Abstract
Patients with advanced malignancies treated with immune checkpoint inhibitors are at increased risk for developing immune-related neurological complications. It is a phenomenon of immunological twist when immunotherapy against co-stimulatory molecules activates previously normal T cells to kill tumor cells but, in so doing, the T cells become unrestrained, triggering other autoimmune diseases for which conventional immunotherapy is needed. The most common autoimmune neurological diseases, usually occurring within 2–12 weeks after immune checkpoint inhibitor initiation, include: inflammatory myopathies, myasthenia gravis, acute and chronic demyelinating polyradiculoneuropathies, vasculitic neuropathies, isolated cranial neuropathies, aseptic meningitis, autoimmune encephalitis, multiple sclerosis and hypophysitis. The neurological events can evolve rapidly, necessitating the need for vigilance at all stages of treatment, even after completion, because early immunotherapeutic interventions are effective. The review addresses these complications and the applied therapies, discusses immune pathomechanisms including triggering preexisting autoimmunity, highlights the distinction between paraneoplastic and autoimmune etiologies, and identifies uncertainties regarding risk factors, use of immune checkpoint inhibitors in patients with known immune diseases or restarting therapy after a neurological event. Although the autoimmune neurological complications are not very common, their incidence will likely increase as the use of immune checkpoint inhibitors in metastatic cancer is growing rapidly.
Keywords: autoimmune neurological disorders, immune checkpoint inhibitors, immunotherapy, immune-related neurological complications, neuro-immunology, neurological side effects
Introduction
There is overwhelming evidence from a number of uncontrolled series that patients receiving treatment with immune checkpoint inhibitors (ICPIs) for advanced malignancies including metastatic cancer, especially melanoma, are at risk for developing immune-related neurological events that predominantly affect the neuromuscular system.1–7 This seems like a ‘double vulnerability’ of the nervous system in cancer patients because, in addition to a potential paraneoplastic effect exerted by the cancer itself and the neurotoxicity of some chemotherapeutic agents, cancer immunotherapies can unleash unrestrained T cells capable of triggering autoimmune neurological diseases. Other organs, such as liver, skin, endocrine and rheumatic tissues can also be affected.1,8–10
The phenomenon is a sophisticated double immunotherapeutic twist: agents against the ‘inhibitory’ co-stimulatory T cell molecules activate T cells to kill the tumor but become unleashed and so ‘uninhibited’ that they also attack healthy tissues, causing autoimmune diseases; in turn, immunotherapy is needed again to reverse the T cell-triggered autoimmunity. The purpose of this review is to highlight the neurological complications associated with ICPIs; increase the awareness of the neurologists to identify them promptly because if treated early they can be reversed; address immunopathogenesis; discuss therapies; and pinpoint a series of evolving uncertainties regarding risk factors and the decision to administer ICPIs in a setting of an active or preexisting autoimmune neurological disease.
Immune tolerance, disturbance of immune balance by ICPIs and immunopathogenesis of ICPI-triggered autoimmunity
The interaction of T cell receptor (TCR) with the target antigen presenting cell (APC)/MHC complex leads to engagement of the co-stimulatory factors CD28, CTLA-4 and PD-1 on T cells, with their respective receptors CD80 (B7-1)/CD86(B7-2) and PD-LI-1/PDL-2 on APCs (Figure 1(a)).11–13 This process activates downstream events leading via the IL-2 promoter to cell proliferation and T cell differentiation.11,12 As shown in Figure 1(a), when CD28 binds to its CD80(B71)/CD86(B7-2) receptor, it exerts positive (+) activating signals; in contrast, the CTLA-4 on activated T cells binds with higher affinity to CD80/CD86, exerting negative (−) inhibitory signals and blocking T cell activation. In autoimmune diseases the concept of target-specific immunotherapies is based on therapeutic monoclonal antibodies or fusion proteins directed against the activating positive CD28 and CD80/86 (B7-1,2) signals,8–13 or in enhancing inhibition, like the CTLA-4 Ig fusion protein (Abatacept) (Figure 1(a)) that is effective in rheumatoid arthritis.8,9
Figure 1.
