Abstract
Background
The aim of this study was to analyze the prognostic value of ARPC5 in patients with multiple myeloma (MM).
Material/Methods
MM gene expression studies GSE6477, GSE31162, GSE24080, and GSE19784 were obtained and analyzed. The expression of ARPC5 was assessed in normal plasma cells, baseline MM cells, and relapsed MM cells. Univariate and multivariable analyses were used to determine the relationship between ARPC5 expression and clinical characteristics and survivals of MM patients. Quantitative PCR was used to detect the expression ARPC5 in bone marrow mononuclear cells of MM patients and normal controls. GSEA was conducted to identify associated mechanisms.
Results
ARPC5 expression was significantly increased in baseline MM cells compared to normal plasma cells (P=0.0414). Meanwhile, ARPC5 was significantly increased in relapsed MM cells compared to baseline MM cells (P<0.0001). ARPC5 expression was significantly associated with β2-microglobin (P=0.047), serum lactate dehydrogenase (P=0.007), and rates of aspirate plasma cells (P=0.007). Meanwhile, patients in the ARPC5 high expression group were associated with poor overall survival (P=0.0027) and event-free survival (P=0.0102) compared to those in the ARPC5 low expression group. Multivariable analysis indicated that ARPC5 was an independent prognostic factor for MM patients. Quantitative PCR demonstrated that ARPC5 was significantly increased in MM patients. GSEA results indicated that ARPC5 might affect cellular growth of myeloma cells through mammalian target of rapamycin (mTOR)C1 signaling pathway.
Conclusions
ARPC5 could be treated as an independent biomarker for patients with MM.
MeSH Keywords: Actin-Related Protein 2-3 Complex, Multiple Myeloma, Prognosis
Background
Multiple myeloma (MM), the second most frequent hematologic malignancy after non-Hodgkin lymphoma, is characterized by the clonal expansion of malignant plasma cells in the bone marrow [1,2]. Nearly 1–5 cases in 1000 persons are diagnosed with MM annually. MM represents about 20% of mortalities of all blood cancers [3]. The availability novel agents including bortezomib, carfilzomib, thalidomide, lenalidomide, and pomalidomide has significantly improved the response rates and survival rates of patients with MM [4–6]. Although MM patients initially respond to treatment, they inevitably relapse due to acquired drug resistance [7]. Although the 5-year survival rate for patients with MM is about 49%, the survival rate of patient with high-risk features is dismal [8]. Therefore, accurate identification of this subset of patients is crucial to the diagnosis, treatment, and prognosis of patients with MM.
ARPC5, also known as actin related protein 2/3 complex subunit 5, encodes 1 of 7 subunits of the human Arp2/3 protein complex [9,10]. Several studies demonstrated that ARPC5 was involved in tumor growth or metastasis of head and neck squamous cell carcinoma and lung squamous cell carcinoma, and cell differentiation [11–12]. Herein, we analyzed the relationship between ARPC5 expression and demographic and tumor characteristics of patients with MM.
Material and Methods
Identification of MM microarray studies
MM microarray GSE24080 [13,14] was contributed by the Myeloma Institute for Research and Therapy at the University of Arkansas for Medical Sciences. Pretreatment bone marrow plasma cells of 559 newly diagnosed MM patients in trial TT2 [15,16] and TT3 [17] were collected and gene expression profiling was conducted for these cases with Affymetrix Human Genome U133 Plus 2.0 gene chip. Demographic and tumor characteristics including age, gender, race, isotype, serum β-2 microglobulin, CRP, creatinine, lactate dehydrogenase, albumin, hemoglobin, rates of aspirate plasma cells, rates of bone marrow biopsy plasma cells, number of magnetic resonance imaging (MRI)-defined focal lesions, cytogenetic abnormalities, event-free survival and overall survival were well documented. GSE6477 [18b19] was contributed by Fonseca et al. A total number of 73 cases of newly diagnosed MM and 15 cases of normal donor were included. Gene expression profiling was conducted for these cases with Affymetrix Human Genome U133A gene chip. GSE31162 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE31162) included gene expression data of 780 newly diagnosed MM and 258 relapsed MM. GSE19784 [20], contributed by Broyl et al., was comprised of 328 previously untreated MM samples and annotated with Affymetrix Human Genome U133 Plus 2.0 Array. We used GSE14784 to perform gene set enrichment analysis (GSEA) [21,22].
