Abstract
At present, chemotherapy and radiotherapy represent the primary modalities of treatment for patients with unresectable esophageal squamous cell carcinoma (ESCC). However, the outcome of patients remains poor owing to radioresistance. The present study aimed to determine the radiosensitizing effect of liriodenine, an aporphine alkaloid derived from Enicosanthellum pulchrum, and investigating the underlying mechanisms in ESCC, using the esophageal cancer ECA-109 cell line. Cellular proliferation was evaluated using the Cell Counting kit-8 assay. Colony formation assay was performed to characterize the radiosensitive effects of liriodenine on ECA-109 cells, and flow cytometry was used to detect the percentage of cells undergoing apoptosis. An immunofluorescence assay was utilized to evaluate the DNA damage repair ability. Western blotting was used to assess the protein levels of caspase-3, B cell lymphoma-2 (Bcl-2) and Bcl-2-associated X (Bax). Liriodenine dose-dependently inhibited ECA-109 cell viability. The clonogenic survival assay demonstrated that liriodenine increased the radiosensitivity of ESCC cells, with a sensitization enhancement ratio of 1.11–1.69. The results of flow cytometry demonstrated that liriodenine induced apoptosis and G2/M arrest. The immunofluorescence assay revealed that liriodenine delays DNA damage repair. The upregulation of Bax and Caspase-3, and the suppression of Bcl-2 confirmed that apoptosis was occurring. Liriodenine radiosensitizes ECA-109 cells by inducing apoptosis and G2/M arrest. The findings of the present study indicated that liriodenine may represent an anticancer agent with promising potential for the treatment of ESCC.
Keywords: liriodenine, esophageal squamous cell carcinoma, radiotherapy, apoptosis, G2/M arrest
Introduction
Esophageal cancer is the eighth most common malignant tumor and the sixth leading cause of cancer-associated mortalities in the world (1–3). Esophageal squamous cell carcinoma (ESCC) is the major histological subtype of esophageal cancer, representing 80% of the incidence of this disease (2). The 5-year survival rate remains poor, at 15–25% following diagnosis (4,5). Although radiotherapy remains the primary treatment modality for unresectable ESCC, this approach is limited by the significant radioresistance exhibited by the neoplasm. As such, the identification of a novel effective anticancer agent may be essential for improving treatment in ESCC by radiation therapy.
8H-benzo (g)-1, 3-benzodioxolo (6,5,4-de)-quinolin-8-one (liriodenine) is a cytotoxic isoquinoline alkaloid that has been isolated from plant species of numerous genera, including Fissistigma glaucescens, Annona glabra and Liriodendron tulipifera (6–8). Previous studies have highlighted the diverse biological properties of liriodenine, including anti-platelet, mutagenic, anti-microbial, anti-fungal and anti-arrhythmic effects (9–11). In addition, in vitro studies have identified that liriodenine exhibits growth inhibition on cancer cell lines (12,13). Specifically, Li et al (12) demonstrated that liriodenine could significantly enhance antitumor effects via upregulation of tumor protein p53 expression, and that this compound could significantly inhibit migration and induce apoptosis in laryngocarcinoma HEp-2 cells. Furthermore, liriodenine led to the suppression of proliferation and concomitant induction of apoptosis in human lung adenocarcinoma A549 cells (13). Liriodenine also induced a G1 cell cycle arrest and repression of DNA synthesis in HepG2 and SK-Hep-1 cells (14).
Taken together, these findings highlight the significant therapeutic potential of liriodenine. However, to the best of our knowledge, no study has yet evaluated the combinatorial effects of liriodenine with radiation therapy. The present study evaluated the use of liriodenine as an anticancer agent, particularly for human ESCC, and characterized the molecular mechanism through which liriodenine acts.
Materials and methods
Cell culture and reagents
The ESCC ECA-109 cell line was obtained from the Type Culture Collection of the Chinese Academy of Science (Shanghai, China). The cells were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences, Little Chalfont, UK), in an incubator with an atmosphere of 5% CO2 at 37°C. Liriodenine powder was obtained from SenBeiJia Biological Technology Co., Ltd. (Nanjing, China) dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 1×105 µM and subsequently added to cell culture media for in vivo experiments. B cell lymphoma-2 (Bcl-2; 15071; dilution, 1:500), Bcl-2-associated X protein (Bax; 5023; dilution, 1:500) and Caspase-3 (9665; dilution, 1:500) antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA), while antibodies targeting GAPDH (5174; dilution, 1:5,000) were purchased from Bioworld Technology, Inc. (St. Louis Park, MN, USA). Biotinylated goat anti-rabbit IgGs (111-035-003) were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). Fluorescein isothiocyanate (FITC)-conjugated donkey anti-mouse IgG (715-097-003), biotinylated anti-mouse IgG (715-035-003) were purchased from Jackson Immuno Research Laboratories, Inc.
