Abstract
Aim of the study:
To evaluate the electroclinical course and the correlation Electroencephalographic (EEG) pattern and epileptic seizures in an infant with Miller Dieker Syndrome (MDS) during the first year of life.
Materials and Methods:
MDS was diagnosed in the infant soon after birth and followed up from six months of life to one year, at the Department of Pediatrics, General Pediatric Operative Unit, Policlinico Vittorio Emanuele, University Hospital, XCatania, Italy, with clinical and serial EEG recording.
Results:
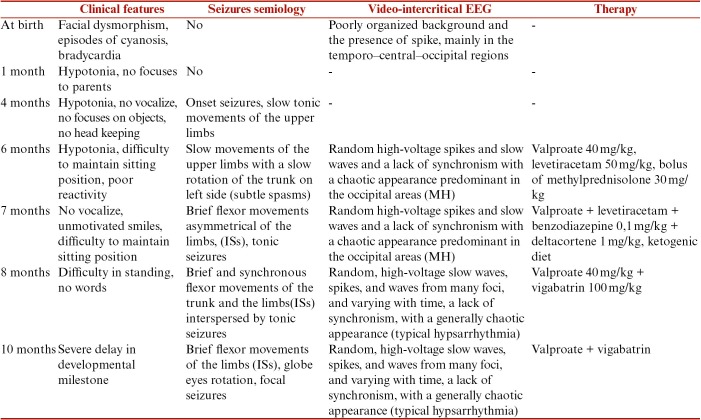
Aside from severe delay in the developmental milestone, the onset of the seizures was first noticed by the parents at the age of 4 months as brief slow tonic movements; at 6 months as tonic movements of the upper limbs with a slow rotations of the trunk, i.e. “subtle spams”; and at 7 months as typical “infantile spams” and tonic seizures. The EEG recording registered pattern of modified hypsarrhythmia (MH) correlated with “subtle spams” at the age of 6 months and at the age of 7 months the same EEG recording of MH associated to clinical expression of classical Infantile Spams (IS).
Conclusions:
In this infant, the EEG pattern and epileptic seizures were widely variable ranging clinically from brief anomalous movements to “subtle spams” and to typical infantile spams. At the same time, the EEG recording manifested first with MH and one month later with classical hypsarrhythmia. The EEG recording MH correlated first with clinical expression of subtle spams and the EEG remaining unchanged with the classical clinical expression of infantile spams.
KEYWORDS: Epileptic seizures, infantile spasms, lissencephaly, Miller–Dieker syndrome, modified hypsarrhythmia
INTRODUCTION
Lissencephaly (LIS) is a malformation of brain cortical development manifested by a smooth cerebral surface (agyria/pachygyria). The development of the human cerebral cortex follows several steps mainly involving cell proliferation, cell migration, and cortical organization. The disruption of any of these steps, including molecular genetic causes, results in abnormal formation of cerebral convolution or gyri.[1] The histopathologic features show incomplete neuronal migration with a widened cortex only organized into layers, and large numbers of heterotopic neurons below the cortex.[2] LIS, together with subcortical band heterotopia (SBH), represents two of the most severe forms of cerebral malformation.[3,4,5] From the discovery of the first genetic causes of LIS involving the deletion 17p13.3 and of the most common causal genes LIS1 (PAFAH1B1) and DCK, more than 19 LIS–SBH associated genes have been reported.[6,7,8,9,10,11] Miller–Dieker syndrome (MDS) is a contiguous gene deletion of the short arm of chromosome 17 (17p13.3) presenting with severe intellectual disability, drug-resistant seizures, and facial dysmorphisms. Body organs may be also affected including the heart, genitalia, and kidneys. The facial dysmorphisms are quite distinctive and include microcephaly, a high and prominent forehead, bitemporal hollowing, short nose with upturned narix, edge lips downward, a thin vermilion border of the upper lip, and microretrognathia.[4,5,6] The steps of development are only poorly reached and severe intellectual disability is the most frequent clinical sign. Most of the affected children die precociously as a result of intractable seizures and aspiration pneumonia. The seizures in MDS are widely variable and may present in several different types but more commonly as “infantile spasms” (ISs).[5]
Herewith we report on the clinical features and video-electroencephalographic (EEG) recording in a child with MDS in the course of the first year of life.
