Abstract
The neurotransmitters dopamine and serotonin participate in specific behavioral neuromuscular mechanisms in the nematode Caenorhabditis elegans. Dopamine is involved in the gentle touch response and serotonin in the pharyngeal pumping rate. In its genome, the worm presents genes encoding dopamine and serotonin receptors orthologous to those of human genes. Risperidone and aripiprazole are a class of drugs known as atypical antipsychotics commonly used to treat schizophrenia, bipolar disorder, and irritability associated with autism. Risperidone is an antagonist of the dopamine D2 and serotonin 5-HT2A receptors. Aripiprazole functions as a partial agonist of the dopamine D2 receptor and as a partial agonist and antagonist of 5-HT1A and 5-HT2A serotonin receptors, respectively. Our results show that risperidone and aripiprazole alter the touch response and pharyngeal pumping in wild-type worm animals. Furthermore, in the presence of the drugs, both behaviors change to varying degrees in dopamine (dop-1, dop-2, and dop-3), serotonin (ser-1), and tyramine (ser-2) receptor-deficient mutants. This variation in response reveals specific targets for these antipsychotics in the nematode. Interestingly, their effect on behavior persisted to some extent in successive generations, indicating that they might induce epigenetic changes throughout development. Sodium butyrate, a histone deacetylase inhibitor, eliminated the consecutive generation effect of both drugs. In addition, these transgenerational effects were also abolished after the dauer stage. These observations suggest that risperidone and aripiprazole, in addition to interacting with specific receptors impairing the function of the nervous system of the nematode, may lead to the deposition of long-lasting epigenetic marks.
Keywords: risperidone, aripiprazole, gentle touch response, pharyngeal pumping rate, transgenerational epigenetics, C elegans
Introduction
Caenorhabditis elegans has several advantages for being used as a model organism to study animal behavior.1 The connectivity between the neurons of the nematode was the first complete connectome published for any nervous system.2,3 The analysis of the neural circuitry allows for detailed models of how neurons function together to generate behavior.4 The hermaphrodite has exactly 302 neurons and 56 glial cells, and unlike most living systems, the number of somatic cells is invariable with 959 somatic cells in total. This fact makes it possible to know the lineage history of each cell, allowing the study of the origin of the 118 morphologically distinct neuron classes during development.5 From a molecular point of view, there are several similarities between the nervous system of C elegans and mammals.6,7 The nematode uses various neuromodulators, including monoamines (dopamine and serotonin) and several neuropeptides.8 Furthermore, the 83% of the C elegans proteome is orthologous to the vertebrate’s proteome.9 This information, together with practicable genetic advantages, a basic anatomy, and different behavioral assays, makes C elegans a valuable model for studying basic behavioral mechanisms.1,10-12 Furthermore, the nematode is used to understand the mechanisms of transgenerational epigenetic inheritance.13
The atypical antipsychotics risperidone and aripiprazole have been reported to be efficacious in treating aggression, self-injurious behavior, and severe tantrums in children and adolescents.14,15 Both medications are FDA (Food and Drug Administration) approved in children and adolescents between 6 and 17 years old. Although second-generation or atypical antipsychotic drugs were developed to reduce the frequency of extrapyramidal syndrome,16 there is still a frequent risk of adverse effects in children and adolescents taking risperidone or aripiprazole. A Bayesian meta-analysis study with children and adolescents treated with risperidone or aripiprazole showed that both increased the risk of somnolence/sedation and produced an increase in weight gain.17 In addition, risperidone increased prolactinemia and glucose levels, and aripiprazole augmented the risk of extrapyramidal syndrome.17
Binding studies in vitro showed that as an antagonist, risperidone had high affinity for serotonin 5-HT2, dopamine D2, α1 and α2 adrenergic, and H1 histaminergic receptors.18 Aripiprazole exhibited partial agonist properties over dopamine D2 receptor and had serotonin 5-HT1A-receptor partial agonist as well as 5-HT2A-receptor antagonist properties.19 Therefore, these drugs exhibit complex mechanisms in their interaction with the nervous system which in several ways remain unknown.
In this article, we study the effect of risperidone and aripiprazole on the gentle touch response and the pharyngeal pumping rate of C elegans. These neuromuscular behaviors are linked to dopamine and serotonin neurotransmission, respectively. These drugs, like others targeting biogenic amine receptors, were developed with humans in mind and may not interact in the same way with the nematode receptors.20 Previous studies have shown that risperidone influenced growth of the nematode21 and that risperidone and aripiprazole produced alterations in the development of some of its neurons.22 Our results show that both drugs alter the behavioral patterns in the wild-type strain.
In addition, we analyzed the effect of risperidone and aripiprazole in different dop- and ser- mutants, including dop-2, dop-3, and ser-1 because they encode receptors with the highest percentage of similarity with the main target genes described for these antipsychotics in humans. These receptors belong to a super-family of 7-transmembrane G protein–coupled receptors orthologous to D2, 5-HT, and adrenergic α-2A human receptors.
In human and mice, risperidone is associated with a decrease in histone acetylation at the GRM2 promoter.23 In relation to this observation, we found that the effects of risperidone and aripiprazole on gentle touch response and pharyngeal pumping behaviors remained, to some extent, in successive generations. Treatment with sodium butyrate (SB) or dauer larvae passage indicates that epigenetic mechanisms could be involved.
Materials and Methods
Experimental design
The experimental design is summarized in Table 1.
Table 1.
Experimental design.
| Worm strains | N2 wild-type strain. Serotonin, dopamine, and tryptamine receptor-deficient mutants. |
| Worm synchronization | L4 larval stage synchronized by egg laying and bleaching methods. |
| Risperidone and aripiprazole NGM plates | Eggs or L1 larvae from bleaching method were incubated on NGM plates with 150 and 300 µM risperidone or aripiprazole + 1% DMSO until they reached L4 stage. Control plates NGM + 1% DMSO. |
| Behavioral assays | Gentle touch response and pharyngeal pumping rate were measured when worms reached L4 stage. In all cases, at least 3 independent experiments were performed with no less than 10 worms each. |
| Transgenerational assays | Eggs or L1 larvae from N2 wild type were incubated on NGM plates with risperidone or aripiprazole (300 µM + 1% DMSO) until they reached L4 stage. Then, behavioral assays were accomplished. When L4 worms reached gravid adult stage, they were placed on NGM plates without drugs. After egg laying, behavioral assays were performed at L4 stage. Up to 3 generations were analyzed. The same procedure was performed in the presence of sodium butyrate (1 mM), a histone deacetylase inhibitor, or by induction of dauer stage by starvation. |
| Statistical analysis | Comparisons in each experiment were done by 1-way analysis of variance using SPSS statistical tool. |
Abbreviations: DMSO, dimethyl sulfoxide; NGM, Nematode Growth Medium.
Strains and maintenance
All nematodes were grown and maintained at 20°C seeded with OP50 under standard conditions on Nematode Growth Medium (NGM) agar plates as described previously.1
The wild-type reference animals for all cases are the N2 Bristol strain. The following strains were used in this work: LX645:dop-1(vs100)X, LX702:dop-2(vs105)V, LX703:dop-3(vs106)X, LX706:dop-1(vs100)X;dop-2(vs105)V, LX705: dop-1(vs100)X;dop-3(vs106)X, LX704:dop-2(vs105)V;dop-3(vs106)X, LX734:dop-1(vs100)X;dop-2(vs105)V;dop-3(vs106)X, DA1814:ser-1(ok345)X, and OH313:ser-2(pk1357)X. The OP50 Escherichia coli strain and all the nematode strains were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA).