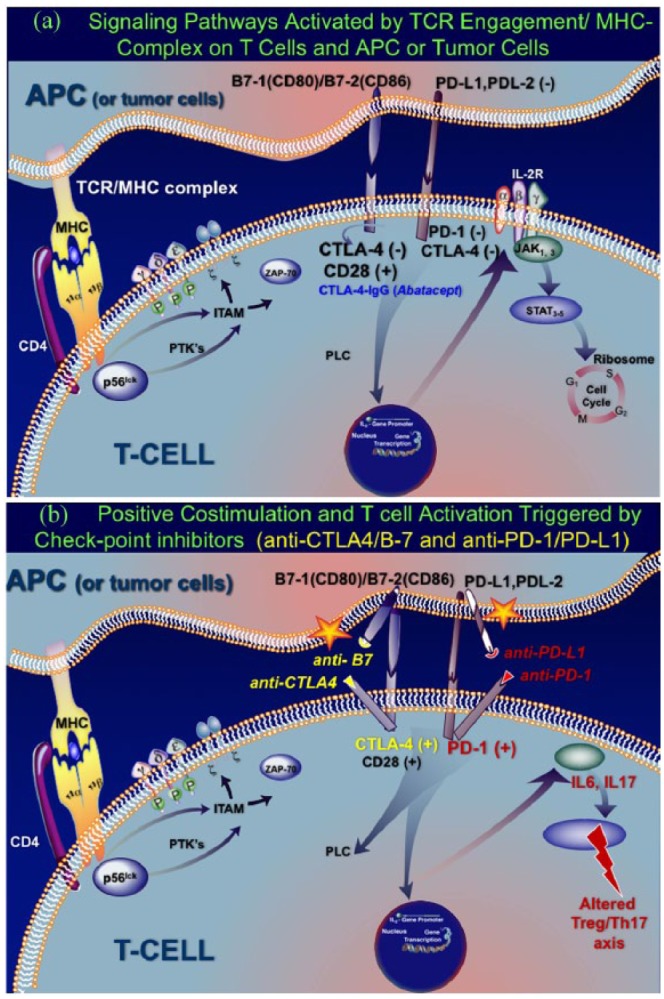
Signaling pathways activated by MHC/ T cell receptor (TCR) engagement and effect of immune checkpoint inhibitors.
(a) Cytotoxic T lymphocyte-associated antigen 4 (CTLA4) exerts negative, inhibitory signals when bind to their ligands Programmed Cell Death-Ligand 1, and 1 (PD-L1/PDL-2) on APCs and tumor cells and do not attack the tumor. The interaction of TCR with the target antigen presenting cell (APC) (the TCR/MHC complex) activates intracellular phosphotyrosine kinases (ZAP-70) that mediate signaling via phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) and various transduction molecules. T cell activation is meditated via key co-stimulatory factors, based on interactions of CD28/CTLA-4 or PD-1 on T cells with their respective ligands B7-1(CD80)/B7-2 (CD86) or PD-L1/PD-L2 on APCs and tumor cells. CD28, when binding to its CD80(B71)/CD86(B7-2) receptor, exerts positive (+) activating signals; in contrast, the CTLA-4 on activated T cells binds with higher affinity to CD80/CD86 and exerts negative (–) inhibitory signals, blocking T cell activation. This interaction activates sequentially downstream events, leading via the IL-2 promoter and Janus kinases to cell proliferation and T cell differentiation. The application of target-specific immunotherapies in autoimmune diseases is based on therapeutic monoclonal antibodies or fusion proteins directed against the positive activating CD28 and CD80/86 (B7-1,2) signals or in enhancing inhibition, via the CTLA-4 Ig fusion protein, like the drug Abatacept,which is effective in rheumatoid arthritis.
(b) Effect of ICPIs: positive co-stimulation and T cell activation. The ICPIs prevent the CTLA-4 or PD-1 from binding to their respective receptors CD80/86 and PDL-1 (noted by *) and, by doing so, they inhibit the inherent ‘inhibitory’ (–) co-stimulatory interactions between T cells and tumor cells. Such inhibition of inhibitory signals results in positive co-stimulation that unleashes a strong and uncontrolled T cell activation ( like ‘taking the brakes off’ the immune system). The ICPI-induced activated T cells enhance Th1 and Th-17 cell responses, increase the production of cytokines, such as IL-6 and IL-17, leading to abnormal T-regulatory (Treg) cell function and altered Treg/Th17 cell axis, which is critical for humoral immunity and development of autoimmune disease.
CTLA-4, cytotoxic T lymphocyte antigen; ICAM, intercellular adhesion molecule; ITAM, immunoreceptor tyrosine-based activation motifs; LFA-1, lymphocyte function antigen 1; LFA-3, lymphocyte function antigen 3; NFAT, nuclear factor of activated T cells; PLC, phospholipase C; PTK, protein tyrosine kinase; STAT, signal transducer and activator of transcription; ZAP-70, zeta-chain associated protein 70.
