α-Syn imaging agent. However, fluorine-bearing compounds # 13, 14, and 15 showed a modest improvement in α-Syn selectivity, most notably #15 which had considerable (5.1-fold) preference for α-Syn fibrils. A fluorobenzoxazole fragment might be employed in the design of future focused libraries employing fluorobenzoxazole fragments produced with the goals of obtaining a more α-Syn selective compound with affinities and molecular properties similar to existing clinical imaging agents.21-22 An advantage of future imaging agents using the fluorobenzoxazole fragment is the possiblilty of an 18F isotopologue for PET imaging. In addition, compounds containing fluorobenzoxazoles (or the closely related fluorobenzothiazoles) might offer an approach to find in vitro tools to enable better characterization of binding site densities in tissues of interest. Worth noting, benzoxazoles and benzothiazoles are often used in the design of compounds binding amyloid targets,23-25 thus supporting this strategy for the identification of potential novel α-Syn imaging agents.
Supplemental Material
Supplementary_Data for The Binding of BF-227-Like Benzoxazoles to Human α-Synuclein and Amyloid β Peptide Fibrils by Lee Josephson, Nancy Stratman, YuTing Liu, Fang Qian, Steven H. Liang, Neil Vasdev, and Shil Patel in Molecular Imaging
Acknowledgments
Neil Vasdev and Lee Josephson thank the Michael J. Fox Foundation and the Rainwater Charitable Foundation (Tau Consortium) for jointly supporting this research collaboration. The authors thank Elena Drummond for her technical assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Lee Josephson and Neil Vasdev are cofounders of MedChem Imaging, LLC.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded in part by a Michael J. Fox Foundation grant (ID: 14605.01).
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Eberling JL, Dave KD, Frasier MA. Alpha-synuclein imaging: a critical need for Parkinson’s disease research. J Parkinsons Dis. 2013;3(4):565–567. [DOI] [PubMed] [Google Scholar]
- 2. Vernon AC, Ballard C, Modo M. Neuroimaging for Lewy body disease: is the in vivo molecular imaging of alpha-synuclein neuropathology required and feasible? Brain Res Rev. 2010;65(1):28–55. [DOI] [PubMed] [Google Scholar]
- 3. Mathis CA, Lopresti BJ, Ikonomovic MD, Klunk WE, Mathis CA. Small-molecule PET tracers for imaging proteinopathies. Semin Nucl Med. 2017;47(5):553–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kudo Y, Okamura N, Furumoto S. , et al. 2-(2-[2-dimethylaminothiazol-5-yl]ethenyl)-6-(2-[fluoro]ethoxy)benzoxazole: a novel PET agent for in vivo detection of dense amyloid plaques in Alzheimer’s disease patients. J Nucl Med. 2007;48(4):553–561. [DOI] [PubMed] [Google Scholar]
- 5. Fodero-Tavoletti MT, Mulligan RS, Okamura N, et al. In vitro characterisation of BF227 binding to alpha-synuclein/Lewy bodies. Eur J Pharmacol. 2009;617(1-3):54–58. [DOI] [PubMed] [Google Scholar]
- 6. Volpicelli-Daley LA, Luk KC, Lee VM. Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat Protoc. 2014;9(9):2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22(23):3099–3108. [DOI] [PubMed] [Google Scholar]
- 8. Vlassenko AG, Benzinger TL, Morris JC. PET amyloid-beta imaging in preclinical Alzheimer’s disease. Biochim Biophys Acta. 2012;1822(3):370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kayed R, Glabe CG. Conformation-dependent anti-amyloid oligomer antibodies. Methods Enzymol. 2006;413:326–344. [DOI] [PubMed] [Google Scholar]
- 10. Roberti MJ, Folling J, Celej MS, Bossi M, Jovin TM, Jares-Erijman EA. Imaging nanometer-sized alpha-synuclein aggregates by superresolution fluorescence localization microscopy. Biophys J. 2012;102(7):1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santa-Maria I, Perez M, Hernandez F, Avila J, Moreno FJ. Characteristics of the binding of thioflavin S to tau paired helical filaments. J Alzheimers Dis. 2006;9(3):279–285. [DOI] [PubMed] [Google Scholar]
- 12. Stankoff B, Wang Y, Bottlaender M, et al. Imaging of CNS myelin by positron-emission tomography. Proc Natl Acad Sci U S A. 2006;103(24):9304–9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng KP, Pascoal TA, Mathotaarachchi S, et al. Monoamine oxidase B inhibitor, selegiline, reduces 18F-THK5351 uptake in the human brain. Alzheimers Res Ther. 2017;9(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harada R, Ishiki A, Kai H, et al. Correlations of 18F-THK5351 PET with post-mortem burden of tau and astrogliosis in Alzheimer’s disease. J Nucl Med. 2018;59(4):671–674. [DOI] [PubMed] [Google Scholar]
- 15. Saint-Aubert L, Lemoine L, Chiotis K, Leuzy A, Rodriguez-Vieitez E, Nordberg A. Tau PET imaging: present and future directions. Mol Neurodegener. 2017;12(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gibbs-Strauss SL, Nasr KA, Fish KM, et al. Nerve-highlighting fluorescent contrast agents for image-guided surgery. Mol Imaging. 2011;10(2):91–101. [PMC free article] [PubMed] [Google Scholar]
- 17. Biancalana M, Makabe K, Koide A, Koide S. Molecular mechanism of thioflavin-T binding to the surface of beta-rich peptide self-assemblies. J Mol Biol. 2009;385(4):1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Groenning M. Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils-current status. J Chem Biol. 2010;3(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krebs MR, Bromley EH, Donald AM. The binding of thioflavin-T to amyloid fibrils: localisation and implications protein particulates: another generic form of protein aggregation? J Struct Biol. 2005;149(1):30–37. [DOI] [PubMed] [Google Scholar]
- 20. Murugan NA, Halldin C, Nordberg A, Laangstroem B, Aagren H. The culprit is in the cave: the core sites explain the binding profiles of amyloid-specific tracers. J Phys Chem Lett. 2016;7(17):3313–3321. [DOI] [PubMed] [Google Scholar]
- 21. Schuffenhauer A, Ruedisser S, Marzinzik AL, et al. Library design for fragment based screening. Curr Top Med Chem. 2005;5(8):751–762. [DOI] [PubMed] [Google Scholar]
- 22. Kumar A, Voet A, Zhang KY. Fragment based drug design: from experimental to computational approaches. Curr Med Chem. 2012;19(30):5128–5147. [DOI] [PubMed] [Google Scholar]
- 23. Noel S, Cadet S, Gras E, Hureau C. The benzazole scaffold: a SWAT to combat Alzheimer’s disease. Chem Soc Rev. 2013;42(19):7747–7762. [DOI] [PubMed] [Google Scholar]
- 24. Razavi H, Powers ET, Purkey HE, et al. Design, synthesis, and evaluation of oxazole transthyretin amyloidogenesis inhibitors. Bioorg Med Chem Lett. 2005;15(4):1075–1078. [DOI] [PubMed] [Google Scholar]
- 25. Eckroat TJ, Mayhoub AS, Garneau-Tsodikova S. Amyloid-beta probes: review of structure-activity and brain-kinetics relationships. Beilstein J Org Chem. 2013;9:1012–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Data for The Binding of BF-227-Like Benzoxazoles to Human α-Synuclein and Amyloid β Peptide Fibrils by Lee Josephson, Nancy Stratman, YuTing Liu, Fang Qian, Steven H. Liang, Neil Vasdev, and Shil Patel in Molecular Imaging