Abstract
Isocitrate dehydrogenase 1/2 (IDH)1/2 mutations are frequently detected in glioma. The aim of the present study was to investigate the association between IDH1/2 mutations and glioma grades. The current study was retrospective and used samples from 206 patients with brain glioma and 9 patients with spinal cord glioma as a control. Patients were diagnosed and graded according to the World Health Organization classification of tumors of the central nervous system. The association of patient age with glioma grade was evaluated, and IDH1/2 mutations were also examined and analyzed in different grades. On average, brain glioma grade tended to increase with increasing patient age; patients with grade IV (primary) gliomas had a significantly higher mean age than those with grades I and II (P<0.05), and patients with grade II glioma had a significantly lower mean age than those with grade III (P<0.05). The majority of brain gliomas with mutations in IDH1/2 in grade II, II–III and III occurred in adults, rather than adolescents. IDH1/2 mutations occurred most frequently in grade II, II–III and III gliomas, and these mutation frequencies differed significantly between brain glioma grades (P<0.001). In summary, mutations in IDH1/2 were associated with grade II, II–III and III brain gliomas, and possibly with the progression of brain glioma from grade II to grade III.
Keywords: isocitrate dehydrogenase 1/2, brain glioma, spinal cord glioma, age, mutation
Introduction
Primary malignant brain tumors have the third highest cancer-associated mortality and morbidity rates among individuals worldwide (1). Malignant glioma is the most frequent intra-axial primary malignant brain tumor (2). There are ~22,500 new cases of primary malignant brain tumor among adults per year in the United States, of which 70% are malignant gliomas (3). Malignant gliomas occur in all age groups, but are most prevalent in adults aged >45 years (4).
Gliomas can be classified as grade I to IV on the basis histological features and genetic alterations, as defined by the World Health Organization (WHO) (5). In general, grade I gliomas are biologically benign and can be removed by surgical resection. Although grade II gliomas are considered to be low-grade malignancies, they may not be totally resectable. Grade III gliomas are invasive and aggressive, characterized by quick progression and poor patient outcome. Grade IV tumors, also known as glioblastoma multiforme (GBM), are the most invasive form and are associated with a poor prognosis (6,7). There are two types of GBM: Primary GBM and secondary GBM. Secondary GBMs are defined as tumors that have clinical, radiologic, or histopathological evidence of malignant progression from a preexisting lower-grade tumor, whereas primary GBMs have no such history and present at diagnosis as advanced cancer (8). Despite the development of various therapeutic strategies, including surgical resection, radiation and adjuvant chemotherapy, the prognosis for patients with malignant glioma remains poor; the median overall survival time for patients with GBM is only 15 months (9). As such, the development of a more effective therapy for malignant glioma is required.
Parsons et al (8) found that ~12% of GBM patients had a mutation in the isocitrate dehydrogenase (IDH) 1 gene; in >90% of these patients, this mutation was R132H. However, there are also other IDH1 mutations at codon 132, including R132S, R132C and R132L (10). IDH1 is located on chromosome 2q33 and encodes the IDH1 enzyme, which catalyzes the oxidative carboxylation of isocitrate to α-ketoglutarate, giving rise to the generation of nicotinamide adenine dinucleotide phosphate (NADPH). A growing body of evidence suggests that high rates of spontaneous mutations are found in the gene encoding cytosolic NADP+-dependent IDH1 in glioma (11,12). Tumors without an IDH1 mutation often exhibit a mutation at amino acid position 172 in the mitochondrial NADP+-dependent IDH2 (R172). The IDH2 gene is located on 15q26.1 and the most frequent mutation is R172K (13). IDH2 mutations at R172 include R172K, R172W and R172M (14). IDH1/2 mutations are associated with poor prognosis in glioma patients (15,16). Mutant IDH1 may boost glioma growth and suppress glioma cell differentiation (17). It is therefore of considerable importance to understand the association between IDH1/2 mutations and glioma progression.
The present study aimed to elucidate the association between IDH1/2 mutations and glioma grades. A total of 206 samples from patients with brain glioma and 9 samples from patients with spinal cord glioma (as a control) were analyzed. IDH1/2 mutations, their frequency in glioma grades and the association between patient age and glioma grade were examined. The data obtained here could aid in improving understanding of the role of IDH1/2 mutations in glioma.