Tumors, like other APCs, also express on their cell surface the inhibitory ligands PD-L1/PDL-2 and B7-1/B7-2, which are respectively engaged with PD-1 and CTLA-4 on T cells, downregulating T cell responses (Figure 1(a)). These receptor–ligand interactions essentially act as an ‘off switch’, which ‘tell the T cells to leave the tumor cells alone’ so T cells do not attack the tumor. As shown in Figure 1(b), the ICPIs prevent the CTLA-4 or PD-1 from binding to their respective receptors CD80/86 and PDL-1 and, by doing so, inhibit the inherent ‘inhibitory’ co-stimulatory interactions between T cells and tumor cells, resulting in positive (+) signals; ICPIs essentially turn the ‘switch’ back on, resulting in positive co-stimulation and strong cell activation, like taking the ‘brakes off’ the immune system (Figure 1(b)).1,7–13 Although this blockade has a positive (therapeutic) effect because the T cells are now able to kill tumor cells, it concurrently has an opposite negative effect as the resulting enhanced co-stimulation causes an uncontrolled T cell activation that disrupts immune tolerance, resulting in immune-related events against various organs.
The exact pathomechanisms by which these blocked interactions lead to diversity of autoimmune complications remain unclear. ICPIs enhance Th1 and Th-17 cell responses and the production of cytokines, such as IL-6 and IL-17 that lead to abnormal T-regulatory (Treg) cell function and humoral immunity (Figure 1(b)).2,13,14 An altered Treg/Th17 cell axis is critical for the development of many autoimmune diseases. Further, both PD-1 and CTLA-4 inhibitions can stimulate antibody production, leading to antibody-mediated autoimmune diseases, especially in patients with preexisting autoimmunity or autoimmune susceptibility (Figure 1(b)). The pathogenicity of some autoantibodies is facilitated by molecular mimicry due to cross-reactivity of certain nervous system antigens with the same antigens expressed by the tumor, especially melanoma, as discussed later.
Commonly used ICPIs
ICPIs are currently FDA-approved for advanced malignancies, especially metastatic melanoma, non-small cell lung cancer (NSCLC) and Hodgkin’s lymphoma (HL).1 A complete list of ICPIs, indicated for each specific malignancy type as approved by the FDA, has been recently published.1 The main drugs currently on the market are directed against the following:
CTLA-4: Ipilimumab (Yervoy), the first ICPI approved for metastatic melanoma.
PD-1: Pembrolizumab (Keytruda) and Nivolumab (Opdivo), with a 26–31% response rate among patients with metastatic melanoma refractory to ipilimumab. These are also approved for a subset of patients with NSCLC, metastatic head and neck cancer, renal cell carcinoma and HL.
PD-L1: Atezolizumab (Tecentriq), Avelumab (Bavencio) and Durvalumab (Imfinzi), all approved for urothelial carcinoma and as second-line therapy for NSCLC.
Some of these agents are also used in combination with even better response rates, but more common immune complications. For example, a 60% response rate was seen using nivolumab and ipilimumab for metastatic melanoma, compared to an 11% response rate for ipilimumab alone.1
Neurological complications of ICPIs
Incidence and risk factors
Immune-related adverse events resulting from enhanced T cell activation essentially affect nearly every organ, with varying degrees of severity (Table 1), including colitis, hepatitis, pneumonitis, hypothyroidism, autoimmune retinopathy, uveitis or iritis and rheumatic or musculoskeletal complications. They most commonly cause a series of autoimmune neurological events affecting muscle, neuromuscular junction, nerves, routes, spinal cord and brain, as described below. These events vary in severity and occur at any point during ICPI administration, but 60–80% occur early, within the first 4 months of therapy initiation.1–10
Table 1.
Common immune complications of ICPIs.
