Abstract
Background:
Hydrocephalus in premature infants is an onerous disease. In such situations, choosing the best option for cerebrospinal fluid (CSF) diversion is difficult. Ventriculosubgaleal shunt is an effective method of temporary CSF diversion in such situations. In this retrospective study, we compare the outcome of ventriculosubgaleal shunt in premature infants with hydrocephalus of infectious and noninfectious etiology.
Materials and Methods:
All premature children with hydrocephalus secondary to various etiologies who underwent ventriculosubgaleal shunt were studied. The participants were grouped into two depending upon the etiology of hydrocephalus: Group 1 (infectious) and Group 2 (non-infectious). The primary outcome was a successful conversion to ventriculoperitoneal shunt (VPS) and the secondary outcome was mortality. Data were entered into statistical software SPSS version 16 and appropriate statistical analysis was performed to conclude any statistical significance between groups.
Results:
The study included 16 infants among whom 9 were in the infectious group and 7 in the non-infectious group. Primary end point of conversion to VPS was achieved in 55.5% of patients in group 1 and 85.7% in group 2. The secondary end point, i.e., mortality was observed in 44.4% of patients in group 1 and 14.2% in group 2. The average duration during which this was achieved was 40 days (range 20–60 days) in group 1 and 25 days (range 20–30 days) in group 2.
Conclusion:
Ventriculosubgaleal shunt is a safe and effective procedure in infants awaiting definitive VPS for hydrocephalus of infectious as well as noninfectious origin. There was no statistical difference in the rate of successful conversion to a permanent VPS from ventriculosubgaleal shunt in hydrocephalus of either etiologies. Complications and time for successful conversion were more in postmeningitic hydrocephalus.
KEYWORDS: Hydrocephalus, premature infants, ventriculosubgaleal shunt
INTRODUCTION
The management of hydrocephalus in premature infants is a conundrum. The etiologies in this age group vary from more common causes such as germinal matrix bleed, infections, and aqueductal stenosis, to less frequent cerebellar hematomas and tumors. Ventriculoperitoneal shunt (VPS) is the definitive management of hydrocephalus. However, cerebrospinal fluid (CSF) diversion by VPS is not always feasible in premature infants due to factors such as abnormal liquor characteristics, unfavorable abdominal status, or local/systemic infections. Hence, some patients need temporary alternative while awaiting conditions that favor long-term shunt patency. CSF diversion in the form of ventriculosubgaleal shunts (VSGSs) has been described for various etiologies ranging from tumors to infections. VSGS have been recommended as a more physiologic and less invasive method of relieving intraventricular pressure with reasonably low morbidity.[1,2] Our aim was to study the outcome of VSGS in treatment of preterm infants with postinfectious hydrocephalus and those with hydrocephalus due to other causes.
MATERIALS AND METHODS
A retrospective review of patient’s records was carried out at our tertiary center during the period January 2012 to January 2015. This study was carried out after obtaining appropriate clearance from the institutional review board.
Inclusion criteria
All patients who underwent VSGS in our institution in the aforementioned period were included in the study. On the basis of the data collected, study participants were divided in two groups—group 1(postinfectious hydrocephalus) and group 2 (noninfectious hydrocephalus). The diagnosis of postinfectious hydrocephalus was made when there was a positive CSF culture for bacteria or CSF findings suggestive of infection in the clinical background of meningitis. Group 2 included patients who presented with hydrocephalus due to noninfectious etiology viz. germinal matrix bleed, aqueductal stenosis, and cerebellar hematomas. Both groups also included patients who underwent secondary VSGS after failure of VPS performed at different institutions. The primary causes of hydrocephalus seen in these patients were of both infectious and noninfectious origin.
Exclusion criteria
Patients who died before the procedure were excluded from the study. Patients in whom the VSGS was not converted to VPS due to lack of consent were also excluded from the analysis of primary outcome.
Outcome
The primary outcome of the study was good outcome, which was defined as successful conversion to VPS, and secondary end point was bad outcome defined as mortality.
Statistical analysis
The principal characteristics studied were age at presentation, birth weight, weight at the time of surgery (VSGS), complications of surgery, weight at conversion to VPS, time to conversion to VPS, and outcome.