Data analysis
To evaluate the expression of ARPC5 in baseline MM, normal plasma, and relapsed MM, 2-sample t-test was conducted and relative expression of ARPC5 was presented as mean ± standard deviation. To determine the correlation between ARPC5 expression and demographic and tumor characteristics of patients with MM, patients in GSE24080 were divided into ARPC5 low expression group and ARPC5 high expression group based on the median of ARPC5 (the corresponding probe ID is 1555797_a_at) expression in GSE24080, and then chi-square test and logistic regression analysis were performed. To investigate the prognostic value of ARPC5 in MM patients, Kaplan-Meier survival analysis and multivariable Cox proportional hazards analysis were conducted. If P values were less than 0.05, the differences were considered statistically significant. Finally, to characterize potentially relevant mechanisms for ARPC5 affected MM cells, GSEA was conducted.
MM sample collection, mononuclear separation, and quantitative PCR
Pretreatment bone marrow aspirates were obtained from10 newly diagnosed MM patients and 10 patients who were finally excluded based on the diagnosis of hematological malignances at Jingzhou Central Hospital. The collection of bone marrows was approved by the Ethics Committee of Jingzhou Central Hospital and the informed consents of the included participants were obtained. Bone marrow mononuclear cells were isolated by using the Ficoll-Hypaque density gradient protocol according to the manufacture’s protocols. Total RNAs were isolated with TRIzol reagent (Invitrogen) according to manufacturer’s instructions. After the synthesis of cDNA, quantitative PCR was performed using SYBR Premix ExTaq (TaKaRa, Japan) on an ABI7500 real-time PCR instrument (ABI Company, Oyster Bay, NY, USA) according to the manufacturer’s instructions. Homo β-actin was treated as an internal control. The PCR detection was performed 3 times. Primers used in this study were as follows: ARPC5 forward: 5′-AAGTTCGTG GACGAGGAGG-3′, ARPC5 reverse 5′-GTAGGGCAGCGGTCATGTTTC-3′; β-actin forward: 5′-AGCGAGCATCCCCCAAAGTT-3′, reverse 5′-GGGCACGAAGGCTCATCATT-3′.
Results
ARPC5 was significantly upregulated in baseline MM and relapsed MM
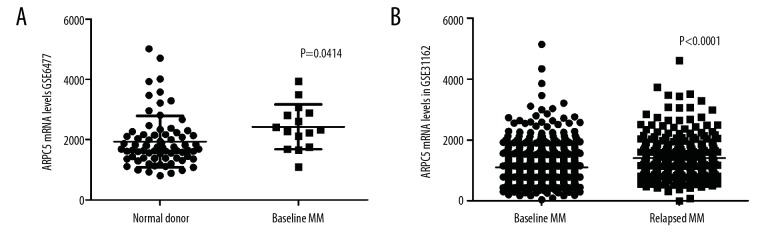
To explore whether ARPC5 was associated with carcinogenesis of MM, we analyzed the expression of ARPC5 in normal plasma cells, baseline MM cells, and relapsed MM cells using GSE6477 and GSE31162. As shown in Figure 1, the expression of ARPC5 was significantly increased in baseline MM cells (n=73) as compared to that in normal plasma cells (n=15) (2425±744.58 vs. 1935±850.61, P=0.0414, Figure 1A). Meanwhile, the expression of ARPC5 was significantly increased in relapsed MM cells (n=258) as compared to that in baseline MM cells (n=780) (1397±703.46 vs. 1096±552.62, P<0.0001, Figure 1B).
Figure 1.
Relative expression of ARPC5 in normal plasma cells, baseline myeloma cells, and relapsed myeloma cells.
Correlations between ARPC5 expression and demographic and tumor characteristics of patients with MM
Then, we analyzed the relationship between ARPC5 expression and clinical characteristics of MM patients. As shown in Table 1, univariate analysis (chi-square test) suggested that pretreatment ARPC5 was associated with gender (P=0.014), serum β2-microglobulin (P<0.0001), serum creatinine (P=0.019), serum lactate dehydrogenase (P=0.019), hemoglobin (P=0.001), rates of aspirate plasma cells (P=0.022), and number of magnetic resonance imaging (MRI)-defined focal lesions (P=0.049). Mutivariable analysis (logistic regression analysis) demonstrated that ARPC5 expression was significantly associated with β2-microglobin (P=0.047), serum lactate dehydrogenase (P=0.007), and rates of aspirate plasma cells (P=0.007). These results indicated that higher expression of ARPC5 expression was associated with poor tumor characteristics of patients with MM.
Table 1.