Cell proliferation assay
A total of 5×104 cells/well were seeded on 96-well culture plates. After 24 h, the cells were treated with liriodenine at 37°C at various concentrations (0, 0.1, 0.5, 1, 2.5, 5, 7.5, 10 and 20 µM). A Cell Counting kit-8 (OBiO Technology, Corp., Ltd., Shanghai, China) was added to wells after 24 and 48 h according to manufacturer's protocol. Living cells were detected at a wavelength of 450 nm using a microplate reader. All experiments were repeated three times.
Clonogenic assay
ECA-109 cells were plated onto 6-well dishes at a density of 1×103 cells/well and treated with liriodenine (0.1 or 1 µM) or control (DMSO) for 24 h. A medical linear accelerator (ElektaPrecise; Elekta Instrument AB, Stockholm, Sweden) was used to irradiate cells with 0, 2, 4, 6 and 8 Gy. Following irradiation, the cells were cultured for 10–14 days. The cells were fixed with methanol (99.99%) for 0.5 h at 37°C and Giemsa-stained for 15 min at 37°C. The numbers of colonies were counted under the TS100 microscope at magnification ×10 (Nikon Corporation, Tokyo, Japan). Each effective colony was greater than 50 cells. All experiments were repeated three times. SER (sensitization enhancement ratio) was calculated according to D0 (mean lethal dose of control group)/D0 (mean lethal dose of Liriodenine treatment group). SF was calculated according to SF=1-(1-e−D/D0)n.
Apoptosis assay
ECA-109 cells were plated on 6-well plates at a density of 1×105 cells/well and divided into one of following groups: Control, liriodenine treatment group, 8 Gy X-rays group, combination group. The numbers of cells undergoing apoptosis were evaluated using annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining using an Annexin V-FITC Apoptosis kit according to the manufacture's protocol (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) for 15 min in the dark at 37°C. The treated cells were detected by FlowJo V10 (FlowJo LLC, Ashland, OR, USA) using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Cell cycle assay
ECA-109 cells were cultured on 6-well plates at a density of 1×105 cells/well and treated with liriodenine (0.1 and 1 µM) or 8 Gy X-rays. Cells were collected and fixed in 75% ice-cold ethanol overnight at 4°C. Cells were stained with 5% PI for 15 min in the dark at 37°C and the cell cycle distribution was assessed using a FACSCalibur flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Immunofluorescence assay
ECA-109 cells were plated onto confocal laser small dishes at a density of 5×104 cells/well and harvested at 2, 8 and 24 h post-irradiation (8 Gy) with or without liriodenine (1 µM). Cells were then fixed in 4% paraformaldehyde at 37°C for 1 h and permeabilized in 0.2% Triton X-100 (Sigma-Aldrich; Merck KGaA) at 37°C for 15 min. Thereafter, cells were blocked with 5% bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.) for 1 h at room temperature and incubated with γH2AX antibody (80312, dilution, 1:1,000, OBbiO Technology, Corp., Ltd.) at room temperature for 1 h. Cells were incubated with an Alexa Fluor 594-conjugated secondary antibody (dilution, 1:5,000, Jackson ImmunoRearch) for 0.5 h at 37°C. Cells were treated with DAPI (Beyotime Institute of Biotechnology, Shanghai, China) for 30 min in the dark at 4°C and visualized using confocal fluorescence microscopy under three fields of view at magnification ×40 (Leica Microsystems, Inc., Buffalo Grove, IL, USA).