Fisher’s exact test was used to determine the significance of association between etiology (infectious vs. noninfectious) and outcome, i.e., conversion to VPS or death. Mann–Whitney U test was employed to analyze whether the time duration of conversion to VPS in both groups was statistically significant.
Surgical procedure
The illustrated case shows postmeningitic hydrocephalus in a preterm infant [Figure 1] whose CSF protein level was 2 gm/L.
Figure 1.
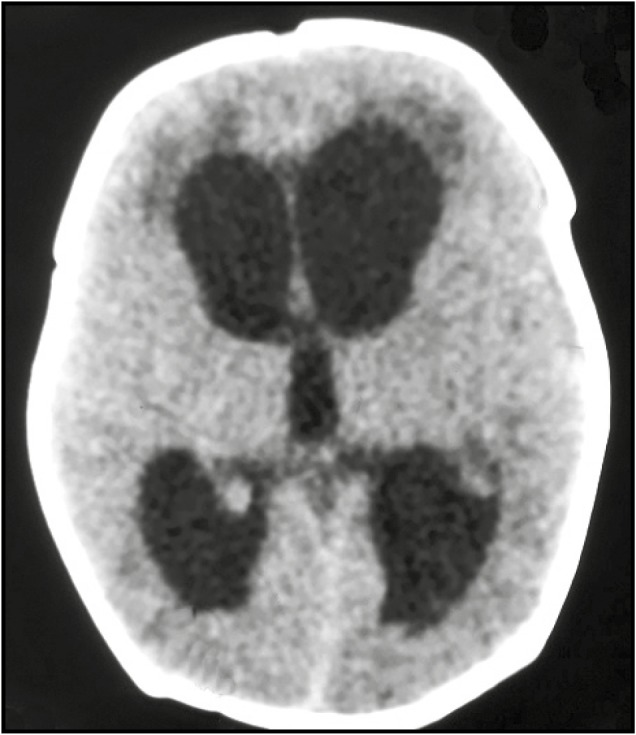
Computed tomography of brain plain—showing pan ventricular dilatation of the ventricle of the brain. Left-sided ventricle is marginally more dilated than the right
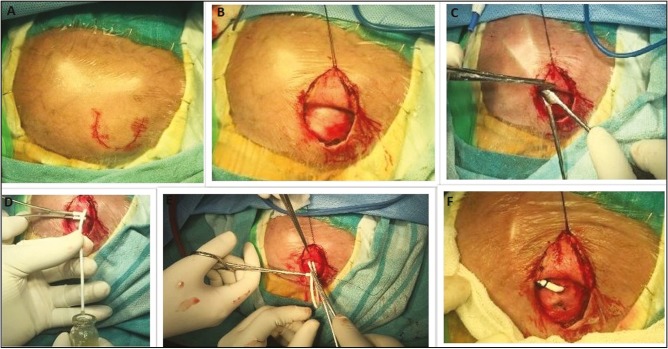
The lateral end of the fontanelle is the preferred site for the ventricular end of VSGS insertion. A curvilinear incision is made at the lateral end of the anterior fontanelle [Figure 2A]. After raising the skin flap, a generous pouch of size 10×10 cm is created occupying the temporal and parieto-occipital region of the scalp [Figure 2B and C]. The lateral end of the fontanelle is punctured with the ventricular end of the catheter, which is further advanced into the lateral ventricle. After collecting CSF for analysis, the tube is connected to a connecter which is anchored in place by suturing it to the periosteum [Figure 2D]. A slit peritoneal end of the medium pressure Chhabra Ventriculoperitoneal shunt is connected to the connector and allowed to be drained in the newly created subgaleal pouch [Figure 2E and F]. The incision is closed in layers. An illustrative case of a successful VSGS procedure is shown in Figure 3 followed by Figure 4 which shows the child after successful conversion of VSGS into VPS.
Figure 2.