Correlations between ARPC5 expression and tumor characteristics of multiple myeloma patients.
| Characteristics | ARPC5 expression | Univariate analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|---|
| High (n=279) | Low (n=280) | Chi-square | P value | OR(95% CI) | P value | ||
| Age (years) | |||||||
| ≤60 | 166 | 157 | 0.214 | 0.644 | 0.986 | (0.966–1.007) | 0.192 |
| >60 | 113 | 123 | |||||
| Gender | |||||||
| Male | 154 | 183 | 6.025 | 0.014 | 0.779 | (0.511–1.188) | 0.246 |
| Female | 125 | 97 | |||||
| Race | |||||||
| White | 246 | 251 | 0.307 | 0.58 | 0.901 | (0.471–1.723) | 0.752 |
| Other | 33 | 29 | |||||
| M protein* | |||||||
| IgG | 141 | 172 | 8.408 | 0.078 | |||
| IgA | 77 | 56 | 0.389 | (0.34–4.631) | 0.462 | ||
| FLC | 47 | 37 | 0.785 | (0.066–9.29) | 0.848 | ||
| IgD | 2 | 1 | 0.533 | (0.044–6.451) | 0.621 | ||
| NS | 3 | 5 | 0.2 | (0.01–3.941) | 0.29 | ||
| β2-microglobin (mg/mL)* | |||||||
| <3.5 | 138 | 181 | 15.42 | P<0.0001 | 1.091 | (1.001–1.188) | 0.047 |
| 3.5–5.5 | 67 | 58 | |||||
| ≥5.5 | 73 | 41 | |||||
| CRP (mg/L)* | |||||||
| <5.8 | 151 | 173 | 3.782 | 0.052 | 0.998 | (0.987–1.009) | 0.704 |
| ≥5.8 | 127 | 104 | |||||
| Creatinine (mg/dL)* | |||||||
| <1.2 | 174 | 200 | 5.522 | 0.019 | 0.93 | (0.707–1.225) | 0.608 |
| ≥1.2 | 104 | 78 | |||||
| LDH (U/L) | |||||||
| <170 | 150 | 178 | 5.544 | 0.019 | 1.005 | (1.001–1.009) | 0.007 |
| ≥170 | 129 | 102 | |||||
| ALB (g/dL) | |||||||
| <3.5 | 46 | 31 | 3.451 | 0.063 | 0.759 | (0.508–1.136) | 0.18 |
| ≥3.5 | 233 | 249 | |||||
| HGB (g/dL) | |||||||
| <11 | 142 | 105 | 10.169 | 0.001 | 0.916 | (0.798–1.050) | 0.207 |
| ≥11 | 137 | 175 | |||||
| ASPC* (%) | |||||||
| <40 | 139 | 116 | 5.244 | 0.022 | 0.983 | (0.972–0.996) | 0.007 |
| ≥40 | 123 | 153 | |||||
| BMPC (%)* | |||||||
| <50 | 143 | 131 | 1.554 | 0.213 | 0.999 | (0.988–1.01) | 0.864 |
| ≥50 | 126 | 143 | |||||
| MRI (Magnetic resonance imaging (MRI)-defined focal lesions (skull, spine, pelvis)* | |||||||
| Yes | 200 | 180 | 3.87 | 0.049 | 1.01 | (0.999–1.028) | 0.078 |
| No | 61 | 81 | |||||
| Cytogenetic abnormalities | |||||||
| Yes | 104 | 103 | 0.014 | 0.904 | 0.98 | (0.64–1.502) | 0.927 |
| No | 175 | 177 | |||||
CRP – C-reactive protein; LDH – lactate dehydrogenase; ALB – albumin; HGB – hemoglobin; ASPC – aspirate plasma cells; BMPC – bone marrow biopsy plasma cells;
indicates that the corresponding data for that clinical phenotype has some missing values.