Western blotting analysis
ECA-109 cells were cultured on 6-well plates at a density of 1×105 cells/well and treated with liriodenine (1 µM) or 8 Gy X-rays. Radioimmunoprecipitation assay buffer (OBiB Technology, Corp., Ltd.) was used to lyse cells, and a Bicinchoninic Acid Protein Quantification kit (Beyotime Institute of Biotechnology, Haimen, China) was used to quantify the amount of protein in the lysates. Equal amounts of 15 µg proteins were separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA). Western blot analysis was performed using the aforementioned rabbit polyclonal primary antibodies to assess the protein levels of caspase-3, Bax, Bcl-2 and GAPDH, followed by horseradish peroxidase-conjugated secondary antibodies (dilution, 1:5,000; Bioworld Technology, Inc.) for 1 h at 37°C. Immunoblotted proteins were analyzed with the ChemiDoc XRS imaging system and QuantityOne software (Version 4.6.9, Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The mean ± standard deviation (SD) from triplicate assays was calculated, and differences between treatment groups were determined using one-way analysis of variance with post hoc Tukey's test. Statistical analysis was performed using SPSS 18 (SPSS, Inc., Chicago, IL, USA) and figures were generated using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Liriodenine significantly promotes radiosensitivity of ECA-109 cells
Liriodenine decreased proliferation of ESCC ECA-109 cells in a dose-dependent manner (Fig. 1A; P<0.01). Liriodenine at a low cytotoxic concentration (0.1 and 1 µM) was selected to assess the sensitizing effects towards radiotherapy. Liriodenine plus radiotherapy exhibited a marked reduction in the colony formation of ESCC cells, as shown in Fig. 1B (P<0.01). The sensitization enhancement ratios of liriodenine concentrations at 0.1 and 1 µM were 1.11 and 1.69, and survival fraction (Gy) were 0.6 and 0.38 in ECA-109 cells according to methods in a clonogenic assay, respectively (Table I). The ability to form colonies was used as an indication of resistance to IR. The combination treatment of liriodenine and radiation significantly promoted the radiosensitivity of ECA-109 cells.
Figure 1.
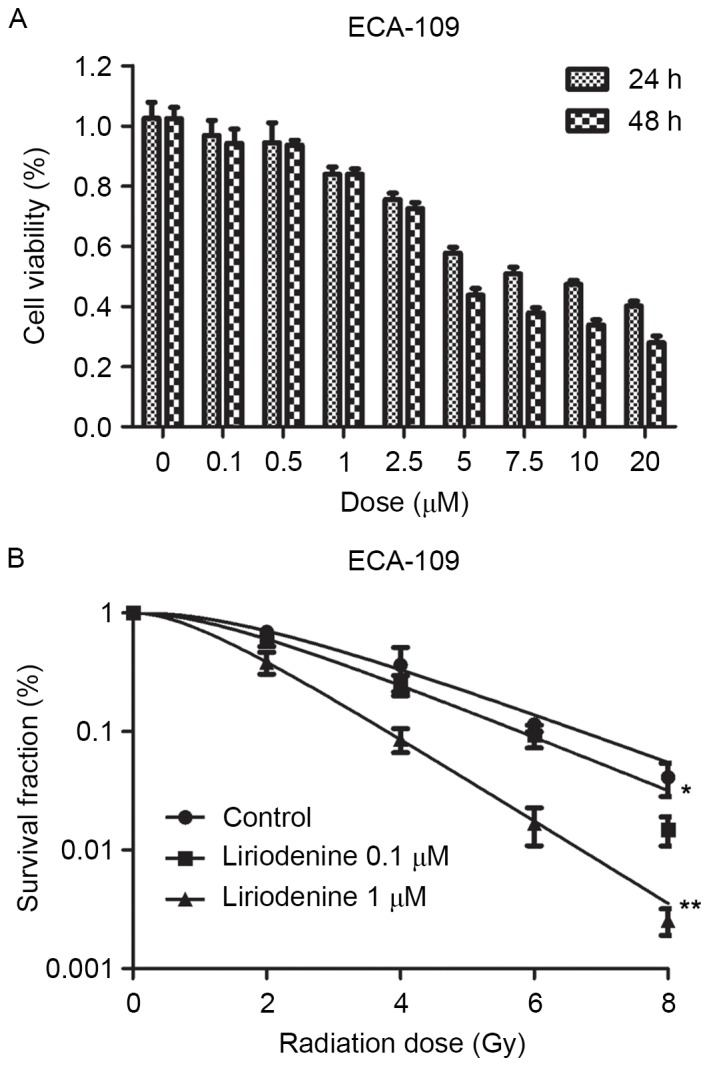
Liriodenine sensitizes ECA-109 cells to IR. (A) ECA-109 cells were treated with liriodenine (0.1, 0.5, 1, 2.5, 5, 7.5, 10 and 20 µM) or dimethyl sulfoxide as a control for 24 or 48 h. Liriodenine suppresses cell viability in a dose- and time-dependent manner in ECA-109 cells. (B) A clonogenic assay was performed to calculate the sensitization enhancement ratio of ECA-109 cells in response to different doses (0.1 and 1 µM) of liriodenine. IR, irradiation. *P<0.05 and **P<0.01
Table I.