|
A. Systemic autoimmune complications
1. Colitis; 2. hepatitis; 3. pneumonitis; 4. hypothyroidism; 5. retinopathy, uveitis or iritis; 6. rheumatic or musculoskeletal events. B. Autoimmune neurological complications i) Mild events (grades 1–2) with nonspecific neurologic symptoms: headaches, dizziness, paresthesias or small-fiber sensory neuropathies. ii) Serious events (grades 3–4), manifested as: 1. inflammatory myopathies; 2. myasthenia gravis; 3. vasculitis; 4. neuropathies; 5. aseptic meningitis; 6. autoimmune encephalitis; 7. multiple sclerosis; 8. hypophysitis. iii) Trigger of preexisting autoimmune neurological diseases |
The overall incidence of neurological complications ranges from 2% to 4%.1–9 Mild events (grades 1–2) occur in up to 6–12% of patients and consist of nonspecific neurological symptoms, such as headaches, dizziness, paresthesias or small-fiber sensory neuropathies that do not overall impact ICPI continuation. More serious events (grades 3–4) occur in fewer than 1%, ranging from 0.4% to 0.2% with nivolumab and pembrolizumab, 0.3–0.8% with ipilimumab and 2.4–14% with the combination of PD-1 and CTLA-4 inhibitors (i.e. ipilimumab with nivolumab).1–9,14–16 In one series, among 347 patients treated with pembrolizumab or nivolumab, 10 (2.9%; 7 on pembrolizumab and 3 on nivolumab) developed neuromuscular complications after a median of 5.5 (range: 1–20) cycles of treatment.6 ICPIs can also precipitate preexisting autoimmune diseases, with an estimated 27–42% risk for mild to moderate exacerbations.7,13,17–20 Ipilimumab seems more commonly associated with neurological events, although PD-1 inhibitors may confer a greater risk over time because of their prolonged administration.
Neurological events
Inflammatory myopathies. Among all the inflammatory myopathy subtypes,21–23 dermatomyositis, polymyositis and especially necrotizing autoimmune myositis (NAM) are the most frequent autoimmune myopathies triggered, especially by pembrolizumab, ipilimumab and nivolumab.6,10,18,20,24–29 NAM, which is emerging as the commonest type of inflammatory myopathy,23 is also the most common subtype associated with ICPIs. In some patients, NAM may coexist with MG, or MG-like symptoms, presenting with head drop, proximal muscle weakness, myalgia, dyspnea, ophthalmoparesis or bulbar weakness. Among 654 patients receiving ICPIs (pembrolizumab = 389; nivolumab = 264; both = 1), 5 on pembrolizumab had biopsy-proven myopathies (2 NAM, 1 dermatomyositis, and 2 nonspecific myopathy).6 Eosinophilic fasciitis28 and orbital myositis30 have been also reported.
ICPIs can also exacerbate polymyositis.29 In one such patient who was stable on IVIg, CK increased within 1 week after starting pembrolizumab, with worsening of painful weakness and further CK elevation after subsequent infusions. Of interest, myositis improved after 10 months of therapy while the patient became tumor-free.
Myasthenia gravis. A number of patients develop ocular MG 7–11 weeks after ICPI initiation, most often using pembrolizumab and nivolumab;31–34 others develop generalized MG, including myasthenic crisis. A common coincidence is the elevated CK,34 as mentioned earlier. ICPIs can also exacerbate preexisting MG.29,32 In one study, the incidence of MG was 0.12%, occurring in 12 patients among 9869 treated with nivolumab.34 MG can evolve rapidly at all stages of these treatments, even after completion, and generally responds well to corticosteroids, IVIg or plasmapheresis.
Vasculitis. Vasculitis occurs either in single organs (retina and uterus), or in the form of giant cell arteritis10,20,24 and polymyalgia rheumatica, as reported in two patients treated with ipilimumab.10
Neuropathies. Neuropathies occur in fewer than 1% and vary in severity from the small-fiber sensory type (as commonly seen with chemotherapies, not affecting the continuation of ICPIs), to more typical immune-mediated, such as Guillain–Barré syndrome (occurring in 0.1–0.2%) and chronic inflammatory demyelinating polyneuropathy (CIDP).2–4,10–13,15,35–39 Because melanocyte antigens, such as GM2, GM3, GD2, and GD3 are present in both, the gangliosides on the myelin sheath and the melanomas,40 molecular mimicry and ICPI-induced disruption of immune homeostasis might explain the development of CIDP.4,24,35 Polyradiculoneuropathies, meningoradiculoneuritis with facial diplegia, muscle weakness and uptake of the caudal nerve fibers have been reported, responding rapidly to high-dose steroids or IVIg.4,24,35,38 Cranial mononeuropathies affecting the optic nerve, abducens or facial nerves, either isolated or in a setting of meningoradiculoneuritis, can also occur3,16,41
Aseptic meningitis. Aseptic meningitis typically presents at 1–7 weeks after the first dose of ICPIs and occurs in approximately 0.1–0.2% of patients.3,16,20,42 Cerebrospinal fluid (CSF) shows lymphocytosis and magnetic resonance imaging (MRI) may show meningeal enhancement.42 Most patients respond to steroids.3,36