The behavioral assays were performed with synchronized worms at the L4 larval stage, identifiable by a white crescent-shaped mark in the vulval region. The larval developmental stage was determined using a ZEISS Discovery V8 Stereo Microscope with a cold light source. Synchronized worms were obtained by 2 different methods, eggs laying and bleaching. The eggs laying strategy consisted in picking about 15 gravid worms, incubate them at 20°C in NGM agar plates, and after the eggs were laid the adults were removed from the plate after 24 hours. Then, the progeny was allowed to grow until the L4 stage for performing the experiments. The second method consisted in bleaching of gravid adult worms.24 Worms are sensitive to bleach, but the eggs are protected by their shells. After the treatment with an alkaline hypochlorite solution (2.5 mL NaOH 1N + 1 mL bleach 4%), the eggs were washed and incubated in M9 buffer. This allows hatching but avoids development after the L1 larvae stage. These L1 stage stocks were used in the next 24 to 48 hours to grow the worms synchronously on NGM plates until the L4 stage for performing the behavioral experiments.
Risperidone and aripiprazole assays
Risperidone powder (Adooq Bioscience LLC, Irvine, CA, USA, and a gift from Janssen-Cilag S.A., Madrid, Spain) and aripiprazole powder (Adooq Bioscience LLC) were diluted in dimethyl sulfoxide (DMSO) to obtain a stock solution (30 mM). From here, NGM agar plates with risperidone and aripiprazole, 150 and 300 µM, were obtained with a final concentration of 1% DMSO. Risperidone and aripiprazole were added into melted autoclaved NGM agar at around 40°C to 50°C. The control plates without the antipsychotics were prepared with 1% of DMSO. Eggs or synchronized L1 larvae stocks of each strain were seeded in these plates, and when they developed to the late L4 larval stage, they were tested for gentle touch response and pharyngeal pumping rate.
Behavioral assays
All the behavioral assays were performed with worms in L4 stage. For each experimental condition, at least 3 independent experiments with at least 10 worms of each strain were performed. The behavioral assays were performed blind to genotype and treatments in an acclimatized laboratory room at 20°C.
Gentle touch response assay
This assay was performed using an eyebrow hair attached to a Pasteur pipette. The phenotype was tested by gently stocking the worm 10 times with the eyebrow hair alternating the anterior (just behind the pharynx) and posterior (just before the anus) parts of the body. The 10 touches were made without pause. The interval between each touch was approximately 1 second. A positive response was scored when the animal moved backward (induced by anterior stimulus) or forward (induced by posterior stimulus).25,26
Pharyngeal pumping assay
Pharyngeal pumping was quantified by counting the number of pharyngeal contractions of individual L4 hermaphrodite worms grown on NGM plates and seeded with OP50. To count the pumping, individual worms were recorded for 30 seconds focusing on the pharynx. The video recording was followed by offline analysis in slow motion (at a speed 5 times slower) counting 10 seconds in each individual worm. Then, the data were extrapolated to pumps per minute.
Transgenerational assays
Synchronized L1 worms obtained from the bleaching method were placed on seeded NGM control plates (NGM + 1% DMSO) and NGM test plates (300 µM risperidone or 300 µM aripiprazole + 1% DMSO) and allowed to lay eggs. The progeny developed in these media until the late L4 larval stage or to the gravid adult stage. The L4 animals were assayed for gentle touch response and pharyngeal pumping rate. However, to analyze the behavior in the following generations without risperidone or aripiprazole, gravid adult animals after grown in media with the antipsychotic (risperidone and aripiprazole) were placed on seeded NGM plates without drugs and then allowed to develop to the late L4 larval stage or to the gravid adult stage. Again, the L4 animals were assayed for gentle touch response and pharyngeal pumping rate and the gravid adult animals were placed on seeded NGM plates without drugs and allowed again to develop to the late L4 larval stage or to the gravid adult stage. This procedure was repeated consecutively.
SB assays
Sodium butyrate powder (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in water to obtain a stock concentration of 100 mM and added to melted NGM medium at 40°C to 50°C to get a final concentration of 1 mM and then poured into plates. L1 worms obtained from bleaching of gravid adults coming from NGM + risperidone plates (300 µM risperidone + 1% DMSO) or NGM + aripiprazole (300 µM + 1% DMSO) were placed or seeded in NGM plates with SB. Then, they were allowed to develop to the late L4 larval stage and tested for gentle touch response and pharyngeal pumping rate.
Dauer stage assays
L1 worms from adults grown in the presence of risperidone or aripiprazole, obtained from the bleaching method, were placed on NGM plates without antipsychotic and seeded with OP50 bacteria and grown until late L4 larval stage (first generation of transgenerational study, n = 1). Then, they were tested for gentle touch response and pharyngeal pumping rate. Alternatively, L1 worms were placed on NGM plates without bacteria to induce the dauer stage by starvation. Later, the dauer larval stage animals were placed on NGM plates seeded with OP50 bacteria and allowed to develop to the late L4 larval stage. Then, they were assayed for gentle touch response and pharyngeal pumping rate.
Statistical analysis
Comparisons shown in each experiment were done by 1-way analysis of variance (ANOVA) using SPSS statistical tool. For comparing the wild type with the mutant strains, we performed the 1-way ANOVA with all the strains and then corrected using the Bonferroni post hoc test. In the case of treatment with risperidone and aripiprazole, a separate 1-way ANOVA was run to test statistical differences of the drugs’ effects in each strain.
Results
Risperidone and aripiprazole alter the touch response in N2 wild-type animals
When the nematode detects a physical stimulus using an eyebrow hair to stimulate the anterior or posterior part of its body, it changes the direction of movement by going backward or forward, respectively.26 In standard conditions, the worm responds 10 times consecutively, alternating gentle touch in the anterior and posterior parts of the body.
As both antipsychotics were insoluble in water, to test whether risperidone and aripiprazole were able to change the response to gentle touch they were solubilized in DMSO. Concentrations higher than 350and 450 µM for risperidone and aripiprazole, respectively, were not possible to test because of solubility problems and because DMSO at concentrations higher than 1% was toxic and disturbed the behavior.
Figure 1 shows that risperidone and aripiprazole reduce the mechanosensory response capability in the N2 wild-type strain. Concentrations higher than 300 µM of both antipsychotics did not significantly decrease the touch response (Figure 1). We also analyzed whether there were differences in the response to gentle touch in the anterior and posterior parts of the body. We found that risperidone or aripiprazole makes the worms fail to respond, mainly to touch in the anterior part of the body (Figure 2).
Figure 1.
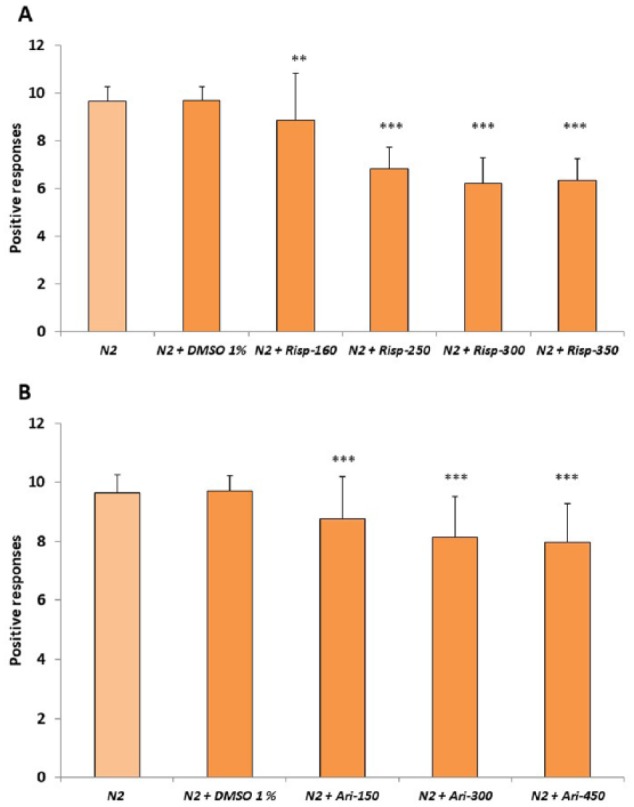
Effect of risperidone and aripiprazole in gentle touch response of Caenorhabditis elegans. Gentle touch response of the N2 wild-type strain was performed either without or with 1% DMSO, and in the presence of different micrometer concentrations of (A) risperidone (Risp) or (B) aripiprazole (Ari) in 1% DMSO. Data were analyzed as the number of positive responses to 10 alternative gentle touches in the anterior and the posterior parts of the body. At least 3 independent experiments were performed with no less than 10 L4 worms per experiment. Bars represent the mean ± SEM. The comparisons were done using 1-way analysis of variance between “N2 + DMSO” and N2 treated with risperidone or aripiprazole at different concentrations.