Operative procedure of the child undergoing VPS. A and B—child is positioned with head turned to the right side. A curvilinear incision is marked at the level of the lateral edge of the anterior fontanelle. A skin flap is raised along the incision line. C—a spacious subgaleal pouch is created along the temporal and parieto-occipital region. D—the lateral end of the fontanelle is tapped with a ventricular catheter. Note the flow of the opalescent CSF into the sample bottle. E and F—the distal end of a peritoneal catheter is connected to a connector to prevent kinking at the edge of the fontanelle, and slit ends of the same are placed into the newly created subgaleal pouch
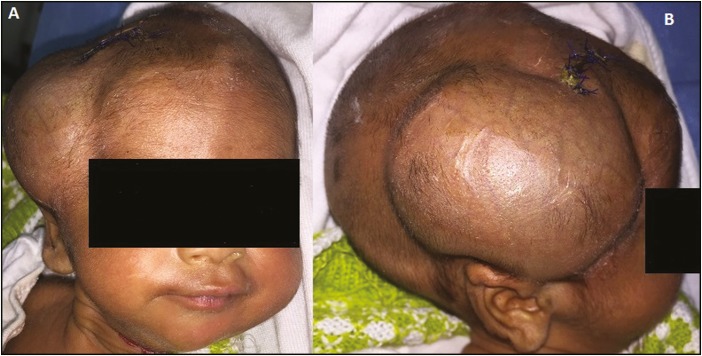
Figure 3.
Postoperative day 3 shows a working ventriculosubgaleal shunt in an infant who underwent the procedure as a result of postmeningitic hydrocephalus
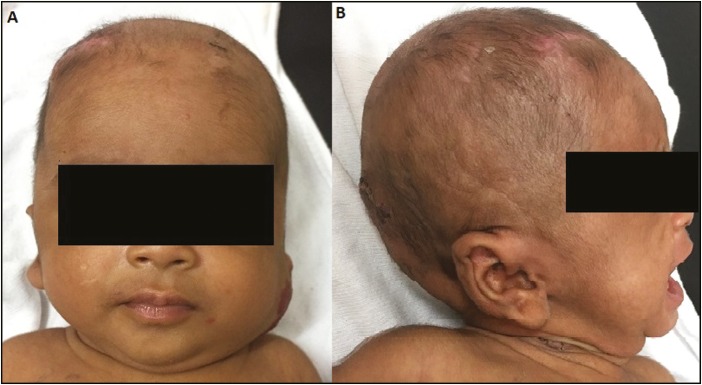
Figure 4.
Collapsed CSF pouch in the patient shown earlier in Figure 3 after successful conversion of VSGS into VPS
RESULTS
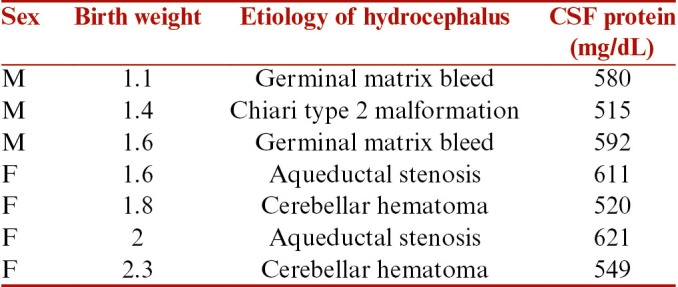
There were total 16 patients who underwent VSGS during the defined period. There were 6 male and 10 female patients. All patients were born preterm. The mean birth weight of the patients was 1.78 kg (range from 0.9 to 2.5 kg). The mean birth weight in infants of group 1 was 1.84 kg and in group 2 was 1.78 kg. All patients were primarily seen in the pediatric emergency and were referred to us after a diagnosis of hydrocephalus was established. Depending on the etiology, the patients were grouped into infectious and noninfectious groups. There were nine patients in group 1(infectious group) and seven patients in group 2 (noninfectious group). There were six patients in this study who underwent a VPS from other institutions and presented with secondary shunt malfunctions. The primary etiologies for hydrocephalus in them were aqueductal stenosis in two, germinal matrix hemorrhage in two, and postmeningitic hydrocephalus in two. Hence, these patients contributed to four patients in group 1 and two patients in group 2. The other etiologies in the group 2 were composed of cerebellar hematoma associated with hydrocephalus and Chiari type 2 malformation associated hydrocephalus. The patient and CSF characteristics in both the groups are summarized in Tables 1 and 2. The CSF protein in all cases was >500 mg%, which was unfavorable for VPS. The mean protein concentration in group 1 was 867.4mg/dL and 569.7mg/dL in group 2.