Autoimmune encephalitis. Autoimmune encephalitis occurs in 0.1–0.2% of patients within days or a few weeks after ICPI initiation, especially with combined treatment of ipilimumab and nivolumab.4,12–16,39,42 A number of patients have NMDA receptor (NDMAR) antibodies, as seen in other autoimmune encephalopathies, or combined with paraneoplastic antibodies such as contactin-associated protein-like 2 or anti-Hu.15 Because NMDAR is expressed on melanocytes and the GRIN2A gene that encodes the NMDAR subunit GluN2A is highly mutated in patients with melanomas,29,43 the encephalitis probably represents molecular mimicry when the ICPI-induced disruption of immune tolerance triggers antibodies against the NMDAR expressed on both melanoma cells and the central nervous system (CNS) synapses.4,13,29,43 Autoimmune encephalitis responds to high-dose steroids or standard immunotherapies.4,13 One case with anti-Hu antibodies responded to natalizumab, suggesting a role of hyperactive T cells.44 Because natalizumab decreases CNS inflammation without compromising the immune reaction against systemic cancer localizations, it was suggested that natalizumab could be a more suitable therapy if continuation of ICPIs is indicated.44
Multiple sclerosis. Exacerbation of MS or de novo occurrence of CNS demyelinating disease, such as optic neuritis, transverse myelitis and acute tumefactive demyelinating lesions, can occur within weeks or months after treatment with ipilimumab, pembrolizumab or nivolumab.13,45–47 Transition from radiologically isolated syndrome to clinically definite multiple sclerosis after ipilimumab initiation was also reported.13 One MS patient after two courses of ipilimumab, given 2 years apart, had complete melanoma regression but developed a transient but massive increase in clinical or MRI activity after each ipilimumab course. In this patient, investigation of TCR gene sequencing revealed two distinct oligoclonal CD4 and CD8 T cell repertoires induced by the anti-CTLA-4 effect of ICPIs; an immune response against the tumor and another against CNS antigens resulted in profound MS disease activity.13,48 Because this patient subsequently remained stable with IFNβ, it was suggested that subclinical or even clinical CNS inflammation may not be a strict contraindication to using ICPIs.13,48 An enhanced in situ CNS inflammation may explain the MS relapses because under inflammatory conditions PDL1 is highly expressed on astrocytes and microglia; blocking the interaction between PDL1 and PD1 on lymphocytes infiltrating the CNS might therefore increase the local inflammatory response.13
Hypophysitis. Hypophysitis may occur in up to 5–10% of patients, usually 6–12 weeks after ICPI initiation, representing an immune-related toxicity. It presents with headache, fatigue, dizziness and multiple anterior pituitary hormone deficiencies (mostly adrenocorticotropic and thyroid-stimulating hormone).4,13 Serum level of pituitary hormones is low and brain MRI may show enhancement and swelling of the pituitary gland.3 Hypophysitis responds to high-dose steroids along with hormonal supplementation.49
Therapies
Immunotherapies are generally guided by the type and severity of the neurological event, the relative risks and benefit of treatment, and any associated comorbidities or potential contraindications. There is no optimal or standardized therapeutic regimen and treatments remain still empirical, based on our existing experience with each of these disorders in non-cancer patients. Intravenous corticosteroids, IVIg and plasmapheresis are the first-line therapies, followed by immunosuppressants, such as mycofenolate, rituximab, methotrexate and cyclophosphamide. Alternative immunosuppressive agents not commonly used in neurologic conditions have been considered in refractory cases including proteasome inhibitors (bortezomib), tacrolimus or IL-17 blockers.7
Many oncologists in a setting of a potentially treatable immune-related neurological complication recommend continuation of ICPIs until the underlying malignancy is halted; others prefer to discontinue ICPIs if complete response has been achieved; and still others believe that if a durable response was observed with only a few doses, the duration of ICPIs can be shortened once the immune system is appropriately primed. In general, in severe cases ICPI therapies are stopped until the neurological event is controlled with prompt immunotherapy; reinstitution of ICPIs remains at the physician’s discretion, considering life expectancy and cancer severity.15,20
Based on anecdotal data, there has been a suggestion that the development of neurological events may be associated with an increase in tumor objective response rate (ORR).15,20,24 ORR was reported in 50–70% of patients who developed neurological events, compared to 20–30% who did not, with an increased overall survival.15,20,24 The significance of these small and uncontrolled but interesting observations is still unclear. Based on the available limited data, however, the immunotherapies applied to treat the neurological complications do not seem to attenuate the efficacy of ICPIs.