Statistical P values: **P < .01; ***P < .001.
Figure 2.
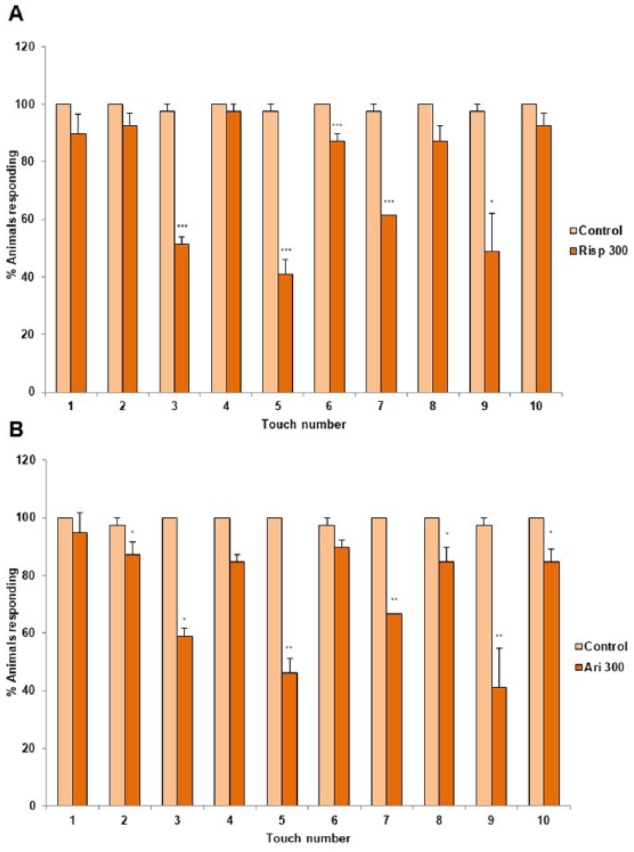
Anterior and posterior gentle touch responses in the presence of risperidone or aripiprazole. Gentle touch response of N2 wild-type strain was performed with 1% DMSO (control, light color), and in the presence of 300 μM of (A) risperidone (Risp) or (B) aripiprazole (Ari) in 1% DMSO (dark color). In the absence of the drugs (light color), control worms respond 5 times alternatively and consecutively to gentle touch in the anterior (1, 3, 5, 7, and 9) and posterior parts of the body (2, 4, 6, 8, and 10). In the presence of 300 µM of antipsychotic (dark color) (A) risperidone or (B) aripiprazole, worms fail to respond significantly to the anterior touches (3, 5, 7, and 9). Data were quantified as percentage of animals responding to 10 alternative gentle touches in the anterior (touches 2, 4, 6, 8, and 10) and the posterior (touches 1, 3, 5, 7, and 9) part of the body. At least 3 independent experiments were performed (at least 10 L4 worms per experiment). Bars represent the mean ± SEM. The statistical analysis was performed comparing the response of each touch, 1, 3, 5, 7, and 9 (anterior part of the body) and 2, 4, 6, 8, and 10 (posterior part of the body), in the absence (light color) and presence (dark color) of the antipsychotic. Statistical significance was calculated by 1-factor analysis of variance.
Statistical P values: ***P < .001; **P < .01; *P < .05 vs “control.”
Comparative effect of risperidone and aripiprazole in the gentle touch response in different dop- and ser- knockout strains of C elegans
To further investigate the underlying mechanism of the effect of risperidone and aripiprazole in gentle touch response, we studied this behavior in different dop- and ser- knockout mutants. Table 2 shows that dop-1, dop-2, and dop-3 single mutants, dop-1; dop-2, dop-1; dop-3; and dop-2; dop-3 double mutants, and dop-1; dop-2; dop-3 triple mutants have a reduced gentle touch response in the absence of antipsychotics, in comparison with the N2 wild-type strain. In contrast, ser-1 and ser-2 mutants behave like the wild-type strain. Except dop-3, dop- and ser-mutants show a diminished response to gentle touch in the presence of risperidone, like the wild-type strain (Table 3). The importance of DOP-3 for the effects seen with risperidone was confirmed in double mutants dop-1; dop-3 and dop-2; dop-3 and in the triple mutant dop-1; dop-2; dop-3, where there was no apparent effect of the antipsychotic (Table 3). These observations indicate that DOP-3 might be a target for risperidone.
Table 2.
Comparative effect in gentle touch response of wild-type and different mutant strains deficient in dop- and ser- genesa,b.
| Strain (I) | Strain (J) | Mean difference (I-J) | P valuec |
|---|---|---|---|
| N2 (n = 80) | dop-1 (n = 90) | 0.40 | .033* |
| dop-2 (n = 90) | 0.56 | .001*** | |
| dop-3 (n = 80) | 0.48 | .004** | |
| dop-1;dop-2 (n = 50) | 0.74 | .001*** | |
| dop-1;dop-3 (n = 50) | 1.23 | .001*** | |
| dop-2;dop-3 (n = 50) | 1.30 | .001*** | |
| dop-1;dop-2;dop-3 (n = 50) | 2.27 | .001*** | |
| ser-1 (n = 40) | −0.07 | 1.00 | |
| ser-2 (n = 50) | −0.06 | 1.00 |
Gentle touch assays comparing the response between the N2 wild-type strain and single-, double-, and triple-mutant strains in NGM agar plates with 1% DMSO.
Bonferroni post hoc analysis after 1-way analysis of variance (sum of squares = 447.232; df = 9; F = 45.239; P = .001) of the wild-type N2 and each mutant strain.
Statistical P values: *P < .1; **P < .01; ***P < .001.
Table 3.
Comparative effect of risperidone in gentle touch response of wild-type and different mutant strains deficient in dop- and ser- genesa,b.
| Strain | Sum of sq. | df | F | P valueb |
|---|---|---|---|---|
| N2 (n = 80)/N2 + risp (n = 80) | 224.16 | 1 | 305.52 | .001*** |
| dop-1 (n = 90)/dop-1+ risp (n = 90) | 68.17 | 1 | 58.72 | .001*** |
| dop-2 (n = 90)/dop-2+ risp (n = 90) | 71.40 | 1 | 61.13 | .001*** |
| dop-3 (n = 80)/dop-3+ risp (n = 80) | 6.16 | 1 | 8.14 | .005** |
| dop-1; dop-2 (n = 50)/dop-1; dop-2 + risp (n = 50) | 18.61 | 1 | 14.25 | .001*** |
| dop-1; dop-3 (n = 50)/dop-1; dop-3 + risp (n = 50) | 6.01 | 1 | 3.58 | .061 |
| dop-2; dop-3 (n = 50)/dop-2; dop-3 + risp (n = 50) | 6.50 | 1 | 3.42 | .067 |
| dop-1; dop-2; dop-3 (n = 50)/dop-1; dop-2; dop-3 + Risp (n = 50) | 0.24 | 1 | 0.13 | .713 |
| ser-1 (n = 40)/ser-1 + risp (n = 40) | 32.05 | 1 | 95.76 | .001*** |
| ser-2 (n = 50)/ser-2 + risp (n = 60) | 24.56 | 1 | 26.14 | .001*** |
Gentle touch assays comparing the response between the N2 wild-type strain and single-, double-, and triple-mutant strains in NGM agar plates in the absence or presence of 300 µM risperidone (Risp).
