Abstract
Objectives:
Our group developed the use of the Candida skin test reagent as an adjuvant of cell-mediated immunity in designing a human papillomavirus therapeutic vaccine. Here, this technology is being applied for designing a prostate cancer immunotherapy.
Methods:
Peptides based on the prostate-specific antigen amino acid sequences were selected, synthesized, and evaluated in terms of their (1) solubility, (2) maturation effects on Langerhans cells by fluorescence-activated cell sorter analysis, and (3) recognition by peripheral immune cells from prostate cancer patients using interferon-γ enzyme-linked immunospot assay.
Results:
The peptides were soluble in 10 mM succinate at pH of 5 with 5% glycine, and they demonstrated no maturation effects on Langerhans cells from healthy donors. On the other hand, peripheral immune cells from 4 of 10 prostate cancer patients examined had positive responses in enzyme-linked immunospot assay to one or more prostate-specific antigen peptides.
Conclusion:
In summary, a design and a formulation of a novel prostate cancer immunotherapy are described. The immunogenicity of prostate-specific antigen peptides in some prostate cancer patients supports further development of this immunotherapy.
Keywords: Prostate-specific antigen, peptides, Candida, Langerhans cells, prostate cancer
Introduction
Prostate cancer is a major health problem in the United States and the world. It continues to be the most common cancer diagnosed and the second leading cause of cancer-related mortality in men in the United States. The American Cancer Society (ACS) estimates that 180,890 men would have been diagnosed with prostate cancer in 2016, comprising 21% of all male cancer diagnoses. The estimated number of lives claimed was 26,120, comprising 8% of all cancer-related mortalities in American men in 2016.1 Most patients in the United States are diagnosed with localized or regional prostate cancer that is treated with surgery (e.g. prostatectomy) and/or radiation therapy, including brachytherapy. Despite local treatment, approximately 30% of these patients develop recurrent disease.2–4 Treatment options for advanced prostate cancer include androgen deprivation therapy. Docetaxel is the first-line chemotherapy.5 There are several new lines of treatment for castrate-resistant prostate cancer including an immunotherapy, sipuleucil-T; an androgen receptor blocker, enzalutamide; an adrenal androgen synthesis inhibitor, abiraterone acetate; a chemotherapy, cabazitaxel; and a radioactive agent, Radium 223.6
Immunotherapy for prostate cancer is an attractive strategy in light of the potential role of the immune system. For example, the correlation described between the presence of tumor-infiltrating lymphocytes and good prognosis speaks well for the potential of immunotherapy.7 Human prostate-specific antigen (PSA) is one of the prostate differentiation antigens which has been used extensively as targets for T-cell-induced immunotherapy for prostate cancer. PSA is synthesized with a 17–amino acid leader sequence (pre-pro PSA) that is cleaved to generate an inactive 244–amino acid precursor protein.8 Cleavage of the N-terminal 7–amino acids from pro PSA generates the active enzyme, which has five intra-chain disulfide bonds, a single asparagine-linked oligosaccharide, and a mass of 33 kilodaltons.9–11 The physiological role of PSA is the liquefaction of the sperm-trapping coagulum consisting of semenogelin I, semenogelin II, and fibronectin, resulting in liberation of the spermatozoa.12
PSA is an ideal tumor antigen, as it is likely expressed exclusively in prostate.13–15 Several vaccine strategies have been based on PSA, including dendritic cells pulsed with PSA,16 recombinant viruses expressing PSA,17–19 recombinant PSA protein,20 PSA peptides,21 and DNA vaccines.22 Specifically, PROSTVAC includes recombinant pox viruses that express PSA with three immune-enhancing co-stimulatory molecules (LFA-3, ICAM-1, and B7.1).19 Other tumor antigens which have been investigated as potential targets were prostate-specific membrane antigen, prostate acid phosphatase, and prostate secretory protein-94.15
Identification of tumor-associated antigen-derived peptides able to elicit anti-tumor T-cell responses is essential for the development of peptide-based cancer vaccines,23 and some PSA-derived human T-cell epitopes have been described.24,25 Recent studies have shown that PSA-derived peptides can cause expansion of interferon-γ (IFN-γ)-secreting CD8+ T-cells in vitro with peripheral blood mononuclear cells (PBMCs) from healthy individuals and prostate cancer patients.26 Cytokines secreted by antigen-presenting cells play important roles in the process of differentiation of T-helper cells into T-helper type 1 (Th1), T-helper type 2, or T-helper type 17 (Th17) cells. Interleukin (IL)-12 p70 directs Th1 response, while IL-1 and IL-6 direct the Th17 response.27,28 Both CD4+ and CD8+ T-cells are required for an optimal tumor rejection to occur.29
The idea of using Candida skin test reagent as an adjuvant came from studies which used it as an intralesional injection therapy for regressing common warts.30–35 The role of T-cells in regression was shown.31,34 In vitro, Candida skin test reagent has been shown to induce T-cell proliferation and IL-12 secretion by Langerhans cells.36,37 A brand of Candida skin test reagent called Candin® (Nielsen BioSciences, San Diego, CA) has been tested as a vaccine adjuvant for an investigational human papillomavirus (HPV) therapeutic vaccine, and an increase in circulating Th1 cells has been demonstrated in the vaccine recipients.38,39 Candin is made from two strains of Candida albicans. They are propagated in media containing inorganic salts, biotin, and sucrose, lyophilized and extracted resulting in a clear solution. Unexpectedly, Candida, as a skin test reagent, did not show any maturation effects on Langerhans cells but the HPV type 16 E6 peptides did.37
Here, we present a design of a novel prostate cancer immunotherapy which includes PSA peptides and Candida. A formulation compatible for human use is described. The maturation effect on Langerhans cells from healthy subjects and the immunogenicity of the PSA peptides in prostate cancer patients were investigated. In addition, proteomes were compared between Langerhans cells treated and untreated with Candida with a goal of gaining further insight into its mechanism of action. As the feasibility of producing this prostate cancer immunotherapy has been demonstrated, further work investigating its safety and efficacy is warranted.