Unresolved issues
As the use of ICPIs grows rapidly, it is likely that the incidence of neurological events will increase. It is hoped that the growing experience will clarify the following issues and questions that remain still unsettled.1
Preexisting autoimmune disease and ICPI treatment. In patients with known but inactive autoimmune disorders, ICPIs, by disturbing immune tolerance, can unravel preexisting autoimmunity leading to disease exacerbations in 27–38% of patients. A classic example is the severe exacerbation of MS during ipilimumab therapy, even though the oncological response was good.45 As many of the neurological events can be successfully managed, the risk–benefit ratio seems to favor using the life-saving ICPIs in patients with subclinical CNS inflammatory disease.19 Until predicting factors are identified, however, and the practical risk–benefit ratio is defined, the use of ICPIs in such settings must be individualized, especially since some of the neurological complications can be severe and life-threatening.
The paraneoplastic component as a contributing substrate. ICPIs could theoretically promote immune-mediated paraneoplastic neurological syndromes, especially when these conditions result from a cross-reactive immune response against a self-antigen expressed on both neural cells and tumor cells.13 In a phase II trial, antineuronal antibodies such as anti-Hu, and anti-Yo (implicated in paraneoplastic cerebellar degeneration and subacute sensory neuropathy) were detectable in 45% of patients with small cell lung cancer treated with ipilimumab and chemotherapy. Although at times difficult, clinicians must be cognizant in distinguishing whether a neurological event is part of the paraneoplastic process or an ICPI-triggered autoimmune disease. Paraneoplastic complications evolve slowly and do not fit the autoimmune and temporal profile of the ICPI-triggered disorders; in contrast, the autoimmune diseases evolve rapidly and occur at all stages of ICPI treatments, even after completion.13
Susceptibility to ICPI-induced autoimmune disease. It remains unclear why autoimmune disorders occur in some patients without preexisting autoimmunity but not in others, even though the T cell activation is always profound. Whether polymorphisms in CTLA-4 play a role, as suggested,4,50 remains to be explored. Screening for common autoantibodies before ICPI initiation may provide some clues, but several of these antibodies are non-pathogenic or very specific and it remains uncertain whether they can predict susceptibility. In a retrospective literature review of 123 patients in 43 different publications with obvious limitations, 75% of patients receiving ICPIs had an exacerbation of preexisting autoimmune disease or immune-related adverse events of varying severity; of interest, patients receiving immunosuppressive therapy at initiation of ICPIs seemed to have fewer adverse events.51 The practicality of this observation remains uncertain, given the significant study limitations.
Conventional immunotherapies in conjunction with ICPIs. It has been questioned whether the immunosuppressants used to treat the ICPI-triggered neurological events can reduce the ICPIs’ antitumor efficacy and whether they increase the risk for opportunistic infections. The latter is of concern because in a setting of cancer and prior ICPI administration, the risk of infections, especially progressive multifocal leukoencephalopathy (PML), may be high, necessitating vigilance and close follow up.
Restarting ICPI treatment after a major immune event. The safety of retreatment with ICPI remains unsettled. Although this decision depends on several factors, especially life expectancy, the severity of the initial immune-related neurological event undoubtedly plays a fundamental role. Using a different ICPI may be an evolving option because immune-related adverse events associated with one class of ICPIs (e.g. anti-CTLA-4) may not necessarily recur with another class (e.g. anti-PD-1). More data are clearly needed.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The author declares that there is no conflict of interest.