Radiosensitization effects of liriodenine on esophageal squamous cell carcinoma ECA-109 cells.
| ECA-109 treatment | D0 | Dq | SF2 | SER |
|---|---|---|---|---|
| Control | 2.11 | 3.13 | 0.69 | |
| Liriodenine 0.1 µM | 1.91 | 2.17 | 0.60 | 1.11 |
| Liriodenine 1 µM | 1.25 | 1.44 | 0.38 | 1.69 |
D0, mean lethal dose; Dq, quasi-threshold dose; SF2, survival fraction (2 Gy); SER, sensitization enhancement ratio.
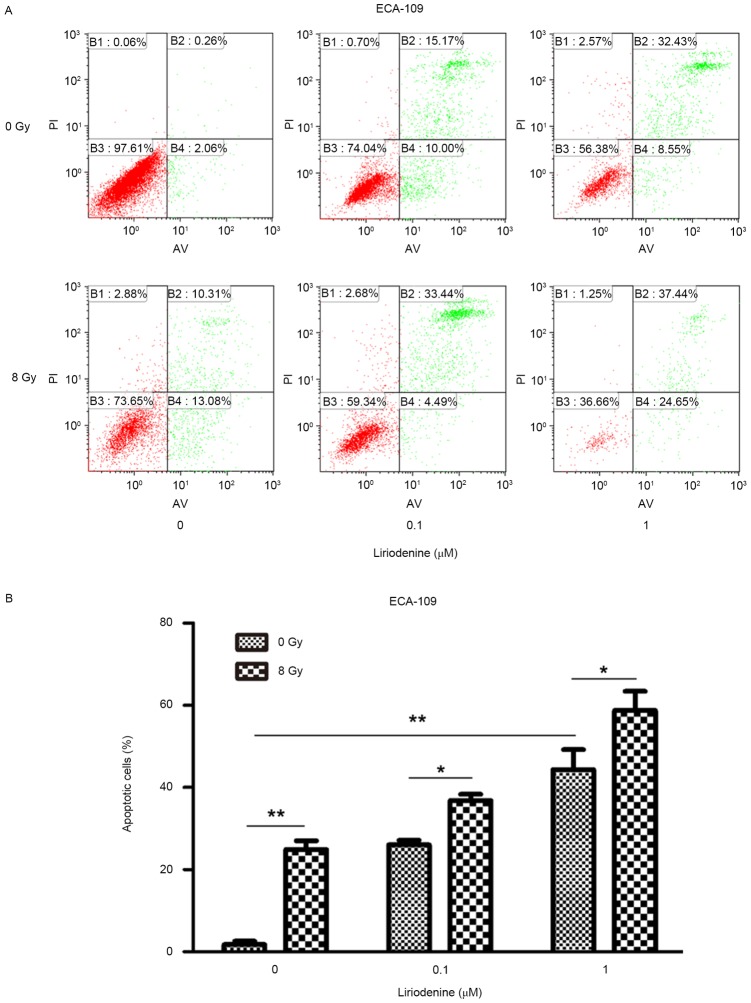
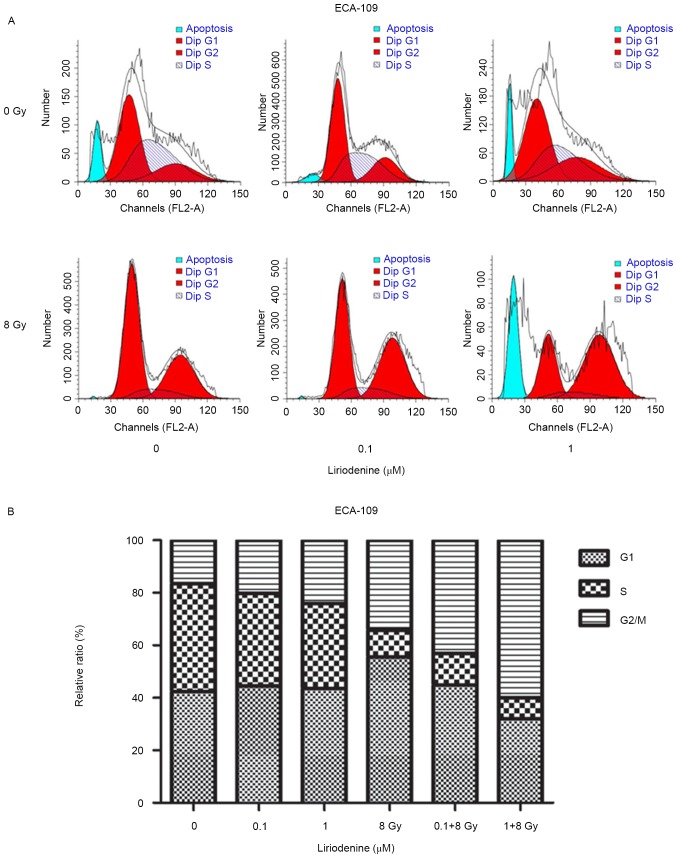
Treatment with radiation and liriodenine together increase apoptosis and modify cell cycle distribution
Whether liriodenine induced radiosensitization due to increased apoptosis and changes to the cell cycle was investigated. ECA-109 cells treated with liriodenine and IR together exhibited significantly increased apoptosis rates compared with either treatment alone (Fig. 2; P<0.01). To investigate the antitumor effects of liriodenine, changes in the cell cycle distribution were evaluated. The variation of the percentage of cells in each phase of the cell cycle is depicted in Fig. 3. Flow cytometry analysis indicated that treatment with liriodenine and IR resulted in a significant proportion of cells arrested in G2/M phase (60.02% for ECA-109) (P<0.01) as compared with single agent treatments. Exposure of the cells to liriodenine or IR alone caused a small degree of accumulation of the cells in G2/M phase, and a slight decrease in the proportion of cells in G0/G1 phase. Therefore, liriodenine appears to induce the arrest of cells in G2/M phase.
Figure 2.