Material and methods
Design and solubility screening of the PSA peptides
The goal of this pilot study was to design a novel prostate cancer vaccine using PSA peptides and Candida that would eventually be suitable for human testing. Beyond the feasibility of being synthesized, one of the criteria for suitability for human testing is the solubility of the peptides, as it makes injection possible and monitoring of peptide stability feasible during the clinical trial phase. Six peptides (40–amino acids in length except for the most C-terminal peptide which is 20–amino acids long) covering 85% of the PSA protein sequence were selected for their likeliness of being solubilized in a single solution (Table 1). They were synthesized by RS Synthesis (Louisville, KY) and were acetylated at N-termini and amidated at C-termini to enhance stability.
Table 1.
Characteristics of the PSA peptides included in the immunotherapy design.
| Peptide sequence | Amino acid position | Amino acid length | Chemical formula | Hydrophobic amino acid residues (%) | Hydrophilic amino acid residues (%) | Molecular weight (grams per mole) | Charge | Attribute |
|---|---|---|---|---|---|---|---|---|
| Ac-MWVPVVFLTLSVTWIGAAPLILSRIVGGWECEKHSQPWQV-NH2 | 1–40 | 40 | C217H329N53O52S2 | 12 | 60 | 4576.5 | 1 | Basic |
| Ac-LVASRGRAVCGGVLVHPQWVLTAAHCIRNKSVILLGRHSL-NH2 | 41–80 | 40 | C192H323N63O47S2 | 20 | 50 | 4330.2 | 8 | Basic |
| Ac-FHPEDTGQVFQVSHSFPHPLYDMSLLKNRFLRPGDDSSHD-NH2 | 81–120 | 40 | C210H305N59O63S1 | 32 | 38 | 4696.2 | 1 | Basic |
| Ac-EPEEFLTPKKLQCVDLHVISNDVCAQVHPQKVTKFMLCAG-NH2 | 161–200 | 40 | C201H323N53O58S4 | 28 | 45 | 4538.4 | 1 | Basic |
| Ac-RWTGGKSTCSGDSGGPLVCNGVLQGITSWGSEPCALPERP-NH2 | 201–240 | 40 | C174H274N52O57S3 | 15 | 32 | 4102.6 | 0 | Neutral |
| Ac-SLYTKVVHYRKWIKDTIVANP-NH2 | 241–261 | 20 | C121H190N32O30 | 29 | 43 | 2573.1 | 4 | Basic |
Six peptides were tested individually for solubility in pH 4 or 5 with 10 mM succinate or 10 mM glutamate. Since five of the six peptides had positive charges, they were expected to be soluble in solutions of lower pH. Turbidity was measured by determining the optical density (OD) at 630 nm using an Epoch Microplate Spectrophotometer (BioTek, Winooski, VT). Turbidity is a measure of the light-transmitting properties of water, and it reflects the amount of suspended material (i.e. insoluble) in the liquid which scatters light. The turbidity of <1.0 was considered to be soluble, and that of <0.2 was considered to be very soluble. For each peptide not fully soluble, the following amino acids were added to enhance solubility: (1) 5% glycine, (2) 2% histidine, (3) 2.5% lysine, (4) 1.5% serine, (5) 1.5% threonine, and (6) 5% arginine. Then, the solubility of all six peptides combined was tested.
Fluorescence-activated cell sorter analysis for assessing maturation effects of the PSA peptides
While it may not be a typical feature of self-antigens, some of them have been shown to be able to increase expression of co-stimulatory molecules on antigen-presenting cells.40 Therefore, the ability of the PSA peptides to induce maturation of Langerhans cells were assessed. PBMCs were purified from apheresis products of healthy donors (n = 6) (Key Biologics, LLC, Memphis, TN) using a ficoll gradient centrifugation method. Monocytes were negatively isolated from PBMC using Monocyte Isolation Kit II (Miltenyi Biotec, Auburn, CA) and were differentiated to Langerhans cells using granulocyte–macrophage colony-stimulating factor, IL-4, and transforming growth factor-β.41 The effectiveness of differentiation to Langerhans cells was previously demonstrated by detecting CD1a, Langerin, and E-cadherin.36,37 Maturation effects on Langerhans cells were examined by detecting increased mean fluorescence intensity of CD40, CD80, CD86, and HLA-DR using antibodies (eBioscience, Inc, San Diego, CA) 48 h after exposure with PSA peptides (10 µg/peptide/ml, individually and combined) or with Candida (150 µl/ml of Candin). Zymosan, a preparation of cell wall from Saccharomyces cerevisiae, (10 µg/ml, InvivoGen, San Diego, CA) was used as a positive control, and media containing no peptide served as a negative control.
Ex vivo IFN-γ enzyme-linked immunospot assay for assessing immunogenicity of the PSA peptides
An additional goal of this pilot study was to assess the immunogenicity of the PSA peptides. Patients were approached by their physician or by a study coordinator at their routine clinic visits to enroll in this study (University of Arkansas for Medical Sciences IRB Protocol Number 204374). After obtaining a written informed consent, 60 ml of whole blood was drawn in tubes containing sodium heparin from patients (n = 10) diagnosed with prostate cancer or with a history of prostate cancer with the following inclusion criteria: (1) histological documented diagnosis of prostate cancer and (2) 18 years of age or greater. A sample size of 10 patients was selected, since the antigenicity of these PSA peptides was expected to be common based on data published by Podrazil et al.42 (i.e. 11 of 23 patients with metastatic, castration-resistant prostate cancer enrolled in a Phase I/II clinical trial of a PSA-based dendritic cell immunotherapy had responses to PSA peptides at baseline prior to vaccination). We excluded subjects who had other malignancies, unless they have been disease free for five or more years prior to the time of enrollment (Table 2).
Table 2.
Characteristics of prostate cancer patients evaluated for immunogenicity of the PSA peptides using IFN-γ ELISPOT assay.