CASE REPORT
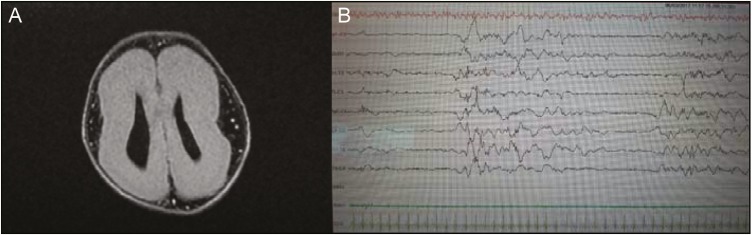
A 6-month-old boy was referred to the Pediatric Clinic Policlinico-Vittorio Emanuele hospital, Catania (Italy), for presenting with drug-resistant seizures with the diagnosis of MDS. He is the second born of healthy, unrelated Italian parents. At the time of gestation, the mother was aged 34 and the father 38 years. The sister (aged 3 years) is healthy. The mother felt normal fetal movements during the gestation. A diagnosis of brain malformation was recorded during the 6 months of gestation as the result of intrauterine ultrasound (US), which disclosed ventriculomegaly, absence of corpus callosum, and intrauterine growth restriction. The mother denied having had any infection diseases or having used drugs or alcohol during her pregnancy. The boy was born at 37 weeks of gestation by spontaneous vaginal hasty delivery. The birth weight was 1800 g, length 45 cm, and head circumference 32 cm. The apgar scores were 5 and 8 at 1 and 5 minutes, respectively. Soon after birth, he was admitted to the neonatal intensive care unit in another city hospital for treatment and clinical evaluation related to facial dimorphisms, cerebral malformation, low weight, and episodes of cyanosis and bradycardia. During this hospitalization, treatment for his respiratory distress and poor alimentation was started. During this period, lasted 26 days, he was submitted to several laboratory investigations, genetic analysis, and neurological investigations. Routine laboratory findings disclosed the presence of mild metabolic acidosis, which was immediately corrected. Brain magnetic resonance imaging (MRI) showed severe LIS consisting of wide-spread agyria and restricted areas of pachygyria Figure 1A]. The EEG showed poorly organized background and the presence of spikes, mainly in the temporo–central–occipital regions [Figure 1B]. At US, colpocephaly and absence of normal cortical gyration with simplified sulci and cerebral scissors were noticed. The parents did not refer anomalous movements from birth to the first 4 months of life when some daily episodes of slow tonic movements of the upper limbs were noticed. General hypotonia and others signs of development delay were still present, and difficulty in maintaining the head upright and persisting episodes of slow movements in the upper limbs were reported by the general pediatrician. During this period, the baby showed a good growth improvement.
Figure 1.