Liriodenine promotes apoptosis in ECA-109 cells. ECA-109 cells treated with liriodenine (0.1 and 1 µM) with/without IR for 24 h. (A) Flow cytometry analysis demonstrates that liriodenine increases apoptosis in ECA-109 cells. (B) Proportion of apoptotic cells in (A) were quantified. Results were obtained from three independent experiments. Error bars represent the standard error of the mean. *P<0.05 and **P<0.01. IR, irradiation; AV, annexin V; PI, propidium iodide.
Figure 3.
Effects of liriodenine on the cell cycle distribution of ECA-109 cells. (A) The cell cycle distribution of ESCC cells was examined by flow cytometry. (B) ECA-109 cells were treated with liriodenine alone or in combination with IR for 24 h. Cell cycle distribution was calculated by flow cytometry. Liriodenine induced arrest in G2/M phase. Results were obtained from three independent experiments. Error bars represent the standard error of the mean. IR, irradiation.
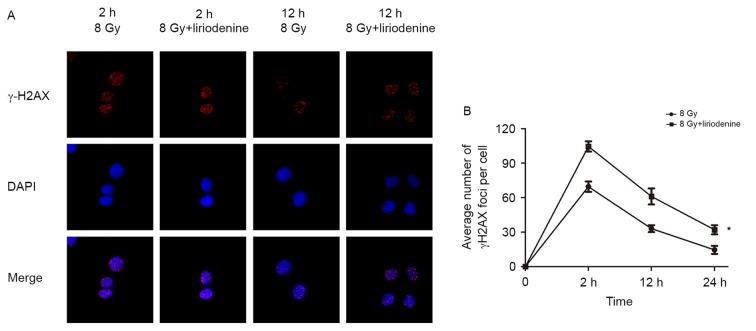
Liriodenine significantly decreases double-strand break (DSB) repair in ECA-109 cells
ECA-109 cells with or without pretreatment with liriodenine were harvested at 2, 12 and 24 h post-irradiation. The mean numbers of γ-H2AX foci were calculated to examine the amount of DSBs in cells. DNA repair following double-stranded DNA breaks requires a variant histone protein called H2A.X. Radiation induces phosphorylation of H2A.X at Ser139 following DNA breaks (15). Liriodenine primarily enhanced the radiosensitivity of cells by delaying the repair of DNA damage. As shown in Fig. 4, treatment with liriodenine plus irradiation significantly delayed DNA DSB repair compared with irradiation alone.