Materials and methods
Tumor samples
The present study was approved by the Ethics Committee of the Chinese People's Liberation Army No. 94 Hospital (Nanchang, China), and all patients provided written informed consent. Tumor tissue was obtained from human brain tumor specimens that were diagnosed in the Neuropathology Departments of Xinqiao Hospital of the Third Military Medical University (Chongqing, China) and The First Affiliated Hospital of Nanchang University (Nanchang, Jiangxi, China) between August 2011 and March 2014. The study included 206 brain glioma samples (the mean age of the patients was 42.06±15.21 years, 102 males and 104 females; 158 from Xinqiao Hospital of the Third Military Medical University and 48 from The First Affiliated Hospital of Nanchang University) and 9 samples of spinal cord glioma (Department of Neurosurgery, Third Military Medical University, Chongqing, China). The tumors were diagnosed and graded according to the WHO classification of tumors of the central nervous system (18). The 206 brain glioma samples comprised 6 cases of grade I glioma, 66 cases of grade II glioma, 26 cases of grade II–III glioma, 61 cases of grade III glioma and 47 cases of grade IV glioma (43 cases of primary GBM and 4 cases of secondary GBM). No patients received preoperative radiotherapy, chemotherapy or other treatment. The tumor tissue sections included 158 frozen specimens and 48 paraffinized specimens.
DNA extraction and polymerase chain reaction (PCR) amplification for IDH1/IDH2 sequencing
Tumor areas in unstained histological sections were manually micro-dissected using sterile scalpels. Using the EZNA Tissue DNA kit (Omega Bio-Tek, Inc., Norcross, GA USA), DNA was isolated from tumor tissue according to the manufacturer's instructions.
For PCR, isolated DNA (1 µl) was added to 100 µl of PCR reaction solution. The primer sequences were as follows: IDH1 forward, 5′-CGGTCTTCAGAGAAGCCATT-3′; IDH1 reverse, 5′-GCAAAATCACATTATTGCCAAC-3′; IDH2 forward, 5′-CCACTATTATCTCTGTCCTC-3′; and IDH2 reverse, 5′GCTAGGCGAGGAGCTCCAGT3′. PCR with IDH1 primers generated a 129-bp product, whereas the IDH2 primers generated a 118-bp product. PCR amplification was conducted using a SYBR Premix Taq™ kit (Takara Bio, Inc., Otsu, Japan). The reaction mixture underwent an initial denaturation step at 95°C for 5 min, and then 40 cycles of amplification, which consisted of 95°C denaturation for 30 sec, 60°C annealing for 30 sec, and 72°C extension for 30 sec.
IDH1/IDH2 were sequenced using a semi-automated sequencer (Applied Biosystems 3100 Genetic Analyzer; Thermo Fisher Scientific, Inc., Waltham, MA, USA) as well as Sequence Pilot version 3.1 software (JSI Medical Systems GmbH, Ettenheim, Germany), as described previously (19).
Statistical analysis
Data are expressed as the mean ± standard deviation. Statistics analysis was performed using SPSS v.16 (SPSS, Inc., Chicago, IL, USA). The association between the disease grade classification and patient age was examined by one-way analysis of variance. A pairwise comparison of means was performed using the least-significant difference test. The associations between the disease grade classification and IDH1/IDH2 mutation frequencies were evaluated using a Wilcoxon rank-sum test. P<0.05 was considered to indicate a statistically significant difference.
Results
Association between brain glioma grade classification and patient age
The ages of the patients grouped according to brain glioma grade were analyzed. The mean ages for patients with tumors of grade I, II, II–III, III, IV (primary GBM) and IV (secondary GBM) were 32.67±15.16, 38.55±14.24, 41.08±14.69, 43.82±15.31, 47.49±15.55 and 35.25±19.84 years, respectively (Table I). Increased glioma grades tended to be association with increased age. Significant differences in mean age were observed between grades I and IV (primary) glioma (P=0.025), between grades II and III glioma (P=0.049), and between grades II and IV (primary) glioma (P=0.003). Thus, patients with grade IV (primary) were significantly older on average than patients with grade I and II disease. Additionally, patients with grade II glioma were significantly younger than those with grade III disease.
Table I.