(A) Brain MRI to 5 days of life: T2-axial fluid attenuated inversion recovery sequence. (B) Intercritical EEG at the age of 5 days showed poorly organized background and the presence of spike, mainly in the temporo–central–occipital regions
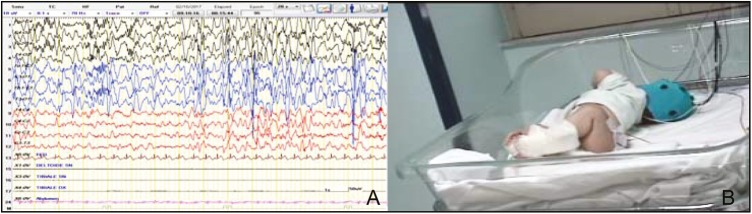
At the age of 6 months, he was admitted for the first time for our observation, due to slow tonic seizures sometimes associated to short phase of desaturation prevalently during the sleep. His weight was 6 kg (third percentile); length 62 cm (>third percentile), and head circumference 40 cm (<third percentile). Mild facial dysmorphism was noticed and consisted of high forehead, slight bitemporal hollowing, epicanthus, small upturned nose, low-set and abnormally shaped ears, slim lips with edge under-down, and retrognathia. The neurological examination showed severe hypotonia with difficulty in maintaining the sitting position and poor reactivity. The anterior fontanel was open 0.5×0.5. Partial bilateral ptosis was noticed. The seizures were mainly localized at the face with blinking and slow tonic movements of the upper limbs with a slow rotation of the trunk afterward the left side appearing prevalently before sleeping. The intercritical video-EEG showed a pattern of modified hypsarrhythmia (MH) consisting of random high-voltage spikes and slow waves predominant in the occipital areas and a lack of synchronism with a chaotic appearance [Figure 2A]. The critical video-EEG showed a typical electrical suppression during an epileptic spasm: the background was variable and mainly consisted of the combination of hypervoltage slow waves with a bout of rapid low amplitude activity or a diffuse attenuation of the trace.
Figure 2.
(A) Intercritical EEG at the age of 6 months showed focal occipital discharge (MH). (B) Clinical features: slow tonic movements of the upper limbs with a slow rotation of the trunk afterward the left side
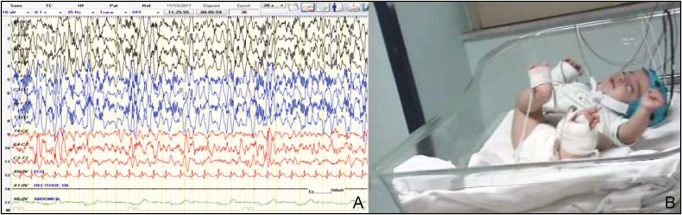
At 4 weeks later, whereas the intercritical EEG remained unchanged [Figure 3A], the types of seizures showed different features, presenting with brief and synchronous movements of the head, trunk, and limbs, typical of ISs [Figure 3B]. These anomalous movements usually lasted about a few seconds and frequently occurred in clusters. The critical video-EEG showed a typical electrical suppression during the epileptic spasms. As the seizures persisted with high frequency, treatment with valproic acid (40 mg/kg/day), levetiracetam (50 mg/kg/day), and vitamin B6 (5 mg/kg/day) was started. Also, the administration of a ketogenic diet failed to give good response. With the gradual addition of midazolam to the treatment regimen, a reduction of the frequency of seizures was achieved, but the seizures still persisted. Routine laboratory analyzes, including blood count, coagulation testing, blood lactate, pyruvate, glucose and ketones, creatine kinase, copper, vitamin D, ceruloplasmin, plasma and urine amino acids, organic urinary acids, purine and pyrimidine, 7-dehydrocholesterol, and total cholesterol were normal. Renal and abdominal US investigations were also normal.
Figure 3.
(A) Intercritical EEG at the age of 7 months showed focal occipital discharge (MH). (B) Clinical features: flexion spasms
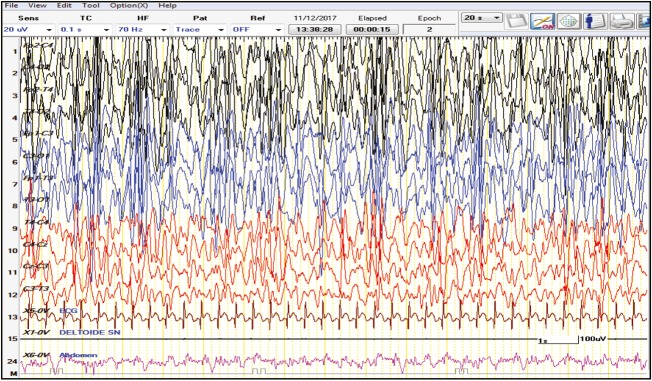
The child was discharged from the hospital and followed up in day hospital. At the age of 8 months, the typical IS seizures were still present and correlated with an EEG intercritical pattern of hypsarrhythmia [Figure 4]. From the age of 8 to 12 months, both clinical phenotypes and EEG recording unchanged, aside from the onset of focal seizures (see Table 1).