| Patients | Age | Race | Disease status at blood draw | TNM stage at diagnosis | Gleason score | PSA at time of diagnosis | PSA at blood draw | Treatments | Androgen dependence | Site(s) of metastasis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | White | Remission, off Tx | Stage I (T1b, N0, M0) | 6 (3 + 3) | 1.2 | 0.2 | RP | No ADT | None |
| 2 | 75 | White | Active disease with metastasis, on Tx | Stage IIB (pT2c, N0, M0) | 7 (3 + 4) | 5 | 6.1 | RP, bicalutamide, ADT, docetaxel | AI | Lung |
| 3 | 68 | White | Remission, on Tx | Stage IV (pT3b, N1, M0) | 7 (3 + 4) | 13.2 | <0.1 | RP with lymph node dissection, ADT, XRT | AD | Lymph node |
| 4 | 73 | White | Recurrence, on Tx | Stage IIB (pT2c, N0, M0) | 6 (3 + 3) | NA | 5.2 | XRT, ADT, abiratone with prednisone | AI | None |
| 5 | 72 | Black | Recurrence, on Tx | Stage IIB (T2c, N0, M0) | NA | NA | 28.1 | Brachytherapy, ADT, bicalutamide, enzalutamide, RCP | AI | Lymph nodes |
| 6 | 83 | White | Active disease, on Tx | Stage IV (Tx, Nx, M1) | 9 (4 + 5) | 201 | 41.3 | ADT, bicalutamide, docetaxel | AD | Bone |
| 7 | 68 | White | Remission, off Tx | Stage IIA (pT2a, N0, M0) | 6 (3 + 3) | 15 | <0.1 | RP | No ADT | None |
| 8 | 64 | Black | Active disease, on Tx | Stage IV (Tx, Nx, M1) | NA | >1500 | <0.1 | ADT, bicalutamide, XRT, enzalutamide, abiraterone with prednisone | AI | Bone, ischial bursa, lymph node |
| 9 | 86 | White | Active disease with metastasis, on Tx | Stage IIA (T2a, N0, M0) | 7 (4 + 3) | NA | 12.4 | Orchiemictomy, prostatectomy, bicalutamide, abiraterone with prednisone | AI | Bone |
| 10 | 69 | White | Active disease with metastatsis, on Tx | Stage IV (Tx, Nx, M1) | 9 (4 + 5) | NA | 4 | ADT, bicalutamide, abiraterone with prednisone, sipuleucil-T | AI | Bone |
Tx: treatment; NA: not applicable; RP: radical prostatectomy; ADT: androgen deprivation therapy; AI: androgen independent; AD: androgen dependent; XRT: radiation therapy; RCP: radical cystoprostatectomy.
PBMCs were isolated as described above. Briefly, 96-well plates (MultiScreen-HA; EMD Millipore, Bedford, MA) were coated overnight with 5 μg/ml of primary anti-IFN-γ monoclonal antibody (Mabtech AB, Stockholm, Sweden). The plates were washed four times with phosphate-buffered saline (PBS) and blocked using Roswell Park Memorial Institute (RPMI) 1640 with 5% pooled human serum for 1 h at 37°C. Three hundred thousand PBMC per well in triplicate were presented with 10 µg/ml each of the PSA peptides described above (individually and combined). Phytohemagglutinin (10 µg/ml) was used as a positive control, while media containing no peptide served as a negative control. Human recombinant IL-2 (R&D Systems, Inc, Minneapolis, MN) at 20 units/ml was added to all wells. After a 40-h incubation at 37°C, the plates were washed four times with PBS containing 0.05% Tween-20. A secondary antibody (biotin-conjugated IFN-γ monoclonal antibody from Mabtech AB) at a final concentration of 1 µg/ml was added, and the plates were incubated for 2 h at 37°C. The plates were washed four times with PBS containing 0.1% Tween-20. Avidin-bound biotinylated horseradish peroxidase H (Vectastain Elite ABC kit; Vector Laboratories Inc, Burlingame, CA) was added, and the plates were incubated for 1 h at 37°C. After four washings with PBS containing 0.1% Tween-20, stable diaminobenzene (Invitrogen, Carlsbad, CA) was added to develop the reaction. The plates were washed with distilled water three times and air-dried overnight. The spots formed by IFN-γ-secreting T-cells were counted with an automated enzyme-linked immunospot (ELISPOT) analyzer (AID ELISPOT Classic Reader; AID Autoimmun Diagnostika GmbH, Strassberg, Germany). The average spot-forming units (SFU) per well were calculated. As previously described, a response was considered to be positive when the average SFU in wells with a given peptide was at least twice that of the average SFU in the no-peptide control wells.43
Epitope prediction
A number of potential epitopes contained in the PSA peptides described above were assessed. Predictions of PSA major histocompatibility complex (MHC) Class I (HLA-A*01:01, -A*02:01, and -A*03:01) and II (HLA-DRB1*04:01, -DRB1*07:01, and -DRB1*11:01) epitopes were made on 28 April 2016 using the Immune Epitope Database and Analysis Resource (IEDB) analysis consensus tool.44 It combines predictions from artificial neural network (ANN) aka NetMHC (3.4),45,46 stabilized matrix method (SMM)47 and Comblib48 for MHC I, and algorithms by Wang et al.49,50 for MHC Class II.
Proteomics
To uncover the mechanisms of how Candida may confer immune stimulation, proteomes differentially expressed in Langerhans cells treated and untreated (PBS) with Candida were examined. The Langerhans cells were generated as described above from healthy donors (n = 10, Key Biologics, LLC) and were treated with Candida (150 µl/ml) or PBS for 24 h which was the duration which most frequently resulted in detection of IL-12.36,37 Candida-treated and PBS-treated cell pellets (3 × 106 each) were lysed in 4% sodium dodecyl sulfate (SDS), 100 mM Tris-HCl pH 7.6, and 0.1 M dithiothreitol. DNA was sheared by sonication, and the lysate was clarified by centrifugation. Then, the DNA was run on NuPAGE 4%–12% denaturing gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (Thermo Fisher Scientific, Waltham, MA). Each SDS-PAGE gel lane was cut into 3-mm slices and subjected to in-gel trypsin digestion as follows. Gel slices were destained in 50% methanol (Thermo Fisher Scientific), 100 mM ammonium bicarbonate (Sigma-Aldrich, St. Louis, MO), followed by reduction in 10 mM Tris [2-carboxyethyl]phosphine (Thermo Fisher Scientific) and alkylation in 50 mM iodoacetamide (Sigma-Aldrich). Gel slices were then dehydrated in acetonitrile (Thermo Fisher Scientific), followed by addition of 100 ng porcine sequencing grade modified trypsin (Promega, Madison, WI) in 100 mM ammonium bicarbonate (Sigma-Aldrich). The gels were incubated at 37°C for 12–16 h. Peptide products were then acidified in 0.1% formic acid (Thermo Fisher Scientific). Tryptic peptides were separated on reverse phase Jupiter Proteo resin (Phenomenex, Torrance, CA) on a 200 × 0.075 mm column, using a nanoAcquity ultra-performance liquid chromatography system (Waters Corporation, Milford, MA). Peptides were eluted using a 30-min gradient from 97:3 to 65:35 buffer A:B ratio (0.1% formic acid and 0.5% acetonitrile in buffer A and 0.1% formic acid and 99.9% acetonitrile in buffer B). Eluted peptides were ionized by electrospray (2.35 kV) followed by mass spectroscopy (MS)/MS analysis using collision induced dissociation on an Orbitrap Fusion Tribrid mass spectrometer (Thermo Fisher Scientific) in top-speed data-dependent mode. MS data were acquired using the Fourier transform mass spectrometry analyzer in profile mode at a resolution of 240,000 full length at half maximum (FWHM) over a range of 375–1500 m/z. MS/MS data were acquired following higher-energy collisional dissociation activation using the ion trap analyzer in centroid mode and normal mass range, with precursor mass-dependent normalized collision energy between 28.0 and 31.0. Tandem mass spectra were extracted by Thermo MS File Reader, version 2.2. Charge-state deconvolution and deisotoping were not performed. All MS/MS samples were analyzed using MaxQuant (Max Planck Institute of Biochemistry, Martinsried, Germany; version 1.5.3.8) to search the UniProt human protein database (20 December 2015 release, 70,625 entries), assuming the digestion enzyme strict trypsin using 1.0% false discovery rate thresholds for both protein and peptide identification. Following an initial re-calibration of peptide masses at 5 ppm tolerance, MaxQuant analysis was performed with a fragment ion mass tolerance of 0.5 Da and a parent ion tolerance of 3 ppm. Carbamidomethyl of cysteine was specified as a fixed modification. Oxidation of methionine and acetylation of protein N-termini were specified as variable modifications.