References
- 1. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378: 158–168. [DOI] [PubMed] [Google Scholar]
- 2. Fellner A, Makranz C, Lotem M, et al. Neurologic complications of immune checkpoint inhibitors. J Neuro-Oncology 2018; 137: 601–609. [DOI] [PubMed] [Google Scholar]
- 3. Astaras C, Michell R, Moura B, et al. Neurological adverse events associated with immune checkpoint inhibitors: diagnosis and management. Curr Neurol Neurosci Rep 2018; 18: 3. [DOI] [PubMed] [Google Scholar]
- 4. Hottinger AF. Neurologic complications of immune checkpoint inhibitors. Curr Opin Neurol 2016; 29: 806–812. [DOI] [PubMed] [Google Scholar]
- 5. Wick W, Hertenstein A, Platten M. Neurological sequelae of cancer immunotherapies and targeted therapies. Lancet Oncol 2016; 17: e529–e541. [DOI] [PubMed] [Google Scholar]
- 6. Kao JC, Liao B, Markovic SN, et al. Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol 2017; 74: 1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Touat M, Talmasov D, Ricard D, et al. Neurological toxicities associated with immune-checkpoint inhibitors. Curr Opin Neurol 2017; 30: 659–668. [DOI] [PubMed] [Google Scholar]
- 8. Suarez-Almazor ME, Kim ST, Abdel-Wahab N, et al. Immune-related adverse events with use of checkpoint inhibitors for immunotherapy of cancer. Arthritis Rheumatol 2017; 69: 687–699. [DOI] [PubMed] [Google Scholar]
- 9. Tak PL, Kalden JR. Advances in rheumatology: new targeted therapeutics. Arthritis Res Ther 2011; 25(13 Suppl. 1): S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cappelli LC, Gutierrez AK, Bingham CO, et al. Rheumatic and musculoskeletal immune-related adverse events due to immune-checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res 2017; 69: 1751–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dalakas MC. Novel future therapeutic options in myasthenia gravis. Autoimmun Rev 2013; 12: 936–941. [DOI] [PubMed] [Google Scholar]
- 12. Hohlfeld R, Dalakas MC. Basic principles of immunotherapy in neurological diseases. Sem Neurol 2003; 23: 121–132. [DOI] [PubMed] [Google Scholar]
- 13. Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol 2017; 13(12):755–763. [DOI] [PubMed] [Google Scholar]
- 14. Feng S, Coward J, McCaffrey E, et al. Pembrolizumab-induced encephalopathy: a review of neurological toxicities with immune checkpoint inhibitors. J Thorac Oncol 2017; 12: 1626–1635. [DOI] [PubMed] [Google Scholar]
- 15. Cuzzubbo S, Javeri F, Tissier M, et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer 2017; 73: 1–8. [DOI] [PubMed] [Google Scholar]
- 16. Larkin J, Chmielowski B, Lao CD, et al. Neurologic serious adverse events associated with nivolumab plus ipilimumab or nivolumab alone in advanced melanoma, including a case series of encephalitis. Oncologist 2017; 22: 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gutzmer R, Koop A, Meier F, et al. Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur J Cancer 2017; 75: 24–32. [DOI] [PubMed] [Google Scholar]
- 18. Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2016; 2: 234–240. [DOI] [PubMed] [Google Scholar]
- 19. Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 2017; 28: 368–376. [DOI] [PubMed] [Google Scholar]
- 20. Spain L, Walls G, Julve M, et al. Neurotoxicity from immune-checkpoint inhibition in the treatment of melanoma: a single centre experience and review of the literature. Ann Oncol 2017; 28: 377–385. [DOI] [PubMed] [Google Scholar]
- 21. Dalakas MC. Polymyositis, dermatomyositis and inclusion body myositis. N Engl J Med 1991; 325: 1487–1498. [DOI] [PubMed] [Google Scholar]
- 22. Dalakas MC. Immunopathogenesis of inflammatory myopathies: a critical review on pathogenesis and therapies. Ann Neurol 1995; 37: 74–86. [DOI] [PubMed] [Google Scholar]
- 23. Dalakas MC. Myositis: are autoantibodies pathogenic in necrotizing myopathy? Nature Rev Rheumatol 2018; 14: 251–252. [DOI] [PubMed] [Google Scholar]
- 24. Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 2016; 60: 210–225. [DOI] [PubMed] [Google Scholar]
- 25. Sheik Ali S, Goddard AL, Luke JJ, et al. Drug-associated dermatomyositis following ipilimumab therapy: a novel immune-mediated adverse event associated with cytotoxic T-lymphocyte antigen 4 blockade. JAMA Dermatol 2015; 151: 195–199. [DOI] [PubMed] [Google Scholar]
- 26. Hunter G, Voll C, Robinson CA. Autoimmune inflammatory myopathy after treatment with ipilimumab. Can J Neurol Sci 2009; 36: 518–520. [DOI] [PubMed] [Google Scholar]