Table 1.
Clinical profile of patients who underwent VSGS for infectious etiology
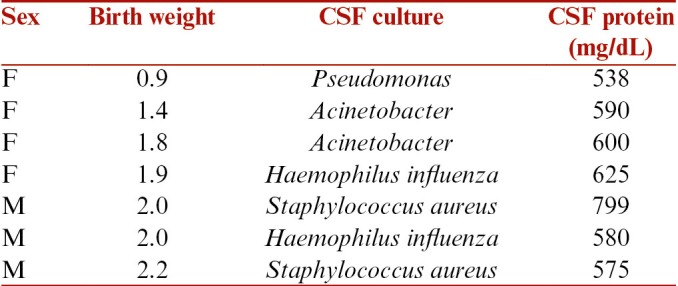
Table 2.
Clinical profile of patients who underwent VSGS for various conditions other than infectious etiology
The average age of the patients in group 1 was 4.1 months (range 1–8 months), and in group 2, it was 5 months (range 2–12 months). All patients in either group underwent VSGS in the manner described earlier. All patients were followed up for improvements in the CSF parameters and body weight. The primary objective of conversion to VPS was achieved in all but one patient with aqueductal stenosis in group 2. The parents of the infant were not willing for any further procedure. This one patient remained asymptomatic as long as 24 months after VSGS and hence was excluded from further analysis. Primary end point of conversion to VPS was achieved in 55.5% of group 1 and 85.7% of group 2 (Fisher’s exact). This outcome was found to be statistically nonsignificant. The average duration during which this was achieved was 40 days (range 20–60 days) in group 1. Excluding one patient, the average duration of conversion in group 2 was 25 days (range 20–30 days). The average weight at conversion to VPS in group 1 was 4.01 kg and in group 2 was 4.39 kg.
The secondary end point, i.e., mortality occurred in 44.4% of group 1 and 14.2% of group 2. Complications occurred in both groups.
Complications
They were in the form of scalp infections, scalp tissue breakdown, CSF leak and subcutaneous pyoceles, pouch collapse, and bone remodeling under the subcutaneous pouch. Sterile CSF leaks were sutured and gross subcutaneous infection prompted a VSGS on the opposite side. Overall complication in this study was 37.5%. The rate of complication in each group was 44.4% (group 1) vs. 28.7% (group 2). This difference was not found to be significant on statistical analysis. Two patients in group 1 underwent bilateral VSGS and one among them was subjected to external ventricular drain (EVD). Minor complications were scalp infection with sterile CSF, and local tissue breakdown was encountered more frequently in group 1. An important complication of failure of VSG was pouch collapse. This is due to nursing the child on the side of the pouch in the immediate postoperative period. This complication may prompt the surgeon to repeat the surgery, but we think it is hardly necessary. We have found a novel technique by which we move the catheter along the subcutaneous plain from outside in an attempt to redissect the collapsed pouch. The immature adhesions of the subcutaneous plain usually yield to the stout distal end of the peritoneal catheter. However, this technique fails if this is performed after the subcutaneous adhesions have already matured and pouch has irreversibly collapsed. Bone remodeling under the tense CSF subgaleal pouch is another complication that we encountered in one patient. This complication was less evident on follow-up due to the skull expansion with growth.