One-way ANOVA analysis between each strain with and without risperidone.
Statistical P values: **P < .01; ***P < .001.
Likewise, quantitative assays with aripiprazole for gentle touch response were also performed in strains of C elegans defective in dop- and ser- genes. Table 4 shows that the effect of aripiprazole on gentle touch response was annulled in dop-1 and dop-2 knockouts but not in the dop-3 mutant. These results indicate that unlike risperidone, aripiprazole might interact with DOP-1 and DOP-2. Double and triple dop-1, dop-2, and dop-3 mutants confirmed the results obtained with single defective strains (Table 4). As in the case of risperidone, ser-1 and ser-2 mutants showed the same response of that in the wild-type strain.
Table 4.
Comparative effect of aripiprazole in gentle touch response of wild-type and different mutant strains deficient in dop- and ser- genesa,b.
| Strain | Sum of sq. | df | F | P valuec |
|---|---|---|---|---|
| N2 (n = 70)/N2 + ari (n = 70) | 95.83 | 1 | 109.85 | .001*** |
| dop-1 (n = 70)/dop-1 + ari (n = 70) | 1.22 | 1 | 1.02 | .314 |
| dop-2 (n = 70)/dop-2 + ari (n = 70) | 1.85 | 1 | 0.99 | .321 |
| dop-3 (n = 70)/dop-3 + ari (n = 70) | 27.85 | 1 | 19.52 | .001*** |
| dop-1; dop-2 (n = 30)/dop-1; dop-2 + ari (n = 30) | 3.26 | 1 | 2.16 | .146 |
| dop-1; dop-3 (n = 30)/dop-1; dop-3 + ari (n = 30) | 0.41 | 1 | 0.15 | .696 |
| dop-2; dop-3 (n = 30)/dop-2; dop-3 + ari (n = 30) | 0.60 | 1 | 0.35 | .555 |
| dop-1; dop-2; dop-3 (n = 30)/dop-1; dop-2; dop-3 + ari (n = 30) | 0.81 | 1 | 0.45 | .503 |
| ser-1 (n = 40)/ser-1 + ari (n = 40) | 44.62 | 1 | 152.74 | .001*** |
| ser-2 (n = 40)/ser-2 + ari (n = 40) | 34.66 | 1 | 69.24 | .001*** |
Gentle touch assays comparing between the N2 wild-type strain and single-, double-, and triple-mutant strains in NGM agar plates in the absence or presence of 300 µM aripiprazole (Ari).
One-way ANOVA analysis between each strain with and without aripiprazole.
Statistical P values: ***P < .001.
Risperidone and aripiprazole modify the pharyngeal pumping rate in N2 wild-type animals
To study whether risperidone or aripiprazole had an effect in other different neuromuscular mechanisms of the worm, we investigated the pharyngeal pumping rate. Pharyngeal pumping consists of a cycle of muscular contraction and relaxation requiring sensory stimulation, response of the pharyngeal nervous system, and action of the pharyngeal muscles.27 We examined the pharyngeal pumping in the absence and presence of risperidone or aripiprazole at a concentration of 300 µM. As in the case of the gentle touch response, both drugs induced a significant reduction in the number of pharyngeal pumping counts (Figure 3).
Figure 3.
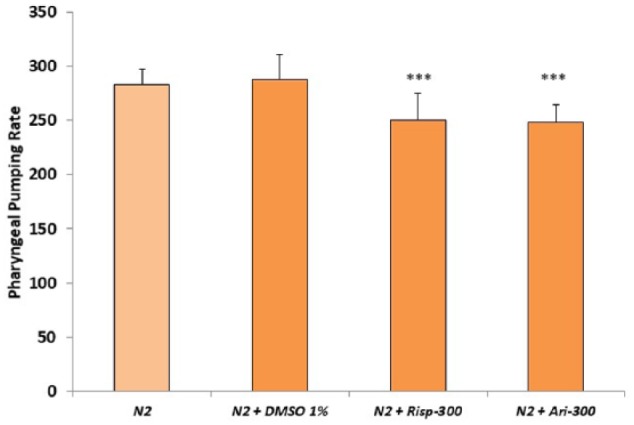
Effect of risperidone and aripiprazole in pharyngeal pumping rate of Caenorhabditis elegans. Pharyngeal pumping rate of the N2 wild-type strain was measured either in the absence (1% DMSO) or presence of 300 µM concentrations of risperidone or aripiprazole. The number of pharyngeal pumps in a minute was counted in slow motion. At least 3 independent experiments were performed with no less than 10 L4 worms per experiment. Bars represent the mean ± SEM. The comparisons were done using 1-way analysis of variance between “N2 + DMSO” and N2 treated with risperidone or aripiprazole. Statistical P values: ***P < .001; **P < .01.
Comparative effect of risperidone and aripiprazole in the pharyngeal pumping of knockout strains in ser-1 and ser-2 receptor genes of C elegans
Previously, it has been shown that the pharyngeal pumping rate of wild type and dopamine receptor loss-of-function dop-1, dop-2, and dop-3 mutants were similar.28 Likewise, the pharyngeal pumping rate of the wild-type strain treated with dopamine was undifferentiated from those of mock-treated animals.28 Our results show that the ser-1 and ser-2 defective mutants displayed an impaired pharyngeal pumping rate compared with the wild-type strain (Figure 3). The mutant ser-1 was insensitive to the effect of risperidone (Figure 4A). The results suggest that SER-1 could be a molecular target of risperidone in C elegans.
Figure 4.
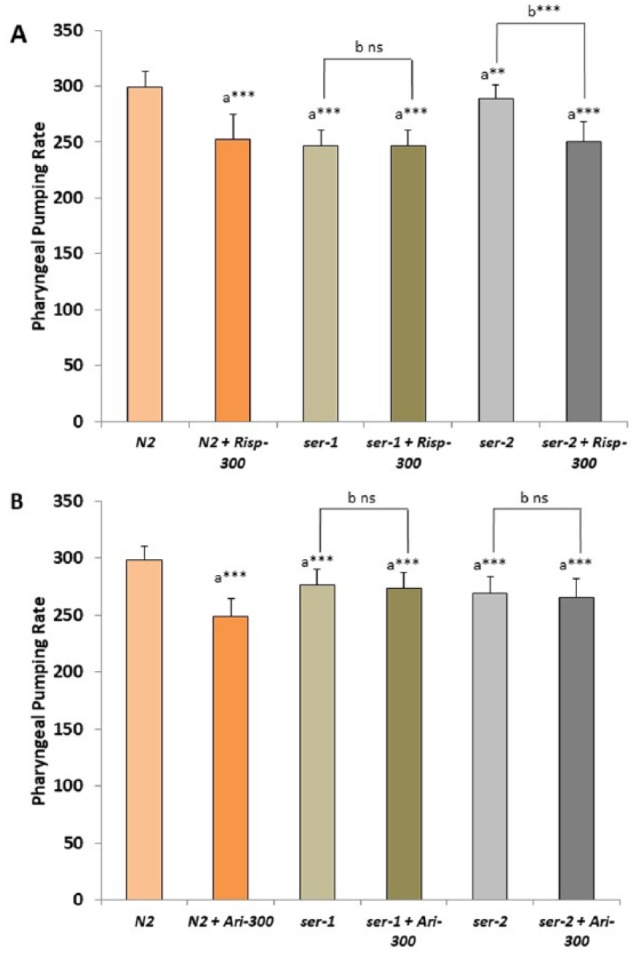
Comparative effect of risperidone and aripiprazole in pharyngeal pumping rate of different mutant strains defective in ser-1 or ser-2 genes. Assays for pharyngeal pumping rate were compared between N2 wild-type strain and ser-1 or ser-2 defective mutant strains in the absence (1% DMSO) or presence of (A) 300 µM risperidone or (B) 300 µM aripiprazole. At least 3 independent experiments were performed with no less than 10 L4 worms per experiment. Bars represent the mean ± SEM. One-way analysis of variance was used to calculate the statistical significance (a) between the N2 wild type and each mutant strain and (b) between each strain with and without the antipsychotic (Supplemental Tables 1 and 2). Statistical P values: ***P < .001.