Figure 4.
Intercritical EEG at the age of 8 months showed hypsarrhythmia
Table 1.
Clinical features, seizures semiology, intercritical EEG, and treatment along the first year of life
GENETIC ANALYSIS
Chromosomal microarray analysis C4×180K (AMADID 022060-Agilent CytoGenomics edition 3.0 [Agilent, Santa Clara, CA]) showed a microduplication of the distal extremities of the long arm of chromosome 1 in the region 1q44 about 4.2 Mb and microdeletion of the distal region of the short arm of chromosome 17 in the region p13.3p13.2 about 3.8 Mb including the critical region for the MDS. The fluorescence in situ hybridization test confirmed the molecular anomaly and allowed us to associate the micro-rearrangement to the chromosome 17 deriving from a de novo unbalance translocation between the long arm of chromosome 1 and the short arm of chromosome 17 with breakpoint in 1q44 and 17p13.2, respectively.
DISCUSSION
The infant presented with the typical clinical features of DMs. Diagnosis of cerebral malformation was made through US during the gestation, at 6 months of intrauterine life. The diagnostic suspicion was confirmed at birth on the presence of microcephaly, facial dysmorphisms, and brain MRI, which disclosed the typical aspect of LIS. The subsequence result of molecular analysis with the microdeletion of one district region of the short arm of chromosome 17 in the region p13.3p13.2 confirmed the diagnosis of MDS. Clinical manifestations in children with MDS involve mainly the brain. Microcephaly may be present at birth, but it may also manifest as the child grows older. Length is usually normal at the birth whereas the birth weight is low. Subsequently, poor feeding and frequent episodes of vomiting are the main cause of poor weight gain. Respiratory distress and infections of the respiratory tract are frequently recorded. Abnormal neurological examination, intellectual disability, and epileptic seizures are the main manifestations of the syndrome. Hypotonia and spasticity are frequently reported and intellectual disability is recorded with a score between severe and profound.[4,5]
In MDS, different phenotypes of seizures, including partial complex and epileptic syndromes such as West syndrome, are recorded.[6,7,8] The patients have intractable epileptic seizures and the onset of the crises is precocious and manifested in a range from a few days to 2 years.[7,8,9,10] In a study by de Wit et al.,[11] five patients had their onset of seizures in the neonatal period, seventeen had ISs, and two suffered from multifocal epilepsy. The prognosis of MDS is severe. According to the survey of de Wit et al.[11] in a long-term (mean length follow-up of 14 years) performed in 24 patients affected by LIS type 1, only 11 were alive at the evaluation. All the patients showed severe intellectual disability, intractable epilepsy, and need of special care and dependency.
In this infant, a clinical and EEG investigation to examine their evolution in the course of the first year of life was performed. The delay and arrest in reaching the normal developmental stages were noticed since the first day of life and persisted through the first year of life. The epileptic seizures were noticed by the parents and revealed by the pediatrician at 4 months of life and they were reported as slow movements of the arms. The epileptic seizures became more evident at the age of 6 months when the infant was admitted to the hospital. The seizures presented first with slow movements of the upper limbs with a slow rotation of the trunk and were classified in the group of “subtle spasms” according to the “consensus statement of the West Delphi group.”[12] They were correlated with the intercritical EEG prototypic pattern defined as “MH” by the “consensus statement.” The MH includes different pattern of hypsarrhythmia with increased interhemispheric synchronization, asymmetry, with consistent focus of abnormal discharge, with episodes of attenuation, and hypsarrhythmia comprising primarily high-voltage slow activity with little sharp-wave or spike activity.[12,13]
In the proband, at the age of 6 months, the video-EEG showed persistent occipital focus discharges suggestive of MH with a corresponding clinical feature of “subtle spasms.” One month later, the clinical features of seizures took the aspect of typical ISs, whereas the EEG pattern of MH remained unchanged. In the following months, the ISs were still present and focal seizures were recorded in correlation with an EEG pattern of hypsarrhythmia.