DISCUSSION
The management of hydrocephalus in prematurity is a conundrum. The ultimate goal of permanent drainage of CSF is not often achieved owing to low birth weight or liquor characteristics. This has led investigators to look for other alternate procedures such as placement of EVD,[3] ventricular access devices (VADs)[4,5,6] such as Ommaya and Rickham’s reservoir and repeated lumbar puncture[7,8] for the relief of hydrocephalus. VSGS is another feasible alternative considered in this particular scenario because of it being more physiologic than other forms of CSF diversions. VSGS acts by absorbing the CSF through the skin of hemicranium, face, and eyelid in the short term and also through the subgaleal pouch. It also protects the brain by dampening the intermittent peaks of the intracranial pressure (ICP) that occurs in hydrocephalus.[9] VSGS procedure has an added advantage of not altering electrolyte and nutrition losses that are inherent to other forms of CSF diversion.[10] It also offers theoretical advantage of back pressure offered by the subgaleal pouch, which can accentuate the natural CSF absorptive mechanisms.[11] Although known for more than 100 years, VSGS procedure is observed to be employed at a much less rate compared with the others.[12]
The amount of CSF drained by VSGS might not be equivalent to the quantity of flow as in VPS but is a preferable option in premature cases. This is because unfavorable conditions such as low birth weight, presence of an immature immune system, inadequate absorption capacity of the abdomen, ineffective elimination of blood products from the CSF flow in the ventricular system, and insufficient thickness of subcutaneous tissue preclude a successful VPS. During the improvement of those parameters, the most important parameter to be followed up carefully is body weight. Therefore, in some institutions, the adequate body weight of 2 kg or even 2.5 kg is a prerequisite before performing a VPS.[4,13]
The optimal CSF characteristics for long-term shunt patency is controversial. As a general belief, CSF with high protein content is considered to favor obstruction, and permanent diversion procedures are deferred until protein levels are normalized.[10,14,15,16] This practice is followed in our unit also. In all our patients, the CSF was hyperproteinorrhachic (>0.5 g/L). On the contrary to this philosophy, there are researchers who have opposed this finding and noted that hyperproteinorrhachia does not influence shunt obstruction.[17,18,19]
It is known that repeated ventricular taps and lumbar punctures in premature newborns can control the rise of ICP but has the disadvantage of increasing the risk of infection and meningitis. Similarly, EVD systems expose the intraventricular environment to the exterior, consequently predispose to infection. In a meta-analysis of literature, Badhiwala et al.[11] found lower rates of infection in VSGS compared to EVD and VADs. The EVD systems also carry the risk of inadvertent over drainage if not closely monitored. Moreover, it is associated with loss of protein and electrolytes, which may be detrimental in already fragile children.[9]
The VSGS is similar to VADs (implanted reservoirs) that it is a closed system within the body. The CSF pouch can be used to monitor the CSF characteristics by aspirating the subcutaneous reservoir. But this is not without risk as repeated punctures can damage the fragile skin and subcutaneous tissue of premature infants, leading to breakdown and erosion with added risk of infection.[20,21] Performing VPS at an early period in premature infants with post hemorrhagic hydrocephalus was associated with increased rates of shunt infection as well as malfunction.[22] Taylor and Peter[14] published a series of 36 cases in 2001 and advocated that VPS should be performed late on premature infants because of the need for clearance of ventricle from blood products, which takes at least 5 weeks.
The VSG shunt is not a new procedure; Von Mikulicz was the first to describe it as early as in 1893.[23] In the last two decades or so, there has been renewed but limited interest in VSG shunting. The indications are not only limited to posthemorrhagic hydrocephalus of prematurity, but has been extrapolated for hydrocephalus of other etiology such as caused by brain tumors, subdural fluid collections, and infections.[2,24,25] The literature is also restricted, and only a few publications are available in which more than 10 patients’ data can be collected.[1,24,25,26,27,28]
Our study was composed of patients who were preterm with comparable average birth weight of 1.84 kg in group 1 and 1.78 kg in group 2. Average age of presentation was 4.1 months and 5 months, respectively. We found that mortality was more in group 1 patients (44.4%) as compared to group 2 patients (14.2%). The mortality in group 1 was primarily due to disease progression leading to uncontrolled infection and septicemia. The mortality in group 2 occurred in one patient with cerebellar hemorrhage in whom surgery was contemplated but had a cardiac arrest succumbing to death. Remaining patients who survived in both the groups were converted to VPS within a duration of 2–2.5 months of VSGS amounting to conversion rate of 55.5% (5/9) in group 1 and 85.7% (6/7) in group 2. This duration is similar to the time duration of viability of VSGS described by Tubbs et al.[29]
One patient from group 2 still continues to be asymptomatic after 2.5 years with patent VSGS. Such patients with long duration of working VSGS has been described earlier in the literature.[2,26,29,30] Awaiting conversion to VPS, complications were seen in 44.4% in group 1 and 28.7% in group 2. The complications observed in both groups were local tissue breakdown and CSF leak. Complications were managed accordingly.