Unlike risperidone, aripiprazole did not produce any apparent effect in the pharyngeal pumping of both ser-1 and ser-2 defective mutants (Figure 4B). Therefore, aripiprazole might present an affinity for both the serotonin SER-1 and the tyramine SER-2 receptors.
Transgenerational epigenetic inheritance of impaired gentle touch assay and pharyngeal pumping induced by risperidone and aripiprazole
We analyzed whether the effect of risperidone and aripiprazole could be maintained in successive generations in the absence of the antipsychotics. The worms were grown in the presence of the drugs for 1 generation. After that, their effect on gentle touch response and pharyngeal pumping were studied over several generations in the absence of the antipsychotics. The effect of risperidone on the gentle touch response was preserved in 2 successive generations in the absence of the drug (Figure 5A). In the case of aripiprazole, no transgenerational effect was observed (Figure 5B). Different results were obtained regarding the pharyngeal pumping rate. The effect of risperidone did not remain in a single generation (Figure 6A), whereas in the case of aripiprazole, its effect was maintained in one successive generation (Figure 6B).
Figure 5.
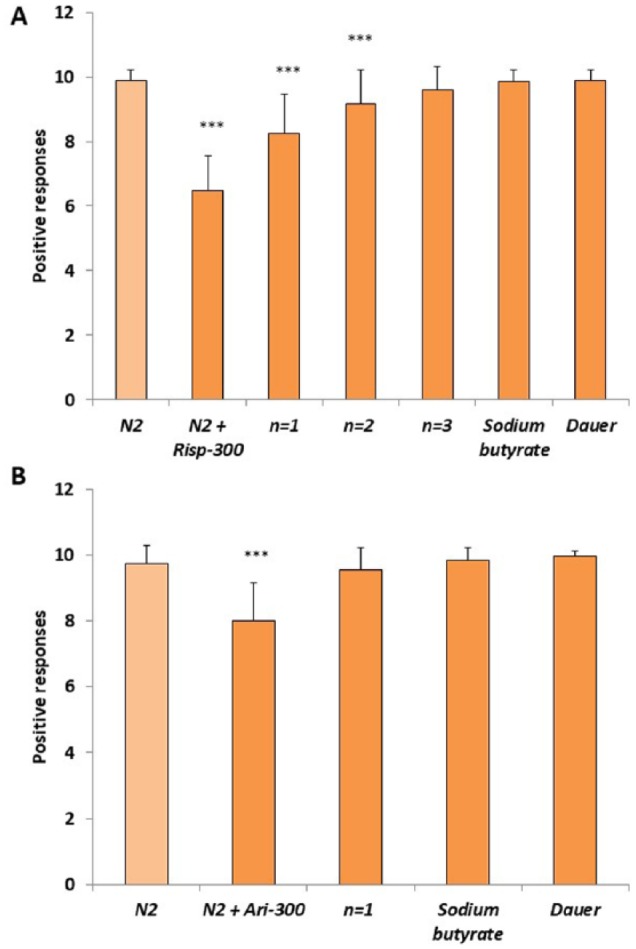
Transgenerational epigenetic inheritance of the effect of risperidone and aripiprazole in the gentle touch response. Transgenerational effect of the (A) risperidone or (B) aripiprazole on gentle touch response in the N2 wild-type strain after several generations (n) in the absence of the antipsychotics. Sodium butyrate (1 mM) or passing the worms through the dauer stage after antipsychotics exposure rescues the impaired effect in gentle touch response. At least 3 independent experiments were carried out with no less than 10 L4 worms per experiment. Bars represent the mean ± SEM. One-way analysis of variance was used to calculate the statistical significance (Supplemental Tables 3 and 4). Statistical P values: ***P < .001.
Figure 6.
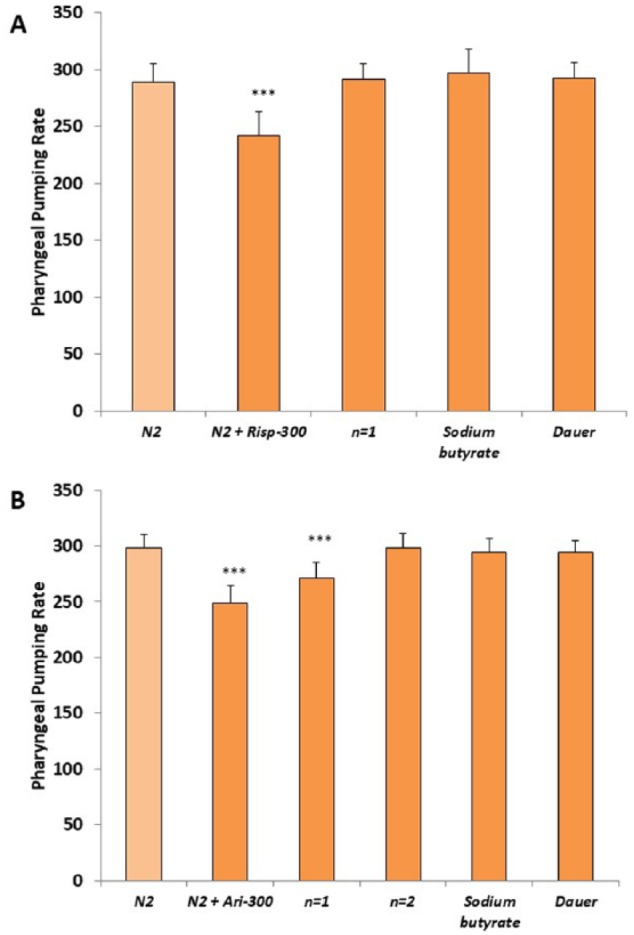
Transgenerational epigenetic inheritance of the effect of risperidone and aripiprazole in the pharyngeal pumping rate. Transgenerational effect of the (A) risperidone or (B) aripiprazole on pharyngeal pumping rate in the N2 wild-type strain after several generations (n) in the absence of the antipsychotics. Sodium butyrate (1 mM) or passing the worms through the dauer stage after antipsychotics exposure rescues the impaired effect in pharyngeal pumping rate. At least 3 independent experiments were performed with no less than 10 L4 worms per experiment. Bars represent the mean ± SEM. One-way analysis of variance was used to calculate the statistical significance (Supplemental Tables 5 and 6). Statistical P values: ***P < .001.