Higher expression of ARPC5 was associated with poor survivals of patients with MM
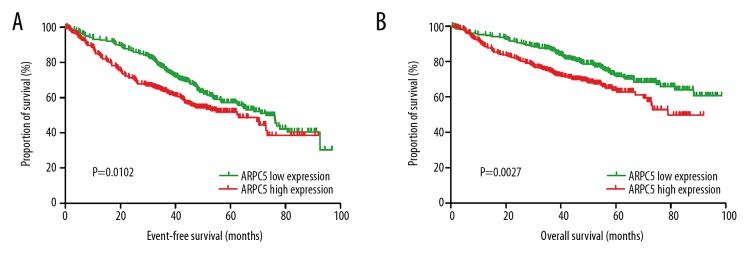
We analyzed the prognostic significance of ARPC5 for MM patients in GSE24080. Patients in GSE20480 were divided into ARPC5 low expression group and ARPC5 high expression group based on the median of ARPC5 expression in GSE24080. Results of Kaplan-Meier survival analyses indicated that patients in the ARPC5 low expression group (n=280) were associated with more superior event-free survival (HR=0.718, 95% CI: 0.5577–0.9245, P=0.0102, Figure 2A) and overall survival (HR=0.6279, 95% CI: 0.4634–0.8508, P=0.0027, Figure 2B) compared with patients in the ARPC5 high expression group (n=279). Multivariable Cox proportional hazard analysis demonstrated that ARPC5 expression was an independent prognosis factor for event-free survival (P=0.006, HR=1.351, 95% CI: 1.092–1.672) and overall survival (P=0.020, HR=1.356, 95% CI: 1.05–1.75) of patients with MM (Table 2).
Figure 2.
Event-free survival (A) and overall survival (B) of patients in the ARPC5 low expression group and the ARPC5 high expression group.
Table 2.
Multivariable Cox proportional hazard analysis on Overall survival and event free survival of patients with multiple myeloma.
| Characteristics | Overall survival | Event free survival | ||||
|---|---|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | |||
| ARPC5 | 0.02 | 1.356 | (1.05–1.75) | 0.006 | 1.351 | (1.092–1.672) |
| Age | 0.271 | 1.01 | (0.992–1.028) | 0.633 | 0.996 | (0.982–1.011) |
| Sex | 0.515 | 1.125 | (0.79–1.602) | 0.274 | 1.176 | (0.879–1.572) |
| Type | 0.884 | 0.023 | ||||
| Type (1) | 0.889 | 1.153 | (0.157–8.481) | 0.143 | 0.415 | (0.128–1.348) |
| Type (2) | 0.995 | 1.006 | (0.136–7.45) | 0.168 | 0.435 | (0.133–1.42) |
| Type (3) | 0.887 | 0.863 | (0.113–6.582) | 0.019 | 0.231 | (0.068–0.789) |
| Type (4) | 0.955 | <0.0001 | (<0.0001–<0.00001) | 0.034 | 0.085 | (0.009–0.835) |
| B2M | <0.0001 | 1.098 | (1.056–1.142) | <0.0001 | 1.088 | (1.05–1.127) |
| CRP | 0.489 | 0.997 | (0.988–1.006) | 0.489 | 0.997 | (0.99–1.005) |
| Creatinine | 0.262 | 0.917 | (0.788–1.067) | 0.604 | 0.965 | (0.845–1.103) |
| LHD | <0.0001 | 1.005 | (1.003–1.007) | 0 | 1.004 | (1.002–1.006) |
| ALB | 0.06 | 0.747 | (0.552–1.012) | 0.407 | 0.900 | (0.701–1.155) |
| HGB | 0.892 | 1.008 | (0.904–1.123) | 0.38 | 0.959 | (0.873–1.053) |
| ASPC | 0.797 | 0.999 | (0.989–1.009) | 0.413 | 0.997 | (0.988–1.005) |
| BMPS | 0.875 | 1.001 | (0.991–1.01) | 0.711 | 1.001 | (0.994–1.009) |
| MRI | 0.076 | 1.010 | (0.999–1.02) | 0.279 | 1.005 | (0.996–1.015) |
| CA | 0.001 | 0.549 | (0.388–0.777) | 0.003 | 0.645 | (0.483–0.863) |
| RACE | 0.846 | 0.948 | (0.554–1.623) | 0.116 | 1.482 | (0.908–2.420) |
| Treatment | 0.32 | 1.244 | (0.809–1.913) | 0.004 | 1.691 | (1.178–2.428) |
CRP – C-reactive protein; LDH – lactate dehydrogenase; ALB – albumin; HGB – haemoglobin; ASPC – aspirate plasma cells; BSPC – bone marrow biopsy plasma cells; CA – vytogenetic abnormalities.
ARPC5 was significantly increased in MM patients
As stated earlier, we detected the expression of ARPC5 in bone marrow mononuclear cells of MM patients and normal donors who were excluded based on hematological malignance. These 10 MM patients included 6 males and 4 females, the median age was 63.5 years; 5 cases were IgG MM, 3 cases were IgA MM, 1 case was light chain MM, and 1 case was IgD MM (Supplementary Table 1). As shown in Figure 3, mRNA levels of ARPC5 were significantly increased in the bone marrow mononuclear cells of these 10 MM patients compared with that in patients with non-malignant bone marrow mononuclear cells (0.8160±0.136 vs. 0.4167±0.074, P<0.0001).