Statistical analysis
Prior to assessing statistical significance, a log2 transformation was applied to the data. The analysis comparing Candida-treated with PBS-treated samples was performed in R, version 3.2.4, using the limma package.51,52 For each protein, linear models accounting for the paired nature of the data were fit to the log2-transformed data. These models are analogous to a paired t-test except that empirical Bayes methods have been used to borrow information between proteins. P-values were adjusted using the Benjamini–Hochberg method to control the false discovery rate.53
Results
Design and solubility screening of the PSA peptides
The attributes of five of the selected PSA peptides are basic and that of PSA peptide with amino acid position (201–240) is neutral (Table 1). PSA (121–160) was excluded from the design due to its acidic nature, making it unlikely to dissolve in the same solution with a low pH, when other peptides are combined. The six peptides were tested individually for solubility at pH 4 or 5 with 10 mM succinate or 10 mM glutamate, and they were soluble in all four solutions except for PSA (1–40). It had the lowest turbidity reading (OD 630 nm of 0.805) in 10 mM succinate at pH 5 solution. The OD 630 nm of the remaining peptides were 0.037 for PSA (41–80), 0.038 for PSA (81–120), 0.041 for PSA (161–200), 0.037 for PSA (201–240), and 0.038 for PSA (241–261). When six amino acids were added individually, the results showed that the PSA (1–40) peptide at 5 mg/ml was most soluble in 10 mM succinate at pH 5.0 with 5% glycine with OD 630 nm of 0.695.
In order to dissolve all six peptides in a single solution, we dissolved the PSA (1–40) peptide at 3 mg/ml in 10 mM succinate at pH 5 with 5% glycine. The remaining PSA peptides were dissolved at 7.5 mg/ml in the same solution. The OD 630 nm of each peptide was 0.695 for PSA (1–40), 0.041 for PSA (41–80), 0.037 for PSA (81–120), 0.039 for PSA (161–200), 0.153 for PSA (201–240), and 0.143 for PSA (241–261). The PSA peptides (41–80), (81–120), (161–200), (201–240), and (241–261) were sequentially added one at a time to the PSA (1–40) peptide with the final concentration of each peptide being 1 mg/ml. The OD 630 nm of the combined PSA peptides was 0.077. Therefore, it is possible to solubilize all six PSA peptides in a solution of 10 mM succinate at pH 5 with 5% glycine.
Assessing phenotypic maturation of Langerhans cells
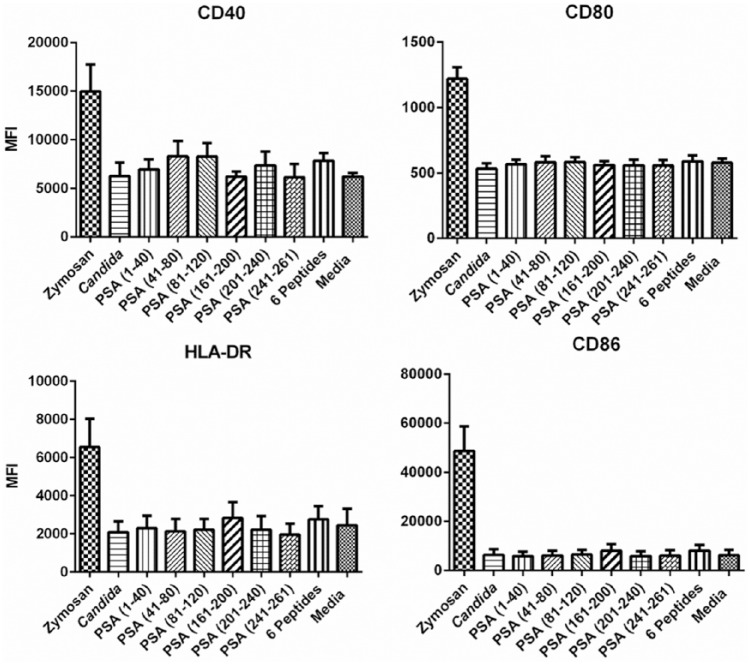
Individual PSA peptides, combined PSA peptides and Candida did not show any increases in mean fluorescent intensities compared to the untreated Langerhans cells (Figure 1). Therefore, no maturation effects on Langerhans cells as determined by expression of CD40, CD80, CD86, and HLA-DR on the cell surface were observed.
Figure 1.
Fluorescence-activated cell sorter analysis of Langerhans cells from healthy donors treated with PSA peptides to assess maturation effects by measuring surface expression of CD40, CD80, CD86, and HLA-DR. The error bars represent standard error of means.