To determine whether risperidone and aripiprazole were inducing epigenetic markers in the worm, we studied whether the histone deacetylase (HDAC) inhibitor SB had any effect on the results. We found that SB (1 mM) abolished the transgenerational effect of risperidone and aripiprazole in the reduction of gentle touch response (Figure 5) and pharyngeal pumping rate (Figure 6). These results suggest that the antipsychotics may be inducing epigenetic changes.
In the C elegans dauer stage, the aging clock is paused and reversed by environmental factors. This event suggests that there is a series of epigenetic mechanisms that could cause drastic changes in gene expression.29 In a favorable environment, there would be a reprogramming of gene expression to activate metabolism and erasing of the epigenetic signature. For this reason, we tested whether the worms recovered the wild-type phenotype response to risperidone and aripiprazole when they pass through the dauer larval stage after growing in media with the antipsychotics. We observed that after passing through the dauer larval stage, the transgenerational effect of risperidone and aripiprazole was eliminated for both the gentle touch response (Figure 5) and pharyngeal pumping rate (Figure 6).
Discussion
Risperidone and aripiprazole reduce the gentle touch response in C elegans
The neurons involved in the detection of gentle mechanical stimuli are well characterized in C elegans. In the gentle touch response, sensory neurons detect and transmit mechanical stimuli by ionic currents to a neural circuit that produces a locomotory response.30 In this circuit, the AVM and ALM neurons exhibit activity in response to touch in the anterior part of the body between the nose and midbody. The PVM and PLM neurons respond to touch in the posterior part of the body between the midbody and the tail.31 Both ALM and PLM neurons have been shown to express the dop-1 receptor gene.32 Our results show that both risperidone and aripiprazole reduced the capability of the N2 wild type to react to gentle touch (Figure 1). These antipsychotics are antagonists33 and partial agonists34 of the human DRD2 dopamine receptor, respectively. Caenorhabditis elegans has 2 orthologues, DOP-2 and DOP-3, for the DRD2 receptor. Therefore, the binding of these drugs to DOP receptors could inhibit the function of the dopaminergic system that regulates the mechanosensory behavior.
The worms failed to respond mainly in the anterior part of the body in the presence of risperidone or aripiprazole (Figure 2). Previous experiments showed that the head touch circuit habituated more rapidly to the tap response than the tail touch circuit.35 Dopamine seems to play a role in the ALM (anterior) touch neurons and not in the PLM (posterior) touch neurons.36 Regarding this observation, it has been reported that DOP-2 functions as a dopamine receptor to modulate anterior touch habituation.37 Although our experiments agree with these observations, we do not know whether the effect of the antipsychotics on touch response can be interpreted as a decreased sensitivity to stimuli instead of habituation. In fact, it was shown that posterior touch can inhibit tap-induced anterior reversal response,38 indicating that the 2 circuits can interact, and therefore it is possible that the response to touch in the anterior part of the body could be influenced by the posterior touches in the presence of the antipsychotics. Another possibility is that as risperidone and aripiprazole have been found to produce effects on neuronal development, including ALM neuroblast migration and PLM axonal outgrowth in C elegans,22 the reduced effect in the touch response could be due to defects in the wiring of the circuit in worms reared on the drug.
Comparative effect of risperidone and aripiprazole in the gentle touch response in C elegans knockout strains in different dop- and ser- receptor genes
Quantitative assays with risperidone for gentle touch response were performed in different strains of C elegans defective in genes encoding DOP-1, DOP-2, DOP-3, SER-1, and SER-2 receptors. The results of Table 2 show that SER-1 and SER-2 are presumably not involved in the gentle touch response, whereas dopamine receptors DOP-132,36,39 and DOP-237 as previously described, as well as DOP-3 are all involved because a reduction in the gentle touch response is observed in the 3 mutants. Only the dop-3 single mutant has a reduced effect of risperidone in the gentle touch response (Table 3). Although knockout of dop-1 and dop-2 genes have no effect on risperidone action, double mutants with dop-3 showed that DOP-1 and DOP-2 can also interact with risperidone to some extent. However, in the case of aripiprazole, DOP-1 and DOP-2 seem to be the main receptors involved in its mechanism of action (Table 4).
DOP-1 is classified as a D1-type receptor, whereas DOP-2 and DOP-3 are classified as D2-type receptors.40 Risperidone has affinity for human D1 and D2 receptors.41 Aripiprazole not only interacts mainly with human D2 receptors but also is able to bind with low affinity to the D1 receptor.34 Therefore, these results are partially in agreement with the mechanism of action proposed in humans. It was expected that the D2-type, dop-2 and dop-3 deficient mutants would be less sensitive to aripiprazole because in human the D2 receptor is the main target of this drug.
Risperidone and aripiprazole reduce the pharyngeal pumping counts in C elegans
The neuromuscular mechanism of the pharyngeal pumping depends on serotonin, octopamine, and tyramine.42,43 It is comparatively different from the gentle touch response that depends on dopamine.28,44 The neuromuscular pump of the worm connects the mouth with the intestine.45 After the pharyngeal muscle has sucked in bacteria by pumping, they are transported through the gut by isthmus peristalsis. The timing of normal rapid feeding is controlled by specific pharyngeal motor neurons.27
Our results indicate that risperidone and aripiprazole may interact specifically with SER-1, an orthologue of HTR2C, a human serotonin receptor. It seems that the effect of risperidone and aripiprazole are ser-1 dependent because there was no effect of these drugs when SER-1 is not functional. The results also indicate that aripiprazole, but not risperidone, seems to bind SER-2, a tyramine receptor46 orthologue of ADRA2A, a human adrenoreceptor α-2A.
Transgenerational epigenetic inheritance of impaired gentle touch response and pharyngeal pumping rate induced by risperidone and aripiprazole
Recently, it has been shown that temperature changes can induce transgenerational information in C elegans that can last for 14 generations.47 This epigenetic memory is associated with repression of set-25 encoding the putative histone methyltransferase that trimethylates histone H3 lysine 9 (H3K9me3). This epigenetic marker is associated with genes that are not expressed or are expressed at very low levels.48 Previously, we had shown that testosterone decreases the gentle touch response and its effect remained for 4 generations in the absence of the hormone.49
It has been reported that risperidone downregulated the transcription of GRM2, which codes for the metabotropic glutamate 2 receptor.23 This result was associated with a decrease in histone acetylation at the GRM2 promoter in the mouse and human frontal cortex. The efficacy of risperidone in schizophrenia was increased, if at the same time the treatment included valproate, a HDAC inhibitor.50 The HDACs repress gene transcription because they remove acetyl groups of histone tails, compacting chromatin structure.
We studied whether risperidone and aripiprazole may induce changes in behavior that were transgenerationally inherited. The effect of these drugs on gentle touch response and pharyngeal pumping was different for the 2 antipsychotics (Figures 5 and 6). In the case of gentle touch response, the effect of risperidone lasted for 2 successive generations. In contrast, the effect on pharyngeal pumping of risperidone and aripiprazole was divergent. Thus, the effect of aripiprazole lasted for 1 generation, whereas no effect of risperidone was observed during the next generation in the absence of the drug.
These results suggest that both antipsychotic drugs could cause the deposition of epigenetic markers to a different extent and probably in different parts of the genome. These changes could not be due to m5C DNA methylation as no conventional DNA methyltransferase has been found in the genome of C elegans.51 Therefore, other mechanisms could be involved, probably changes in chromatin histones.
Sodium butyrate inhibits HDACs in different organisms, including plants,52 Saccharomyces cerevisiae,53 mammalian cells,54,55 Drosophila56 and C elegans,55,57 and therefore we studied whether SB had effects on our experiments.