Figure 3.
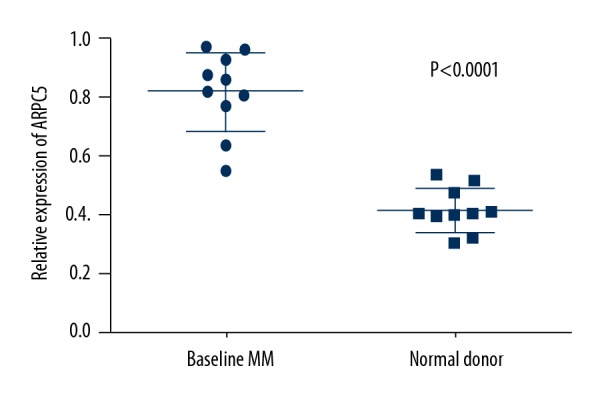
ARPC5 expression in bone marrow mononuclear cells from newly diagnosed myeloma patients and normal donors.
Results of gene set enrichment analysis
In order to identify related molecular mechanisms involved in the function of ARPC5 in MM cells, GSEA analysis was conducted using another independent MM microarray study GSE19784 (MM samples in GSE19784 were divided into ARPC5 low expression group and ARPC5 high expression group according to the median of ARPC5 expression), as shown in Figure 4, the MM samples in ARPC5 mammalian target of rapamycin (mTOR)C1signaling were: nominal P<0.0001, FDR=1.47%, enrichment score=0.57, normalized enrichment score=1.75. This result indicated that ARPC5 might promote the growth of MM cells through mTORC1 signaling.
Figure 4.
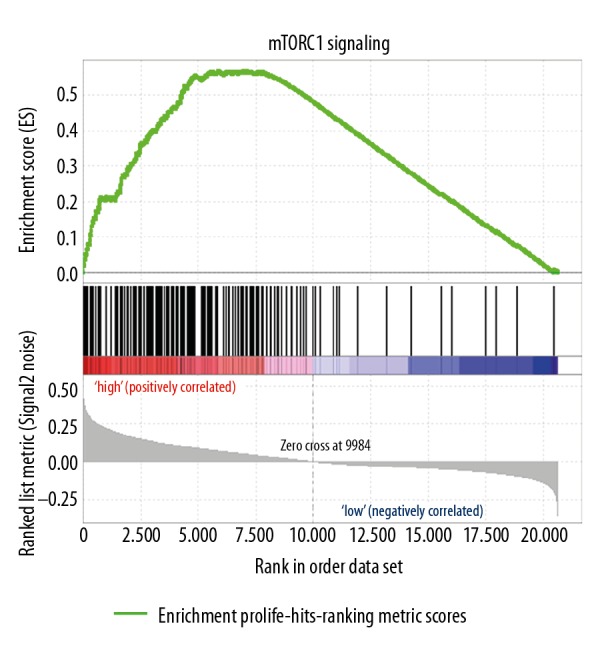
Samples in ARPC5 high expression group were significantly enrichment in mTORC1 signaling pathway.
Discussion
Although significant improvements have been made regarding the diagnosis and treatment of patients with MM, the disease remains incurable due to acquired drug resistance [23]. In the present study, we demonstrated that ARPC5 might be an independent risk factor of patients with MM.
The inclusion criteria for MM microarray studies resulted in a total number of 4 MM gene expression microarray studies that included more than 2055 participants who were selected for our analysis. GSE6477 included 73 samples of newly diagnosed MM and 15 samples of normal donor, thus, it was used to evaluate the expression of ARPC5 normal plasma cells and myeloma cells. GSE31162 included a great number of relapsed and newly diagnosed MM samples (780 relapsed MM and 258 newly diagnosed MM), thus, it was used to evaluate the expression ARPC5 in relapsed MM cells and newly diagnosed MM cells. Thanks to the large quantity of pretreatment MM samples and detailed clinical information of MM patients, GSE24080 was selected to analyze the relationship between ARPC5 expression and clinical features of patients with MM. The reason that GSE19784 was selected to perform GSEA analysis was attributed to the relative large number of MM samples in this dataset. In summary, the results of our study were based on the relative large number of participants in corresponding analyses.