Analysis of variance for the associations between patient age and different grades of brain glioma.
| P-value for pairwise comparison | ||||||
|---|---|---|---|---|---|---|
| Grade | Age, years (mean ± SD), range | Grade II | Grade II–III | Grade III | Grade IV (primary) | Grade IV (secondary) |
| I | 32.67±15.16, (13–48) | 0.360 | 0.218 | 0.084 | 0.025a | 0.790 |
| II | 38.55±14.24, (7–71) | – | 0.468 | 0.049a | 0.003a | 0.671 |
| II–III | 41.08±14.69, (26–69) | – | – | 0.437 | 0.087 | 0.471 |
| III | 43.82±15.31, (8–86) | – | – | – | 0.221 | 0.270 |
| IV (primary) | 47.49±15.55 (13–78) | – | – | – | – | 0.121 |
| IV (secondary) | 35.25±19.84, (25–65) | – | – | – | – | – |
Statistically significant (P<0.05). SD, standard deviation. P-values represent pairwise comparisons between the different grades according to mean age. A pairwise comparison of means was performed using the least-significant difference test.
IDH1/IDH2 mutation frequencies
IDH1/2 mutations were detected in tissue specimens from each patient (Fig. 1), and the mutation frequencies were determined (Table II). No mutations in IDH1 or IDH2 were detected in samples from patients with grade I brain glioma (n=6). In grade II samples (n=66), IDH1 mutations were observed in 44 cases (66.67%), and IDH2 mutations were detected in 4 cases (6.06%). There were 11 instances of IDH1 mutations (42.31%) and 4 cases of IDH2 mutations (15.38%) among the grade II–III gliomas (n=26). Of the 61 cases of grade III glioma, 34 and 3 cases exhibited IDH1 and IDH2 mutations, respectively (55.74 and 4.92%). The total frequencies of IDH1/2 mutations in grade II, II–III and III gliomas were 72.73, 57.69 and 60.66%, respectively. The overall IDH1/2 mutation frequency in grade II, II–III and III gliomas was 65.36%.
Figure 1.
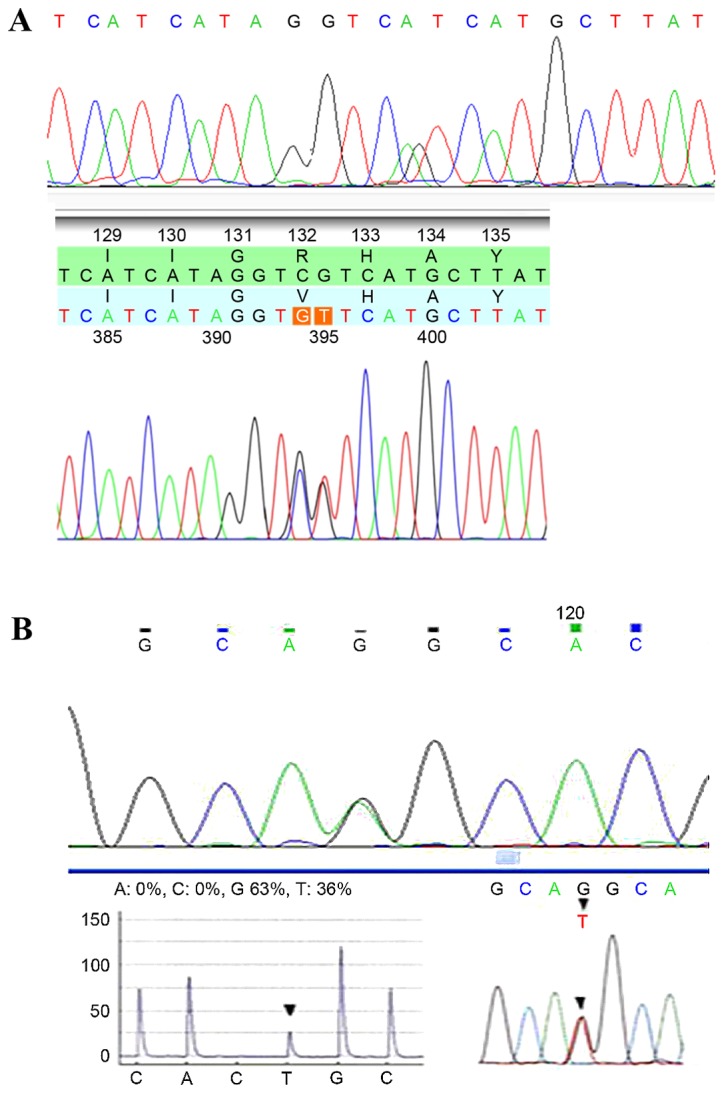
Examples of IDH1 and IDH2 sequencing results. (A) Substitution in IDH1 at amino acid residue 132 (R132H). (B) Substitution in IDH2 at amino acid residue 172 (R172K). IDH, isocitrate dehydrogenase.