Figure 4.
Liriodenine significantly decreases DSB repair in ECA-109 cells. (A) γ-H2AX foci was evaluated by immunofluorescence. (B) Irradiated ECA-109 cells were treated with or without liriodenine for 2, 12 and 24 h. The mean numbers of γ-H2AX foci were calculated to show the degree of DSBs. Cells were co-stained with DAPI to visualize nuclei. Magnification ×40. *P<0.05 vs. 8 Gy at 2 h. DSB, double strand break; γ-H2AX, γ-histone 2AX.
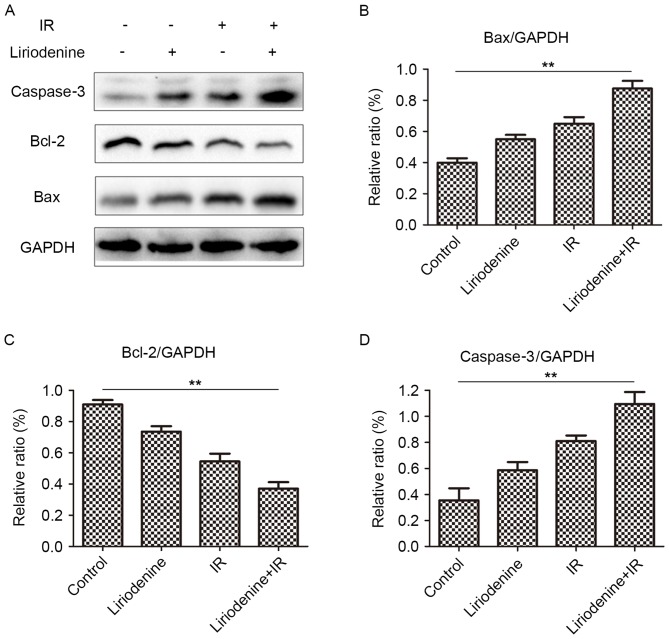
Liriodenine radiosensitizes ESCC cells by regulating protein expression of Bax, Bcl-2 and Caspase-3
ESCC was radiosensitized by liriodenine through the regulation of Bax, Bcl-2 and caspase-3 expression. Following combinatorial treatment with liriodenine and radiation, changes in the expression of Bax, Bcl-2 and caspase-3 were observed (Fig. 5). The expression of Bcl-2 protein decreased, whereas that of Bax and caspase-3 increased in cells that received combined treatment, compared with the cells treated with liriodenine or radiation alone. These results indicated that liriodenine induced changes in cell proliferation and apoptosis.
Figure 5.
Expression of markers of apoptosis evaluated by western blot analysis. Cells were treated with liriodenine and IR for 24 h. (A) Western blot analysis of Bax, Bcl-2 and caspase-3. (B) Relative ratio of Bax/GAPDH. (C) Relative ratio of Bcl-2/GAPDH. (D) Relative ratio of caspase-3/GAPDH. **P<0.01. IR, irradiation; Bcl-2, B cell lymphoma-2; Bax, Bcl-2-associated X protein.
Discussion
The aim of the present study was to evaluate the radiosensitizing effect of liriodenine, an aporphine alkaloid from Enicosanthellum pulchrum, Fissistigma glaucescens, Annona glabra, Liriodendron tulipifera, and to investigate the underlying molecular mechanisms through which this agent functions. Liriodenine was first reported to exhibit tumor suppressive properties in 1969 (6). In previous studies, liriodenine was shown to inhibit cell proliferation in hepatoma, ovarian, laryngocarcinoma and lung cancer (12–14). Similarly, liriodenine significantly inhibited the proliferation of melanoma cells in a cell viability assay (16). Results demonstrated that liriodenine, particularly when combined with radiation therapy, induced a significant decrease in the viability and proliferation of ESCC cells in a dose-dependent manner in vitro.