Our results showed that ARPC5 was increased in baseline MM cells relative to normal plasma cells, and it was also increased in relapsed MM cells when compared with that in baseline MM cells, indicating that ARPC5 was associated with the carcinogenesis and progression of MM. The results of our correlation analysis, survival analysis, and clinical validation were in accordance with this conclusion. Results of GSEA indicated that ARPC5 might affect the proliferation of MM cells through mTORC1 signaling.
Moriya et al. suggested that the knockdown of microRNA-133a was associated with downregulation of APRC5, knockdown of ARPC5 inhibited the proliferation of lung squamous cell carcinoma and they concluded that ARPC5 might function as oncogenes in the development of lung squamous cell carcinoma [24]. Liu et al. demonstrated that microRNA-141 inhibited the proliferation of prostate cancer stem cells by regulating ARPC5 as well as other Rho GTPase family members including (CDC42, CDC42EP3, RAC1) [11]. Kinoshita et al. suggested that ARPC5 was a candidate target of miR-133a, and ARPC5 was significantly increased in head and neck squamous cell carcinoma compared with that in non-cancer tissues, and ARPC5 was also significantly increased in invasive cancer cells. Thus, they concluded that ARPC5 contributed to cancer cell migration and invasion in head and neck squamous cell carcinoma and this gene was directly regulated by miR-133a [12]. In human osteosarcoma, Bernardini et al. demonstrated that the SI-83 induced dephosphorylation of ARPC5L, a subunit of the actin related Arp2/3 complex, and the decrease of other cytoskeleton proteins [25]. These studies indicated that ARPC5 might play a role as oncogene in prostate cancer and head and neck squamous cell carcinoma.
Mammalian target of rapamycin (mTOR) represents a downstream serine/threonine kinase of the PI3K/Akt pathway that integrates signals from the tumor microenvironment to regulate multiple cellular processes, and TORC1was comprised of the mTOR catalytic subunit and 3 associated proteins, raptor, PRAS40, and mLST8/GβL [26]. Maiso et al. demonstrated that downregulation TORC1 was associated with significant suppression of the growth of myeloma cells [26]. Xiang et al. demonstrated that MK2206 combined with bufalin inhibited the proliferation and stimulated apoptosis of myeloma cells through the inhibition of the AKT/mTOR pathway [27]. Yang et al. demonstrated that polydatin inhibited cell proliferation and induced apoptosis and autophagy of myeloma cells through the mTOR/p70s6k signaling pathway [28]. These results indicated that the inhibition of mTOR signaling pathway was associated with cellular growth of MM cells. Our GSEA results indicated that APCR5 might inhibit the proliferation of MM cells through regulation of mTORC1 signaling pathway. Taking all these results into consideration, it could be speculated that ARPC5 might affect the growth of myeloma cells through regulation of mTOR signaling pathway.
Although several previous studies have evaluated the expression of some molecular using bone marrow mononuclear cells from MM patients and those conclusions have been widely accepted, our study has limitations. We analyzed the expression of ARPC5 in bone marrow mononuclear cells rather than CD138+ MM cells from MM patients; the expression of ARPC5 in bone marrow mononuclear cells might not fully reflect its expression in MM cells. Thus, the conclusions of clinical validation should be interpreted with caution, and we suggest future studies should detect ARPC5 expression in CD138+ MM cells.
Conclusions
Our results indicated that ARPC5 was increased in myeloma cells, correlated with tumor characteristics of myeloma cells, therefore, ARPC5 could be treated as an independent risk factor of patients with MM.
Supplementary Table
Supplementary Table 1.
The clinical characteristics of ten MM patients.