- 27. Yoshioka M, Kambe N, Yamamoto Y, et al. Case of respiratory discomfort due to myositis after administration of nivolumab [letter]. J Dermatol 2015; 42: 1008–1009. [DOI] [PubMed] [Google Scholar]
- 28. Khoja L, Maurice C, Chappell M, et al. Eosinophilic fasciitis and acute encephalopathy toxicity from pembrolizumab treatment of a patient with metastatic melanoma. Cancer Immunol Res 2016; 4: 175–178. [DOI] [PubMed] [Google Scholar]
- 29. Liewluck T, Kao JC, Mauremann ML. PD-1 inhibitor-associated myopathies: emerging immune-mediated myopathies. J Immunother 2018; 41: 208–211. [DOI] [PubMed] [Google Scholar]
- 30. Henderson AD, Thomas DA. A case report of orbital inflammatory syndrome secondary to ipilimumab. Ophthal Plast Reconstr Surg 2015; 31: e68–e70. [DOI] [PubMed] [Google Scholar]
- 31. Nguyen BH, Kuo J, Budiman A, et al. Two cases of clinical myasthenia gravis associated with pembrolizumab use in responding melanoma patients. Melanoma Res 2017; 27: 152–154. [DOI] [PubMed] [Google Scholar]
- 32. Gonzalez NL, Puwanant A, Lu A, et al. Myasthenia triggered by immune checkpoint inhibitors: new case and literature review. Neuromuscul Disord 2017; 27: 266–268. [DOI] [PubMed] [Google Scholar]
- 33. Lau KH, Kumar A, Yang IH, et al. Exacerbation of myasthenia gravis in a patient with melanoma treated with pembrolizumab. Muscle Nerve 2016; 54: 157–161. [DOI] [PubMed] [Google Scholar]
- 34. Suzuki S, Ishikawa N, Konoeda F, et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 2017; 89: 1–8. [DOI] [PubMed] [Google Scholar]
- 35. Liao B, Shroff S, Kamiya-Matsuoka C, et al. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol 2014; 16: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thaipisuttikul I, Chapman P, Avila EK. Peripheral neuropathy associated with ipilimumab: a report of 2 cases. J Immunother 2015; 38: 77–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilgenhof S, Neyns B. Anti-CTLA-4 antibody-induced Guillain–Barre syndrome in a melanoma patient. Ann Oncol 2011; 22: 991–993. [DOI] [PubMed] [Google Scholar]
- 38. Bompaire F, Mateus C, Taillia H, et al. Severe meningo-radiculo-neuritis associated with ipilimumab. Invest New Drugs 2012; 30: 2407–2410. [DOI] [PubMed] [Google Scholar]
- 39. Wilgenhof S, Neyns B. Anti-CTLA-4 antibody-induced Guillain–Barré syndrome in a melanoma patient. Ann Oncol 2011; 22: 991–993. [DOI] [PubMed] [Google Scholar]
- 40. Weiss MD, Luciano CA, Semino-Mora C, et al. Molecular mimicry in chronic inflammatory demyelinating polyneuropathy and melanoma. Neurology 1998; 51: 1738–1741. [DOI] [PubMed] [Google Scholar]
- 41. Altman AL, Golub JS, Pensak ML, et al. Bilateral facial palsy following ipilimumab infusion for melanoma. Otolaryngol Head Neck Surg 2015; 153: 894–895. [DOI] [PubMed] [Google Scholar]
- 42. Yeh OL, Francis CE. Ipilimumab-associated bilateral optic neuropathy. J Neuroophthalmol 2015; 35: 144–147. [DOI] [PubMed] [Google Scholar]
- 43. Wei X, Walia V, Lin JC, et al. Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nat Genet 2011; 43: 442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hottinger AF, de Micheli R, Guido V, et al. Natalizumab may control immune checkpoint inhibitor-induced limbic encephalitis. Neurol Neuroimmunol Neuroinflamm 2018; 5: e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gettings EJ, Hackett CT, Scott TF. Severe relapse in a multiple sclerosis patient associated with ipilimumab treatment of melanoma. Mult Scler 2015; 21: 670. [DOI] [PubMed] [Google Scholar]
- 46. Wilson MA, Guld K, Galetta S, et al. Acute visual loss after ipilimumab treatment for metastatic melanoma. J Immunother Cancer 2016; 4: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cao Y, Goods BA, Raddassi K, et al. CNS demyelination and enhanced myelin-reactive responses after ipilimumab treatment. Neurology 2016; 86: 1553–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gerdes LA, Held K, Beltran E, et al. CTLA4 as immunological checkpoint in the development of multiple sclerosis. Ann Neurol 2016; 80: 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Corsello SM, Barnabei A, Marchetti P, et al. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab 2013; 98: 1361–1375. [DOI] [PubMed] [Google Scholar]
- 50. Liu SM, Sutherland AP, Zhang Z, et al. Overexpression of the CTLA-4 isoform lacking exons 2 and 3 causes autoimmunity. J Immunol 2012; 188: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abdel-Wahab N, Shah M, Lopez-Olivo M, et al. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease. Ann Intern Med 2018; 168: 121–130. [DOI] [PubMed] [Google Scholar]