It has previously been reported that liriodenine affects cell cycle distribution. Li et al (16) revealed that liriodenine and cisplatin significantly induced cell cycle arrest at the G2/M phase and the S phase in human hepatoma BEL-7404 cells, while cell cycle arrest at G2/M was also observed in human lung adenocarcinoma A549 cells treated with liriodenine (13). In addition, combination treatment with liriodenine and glaucine induced a moderate accumulation of cells in G2/M phase in the human melanoma A375.S2 cells (17). In the present study, it was revealed that IR and nanomolar concentration of liriodenine significantly induced cell cycle arrest at the G2/M phase. The present results are thus in agreement with those of previous studies.
Radiotherapy represents the primary modality of treatment for patients with unresectable ESCC. However, despite this treatment, 5-year survival rates remain poor, which is attributed to radiation resistance (18). Previous studies have reported that liriodenine may induce apoptosis in human lung, ovarian and laryngocarcinoma cancer cells (12–14). In the present study, compared with liriodenine or IR alone, liriodenine and IR together strongly induced apoptosis in ESCC cells. The radiosensitizing effect of liriodenine may be mediated by numerous molecular pathways. The results of the present study indicated that cell cycle arrest and apoptosis represent the primary effects of liriodenine treatment.
High Bcl-2 expression levels have been shown to lead to radioresistance in cancer patients, and radiation-induced activity of Bcl-2 family members are known to cause radioresistance in human prostate cancer cell lines (19). Similarly, high expression of Bax may sensitize patients with head and neck cancer to radiotherapy (20). As such, several direct Bax activators have been use to overcome resistance to chemotherapy and radiotherapy (21). Caspases are central for the induction of apoptosis (22). Hypofractionated RT induces cell death only in caspase-3-proficient breast cancer cells (23). In the present study, increased expression of Bax and caspase-3 was observed following treatment with liriodenine and IR, whereas that of Bcl-2 was significantly decreased. As such, these results indicated that liriodenine may induce apoptosis of radioresistant ESCC cells by regulating the level of the key apoptotic-associated proteins.
In summary, the present study demonstrated that liriodenine affects ESCC cell viability and proliferation, which was associated with changes in cell cycle distribution. Liriodenine may also induce apoptosis of radioresistant ESCC cell lines by increasing expression of Bax and caspase-3, and decreasing that of Bcl-2, indicating that liriodenine may be a promising anticancer agent in ESCC radiation therapy.
Acknowledgements
The authors would like to thank Professor Xinchen Sun (The First Affiliated Hospital of Nanjing Medical University, Nanjing, China) for his technical support.
Funding
The present study was supported by Key Academic Discipline of Jiangsu Province ‘Medical Aspects of Specific Environments’, A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (grant no. JX10231801), A Phase III Clinical Trial Funded by Wuxi Hospital Management Center (grant no. YGZXM14033).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
GW and GC performed the experiments, analyzed the data and wrote the manuscript. JZ participated in flow cytometry analysis. JZ and JC helped with western blot analysis and data collection. HZ and FZ conceived the idea, designed the study and helped draft the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to publish
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 4.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 5.Pennathur A, Farkas A, Krasinskas AM, Ferson PF, Gooding WE, Gibson MK, Schuchert MJ, Landreneau RJ, Luketich JD. Esophagectomy for T1 esophageal cancer: Outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg. 2009;87:1054–1055. doi: 10.1016/j.athoracsur.2008.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warthen D, Gooden EL, Jacobson M. Tumor inhibitors: Liriodenine, a cytotoxic alkaloid from Annona glabra. J Pharm Sci. 1969;58:637–638. doi: 10.1002/jps.2600580533. [DOI] [PubMed] [Google Scholar]