| Patients ID | Gender | Age | M-protein type | ISS stage | Bone marrow biopsy plasma cells (%) | Relative expression of ARPC5 |
|---|---|---|---|---|---|---|
| 1 | Female | 68 | IgA-Kap | 3A | 88 | 0.547445 |
| 2 | Male | 70 | IgA-Kap | 3A | 70 | 0.63868 |
| 3 | Male | 64 | IgA-Lam | 2B | 73 | 0.767532 |
| 4 | Female | 59 | IgD-Lam | 3B | 75 | 0.861735 |
| 5 | Male | 51 | IgG-Kap | 3B | 59 | 0.923884 |
| 6 | Female | 60 | IgG-Kap | 3A | 60 | 0.805834 |
| 7 | Female | 63 | IgG-Kap | 3A | 65 | 0.870867 |
| 8 | Male | 64 | IgG-Kap | 3A | 90 | 0.816188 |
| 9 | Male | 48 | IgG-Lam | 3A | 85 | 0.95939 |
| 10 | Male | 66 | Light Chain-Lam | 3A | 68 | 0.96821 |
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Panaroni C, Yee AJ, Raje NS. Myeloma and bone disease. Curr Osteoporos Rep. 2017;15(5):483–98. doi: 10.1007/s11914-017-0397-5. [DOI] [PubMed] [Google Scholar]
- 2.Joseph NS, Gentili S, Kaufman JL, et al. High-risk multiple myeloma: Definition and management. Clin Lymphoma Myeloma Leuk. 2017;17S:S80–87. doi: 10.1016/j.clml.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Kumar SK, Rajkumar V, Kyle RA, et al. Multiple myeloma. Nat Rev Dis Primers. 2017;3:17046. doi: 10.1038/nrdp.2017.46. [DOI] [PubMed] [Google Scholar]
- 4.Larocca A, Mina R, Gay F, et al. Emerging drugs and combinations to treat multiple myeloma. Oncotarget. 2017;8:60656–72. doi: 10.18632/oncotarget.19269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Chen J, He YA, et al. Comparing efficacy and survivals of initial treatments for elderly patients with newly diagnosed multiple myeloma: A network meta-analysis of randomized controlled trials. Onco Targets Ther. 2017;10:121–28. doi: 10.2147/OTT.S123680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, He CK, Meng X, et al. Bortezomib-based vs. non-bortezomib-based post-transplantation treatment in multiple myeloma patients: A systematic review and meta-analysis of Phase III randomized controlled trials. Onco Targets Ther. 2015;8:1459–69. doi: 10.2147/OTT.S84828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dingli D, Ailawadhi S, Bergsagel PL, et al. Therapy for relapsed multiple myeloma: Guidelines from the mayo stratification for myeloma and risk-adapted therapy. Mayo Clin Proc. 2017;92:578–98. doi: 10.1016/j.mayocp.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Lairson DR, Chan W, Du XL. Improved survival in Medicare patients with multiple myeloma: Findings from a large nationwide and population-based cohort. Med Oncol. 2017;34:153. doi: 10.1007/s12032-017-1001-7. [DOI] [PubMed] [Google Scholar]
- 9.Liu CR, Miao J, Zhang YL, et al. Effects of hypothyroidism on expression of CRMP2B and ARPC5 during development of the rat frontal cortex. Int J Biol Sci. 2013;9:209–18. doi: 10.7150/ijbs.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalhaimer P, Pollard TD. Molecular dynamics simulations of Arp2/3 complex activation. Biophys J. 2010;99:2568–76. doi: 10.1016/j.bpj.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu C, Liu R, Zhang D, et al. MicroRNA-141 suppresses prostate cancer stem cells and metastasis by targeting a cohort of pro-metastasis genes. Nat Commun. 2017;8:14270. doi: 10.1038/ncomms14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinoshita T, Nohata N, Watanabe-Takano H, et al. Actin-related protein 2/3 complex subunit 5 (ARPC5) contributes to cell migration and invasion and is directly regulated by tumor-suppressive microRNA-133a in head and neck squamous cell carcinoma. Int J Oncol. 2012;40:1770–78. doi: 10.3892/ijo.2012.1390. [DOI] [PubMed] [Google Scholar]
- 13.Popovici V, Chen W, Gallas BG, et al. Effect of training-sample size and classification difficulty on the accuracy of genomic predictors. Breast Cancer Res. 2010;12:R5. doi: 10.1186/bcr2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi L, Campbell G, Jones WD, et al. The MicroArray Quality Control (MAQC)-II study of common practices for the development and validation of microarray-based predictive models. Nat Biotechnol. 2010;28:827–38. doi: 10.1038/nbt.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlogie B, Pineda-Roman M, van Rhee F, Haessler J, Anaissie E, Hollmig K, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008;112:3115–21. doi: 10.1182/blood-2008-03-145235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta J. Total therapy 2 in treatment of multiple myeloma: Questions about gene expression profiling and treatment-related mortality. J Clin Oncol. 2011;29:e124. doi: 10.1200/JCO.2010.32.1869. author reply e125–26. [DOI] [PubMed] [Google Scholar]