CSF leak, pus discharge at local site and subgaleal swelling, and erythema were more common in group 1. On the contrary, four patients in group 2 who had already undergone VPS followed by malfunction and hydrocephalus; and later subjected to VSGS did not showed any major complication and did fairly well till their conversion to VPS. Though overall complication rate in group 2 was 14.2% but the infection rate was nil. On the other hand, group 1 patients had higher incidence of infections complications. This findings have been mirrored earlier in the study by Nagy et al.[31] who found an infection rate of 8.33% with VSGS in post haemorrhagic hydrocephalus group and 20.0% in the post infectious hydrocephalus group. However, this difference was not found to be statistically significant (P = 0.23). The bone remodeling that we encountered in a patient with an overlying tense CSF pouch has been well described in the literature.[29] This complication is self-limiting and disappears with normal skull expansion as the infant grows. Serious intracranial hemorrhage has been described in the literature[29] following insertion of VSGS but this was not see in our series.
Also, preterm children with noninfectious hydrocephalus who had undergone VPS from other institutions and presented to us with malfunction were operated during their early infancy. This may question the decision of performing VPS in the early neonatal period for noninfectious hydrocephalus. Out of the four patients who expired in group 1, three of them had undergone VSGS primarily without previous VPS.
Limitation of study
The retrospective nature of the study among the small cohort of patients failed to derive any specific conclusion in the rates of successful conversion between hydrocephalus of infectious and noninfectious etiology. Heterogeneous etiology in the noninfectious groups was also a limitation of the study.
CONCLUSION
VSGS is a feasible temporary CSF diversion procedure in the management of hydrocephalus till the child optimizes itself for a permanent diversion procedure. The conversion rates into permanent VPS are more favorable in noninfectious hydrocephalus. Also treating a preterm with noninfectious hydrocephalus by VPS first may not prove a good option due to increased risk of VPS malfunction and higher chances of revision.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rahman S, Teo C, Morris W, Lao D, Boop FA. Ventriculosubgaleal shunt: a treatment option for progressive posthemorrhagic hydrocephalus. Childs Nerv Syst. 1995;11:650–4. doi: 10.1007/BF00300724. [DOI] [PubMed] [Google Scholar]
- 2.Tubbs RS, Smyth MD, Wellons JC, 3rd, Blount JP, Grabb PA, Oakes WJ. Alternative uses for the subgaleal shunt in pediatric neurosurgery. Pediatr Neurosurg. 2003;39:22–4. doi: 10.1159/000070875. [DOI] [PubMed] [Google Scholar]
- 3.Cornips E, Van Calenbergh F, Plets C, Devlieger H, Casaer P. Use of external drainage for posthemorrhagic hydrocephalus in very low birth weight premature infants. Childs Nerv Syst. 1997;13:369–74. doi: 10.1007/s003810050102. [DOI] [PubMed] [Google Scholar]
- 4.Lam HP, Heilman CB. Ventricular access device versus ventriculosubgaleal shunt in post hemorrhagic hydrocephalus associated with prematurity. J Matern Fetal Neonatal Med. 2009;22:1097–101. doi: 10.3109/14767050903029576. [DOI] [PubMed] [Google Scholar]