Assessing immunogenicity of the PSA peptides
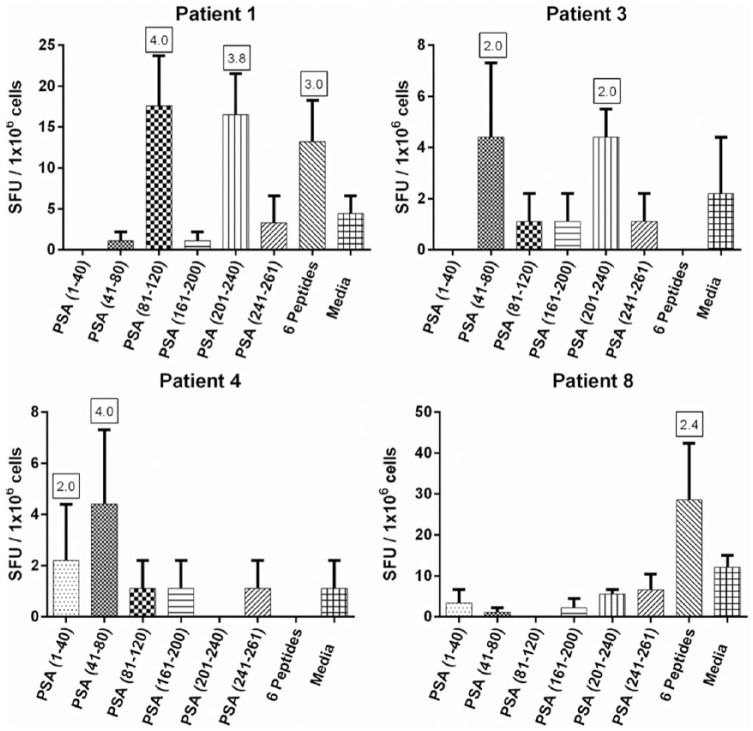
We enrolled the patients from September 2015 through January 2016 (Table 2). Peripheral immune cells from 4 of 10 prostate cancer patients examined had positive responses in ELISPOT assay to one or more PSA peptides (Figure 2). For the remaining six prostate cancer patients, no positive responses were detected except to the phytohemagglutinin-positive control (positivity indices of 57.3, 197.0, 154.5, 131.4, 186.0, and 253.3, respectively for patients 2, 5, 6, 7, 9, and 10). Therefore, immune responses to the PSA peptides are detectable in some prostate cancer patients without vaccination.
Figure 2.
IFN-γ ELISPOT assay results assessing the immunogenicity of the PSA peptides in prostate cancer patients (n = 10). The results of patients with positive response to at least one PSA peptide or peptide pool are shown (n = 4). The error bars represent standard error of means, and the boxed numbers represent positivity indices, which are calculated by dividing the mean SFUs in peptide wells with the mean SFUs in media only wells.
Epitope prediction
Using the IEDB analysis consensus tool, 759 potential PSA MHC Class I epitopes (Table S1) and 741 potential Class II epitopes (Table S2) were identified. Of those, 144 Class I epitopes and 162 Class II epitopes would be lost by not including the PSA (121–160) peptide resulting in 615 potential Class I epitopes and 579 potential Class II epitopes.
A comparison of proteomes of Langerhans cells treated and untreated with Candida
Candida- and PBS-treated Langerhans cell pellet samples were obtained from 10 subjects, from which spectral count information was captured. In total, 4637 proteins that were detected in at least one of the 20 samples were identified. As a consequence, some proteins exhibited zero counts or very low counts for almost all samples. In order to ensure model stability, the data were filtered so that only proteins having spectral counts ⩾5 for at least 10 samples were included in the analysis. This reduced the number of proteins to 2277. Twelve proteins (filaggrin-2, zinc finger RNA-binding protein, Ras-related protein Rab-33B, endophilin-A2, mitochondrial electron transfer flavoprotein-ubiquinone oxidoreductase, four types of keratin, cytochrome b-c1 complex subunit 7, REST corepressor 1, and microtubule-associated protein 4) had unadjusted p-values less than 5%, and none of the adjusted p-values were found to be significant at the 5% level of significance.
Discussion
PSA is an ideal tumor antigen to target with immunotherapy because it is likely exclusively expressed in prostate.13,14 In order to assess whether cross-reactivity with closely related Kallikrein-related peptidases (KLKs) may be of a concern, comparisons of PSA amino acid sequence with the published sequences of serine proteases were evaluated from available literature. A strong homology between PSA and the various enzymes in the kallikrein family was uncovered. The sequence identity of PSA with γ-nerve growth factor is 56%. Homology was also seen with other serine proteases, including tonin (54%), epidermal growth factor-binding protein (53%), a nerve growth factor (51%), trypsin (42%), and chymotrypsin (35%).14 Possible cross-recognition of these proteins with amino acids sequence homology is of concern in developing the PSA-based immunotherapy, although the overall homology does not appear to be high enough to cause adverse effects. Nevertheless, a close monitoring for adverse events is warranted in future clinical trials.
This study is considered the first in development of a new prostate cancer treatment by administering Candida as an adjuvant with PSA peptides, although other PSA-based vaccines have been described as mentioned earlier. We were also the first to use it as a vaccine adjuvant in humans.38,39 The Phase I clinical trial of the HPV therapeutic vaccine enrolled 52 subjects, and 34 subjects qualified for vaccination, as they were confirmed to have biopsy-proven high-grade squamous intraepithelial lesions. The vaccine consisted of four current good manufacturing-grade HPV type 16 E6 synthetic peptides and Candida. A total of 132 injections of the HPV therapeutic vaccine have been administered to 34 subjects, and no serious toxicity was observed. The injections were administered intradermally in any limb. Most frequently forearms were chosen, but they were also given in outer thighs. A total of four injections were given every 3 weeks. Histological regression was observed in about half of those who completed the study which is double the rate of spontaneous regression shown in a historical placebo group.38,39 Immunological response has been detected in 61% of the vaccine recipients.38
Evaluation of the PSA peptide solubility was the main challenge. Some of the selected peptides contain high percentage of hydrophobic residues. To overcome this problem, we added hydrophilic or charged amino acids to enhance solubility. In order to be able to solubilize all peptides in a single solution, PSA (161–200) which was the only acidic peptide was not included. While this resulted in decreased number of potential epitopes that can be recognized by T-cells, sufficiently larger number of potential epitopes seems to exist in the remaining portions of the PSA. Furthermore, the sequence of how peptides are combined seemed to be critical.