Table II.
Frequencies of IDH1 and IDH2 mutations in brain glioma.
| Frequency per grade, n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | I | II | II–III | III | II, II–III and III | IV (primary) | IV (secondary) | Z-valuea | P-value |
| Total patients | 6 | 66 | 26 | 61 | 153 | 43 | 4 | – | – |
| IDH1 mutation | 0 (0.00) | 44 (66.67) | 11 (42.31) | 34 (55.74) | 89 (58.17) | 3 (6.98) | 2 (50.00) | – | – |
| IDH2 mutation | 0 (0.00) | 4 (6.06) | 4 (15.38) | 3 (4.92) | 11 (7.19) | 2 (4.65) | 0 (0.00) | – | – |
| IDH1/2 mutations | 0 (0.00) | 48 (72.73) | 15 (57.69) | 37 (60.66) | 100 (65.36) | 5 (11.63) | 2 (50.00) | −4.388 | <0.001 |
Wilcoxon rank sum test was used to analyze the association between IDH1/2 mutation frequency and glioma grade. IDH, isocitrate dehydrogenase.
In the 43 samples of grade IV primary GBM, there were 3 and 2 cases that exhibited mutations in IDH1 (6.98%) and IDH2 (4.65%), respectively. By contrast, in the 4 samples of grade IV secondary GBM, there were 2 IDH1 mutations (50.00%) and no IDH2 mutations observed. The total frequencies of IDH1/2 mutations for grade IV primary GBM and grade IV secondary GBM were 11.63 and 50.00%, respectively.
It should be noted that IDH1 and IDH2 mutation frequencies in grade II, II–III and III brain gliomas were higher than those in grades I and IV. By contrast, no mutations in IDH1 or IDH2 were observed in any of the 9 spinal cord gliomas. These data suggested that IDH1 and IDH2 mutations were more likely to occur in grade II, II–III and III brain gliomas.
Association between IDH1/2 mutation frequencies and brain glioma grade
Next, the association between IDH1/IDH2 mutation frequency and brain glioma grade was analyzed. Wilcoxon rank sum test revealed that IDH1/2 mutation frequencies differed significantly between the different grades of brain glioma (Z=−4.388, P<0.001; Table II). The frequency of IDH1/2 mutations was higher in grade II gliomas than in grade II–III and III gliomas, yet was higher in grade III than in grade II–III gliomas. The data suggested that IDH1/2 mutations were associated with grade II, II–III and III brain gliomas, and may be involved with the progression of brain glioma from grade II to III.
Discussion
Malignant glioma remains a considerable threat to human health worldwide, and the prognosis of patients with high-grade malignant glioma is poor. Since IDH1/2 mutations have been detected in a number of GBM patients (9), the functions of IDH1/2 mutations in glioma are of interest. The present study found that IDH1/2 mutations were frequent in grade II, II–III and III brain gliomas. Furthermore, IDH1/IDH2 mutation frequencies were significantly different among grade II, II–III and III, suggesting the mutations may be associated with the progression of brain glioma from grade II to grade III.
IDH1/2-mutant tumors are reported to primarily occur in adolescents rather than younger children or adults (19,20). However, the present study found that the mean ages of patients with grade II, II–III and III disease were 38.55±14.24, 41.08±14.69 and 43.82±15.31 years, respectively, suggesting that the majority of IDH1/2-mutated gliomas occurred in adults. Ethnic and regional differences between study groups may explain these paradoxical results, while limited sample size could be another reason. Increasing glioma grade appeared to be somewhat correlated with increasing patient age. On average, patients with grade IV (primary) gliomas were significantly older than those with grade I and II gliomas, and patients with grade III gliomas were significantly older than those with grade II gliomas. However, a larger sample size is required to validate the results of the present study.