- 5.Wang JY, Amin AG, Jallo GI, Ahn ES. Ventricular reservoir versus ventriculosubgaleal shunt for posthemorrhagic hydrocephalus in preterm infants: infection risks and ventriculoperitoneal shunt rate. J Neurosurg Pediatr. 2014;14:447–54. doi: 10.3171/2014.7.PEDS13552. [DOI] [PubMed] [Google Scholar]
- 6.Fountain DM, Chari A, Allen D, James G. Comparison of the use of ventricular access devices and ventriculosubgaleal shunts in posthaemorrhagic hydrocephalus: systematic review and meta-analysis. Childs Nerv Syst. 2016;32:259–67. doi: 10.1007/s00381-015-2951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreusser KL, Tarby TJ, Kovnar E, Taylor DA, Hill A, Volpe JJ. Serial lumbar punctures for at least temporary amelioration of neonatal posthemorrhagic hydrocephalus. Pediatrics. 1985;75:719–24. [PubMed] [Google Scholar]
- 8.Papile LA, Burstein J, Burstein R, Koffler H, Koops BL, Johnson JD. Posthemorrhagic hydrocephalus in low-birth-weight infants: treatment by serial lumbar punctures. J Pediatr. 1980;97:273–7. doi: 10.1016/s0022-3476(80)80494-x. [DOI] [PubMed] [Google Scholar]
- 9.Narayan P, Mapstone TB. Shunting techniques: ventriculosubgaleal shunting. Techniques in Neurosurgery. 2001;7:212–15. [Google Scholar]
- 10.Reinprecht A, Dietrich W, Berger A, Bavinzski G, Weninger M, Czech T. Posthemorrhagic hydrocephalus in preterm infants: long-term follow-up and shunt-related complications. Childs Nerv Syst. 2001;17:663–9. doi: 10.1007/s00381-001-0519-2. [DOI] [PubMed] [Google Scholar]
- 11.Badhiwala JH, Hong CJ, Nassiri F, Hong BY, Riva-Cambrin J, Kulkarni A V. Treatment of posthemorrhagic ventricular dilation in preterm infants: a systematic review and meta-analysis of outcomes and complications. J Neurosurg Pediatr. 2015;16:545–55. doi: 10.3171/2015.3.PEDS14630. [DOI] [PubMed] [Google Scholar]
- 12.Kazan S, Güra A, Uçar T, Korkmaz E, Ongun H, Akyuz M. Hydrocephalus after intraventricular hemorrhage in preterm and low-birth weight infants: analysis of associated risk factors for ventriculoperitoneal shunting. Surg Neurol. 2005;64(suppl 2):S77–81. doi: 10.1016/j.surneu.2005.07.035. discussion S81. [DOI] [PubMed] [Google Scholar]
- 13.Horinek D, Cihar M, Tichy M. Current methods in the treatment of posthemorrhagic hydrocephalus in infants. Bratisl Lek Listy. 2003;104:347–51. [PubMed] [Google Scholar]
- 14.Taylor AG, Peter JC. Advantages of delayed VP shunting in post-haemorrhagic hydrocephalus seen in low-birth-weight infants. Childs Nerv Syst. 2001;17:328–33. doi: 10.1007/s003810000429. [DOI] [PubMed] [Google Scholar]
- 15.Petraglia AL, Moravan MJ, Dimopoulos VG, Silberstein HJ. Ventriculosubgaleal shunting–a strategy to reduce the incidence of shunt revisions and slit ventricles: an institutional experience and review of the literature. Pediatr Neurosurg. 2011;47:99–107. doi: 10.1159/000330539. [DOI] [PubMed] [Google Scholar]
- 16.Seeburg D, Ahn E, Huisman T. Secondary pediatric encephalocele after ventriculosubgaleal shunting for posthemorrhagic hydrocephalus. Neuropediatrics. 2014;45:252–5. doi: 10.1055/s-0033-1363298. [DOI] [PubMed] [Google Scholar]
- 17.Fulkerson DH, Vachhrajani S, Bohnstedt BN, Patel NB, Patel AJ, Fox BD, et al. Analysis of the risk of shunt failure or infection related to cerebrospinal fluid cell count, protein level, and glucose levels in low-birth-weight premature infants with posthemorrhagic hydrocephalus. J Neurosurg Pediatr. 2011;7:147–51. doi: 10.3171/2010.11.PEDS10244. [DOI] [PubMed] [Google Scholar]