Wang et al.37 have demonstrated that the HPV type 16 E6 peptides can significantly increase expression of CD40 and CD80 of Langerhans cells in vitro. However, the PSA peptide did not show such partial maturation effects, and only a few self-antigens have been shown to have such properties.40
Because PSA is a self-antigen, many PSA-specific T-cells are likely to have been deleted during the negative selection process in the thymus resulting in no immunogenicity of PSA. However, anti-PSA immune response has been demonstrated in a clinical trial of recombinant vaccinia virus expressing PSA.54 Using an IFN-γ ELISPOT assay, peripheral immune cells from 4 of 10 prostate cancer patients examined had positive responses to one or more PSA peptide pools, further supporting the use of PSA as a tumor antigen target in immunotherapy. Many responses were detectable but weak. This was expected as we used ex vivo ELISPOT assay without in vitro stimulation, and T-cells from periphery were tested (not from the prostate gland). Although responses with positivity indices of around 2 are not robust, our group has been able to show that they represent true positives in many occasions by isolating peptide-specific T-cell clones.55–59 As long as there is some detectable antigenicity, the novel immunotherapy would likely be able to enhance the T-cell responses when they are tested in clinical trials. Future studies should include addressing which type(s) of T-cells (CD4-positive versus CD8-positive) is being stimulated by the immunotherapy as well as examination of any role B-cells may play.
Proteomics is the large-scale study of proteins, particularly their structures and functions.60 Visualization methods for protein detection following one- or two-dimensional gel electrophoresis separation represent a critical step in quantitative proteome analysis.61 Most techniques currently used in proteomics use a variety of fractionation and separation steps prior to analysis by MS.62 With the aim of uncovering additional insights as to how Candida may be stimulating cell-mediated immune responses, we analyzed the proteomes of Langerhans cells treated and untreated with Candida. While 12 proteins differentially expressed using unadjusted p-values were identified, none of them was significant after correction for multiple analyses. Therefore, the mechanisms of immune activation may only exist in secreted proteins such as IL-12 (not in cell pellet) previously demonstrated to be secreted by Candida-treated Langerhans cells.36,37 Alternatively, the sensitivity of proteomic detection may not have been sufficient for identifying cytokines such as IL-12, which was the most commonly detected cytokine by Candida-treated Langerhans cells using a quantitative reverse transcription—polymerase chain reaction method.36,37 Indeed, IL-12 was not detected in any of the samples tested (data not shown). After all, 2277 proteins detected and entered into analysis would be a fraction of 18,000 human proteins that could be expressed.63,64 We have previously examined the induction of Th1, T-helper type 2 (Th2), and Th17 responses by intracellular cytokine staining of CD4 cells exposed to Candida-pulsed Langerhans cells. IFN-γ secretion was increased and IL-4 secretion was decreased in CD4 cells of a few healthy subjects, but IL-17A was essentially unchanged upon Candida treatment.
Sipuleucil-T is an already Food and Drug Administration (FDA)-approved immunotherapy for prostate cancer. Furthermore, PROSTVAC and DCVAC are in late stages of clinical trials (NCT01322490 and NCT02111577). Ultimately, the clinical efficacy of our Candida-based prostate cancer immunotherapy would need to be tested in clinical trials as well; however, there are some obvious advantages it may have over sipuleucil-T and DCVAC (both require preparing dendritic cells from the patients) as our product will be an off-the-shelf agent which does not need to be individually produced. It also does not contain an infectious agent like PROSTVAC which contains recombinant pox virus as Candida extract rather than live Candida is used. While some peptide vaccines are designed to be administered to patients with selected HLA types,21 our PSA peptides were predicted to be presented by a large number of HLA types likely making such selection unnecessary. However, a possibility of potential competition in HLA binding and whether these peptides are naturally processed by antigen-presenting cells would need to be investigated. The limitations of the current study were due to its preclinical nature. Therefore, it was beyond the scope of this work to test this immunotherapy in a human clinical trial nor to compare its efficacy with other modalities of prostate cancer immunotherapy.
Conclusion
We described a human preclinical study of a novel prostate cancer immunotherapy consisting of PSA peptides and Candida skin test reagent as an adjuvant. As solubility and formulation have been developed, it would be feasible to further evaluate the utility of this new therapy particularly when a proportion of prostate cancer patients seem to have immune cells with the ability to recognize these PSA peptides already. Therefore, whether this immunotherapy may enhance immune responses to PSA leading to tumor regression should be examined.
Supplemental Material
Supplemental material, Table_S1._Predicted_MHC_class_I_PSA_epitopes for A novel prostate cancer immunotherapy using prostate-specific antigen peptides and Candida skin test reagent as an adjuvant by Al-Ola Abdallah, Hannah Coleman, Mohamed Kamel, Rodney Davis, Teri Landrum, Horace Spencer, Sam Mackintosh, Fade A Mahmoud, Natasa Milojkovic, Chester Wicker, Konstantinos Arnaoutakis and Mayumi Nakagawa in SAGE Open Medicine
Supplemental Material
Supplemental material, Table_S2._Predicted_MHC_class_II_epitopes for A novel prostate cancer immunotherapy using prostate-specific antigen peptides and Candida skin test reagent as an adjuvant by Al-Ola Abdallah, Hannah Coleman, Mohamed Kamel, Rodney Davis, Teri Landrum, Horace Spencer, Sam Mackintosh, Fade A Mahmoud, Natasa Milojkovic, Chester Wicker, Konstantinos Arnaoutakis and Mayumi Nakagawa in SAGE Open Medicine
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.-O.A. and M.N. are inventors named in a patent application describing the prostate cancer immunotherapy.
Ethical approval: Ethical approval for this study was obtained from the University of Arkansas for Medical Sciences Institutional Review Board #204374 “A Pilot Study to Assess the Immunogenicity of Candidate PSA Peptides For a Prostate Cancer Vaccine.”
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Most of the work described in this paper was supported by the Winthrop P. Rockefeller Cancer Institute Intramural Support. The study of proteomes was funded by a number of grants from the National Institutes of Health: (grants no.: R01CA143130, P20GM103429, P30GM103450, P20GM103625, and S10OD018445) and the Arkansas Biosciences Institute, which is supported by the Tobacco Settlement Proceeds Act of 2000.
Informed consent: Written informed consent was obtained from all subjects before the study.
Trial registration: This study was registered in clinicaltrials.gov with a registration number of NCT 02485964.