- 7.Bentley KW. Beta-phenylethylamines and the isoquinoline alkaloids. Nat Prod Rep. 2006;23:444–463. doi: 10.1039/B509523A. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh TJ, Liu TZ, Chern CL, Tsao DA, Lu FJ, Syu YH, Hsieh PY, Hu HS, Chang TT, Chen CH. Liriodenine inhibits the proliferation of human hepatoma cell lines by blocking cell cycle progression and nitric oxide mediated activation of p53 expression. Food Chem Toxicol. 2005;43:1117–1126. doi: 10.1016/j.fct.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Chang GJ, Wu MH, Wu YC, Su MJ. Electrophysiological mechanisms for antiarrhythmic efficacy and positive inotropy of liriodenine, a natural aporphine alkaloid from Fissistigma glaucescens. Br J Pharmacol. 1996;118:1571–1583. doi: 10.1111/j.1476-5381.1996.tb15577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark AM, Watson ES, Ashfaq MK, Hufford CD. In vivo efficacy of antifungal oxoaporphine alkaloids in experimental disseminated candidiasis. Pharm Res. 1987;4:495–498. doi: 10.1023/A:1016479622383. [DOI] [PubMed] [Google Scholar]
- 11.Hufford CD, Funderburk MJ, Morgan JM, Robertson LW. Two antimicrobial alkaloids from heartwood of Liriodendron tulipifera L. J Pharm Sci. 1975;64:789–792. doi: 10.1002/jps.2600640512. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Xu Y, Wang B. Liriodenine induces the apoptosis of human laryngocarcinoma cells via the upregulation of p53 expression. Oncol Lett. 2015;9:1121–1127. doi: 10.3892/ol.2014.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang HC, Chang FR, Wu YC, Lai YH. Anti-cancer effect of liriodenine on human lung cancer cells. Kaohsiung J Med Sci. 2004;20:365–371. doi: 10.1016/S1607-551X(09)70172-X. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh TJ, Liu TZ, Chern CL, Tsao DA, Lu FJ, Syu YH, Hsieh PY, Hu HS, Chang TT, Chen CH. Liriodenine inhibits the proliferation of human hepatoma cell lines by blocking cell cycle progression and nitric oxide-mediated activation of p53 expression. Food Chem Toxicol. 2005;43:1117–1126. doi: 10.1016/j.fct.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Almeida R, Fernández-Justel JM, Santa-María C, Cadoret JC, Cano-Aroca L, Lombraña R, Herranz G, Agresti A, Gómez M. Chromatin conformation regulates the coordination between DNA replication and transcription. Nat Commun. 2018;9:1590. doi: 10.1038/s41467-018-03539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YL, Qin QP, Liu YC, Chen ZF, Liang H. A platinum (II) complex of liriodenine from traditional Chinese medicine (TCM): Cell cycle arrest, cell apoptosis induction and telomerase inhibition activity via G-quadruplex DNA stabilization. J Inorg Biochem. 2014;137:12–21. doi: 10.1016/j.jinorgbio.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Chiu CC, Chou HL, Wu PF, Chen HL, Wang HM, Chen CY. Bio-functional constituents from the stems of liriodendron tulipifera. Molecules. 2012;17:4357–4372. doi: 10.3390/molecules17044357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Efimova EV, Liang H, Pitroda SP, Labay E, Darga TE, Levina V, Lokshin A, Roizman B, Weichselbaum RR, Khodarev NN. Radioresistance of Stat1 overexpressingtumour cellsis associated with suppressed apoptotic responseto cytotoxic agents and increased IL6-IL8 signalling. Int J Radiat Biol. 2009;85:421–431. doi: 10.1080/09553000902838566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezekwudo D, Shashidharamurthy R, Devineni D, Bozeman E, Palaniappan R, Selvaraj P. Inhibition ofexpression of anti-apoptotic protein Bcl-2 and induction of cell death in radioresistant human prostate adenocarcinoma cell line (PC-3) bymethyl jasmonate. Cancer Lett. 2008;270:277–285. doi: 10.1016/j.canlet.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Csuka O, Remenár E, Koronczay K, Doleschall Z, Németh G. Predictive value of p53, Bcl2 and bax in the radiotherapy of head and neck cancer. Pathol Oncol Res. 1997;3:204–210. doi: 10.1007/BF02899922. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Ding Y, Ye N, Wild C, Chen H, Zhou J. Direct activation of bax protein for cancer therapy. Med Res Rev. 2016;36:313–341. doi: 10.1002/med.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modjtahedi N, Giordanetto F, Madeo F, Kroemer G. Apoptosis-inducing factor: Vital and lethal. Trends Cell Biol. 2006;16:264–272. doi: 10.1016/j.tcb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Kötter B, Frey B, Winderl M, Rubner Y, Scheithauer H, Sieber R, Fietkau R, Gaipl US. The in vitro immunogenic potential of caspase-3 proficient breast cancer cells with basal low immunogenicity is increased by hypofractionated irradiation. Radiat Oncol. 2015;10:197. doi: 10.1186/s13014-015-0506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.