Our results show that SB eliminates the transgenerational effect of both antipsychotic drugs on gentle touch response and pharyngeal pumping rate. Clinical studies have suggested that drugs such as valproate are effective when administrated chronically in combination with risperidone.58 Valproate can function as a nonspecific HDAC inhibitor.50 The HDAC eliminates acetyl groups from lysine residues in the N-terminal tails of histones and this produces chromatin condensation and silencing of gene expression.59,60 Specifically, SB administration in tissues of mice increased the acetylation of histone H3 lysine 14 and histone H4 lysine 8.61 Other clinical studies in patients with schizophrenia established that HDAC inhibitors are more efficient when they are administrated together with atypical antipsychotics because HDAC inhibitors avoid the repressive histone modifications at the mGlu2 promoter by risperidone, and it is thought that it is the cause of an increase in the therapeutic-like effects in this mental disorder.23
There are mechanisms that can reset the epigenetic modifications of the genome.62 Although the life expectancy of C elegans is only about 2 weeks, under unfavorable environmental conditions, such as in the absence of food, the developing larva can adopt a form of resistance (dauer larva). During this stage, which can last several months, the larva does not feed and metabolically is practically inactive.63 The fact that the metabolism changes so drastically in the dauer larva and that environmental factors can reverse it suggests that there may be epigenetic factors regulating this process. On one hand, epigenetic markers will induce the repression of certain genes and, on the other hand, a reprogramming would take place erasing these markers when the worm leaves the dauer stage. In this scenario, it is possible that when the worm passes through the dauer stage, the epigenetic signals caused by risperidone and aripiprazole could be removed. This may be the reason why, after going through the dauer larva, the gentle touch response and pharyngeal pumping were like those of the control worms grown in the absence of antipsychotic drugs.
In conclusion, it is possible that risperidone and aripiprazole could trigger stable epigenetic changes. If this were the case, it would be interesting to further investigate what are the exact mechanisms that control the action of these drugs in the epigenome.
Supplemental Material
Supplemental material, Supplementary_Tables for Behavioral Mechanisms That Depend on Dopamine and Serotonin in Caenorhabditis elegans Interact With the Antipsychotics Risperidone and Aripiprazole by Jaime Osuna-Luque, Ángel Rodríguez-Ramos, María del Mar GÁmez-del-Estal and Manuel Ruiz-Rubio in Journal of Experimental Neuroscience
Acknowledgments
The authors thank the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA) for the worm strains and Janssen-Cilag S.A., Madrid, Spain, for the gift of pure risperidone powder to perform experiments. They are grateful to Ms Nuria Cascales Picó for her collaboration in some of the experiments with aripiprazole and also thank Dr Juan Antonio Moriana (Department of Psychology, University of Córdoba, Spain) for his help in the statistical analysis and Dr Jennifer Semple and Mr Martin Jakob (Institut für Zellbiologie in Bern, Switzerland) for editing and critical reading of the manuscript.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: ITC-20111029 (Centro para el Desarrollo Tecnológico e Industrial, Spain) and “Plan Propio de Investigación de la Universidad de Córdoba,” Spain.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: J O-L performed most of the experiments; J O-L, A R-R, MM G-D-E and M R-R analyzed the data; A R-R performed most of the statistical analysis; M R-R and MM G-D-E conceived and designed most of the experiments; M R-R wrote the paper.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs: Ángel Rodríguez-Ramos  https://orcid.org/0000-0002-4461-9783
https://orcid.org/0000-0002-4461-9783
Manuel Ruiz-Rubio  https://orcid.org/0000-0001-8733-4377
https://orcid.org/0000-0001-8733-4377
References
- 1. Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jarrell TA, Wang Y, Bloniarz AE, et al. The connectome of a decision-making neural network. Science. 2012;337:437-444. [DOI] [PubMed] [Google Scholar]
- 3. White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1-340. [DOI] [PubMed] [Google Scholar]
- 4. Jin Y, Qi YB. Building stereotypic connectivity: mechanistic insights into structural plasticity from C. elegans. Curr Opin Neurobiol. 2018;48:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hobert O. Neurogenesis in the nematode Caenorhabditis elegans. WormBook. 2010:1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benard H, Hobert O. Looking beyond development: maintaining nervous system architecture. Curr Top Dev Biol. 2009;87:175-194. [DOI] [PubMed] [Google Scholar]
- 7. Flames N, Hobert O. Transcriptional control of the terminal fate of monoaminergic neurons. Annu Rev Neurosci. 2011;34:153-184. [DOI] [PubMed] [Google Scholar]
- 8. Rand JB, Nonet ML. Neurotransmitter Assignments for Specific Neurons. New York, NY: Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- 9. Lai CH, Chou CY, Ch’ang LY, Liu CS, Lin W. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 2000;10:703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bessa C, Maciel P, Rodrigues AJ. UsingC. elegans to decipher the cellular and molecular mechanisms underlying neurodevelopmental disorders. Mol Neurobiol. 2013;48:465-489. [DOI] [PubMed] [Google Scholar]
- 11. Calahorro F, Ruiz-Rubio M. Caenorhabditis elegans as an experimental tool for the study of complex neurological diseases: Parkinson’s disease, Alzheimer’s disease and autism spectrum disorder. Invert Neurosci. 2011;11:73-83. [DOI] [PubMed] [Google Scholar]
- 12. Ruiz-Rubio M, Calahorro F, Gámez-del-Estal MM. Invertebrate models of synaptic transmission in autism spectrum disorders. In: Roubertoux PL, ed. Organism Models of Autism Spectrum Disorders (Neuromethods Vol. 100). New York, NY: Springer and Humana Press; 2015:157-182. [Google Scholar]
- 13. Minkina O, Hunter CP. Intergenerational transmission of gene regulatory information in Caenorhabditis elegans. Trends Genet. 2018;34:54-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Accordino RE, Kidd C, Politte LC, Henry CA, McDougle CJ. Psychopharmacological interventions in autism spectrum disorder. Expert Opin Pharmacother. 2016;17:937-952. [DOI] [PubMed] [Google Scholar]
- 15. Ishitobi M, Kosaka H, Takahashi T, et al. Effectiveness and tolerability of switching to aripiprazole from risperidone in subjects with autism spectrum disorders: a prospective open-label study. Clin Neuropharmacol. 2013;36:151-156. [DOI] [PubMed] [Google Scholar]
- 16. Worrel JA, Marken PA, Beckman SE, Ruehter VL. Atypical antipsychotic agents: a critical review. Am J Health Syst Pharm. 2000;57:238-255. [DOI] [PubMed] [Google Scholar]
- 17. Cohen D, Bonnot O, Bodeau N, Consoli A, Laurent C. Adverse effects of second-generation antipsychotics in children and adolescents: a Bayesian meta-analysis. J Clin Psychopharmacol. 2012;32:309-316. [DOI] [PubMed] [Google Scholar]
- 18. Germann D, Kurylo N, Han F. Risperidone. Profiles Drug Subst Excip Relat Methodol. 2012;37:313-361. [DOI] [PubMed] [Google Scholar]
- 19. Ardiana F, Lestari ML, Indrayanto G. Aripiprazole. Profiles Drug Subst Excip Relat Methodol. 2013;38:35-85. [DOI] [PubMed] [Google Scholar]