- 18.Brydon HL, Hayward R, Harkness W, Bayston R. Does the cerebrospinal fluid protein concentration increase the risk of shunt complications? Br J Neurosurg. 1996;10:267–73. doi: 10.1080/02688699650040124. [DOI] [PubMed] [Google Scholar]
- 19.Brydon HL, Hayward R, Harkness W, Bayston R. Physical properties of cerebrospinal fluid of relevance to shunt function.1: The effect of protein upon CSF viscosity. Br J Neurosurg. 1995;9:639–44. doi: 10.1080/02688699550040927. [DOI] [PubMed] [Google Scholar]
- 20.Fulmer BB, Grabb PA, Oakes WJ, Mapstone TB. Neonatal ventriculosubgaleal shunts. Neurosurgery. 2000;47:80–3. doi: 10.1097/00006123-200007000-00018. discussion 83-4. [DOI] [PubMed] [Google Scholar]
- 21.Hudgins RJ, Boydston WR, Gilreath CL. Treatment of posthemorrhagic hydrocephalus in the preterm infant with a ventricular access device. Pediatr Neurosurg. 1998;29:309–13. doi: 10.1159/000028744. [DOI] [PubMed] [Google Scholar]
- 22.Kadri H, Mawla AA, Kazah J. The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Childs Nerv Syst. 2006;22:1086–90. doi: 10.1007/s00381-006-0050-6. [DOI] [PubMed] [Google Scholar]
- 23.Aschoff A, Kremer P, Hashemi B, Kunze S. The scientific history of hydrocephalus and its treatment. Neurosurg Rev. 1999;22:67–93. doi: 10.1007/s101430050035. discussion 94-5. [DOI] [PubMed] [Google Scholar]
- 24.Kariyattil R, Mariswamappa K, Panikar D. Ventriculosubgaleal shunts in the management of infective hydrocephalus. Childs Nerv Syst. 2008;24:1033–5. doi: 10.1007/s00381-008-0628-2. [DOI] [PubMed] [Google Scholar]
- 25.Steinbok P, Cochrane DD. Ventriculosubgaleal shunt in the management of recurrent ventriculoperitoneal shunt infection. Childs Nerv Syst. 1994;10:536–9. doi: 10.1007/BF00335079. [DOI] [PubMed] [Google Scholar]
- 26.Karas CS, Baig MN, Elton SW. Ventriculosubgaleal shunts at Columbus Children’s Hospital: neurosurgical implant placement in the neonatal intensive care unit. J Neurosurg. 2007;107:220–3. doi: 10.3171/PED-07/09/220. [DOI] [PubMed] [Google Scholar]
- 27.Köksal V, Öktem S. Ventriculosubgaleal shunt procedure and its long-term outcomes in premature infants with post-hemorrhagic hydrocephalus. Childs Nerv Syst. 2010;26:1505–15. doi: 10.1007/s00381-010-1118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wellons JC, Shannon CN, Kulkarni AV, Simon TD, Riva-Cambrin J, Whitehead WE, et al. Hydrocephalus Clinical Research Network. A multicenter retrospective comparison of conversion from temporary to permanent cerebrospinal fluid diversion in very low birth weight infants with posthemorrhagic hydrocephalus. J Neurosurg Pediatr. 2009;4:50–5. doi: 10.3171/2009.2.PEDS08400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tubbs RS, Banks JT, Soleau S, Smyth MD, Wellons JC, 3rd, Blount JP, et al. Complications of ventriculosubgaleal shunts in infants and children. Childs Nerv Syst. 2005;21:48–51. doi: 10.1007/s00381-004-0967-6. [DOI] [PubMed] [Google Scholar]
- 30.Willis BK, Kumar CR, Wylen EL, Nanda A. Ventriculosubgaleal shunts for posthemorrhagic hydrocephalus in premature infants. Pediatr Neurosurg. 2005;41:178–85. doi: 10.1159/000086558. [DOI] [PubMed] [Google Scholar]
- 31.Nagy A, Bognar L, Pataki I, Barta Z, Novak L. Ventriculosubgaleal shunt in the treatment of posthemorrhagic and postinfectious hydrocephalus of premature infants. Childs Nerv Syst. 2013;29:413–8. doi: 10.1007/s00381-012-1968-5. [DOI] [PubMed] [Google Scholar]