ORCID iD: Mayumi Nakagawa  https://orcid.org/0000-0002-0377-4613
https://orcid.org/0000-0002-0377-4613
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. D’Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA 2008; 299: 289–295. [DOI] [PubMed] [Google Scholar]
- 3. Zelefsky MJ, Eastham JA, Cronin AM, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol 2010; 28: 1508–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol 2010; 28: 1106–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petrylak DP. Practical guide to the use of chemotherapy in castration resistant prostate cancer. Can J Urol 2014; 21: 77–83. [PubMed] [Google Scholar]
- 6. Corfield J, Crozier J, Joshua A, et al. Understanding the role of new systemic agents in the treatment of prostate cancer. BJU Int 2016; 118(Suppl. 3): 8–13. [DOI] [PubMed] [Google Scholar]
- 7. Vesalainen S, Lipponen P, Talja M, et al. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer 1994; 30A: 1797–1803. [DOI] [PubMed] [Google Scholar]
- 8. Lundwall A, Lilja H. Molecular cloning of human prostate specific antigen cDNA. FEBS Lett 1987; 214: 317–322. [DOI] [PubMed] [Google Scholar]
- 9. Kumar A, Mikolajczyk SD, Goel AS, et al. Expression of pro form of prostate-specific antigen by mammalian cells and its conversion to mature, active form by human kallikrein 2. Cancer Res 1997; 57: 3111–3114. [PubMed] [Google Scholar]
- 10. Lovgren J, Rajakoski K, Karp M, et al. Activation of the zymogen form of prostate-specific antigen by human glandular kallikrein 2. Biochem Biophys Res Commun 1997; 238: 549–555. [DOI] [PubMed] [Google Scholar]
- 11. Takayama TK, Fujikawa K, Davie EW. Characterization of the precursor of prostate-specific antigen. Activation by trypsin and by human glandular kallikrein. J Biol Chem 1997; 272: 21582–21588. [DOI] [PubMed] [Google Scholar]
- 12. Lilja H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest 1985; 76: 1899–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 1987; 317: 909–916. [DOI] [PubMed] [Google Scholar]
- 14. Watt KW, Lee PJ, M’Timkulu T, et al. Human prostate-specific antigen: structural and functional similarity with serine proteases. Proc Natl Acad Sci U S A 1986; 83: 3166–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horig H, Lee CS, Kaufman HL. Prostate-specific antigen vaccines for prostate cancer. Expert Opin Biol Ther 2002; 2: 395–408. [DOI] [PubMed] [Google Scholar]
- 16. Heiser A, Coleman D, Dannull J, et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest 2002; 109: 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gulley J, Chen AP, Dahut W, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate 2002; 53: 109–117. [DOI] [PubMed] [Google Scholar]
- 18. Hodge JW, Schlom J, Donohue SJ, et al. A recombinant vaccinia virus expressing human prostate-specific antigen (PSA): safety and immunogenicity in a non-human primate. Int J Cancer 1995; 63: 231–237. [DOI] [PubMed] [Google Scholar]
- 19. Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010; 28: 1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meidenbauer N, Harris DT, Spitler LE, et al. Generation of PSA-reactive effector cells after vaccination with a PSA-based vaccine in patients with prostate cancer. Prostate 2000; 43: 88–100. [DOI] [PubMed] [Google Scholar]
- 21. Yoshimura K, Minami T, Nozawa M, et al. A phase 2 randomized controlled trial of personalized peptide vaccine immunotherapy with low-dose dexamethasone versus dexamethasone alone in chemotherapy-naive castration-resistant prostate cancer. Eur Urol 2016; 70: 35–41. [DOI] [PubMed] [Google Scholar]
- 22. Kim JJ, Trivedi NN, Wilson DM, et al. Molecular and immunological analysis of genetic prostate specific antigen (PSA) vaccine. Oncogene 1998; 17: 3125–3135. [DOI] [PubMed] [Google Scholar]
- 23. Celis E, Sette A, Grey HM. Epitope selection and development of peptide based vaccines to treat cancer. Semin Cancer Biol 1995; 6: 329–336. [DOI] [PubMed] [Google Scholar]
- 24. Corman JM, Sercarz EE, Nanda NK. Recognition of prostate-specific antigenic peptide determinants by human CD4 and CD8 T cells. Clin Exp Immunol 1998; 114: 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Correale P, Walmsley K, Zaremba S, et al. Generation of human cytolytic T lymphocyte lines directed against prostate-specific antigen (PSA) employing a PSA oligoepitope peptide. J Immunol 1998; 161: 3186–3194. [PubMed] [Google Scholar]
- 26. Elkord E, Williams PE, Kynaston H, et al. Differential CTLs specific for prostate-specific antigen in healthy donors and patients with prostate cancer. Int Immunol 2005; 17: 1315–1325. [DOI] [PubMed] [Google Scholar]
- 27. Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 2010; 327: 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009; 30: 646–655. [DOI] [PubMed] [Google Scholar]
- 29. Hung K, Hayashi R, Lafond-Walker A, et al. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 1998; 188: 2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clifton MM, Johnson SM, Roberson PK, et al. Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol 2003; 20: 268–271. [DOI] [PubMed] [Google Scholar]
- 31. Horn TD, Johnson SM, Helm RM, et al. Intralesional immunotherapy of warts with mumps, Candida, and trichophyton skin test antigens: a single-blinded, randomized, and controlled trial. Arch Dermatol 2005; 141: 589–594. [DOI] [PubMed] [Google Scholar]
- 32. Johnson SM, Horn TD. Intralesional immunotherapy for warts using a combination of skin test antigens: a safe and effective therapy. J Drugs Dermatol 2004; 3: 263–265. [PubMed] [Google Scholar]
- 33. Johnson SM, Roberson PK, Horn TD. Intralesional injection of mumps or Candida skin test antigens: a novel immunotherapy for warts. Arch Dermatol 2001; 137: 451–455. [PubMed] [Google Scholar]
- 34. Kim KH, Horn TD, Pharis J, et al. Phase 1 clinical trial of intralesional injection of Candida antigen for the treatment of warts. Arch Dermatol 2010; 146: 1431–1433. [DOI] [PubMed] [Google Scholar]
- 35. Phillips RC, Ruhl TS, Pfenninger JL, et al. Treatment of warts with Candida antigen injection. Arch Dermatol 2000; 136: 1274–1275. [DOI] [PubMed] [Google Scholar]