- 20. Chase DL, Koelle MR. Biogenic amine neurotransmitters in C. elegans. Wormbook. 2007:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donohoe DR, Aamodt EJ, Osborn E, Dwyer DS. Antipsychotic drugs disrupt normal development in Caenorhabditis elegans via additional mechanisms besides dopamine and serotonin receptors. Pharmacol Res. 2006;54:361-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donohoe DR, Weeks K, Aamodt EJ, Dwyer DS. Antipsychotic drugs alter neuronal development including ALM neuroblast migration and PLM axonal outgrowth in Caenorhabditis elegans. Int J Dev Neurosci. 2008;26:371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kurita M, Holloway T, Garcia-Bea A, et al. HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat Neurosci. 2012;15:1245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Porta de la Riva M, Fontrodona L, Villanueva A, Cerón J. Basic Caenorhabditis elegans methods: synchronization and observation. J Vis Exp. 2012;64:e4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bounoutas A, Chalfie M. Touch sensitivity in Caenorhabditis elegans. Pflugers Arch. 2007;454:691-702. [DOI] [PubMed] [Google Scholar]
- 26. Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Avery L, Horvitz HR. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron. 1989;3:473-485. [DOI] [PubMed] [Google Scholar]
- 28. Barros AG, Bridi JC, de Souza BR, et al. Dopamine signaling regulates fat content through β-oxidation in Caenorhabditis elegans. PLoS ONE. 2014;9:e85874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gonzalez-Aguilera C, Palladino F, Askjaer P. C. elegans epigenetic regulation in development and aging. Brief Funct Genomics. 2014;13:223-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci. 2005;8:43-50. [DOI] [PubMed] [Google Scholar]
- 31. Schafer WR. Mechanosensory molecules and circuits in C. elegans. Pflugers Arch. 2015;467:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanyal S, Wintle RF, Kindt KS, et al. Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J. 2004;23:473-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muly EC, Votaw JR, Ritchie J, Howell LL. Relationship between dose, drug levels, and D2 receptor occupancy for the atypical antipsychotics risperidone and paliperidone. J Pharmacol Exp Ther. 2012;341:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Bartolomeis A, Tomasetti C, Iasevoli F. Update on the mechanism of action of aripiprazole: translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs. 2015;29:773-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wicks SR, Rankin CH. The integration of antagonistic reflexes revealed by laser ablation of identified neurons determines habituation kinetics of the Caenorhabditis elegans tap withdrawal response. J Comp Physiol A. 1996;179:675-685. [DOI] [PubMed] [Google Scholar]
- 36. Kindt KS, Quast KB, Giles AC, et al. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron. 2007;55:662-676. [DOI] [PubMed] [Google Scholar]
- 37. Mersha M, Formisano R, McDonald R, Pandey P, Tavernarakis N, Harbinder S. GPA-14, a Gαi subunit mediates dopaminergic behavioral plasticity in C. elegans. Behav Brain Funct. 2013;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rankin CH. Interactions between two antagonistic reflexes in the nematode Caenorhabditis elegans. J Comp Physiol A. 1991;169:59-67. [DOI] [PubMed] [Google Scholar]
- 39. Rose JK, Rankin CH. Analyses of habituation in Caenorhabditis elegans. Learn Mem. 2001;8:63-69. [DOI] [PubMed] [Google Scholar]
- 40. Sugiura M, Fuke S, Suo S, Sasagawa N, Van Tol HH, Ishiura S. Characterization of a novel D2-like dopamine receptor with a truncated splice variant and a D1-like dopamine receptor unique to invertebrates from Caenorhabditis elegans. J Neurochem. 2005;94:1146-1157. [DOI] [PubMed] [Google Scholar]
- 41. Tauscher J, Hussain T, Agid O, et al. Equivalent occupancy of dopamine D1 and D2 receptors with clozapine: differentiation from other atypical antipsychotics. Am J Psychiatry. 2004;161:1620-1625. [DOI] [PubMed] [Google Scholar]
- 42. Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012-1014. [DOI] [PubMed] [Google Scholar]
- 43. Rex E, Molitor SC, Hapiak V, Xiao H, Henderson M, Komuniecki R. Tyramine receptor (SER-2) isoforms are involved in the regulation of pharyngeal pumping and foraging behavior in Caenorhabditis elegans. J Neurochem. 2004;91:1104-1115. [DOI] [PubMed] [Google Scholar]
- 44. Nagashima T, Oami E, Kutsuna N, Ishiura S, Suo S. Dopamine regulates body size in Caenorhabditis elegans. Dev Biol. 2016;412:128-138. [DOI] [PubMed] [Google Scholar]
- 45. Bean CJ, Schaner CE, Kelly WG. Meiotic pairing and imprinted X chromatin assembly in Caenorhabditis elegans. Nat Genet. 2004;36:100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rex E, Komuniecki RW. Characterization of a tyramine receptor from Caenorhabditis elegans. J Neurochem. 2002;82:1352-1359. [DOI] [PubMed] [Google Scholar]
- 47. Klosin A, Casas E, Hidalgo-Carcedo C, Vavouri T, Lehner B. Transgenerational transmission of environmental information in C. elegans. Science. 2017;356:320-323. [DOI] [PubMed] [Google Scholar]
- 48. Kolasinska-Zwierz P, Down T, Latorre I, Liu T, Liu XS, Ahringer J. Differential chromatin marking of introns and expressed exons by H3K36me3. Nat Genet. 2009;41:376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gamez-Del-Estal MM, Contreras I, Prieto-Perez R, Ruiz-Rubio M. Epigenetic effect of testosterone in the behavior of C. elegans. A clue to explain androgen-dependent autistic traits? Front Cell Neurosci. 2014;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Loscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31-59. [DOI] [PubMed] [Google Scholar]
- 51. Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6-21. [DOI] [PubMed] [Google Scholar]
- 52. Chua YL, Watson LA, Gray JC. The transcriptional enhancer of the pea plastocyanin gene associates with the nuclear matrix and regulates gene expression through histone acetylation. Plant Cell. 2003;15:1468-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu Y, Teng Y, Liu H, Reed SH, Waters R. UV irradiation stimulates histone acetylation and chromatin remodeling at a repressed yeast locus. Proc Natl Acad Sci U S A. 2005;102:8650-8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Candido EP, Reeves R, Davie JR. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978;14:105-113. [DOI] [PubMed] [Google Scholar]
- 55. Catoire H, Pasco MY, Abu-Baker A, et al. Sirtuin inhibition protects from the polyalanine muscular dystrophy protein PABPN1. Hum Mol Genet. 2008;17:2108-2117. [DOI] [PubMed] [Google Scholar]
- 56. Tie F, Banerjee R, Stratton CA, et al. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development. 2009;136:3131-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang M, Poplawski M, Yen K, et al. Role of CBP and SATB-1 in aging, dietary restriction, and insulin-like signaling. PLoS Biol. 2009;7:e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Citrome L, Casey DE, Daniel DG, Wozniak P, Kochan LD, Tracy KA. Adjunctive divalproex and hostility among patients with schizophrenia receiving olanzapine or risperidone. Psychiatr Serv. 2004;55:290-294. [DOI] [PubMed] [Google Scholar]
- 59. Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov. 2012;11:384-400. [DOI] [PubMed] [Google Scholar]
- 60. Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Itzhak Y, Liddie S, Anderson KL. Sodium butyrate-induced histone acetylation strengthens the expression of cocaine-associated contextual memory. Neurobiol Learn Mem. 2013;102:34-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Apostolou E, Hochedlinger K. Chromatin dynamics during cellular reprogramming. Nature. 2013;502:462-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22:2149-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Tables for Behavioral Mechanisms That Depend on Dopamine and Serotonin in Caenorhabditis elegans Interact With the Antipsychotics Risperidone and Aripiprazole by Jaime Osuna-Luque, Ángel Rodríguez-Ramos, María del Mar GÁmez-del-Estal and Manuel Ruiz-Rubio in Journal of Experimental Neuroscience