There is growing interest in the frequency of IDH1/2 mutations in different types of glioma (21). A few studies have reported on IDH1/2 mutation frequencies in different grades of oligodendroglioma and astrocytoma (19,22,23). Unlike these studies, the present research specifically focused on the association between IDH1/2 mutation frequency and different grades of brain glioma, regardless of the histopathological type. IDH1/2 mutations occurred more often in grade II, II–III and III gliomas (72.73, 57.69 and 60.66%, respectively) than in grade I or IV gliomas. The overall IDH1/2 mutation frequency for grades II, II–III and III was 65.36%. No mutations were detected in grade I gliomas. The present results revealed that IDH1/IDH2 mutation frequencies were significantly different between glioma grades II, II–III and III, suggesting that the mutations may be associated with the progression of brain gliomas from grade II to grade III.
A previous study examining 321 gliomas revealed that IDH1 mutations were present in 82% of patients with secondary GBM (n=34), but only 5% of patients with primary GBM (n=59) (24). Similarly, the present study also revealed a sharp discrepancy in IDH1 mutation frequency between secondary and primary GBM. IDH1 mutation was detected in 2 (50.00%) of 4 secondary GBM cases, and 3 (6.98%) of 43 primary GBM cases. The discrepancy in IDH1 mutation frequency in the same GBM types between the two studies may be attributed to limited sample size or differences in ethnicity.
The IDH1 and IDH2 enzymes are localized in the cytoplasm, peroxisomes and mitochondria. IDH1/2 play a part in the conversion of isocitrate to α-ketoglutarate and block the reduction of NADP+ to NADPH. These enzymes also protect the cell against oxidative stress. There is evidence that IDH1 (R132) mutation is an event shared by all recurrent gliomas early in tumorigenesis (23). Certain studies have reported that gliomas with mutations in one of the IDH genes have a better patient outcome than those with other gene mutations (15,16), and IDH1/2 mutations are recognized as positive prognostic biomarkers (25,26). The present study found that IDH1/2 mutations were most frequent in grade II, II–III and III gliomas. It is therefore reasonable to speculate that patients with gliomas of these grades with IDH1/2 mutations have a better prognosis than those without these mutations. The mutations may increase the survival of patients by enhancing cellular oxidative stress and reducing NADPH levels (10,27). Nevertheless, the large quantity of 2-hydroxyglutarate (2-HG) that is produced concomitantly by IDH mutant enzymes could facilitate malignant progression (28,29). However, the negative effect of 2-HG may be abrogated by the beneficial effect of IDH mutations. These findings indicate that mutant IDH enzymes may affect multiple pathways in glioma, which coordinate to influence patient prognosis.
The present study is a preliminary one. A larger sample size is required to validate the results of this study, and more studies to identify molecular targets and pathways that are influenced by IDH1/2 mutations are underway. Further information regarding the underlying mechanisms of these mutations in glioma is therefore expected in the future.
In summary, the present study provides further information regarding the role of IDH1/IDH2 mutations in brain gliomas in Chinese patients. IDH1/2 mutations are associated with grade II, II–III and III brain gliomas, and are possibly involved with the progression of brain gliomas from grade II to grade III. The majority of IDH1/2-mutant brain gliomas affected adults, rather than adolescents. Further studies are required to clarify the precise roles of these mutations in brain gliomas.
Acknowledgements
Not applicable.