Some indications are raised by the report on this child regarding the clinical and EEG pattern during the course of the first year of life: subtle spasms in patients with MDS have not been commonly reported. Moreover, to note, the correlation of MH and focal discharges associated first with phenotype seizures of subtle spasms and subsequently of IS.
We hypothesize that the clinical and EEG anomalies reported in this patient are to be related to the areas of cerebral malformation prevalently acting and to the time of the brain development.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors wish to thank American Manuscript Editors (USA) for editing the manuscript. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Kato M, Dobyns WB. Lissencephaly and the molecular basis of neuronal migration. Hum Mol Genet. 2003;12:R89–96. doi: 10.1093/hmg/ddg086. [DOI] [PubMed] [Google Scholar]
- 2.Olson EC, Walsh CA. Smooth, rough and upside-down neocortical development. Curr Opin Genet Dev. 2002;12:320–7. doi: 10.1016/s0959-437x(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 3.Guerrini R, Dobyns WB, Barkovich AJ. Abnormal development of the human cerebral cortex: genetics, functional consequences and treatment options. Trends Neurosci. 2008;31:154–62. doi: 10.1016/j.tins.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Pavone L, Rizzo R, Dobyns WB. Clinical manifestations and evaluation of isolated lissencephaly. Childs Nerv Syst. 1993;9:387–90. doi: 10.1007/BF00306189. [DOI] [PubMed] [Google Scholar]
- 5.Pavone L, Corsello G, Pavone P, Iannetti P. Lissencephalic syndromes: brain and beyond. Front Biosci (Schol Ed) 2010;2:85–95. doi: 10.2741/s47. [DOI] [PubMed] [Google Scholar]
- 6.Dobyns WB, Stratton RF, Parke JT, Greenberg F, Nussbaum RL, Ledbetter DH. Miller-Dieker syndrome: lissencephaly and monosomy 17p. J Pediatr. 1983;102:552–8. doi: 10.1016/s0022-3476(83)80183-8. [DOI] [PubMed] [Google Scholar]
- 7.Di Donato N, Chiari S, Mirzaa GM, Aldinger K, Parrini E, Olds C, et al. Lissencephaly: expanded imaging and clinical classification. Am J Med Genet A. 2017;173:1473–88. doi: 10.1002/ajmg.a.38245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobyns WB, Curry CJ, Hoyme HE, Turlington L, Ledbetter DH. Clinical and molecular diagnosis of Miller-Dieker syndrome. Am J Hum Genet. 1991;48:584–94. [PMC free article] [PubMed] [Google Scholar]
- 9.Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, et al. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–21. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- 10.des Portes V, Francis F, Pinard JM, Desguerre I, Moutard ML, Snoeck I, et al. Doublecortin is the major gene causing X-linked subcortical laminar heterotopia (SCLH) Hum Mol Genet. 1998;7:1063–70. doi: 10.1093/hmg/7.7.1063. [DOI] [PubMed] [Google Scholar]
- 11.de Wit MC, de Rijk-van Andel J, Halley DJ, Poddighe PJ, Arts WF, de Coo IF, et al. Long-term follow-up of type 1 lissencephaly: survival is related to neuroimaging abnormalities. Dev Med Child Neurol. 2011;53:417–21. doi: 10.1111/j.1469-8749.2011.03937.x. [DOI] [PubMed] [Google Scholar]
- 12.Lux AL, Osborne JP. A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: consensus statement of the West Delphi group. Epilepsia. 2004;45:1416–28. doi: 10.1111/j.0013-9580.2004.02404.x. [DOI] [PubMed] [Google Scholar]
- 13.Hrachovy RA, Frost JD Jr, Kellaway P. Hypsarrhythmia: variations on the theme. Epilepsia. 1984;25:317–25. doi: 10.1111/j.1528-1157.1984.tb04195.x. [DOI] [PubMed] [Google Scholar]