- 17.van Rhee F, Szymonifka J, Anaissie E, et al. Total therapy 3 for multiple myeloma: Prognostic implications of cumulative dosing and premature discontinuation of VTD maintenance components, bortezomib, thalidomide, and dexamethasone, relevant to all phases of therapy. Blood. 2010;116:1220–27. doi: 10.1182/blood-2010-01-264333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chng WJ, Kumar S, Vanwier S, et al. Molecular dissection of hyperdiploid multiple myeloma by gene expression profiling. Cancer Res. 2007;67:2982–89. doi: 10.1158/0008-5472.CAN-06-4046. [DOI] [PubMed] [Google Scholar]
- 19.Tiedemann RE, Zhu YX, Schmidt J, et al. Kinome-wide RNAi studies in human multiple myeloma identify vulnerable kinase targets, including a lymphoid-restricted kinase, GRK6. Blood. 2010;115:1594–604. doi: 10.1182/blood-2009-09-243980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broyl A, Hose D, Lokhorst H, et al. Gene expression profiling for molecular classification of multiple myeloma in newly diagnosed patients. Blood. 2010;116:2543–53. doi: 10.1182/blood-2009-12-261032. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 23.Richardson PG, Kumar S, Laubach JP, et al. New developments in the management of relapsed/refractory multiple myeloma – the role of ixazomib. J Blood Med. 2017;8:107–21. doi: 10.2147/JBM.S102328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriya Y, Nohata N, Kinoshita T, et al. Tumor suppressive microRNA-133a regulates novel molecular networks in lung squamous cell carcinoma. J Hum Genet. 2012;57(1):38–45. doi: 10.1038/jhg.2011.126. [DOI] [PubMed] [Google Scholar]
- 25.Bernardini G, Laschi M, Serchi T, et al. Proteomics and phosphoproteomics provide insights into the mechanism of action of a novel pyrazolo[3,4-d]pyrimidine Src inhibitor in human osteosarcoma. Mol Biosyst. 2014;10(6):1305–12. doi: 10.1039/c3mb70328b. [DOI] [PubMed] [Google Scholar]
- 26.Maiso P, Liu Y, Morgan B, et al. Defining the role of TORC1/2 in multiple myeloma. Blood. 2011;118:6860–70. doi: 10.1182/blood-2011-03-342394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang RF, Wang Y, Zhang N, et al. MK2206 enhances the cytocidal effects of bufalin in multiple myeloma by inhibiting the AKT/mTOR pathway. Cell Death Dis. 2017;8:e2776. doi: 10.1038/cddis.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang B, Zhao S. Polydatin regulates proliferation, apoptosis and autophagy in multiple myeloma cells through mTOR/p70s6k pathway. Onco Targets Ther. 2017;10:935–44. doi: 10.2147/OTT.S123398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang XT, He YC, Zhou SY, et al. Bone marrow plasma macrophage inflammatory protein protein-1 alpha(MIP-1 alpha) and sclerostin in multiple myeloma: Relationship with bone disease and clinical characteristics. Leuk Res. 2014;38(5):525–31. doi: 10.1016/j.leukres.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Ma Y, Liu W, Zhang L, Jia G. Effects of histone deacetylase inhibitor panobinostat (LBH589) on bone marrow mononuclear cells of relapsed or refractory multiple myeloma patients and its mechanisms. Med Sci Monit. 2017;23:5150–57. doi: 10.12659/MSM.904232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan J, Sun Y, Zhang N, et al. Characteristics of BAFF and APRIL factor expression in multiple myeloma and clinical significance. Oncol Lett. 2017;14(3):2657–62. doi: 10.3892/ol.2017.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
The clinical characteristics of ten MM patients.
| Patients ID | Gender | Age | M-protein type | ISS stage | Bone marrow biopsy plasma cells (%) | Relative expression of ARPC5 |
|---|---|---|---|---|---|---|
| 1 | Female | 68 | IgA-Kap | 3A | 88 | 0.547445 |
| 2 | Male | 70 | IgA-Kap | 3A | 70 | 0.63868 |
| 3 | Male | 64 | IgA-Lam | 2B | 73 | 0.767532 |
| 4 | Female | 59 | IgD-Lam | 3B | 75 | 0.861735 |
| 5 | Male | 51 | IgG-Kap | 3B | 59 | 0.923884 |
| 6 | Female | 60 | IgG-Kap | 3A | 60 | 0.805834 |
| 7 | Female | 63 | IgG-Kap | 3A | 65 | 0.870867 |
| 8 | Male | 64 | IgG-Kap | 3A | 90 | 0.816188 |
| 9 | Male | 48 | IgG-Lam | 3A | 85 | 0.95939 |
| 10 | Male | 66 | Light Chain-Lam | 3A | 68 | 0.96821 |