Funding
This study work was supported by a grant from the Natural Science Foundation of China (grant no. H1618).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author's contributions
LD and PX were responsible for conception and design of the research and drafting of the manuscript. YL and SL performed data acquisition. XB and WZ were responsible tor data analysis and interpretation. SL performed statistical analysis. SQ and SL conducted the experiments. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cha S. Perfusion MR imaging of brain tumors. Top Magn Reson Imaging. 2004;15:279–289. doi: 10.1097/00002142-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Altieri R, Agnoletti A, Quattrucci F, Garbossa D, Calamo Specchia FM, Bozzaro M, Fornaro R, Mencarani C, Lanotte M, Spaziante R, Ducati A. Molecular biology of gliomas: Present and future challenges. Transl Med UniSa. 2014;10:29–37. [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol. 2010;12:520–527. doi: 10.1093/neuonc/nop066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 7.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 8.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labussiere M, Sanson M, Idbaih A, Delattre JY. IDH1 gene mutations: A new paradigm in glioma prognosis and therapy? Oncologist. 2010;15:196–199. doi: 10.1634/theoncologist.2009-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohrenz IV, Antonietti P, Pusch S, Capper D, Balss J, Voigt S, Weissert S, Mukrowsky A, Frank J, Senft C, et al. Isocitrate dehydrogenase 1 mutant R132H sensitizes glioma cells to BCNU-induced oxidative stress and cell death. Apoptosis. 2013;18:1416–1425. doi: 10.1007/s10495-013-0877-8. [DOI] [PubMed] [Google Scholar]
- 11.Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 12.Ichimura K, Pearson DM, Kocialkowski S, Bäcklund LM, Chan R, Jones DT, Collins VP. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11:341–347. doi: 10.1215/15228517-2009-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horbinski C. What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol. 2013;125:621–636. doi: 10.1007/s00401-013-1106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Tao Q, Lei Y, Si G, Yan Qing D, Hui Xia H, Xue Lin Z, LanXiao W, Fei Y. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci. 2012;103:269–273. doi: 10.1111/j.1349-7006.2011.02134.x. [DOI] [PubMed] [Google Scholar]
- 16.Zou P, Xu H, Chen P, Yan Q, Zhao L, Zhao P, Gu A. IDH1/IDH2 mutations define the prognosis and molecular profiles of patients with gliomas: A meta-analysis. PLoS One. 2013;8:e68782. doi: 10.1371/journal.pone.0068782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B, Komisopoulou E, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen N, Weller RO. Who classification of tumours of the central nervous system. Wiley Online Library; 2007. [Google Scholar]
- 19.Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, et al. Type and frequency of IDH1IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: A study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 20.Pollack IF, Hamilton RL, Sobol RW, Nikiforova MN, Lyons-Weiler MA, LaFramboise WA, Burger PC, Brat DJ, Rosenblum MK, Holmes EJ, et al. IDH1 mutations are common in malignant gliomas arising in adolescents: A report from the children's oncology group. Childs Nerv Syst. 2011;27:87–94. doi: 10.1007/s00381-010-1264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Megova M, Drabek J, Koudelakova V, Trojanec R, Kalita O, Hajduch M. Isocitrate dehydrogenase 1 and 2 mutations in gliomas. J Neurosci Res. 2014;92:1611–1620. doi: 10.1002/jnr.23456. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: Implications for classification of gliomas. Acta Neuropathol. 2010;120:707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 23.Lass U, Nümann A, von Eckardstein K, Kiwit J, Stockhammer F, Horaczek JA, Veelken J, Herold-Mende C, Jeuken J, von Deimling A, Mueller W. Clonal analysis in recurrent astrocytic, oligoastrocytic and oligodendroglial tumors implicates IDH1-mutation as common tumor initiating event. PLoS One. 2012;7:e41298. doi: 10.1371/journal.pone.0041298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metellus P, Coulibaly B, Colin C, de Paula AM, Vasiljevic A, Taieb D, Barlier A, Boisselier B, Mokhtari K, Wang XW, et al. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010;120:719–729. doi: 10.1007/s00401-010-0777-8. [DOI] [PubMed] [Google Scholar]
- 26.Gorlia T, Delattre JY, Brandes AA, Kros JM, Taphoorn MJ, Kouwenhoven MC, Bernsen H, Frénay M, Tijssen CC, Lacombe D, van den Bent MJ. New clinical, pathological and molecular prognostic models and calculators in patients with locally diagnosed anaplastic oligodendroglioma or oligoastrocytoma. A prognostic factor analysis of European organisation for research and treatment of cancer brain tumour group study 26951. Eur J Cancer. 2013;49:3477–3485. doi: 10.1016/j.ejca.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Chou AP, Chen W, Chen R, Deng Y, Phillips HS, Selfridge J, Zurayk M, Lou JJ, Everson RG, et al. Overexpression of isocitrate dehydrogenase mutant proteins renders glioma cells more sensitive to radiation. Neuro Oncol. 2013;15:57–68. doi: 10.1093/neuonc/nos261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin G, Reitman ZJ, Spasojevic I, Batinic-Haberle I, Yang J, Schmidt-Kittler O, Bigner DD, Yan H. 2-hydroxyglutarate production, but not dominant negative function, is conferred by glioma-derived NADP+-dependent isocitrate dehydrogenase mutations. PLoS One. 2011;6:e16812. doi: 10.1371/journal.pone.0016812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