- 36. Nakagawa M, Coleman HN, Wang X, et al. IL-12 secretion by Langerhans cells stimulated with Candida skin test reagent is mediated by dectin-1 in some healthy individuals. Cytokine 2014; 65: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X, Coleman HN, Nagarajan U, et al. Candida skin test reagent as a novel adjuvant for a human papillomavirus peptide-based therapeutic vaccine. Vaccine 2013; 31: 5806–5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coleman HN, Greenfield WW, Stratton SL, et al. Human papillomavirus type 16 viral load is decreased following a therapeutic vaccination. Cancer Immunol Immunother 2016; 65: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greenfield WW, Stratton SL, Myrick RS, et al. A phase I dose-escalation clinical trial of a peptide-based human papillomavirus therapeutic vaccine with Candida skin test reagent as a novel vaccine adjuvant for treating women with biopsy-proven cervical intraepithelial neoplasia 2/3. Oncoimmunology 2015; 4: e1031439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Galdiero M, Pisciotta MG, Gorga F, et al. Modulation of costimulatory molecules CD80/CD86 on B cells and macrophages by stress proteins GroEL, GroES and DnaK. Int J Immunopathol Pharmacol 2005; 18: 637–644. [DOI] [PubMed] [Google Scholar]
- 41. Fahey LM, Raff AB, Da Silva DM, et al. Reversal of human papillomavirus-specific T cell immune suppression through TLR agonist treatment of Langerhans cells exposed to human papillomavirus type 16. J Immunol 2009; 182: 2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Podrazil M, Horvath R, Becht E, et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2015; 6: 18192–18205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaul R, Dong T, Plummer FA, et al. CD8(+) lymphocytes respond to different HIV epitopes in seronegative and infected subjects. J Clin Invest 2001; 107: 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim Y, Ponomarenko J, Zhu Z, et al. Immune epitope database analysis resource. Nucleic Acids Res 2012; 40: W525–W530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lundegaard C, Lamberth K, Harndahl M, et al. NetMHC-3.0: accurate web accessible predictions of human, mouse and monkey MHC class I affinities for peptides of length 8-11. Nucleic Acids Res 2008; 36: W509–W512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nielsen M, Lundegaard C, Worning P, et al. Reliable prediction of T-cell epitopes using neural networks with novel sequence representations. Protein Sci 2003; 12: 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peters B, Sette A. Generating quantitative models describing the sequence specificity of biological processes with the stabilized matrix method. BMC Bioinformatics 2005; 6: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sidney J, Assarsson E, Moore C, et al. Quantitative peptide binding motifs for 19 human and mouse MHC class I molecules derived using positional scanning combinatorial peptide libraries. Immunome Res 2008; 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang P, Sidney J, Dow C, et al. A systemic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. PLoS Comput Biol 2008; 4: e1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang P, Sidney J, Kim Y, et al. Peptide binding predictions for HLA DR, DP and DQ molecules. BMC Bioinformatics 2010; 11: 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Phipson B, Lee S, Majewski IJ, et al. Robust hyperparameter estimation protects against hypervariable genes and improves power to detect differential expression. Ann Appl Stat 2016; 10: 946–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 2004; 3: 3. [DOI] [PubMed] [Google Scholar]
- 53. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B: Met 1995; 57: 289–300. [Google Scholar]
- 54. Eder JP, Kantoff PW, Roper K, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res 2000; 6: 1632–1638. [PubMed] [Google Scholar]
- 55. Coleman HN, Wang X, Greenfield WW, et al. A human papillomavirus type 16 E6 52-62 CD4 T-Cell epitope restricted by the HLA-DR11 molecule described in an epitope hotspot. MOJ Immunology 2014; 1: 00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nakagawa M, Kim KH, Gillam TM, et al. HLA class I binding promiscuity of the CD8 T-cell epitopes of human papillomavirus type 16 E6 protein. J Virol 2007; 81: 1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang X, Greenfield WW, Coleman HN, et al. Use of interferon-γ enzyme-linked immunospot assay to characterize novel T-cell epitopes of human papillomavirus. J Vis Exp 2012; 61: 3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang X, Moscicki AB, Tsang L, et al. Memory T cells specific for novel human papillomavirus type 16 (HPV16) E6 epitopes in women whose HPV16 infection has become undetectable. Clin Vaccine Immunol 2008; 15: 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang X, Santin AD, Bellone S, et al. A novel CD4 T-cell epitope described from one of the cervical cancer patients vaccinated with HPV 16 or 18 E7-pulsed dendritic cells. Cancer Immunol Immunother 2009; 58: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Anderson NL, Anderson NG. Proteome and proteomics: new technologies, new concepts, and new words. Electrophoresis 1998; 19: 1853–1861. [DOI] [PubMed] [Google Scholar]
- 61. Dreger M. Proteome analysis at the level of subcellular structures. Eur J Biochem 2003; 270: 589–599. [DOI] [PubMed] [Google Scholar]
- 62. Patton WF. Detection technologies in proteome analysis. J Chromatogr B 2002; 771: 3–31. [DOI] [PubMed] [Google Scholar]
- 63. Kim MS, Pinto SM, Getnet D, et al. A draft map of the human proteome. Nature 2014; 509: 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wilhelm M, Schlegl J, Hahne H, et al. Mass-spectrometry-based draft of the human proteome. Nature 2014; 509: 582–587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Table_S1._Predicted_MHC_class_I_PSA_epitopes for A novel prostate cancer immunotherapy using prostate-specific antigen peptides and Candida skin test reagent as an adjuvant by Al-Ola Abdallah, Hannah Coleman, Mohamed Kamel, Rodney Davis, Teri Landrum, Horace Spencer, Sam Mackintosh, Fade A Mahmoud, Natasa Milojkovic, Chester Wicker, Konstantinos Arnaoutakis and Mayumi Nakagawa in SAGE Open Medicine
Supplemental material, Table_S2._Predicted_MHC_class_II_epitopes for A novel prostate cancer immunotherapy using prostate-specific antigen peptides and Candida skin test reagent as an adjuvant by Al-Ola Abdallah, Hannah Coleman, Mohamed Kamel, Rodney Davis, Teri Landrum, Horace Spencer, Sam Mackintosh, Fade A Mahmoud, Natasa Milojkovic, Chester Wicker, Konstantinos Arnaoutakis and Mayumi Nakagawa in SAGE Open Medicine